Expression of Growth Hormone-Releasing Hormone and Its Receptor Splice Variants in a Cohort of Hungarian Pediatric Patients with Hematological and Oncological Disorders: A Pilot Study
Abstract
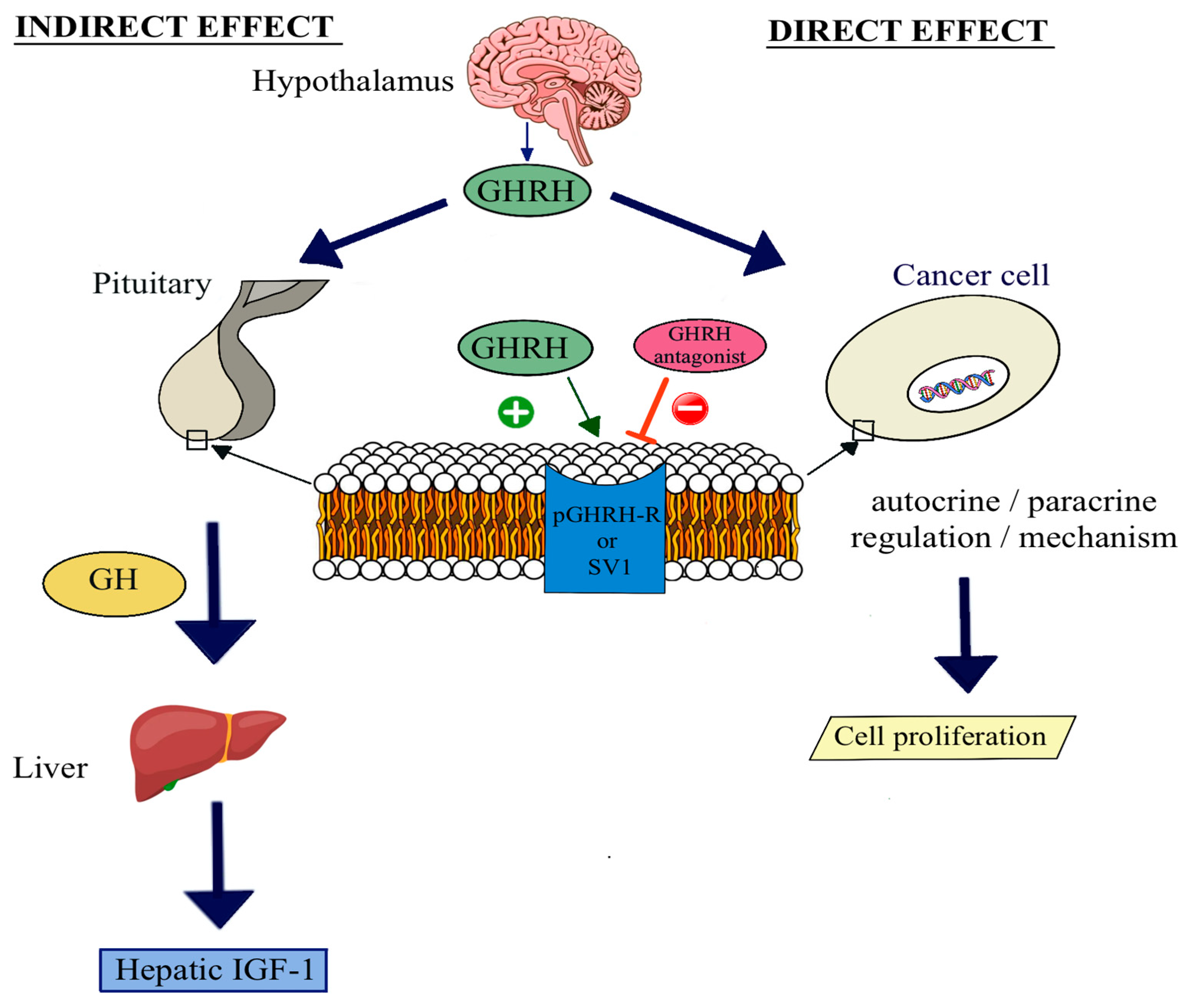
:1. Introduction
2. Results
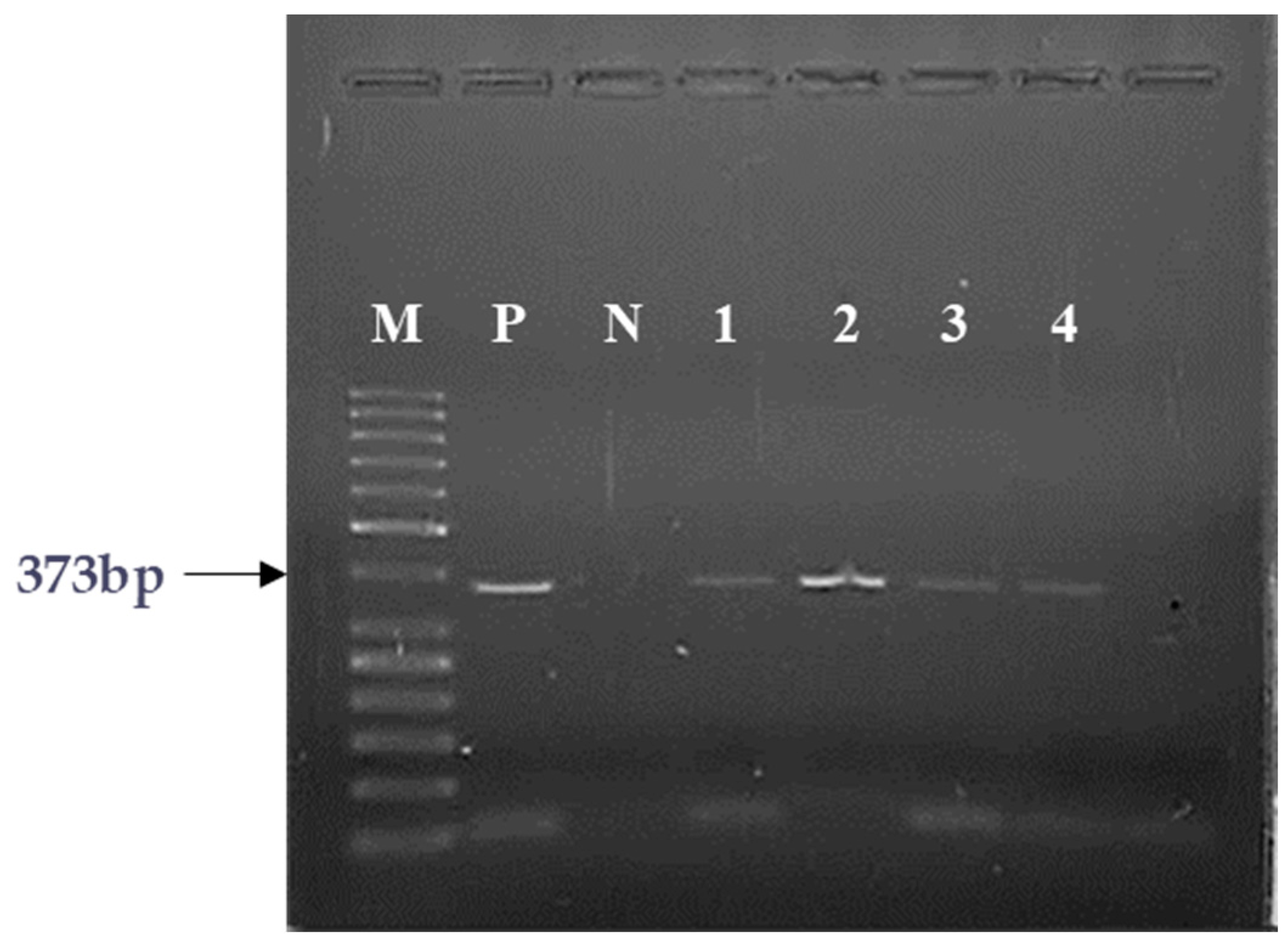
2.1. PCR Analyses of the Expression of GHRH and SV1 of GHRH-Rs
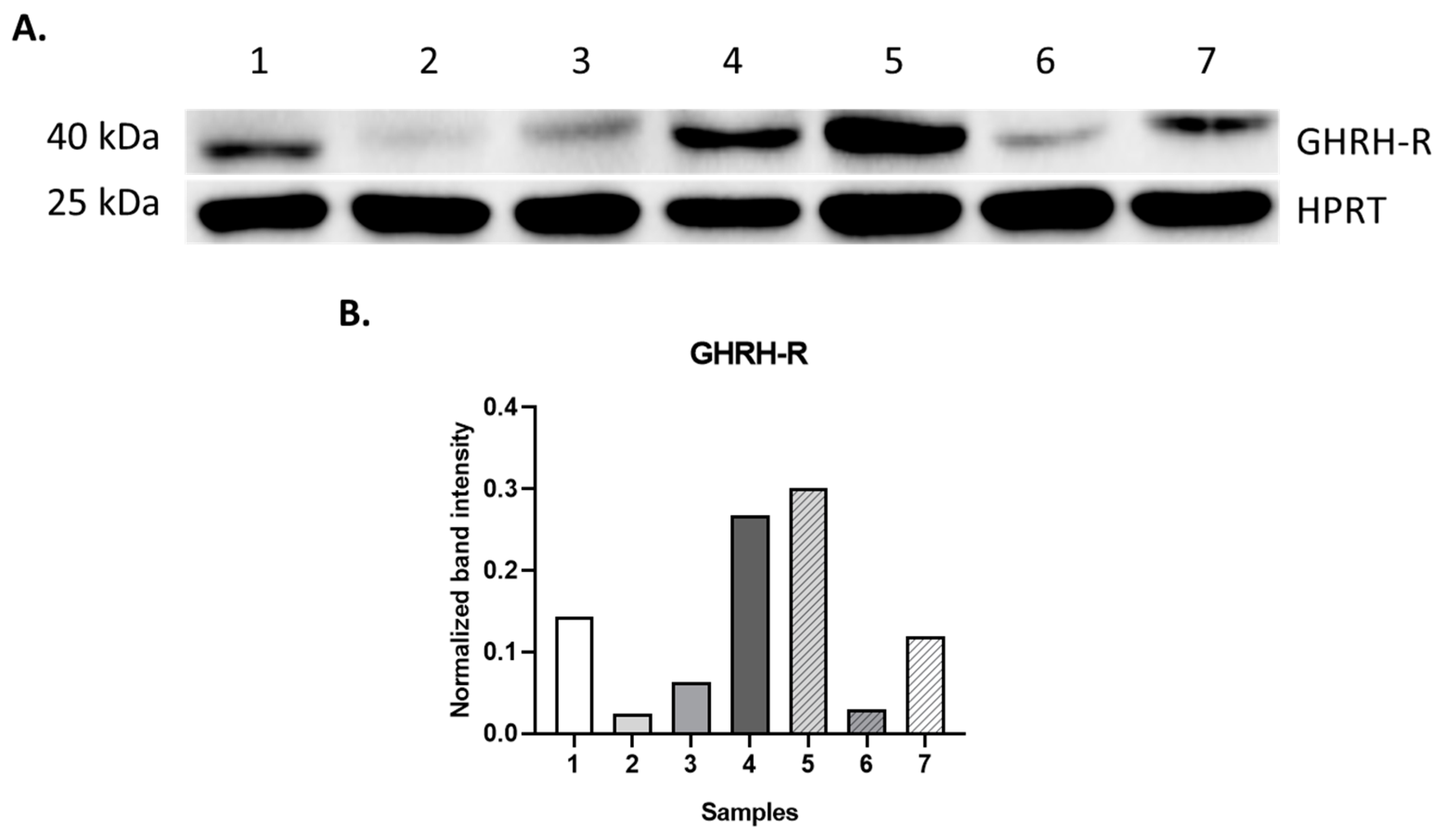
2.2. Western Blot Analysis
2.3. GHRH Receptor Binding Studies
3. Discussion
4. Materials and Methods
4.1. Sample Collecting
4.2. RNA Isolation and Reverse Transcription Reaction (RT-PCR)
4.3. Reverse Transcription PCR (RT-PCR)
4.4. RT-PCR Reaction for GHRH and SV1 of GHRH-R
4.5. Western Blot Analysis of GHRH-R Protein
4.6. Preparation of Membranes and Radioligand Binding Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schally, A.V.; Varga, J.L.; Engel, J.B. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nature clinical practice. Endocrinol. Metab. 2008, 4, 33–43. [Google Scholar]
- Mayo, K.E. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol. Endocrinol. 1992, 6, 1734–1744. [Google Scholar] [PubMed]
- Lin-Su, K.; Wajnrajch, M.P. Growth Hormone Releasing Hormone (GHRH) and the GHRH Receptor. Rev. Endocr. Metab. Disord. 2002, 3, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Comaru-Schally, A.M.; Nagy, A.; Kovacs, M.; Szepeshazi, K.; Plonowski, A.; Varga, J.L.; Halmos, G. Hypothalamic hormones and cancer. Front. Neuroendocrinol. 2001, 22, 248–291. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Unniappan, S. A Comparative Update on the Neuroendocrine Regulation of Growth Hormone in Vertebrates. Front. Endocrinol. 2020, 11, 614981. [Google Scholar] [CrossRef]
- Ranke, M.B.; Wit, J.M. Growth hormone-past, present and future. Nat. Rev. Endocrinol. 2018, 14, 285–300. [Google Scholar] [CrossRef]
- Halmos, G.; Szabo, Z.; Juhasz, E.; Schally, A.V. Signaling mechanism of growth hormone-releasing hormone receptor. Vitam. Horm. 2023, 123, 1–26. [Google Scholar]
- Siejka, A.; Lawnicka, H.; Melen-Mucha, G.; Motylewska, E.; Komorowski, J.; Stepien, H. Antineoplastic action of growth hormone-releasing hormone (GHRH) antagonists. Recent. Pat. Anticancer Drug Discov. 2012, 7, 56–63. [Google Scholar] [CrossRef]
- Xiong, X.; Ke, X.; Wang, L.; Yao, Z.; Guo, Y.; Zhang, X.; Chen, Y.; Pang, C.P.; Schally, A.V.; Zhang, H. Splice variant of growth hormone-releasing hormone receptor drives esophageal squamous cell carcinoma conferring a therapeutic target. Proc. Natl. Acad. Sci. USA 2020, 117, 6726–6732. [Google Scholar] [CrossRef]
- Cong, Z.; Zhou, F.; Zhang, C.; Zou, X.; Zhang, H.; Wang, Y.; Zhou, Q.; Cai, X.; Liu, Q.; Li, J.; et al. Constitutive signal bias mediated by the human GHRHR splice variant 1. Proc. Natl. Acad. Sci. USA 2021, 118, e2106606118. [Google Scholar] [CrossRef]
- Szabo, Z.; Juhasz, E.; Schally, A.V.; Dezso, B.; Huga, S.; Hernadi, Z.; Halmos, G.; Kiss, C. Expression of Growth Hormone-Releasing Hormone and Its Receptor Splice Variants in Primary Human Endometrial Carcinomas: Novel Therapeutic Approaches. Molecules 2022, 27, 2671. [Google Scholar] [CrossRef] [PubMed]
- Rekasi, Z.; Czompoly, T.; Schally, A.V.; Halmos, G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc. Natl. Acad. Sci. USA 2000, 97, 10561–10566. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Siegel, D.A.; King, J.B.; Lupo, P.J.; Durbin, E.B.; Tai, E.; Mills, K.; Van Dyne, E.; Buchanan Lunsford, N.; Henley, S.J.; Wilson, R.J. Counts, incidence rates, and trends of pediatric cancer in the United States, 2003–2019. J. Natl. Cancer Inst. 2023, 115, 1337–1354. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Pediatric Cancers: Insights and Novel Therapeutic Approaches. Cancers 2023, 15, 3537. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, S.; Linabery, A.M.; Charbonneau, B.; Ross, J.A. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975-2005. Cancer 2009, 115, 4218–4226. [Google Scholar] [CrossRef]
- Coppit, G.L., 3rd; Perkins, J.A.; Manning, S.C. Nasopharyngeal teratomas and dermoids: A review of the literature and case series. Int. J. Pediatr. Otorhinolaryngol. 2000, 52, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Esparza, S.D.; Sakamoto, K.M. Topics in pediatric leukemia—Acute lymphoblastic leukemia. MedGenMed Medscape Gen. Med. 2005, 7, 23. [Google Scholar]
- Bernt, K.M.; Hunger, S.P. Current concepts in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia. Front. Oncol. 2014, 4, 54. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Feller, L.; Wood, N.H.; Khammissa, R.A.; Lemmer, J.; Raubenheimer, E.J. The nature of fibrous dysplasia. Head. Face Med. 2009, 5, 22. [Google Scholar] [CrossRef]
- Khan, M.R.; Binkovitz, L.A.; Smyrk, T.C.; Potter, D.D., Jr.; Furuya, K.N. Mesenchymal Hamartoma in Children: A Diagnostic Challenge. Case Rep. Pediatr. 2019, 2019, 4132842. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, J.; Cao, X.; Yang, M.; Zhu, J.; Zhao, G. Solitary infantile myofibromatosis in the bones of the upper extremities: Two rare cases and a review of the literature. Oncol. Lett. 2013, 6, 1406–1408. [Google Scholar] [CrossRef] [PubMed]
- Mashiah, J.; Hadj-Rabia, S.; Dompmartin, A.; Harroche, A.; Laloum-Grynberg, E.; Wolter, M.; Amoric, J.C.; Hamel-Teillac, D.; Guero, S.; Fraitag, S.; et al. Infantile myofibromatosis: A series of 28 cases. J. Am. Acad. Dermatol. 2014, 71, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Letelier, C.; Gunther, M.; Alarcon, A.; Vera, P.; Kakarieka, E.; Pantoja, R. Agressive pediatric myofibromatosis in a two-year-old child. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 216–219. [Google Scholar] [CrossRef]
- Larralde, M.; Ferrari, B.; Martinez, J.P.; Barbieri, M.A.F.; Méndez, J.H.; Casas, J. Infantile myofibromatosis. An. Bras. Dermatol. 2017, 92, 854–857. [Google Scholar] [CrossRef]
- James, R.M.; Kinsey, S.E. The investigation and management of chronic neutropenia in children. Arch. Dis. Child. 2006, 91, 852–858. [Google Scholar] [CrossRef]
- Dale, D.C. How I manage children with neutropenia. Br. J. Haematol. 2017, 178, 351–363. [Google Scholar] [CrossRef]
- Terrell, D.R.; Beebe, L.A.; Vesely, S.K.; Neas, B.R.; Segal, J.B.; George, J.N. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am. J. Hematol. 2010, 85, 174–180. [Google Scholar] [CrossRef]
- Munir, F.; Hardit, V.; Sheikh, I.N.; AlQahtani, S.; He, J.; Cuglievan, B.; Hosing, C.; Tewari, P.; Khazal, S. Classical Hodgkin Lymphoma: From Past to Future-A Comprehensive Review of Pathophysiology and Therapeutic Advances. Int. J. Mol. Sci. 2023, 24, 10095. [Google Scholar] [CrossRef] [PubMed]
- Garami, M.; Schuler, D.; Jakab, Z. Importance of the National Childhood Cancer Registry in the field of paediatric oncology care in Hungary. Orv. Hetil. 2014, 155, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; DelCanto, G.M.; Popovics, P.; Perez, A.; Vila Granda, A.; Vidaurre, I.; Cai, R.Z.; Rick, F.G.; Swords, R.T.; Schally, A.V. A new approach to the treatment of acute myeloid leukaemia targeting the receptor for growth hormone-releasing hormone. Br. J. Haematol. 2018, 181, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.; Ludwig, K.; Pacenta, H.L.; Klesse, L.J.; Watt, T.C.; Laetsch, T.W. Recent progress in the treatment of cancer in children. CA Cancer J. Clin. 2021, 71, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Adamczewska-Wawrzynowicz, K.; Wiącek, A.; Kozłowska, A.; Mikosza, K.; Szefler, L.; Dudlik, W.; Dey, S.; Varghese, N.; Derwich, K. Modern treatment strategies in pediatric oncology and hematology. Discov. Oncol. 2023, 14, 98. [Google Scholar] [CrossRef]
- Helms, L.; Guimera, A.E.; Janeway, K.A.; Bailey, K.M. Innovations in Cancer Treatment of Children. Pediatrics 2023, 152, e2023061539. [Google Scholar] [CrossRef]

| Models Investigated | |
|---|---|
| Breast | Renal |
| Ovarian | Osteosarcoma |
| Endometrial | Ewing sarcoma |
| Prostate | Glioblastoma |
| Lung | Esophageal squamous cell carcinoma |
| Pancreatic | Pleural mesothelioma |
| Colorectal | Acute myeloid leukemia |
| Sample | Clinical Condition | GHRH mRNA | SV1 mRNA |
|---|---|---|---|
| 1 | HL | + | + |
| 2 | RMS | + | + |
| 3 | RMS | + | + |
| 4 | TR/adenocarcinoma components | + | + |
| 5 | ALL | + | + |
| 6 | ALL | + | + |
| 7 | ALL | + | + |
| 8 | ALL | − | - |
| 9 | ALL | + | + |
| 10 | BMH | + | + |
| 11 | FD | + | + |
| 12 | JM | + | + |
| 13 | CBN | − | − |
| 14 | HS | − | − |
| 15 | ITP | − | − |
| Sample | Clinical Condition | GHRH-R Protein | GHRH-R Binding | |
|---|---|---|---|---|
| Kd (nM) | Bmax (fmol/mg Prot.) | |||
| 1 | HL | + | 1.37 | 398.7 |
| 2 | RMS | + | 3.84 | 222.1 |
| 3 | RMS | + | 4.38 | 245.0 |
| 4 | TR/adenocarcinoma components | + | 8.99 | 475.0 |
| 5 | ALL | N/A | N/A | |
| 6 | ALL | N/A | N/A | |
| 7 | ALL | N/A | N/A | |
| 8 | ALL | N/A | N/A | |
| 9 | ALL | N/A | N/A | |
| 10 | BMH | + | 7.27 | 733.0 |
| 11 | FD | + | 4.81 | 261.4 |
| 12 | JM | + | 1.35 | 294.6 |
| 13 | CBN | N/A | N/A | |
| 14 | HS | N/A | N/A | |
| 15 | ITP | N/A | N/A | |
| Sample | Clinical Condition | Sex | Sample Origin | Age at Sampling | Disease Outcome |
|---|---|---|---|---|---|
| 1 | HL | F | Lymph node | 15 years | Alive |
| 2 | RMS | M | Pelvic tumor tissue | 12.5 years | † Died |
| 3 | RMS | F | Neck mass biopsy | 8 years | † Died |
| 4 | TR/adenocarcinoma components | M | Mediastinal tumor tissue | 14.5 years | † Died |
| 5 | ALL | F | Bone marrow | 5 years | Alive |
| 6 | ALL | M | Bone marrow | 13 years | Alive |
| 7 | ALL | M | Bone marrow | 9.5 years | † Died |
| 8 | ALL | M | Bone marrow | 6.5 years | Alive |
| 9 | ALL | M | Peripheral blood | 15 years | † Died |
| 10 | BMH | M | Mediastinal mass | 4 years | Alive |
| 11 | FD | F | Bone tumor tissue | 10.5 years | Alive |
| 12 | JM | M | Right pelvic bone tumor tissue | 2 years | Alive |
| 13 | CBN | M | Bone marrow | 12.5 months | Alive |
| 14 | HS | F | Bone marrow | 9 months | Alive |
| 15 | ITP | M | Bone marrow | 10 months | Alive |
| Primers | Forward Sequences | Reverse Sequences | Tm (°C) Annealing Temperature | Product Size (bp) |
|---|---|---|---|---|
| ACTB | 5′-GGCATCCTCACCCCCTGAAGTA-3′ | 5′-GGGGTGAAGGTCTCAAA-3′ | 60 °C | 203 bp |
| GHRH | 5′-GGAGTTGTGGCTAGAGAG-3′ | 5′-TGTCTGTCTACCTGACGACCAA-3′ | 64 °C | 150 bp |
| SV1 | 5′-GGAGTTGTGGCTAGAGAG-3′ | 5′-GCATAGAACAGTGGAGAAG-3′ | 67 °C | 373 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, É.; Szabó, Z.; Schally, A.V.; Király, J.; Fodor, P.; Kónya, G.; Dezső, B.; Szabó, E.; Halmos, G.; Kiss, C. Expression of Growth Hormone-Releasing Hormone and Its Receptor Splice Variants in a Cohort of Hungarian Pediatric Patients with Hematological and Oncological Disorders: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 8831. https://doi.org/10.3390/ijms25168831
Juhász É, Szabó Z, Schally AV, Király J, Fodor P, Kónya G, Dezső B, Szabó E, Halmos G, Kiss C. Expression of Growth Hormone-Releasing Hormone and Its Receptor Splice Variants in a Cohort of Hungarian Pediatric Patients with Hematological and Oncological Disorders: A Pilot Study. International Journal of Molecular Sciences. 2024; 25(16):8831. https://doi.org/10.3390/ijms25168831
Chicago/Turabian StyleJuhász, Éva, Zsuzsanna Szabó, Andrew V. Schally, József Király, Petra Fodor, Gábor Kónya, Balázs Dezső, Erzsébet Szabó, Gábor Halmos, and Csongor Kiss. 2024. "Expression of Growth Hormone-Releasing Hormone and Its Receptor Splice Variants in a Cohort of Hungarian Pediatric Patients with Hematological and Oncological Disorders: A Pilot Study" International Journal of Molecular Sciences 25, no. 16: 8831. https://doi.org/10.3390/ijms25168831








