Obesity-Associated Colorectal Cancer
Abstract
1. Introduction
2. Obesity-Associated Colorectal Cancer Epidemiology
3. Risk Factors for Obesity-Associated Colorectal Cancer
4. Molecular Factors Involved in the Development and Progression of Obesity-Associated Colorectal Cancer
4.1. Genome Instability and Mutation and Non-Mutational Epigenetic Reprogramming
4.2. Enabling Replicative Immortality
4.3. Sustaining Proliferative Signaling
4.4. Deregulating Cellular Energetics
4.5. Promoting Tumor Inflammation
4.6. Inducing Angiogenesis and Activating Invasion and Metastasis
4.7. Polymorphic Microbiomes
5. Current Treatments of Obesity-Related Colorectal Cancer: Perspective and Challenges
5.1. Surgery and Radiotherapy
5.2. Chemotherapy
5.2.1. Fluoropyrimidines
5.2.2. Topoisomerase I Inhibitors
5.2.3. Platinum-Based Drugs
5.3. Combined Therapies
5.4. Targeted Therapy
5.4.1. VEGF Inhibitors
5.4.2. BRAF Inhibitors
5.4.3. EGFR Inhibitors
5.4.4. Immune Checkpoint Inhibitors
6. New Targets for Obesity-Associated Colorectal Cancer
6.1. Thiazolidinediones
6.2. Metformin
6.3. Sulfonylureas
6.4. DPPA Inhibitors and the GLP-1 Mimetic Semaglutide
6.5. Alpha-Glucosidase Inhibitor
6.6. SGLT2 Inhibitors
6.7. Statins
6.8. Fenofibrates
6.9. Leptin Inhibitors
6.10. Rapalogues and PI3K/AKT Inhibitors
6.11. ACBP/DBI Blocks
6.12. Pre-Pro Biotics/FMT
6.13. Epigenetic Therapies
7. Impact of Dietary Compounds on the Prevention and Treatment of Obesity-Associated Colorectal Cancer
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Atchade, A.M.; Williams, J.L.; Mermelstein, L.; Nemesure, B. Unraveling the complexities of early-onset colorectal cancer: A perspective on dietary and microbial influences. Front. Public Health 2024, 12, 1370108. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary patterns and risk of colorectal cancer: Analysis by tumor location and molecular subtypes. Gastroenterology 2017, 152, 1944–1953.e1. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2024).
- Collaborators, G.B.D.R.F. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar]
- Jacobs, E.T.; Ahnen, D.J.; Ashbeck, E.L.; Baron, J.A.; Greenberg, E.R.; Lance, P.; Lieberman, D.A.; McKeown-Eyssen, G.; Schatzkin, A.; Thompson, P.A.; et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: A pooling study. Am. J. Epidemiol. 2009, 169, 657–666. [Google Scholar] [CrossRef]
- Seo, J.Y.; Jin, E.H.; Chung, G.E.; Kim, Y.S.; Bae, J.H.; Yim, J.Y.; Han, K.D.; Yang, S.Y. The risk of colorectal cancer according to obesity status at four-year intervals: A nationwide population-based cohort study. Sci. Rep. 2023, 13, 8928. [Google Scholar] [CrossRef]
- Wilson, R.B.; Lathigara, D.; Kaushal, D. Systematic review and meta-analysis of the impact of bariatric surgery on future cancer risk. Int. J. Mol. Sci. 2023, 24, 6192. [Google Scholar] [CrossRef]
- Liu, Y.N.; Gu, J.F.; Zhang, J.; Xing, D.Y.; Wang, G.Q. Bariatric surgery reduces colorectal cancer incidence in obese individuals: Systematic review and meta-analysis. World J. Gastrointest. Surg. 2023, 15, 2331–2342. [Google Scholar] [CrossRef]
- Chierici, A.; Amoretti, P.; Drai, C.; De Fatico, S.; Barriere, J.; Schiavo, L.; Iannelli, A. Does bariatric surgery reduce the risk of colorectal cancer in individuals with morbid obesity? A systematic review and meta-analysis. Nutrients 2023, 15, 467. [Google Scholar] [CrossRef] [PubMed]
- Pitot, H.C. The molecular biology of carcinogenesis. Cancer 1993, 72, 962–970. [Google Scholar] [CrossRef]
- Duan, B.; Zhao, Y.; Bai, J.; Wang, J.; Duan, X.; Luo, X.; Zhang, R.; Pu, Y.; Kou, M.; Lei, J.; et al. Colorectal cancer: An overview. In Gastrointestinal Cancers; Morgado-Diaz, J.A., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Kim, S.E.; Paik, H.Y.; Yoon, H.; Lee, J.E.; Kim, N.; Sung, M.K. Sex- and gender-specific disparities in colorectal cancer risk. World J. Gastroenterol. 2015, 21, 5167–5175. [Google Scholar] [CrossRef]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal cancer: Epidemiology, risk factors, and prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Im, J.P.; Kim, D.; Han, Y.M.; Soh, H.; Song, J.H.; Yang, S.Y.; Kim, Y.S.; Yim, J.Y.; Lim, S.H.; et al. Increasing changes in visceral adiposity is associated with higher risk for colorectal adenoma: Multilevel analysis in a prospective cohort. J. Gastroenterol. Hepatol. 2021, 36, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Im, J.P.; Kim, D.; Chung, S.J.; Jin, E.H.; Han, Y.M.; Park, M.J.; Song, J.H.; Yang, S.Y.; Kim, Y.S.; Yim, J.Y.; et al. Visceral obesity as a risk factor for colorectal adenoma occurrence in surveillance colonoscopy. Gastrointest. Endosc. 2018, 88, 119–127.e4. [Google Scholar] [CrossRef]
- Kang, H.W.; Kim, D.; Kim, H.J.; Kim, C.H.; Kim, Y.S.; Park, M.J.; Kim, J.S.; Cho, S.H.; Sung, M.W.; Jung, H.C.; et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: A cross-sectional, case-control study. Am. J. Gastroenterol. 2010, 105, 178–187. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Kim, R.; Greenwood, D.C.; Giovannucci, E.L. Visceral adiposity and colorectal adenomas: Dose-response meta-analysis of observational studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1101–1109. [Google Scholar] [CrossRef]
- Ben, Q.; An, W.; Jiang, Y.; Zhan, X.; Du, Y.; Cai, Q.C.; Gao, J.; Li, Z. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 2012, 142, 762–772. [Google Scholar] [CrossRef]
- Yun, K.E.; Chang, Y.; Jung, H.S.; Kim, C.W.; Kwon, M.J.; Park, S.K.; Sung, E.; Shin, H.; Park, H.S.; Ryu, S. Impact of body mass index on the risk of colorectal adenoma in a metabolically healthy population. Cancer Res. 2013, 73, 4020–4027. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.H.; Min, Y.W.; Son, H.J.; Rhee, P.L.; Paik, S.W.; Hong, S.N.; Gwak, G.Y. Metabolically-healthy obesity is associated with higher prevalence of colorectal adenoma. PLoS ONE 2017, 12, e0179480. [Google Scholar] [CrossRef]
- Carethers, J.M.; Jung, B.H. Genetics and genetic biomarkers in sporadic colorectal cancer. Gastroenterology 2015, 149, 1177–1190.e3. [Google Scholar] [CrossRef]
- Rex, D.K.; Ponugoti, P.L. Calculating the adenoma detection rate in screening colonoscopies only: Is it necessary? Can it be gamed? Endoscopy 2017, 49, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Vasen, H.F.; van der Meulen-de Jong, A.E.; de Vos Tot Nederveen Cappel, W.H.; Oliveira, J.; Group, E.G.W. Familial colorectal cancer risk: Esmo clinical recommendations. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20 (Suppl. S4), 51–53. [Google Scholar] [CrossRef]
- Vasen, H.F.; Boland, C.R. Progress in genetic testing, classification, and identification of lynch syndrome. JAMA 2005, 293, 2028–2030. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Bishop, D.T.; Macrae, F.; Mecklin, J.P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L.; et al. Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: A prospective investigation in the capp2 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3591–3597. [Google Scholar] [CrossRef]
- Lazzeroni, M.; Bellerba, F.; Calvello, M.; Macrae, F.; Win, A.K.; Jenkins, M.; Serrano, D.; Marabelli, M.; Cagnacci, S.; Tolva, G.; et al. A meta-analysis of obesity and risk of colorectal cancer in patients with lynch syndrome: The impact of sex and genetics. Nutrients 2021, 13, 1736. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Boardman, L.A.; Thibodeau, S.N.; Schaid, D.J.; Lindor, N.M.; McDonnell, S.K.; Burgart, L.J.; Ahlquist, D.A.; Podratz, K.C.; Pittelkow, M.; Hartmann, L.C. Increased risk for cancer in patients with the peutz-jeghers syndrome. Ann. Intern. Med. 1998, 128, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Greene, M.W.; Abraham, P.T.; Kuhlers, P.C.; Lipke, E.A.; Heslin, M.J.; Wijaya, S.T.; Odeniyi, I. Consensus molecular subtype differences linking colon adenocarcinoma and obesity revealed by a cohort transcriptomic analysis. PLoS ONE 2022, 17, e0268436. [Google Scholar] [CrossRef]
- Capece, D.; Franzoso, G. Rewired lipid metabolism as an actionable vulnerability of aggressive colorectal carcinoma. Mol. Cell. Oncol. 2022, 9, 2024051. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef]
- Sanchez, N.F.; Stierman, B.; Saab, S.; Mahajan, D.; Yeung, H.; Francois, F. Physical activity reduces risk for colon polyps in a multiethnic colorectal cancer screening population. BMC Res. Notes 2012, 5, 312. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.S.; Yang, S.Y.; Chung, S.J.; Park, M.J.; Lim, S.H.; Yim, J.Y.; Kim, J.S.; Jung, H.C. Physical activity and other lifestyle factors in relation to the prevalence of colorectal adenoma: A colonoscopy-based study in asymptomatic Koreans. Cancer Causes Control CCC 2013, 24, 1717–1726. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.; Kim, J.E.; Lee, M.; Kang, D.; Shin, A.; Choi, J.Y. Associations between physical activity and incidence of cancer among overweight adults in korea: Results from the health examinees-g study. Cancer Prev. Res. 2023, 16, 405–418. [Google Scholar] [CrossRef]
- Sun, M.; Bjorge, T.; Teleka, S.; Engeland, A.; Wennberg, P.; Haggstrom, C.; Stocks, T. Interaction of leisure-time physical activity with body mass index on the risk of obesity-related cancers: A pooled study. Int. J. Cancer 2022, 151, 859–868. [Google Scholar] [CrossRef]
- Haydon, A.M.; Macinnis, R.J.; English, D.R.; Giles, G.G. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 2006, 55, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Baena, R.; Salinas, P. Diet and colorectal cancer. Maturitas 2015, 80, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.S.; Kirsh, V.A.; Rohan, T.E. The association of the healthy eating index with risk of colorectal cancers (overall and by subsite) among Canadians. Cancer Epidemiol. 2023, 87, 102454. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Paragomi, P.; Wang, R.; Jin, A.; Schoen, R.E.; Sheng, L.T.; Pan, A.; Koh, W.P.; Yuan, J.M.; Luu, H.N. Composite dietary antioxidant index and the risk of colorectal cancer: Findings from the singapore chinese health study. Int. J. Cancer 2022, 150, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Fasanelli, F.; Zugna, D.; Giraudo, M.T.; Krogh, V.; Grioni, S.; Panico, S.; Mattiello, A.; Masala, G.; Caini, S.; Tumino, R.; et al. Abdominal adiposity is not a mediator of the protective effect of mediterranean diet on colorectal cancer. Int. J. Cancer 2017, 140, 2265–2271. [Google Scholar] [CrossRef] [PubMed]
- Bamia, C.; Lagiou, P.; Buckland, G.; Grioni, S.; Agnoli, C.; Taylor, A.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjonneland, A.; et al. Mediterranean diet and colorectal cancer risk: Results from a european cohort. Eur. J. Epidemiol. 2013, 28, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Torres Stone, R.A.; Waring, M.E.; Cutrona, S.L.; Kiefe, C.I.; Allison, J.; Doubeni, C.A. The association of dietary quality with colorectal cancer among normal weight, overweight and obese men and women: A prospective longitudinal study in the USA. BMJ Open 2017, 7, e015619. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Social determinants of colorectal cancer risk, stage, and survival: A systematic review. Int. J. Color. Dis. 2020, 35, 985–995. [Google Scholar] [CrossRef]
- Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal cancer: Disease process, current treatment options, and future perspectives. Pharmaceutics 2023, 15, 2620. [Google Scholar] [CrossRef]
- Johansen, M.P.; Wewer, M.D.; Nordholm-Carstensen, A.; Burisch, J. Perianal crohn’s disease and the development of colorectal and anal cancer: A systematic review and meta-analysis. J. Crohn’s Colitis 2023, 17, 361–368. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Shao, L. Correlation of ulcerative colitis and colorectal cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2021, 12, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctology 2019, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Yang, C.M.; Shi, B.M. Body fatness at an early age and risk of colorectal cancer. Int. J. Cancer 2018, 142, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Hawwash, N.; Martin, G.P.; Sperrin, M.; Renehan, A.G. Link between obesity and early-onset colorectal cancers (eocrc): Importance of accounting for bmi trajectories in early life. Am. J. Gastroenterol. 2022, 117, 812. [Google Scholar] [CrossRef] [PubMed]
- Mandic, M.; Li, H.; Safizadeh, F.; Niedermaier, T.; Hoffmeister, M.; Brenner, H. Is the association of overweight and obesity with colorectal cancer underestimated? An umbrella review of systematic reviews and meta-analyses. Eur. J. Epidemiol. 2023, 38, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Nimptsch, K.; Pischon, T. Obesity and colorectal cancer. Front. Biosci. 2013, 5, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Rodriguez, R.M.; Segura-Sampedro, J.J.; Ochogavia-Segui, A.; Romaguera, D.; Barcelo-Coblijn, G. Insights behind the relationship between colorectal cancer and obesity: Is visceral adipose tissue the missing link? Int. J. Mol. Sci. 2022, 23, 13128. [Google Scholar] [CrossRef]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Madka, V.; Chiliveru, S.; Panneerselvam, J.; Pathuri, G.; Zhang, Y.; Stratton, N.; Kumar, N.; Sanghera, D.K.; Rao, C.V. Targeting il-23 for the interception of obesity-associated colorectal cancer. Neoplasia 2023, 45, 100939. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Loidl, A.; Hermetter, A. Oxidized phospholipids: From molecular properties to disease. Biochim. Biophys. Acta 2007, 1772, 718–736. [Google Scholar] [CrossRef]
- West, J.D.; Ji, C.; Duncan, S.T.; Amarnath, V.; Schneider, C.; Rizzo, C.J.; Brash, A.R.; Marnett, L.J. Induction of apoptosis in colorectal carcinoma cells treated with 4-hydroxy-2-nonenal and structurally related aldehydic products of lipid peroxidation. Chem. Res. Toxicol. 2004, 17, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-hne) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hu, W.; Tang, M.S. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: A possible mechanism for lipid peroxidation-induced carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 8598–8602. [Google Scholar] [CrossRef]
- Zarrouki, B.; Soares, A.F.; Guichardant, M.; Lagarde, M.; Geloen, A. The lipid peroxidation end-product 4-hne induces cox-2 expression through p38mapk activation in 3t3-l1 adipose cell. FEBS Lett. 2007, 581, 2394–2400. [Google Scholar] [CrossRef]
- Martinez-Useros, J.; Garcia-Foncillas, J. Obesity and colorectal cancer: Molecular features of adipose tissue. J. Transl. Med. 2016, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Niedernhofer, L.J.; Daniels, J.S.; Rouzer, C.A.; Greene, R.E.; Marnett, L.J. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003, 278, 31426–31433. [Google Scholar] [CrossRef]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Ahechu, P.; Zozaya, G.; Marti, P.; Hernandez-Lizoain, J.L.; Baixauli, J.; Unamuno, X.; Fruhbeck, G.; Catalan, V. Nlrp3 inflammasome: A possible link between obesity-associated low-grade chronic inflammation and colorectal cancer development. Front. Immunol. 2018, 9, 2918. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Morcillo, S.; Clemente-Postigo, M.; Crujeiras, A.B.; Fernandez-Garcia, J.C.; Torres, E.; Tinahones, F.J.; Macias-Gonzalez, M. Adipose tissue inflammation and vdr expression and methylation in colorectal cancer. Clin. Epigenet. 2018, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.T. Roles of nf-kappab in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and cancer: Existing and new hypotheses for a causal connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. Stats in cancer inflammation and immunity: A leading role for stat3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The role of jnk signaling pathway in obesity-driven insulin resistance. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1399–1406. [Google Scholar] [CrossRef]
- Montegut, L.; Lopez-Otin, C.; Magnan, C.; Kroemer, G. Old paradoxes and new opportunities for appetite control in obesity. Trends Endocrinol. Metab. TEM 2021, 32, 264–294. [Google Scholar] [CrossRef]
- Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nunez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin signaling in obesity and colorectal cancer. Int. J. Mol. Sci. 2022, 23, 4713. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the national lipid association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In Statpearls; Statpearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Karra, P.; Winn, M.; Pauleck, S.; Bulsiewicz-Jacobsen, A.; Peterson, L.; Coletta, A.; Doherty, J.; Ulrich, C.M.; Summers, S.A.; Gunter, M.; et al. Metabolic dysfunction and obesity-related cancer: Beyond obesity and metabolic syndrome. Obesity 2022, 30, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Zong, X.; Li, Z.; Li, N.; Hur, J.; Fritz, C.D.; Chapman, W., Jr.; Nickel, K.B.; Tipping, A.; et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2021, 70, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Crudele, L.; De Matteis, C.; Novielli, F.; Petruzzelli, S.; Di Buduo, E.; Graziano, G.; Cariello, M.; Piccinin, E.; Gadaleta, R.M.; Moschetta, A. Fasting hyperglycaemia and fatty liver drive colorectal cancer: A retrospective analysis in 1145 patients. Intern. Emerg. Med. 2024; online ahead of print. [Google Scholar]
- Yoon, Y.S.; Keum, N.; Zhang, X.; Cho, E.; Giovannucci, E.L. Hyperinsulinemia, insulin resistance and colorectal adenomas: A meta-analysis. Metab. Clin. Exp. 2015, 64, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, C.; Randazzo, C.; Barile, A.M.; Bo, S.; Ponzo, V.; Caldarella, R.; Malavazos, A.E.; Caruso, R.; Colombrita, P.; Lombardo, M.; et al. Factors associated with body weight gain and insulin-resistance: A longitudinal study. Nutr. Diabetes 2024, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.P.; Thompson, C.L.; Chak, A.; Berger, N.A.; Li, L. Insulin resistance, central obesity, and risk of colorectal adenomas. Cancer 2012, 118, 1774–1781. [Google Scholar] [CrossRef]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef]
- Nimptsch, K.; Konigorski, S.; Pischon, T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metab. Clin. Exp. 2019, 92, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Cleveland, R.; Norat, T.; Biessy, C.; Rohrmann, S.; Linseisen, J.; Boeing, H.; Pischon, T.; Panico, S.; Agnoli, C.; et al. Serum levels of igf-i, igfbp-3 and colorectal cancer risk: Results from the epic cohort, plus a meta-analysis of prospective studies. Int. J. Cancer 2010, 126, 1702–1715. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Meyerhardt, J.A.; Chan, A.T.; Ng, K.; Chan, J.A.; Wu, K.; Pollak, M.N.; Giovannucci, E.L.; Fuchs, C.S. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 176–185. [Google Scholar] [CrossRef]
- Szablewski, L. Insulin resistance: The increased risk of cancers. Curr. Oncol. 2024, 31, 998–1027. [Google Scholar] [CrossRef]
- Mouillet-Richard, S.; Cazelles, A.; Sroussi, M.; Gallois, C.; Taieb, J.; Laurent-Puig, P. Clinical challenges of consensus molecular subtype cms4 colon cancer in the era of precision medicine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Longo, S.; Stefanelli, G.; Viscido, A.; Latella, G. Dietary factors modulating colorectal carcinogenesis. Nutrients 2021, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Kompella, P.; Tinahones, F.J.; Macias-Gonzalez, M. An overview of vitamins as epidrugs for colorectal cancer prevention. Nutr. Rev. 2023, 81, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, D.; Mertens, B.; De Smet, S.; Ulens, M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2747–2766. [Google Scholar] [CrossRef] [PubMed]
- Formigaro, C.; Henriquez-Hernandez, L.A.; Zaccaroni, A.; Garcia-Hartmann, M.; Camacho, M.; Boada, L.D.; Zumbado, M.; Luzardo, O.P. Assessment of current dietary intake of organochlorine contaminants and polycyclic aromatic hydrocarbons in killer whales (orcinus orca) through direct determination in a group of whales in captivity. Sci. Total Environ. 2014, 472, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Red meat and processed meat. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2018; Volume 114. [Google Scholar]
- Rato, C.; Amirova, S.R.; Bates, D.G.; Stansfield, I.; Wallace, H.M. Translational recoding as a feedback controller: Systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift. Nucleic Acids Res. 2011, 39, 4587–4597. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.G.; Hernandez-Morales, M.; Nunez, L.; Villalobos, C. Inhibition of polyamine biosynthesis reverses ca(2+) channel remodeling in colon cancer cells. Cancers 2019, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Bello-Fernandez, C.; Packham, G.; Cleveland, J.L. The ornithine decarboxylase gene is a transcriptional target of c-myc. Proc. Natl. Acad. Sci. USA 1993, 90, 7804–7808. [Google Scholar] [CrossRef]
- Ignatenko, N.A.; Holubec, H.; Besselsen, D.G.; Blohm-Mangone, K.A.; Padilla-Torres, J.L.; Nagle, R.B.; de Alboranc, I.M.; Guillen, R.J.; Gerner, E.W. Role of c-myc in intestinal tumorigenesis of the apcmin/+ mouse. Cancer Biol. Ther. 2006, 5, 1658–1664. [Google Scholar] [CrossRef]
- Gobert, A.P.; Latour, Y.L.; Asim, M.; Barry, D.P.; Allaman, M.M.; Finley, J.L.; Smith, T.M.; McNamara, K.M.; Singh, K.; Sierra, J.C.; et al. Protective role of spermidine in colitis and colon carcinogenesis. Gastroenterology 2022, 162, 813–827.e8. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Simon, A.K.; Bergmann, M.; Eisenberg, T.; Kroemer, G.; Madeo, F. Mechanisms of spermidine-induced autophagy and geroprotection. Nat. Aging 2022, 2, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Del Corno, M.; Vari, R.; Scazzocchio, B.; Varano, B.; Masella, R.; Conti, L. Dietary fatty acids at the crossroad between obesity and colorectal cancer: Fine regulators of adipose tissue homeostasis and immune response. Cells 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.G.; Wan, F.; Housseau, F.; Sears, C.L. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterology 2018, 155, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Ghias, K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol. Med. 2016, 13, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of colorectal carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Sakthianandeswaren, A.; Parsons, M.J.; Mouradov, D.; MacKinnon, R.N.; Catimel, B.; Liu, S.; Palmieri, M.; Love, C.; Jorissen, R.N.; Li, S.; et al. Macrod2 haploinsufficiency impairs catalytic activity of parp1 and promotes chromosome instability and growth of intestinal tumors. Cancer Discov. 2018, 8, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular mechanisms of colon cancer progression and metastasis: Recent insights and advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.M. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef]
- Cunningham, J.M.; Christensen, E.R.; Tester, D.J.; Kim, C.Y.; Roche, P.C.; Burgart, L.J.; Thibodeau, S.N. Hypermethylation of the hmlh1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998, 58, 3455–3460. [Google Scholar] [PubMed]
- Goel, A.; Arnold, C.N.; Niedzwiecki, D.; Carethers, J.M.; Dowell, J.M.; Wasserman, L.; Compton, C.; Mayer, R.J.; Bertagnolli, M.M.; Boland, C.R. Frequent inactivation of pten by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004, 64, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Nassif, N.T.; Lobo, G.P.; Wu, X.; Henderson, C.J.; Morrison, C.D.; Eng, C.; Jalaludin, B.; Segelov, E. Pten mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene 2004, 23, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Serebriiskii, I.G.; Pavlov, V.A.; Andrianov, G.V.; Litwin, S.; Basickes, S.; Newberg, J.Y.; Frampton, G.M.; Meyer, J.E.; Golemis, E.A. Source, co-occurrence, and prognostic value of pten mutations or loss in colorectal cancer. NPJ Genom. Med. 2023, 8, 40. [Google Scholar]
- Goel, A.; Nagasaka, T.; Arnold, C.N.; Inoue, T.; Hamilton, C.; Niedzwiecki, D.; Compton, C.; Mayer, R.J.; Goldberg, R.; Bertagnolli, M.M.; et al. The cpg island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology 2007, 132, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Ma, L.; Ma, G.H.; Ren, H. Genome-wide analysis reveals DNA methylation alterations in obesity associated with high risk of colorectal cancer. Sci. Rep. 2019, 9, 5100. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Morcillo, S.; Diaz-Lagares, A.; Sandoval, J.; Castellano-Castillo, D.; Torres, E.; Hervas, D.; Moran, S.; Esteller, M.; Macias-Gonzalez, M.; et al. Identification of an episignature of human colorectal cancer associated with obesity by genome-wide DNA methylation analysis. Int. J. Obes. 2019, 43, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.L.; Millard, M.; Bosenberg, M.W.; DePinho, R.A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat. Genet. 2001, 28, 155–159. [Google Scholar] [CrossRef]
- Meeker, A.K.; Hicks, J.L.; Iacobuzio-Donahue, C.A.; Montgomery, E.A.; Westra, W.H.; Chan, T.Y.; Ronnett, B.M.; De Marzo, A.M. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 3317–3326. [Google Scholar] [CrossRef]
- Roger, L.; Jones, R.E.; Heppel, N.H.; Williams, G.T.; Sampson, J.R.; Baird, D.M. Extensive telomere erosion in the initiation of colorectal adenomas and its association with chromosomal instability. J. Natl. Cancer Inst. 2013, 105, 1202–1211. [Google Scholar] [CrossRef]
- Karami, S.; Han, Y.; Pande, M.; Cheng, I.; Rudd, J.; Pierce, B.L.; Nutter, E.L.; Schumacher, F.R.; Kote-Jarai, Z.; Lindstrom, S.; et al. Telomere structure and maintenance gene variants and risk of five cancer types. Int. J. Cancer 2016, 139, 2655–2670. [Google Scholar] [CrossRef] [PubMed]
- LaBella, K.A.; Hsu, W.H.; Li, J.; Qi, Y.; Liu, Y.; Liu, J.; Wu, C.C.; Liu, Y.; Song, Z.; Lin, Y.; et al. Telomere dysfunction alters intestinal stem cell dynamics to promote cancer. Dev. Cell 2024, 59, 1475–1486.e5. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, S.; Gonzalez-Gamo, D.; Fernandez-Marcelo, T.; Tesolato, S.; De La Serna, S.; Dominguez-Serrano, I.; Cano-Valderrama, O.; Barabash, A.; De Juan, C.; Torres-Garcia, A.; et al. Obesity and telomere status in the prognosis of patients with colorectal cancer submitted to curative intention surgical treatment. Mol. Clin. Oncol. 2021, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.J.; Toden, S.; Bird, A.R.; Topping, D.L.; Fenech, M.; Conlon, M.A. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch. Clin. Nutr. 2012, 31, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, S.; Gonzalez-Gamo, D.; Tesolato, S.E.; Barabash, A.; de la Serna, S.C.; Dominguez-Serrano, I.; Dziakova, J.; Rivera, D.; Torres, A.J.; Iniesta, P. Telomere length and telomerase activity in subcutaneous and visceral adipose tissues from obese and non-obese patients with and without colorectal cancer. Cancers 2022, 15, 273. [Google Scholar] [CrossRef]
- Behrens, J.; Lustig, B. The wnt connection to tumorigenesis. Int. J. Dev. Biol. 2004, 48, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Stefani, C.; Miricescu, D.; Stanescu, S., II; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth factors, pi3k/akt/mtor and mapk signaling pathways in colorectal cancer pathogenesis: Where are we now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Chen, J.Z. Obesity, the pi3k/akt signal pathway and colon cancer. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2009, 10, 610–616. [Google Scholar] [CrossRef]
- Chen, J. Multiple signal pathways in obesity-associated cancer. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2011, 12, 1063–1070. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, S.; Sebastian, C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin. Cell Dev. Biol. 2020, 98, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.K.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef]
- Bu, P.; Chen, K.Y.; Xiang, K.; Johnson, C.; Crown, S.B.; Rakhilin, N.; Ai, Y.; Wang, L.; Xi, R.; Astapova, I.; et al. Aldolase b-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell Metab. 2018, 27, 1249–1262.e4. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pu, X.; Wang, X.; Xu, M. Reprogramming of lipid metabolism in the tumor microenvironment: A strategy for tumor immunotherapy. Lipids Health Dis. 2024, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhu, C.; Du, L.; Huang, J.; Lu, J.; Yang, J.; Tong, Y.; Zhu, M.; Song, C.; Shen, C.; et al. A metabolomic signature of obesity and risk of colorectal cancer: Two nested case-control studies. Metabolites 2023, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ung, T.T.; Kim, N.H.; Jung, Y.D. Role of bile acids in colon carcinogenesis. World J. Clin. Cases 2018, 6, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, A.E.; Makinen, M.J.; Vayrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. Ikkbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef]
- Schwitalla, S.; Fingerle, A.A.; Cammareri, P.; Nebelsiek, T.; Goktuna, S.I.; Ziegler, P.K.; Canli, O.; Heijmans, J.; Huels, D.J.; Moreaux, G.; et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 2013, 152, 25–38. [Google Scholar] [CrossRef]
- Wozniakova, M.; Skarda, J.; Raska, M. The role of tumor microenvironment and immune response in colorectal cancer development and prognosis. Pathol. Oncol. Res. POR 2022, 28, 1610502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef] [PubMed]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, A.; Gruber, D.C.; Pisarsky, L.; Heck, C.; Kunita, A.; Yilmaz, M.; Meyer-Schaller, N.; Cornille, K.; Hopfer, U.; Bentires-Alj, M.; et al. Vegf-mediated angiogenesis links emt-induced cancer stemness to tumor initiation. Cancer Res. 2014, 74, 1566–1575. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kornmann, M.; Traub, B. Role of epithelial to mesenchymal transition in colorectal cancer. Int. J. Mol. Sci. 2023, 24, 14815. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.H.; Yao, Y.; Yu, S.; Han, L.L.; Wang, W.J.; Guo, H.; Tian, T.; Ruan, Z.P.; Kang, X.M.; Wang, J.; et al. Sdf-1/cxcr4 promotes epithelial-mesenchymal transition and progression of colorectal cancer by activation of the wnt/beta-catenin signaling pathway. Cancer Lett. 2014, 354, 417–426. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, X.F.; Fang, Y.; Li, M.L.; Shu, R.; Gong, Y.; Luo, H.Y.; Tian, Y. A possible genetic association between obesity and colon cancer in females. Front. Endocrinol. 2023, 14, 1189570. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zheng, Y.; Chen, H.; Luo, M.; Yang, C.; Ren, D.; Qin, P.; Zhang, H.; Lin, H. Single-cell transcriptome analysis reveals immunosuppressive landscape in overweight and obese colorectal cancer. J. Transl. Med. 2024, 22, 134. [Google Scholar] [CrossRef]
- Schwitalla, S.; Ziegler, P.K.; Horst, D.; Becker, V.; Kerle, I.; Begus-Nahrmann, Y.; Lechel, A.; Rudolph, K.L.; Langer, R.; Slotta-Huspenina, J.; et al. Loss of p53 in enterocytes generates an inflammatory microenvironment enabling invasion and lymph node metastasis of carcinogen-induced colorectal tumors. Cancer Cell 2013, 23, 93–106. [Google Scholar] [CrossRef]
- Xu, P.; Tao, Z.; Yang, H.; Zhang, C. Obesity and early-onset colorectal cancer risk: Emerging clinical evidence and biological mechanisms. Front. Oncol. 2024, 14, 1366544. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Heo, G.; Lee, Y.; Im, E. Interplay between the gut microbiota and inflammatory mediators in the development of colorectal cancer. Cancers 2021, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.R.J.; Patel, K.; Putnam, W.C.; Kapur, P.; Rakheja, D. Oncometabolites: A new paradigm for oncology, metabolism, and the clinical laboratory. Clin. Chem. 2017, 63, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) e. Coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Messaritakis, I.; Koulouris, A.; Boukla, E.; Vogiatzoglou, K.; Lagkouvardos, I.; Intze, E.; Sfakianaki, M.; Chondrozoumaki, M.; Karagianni, M.; Athanasakis, E.; et al. Exploring gut microbiome composition and circulating microbial DNA fragments in patients with stage ii/iii colorectal cancer: A comprehensive analysis. Cancers 2024, 16, 1923. [Google Scholar] [CrossRef] [PubMed]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- John Kenneth, M.; Tsai, H.C.; Fang, C.Y.; Hussain, B.; Chiu, Y.C.; Hsu, B.M. Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer. J. Adv. Res. 2023, 52, 45–57. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The intestinal microbiota and colorectal cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal. CCS 2023, 21, 212. [Google Scholar] [CrossRef]
- Pearson, J.R.; Gill, C.I.; Rowland, I.R. Diet, fecal water, and colon cancer-development of a biomarker. Nutr. Rev. 2009, 67, 509–526. [Google Scholar] [CrossRef]
- Yokota, A.; Fukiya, S.; Islam, K.B.; Ooka, T.; Ogura, Y.; Hayashi, T.; Hagio, M.; Ishizuka, S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes 2012, 3, 455–459. [Google Scholar] [CrossRef]
- Centuori, S.M.; Gomes, C.J.; Trujillo, J.; Borg, J.; Brownlee, J.; Putnam, C.W.; Martinez, J.D. Deoxycholic acid mediates non-canonical egfr-mapk activation through the induction of calcium signaling in colon cancer cells. Biochim. Biophys. Acta 2016, 1861, 663–670. [Google Scholar] [CrossRef]
- Cavestro, G.M.; Mannucci, A.; Balaguer, F.; Hampel, H.; Kupfer, S.S.; Repici, A.; Sartore-Bianchi, A.; Seppala, T.T.; Valentini, V.; Boland, C.R.; et al. Delphi initiative for early-onset colorectal cancer (direct) international management guidelines. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 581–603.e33. [Google Scholar] [CrossRef]
- Hong, Y.R.; Huo, J.; Desai, R.; Cardel, M.; Deshmukh, A.A. Excess costs and economic burden of obesity-related cancers in the united states. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2019, 22, 1378–1386. [Google Scholar] [CrossRef]
- Wick, E.C.; Hirose, K.; Shore, A.D.; Clark, J.M.; Gearhart, S.L.; Efron, J.; Makary, M.A. Surgical site infections and cost in obese patients undergoing colorectal surgery. Arch. Surg. 2011, 146, 1068–1072. [Google Scholar] [CrossRef]
- Du, M.; Griecci, C.F.; Cudhea, F.F.; Eom, H.; Kim, D.D.; Wilde, P.; Wong, J.B.; Wang, Y.C.; Michaud, D.S.; Mozaffarian, D.; et al. Cost-effectiveness analysis of nutrition facts added-sugar labeling and obesity-associated cancer rates in the us. JAMA Netw. Open 2021, 4, e217501. [Google Scholar] [CrossRef]
- Yeoh, A.; Mannalithara, A.; Ladabaum, U. Cost-effectiveness of earlier or more intensive colorectal cancer screening in overweight and obese patients. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 507–519. [Google Scholar] [CrossRef]
- van de Velde, C.J.; Aristei, C.; Boelens, P.G.; Beets-Tan, R.G.; Blomqvist, L.; Borras, J.M.; van den Broek, C.B.; Brown, G.; Coebergh, J.W.; Cutsem, E.V.; et al. Eurecca colorectal: Multidisciplinary mission statement on better care for patients with colon and rectal cancer in europe. Eur. J. Cancer 2013, 49, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, V. 14—Open surgical techniques in colorectal cancer. In Early Diagnosis and Treatment of Cancer Series: Colorectal Cancer; Elsevier: Amsterdam, The Netherlands, 2011; pp. 145–165. [Google Scholar]
- Petrou, N.A.; Rafique, H.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. Colorectal cancer and the obese patient: A call for guidelines. Cancers 2022, 14, 5255. [Google Scholar] [CrossRef] [PubMed]
- Amri, R.; Bordeianou, L.G.; Sylla, P.; Berger, D.L. Obesity, outcomes and quality of care: Body mass index increases the risk of wound-related complications in colon cancer surgery. Am. J. Surg. 2014, 207, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Twelves, C.; Sun, W.; O’Connell, M.J.; Cartwright, T.; McKenna, E.; Saif, M.; Lee, S.; Yothers, G.; Haller, D. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage iii colon cancer and the effect of oxaliplatin on post-relapse survival: A pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014, 15, 1481–1492. [Google Scholar] [PubMed]
- Costas-Chavarri, A.; Nandakumar, G.; Temin, S.; Lopes, G.; Cervantes, A.; Cruz Correa, M.; Engineer, R.; Hamashima, C.; Ho, G.F.; Huitzil, F.D.; et al. Treatment of patients with early-stage colorectal cancer: Asco resource-stratified guideline. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.H.; Gogineni, K.; Subhedar, P.D.; Lin, J.Y.; McCullough, L.E. Obesity and cancer treatment efficacy: Existing challenges and opportunities. Cancer 2019, 125, 1588–1592. [Google Scholar] [PubMed]
- Tabernero, J.; Taieb, J.; Prager, G.W.; Ciardiello, F.; Fakih, M.; Leger, C.; Fougeray, R.; Amellal, N.; van Cutsem, E. Trifluridine/tipiracil plus bevacizumab for third-line management of metastatic colorectal cancer: Sunlight study design. Future Oncol. 2021, 17, 1977–1985. [Google Scholar] [CrossRef]
- Jegadeesan, R.; Aziz, M.; Desai, M.; Sundararajan, T.; Gorrepati, V.S.; Chandrasekar, V.T.; Jayaraj, M.; Singh, P.; Saeed, A.; Rai, T.; et al. Hot snare vs. Cold snare polypectomy for endoscopic removal of 4–10 mm colorectal polyps during colonoscopy: A systematic review and meta-analysis of randomized controlled studies. Endosc. Int. Open 2019, 7, E708–E716. [Google Scholar] [CrossRef]
- Lim, S.H.; Levenick, J.M.; Mathew, A.; Moyer, M.T.; Dye, C.E.; McGarrity, T.J. Endoscopic management of large (>/=2 cm) non-pedunculated colorectal polyps: Impact of polyp morphology on outcomes. Dig. Dis. Sci. 2016, 61, 3572–3583. [Google Scholar] [CrossRef]
- Dekkers, N.; Dang, H.; Vork, K.; Langers, A.M.J.; van der Kraan, J.; Westerterp, M.; Peeters, K.; Holman, F.A.; Koch, A.D.; de Graaf, W.; et al. Outcome of completion surgery after endoscopic submucosal dissection in early-stage colorectal cancer patients. Cancers 2023, 15, 4490. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, W.; Chen, M.; Wang, Z.; Wu, J.; Yang, J.; Yang, L.; Deng, K. Long-term outcomes of local resection versus surgical resection for high-risk t1 colorectal cancer: A systematic review and meta-analysis. Gastrointest. Endosc. 2023, 97, 1016–1030.e14. [Google Scholar] [CrossRef]
- Ziati, J.; Souadka, A.; Benkabbou, A.; Boutayeb, S.; Ahmadi, B.; Amrani, L.; Mohsine, R.; Anass Majbar, M. Transanal total mesorectal excision for patients with rectal cancer: A systematic review and meta-analysis. Gulf J. Oncol. 2021, 1, 66–76. [Google Scholar]
- Cuffy, M.; Abir, F.; Audisio, R.A.; Longo, W.E. Colorectal cancer presenting as surgical emergencies. Surg. Oncol. 2004, 13, 149–157. [Google Scholar] [CrossRef]
- Hany, T.S.; Jadav, A.M.; Lamoon, C.; Cassidy, K.; Bhowmick, A.K. The extraperitoneal approach to left-sided colorectal rectal resections: Experts procedure. Ann. Surg. Oncol. 2023, 30, 1739–1740. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The rolarr randomized clinical trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Curtis, N.J.; Thomas, C.; Dennison, G.; Ockrim, J.B.; Conti, J.A.; Dalton, R.; Allison, A.S.; Francis, N.K. Factors predicting operative difficulty of laparoscopic total mesorectal excision. Dis. Colon Rectum 2019, 62, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Wee, I.J.Y.; Kuo, L.J.; Ngu, J.C. The impact of robotic colorectal surgery in obese patients: A systematic review, meta-analysis, and meta-regression. Surg. Endosc. 2019, 33, 3558–3566. [Google Scholar] [CrossRef]
- Tam, S.Y.; Wu, V.W.C. A review on the special radiotherapy techniques of colorectal cancer. Front. Oncol. 2019, 9, 208. [Google Scholar] [CrossRef]
- Haddock, M.G. Intraoperative radiation therapy for colon and rectal cancers: A clinical review. Radiat. Oncol. 2017, 12, 11. [Google Scholar] [CrossRef]
- Guren, M.G. The global challenge of colorectal cancer. Lancet Gastroenterol. Hepatol. 2019, 4, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Singh, H.; Thareja, S.; Kumar, P. Regulation of thymidylate synthase: An approach to overcome 5-fu resistance in colorectal cancer. Med. Oncol. 2022, 40, 3. [Google Scholar] [CrossRef] [PubMed]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.; Bracher, F. Pharmacokinetic enhancers (boosters)-escort for drugs against degrading enzymes and beyond. Sci. Pharm. 2018, 86, 43. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Nishina, T.; Mizuta, M.; Tsuji, A.; Watanabe, R.; Takahashi, I.; Watanabe, Y.; Moriwaki, T.; Maeba, T.; Hyodo, I. Phase ii study of first-line chemotherapy with uracil-tegafur plus oral leucovorin in elderly (>/=75 years) Japanese patients with metastatic colorectal cancer: Sgosg-cr0501 study. Int. J. Clin. Oncol. 2015, 20, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Dhillon, S.; Deeks, E.D. Trifluridine/tipiracil: A review in metastatic gastric cancer. Drugs 2019, 79, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.C.; Rosen, L.S.; Mayer, R.J.; Goldman, J.W.; Infante, J.R.; Benedetti, F.; Lin, D.; Mizuguchi, H.; Zergebel, C.; Patel, M.R. Phase 1 study of oral tas-102 in patients with refractory metastatic colorectal cancer. Cancer Chemother. Pharmacol. 2015, 76, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Marciniak, B.; Kontek, R. Irinotecan-still an important player in cancer chemotherapy: A comprehensive overview. Int. J. Mol. Sci. 2020, 21, 4919. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Wainberg, Z.; Elez, E.; et al. Final results of destiny-crc01 investigating trastuzumab deruxtecan in patients with her2-expressing metastatic colorectal cancer. Nat. Commun. 2023, 14, 3332. [Google Scholar] [CrossRef]
- Graham, J.; Mushin, M.; Kirkpatrick, P. Oxaliplatin. Nat. Rev. Drug Discov. 2004, 3, 11–12. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef] [PubMed]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Fan, F.; Wang, R.; Ye, X.; Xia, L.; Boulbes, D.; Ellis, L.M. Intracrine vegf signalling mediates colorectal cancer cell migration and invasion. Br. J. Cancer 2017, 117, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Jacobs, I.A.; Burkes, R.L. Bevacizumab in colorectal cancer: Current role in treatment and the potential of biosimilars. Target. Oncol. 2017, 12, 599–610. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Minhajat, R.; Harjianti, T.; Islam, I.C.; Winarta, S.; Liyadi, Y.N.; Bamatraf, N.P.; Amanuddin, R. Bevacizumab side effects and adverse clinical complications in colorectal cancer patients: Review article. Ann. Med. Surg. 2023, 85, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Strasma, A.; Coke, H.; Mamlouk, O.; Tchakarov, A.; Mandayam, S. Lupus-like glomerulonephritis associated with regorafenib, a multikinase inhibitor. Kidney Med. 2021, 3, 294–298. [Google Scholar] [CrossRef]
- Kim, R.D.; Kovari, B.P.; Martinez, M.; Xie, H.; Sahin, I.H.; Mehta, R.; Strosberg, J.; Imanirad, I.; Ghayouri, M.; Kim, Y.C.; et al. A phase i/ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur. J. Cancer 2022, 169, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.; Albarran, V.; San Roman, M.; Gonzalez-Merino, C.; Garcia de Quevedo, C.; Moreno, J.; Calvo, J.C.; Gonzalez, G.; Orejana, I.; Chamorro, J.; et al. Braf inhibitors in metastatic colorectal cancer and mechanisms of resistance: A review of the literature. Cancers 2023, 15, 5243. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, binimetinib, and cetuximab in braf v600e-mutated colorectal cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Yarom, N.; Jonker, D.J. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov. Med. 2011, 11, 95–105. [Google Scholar] [PubMed]
- Garcia-Foncillas, J.; Sunakawa, Y.; Aderka, D.; Wainberg, Z.; Ronga, P.; Witzler, P.; Stintzing, S. Distinguishing features of cetuximab and panitumumab in colorectal cancer and other solid tumors. Front. Oncol. 2019, 9, 849. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.J.; Skelton, W.P.; Starr, J.S.; Parekh, H.; Lee, J.J.; Overman, M.J.; Allegra, C.; George, T.J. Immunotherapy for colorectal cancer: A review of current and novel therapeutic approaches. J. Natl. Cancer Inst. 2019, 111, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, S.; Asadzadeh, Z.; Hemmat, N.; Baghbanzadeh, A.; Sgambato, A.; Ghorbaninezhad, F.; Safarpour, H.; Argentiero, A.; Brunetti, O.; Bernardini, R.; et al. Immune checkpoint inhibitors in colorectal cancer: Challenges and future prospects. Biomedicines 2021, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Harrison, M. Folfoxiri reintroduction in metastatic colorectal cancer. Lancet Oncol. 2020, 21, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmuller, C.; Kahl, C.; Seipelt, G.; et al. Folfiri plus cetuximab versus folfiri plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (fire-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront folfoxiri plus bevacizumab and reintroduction after progression versus mfolfox6 plus bevacizumab followed by folfiri plus bevacizumab in the treatment of patients with metastatic colorectal cancer (tribe2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507. [Google Scholar] [PubMed]
- Qin, S.; Li, J.; Wang, L.; Xu, J.; Cheng, Y.; Bai, Y.; Li, W.; Xu, N.; Lin, L.Z.; Wu, Q.; et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (folfox-4) versus folfox-4 in patients with ras wild-type metastatic colorectal cancer: The open-label, randomized, phase iii tailor trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Galais, M.P.; Raoul, J.L.; Bouche, O.; Gourgou-Bourgade, S.; Douillard, J.Y.; Etienne, P.L.; Boige, V.; Martel-Lafay, I.; Michel, P.; et al. Definitive chemoradiotherapy with folfox versus fluorouracil and cisplatin in patients with oesophageal cancer (prodige5/accord17): Final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014, 15, 305–314. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in mmr-proficient and mmr-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Chatila, W.K.; Kim, J.K.; Walch, H.; Marco, M.R.; Chen, C.T.; Wu, F.; Omer, D.M.; Khalil, D.N.; Ganesh, K.; Qu, X.; et al. Genomic and transcriptomic determinants of response to neoadjuvant therapy in rectal cancer. Nat. Med. 2022, 28, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Chan, T.A.; Eggermont, A.M.M.; Galluzzi, L. Immunosurveillance in clinical cancer management. CA Cancer J. Clin. 2024, 74, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wang, Y.; Wang, H.; Shen, L.; Xiang, Z.; Zhao, Y.; Zhang, H.; Wan, J.; Zhang, H.; Wang, Y.; et al. Randomized phase ii trial of immunotherapy-based total neoadjuvant therapy for proficient mismatch repair or microsatellite stable locally advanced rectal cancer (torch). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, JCO2302261. [Google Scholar] [CrossRef]
- Larson, E.A.; Dalamaga, M.; Magkos, F. The role of exercise in obesity-related cancers: Current evidence and biological mechanisms. Semin. Cancer Biol. 2023, 91, 16–26. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European guidelines for obesity management in adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22 (Suppl. S3), 1–203. [Google Scholar]
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boule, N.; et al. Obesity in adults: A clinical practice guideline. CMAJ Can. Med. Assoc. J. = J. L’association Medicale Can. 2020, 192, E875–E891. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Dicker, D.; Fruhbeck, G.; Halford, J.C.G.; Sbraccia, P.; Yumuk, V.; Goossens, G.H. A new framework for the diagnosis, staging and management of obesity in adults. Nat. Med. 2024; online ahead of print. [Google Scholar]
- Almazeedi, S.; El-Abd, R.; Al-Khamis, A.; Albatineh, A.N.; Al-Sabah, S. Role of bariatric surgery in reducing the risk of colorectal cancer: A meta-analysis. Br. J. Surg. 2020, 107, 348–354. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef]
- Harborg, S.; Kjaergaard, K.A.; Thomsen, R.W.; Borgquist, S.; Cronin-Fenton, D.; Hjorth, C.F. New horizons: Epidemiology of obesity, diabetes mellitus, and cancer prognosis. J. Clin. Endocrinol. Metab. 2024, 109, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Michaud, D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007, 132, 2208–2225. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Nino, M.E. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013, 2013, 697521. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, K. Effect of hyperglycemia on the occurrence and prognosis of colorectal cancer. Am. J. Transl. Res. 2024, 16, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, S.; Wang, W.; Liu, E.; Guo, S.; Zhao, C.; Niu, J.; Zhang, Z. Hyperglycemia promotes liver metastasis of colorectal cancer via upregulation of integrin alphavbeta6. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e930921. [Google Scholar]
- Zhang, C.; Zhang, Y.; Dong, Y.; Zi, R.; Wang, Y.; Chen, Y.; Liu, C.; Wang, J.; Wang, X.; Li, J.; et al. Non-alcoholic fatty liver disease promotes liver metastasis of colorectal cancer via fatty acid synthase dependent egfr palmitoylation. Cell Death Discov. 2024, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Balcar, L.; Wernly, S.; Volkerer, A.; Semmler, L.; Hauptmann, L.; Wernly, B.; Aigner, E.; Niederseer, D.; Datz, C. Insulin resistance and central obesity determine hepatic steatosis and explain cardiovascular risk in steatotic liver disease. Front. Endocrinol. 2023, 14, 1244405. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Jensen, A.B. Drug-induced liver injury caused by capecitabine: A case report and a literature review. Case Rep. Oncol. 2023, 16, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Syu, D.K.; Chen, Y.C.; Liu, C.K.; Sun, C.A.; Chen, M. The association between hypertriglyceridemia and colorectal cancer: A long-term community cohort study in taiwan. Int. J. Environ. Res. Public Health 2022, 19, 7804. [Google Scholar] [CrossRef]
- Chang, Y.H.; Shin, C.M.; Han, K.; Jung, J.H.; Jin, E.H.; Lim, J.H.; Kang, S.J.; Choi, Y.J.; Yoon, H.; Park, Y.S.; et al. The persistence of hypertriglyceridemia and the risk of early onset colorectal cancer according to tumor subsites: A nationwide population-based study. Cancer Res. Treat. 2024, 56, 825–837. [Google Scholar] [CrossRef]
- Murphy, N.; Song, M.; Papadimitriou, N.; Carreras-Torres, R.; Langenberg, C.; Martin, R.M.; Tsilidis, K.K.; Barroso, I.; Chen, J.; Frayling, T.M.; et al. Associations between glycemic traits and colorectal cancer: A mendelian randomization analysis. J. Natl. Cancer Inst. 2022, 114, 740–752. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, P.P.; Sun, X.C.; Hu, T.T. Thiazolidinediones and risk of colorectal cancer in patients with diabetes mellitus: A meta-analysis. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2018, 24, 75–81. [Google Scholar]
- Palacios-Ramirez, R.; Hernanz, R.; Martin, A.; Perez-Giron, J.V.; Barrus, M.T.; Gonzalez-Carnicero, Z.; Aguado, A.; Jaisser, F.; Briones, A.M.; Salaices, M.; et al. Pioglitazone modulates the vascular contractility in hypertension by interference with et-1 pathway. Sci. Rep. 2019, 9, 16461. [Google Scholar] [CrossRef] [PubMed]
- Schockel, L.; Woischke, C.; Surendran, S.A.; Michl, M.; Schiergens, T.; Holscher, A.; Glass, F.; Kreissl, P.; Klauschen, F.; Gunther, M.; et al. Pparg activation promotes the proliferation of colorectal cancer cell lines and enhances the antiproliferative effect of 5-fluorouracil. BMC Cancer 2024, 24, 234. [Google Scholar] [CrossRef]
- Pouya, F.D.; Salehi, R.; Rasmi, Y.; Kheradmand, F.; Fathi-Azarbayjani, A. Combination chemotherapy against colorectal cancer cells: Co-delivery of capecitabine and pioglitazone hydrochloride by polycaprolactone-polyethylene glycol carriers. Life Sci. 2023, 332, 122083. [Google Scholar] [CrossRef] [PubMed]
- Tikoo, K.; Kumar, P.; Gupta, J. Rosiglitazone synergizes anticancer activity of cisplatin and reduces its nephrotoxicity in 7, 12-dimethyl benzaanthracene (dmba) induced breast cancer rats. BMC Cancer 2009, 9, 107. [Google Scholar] [CrossRef]
- Zanardelli, M.; Micheli, L.; Cinci, L.; Failli, P.; Ghelardini, C.; Di Cesare Mannelli, L. Oxaliplatin neurotoxicity involves peroxisome alterations. Ppargamma agonism as preventive pharmacological approach. PLoS ONE 2014, 9, e102758. [Google Scholar] [CrossRef] [PubMed]
- Lawler, T.; Walts, Z.L.; Giurini, L.; Steinwandel, M.; Lipworth, L.; Murff, H.J.; Zheng, W.; Warren Andersen, S. Metformin’s role in lowering colorectal cancer risk among individuals with diabetes from the southern community cohort study. Cancer Epidemiol. 2024, 90, 102566. [Google Scholar] [CrossRef]
- Xu, Y.; Che, H.; Liu, J.; Ye, P. Association of metformin and statin uses with the prognosis of colon cancer: A meta-analysis. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2024, 33, 414–424. [Google Scholar] [CrossRef]
- Xie, J.; Xia, L.; Xiang, W.; He, W.; Yin, H.; Wang, F.; Gao, T.; Qi, W.; Yang, Z.; Yang, X.; et al. Metformin selectively inhibits metastatic colorectal cancer with the kras mutation by intracellular accumulation through silencing mate1. Proc. Natl. Acad. Sci. USA 2020, 117, 13012–13022. [Google Scholar] [CrossRef]
- Coronel-Hernandez, J.; Salgado-Garcia, R.; Cantu-De Leon, D.; Jacobo-Herrera, N.; Millan-Catalan, O.; Delgado-Waldo, I.; Campos-Parra, A.D.; Rodriguez-Morales, M.; Delgado-Buenrostro, N.L.; Perez-Plasencia, C. Combination of metformin, sodium oxamate and doxorubicin induces apoptosis and autophagy in colorectal cancer cells via downregulation hif-1alpha. Front. Oncol. 2021, 11, 594200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, L.; Xu, G.; He, H.; Zhao, H.; Liu, T. Co-delivery of sorafenib and metformin from amphiphilic polypeptide-based micelles for colon cancer treatment. Front. Med. 2022, 9, 1009496. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.L.; Tsai, T.N.; Yang, I.P.; Miao, Z.F.; Chen, Y.C.; Li, C.C.; Su, W.C.; Chang, T.K.; Huang, C.W.; Tsai, H.L.; et al. Metformin enhancement of therapeutic effects of 5-fluorouracil and oxaliplatin in colon cancer cells and nude mice. Biomedicines 2022, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.P.; Miao, Z.F.; Huang, C.W.; Tsai, H.L.; Yeh, Y.S.; Su, W.C.; Chang, T.K.; Chang, S.F.; Wang, J.Y. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage iii colorectal cancer receiving adjuvant folfox6 chemotherapy. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866964. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.S.; Lin, C.T.; Chen, C.N.; Chang, S.F.; Chang, H.I.; Lee, K.C. Metformin increases the cytotoxicity of oxaliplatin in human dld-1 colorectal cancer cells through down-regulating hmgb1 expression. J. Cell. Biochem. 2018, 119, 6943–6952. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hao, Y.; Wang, C.; Han, Y.; Zhu, Y.; Feng, L.; Miao, L.; Liu, Z. Liposomal oxaliplatin prodrugs loaded with metformin potentiate immunotherapy for colorectal cancer. J. Control. Release Off. J. Control. Release Soc. 2022, 350, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of pd-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Akce, M.; Farran, B.; Switchenko, J.M.; Rupji, M.; Kang, S.; Khalil, L.; Ruggieri-Joyce, A.; Olson, B.; Shaib, W.L.; Wu, C.; et al. Phase ii trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer. J. Immunother. Cancer 2023, 11, e007235. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kim, B.C.; Hong, S.P.; Seo, Y.; Lee, H.S.; Park, Y.S.; Na, S.Y.; Park, S.C.; Park, J.; Kim, J.H.; et al. The effect of metformin in treatment of adenomas in patients with familial adenomatous polyposis. Cancer Prev. Res. 2021, 14, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.M.; Kim, N.; Han, K.; Kim, B.; Jung, J.H.; Oh, T.J.; Lee, D.H. Anti-diabetic medications and the risk for colorectal cancer: A population-based nested case-control study. Cancer Epidemiol. 2020, 64, 101658. [Google Scholar] [CrossRef]
- Karagiannis, T.; Boura, P.; Tsapas, A. Safety of dipeptidyl peptidase 4 inhibitors: A perspective review. Ther. Adv. Drug Saf. 2014, 5, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Nreu, B.; Montereggi, C.; Mannucci, E.; Monami, M. Risk of cancer in patients treated with dipeptidyl peptidase-4 inhibitors: An extensive meta-analysis of randomized controlled trials. Acta Diabetol. 2020, 57, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the select trial. Nat. Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef]
- Suzuki, S.; Goto, A.; Nakatochi, M.; Narita, A.; Yamaji, T.; Sawada, N.; Katagiri, R.; Iwagami, M.; Hanyuda, A.; Hachiya, T.; et al. Body mass index and colorectal cancer risk: A mendelian randomization study. Cancer Sci. 2021, 112, 1579–1588. [Google Scholar] [CrossRef]
- Wang, L.; Xu, R.; Kaelber, D.C.; Berger, N.A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 2024, 7, e2421305. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Dian, Y.; Zeng, F.; Deng, G.; Lei, S. Association of glucagon-like peptide-1 receptor agonists with risk of cancers-evidence from a drug target mendelian randomization and clinical trials. Int. J. Surg. 2024; online ahead of print. [Google Scholar]
- Shah, C.; Hong, Y.R.; Bishnoi, R.; Ali, A.; Skelton, W.P.t.; Dang, L.H.; Huo, J.; Dang, N.H. Impact of dpp4 inhibitors in survival of patients with prostate, pancreas, and breast cancer. Front. Oncol. 2020, 10, 405. [Google Scholar] [CrossRef]
- Kosowska, A.; Garczorz, W.; Klych-Ratuszny, A.; Aghdam, M.R.F.; Kimsa-Furdzik, M.; Simka-Lampa, K.; Francuz, T. Sitagliptin modulates the response of ovarian cancer cells to chemotherapeutic agents. Int. J. Mol. Sci. 2020, 21, 8976. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Moffitt, L.R.; Wilson, K.L.; Bilandzic, M.; Wright, M.D.; Gorrell, M.D.; Oehler, M.K.; Plebanski, M.; Stephens, A.N. Dpp4 inhibitor sitagliptin enhances lymphocyte recruitment and prolongs survival in a syngeneic ovarian cancer mouse model. Cancers 2021, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Tsan, Y.T.; Chan, W.C.; Sheu, W.H.; Chen, P.C. Use of an alpha-glucosidase inhibitor and the risk of colorectal cancer in patients with diabetes: A nationwide, population-based cohort study. Diabetes Care 2015, 38, 2068–2074. [Google Scholar] [CrossRef]
- Horibe, Y.; Adachi, S.; Ohno, T.; Goto, N.; Okuno, M.; Iwama, M.; Yamauchi, O.; Kojima, T.; Saito, K.; Ibuka, T.; et al. Alpha-glucosidase inhibitor use is associated with decreased colorectal neoplasia risk in patients with type 2 diabetes mellitus receiving colonoscopy: A retrospective study. Oncotarget 2017, 8, 97862–97870. [Google Scholar] [CrossRef][Green Version]
- Scheen, A.J. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (sglt2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Dai, Q.; Shi, W.; Zhai, S.; Song, Y.; Han, J. Sglt2 inhibitors and risk of cancer in type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Diabetologia 2017, 60, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.N.C.; Chan, R.N.F.; Chou, O.H.I.; Tse, G.; Lee, S. Lower risks of incident colorectal cancer in sglt2i users compared to dpp4i users: A propensity score-matched study with competing risk analysis. Eur. J. Intern. Med. 2023, 110, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Okada, J.; Matsumoto, S.; Kaira, K.; Saito, T.; Yamada, E.; Yokoo, H.; Katoh, R.; Kusano, M.; Okada, S.; Yamada, M. Sodium glucose cotransporter 2 inhibition combined with cetuximab significantly reduced tumor size and carcinoembryonic antigen level in colon cancer metastatic to liver. Clin. Color. Cancer 2018, 17, e45–e48. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.T.K.; Ng, L.; Wong, J.W.H.; Loong, H.H.F.; Chan, W.W.L.; Lee, C.H.; Wong, C.K.H. Repurposing sodium-glucose co-transporter 2 inhibitors (sglt2i) for cancer treatment—A review. Rev. Endocr. Metab. Disord. 2021, 22, 1121–1136. [Google Scholar] [CrossRef]
- Anastasio, C.; Donisi, I.; Del Vecchio, V.; Colloca, A.; Mele, L.; Sardu, C.; Marfella, R.; Balestrieri, M.L.; D’Onofrio, N. Sglt2 inhibitor promotes mitochondrial dysfunction and er-phagy in colorectal cancer cells. Cell. Mol. Biol. Lett. 2024, 29, 80. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, J.; Sun, W.; Li, X.; Wang, Z.; Sun, L.; Wang, Y. Unveiling the anticancer effects of sglt-2i: Mechanisms and therapeutic potential. Front. Pharmacol. 2024, 15, 1369352. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Okada, S.; Yamada, E.; Shimoda, Y.; Osaki, A.; Tagaya, Y.; Shibusawa, R.; Okada, J.; Yamada, M. Effect of dapagliflozin on colon cancer cell [rapid communication]. Endocr. J. 2015, 62, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Dutka, M.; Bobinski, R.; Francuz, T.; Garczorz, W.; Zimmer, K.; Ilczak, T.; Cwiertnia, M.; Hajduga, M.B. Sglt-2 inhibitors in cancer treatment-mechanisms of action and emerging new perspectives. Cancers 2022, 14, 5811. [Google Scholar] [CrossRef]
- Villani, L.A.; Smith, B.K.; Marcinko, K.; Ford, R.J.; Broadfield, L.A.; Green, A.E.; Houde, V.P.; Muti, P.; Tsakiridis, T.; Steinberg, G.R. The diabetes medication canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-i supported respiration. Mol. Metab. 2016, 5, 1048–1056. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, P.; Xu, N.; Liao, M.; Xu, C.; Ding, Y.; Cai, J.; Zhang, Y.; Xie, W. Canagliflozin inhibits p-gp function and early autophagy and improves the sensitivity to the antitumor effect of doxorubicin. Biochem. Pharmacol. 2020, 175, 113856. [Google Scholar] [CrossRef] [PubMed]
- Biziotis, O.D.; Tsakiridis, E.E.; Ali, A.; Ahmadi, E.; Wu, J.; Wang, S.; Mekhaeil, B.; Singh, K.; Menjolian, G.; Farrell, T.; et al. Canagliflozin mediates tumor suppression alone and in combination with radiotherapy in non-small cell lung cancer (nsclc) through inhibition of hif-1alpha. Mol. Oncol. 2023, 17, 2235–2256. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, J.; Wei, Q.; Dong, G.; Dong, Z. Canagliflozin reduces cisplatin uptake and activates akt to protect against cisplatin-induced nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2020, 318, F1041–F1052. [Google Scholar] [CrossRef]
- Longaray, J.B.; Dias, C.K.; Scholl, J.N.; Battastini, A.M.O.; Figueiro, F. Investigation of co-treatment multi-targeting approaches in breast cancer cell lines. Eur. J. Pharmacol. 2024, 966, 176328. [Google Scholar] [CrossRef]
- Akingbesote, N.D.; Norman, A.; Zhu, W.; Halberstam, A.A.; Zhang, X.; Foldi, J.; Lustberg, M.B.; Perry, R.J. A precision medicine approach to metabolic therapy for breast cancer in mice. Commun. Biol. 2022, 5, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, Y.; Luo, D.; Wang, J.; Liu, J.; Luo, Y.; Zhou, W.; Zhuo, Z.; Guo, K.; Zeng, R.; et al. Association between cardiovascular risk factors and colorectal cancer: A systematic review and meta-analysis of prospective cohort studies. EClinicalMedicine 2021, 34, 100794. [Google Scholar] [CrossRef]
- Fang, Z.; He, M.; Song, M. Serum lipid profiles and risk of colorectal cancer: A prospective cohort study in the uk biobank. Br. J. Cancer 2021, 124, 663–670. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; He, J. Role of dyslipidemia in accelerating inflammation, autoimmunity, and atherosclerosis in systemic lupus erythematosus and other autoimmune diseases. Discov. Med. 2020, 30, 49–56. [Google Scholar] [PubMed]
- Filaferro, L.; Zaccarelli, F.; Niccolini, G.F.; Colizza, A.; Zoccali, F.; Grasso, M.; Fusconi, M. Are statins onco- suppressive agents for every type of tumor? A systematic review of literature. Expert Rev. Anticancer Ther. 2024, 24, 435–445. [Google Scholar] [CrossRef]
- Huang, X.; Liang, N.; Zhang, F.; Lin, W.; Ma, W. Lovastatin-induced mitochondrial oxidative stress leads to the release of mtdna to promote apoptosis by activating cgas-sting pathway in human colorectal cancer cells. Antioxidants 2024, 13, 679. [Google Scholar] [CrossRef]
- Loffler, L.; Gogenur, I.; Gogenur, M. Correlations between preoperative statin treatment with short- and long-term survival following colorectal cancer surgery: A propensity score-matched national cohort study. Int. J. Color. Dis. 2024, 39, 60. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Bhendwal, S.; Halmos, B.; Moss, S.F.; Ramey, W.G.; Holt, P.R. Lovastatin augments apoptosis induced by chemotherapeutic agents in colon cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 2223–2229. [Google Scholar]
- Wei, B.; Xu, L.; Guo, W.; Wang, Y.; Wu, J.; Li, X.; Cai, X.; Hu, J.; Wang, M.; Xu, Q.; et al. Shp2-mediated inhibition of DNA repair contributes to cgas-sting activation and chemotherapeutic sensitivity in colon cancer. Cancer Res. 2021, 81, 3215–3228. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, J.; Hu, J.; Zhou, Y.; Zheng, J.; Deng, W.; Inam, M.; Guo, J.; Xie, Y.; Li, Y.; et al. Lovastatin/sn38 co-loaded liposomes amplified icb therapeutic effect via remodeling the immunologically-cold colon tumor and synergized stimulation of cgas-sting pathway. Cancer Lett. 2024, 588, 216765. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Takeda, T.; Matsuda, T.; Kishimoto, K.; Takefuji, H.; Taniwaki, Y.; Ueda, M.; Hoshida, T.; Tanabe, K.; Nishida, S. Statins enhances antitumor effect of oxaliplatin in kras-mutated colorectal cancer cells and inhibits oxaliplatin-induced neuropathy. Cancer Cell Int. 2023, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Wu, R.; Wang, W.; Liu, Y.; Kong, W.; Yang, B.; He, Q.; Zhu, H. Synergistic antitumor activity of regorafenib and rosuvastatin in colorectal cancer. Front. Pharmacol. 2023, 14, 1136114. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Shi, J.; Xia, H.; Wei, X. A novel ruthenium-fluvastatin complex downregulates sncg expression to modulate breast carcinoma cell proliferation and apoptosis via activating the pi3k/akt/mtor/vegf/mmp9 pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5537737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liang, X.; Gao, C.; Chen, M.; Zhou, Y.; Krueger, C.J.; Bao, G.; Gong, Z.; Dai, Z. Loading lovastatin into camptothecin-floxuridine conjugate nanocapsules for enhancing anti-metastatic efficacy of cocktail chemotherapy on triple-negative breast cancer. ACS Appl. Mater. Interfaces 2018, 10, 29385–29397. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Esparza, Y.C.; Wu, S.; Huang, L.; Buquet, C.; Shen, R.; Sanchez-Gonzalez, B.; Garcia Latorre, E.A.; Boyer, O.; Varin, R.; Jimenez-Zamudio, L.A.; et al. Synergistic promoting effects of pentoxifylline and simvastatin on the apoptosis of triple-negative mda-mb-231 breast cancer cells. Int. J. Oncol. 2018, 52, 1246–1254. [Google Scholar] [CrossRef]
- Marti, J.L.G.; Beckwitt, C.H.; Clark, A.M.; Wells, A. Atorvastatin facilitates chemotherapy effects in metastatic triple-negative breast cancer. Br. J. Cancer 2021, 125, 1285–1298. [Google Scholar] [CrossRef]
- Yulian, E.D.; Siregar, N.C.; Bajuadji. Combination of simvastatin and fac improves response to neoadjuvant chemotherapy in locally advanced breast cancer. Cancer Res. Treat. 2021, 53, 1072–1083. [Google Scholar] [CrossRef]
- Chen, J.; Lan, T.; Hou, J.; Zhang, J.; An, Y.; Tie, L.; Pan, Y.; Liu, J.; Li, X. Atorvastatin sensitizes human non-small cell lung carcinomas to carboplatin via suppression of akt activation and upregulation of timp-1. Int. J. Biochem. Cell Biol. 2012, 44, 759–769. [Google Scholar] [CrossRef]
- Martirosyan, A.; Clendening, J.W.; Goard, C.A.; Penn, L.Z. Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: Potential therapeutic relevance. BMC Cancer 2010, 10, 103. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Zhu, J.; Zheng, C.; Wu, M.; Xue, L.; He, G.; Fu, S.; Deng, X. Lovastatin enhances chemosensitivity of paclitaxel-resistant prostate cancer cells through inhibition of cyp2c8. Biochem. Biophys. Res. Commun. 2022, 589, 85–91. [Google Scholar] [CrossRef]
- Rao, Y.; Samuels, Z.; Carter, L.M.; Monette, S.; Panikar, S.S.; Pereira, P.M.R.; Lewis, J.S. Statins enhance the efficacy of her2-targeting radioligand therapy in drug-resistant gastric cancers. Proc. Natl. Acad. Sci. USA 2023, 120, e2220413120. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Moriya, K.; Kaji, K.; Saikawa, S.; Sato, S.; Nishimura, N.; Namisaki, T.; Akahane, T.; Mitoro, A.; Yoshiji, H. Atorvastatin augments gemcitabine-mediated anti-cancer effects by inhibiting yes-associated protein in human cholangiocarcinoma cells. Int. J. Mol. Sci. 2020, 21, 7588. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Koleini, N.; Samiei, E.; Aghaei, M.; Cole, L.K.; Alizadeh, J.; Islam, M.I.; Vosoughi, A.R.; Albokashy, M.; Butterfield, Y.; et al. Simvastatin increases temozolomide-induced cell death by targeting the fusion of autophagosomes and lysosomes. FEBS J. 2020, 287, 1005–1034. [Google Scholar] [CrossRef]
- Anderson, C.C.; Khatri, M.; Roede, J.R. Time-dependent simvastatin administration enhances doxorubicin toxicity in neuroblastoma. Toxicol. Rep. 2020, 7, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kim, T.W.; Hong, Y.S.; Han, S.W.; Lee, K.H.; Kang, H.J.; Hwang, I.G.; Lee, J.Y.; Kim, H.S.; Kim, S.T.; et al. A randomised, double-blind, placebo-controlled multi-centre phase iii trial of xeliri/folfiri plus simvastatin for patients with metastatic colorectal cancer. Br. J. Cancer 2015, 113, 1421–1426. [Google Scholar] [CrossRef]
- Kim, S.T.; Kang, J.H.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Hwang, I.G.; Lee, S.C.; Park, K.W.; et al. Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: A double-blind randomised phase 3 study. Eur. J. Cancer 2014, 50, 2822–2830. [Google Scholar] [CrossRef]
- Seckl, M.J.; Ottensmeier, C.H.; Cullen, M.; Schmid, P.; Ngai, Y.; Muthukumar, D.; Thompson, J.; Harden, S.; Middleton, G.; Fife, K.M.; et al. Multicenter, phase iii, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (lungstar). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Jouve, J.L.; Lecomte, T.; Bouche, O.; Barbier, E.; Khemissa Akouz, F.; Riachi, G.; Nguyen Khac, E.; Ollivier-Hourmand, I.; Debette-Gratien, M.; Faroux, R.; et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, M.; Yang, K.; Chi, T.; Liao, Z.; Wei, P. Ppar-alpha modulators as current and potential cancer treatments. Front. Oncol. 2021, 11, 599995. [Google Scholar] [CrossRef] [PubMed]
- Luty, M.; Piwowarczyk, K.; Labedz-Maslowska, A.; Wrobel, T.; Szczygiel, M.; Catapano, J.; Drabik, G.; Ryszawy, D.; Kedracka-Krok, S.; Madeja, Z.; et al. Fenofibrate augments the sensitivity of drug-resistant prostate cancer cells to docetaxel. Cancers 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. AMS 2013, 9, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Parmesh, P.; Dinesh, U.S.; Khandagale, A.S.; Bapu, A.B.; Sadashiv, R.; Reddy, P. Correlation of leptin and adiponectin receptor expression with clinicopathological parameters in colorectal carcinoma—A cross-sectional prospective study. Arq. Gastroenterol. 2024, 61, e24016. [Google Scholar] [CrossRef] [PubMed]
- Taguri, M.; Kuchiba, A.; Yamaji, T.; Sawada, N.; Goto, A.; Iwasaki, M.; Tsugane, S. Importance of circulating leptin and adiponectin in the causal pathways between obesity and the development of colorectal cancer in Japanese men. J. Epidemiol. 2024, JE20230148. [Google Scholar] [CrossRef]
- Greco, M.; De Santo, M.; Comande, A.; Belsito, E.L.; Ando, S.; Liguori, A.; Leggio, A. Leptin-activity modulators and their potential pharmaceutical applications. Biomolecules 2021, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, Y.; Vallis, J.; Shariati, M.; Parfrey, P.S.; McLaughlin, J.R.; Wang, P.P.; Zhu, Y. Associations between polymorphisms in leptin and leptin receptor genes and colorectal cancer survival. Cancer Biol. Med. 2023, 20, 438–451. [Google Scholar] [CrossRef]
- Chun, K.A.; Kocarnik, J.M.; Hardikar, S.S.; Robinson, J.R.; Berndt, S.I.; Chan, A.T.; Figueiredo, J.C.; Lindor, N.M.; Song, M.; Schoen, R.E.; et al. Leptin gene variants and colorectal cancer risk: Sex-specific associations. PLoS ONE 2018, 13, e0206519. [Google Scholar] [CrossRef]
- Harbuzariu, A.; Rampoldi, A.; Daley-Brown, D.S.; Candelaria, P.; Harmon, T.L.; Lipsey, C.C.; Beech, D.J.; Quarshie, A.; Ilies, G.O.; Gonzalez-Perez, R.R. Leptin-notch signaling axis is involved in pancreatic cancer progression. Oncotarget 2017, 8, 7740–7752. [Google Scholar] [CrossRef]
- Harbuzariu, A.; Gonzalez-Perez, R.R. Leptin-notch axis impairs 5-fluorouracil effects on pancreatic cancer. Oncotarget 2018, 9, 18239–18253. [Google Scholar] [CrossRef] [PubMed]
- Daley-Brown, D.; Harbuzariu, A.; Kurian, A.A.; Oprea-Ilies, G.; Gonzalez-Perez, R.R. Leptin-induced notch and il-1 signaling crosstalk in endometrial adenocarcinoma is associated with invasiveness and chemoresistance. World J. Clin. Oncol. 2019, 10, 222–233. [Google Scholar] [CrossRef]
- Harmon, T.; Harbuzariu, A.; Lanier, V.; Lipsey, C.C.; Kirlin, W.; Yang, L.; Gonzalez-Perez, R.R. Nanoparticle-linked antagonist for leptin signaling inhibition in breast cancer. World J. Clin. Oncol. 2017, 8, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Endo, H.; Tomimoto, A.; Sugiyama, M.; Takahashi, H.; Saito, S.; Inamori, M.; Nakajima, N.; Watanabe, M.; Kubota, N.; et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 2008, 57, 1531–1538. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Guiu, B.; Chauffert, B.; Ladoire, S. Sirolimus, bevacizumab, 5-fluorouracil and irinotecan for advanced colorectal cancer: A pilot study. World J. Gastroenterol. 2009, 15, 4278–4283. [Google Scholar] [CrossRef]
- Fung, A.S.; Wu, L.; Tannock, I.F. Concurrent and sequential administration of chemotherapy and the mammalian target of rapamycin inhibitor temsirolimus in human cancer cells and xenografts. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 5389–5395. [Google Scholar] [CrossRef] [PubMed]
- Yardley, D.A. Combining mtor inhibitors with chemotherapy and other targeted therapies in advanced breast cancer: Rationale, clinical experience, and future directions. Breast Cancer Basic Clin. Res. 2013, 7, 7–22. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, B.; Sun, X.; Rong, G.; Wang, W.; Li, H.; Guan, X.; Li, L.; Zhai, J.; Li, C.; et al. Safety and efficacy of sirolimus combined with endocrine therapy in patients with advanced hormone receptor-positive breast cancer and the exploration of biomarkers. Breast 2020, 52, 17–22. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., 3rd; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Bachelot, T.; Cottu, P.; Chabaud, S.; Dalenc, F.; Allouache, D.; Delaloge, S.; Jacquin, J.P.; Grenier, J.; Venat Bouvet, L.; Jegannathen, A.; et al. Everolimus added to adjuvant endocrine therapy in patients with high-risk hormone receptor-positive, human epidermal growth factor receptor 2-negative primary breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3699–3708. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Poon, A.C.; Tam, P.M.; Mutsaers, A.J. Investigation of the effects of mtor inhibitors rapamycin and everolimus in combination with carboplatin on canine malignant melanoma cells. BMC Vet. Res. 2021, 17, 382. [Google Scholar] [CrossRef] [PubMed]
- Beuvink, I.; Boulay, A.; Fumagalli, S.; Zilbermann, F.; Ruetz, S.; O’Reilly, T.; Natt, F.; Hall, J.; Lane, H.A.; Thomas, G. The mtor inhibitor rad001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 2005, 120, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Leite, R.; Arantes-Rodrigues, R.; Palmeira, C.; Colaco, B.; Lopes, C.; Colaco, A.; Costa, C.; da Silva, V.M.; Oliveira, P.; Santos, L. Everolimus combined with cisplatin has a potential role in treatment of urothelial bladder cancer. Biomed. Pharmacother. Biomed. Pharmacother. 2013, 67, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Leite, R.; Arantes-Rodrigues, R.; Ferreira, R.; Palmeira, C.; Colaco, A.; Moreira da Silva, V.; Oliveira, P.; Lara Santos, L. Temsirolimus improves cytotoxic efficacy of cisplatin and gemcitabine against urinary bladder cancer cell lines. Urol. Oncol. 2014, 32, 41.e11–41.e22. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, M.; Yang, G.; Cao, Z.; Liu, Y.; You, L.; Zhang, T. Mtor inhibitor, gemcitabine and pd-l1 antibody blockade combination therapy suppresses pancreatic cancer progression via metabolic reprogramming and immune microenvironment remodeling in trp53(flox/+)lsl-kras(g12d/+)pdx-1-cre murine models. Cancer Lett. 2023, 554, 216020. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Preusser, M.; Woehrer, A.; Sieghart, W.; Strommer, S.; Werzowa, J.; Fuereder, T.; Wacheck, V. Everolimus (rad001) and anti-angiogenic cyclophosphamide show long-term control of gastric cancer growth in vivo. Cancer Biol. Ther. 2008, 7, 1377–1385. [Google Scholar] [CrossRef]
- Cejka, D.; Preusser, M.; Fuereder, T.; Sieghart, W.; Werzowa, J.; Strommer, S.; Wacheck, V. Mtor inhibition sensitizes gastric cancer to alkylating chemotherapy in vivo. Anticancer Res. 2008, 28, 3801–3808. [Google Scholar] [PubMed]
- Pal, K.; Madamsetty, V.S.; Dutta, S.K.; Mukhopadhyay, D. Co-delivery of everolimus and vinorelbine via a tumor-targeted liposomal formulation inhibits tumor growth and metastasis in rcc. Int. J. Nanomed. 2019, 14, 5109–5123. [Google Scholar] [CrossRef]
- Cao, P.; Maira, S.M.; Garcia-Echeverria, C.; Hedley, D.W. Activity of a novel, dual pi3-kinase/mtor inhibitor nvp-bez235 against primary human pancreatic cancers grown as orthotopic xenografts. Br. J. Cancer 2009, 100, 1267–1276. [Google Scholar] [CrossRef]
- Lin, S.F.; Huang, Y.Y.; Lin, J.D.; Chou, T.C.; Hsueh, C.; Wong, R.J. Utility of a pi3k/mtor inhibitor (nvp-bez235) for thyroid cancer therapy. PLoS ONE 2012, 7, e46726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.Y.; Wu, H.C.; Wu, J.E.; Huang, K.Y.; Yang, S.C.; Chen, S.X.; Tsao, C.J.; Hsu, K.F.; Chen, Y.L.; Hong, T.M. The dual pi3k/mtor inhibitor bez235 restricts the growth of lung cancer tumors regardless of egfr status, as a potent accompanist in combined therapeutic regimens. J. Exp. Clin. Cancer Res. CR 2019, 38, 282. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Wang, T.; Zang, R.; Zang, Y.; Feng, Y.; Yan, S.; Geng, C.; Zhu, N.; Wang, Q. Dual pi3k/mtor inhibitor pf-04979064 regulates tumor growth in gastric cancer and enhances drug sensitivity of gastric cancer cells to 5-fu. Biomed. Pharmacother. Biomed. Pharmacother. 2024, 170, 116086. [Google Scholar] [CrossRef] [PubMed]
- Montegut, L.; Joseph, A.; Chen, H.; Abdellatif, M.; Ruckenstuhl, C.; Martins, I.; Madeo, F.; Kroemer, G. Dbi/acbp is a targetable autophagy checkpoint involved in aging and cardiovascular disease. Autophagy 2023, 19, 2166–2169. [Google Scholar] [CrossRef] [PubMed]
- Pedro, J.M.B.; Sica, V.; Madeo, F.; Kroemer, G. Acyl-coa-binding protein (acbp): The elusive ‘hunger factor’ linking autophagy to food intake. Cell Stress 2019, 3, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Alquier, T.; Christian-Hinman, C.A.; Alfonso, J.; Faergeman, N.J. From benzodiazepines to fatty acids and beyond: Revisiting the role of acbp/dbi. Trends Endocrinol. Metab. TEM 2021, 32, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Montegut, L.; Abdellatif, M.; Motino, O.; Madeo, F.; Martins, I.; Quesada, V.; Lopez-Otin, C.; Kroemer, G. Acyl coenzyme a binding protein (acbp): An aging- and disease-relevant “autophagy checkpoint”. Aging Cell 2023, 22, e13910. [Google Scholar] [CrossRef]
- Joseph, A.; Chen, H.; Anagnostopoulos, G.; Montegut, L.; Lafarge, A.; Motino, O.; Castedo, M.; Maiuri, M.C.; Clement, K.; Terrisse, S.; et al. Effects of acyl-coenzyme a binding protein (acbp)/diazepam-binding inhibitor (dbi) on body mass index. Cell Death Dis. 2021, 12, 599. [Google Scholar] [CrossRef]
- Bravo-San Pedro, J.M.; Sica, V.; Martins, I.; Pol, J.; Loos, F.; Maiuri, M.C.; Durand, S.; Bossut, N.; Aprahamian, F.; Anagnostopoulos, G.; et al. Acyl-coa-binding protein is a lipogenic factor that triggers food intake and obesity. Cell Metab. 2019, 30, 754–767.e9. [Google Scholar] [CrossRef]
- Motino, O.; Lambertucci, F.; Anagnostopoulos, G.; Li, S.; Nah, J.; Castoldi, F.; Senovilla, L.; Montegut, L.; Chen, H.; Durand, S.; et al. Acbp/dbi protein neutralization confers autophagy-dependent organ protection through inhibition of cell loss, inflammation, and fibrosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2207344119. [Google Scholar] [CrossRef]
- Anagnostopoulos, G.; Saavedra, E.; Lambertucci, F.; Motino, O.; Dimitrov, J.; Roiz-Valle, D.; Quesada, V.; Alvarez-Valadez, K.; Chen, H.; Sauvat, A.; et al. Inhibition of acyl-coa binding protein (acbp) by means of a gaba(a)rgamma2-derived peptide. Cell Death Dis. 2024, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, G.; Motino, O.; Li, S.; Carbonnier, V.; Chen, H.; Sica, V.; Durand, S.; Bourgin, M.; Aprahamian, F.; Nirmalathasan, N.; et al. An obesogenic feedforward loop involving ppargamma, acyl-coa binding protein and gaba(a) receptor. Cell Death Dis. 2022, 13, 356. [Google Scholar] [CrossRef]
- Duman, C.; Yaqubi, K.; Hoffmann, A.; Acikgoz, A.A.; Korshunov, A.; Bendszus, M.; Herold-Mende, C.; Liu, H.K.; Alfonso, J. Acyl-coa-binding protein drives glioblastoma tumorigenesis by sustaining fatty acid oxidation. Cell Metab. 2019, 30, 274–289.e5. [Google Scholar] [CrossRef] [PubMed]
- Duman, C.; Di Marco, B.; Nevedomskaya, E.; Ulug, B.; Lesche, R.; Christian, S.; Alfonso, J. Targeting fatty acid oxidation via acyl-coa binding protein hinders glioblastoma invasion. Cell Death Dis. 2023, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Liu, Y.; Yang, J.; Li, M.; Li, S.; Zhang, B.; Yang, R.; Zhang, Y.; Cui, X.; Feng, C. Single-cell sequencing reveals that dbi is the key gene and potential therapeutic target in quiescent bladder cancer stem cells. Front. Genet. 2022, 13, 904536. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.B.; Wei, K.C.; Bepler, G.; Reyes, J.D.; Cani, A.; Polin, L.; White, K.; Kim, S.; Viola, N.; McGrath, J.; et al. Identification of actionable targets for breast cancer intervention using a diversity outbred mouse model. iScience 2023, 26, 106320. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.T.; Rahman, S.M.; Hassanein, M.; Qian, J.; Hoeksema, M.D.; Chen, H.; Eisenberg, R.; Chaurand, P.; Caprioli, R.M.; Shiota, M.; et al. Acyl-coenzyme a-binding protein regulates beta-oxidation required for growth and survival of non-small cell lung cancer. Cancer Prev. Res. 2014, 7, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Cavalloni, G.; Peraldo-Neia, C.; Massa, A.; Bergamini, C.; Trentini, A.; De Rosa, G.; Daniele, L.; Ciccosanti, F.; Cervellati, C.; Leone, F.; et al. Proteomic analysis identifies deregulated metabolic and oxidative-associated proteins in italian intrahepatic cholangiocarcinoma patients. BMC Cancer 2021, 21, 865. [Google Scholar] [CrossRef] [PubMed]
- Venturini, I.; Zeneroli, M.L.; Corsi, L.; Baraldi, C.; Ferrarese, C.; Pecora, N.; Frigo, M.; Alho, H.; Farina, F.; Baraldi, M. Diazepam binding inhibitor and total cholesterol plasma levels in cirrhosis and hepatocellular carcinoma. Regul. Pept. 1998, 74, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Atak, A.; Khurana, S.; Gollapalli, K.; Reddy, P.J.; Levy, R.; Ben-Salmon, S.; Hollander, D.; Donyo, M.; Heit, A.; Hotz-Wagenblatt, A.; et al. Quantitative mass spectrometry analysis reveals a panel of nine proteins as diagnostic markers for colon adenocarcinomas. Oncotarget 2018, 9, 13530–13544. [Google Scholar] [CrossRef]
- Gossett, R.E.; Schroeder, F.; Gunn, J.M.; Kier, A.B. Expression of fatty acyl-coa binding proteins in colon cells: Response to butyrate and transformation. Lipids 1997, 32, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, T.L.; Tsai, Y.L.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.J.; Won, S.M.; Kwon, M.J.; Song, J.H.; Lee, E.B.; Cho, J.H.; Park, K.W.; Yoon, J.H. Screening of lactic acid bacteria with anti-adipogenic effect and potential probiotic properties from grains. Sci. Rep. 2023, 13, 11022. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Holma, R.; El-Nezami, H.; Suomalainen, T.; Kuisma, M.; Saxelin, M.; Poussa, T.; Mykkanen, H.; Korpela, R. The influence of lactobacillus rhamnosus lc705 together with propionibacterium freudenreichii ssp. Shermanii js on potentially carcinogenic bacterial activity in human colon. Int. J. Food Microbiol. 2008, 128, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (isapp) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Qamar, T.R.; Iqbal, S.; Syed, F.; Nasir, M.; Rehman, H.; Iqbal, M.A.; Liu, R.H. Impact of novel prebiotic galacto-oligosaccharides on various biomarkers of colorectal cancer in wister rats. Int. J. Mol. Sci. 2017, 18, 1785. [Google Scholar] [CrossRef] [PubMed]
- Qamar, T.R.; Syed, F.; Nasir, M.; Rehman, H.; Zahid, M.N.; Liu, R.H.; Iqbal, S. Novel combination of prebiotics galacto-oligosaccharides and inulin-inhibited aberrant crypt foci formation and biomarkers of colon cancer in wistar rats. Nutrients 2016, 8, 465. [Google Scholar] [CrossRef]
- Kim, C.E.; Yoon, L.S.; Michels, K.B.; Tranfield, W.; Jacobs, J.P.; May, F.P. The impact of prebiotic, probiotic, and synbiotic supplements and yogurt consumption on the risk of colorectal neoplasia among adults: A systematic review. Nutrients 2022, 14, 4937. [Google Scholar] [CrossRef]
- Senesse, P.; Boutron-Ruault, M.C.; Faivre, J.; Chatelain, N.; Belghiti, C.; Meance, S. Foods as risk factors for colorectal adenomas: A case-control study in burgundy (france). Nutr. Cancer 2002, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Willett, W.C.; Vaidya, R.; Zhang, X.; Giovannucci, E. Yogurt consumption and colorectal cancer incidence and mortality in the nurses’ health study and the health professionals follow-up study. Am. J. Clin. Nutr. 2020, 112, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the italian european prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Olsen, A.; Tjonneland, A.; Dahm, C.C.; Overvad, K.; et al. Consumption of dairy products and colorectal cancer in the european prospective investigation into cancer and nutrition (epic). PLoS ONE 2013, 8, e72715. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Bird, A.R.; Jackson, M.; Esterman, A.; Young, G.P. A synbiotic combination of resistant starch and bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J. Nutr. 2005, 135, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Hu, Y.; Brown, I.L.; Woodman, R.J.; Young, G.P. Synbiotic intervention of bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis 2010, 31, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Luceri, C.; Dolara, P.; Giannini, A.; Biggeri, A.; Salvadori, M.; Clune, Y.; Collins, K.J.; Paglierani, M.; Caderni, G. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics lactobacillus rhamnosus and bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis 2002, 23, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, B.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef]
- Fratila, T.D.; Ismaiel, A.; Dumitrascu, D.L. Microbiome modulation in the prevention and management of colorectal cancer: A systematic review of clinical interventions. Med. Pharm. Rep. 2023, 96, 131–145. [Google Scholar] [CrossRef]
- Xu, M.Q.; Cao, H.L.; Wang, W.Q.; Wang, S.; Cao, X.C.; Yan, F.; Wang, B.M. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J. Gastroenterol. 2015, 21, 102–111. [Google Scholar] [CrossRef]
- Lang, T.; Zhu, R.; Zhu, X.; Yan, W.; Li, Y.; Zhai, Y.; Wu, T.; Huang, X.; Yin, Q.; Li, Y. Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy. Nat. Commun. 2023, 14, 4746. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, J.; Zhao, R.; Li, H.; Cui, H.; Yuan, Z.; Ren, H.; Cao, B.; Wei, B. Akkermansia muciniphila-derived pentadecanoic acid enhances oxaliplatin sensitivity in gastric cancer by modulating glycolysis. Pharmacol. Res. 2024, 206, 107278. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.H.; Wang, X.G.; Zhang, H. Overcome cancer drug resistance by targeting epigenetic modifications of centrosome. Cancer Drug Resist. 2019, 2, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Raynal, N.J.; Da Costa, E.M.; Lee, J.T.; Gharibyan, V.; Ahmed, S.; Zhang, H.; Sato, T.; Malouf, G.G.; Issa, J.J. Repositioning fda-approved drugs in combination with epigenetic drugs to reprogram colon cancer epigenome. Mol. Cancer Ther. 2017, 16, 397–407. [Google Scholar] [CrossRef]
- Sharma, A.; Vatapalli, R.; Abdelfatah, E.; Wyatt McMahon, K.; Kerner, Z.; Guzzetta, A.A.; Singh, J.; Zahnow, C.; Baylin, S.B.; Yerram, S.; et al. Hypomethylating agents synergize with irinotecan to improve response to chemotherapy in colorectal cancer cells. PLoS ONE 2017, 12, e0176139. [Google Scholar] [CrossRef] [PubMed]
- Flis, S.; Gnyszka, A.; Flis, K. DNA methyltransferase inhibitors improve the effect of chemotherapeutic agents in sw48 and ht-29 colorectal cancer cells. PLoS ONE 2014, 9, e92305. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Ke, T.W.; Lai, C.Y.; Hong, W.Z.; Chang, H.Y.; Lee, C.Y.; Wu, C.H.; Chiang, S.F.; Liang, J.A.; Chen, J.Y.; et al. Inhibition of dnmts increases neoantigen-reactive t-cell toxicity against microsatellite-stable colorectal cancer in combination with radiotherapy. Biomed. Pharmacother. Biomed. Pharmacother. 2024, 177, 116958. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Chiang, S.F.; Ke, T.W.; Chen, T.W.; Hu, C.H.; Yang, P.C.; Chang, H.Y.; Liang, J.A.; Chen, W.T.; Chao, K.S.C. Dnmt1 constrains ifnbeta-mediated anti-tumor immunity and pd-l1 expression to reduce the efficacy of radiotherapy and immunotherapy. Oncoimmunology 2021, 10, 1989790. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jin, Y.; Wang, M.; Luo, H.Y.; Fang, W.J.; Wang, Y.N.; Chen, Y.X.; Huang, R.J.; Guan, W.L.; Li, J.B.; et al. Combined anti-pd-1, hdac inhibitor and anti-vegf for mss/pmmr colorectal cancer: A randomized phase 2 trial. Nat. Med. 2024, 30, 1035–1043. [Google Scholar] [CrossRef]
- Liang, X.L.; Ouyang, L.; Yu, N.N.; Sun, Z.H.; Gui, Z.K.; Niu, Y.L.; He, Q.Y.; Zhang, J.; Wang, Y. Histone deacetylase inhibitor pracinostat suppresses colorectal cancer by inducing cdk5-drp1 signaling-mediated peripheral mitofission. J. Pharm. Anal. 2023, 13, 1168–1182. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. Omega-3 and omega-6 polyunsaturated fatty acids, obesity and cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef] [PubMed]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Rossmeisl, M.; Bryhn, M.; Kopecky, J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin. Sci. 2009, 116, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (n-3) fatty acids alleviate adipose tissue inflammation and insulin resistance: Mechanistic insights. Adv. Nutr. 2011, 2, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.V.; Van Horn, L.; Hsia, J.; Manson, J.E.; Stefanick, M.L.; Wassertheil-Smoller, S.; Kuller, L.H.; LaCroix, A.Z.; Langer, R.D.; Lasser, N.L.; et al. Low-fat dietary pattern and risk of cardiovascular disease: The women’s health initiative randomized controlled dietary modification trial. JAMA 2006, 295, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, C.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Giurdanella, M.C.; Pala, V.; et al. Italian mediterranean index and risk of colorectal cancer in the italian section of the epic cohort. Int. J. Cancer 2013, 132, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Cade, J.E.; Evans, C.E.L.; Hancock, N.; Greenwood, D.C. The mediterranean diet and risk of colorectal cancer in the uk women’s cohort study. Int. J. Epidemiol. 2017, 46, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Jafari Nasab, S.; Clark, C.C.T.; Entezari, M. Mediterranean diet and colorectal adenomas: A systematic review and meta-analysis of observational studies. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2024, 33, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Algamas-Dimantov, A.; Yehuda-Shnaidman, E.; Hertz, R.; Peri, I.; Bar-Tana, J.; Schwartz, B. Prevention of diabetes-promoted colorectal cancer by (n-3) polyunsaturated fatty acids and (n-3) pufa mimetic. Oncotarget 2014, 5, 9851–9863. [Google Scholar] [CrossRef]
- Molendi-Coste, O.; Legry, V.; Leclercq, I.A. Why and how meet n-3 pufa dietary recommendations? Gastroenterol. Res. Pract. 2011, 2011, 364040. [Google Scholar] [CrossRef]
- Eaton, S.B.; Konner, M.J.; Cordain, L. Diet-dependent acid load, paleolithic [corrected] nutrition, and evolutionary health promotion. Am. J. Clin. Nutr. 2010, 91, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Khan, S.; Sup Lee, Y. Curcumin molecular targets in obesity and obesity-related cancers. Future Oncol. 2012, 8, 179–190. [Google Scholar] [CrossRef]
- Johnson, S.M.; Gulhati, P.; Arrieta, I.; Wang, X.; Uchida, T.; Gao, T.; Evers, B.M. Curcumin inhibits proliferation of colorectal carcinoma by modulating akt/mtor signaling. Anticancer Res. 2009, 29, 3185–3190. [Google Scholar]
- Dou, H.; Shen, R.; Tao, J.; Huang, L.; Shi, H.; Chen, H.; Wang, Y.; Wang, T. Curcumin suppresses the colon cancer proliferation by inhibiting wnt/beta-catenin pathways via mir-130a. Front. Pharmacol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Johnson, J.J.; Mukhtar, H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007, 255, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pfalzer, A.C.; Koh, G.Y.; Tang, S.; Crott, J.W.; Thomas, M.J.; Meydani, M.; Mason, J.B. Curcumin and salsalate suppresses colonic inflammation and procarcinogenic signaling in high-fat-fed, azoxymethane-treated mice. J. Agric. Food Chem. 2017, 65, 7200–7209. [Google Scholar] [CrossRef]
- Wu, X.; Koh, G.Y.; Huang, Y.; Crott, J.W.; Bronson, R.T.; Mason, J.B. The combination of curcumin and salsalate is superior to either agent alone in suppressing pro-cancerous molecular pathways and colorectal tumorigenesis in obese mice. Mol. Nutr. Food Res. 2019, 63, e1801097. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase iia clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef]
- Moetlediwa, M.T.; Ramashia, R.; Pheiffer, C.; Titinchi, S.J.J.; Mazibuko-Mbeje, S.E.; Jack, B.U. Therapeutic effects of curcumin derivatives against obesity and associated metabolic complications: A review of in vitro and in vivo studies. Int. J. Mol. Sci. 2023, 24, 14366. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Reddivari, L.; Radhakrishnan, S.; Tarver, C. Resveratrol suppresses igf-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of igf-1r/wnt and activation of p53 signaling pathways. BMC Cancer 2010, 10, 238. [Google Scholar] [CrossRef]
- Vanamala, J.; Radhakrishnan, S.; Reddivari, L.; Bhat, V.B.; Ptitsyn, A. Resveratrol suppresses human colon cancer cell proliferation and induces apoptosis via targeting the pentose phosphate and the talin-fak signaling pathways-a proteomic approach. Proteome Sci. 2011, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via malat1 mediated wnt/beta-catenin signal pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Adachi, S.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. (-)-epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male c57bl/ksj-db/db mice. Cancer Prev. Res. 2008, 1, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, S.N.; Kala, R.; Tollefsbol, T.O. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp. Cell Res. 2014, 324, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Jung, J.H.; Shin, E.A.; Park, J.E.; Park, W.Y.; Kim, S.H. Epigallocatechin-3-gallate induces apoptosis as a trail sensitizer via activation of caspase 8 and death receptor 5 in human colon cancer cells. Biomedicines 2020, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Yasui, Y.; Ohigashi, H.; Tanaka, T.; Murakami, A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male c57bl/ksj-db/db mice. Chem.-Biol. Interact. 2010, 183, 276–283. [Google Scholar] [CrossRef]
- Benito, I.; Encio, I.J.; Milagro, F.I.; Alfaro, M.; Martinez-Penuela, A.; Barajas, M.; Marzo, F. Microencapsulated bifidobacterium bifidum and lactobacillus gasseri in combination with quercetin inhibit colorectal cancer development in apc(min/+) mice. Int. J. Mol. Sci. 2021, 22, 4906. [Google Scholar] [CrossRef]
- Fike, L.T.; Munro, H.; Yu, D.; Dai, Q.; Shrubsole, M.J. Dietary polyphenols and the risk of colorectal cancer in the prospective southern community cohort study. Am. J. Clin. Nutr. 2022, 115, 1155–1165. [Google Scholar] [CrossRef]

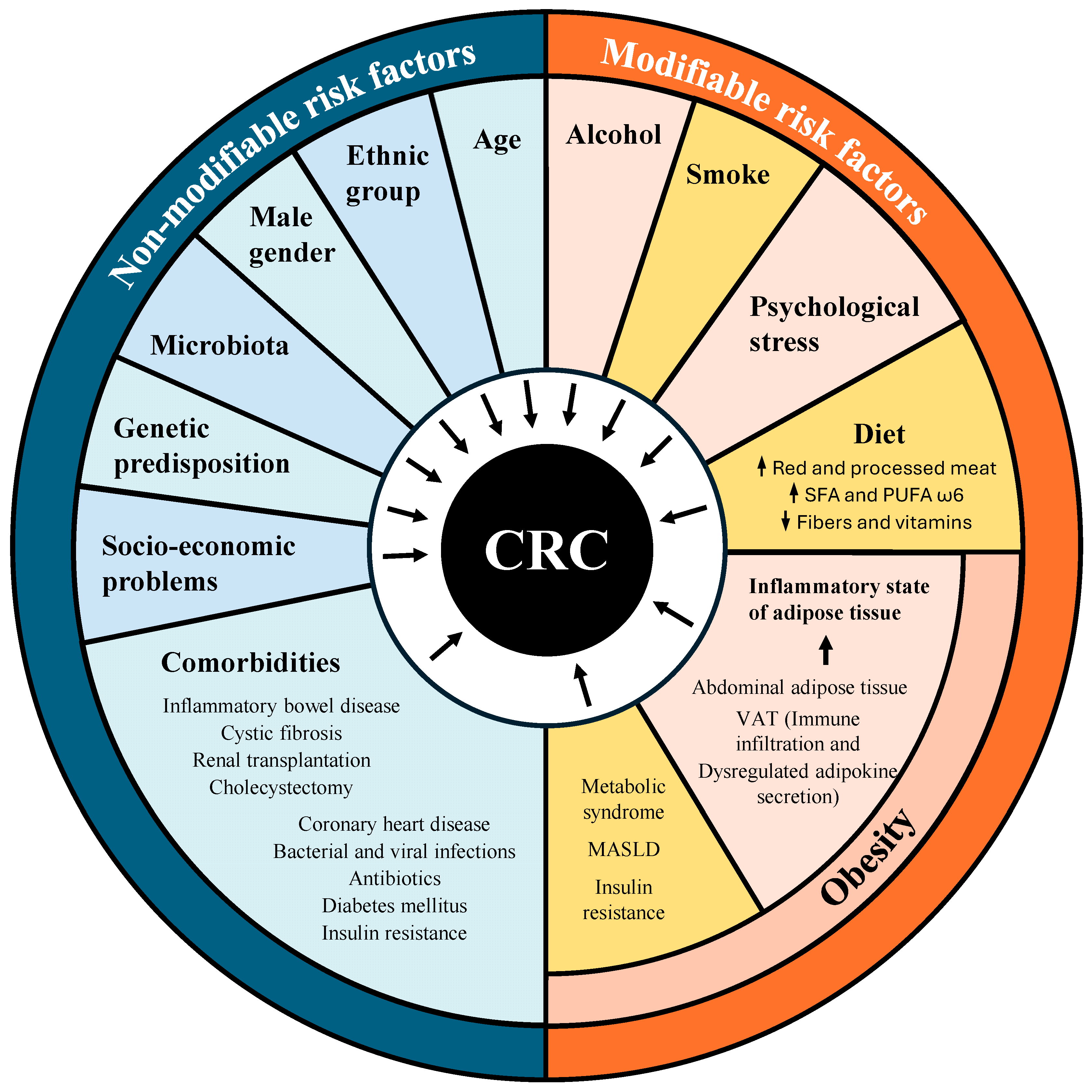

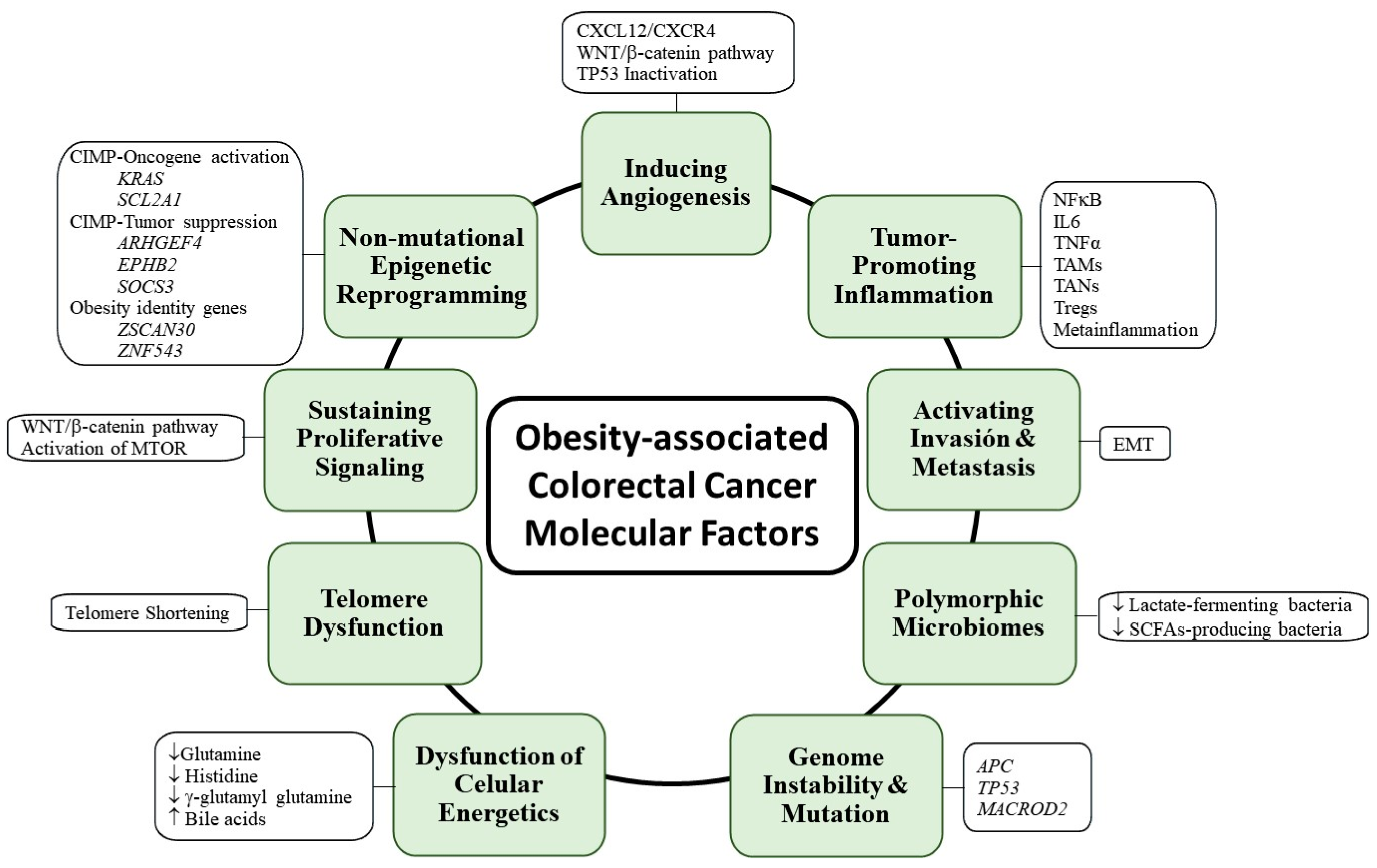
| Treatment | Techniques/Drugs | Stages | References | |
|---|---|---|---|---|
| Surgical resection | Polypectomy | Forceps or snare | CRA | [185] |
| ESD | - | CRA > 2 cm and early-stage | [186,187] | |
| Local Excision | - | Early-stages | [188] | |
| Colon Resection | Colectomy- anastomosis | III–V | [190] | |
| Colostomy | ||||
| Radiation | External (X-rays) | - | Various stages | [195] |
| Intraoperative | [196] | |||
| Chemotherapy | Fluoropyrimidines | 5-FU/Leucovorin | First line, III–IV (II high-risk patients) | [198] |
| Capecitabine | First line, III–IV | [199,200,201] | ||
| Tegafur/Uracil | Third or fourth line, III–IV | [202,203] | ||
| Trifluridine/Tipiracil | Third or fourth line, III–IV | [202,203] | ||
| Topoisomerase I Inhibitors | Irinotecan | III–V | [204] | |
| Deruxtecan | III–V | [205] | ||
| Platinum-based drugs | Oxaliplatin | First line, III–V | [206,207] | |
| Targeted Therapy | VEGF Inhibitors | Bevacizumab | First and second line, IV–V | [7,208,209,210,211] |
| Aflibercept | Second line, IV–V | [211,212] | ||
| Ramucirumab | Second line, IV–V | [211,212] | ||
| Regorafenib | IV–V | [213,214] | ||
| BRAF Inhibitors | Vemurafenib/Dabrafenib/Encorafenib | IV–V | [215,216] | |
| EGFR Inhibitors | Cetuximab/Panitumumab | First or second line | [217,218] | |
| Immune Checkpoint Inhibitors | Nivolumab, Pembrozizumab, Iplizumab, Tremelimumab, Atezolizumab, Durvalumab | IV–V | [219,220] | |
| Combined Therapies | - | FOLFOX | Different stages | [221] |
| FOLFIRI | [222,223] | |||
| FOLFOXIRI | [224,225] | |||
| Treatment | Actionable Target | Disease-Associated CRC | References |
|---|---|---|---|
| ACBP/DBI blocks | Anorexigenic activation/lipid synthesis inhibition | Metabolic syndrome | [352,353,354] |
| α-glucosidase inhibitor | Chemical digestion of glucide inhibition | T2D | [277,278] |
| DPP4i | Incretins availability | T2D | [268] |
| Empagliflozin | SGLT2 inhibition | T2D | [279,280,281,282,284,285,287] |
| Epigenetic inhibitors | DNMTi and HDACi | T2D and obesity | [392] |
| Leptin inhibitor | Appetite inhibition/energy metabolism | Obesity | [326] |
| Metformin | AMPK and SIRT1 activator | T2D and obesity | [256,287] |
| Pre-pro biotics/FMT | Alteration in microbiota composition | Metabolic syndrome | [164,389] |
| Rapalogues | mTOR inhibition | Obesity | [135] |
| Semaglutide | GLP-1 receptor agonist | T2D and obesity | [270,271] |
| Statins | HMG-CoA reductase inhibitor | Hypercholesteronemia/Dyslipidemia | [297,298,299] |
| Sulfonylureas | Insulin release | T2D | [267] |
| TZDs | PPARγ agonist | T2D | [250,271] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Gutierrez, L.; Motiño, O.; Barriuso, D.; de la Puente-Aldea, J.; Alvarez-Frutos, L.; Kroemer, G.; Palacios-Ramirez, R.; Senovilla, L. Obesity-Associated Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 8836. https://doi.org/10.3390/ijms25168836
Gonzalez-Gutierrez L, Motiño O, Barriuso D, de la Puente-Aldea J, Alvarez-Frutos L, Kroemer G, Palacios-Ramirez R, Senovilla L. Obesity-Associated Colorectal Cancer. International Journal of Molecular Sciences. 2024; 25(16):8836. https://doi.org/10.3390/ijms25168836
Chicago/Turabian StyleGonzalez-Gutierrez, Lucia, Omar Motiño, Daniel Barriuso, Juan de la Puente-Aldea, Lucia Alvarez-Frutos, Guido Kroemer, Roberto Palacios-Ramirez, and Laura Senovilla. 2024. "Obesity-Associated Colorectal Cancer" International Journal of Molecular Sciences 25, no. 16: 8836. https://doi.org/10.3390/ijms25168836
APA StyleGonzalez-Gutierrez, L., Motiño, O., Barriuso, D., de la Puente-Aldea, J., Alvarez-Frutos, L., Kroemer, G., Palacios-Ramirez, R., & Senovilla, L. (2024). Obesity-Associated Colorectal Cancer. International Journal of Molecular Sciences, 25(16), 8836. https://doi.org/10.3390/ijms25168836








