Relationship between Gut, Blood, Aneurysm Wall and Thrombus Microbiome in Abdominal Aortic Aneurysm Patients
Abstract
1. Introduction
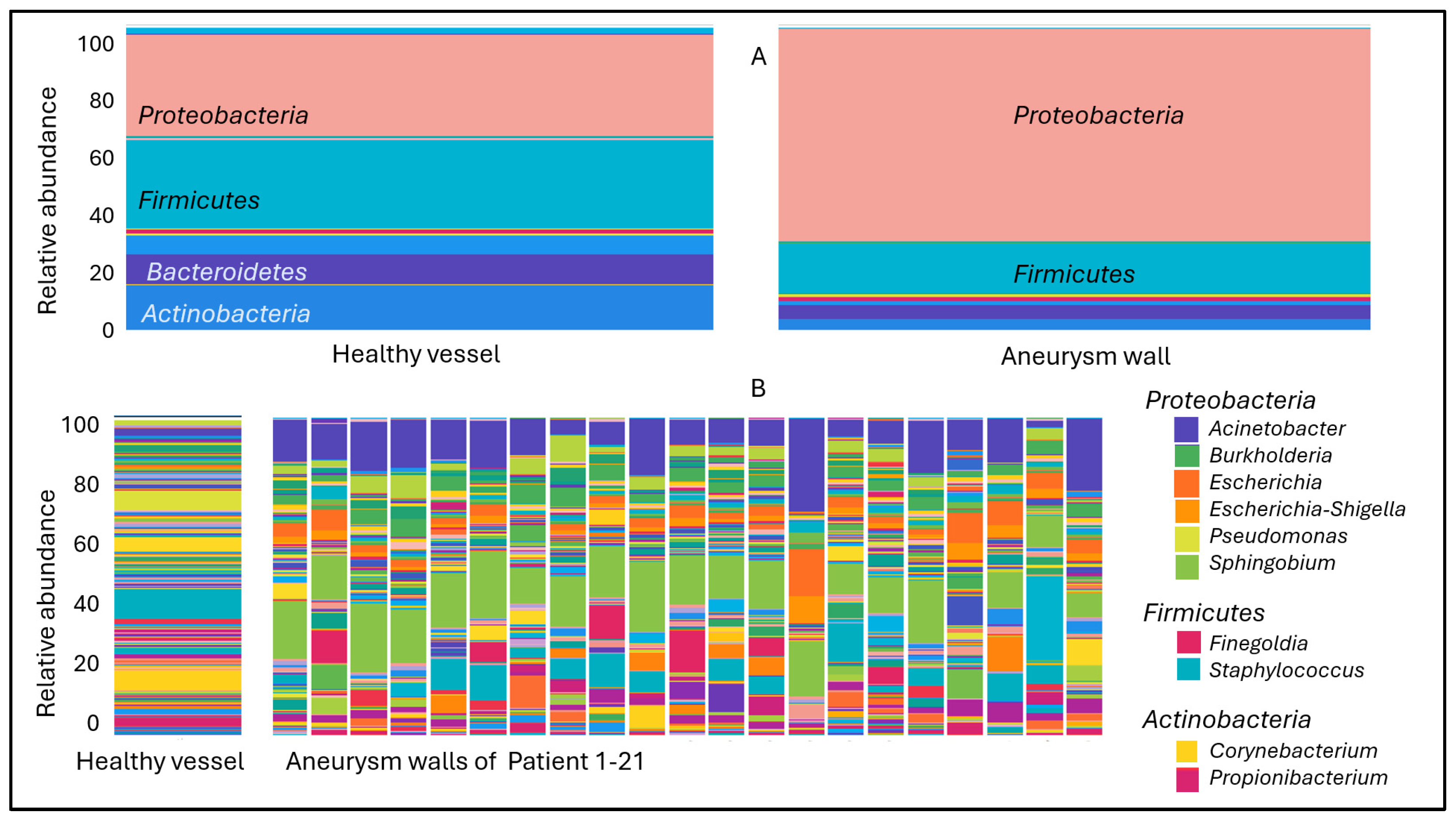
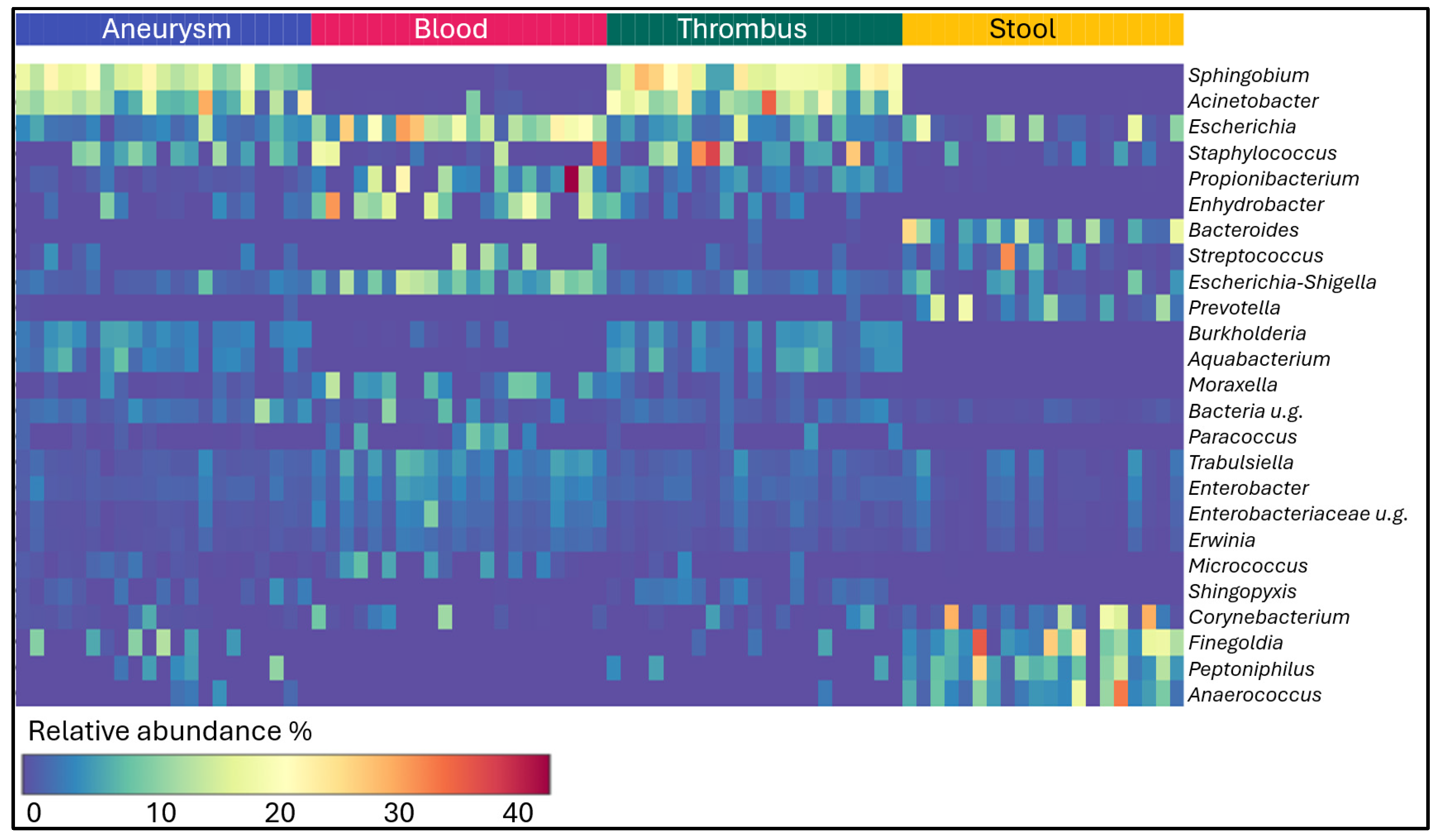
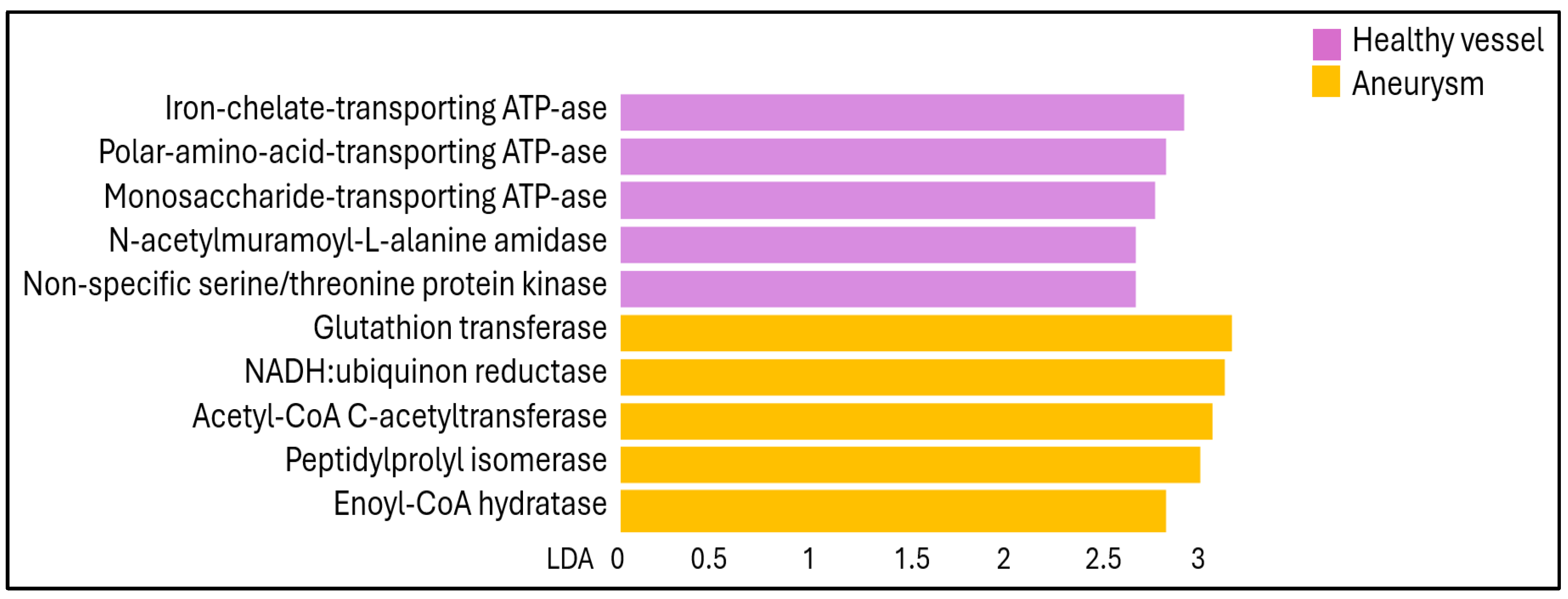
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. DNA Extraction
4.3. 16S rRNA Microbiota Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; He, Y.; Adeloye, D.; Zhu, Y.; Ye, X.; Yi, Q.; Rahimi, K.; Rudan, I.; Global Health Epidemiology Research, G. The Global and Regional Prevalence of Abdominal Aortic Aneurysms: A Systematic Review and Modeling Analysis. Ann. Surg. 2023, 277, 912–919. [Google Scholar] [CrossRef]
- Marquez-Sanchez, A.C.; Koltsova, E.K. Immune and inflammatory mechanisms of abdominal aortic aneurysm. Front. Immunol. 2022, 13, 989933. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Ohki, T.; Toya, N.; Nakagawa, H.; Horigome, A.; Odamaki, T.; Xiao, J.Z.; Koido, S.; Nishikawa, Y.; Ohkusa, T.; et al. Impact of Bifidobacterium adolescentis in patients with abdominal aortic aneurysm: A cross-sectional study. Biosci. Microbiota Food Health 2023, 42, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, Y.; Zheng, Z.; Zhang, M.; Zhang, T.; Jin, J.; Zhang, X.; Yao, G.; Kong, D.; Zhang, C.; et al. Gut microbiome dysbiosis contributes to abdominal aortic aneurysm by promoting neutrophil extracellular trap formation. Cell Host Microbe 2022, 30, 1450–1463.e8. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, S.; Huang, Y.; Qin, X.; Qu, K.; Chen, Y.; Chen, L.; Qiu, J.; Hao, Y.; Wang, G. Alterations in gut microbiota and physiological factors associated with abdominal aortic aneurysm. Med. Nov. Technol. Devices 2022, 14, 9. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef]
- Ling, X.; Jie, W.; Qin, X.; Zhang, S.; Shi, K.; Li, T.; Guo, J. Gut microbiome sheds light on the development and treatment of abdominal aortic aneurysm. Front. Cardiovasc. Med. 2022, 9, 1063683. [Google Scholar] [CrossRef]
- Benson, T.W.; Conrad, K.A.; Li, X.S.; Wang, Z.; Helsley, R.N.; Schugar, R.C.; Coughlin, T.M.; Wadding-Lee, C.; Fleifil, S.; Russell, H.M.; et al. Gut Microbiota-Derived Trimethylamine N-Oxide Contributes to Abdominal Aortic Aneurysm Through Inflammatory and Apoptotic Mechanisms. Circulation 2023, 147, 1079–1096. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhou, H.; Wu, K. Akkermansia muciniphila Alters Gut Microbiota and Immune System to Improve Cardiovascular Diseases in Murine Model. Front. Microbiol. 2022, 13, 906920. [Google Scholar] [CrossRef]
- Shinohara, R.; Nakashima, H.; Emoto, T.; Yamashita, T.; Saito, Y.; Yoshida, N.; Inoue, T.; Yamanaka, K.; Okada, K.; Hirata, K.I. Gut Microbiota Influence the Development of Abdominal Aortic Aneurysm by Suppressing Macrophage Accumulation in Mice. Hypertension 2022, 79, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, F.; Zhou, Q.; Qiu, Y.; Zhang, J.; Tu, Q.; Zhou, Z.; Shao, Y.; Xu, S.; Wang, Y.; et al. Berberine Improves Vascular Dysfunction by Inhibiting Trimethylamine-N-oxide via Regulating the Gut Microbiota in Angiotensin II-Induced Hypertensive Mice. Front. Microbiol. 2022, 13, 814855. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wei, Z.; Yang, C.; Dai, S.; Wang, X.; Shang, Y. The gut microbiota in experimental abdominal aortic aneurysm. Front. Cardiovasc. Med. 2023, 10, 1051648. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lu, W.; Zhong, L.; Hu, Y.; Li, Q.; Ding, R.; Zhong, Z.; Liu, Z.; Xiao, H.; Xie, D.; et al. Alterations in gut microbiota of abdominal aortic aneurysm mice. BMC Cardiovasc. Disord. 2020, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Z.; Chen, B.Y.; Qiu, P.; Zhou, L.J.; Li, Y.L.; Du, L.J.; Liu, Y.; Wang, Y.L.; Zhu, H.; Wu, X.Y.; et al. Altered salivary microbiota profile in patients with abdominal aortic aneurysm. Heliyon 2023, 9, e23040. [Google Scholar] [CrossRef] [PubMed]
- Salhi, L.; Rijkschroeff, P.; Van Hede, D.; Laine, M.L.; Teughels, W.; Sakalihasan, N.; Lambert, F. Blood Biomarkers and Serologic Immunological Profiles Related to Periodontitis in Abdominal Aortic Aneurysm Patients. Front. Cell Infect. Microbiol. 2021, 11, 766462. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Furuyama, T.; Matsubara, Y.; Morisaki, K.; Onohara, T.; Ikeda, T.; Yoshizumi, T. Gut dysbiosis and bacterial translocation in the aneurysmal wall and blood in patients with abdominal aortic aneurysm. PLoS ONE 2022, 17, e0278995. [Google Scholar] [CrossRef]
- Figuero, E.; Lindahl, C.; Marin, M.J.; Renvert, S.; Herrera, D.; Ohlsson, O.; Wetterling, T.; Sanz, M. Quantification of periodontal pathogens in vascular, blood, and subgingival samples from patients with peripheral arterial disease or abdominal aortic aneurysms. J. Periodontol. 2014, 85, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Kregielczak, A.; Dorocka-Bobkowska, B.; Slomski, R.; Oszkinis, G.; Krasinski, Z. Periodontal status and the incidence of selected bacterial pathogens in periodontal pockets and vascular walls in patients with atherosclerosis and abdominal aortic aneurysms. PLoS ONE 2022, 17, e0270177. [Google Scholar] [CrossRef]
- Kurihara, N.; Inoue, Y.; Iwai, T.; Umeda, M.; Huang, Y.; Ishikawa, I. Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 553–558. [Google Scholar] [CrossRef]
- Hidi, L.; Kovacs, G.I.; Szabo, D.; Makra, N.; Penzes, K.; Juhasz, J.; Sotonyi, P.; Ostorhazi, E. Human blood vessel microbiota in healthy adults based on common femoral arteries of brain-dead multi-organ donors. Front. Cell Infect. Microbiol. 2022, 12, 1056319. [Google Scholar] [CrossRef]
- Yan, Q.; Wi, Y.M.; Thoendel, M.J.; Raval, Y.S.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Jeraldo, P.R.; Chia, N.; Patel, R. Evaluation of the CosmosID Bioinformatics Platform for Prosthetic Joint-Associated Sonicate Fluid Shotgun Metagenomic Data Analysis. J. Clin. Microbiol. 2019, 57, e01182-18. [Google Scholar] [CrossRef] [PubMed]
- Szabo, B.G.; Kiss, R.; Makra, N.; Penzes, K.; Vad, E.; Kamotsay, K.; Szabo, D.; Ostorhazi, E. Composition and changes of blood microbiota in adult patients with community-acquired sepsis: A pilot study from bench to bedside. Front. Cell Infect. Microbiol. 2022, 12, 1067476. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Ishibashi, T.; Asano, R.; Tonomura, S.; Maeda, Y.; Motooka, D.; Ueda, J.; Yanagawa, M.; Edamoto-Taira, Y.; Chikaishi-Kirino, T.; et al. Gut dysbiosis is associated with aortic aneurysm formation and progression in Takayasu arteritis. Arthritis Res. Ther. 2023, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Castelino, M.; Eyre, S.; Moat, J.; Fox, G.; Martin, P.; Ho, P.; Upton, M.; Barton, A. Optimisation of methods for bacterial skin microbiome investigation: Primer selection and comparison of the 454 versus MiSeq platform. BMC Microbiol. 2017, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Almoughrabie, S.; Cau, L.; Cavagnero, K.; O’Neill, A.M.; Li, F.; Roso-Mares, A.; Mainzer, C.; Closs, B.; Kolar, M.J.; Williams, K.J.; et al. Commensal Cutibacterium acnes induce epidermal lipid synthesis important for skin barrier function. Sci. Adv. 2023, 9, eadg6262. [Google Scholar] [CrossRef]
- Al Atrouni, A.; Joly-Guillou, M.L.; Hamze, M.; Kempf, M. Reservoirs of Non-baumannii Acinetobacter Species. Front. Microbiol. 2016, 7, 49. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Yun, Y.; Peng, Y. Bacterial community of a spider, Marpiss magister (Salticidae). 3 Biotech. 2017, 7, 371. [Google Scholar] [CrossRef]
- Satari, L.; Iglesias, A.; Porcar, M. The Microbiome of Things: Appliances, Machines, and Devices Hosting Artificial Niche-Adapted Microbial Communities. Microorganisms 2023, 11, 1507. [Google Scholar] [CrossRef]
- Callewaert, C.; Van Nevel, S.; Kerckhof, F.M.; Granitsiotis, M.S.; Boon, N. Bacterial Exchange in Household Washing Machines. Front. Microbiol. 2015, 6, 1381. [Google Scholar] [CrossRef]
- Mei, Q.X.; Huang, C.L.; Luo, S.Z.; Zhang, X.M.; Zeng, Y.; Lu, Y.Y. Characterization of the duodenal bacterial microbiota in patients with pancreatic head cancer vs. healthy controls. Pancreatology 2018, 18, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Gajic, M.; Zecevic, A.; Milivojevic, I.; Buha, I. Pneumonia caused by Sphingomonas paucimobilis infection after a dental intervention. J. Infect. Dev. Ctries. 2023, 17, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, D.H.; Jung, J.Y.; Kim, J.C.; Jeon, C.O. Comparative ocular microbial communities in humans with and without blepharitis. Invest. Ophthalmol. Vis. Sci. 2012, 53, 5585–5593. [Google Scholar] [CrossRef]
- Liang, C.; Wang, L.; Wang, X.; Jia, Y.; Xie, Q.; Zhao, L.; Yuan, H. Altered ocular surface microbiota in obesity: A case-control study. Front. Cell Infect. Microbiol. 2024, 14, 1356197. [Google Scholar] [CrossRef] [PubMed]
- Shovman, O.; Tiosano, S.; Comaneshter, D.; Cohen, A.D.; Amital, H.; Sherf, M. Aortic aneurysm associated with rheumatoid arthritis: A population-based cross-sectional study. Clin. Rheumatol. 2016, 35, 2657–2661. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Lundmark, A.; Delgado, L.F.; Hu, Y.O.O.; Fei, G.; Lee, L.; Fei, C.; Catrina, A.I.; Jansson, L.; Andersson, A.F.; et al. Salivary Microbiota and Host-Inflammatory Responses in Periodontitis Affected Individuals With and Without Rheumatoid Arthritis. Front. Cell Infect. Microbiol. 2022, 12, 841139. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Hao, H.; Naito, Y.; Oboshi, M.; Hirotani, S.; Mitsuno, M.; Miyamoto, Y.; Hirota, S.; Masuyama, T. Aortic iron overload with oxidative stress and inflammation in human and murine abdominal aortic aneurysm. Arter. Thromb. Vasc. Biol. 2015, 35, 1507–1514. [Google Scholar] [CrossRef]
- Dang, G.; Li, T.; Yang, D.; Yang, G.; Du, X.; Yang, J.; Miao, Y.; Han, L.; Ma, X.; Song, Y.; et al. T lymphocyte-derived extracellular vesicles aggravate abdominal aortic aneurysm by promoting macrophage lipid peroxidation and migration via pyruvate kinase muscle isozyme 2. Redox Biol. 2022, 50, 102257. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wan, S.; Han, M.; Zhao, Y.; Li, C.; Cai, G.; Zhang, S.; Sun, Z.; Hu, X.; Cao, H.; et al. Plasma Metabolomics Analysis Identifies Abnormal Energy, Lipid, and Amino Acid Metabolism in Abdominal Aortic Aneurysms. Med. Sci. Monit. 2020, 26, e926766. [Google Scholar] [CrossRef]
- Hosie, A.H.; Poole, P.S. Bacterial ABC transporters of amino acids. Res. Microbiol. 2001, 152, 259–270. [Google Scholar] [CrossRef]
- Liang, E.S.; Cheng, W.; Yang, R.X.; Bai, W.W.; Liu, X.; Zhao, Y.X. Peptidyl-prolyl isomerase Pin1 deficiency attenuates angiotensin II-induced abdominal aortic aneurysm formation in ApoE(-/-) mice. J. Mol. Cell Cardiol. 2018, 114, 334–344. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes-Nikodém, É.; Gyurok, G.P.; Dunai, Z.A.; Makra, N.; Hofmeister, B.; Szabó, D.; Sótonyi, P.; Hidi, L.; Szappanos, Á.; Kovács, G.; et al. Relationship between Gut, Blood, Aneurysm Wall and Thrombus Microbiome in Abdominal Aortic Aneurysm Patients. Int. J. Mol. Sci. 2024, 25, 8844. https://doi.org/10.3390/ijms25168844
Nemes-Nikodém É, Gyurok GP, Dunai ZA, Makra N, Hofmeister B, Szabó D, Sótonyi P, Hidi L, Szappanos Á, Kovács G, et al. Relationship between Gut, Blood, Aneurysm Wall and Thrombus Microbiome in Abdominal Aortic Aneurysm Patients. International Journal of Molecular Sciences. 2024; 25(16):8844. https://doi.org/10.3390/ijms25168844
Chicago/Turabian StyleNemes-Nikodém, Éva, Gergő Péter Gyurok, Zsuzsanna A. Dunai, Nóra Makra, Bálint Hofmeister, Dóra Szabó, Péter Sótonyi, László Hidi, Ágnes Szappanos, Gergely Kovács, and et al. 2024. "Relationship between Gut, Blood, Aneurysm Wall and Thrombus Microbiome in Abdominal Aortic Aneurysm Patients" International Journal of Molecular Sciences 25, no. 16: 8844. https://doi.org/10.3390/ijms25168844
APA StyleNemes-Nikodém, É., Gyurok, G. P., Dunai, Z. A., Makra, N., Hofmeister, B., Szabó, D., Sótonyi, P., Hidi, L., Szappanos, Á., Kovács, G., & Ostorházi, E. (2024). Relationship between Gut, Blood, Aneurysm Wall and Thrombus Microbiome in Abdominal Aortic Aneurysm Patients. International Journal of Molecular Sciences, 25(16), 8844. https://doi.org/10.3390/ijms25168844





