5β-Dihydrosteroids: Formation and Properties
Abstract
1. Introduction
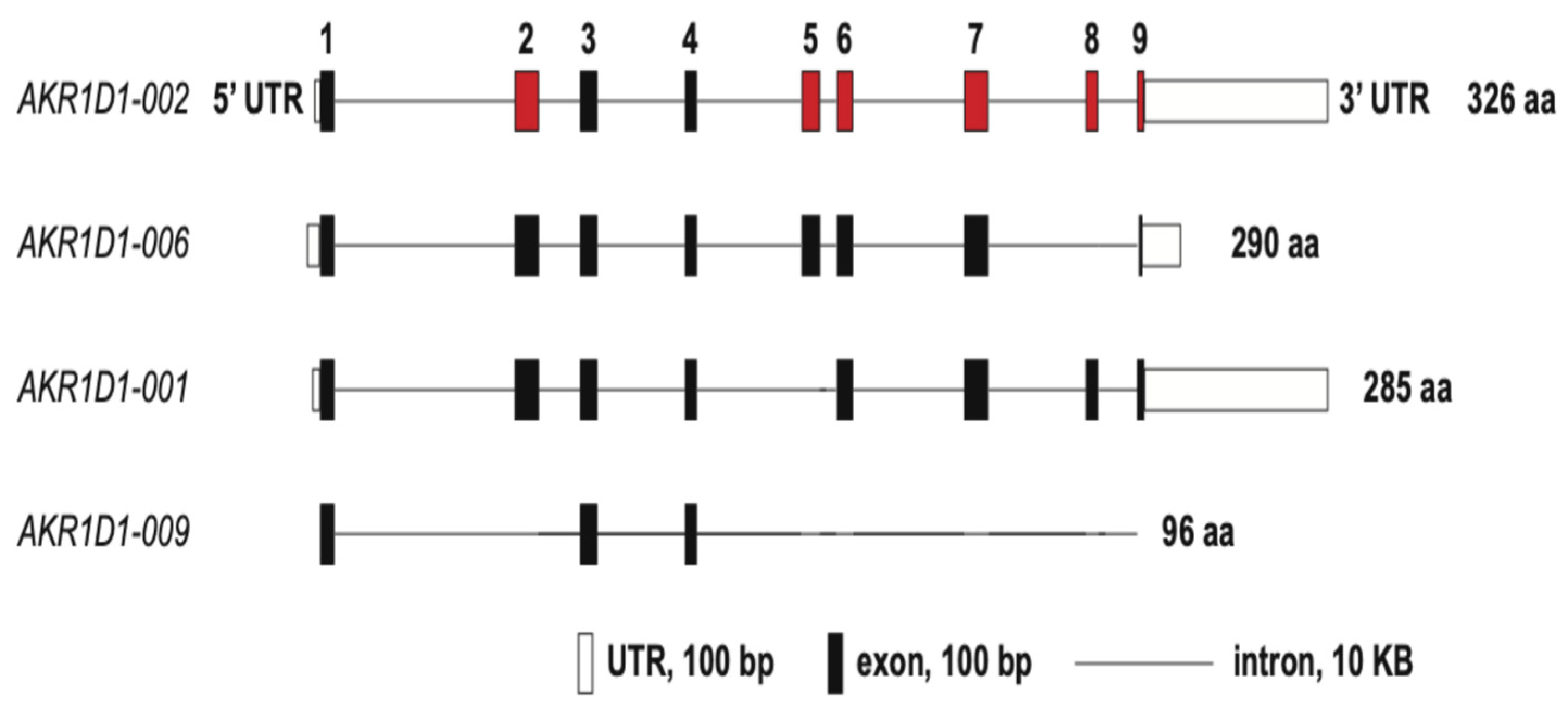
2. Steroid 5β-Reductase Gene
3. Steroid 5β-Reductase Enzymology
4. Steroid 5β-Reductase Deficiency
5. AKR1D4 Knockout Mice
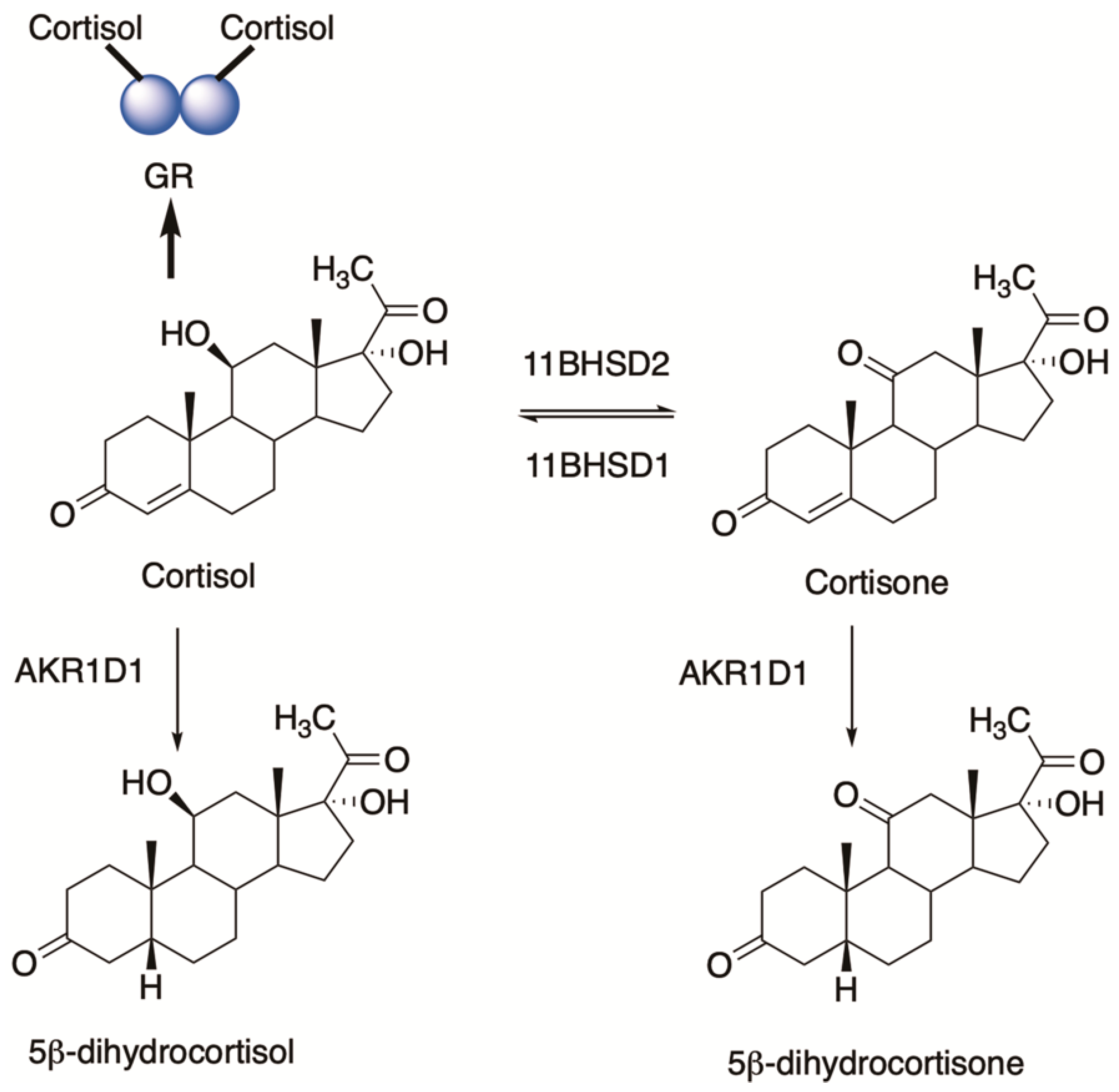
6. Glucocorticoids and 5β-dihydroglucocorticoids
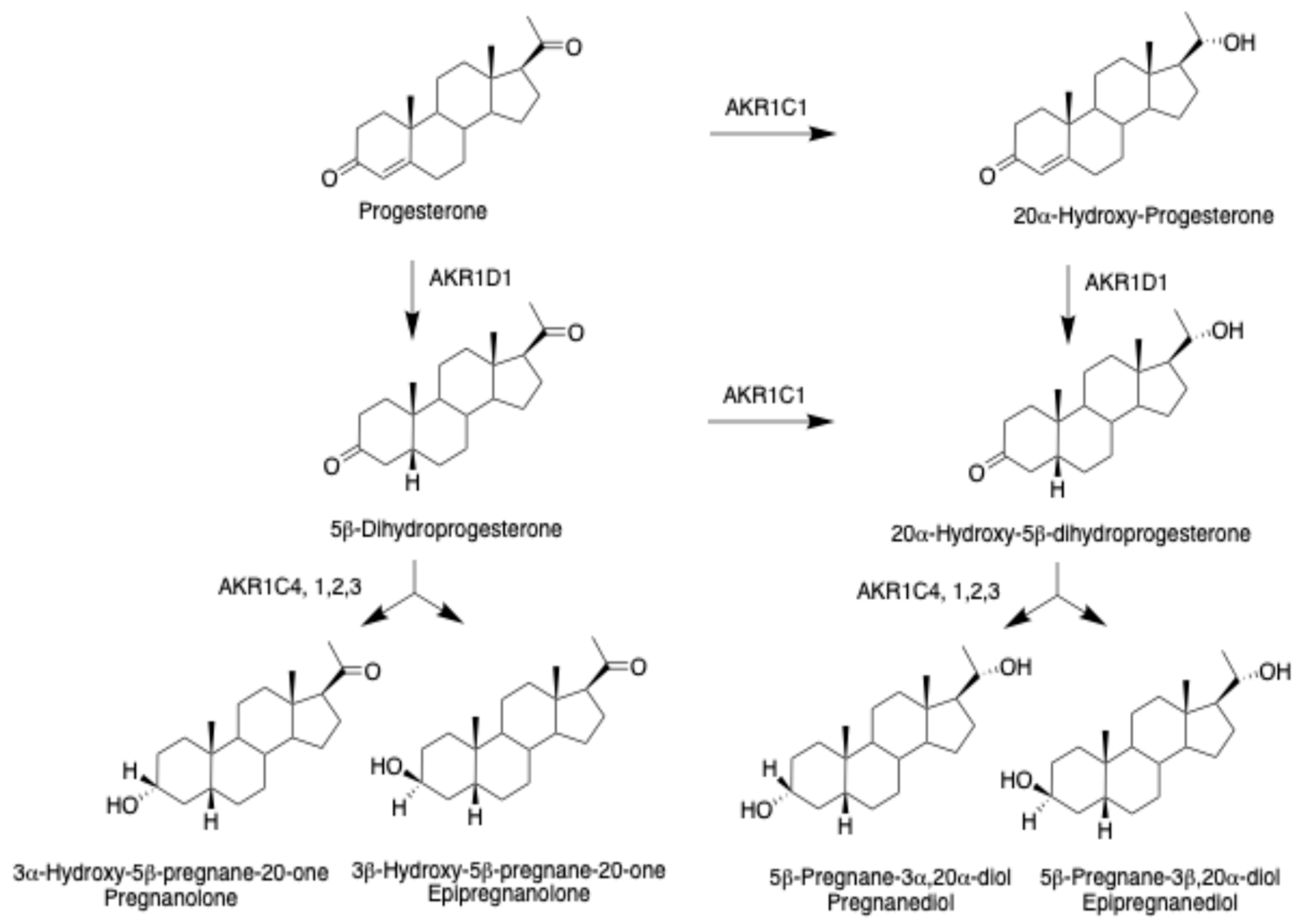
7. Progestins and 5β-Pregnanes
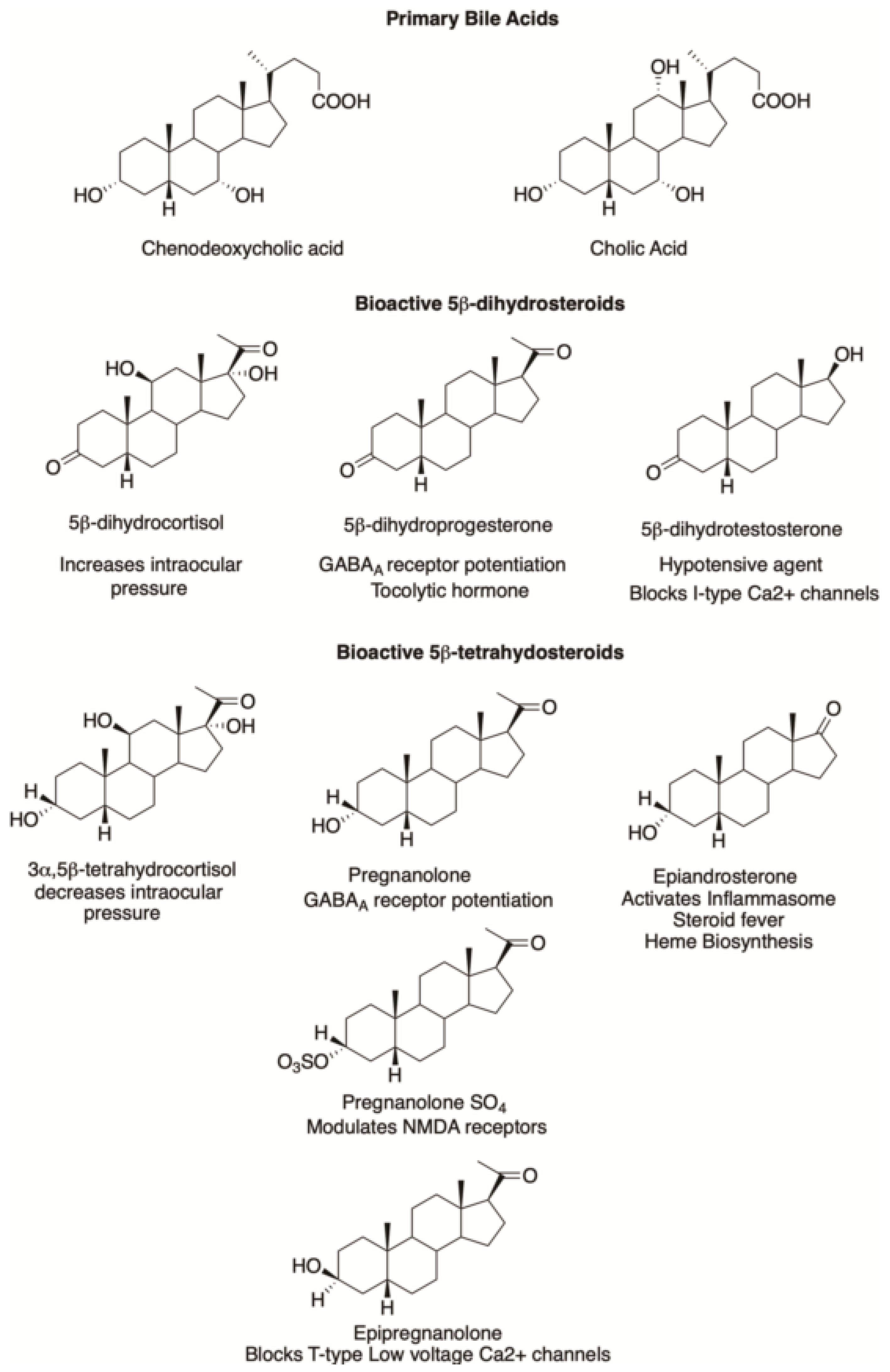
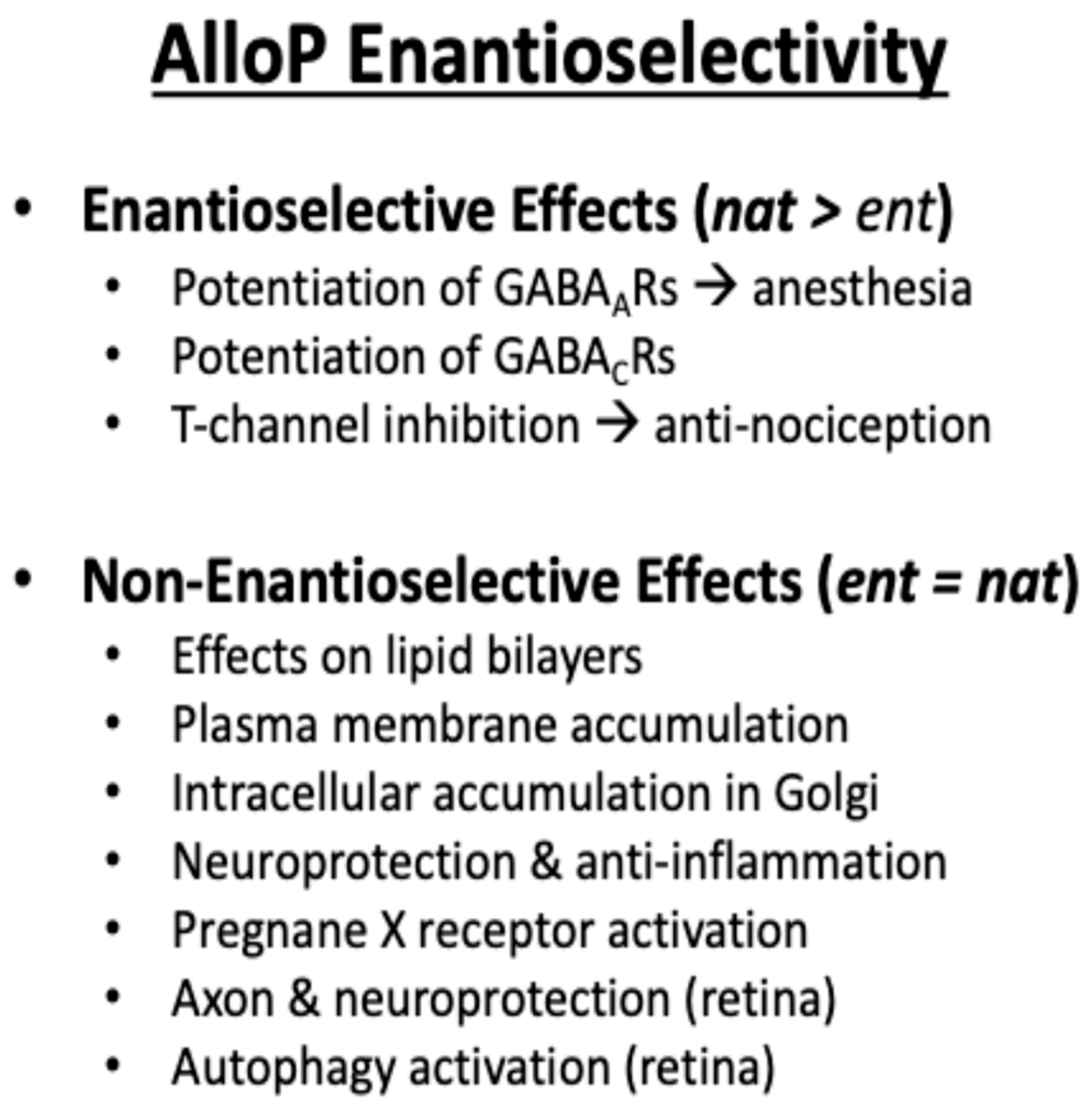
7.1. Neuroactive Steroids
7.2. Tocolytic Hormones
7.3. Platelet Activation
8. 5β-Androstanes
9. Farnesoid X Receptor (FXR) Ligands
10. Pregnane X Receptor (PXR) Ligands
11. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russell, D.W.; Wilson, J.D. Steroid 5α-reductase: Two genes/two enzymes. Annu. Rev. Biochem. 1994, 63, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Drury, J.E.; Penning, T.M. Substrate specificity and inhibitor analyses of human steroid 5β-reductase (AKR1D1). Steroids 2011, 76, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M.; Burczynski, M.E.; Jez, J.M.; Hung, C.F.; Lin, H.K.; Ma, H.; Moore, M.; Palackal, N.; Ratnam, K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: Functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000, 351, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Steckelbroeck, S.; Jin, Y.; Gopishetty, S.; Oyesanmi, B.; Penning, T.M. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: Implications for steroid hormone metabolism and action. J. Biol. Chem. 2004, 279, 10784–10795. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M.; Wangtrakuldee, P.; Auchus, R.J. Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes. Endocr. Rev. 2019, 40, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.L.; Mendonca, B.B. The Molecular Basis of 5α-Reductase Type 2 Deficiency. Sex. Dev. 2022, 16, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, B.B.; Batista, R.L.; Domenice, S.; Costa, E.M.; Arnhold, I.J.; Russell, D.W.; Wilson, J.D. Steroid 5α-reductase 2 deficiency. J. Steroid Biochem. Mol. Biol. 2016, 163, 206–211. [Google Scholar] [CrossRef]
- Daugherty, C.C.; Setchell, K.D.; Heubi, J.E.; Balistreri, W.F. Resolution of liver biopsy alterations in three siblings with bile acid treatment of an inborn error of bile acid metabolism (delta-4-3-oxosteroid 5β-reductase deficiency). Hepatology 1993, 18, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Lemonde, H.A.; Custard, E.J.; Bouquet, J.; Duran, M.; Overmars, H.; Scambler, P.J.; Clayton, P.T. Mutations in SRD5B1 (AKR1D1), the gene encoding delta(4)-3-oxosteroid 5β-reductase, in hepatitis and liver failure in infancy. Gut 2003, 52, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Suchy, F.J.; Welsh, M.B.; Zimmer-Nechemias, L.; Heubi, J.; Balistreri, W.F. Delta-4-3-oxosteroid 5 β-reductase deficiency described in identical twins with neonatal hepatitis. A new inborn error in bile acid synthesis. J. Clin. Investig. 1988, 82, 2148–2157. [Google Scholar] [CrossRef]
- Makishima, M.; Okamoto, A.Y.; Repa, J.J.; Tu, H.; Learned, R.M.; Luk, A.; Hull, M.V.; Lustig, K.D.; Mangelsdorf, D.J.; Shan, B. Identification of a nuclear receptor for bile acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.J.; Blanchard, S.G.; Bledsoe, R.K.; Chandra, G.; Consler, T.G.; Kliewer, S.A.; Stimmel, J.B.; Willson, T.M.; Zavacki, A.M.; Moore, D.D.; et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Juřica, J.; Dovrtělová, G.; Nosková, K.; Zendulka, O. Bile acids, nuclear receptors and cytochrome P450. Physiol. Res. 2016, 65, S427–S440. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Penning, T.M. 5β-Reduced steroids and human Δ(4)-3-ketosteroid 5β-reductase (AKR1D1). Steroids 2014, 83, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Appanna, N.; Gibson, H.; Gangitano, E.; Dempster, N.J.; Morris, K.; George, S.; Arvaniti, A.; Gathercole, L.L.; Keevil, B.; Penning, T.M.; et al. Differential activity and expression of human 5β-reductase (AKR1D1) splice variants. J. Mol. Endocrinol. 2021, 66, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Okuda, A.; Okuda, K. Purification and characterization of delta 4-3-ketosteroid 5 β-reductase. J. Biol. Chem. 1984, 259, 7519–7524. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.H.; Kai, M.H.; Setoguchi, Y.; Eggertsen, G.; Sjoblom, P.; Setoguchi, T.; Okuda, K.I.; Bjorkhem, I. Cloning and expression of cDNA of human delta 4-3-oxosteroid 5 β-reductase and substrate specificity of the expressed enzyme. Eur. J. Biochem. 1994, 219, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997, 326(Part 3), 625–636. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. Steroid 5β-reductase (AKR1D1): Purification and characterization. Methods Enzymol. 2023, 689, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, L.; Drury, J.E.; Penning, T.M.; Christianson, D.W. Crystal structure of human liver Delta4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J. Biol. Chem. 2008, 283, 16830–16839. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, L.; Drury, J.E.; Christianson, D.W.; Penning, T.M. Structure and catalytic mechanism of human steroid 5β-reductase (AKR1D1). Mol. Cell Endocrinol. 2009, 301, 191–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shneider, B.L.; Setchell, K.D.; Whitington, P.F.; Neilson, K.A.; Suchy, F.J. Delta 4-3-oxosteroid 5 β-reductase deficiency causing neonatal liver failure and hemochromatosis. J. Pediatr. 1994, 124, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Andreou, E.R.; Prokipcak, R.D. Analysis of human CYP7A1 mRNA decay in HepG2 cells by reverse transcription-polymerase chain reaction. Arch. Biochem. Biophys. 1998, 357, 137–146. [Google Scholar] [CrossRef]
- Gonzales, E.; Cresteil, D.; Baussan, C.; Dabadie, A.; Gerhardt, M.F.; Jacquemin, E. SRD5B1 (AKR1D1) gene analysis in delta(4)-3-oxosteroid 5β-reductase deficiency: Evidence for primary genetic defect. J. Hepatol. 2004, 40, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Ueki, I.; Kimura, A.; Chen, H.L.; Yorifuji, T.; Mori, J.; Itoh, S.; Maruyama, K.; Ishige, T.; Takei, H.; Nittono, H.; et al. SRD5B1 gene analysis needed for the accurate diagnosis of primary 3-oxo-Delta4-steroid 5β-reductase deficiency. J. Gastroenterol. Hepatol. 2009, 24, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.E.; Mindnich, R.; Penning, T.M. Characterization of disease-related 5β-reductase (AKR1D1) mutations reveals their potential to cause bile acid deficiency. J. Biol. Chem. 2010, 285, 24529–24537. [Google Scholar] [CrossRef]
- Chen, M.; Jin, Y.; Penning, T.M. In-Depth Dissection of the P133R Mutation in Steroid 5β-Reductase (AKR1D1): A Molecular Basis of Bile Acid Deficiency. Biochemistry 2015, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Gardin, A.; Ruiz, M.; Beime, J.; Cananzi, M.; Rathert, M.; Rohmer, B.; Grabhorn, E.; Almes, M.; Logarajah, V.; Peña-Quintana, L.; et al. ∆(4)-3-oxo-5β-reductase deficiency: Favorable outcome in 16 patients treated with cholic acid. Orphanet J. Rare Dis. 2023, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Mori, J.; Pham, A.N.; Thi, K.B.; Takei, H.; Murai, T.; Hayashi, H.; Nittono, H. Healthy Patients with AKR1D1 Mutation Not Requiring Primary Bile Acid Therapy: A Case Series. JPGN Rep. 2023, 4, e372. [Google Scholar] [CrossRef] [PubMed]
- Gathercole, L.L.; Nikolaou, N.; Harris, S.E.; Arvaniti, A.; Poolman, T.M.; Hazlehurst, J.M.; Kratschmar, D.V.; Todorčević, M.; Moolla, A.; Dempster, N.; et al. AKR1D1 knockout mice develop a sex-dependent metabolic phenotype. J. Endocrinol. 2022, 253, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, N.; Gathercole, L.L.; Kirkwood, L.; Dunford, J.E.; Hughes, B.A.; Gilligan, L.C.; Oppermann, U.; Penning, T.M.; Arlt, W.; Hodson, L.; et al. AKR1D1 regulates glucocorticoid availability and glucocorticoid receptor activation in human hepatoma cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M.; Krozowski, Z.S. 11 β-Hydroxysteroid dehydrogenase. Vitam. Horm. 1999, 57, 249–324. [Google Scholar] [PubMed]
- Nikolaou, N.; Arvaniti, A.; Appanna, N.; Sharp, A.; Hughes, B.A.; Digweed, D.; Whitaker, M.J.; Ross, R.; Arlt, W.; Penning, T.M.; et al. Glucocorticoids regulate AKR1D1 activity in human liver in vitro and in vivo. J. Endocrinol. 2020, 245, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. Indomethacin and glucocorticoid metabolism in rat liver cytosol. Biochem. Pharmacol. 1986, 35, 4203–4209. [Google Scholar] [CrossRef] [PubMed]
- Southren, A.L.; Gordon, G.G.; l‘Hommedieu, D.; Ravikumar, S.; Dunn, M.W.; Weinstein, B.I. 5β-Dihydrocortisol: Possible mediator of the ocular hypertension in glaucoma. Investig. Ophthalmol. Vis. Sci. 1985, 26, 393–395. [Google Scholar]
- Weinstein, B.I.; Gordon, G.G.; Southren, A.L. Potentiation of glucocorticoid activity by 5β-dihydrocortisol: Its role in glaucoma. Science 1983, 222, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Southren, A.L.; l‘Hommedieu, D.; Gordon, G.G.; Weinstein, B.I. Intraocular hypotensive effect of a topically applied cortisol metabolite: 3α, 5β-tetrahydrocortisol. Investig. Ophthalmol. Vis. Sci. 1987, 28, 901–903. [Google Scholar]
- Lan, N.C.; Gee, K.W.; Bolger, M.B.; Chen, J.S. Differential responses of expressed recombinant human gamma-aminobutyric acidA receptors to neurosteroids. J. Neurochem. 1991, 57, 1818–1821. [Google Scholar] [CrossRef]
- Manzella, F.M.; Cabrera, O.H.; Wilkey, D.; Fine-Raquet, B.; Klawitter, J.; Krishnan, K.; Covey, D.F.; Jevtovic-Todorovic, V.; Todorovic, S.M. Sex-specific hypnotic effects of the neuroactive steroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile are mediated by peripheral metabolism into an active hypnotic steroid. Br. J. Anaesth. 2023, 130, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Nyagan, A.G.; Do-Rego, J.L.; Beaujean, D.; Luu-The, V.; Pelletier, G.; Vaudry, H. Neurosteroids: Expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 1999, 51, 63–81. [Google Scholar] [PubMed]
- Robel, P.; Baulieu, E.E. Neurosteroids Biosynthesis and function. Trends Endocrinol. Metab. 1994, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Mesaros, A.C.; Blair, I.A.; Penning, T.M. Stereospecific reduction of 5β-reduced steroids by human ketosteroid reductases of the AKR (aldo-keto reductase) superfamily: Role of AKR1C1-AKR1C4 in the metabolism of testosterone and progesterone via the 5β-reductase pathway. Biochem. J. 2011, 437, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Callachan, H.; Cottrell, G.A.; Hather, N.Y.; Lambert, J.J.; Nooney, J.M.; Peters, J.A. Modulation of the GABAA receptor by progesterone metabolites. Proc. R. Soc. Lond. B Biol. Sci. 1987, 231, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.S.; Gibbs, T.T.; Farb, D.H. Pregnenolone sulfate: A positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol. Pharmacol. 1991, 40, 333–336. [Google Scholar] [PubMed]
- Weaver, C.E.; Land, M.B.; Purdy, R.H.; Richards, K.G.; Gibbs, T.T.; Farb, D.H. Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca(2+) accumulation and cell death. J. Pharmacol. Exp. Ther. 2000, 293, 747–754. [Google Scholar]
- Szczurowska, E.; Szanti-Pinter, E.; Randakova, A.; Jakubik, J.; Kudova, E. Allosteric Modulation of Muscarinic Receptors by Cholesterol, Neurosteroids and Neuroactive Steroids. Int. J. Mol. Sci. 2022, 23, 13075. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D.; Harrison, N.L.; Schwartz, R.D.; Barker, J.L.; Paul, S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986, 232, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABA(A) receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Krishnan, K.; Cai, Z.Y.; Manion, B.D.; Benz, A.; Taylor, A.; Evers, A.S.; Zorumski, C.F.; Mennerick, S.; Covey, D.F. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens. Eur. J. Med. Chem. 2008, 43, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Tateiwa, H.; Chintala, S.M.; Chen, Z.; Wang, L.; Amtashar, F.; Bracamontes, J.; Germann, A.L.; Pierce, S.R.; Covey, D.F.; Akk, G.; et al. The Mechanism of Enantioselective Neurosteroid Actions on GABA(A) Receptors. Biomolecules 2023, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Hering, W.J.; Ihmsen, H.; Langer, H.; Uhrlau, C.; Dinkel, M.; Geisslinger, G.; Schüttler, J. Pharmacokinetic-pharmacodynamic modeling of the new steroid hypnotic eltanolone in healthy volunteers. Anesthesiology 1996, 85, 1290–1299. [Google Scholar] [CrossRef]
- Martinez Botella, G.; Salituro, F.G.; Harrison, B.L.; Beresis, R.T.; Bai, Z.; Blanco, M.J.; Belfort, G.M.; Dai, J.; Loya, C.M.; Ackley, M.A.; et al. Neuroactive Steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1’-yl)-19-nor-5β-pregnan-20-one (SAGE-217): A Clinical Next Generation Neuroactive Steroid Positive Allosteric Modulator of the (gamma-Aminobutyric Acid)(A) Receptor. J. Med. Chem. 2017, 60, 7810–7819. [Google Scholar] [CrossRef] [PubMed]
- Coulter, I.; Timic Stamenic, T.; Eggan, P.; Fine, B.R.; Corrigan, T.; Covey, D.F.; Yang, L.; Pan, J.Q.; Todorovic, S.M. Different roles of T-type calcium channel isoforms in hypnosis induced by an endogenous neurosteroid epipregnanolone. Neuropharmacology 2021, 197, 108739. [Google Scholar] [CrossRef] [PubMed]
- Vyklicky, V.; Korinek, M.; Smejkalova, T.; Balik, A.; Krausova, B.; Kaniakova, M.; Lichnerova, K.; Cerny, J.; Krusek, J.; Dittert, I.; et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol. Res. 2014, 63 (Suppl. 1), S191–S203. [Google Scholar] [CrossRef] [PubMed]
- Smejkalova, T.; Korinek, M.; Krusek, J.; Hrcka Krausova, B.; Candelas Serra, M.; Hajdukovic, D.; Kudova, E.; Chodounska, H.; Vyklicky, L. Endogenous neurosteroids pregnanolone and pregnanolone sulfate potentiate presynaptic glutamate release through distinct mechanisms. Br. J. Pharmacol. 2021, 178, 3888–3904. [Google Scholar] [CrossRef] [PubMed]
- Kussius, C.L.; Kaur, N.; Popescu, G.K. Pregnanolone sulfate promotes desensitization of activated NMDA receptors. J. Neurosci. 2009, 29, 6819–6827. [Google Scholar] [CrossRef]
- Kysilov, B.; Hrcka Krausova, B.; Vyklicky, V.; Smejkalova, T.; Korinek, M.; Horak, M.; Chodounska, H.; Kudova, E.; Cerny, J.; Vyklicky, L. Pregnane-based steroids are novel positive NMDA receptor modulators that may compensate for the effect of loss-of-function disease-associated GRIN mutations. Br. J. Pharmacol. 2022, 179, 3970–3990. [Google Scholar] [CrossRef] [PubMed]
- Steckelbroeck, S.; Lütjohann, D.; Bauman, D.R.; Ludwig, M.; Friedl, A.; Hans, V.H.; Penning, T.M.; Klingmüller, D. Non-stereo-selective cytosolic human brain tissue 3-ketosteroid reductase is refractory to inhibition by AKR1C inhibitors. Biochim. Biophys. Acta 2010, 1801, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Covey, D.F. ent-Steroids: Novel tools for studies of signaling pathways. Steroids 2009, 74, 577–585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Covey, D.F.; Evers, A.S.; Izumi, Y.; Maguire, J.L.; Mennerick, S.J.; Zorumski, C.F. Neurosteroid enantiomers as potentially novel neurotherapeutics. Neurosci. Biobehav. Rev. 2023, 149, 105191. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.F.; Mitchell, J.M.; Chowdhury, J.; Tougas, M.; Engelen, S.M.; Senff, N.; Heijnen, I.; Moore, J.T.; Goodwin, B.; Wong, S.; et al. Metabolites of progesterone and the pregnane X receptor: A novel pathway regulating uterine contractility in pregnancy? Am. J. Obstet. Gynecol. 2005, 192, 1304–1313; discussion 1313–1305. [Google Scholar] [CrossRef] [PubMed]
- Grazzini, E.; Guillon, G.; Mouillac, B.; Zingg, H.H. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 1998, 392, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.M. A possible role for progesterone metabolites in human parturition. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.M.; Rice, G.E.; Moses, E.K.; Brennecke, S.P. 5β-dihydroprogesterone and steroid 5β-reductase decrease in association with human parturition at term. Mol. Hum. Reprod. 2005, 11, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Zicari, A.; Realacci, M.; Di Vito, M.; Denora, P.; Narcisi, M.; Russo, M.A.; Piccione, E. Oxytocin modulates nitric oxide generation by human fetal membranes at term pregnancy. Am. J. Reprod. Immunol. 2004, 52, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, P.F. Progesterone metabolites rapidly stimulate calcium influx in human platelets by a src-dependent pathway. Steroids 2008, 73, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Perusquia, M.; Hanson, A.E.; Meza, C.M.; Kubli, C.; Herrera, N.; Stallone, J.N. Antihypertensive responses of vasoactive androgens in an in vivo experimental model of preeclampsia. J. Steroid Biochem. Mol. Biol. 2018, 178, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.M.; Flores-Soto, E.; Sommer, B.; Solis-Chagoyan, H.; Perusquia, M. Androgens are effective bronchodilators with anti-inflammatory properties: A potential alternative for asthma therapy. Steroids 2020, 153, 108509. [Google Scholar] [CrossRef] [PubMed]
- Imperato-McGinley, J.; Peterson, R.E.; Gautier, T.; Arthur, A.; Shackleton, C. Decreased urinary C19 and C21 steroid 5α-metabolites in parents of male pseudohermaphrodites with 5α-reductase deficiency: Detection of carriers. J. Clin. Endocrinol. Metab. 1985, 60, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Stoll, A.; Iannone, M.; De Gregorio, G.; Molaioni, F.; de la Torre, X.; Botrè, F.; Parr, M.K. Influence of Indomethacin on Steroid Metabolism: Endocrine Disruption and Confounding Effects in Urinary Steroid Profiling of Anti-Doping Analyses. Metabolites 2020, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, F.; Chirita, D.; Dalmon, S.; Martin, A.; Bronnec, P.; Sousa, J.; Helynck, O.; Lee, W.; Kastner, D.L.; Chae, J.J.; et al. Steroid hormone catabolites activate the pyrin inflammasome through a non-canonical mechanism. Cell Rep. 2022, 41, 111472. [Google Scholar] [CrossRef] [PubMed]
- Incefy, G.S.; Kappas, A. Enhancement of RNA synthesis in avian liver cell cultures by a 5β-steroid metabolite during induction of δ-aminolevulinate synthase. Proc. Natl. Acad. Sci. USA 1974, 71, 2290–2294. [Google Scholar] [CrossRef] [PubMed]
- Levere, R.D.; Granick, S. Control of hemoglobin synthesis in the cultured chick blastoderm. J. Biol. Chem. 1967, 242, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Zolkowska, D.; Dhir, A.; Krishnan, K.; Covey, D.F.; Rogawski, M.A. Anticonvulsant potencies of the enantiomers of the neurosteroids androsterone and etiocholanolone exceed those of the natural forms. Psychopharmacology 2014, 231, 3325–3332. [Google Scholar] [CrossRef]
- Tu, H.; Okamoto, A.Y.; Shan, B. FXR, a bile acid receptor and biological sensor. Trends Cardiovasc. Med. 2000, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Hollister, K.; Sowers, L.C.; Forman, B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 1999, 3, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.T.; Makishima, M.; Repa, J.J.; Schoonjans, K.; Kerr, T.A.; Auwerx, J.; Mangelsdorf, D.J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell 2000, 6, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Anant, S.; Covey, D.F.; Stenson, W.F. Characterization of enantiomeric bile acid-induced apoptosis in colon cancer cell lines. J. Biol. Chem. 2009, 284, 3354–3364. [Google Scholar] [CrossRef] [PubMed]
- Dussault, I.; Yoo, H.D.; Lin, M.; Wang, E.; Fan, M.; Batta, A.K.; Salen, G.; Erickson, S.K.; Forman, B.M. Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance. Proc. Natl. Acad. Sci. USA 2003, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Drocourt, L.; Pascussi, J.M.; Assenat, E.; Fabre, J.M.; Maurel, P.; Vilarem, M.J. Calcium channel modulators of the dihydropyridine family are human pregnane X receptor activators and inducers of CYP3A, CYP2B, and CYP2C in human hepatocytes. Drug Metab. Dispos. 2001, 29, 1325–1331. [Google Scholar] [PubMed]
- Waxman, D.J. P450 gene induction by structurally diverse xenochemicals: Central role of nuclear receptors CAR, PXR, and PPAR. Arch. Biochem. Biophys. 1999, 369, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Chang, C.; Mani, S.; Krasowski, M.D.; Reschly, E.J.; Iyer, M.; Kholodovych, V.; Ai, N.; Welsh, W.J.; Sinz, M.; et al. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol. Pharmacol. 2007, 72, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Abramova, V.; Leal Alvarado, V.; Hill, M.; Smejkalova, T.; Maly, M.; Vales, K.; Dittert, I.; Bozikova, P.; Kysilov, B.; Hrcka Krausova, B.; et al. Effects of Pregnanolone Glutamate and Its Metabolites on GABA(A) and NMDA Receptors and Zebrafish Behavior. ACS Chem. Neurosci. 2023, 14, 1870–1883. [Google Scholar] [CrossRef] [PubMed]


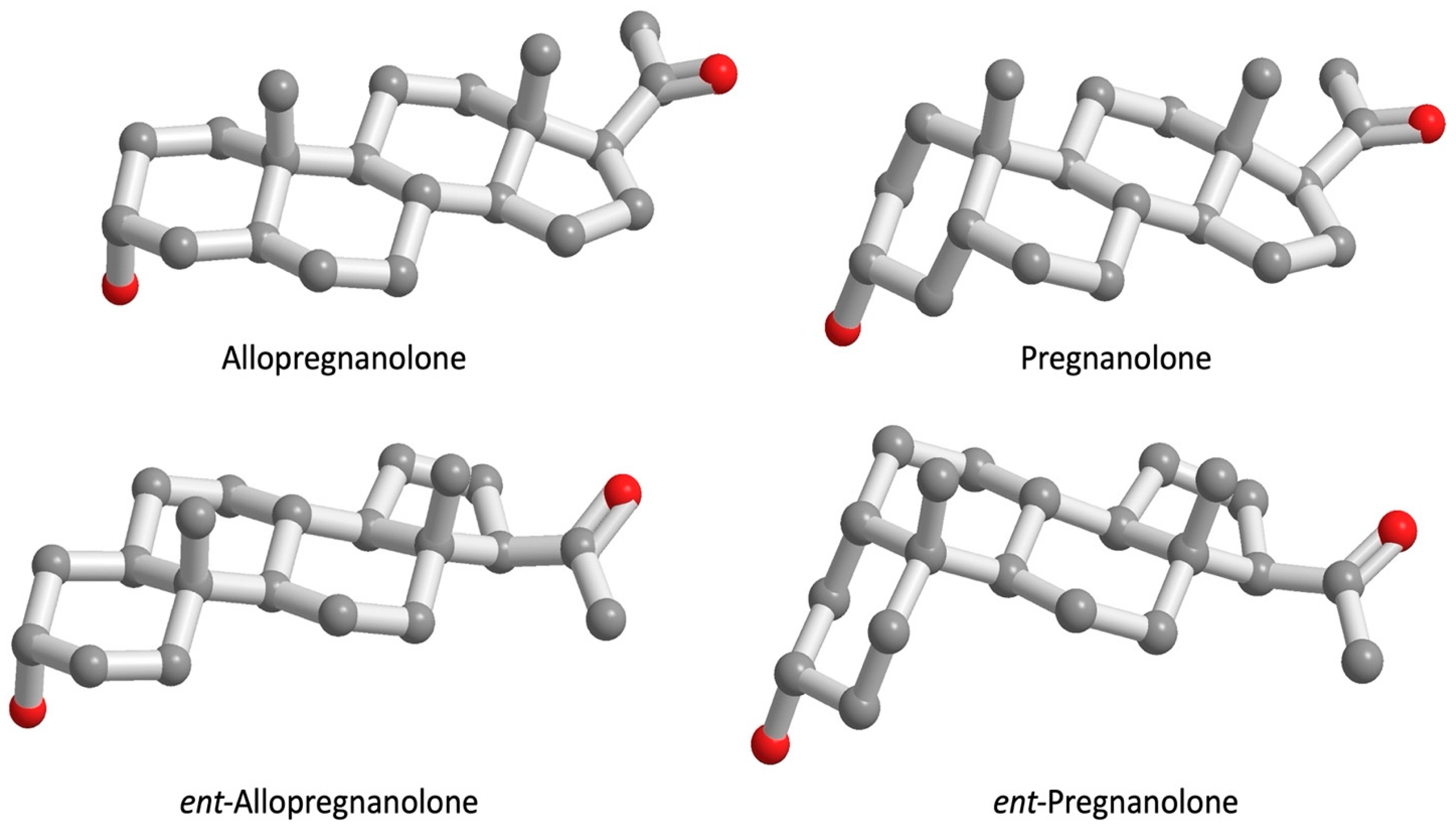
| Inhibition of [3H] Oxytocin Binding to OTR by Progesterone Derivatives | ||
|---|---|---|
| Steroid Compound | Ki (nM) * | |
| Rat OTR | Human OTR | |
| Progesterone | 19 ± 3 | None † |
| R5020 | 59 ± 7 | None † |
| RU486 | 46 ± 5 | None † |
| 5β-pregnane-3,20-dione | None † | 32 ± 5 |
| Progesterone Metabolite | nM |
|---|---|
| 4-Pregnene-3,20-dione (progesterone) | 5 ± 4 |
| 5α-Pregnane-3α,21-diol-20-one (allo THDOC) | 21 ± 2 |
| 5α-Pregnane-3,20-dione (5α-dihydroprogesterone) | 26 ± 5 |
| 5α-Pregnan-3α-ol-20-one (allopregnanolone) | 26 ± 4 |
| 4-Pregnen-20α-ol-3-one (20α-hydroxyprogesterone) | 28 ± 4 |
| 5β-Pregnane-3α, 21-diol-20-one (THDOC) | 44 ± 5 |
| 5α-Pregnan-3β-ol-20-one (isopregnanolone) | 58 ± 19 |
| 5β-Pregnane-3,20-dione (5β-dihydroprogesterone) | 68 ±14 |
| 5α-Pregnane-3α,20α-diol (allo-pregnanediol) | 100 ± 10 |
| 5β-Pregnan-3β-ol-20-one (epipregnanolone) | 107 ± 13 |
| 5β-Pregnan-3α-ol-20-one (pregnanolone) | 142 ± 20 |
| 5α-Pregnane-3β,20α-diol (isopregnanediol) | 215 ± 18 |
| 5β-Pregnane-3α,20α-diol (pregnanediol) | 413 ± 116 |
| Thrombin (0.01 U/mL) | 417 ± 31 |
| 5β-Steroid | EC50 (μM) | % Efficacy/100 |
|---|---|---|
| 5β-pregnane-3,20-dione | 2.6 ± 0.2 | 0.97 |
| 5β-pregnane-3α,20β-diol | 3.81 ± 0.3 | 0.94 |
| 5β-androstan-3α-ol | 1.41 ± 0.01 | 1.12 |
| Lithocholic acid acetate | 1.2 ± 0.2 | 0.54 |
| Lithocholic acid | 10.0 ± 0.1 | 0.15 |
| 7-Ketolithocholic acid | 21.5 ± 1.4 | 0.58 |
| 12-Ketolithocholic acid | 31.3 ± 5.8 | 0.86 |
| Taurochenodeoxycholate | 104 ± 0.8 | 0.5 |
| 5β-cholan-3α,7α,12α,24-tetrol | >100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penning, T.M.; Covey, D.F. 5β-Dihydrosteroids: Formation and Properties. Int. J. Mol. Sci. 2024, 25, 8857. https://doi.org/10.3390/ijms25168857
Penning TM, Covey DF. 5β-Dihydrosteroids: Formation and Properties. International Journal of Molecular Sciences. 2024; 25(16):8857. https://doi.org/10.3390/ijms25168857
Chicago/Turabian StylePenning, Trevor M., and Douglas F. Covey. 2024. "5β-Dihydrosteroids: Formation and Properties" International Journal of Molecular Sciences 25, no. 16: 8857. https://doi.org/10.3390/ijms25168857
APA StylePenning, T. M., & Covey, D. F. (2024). 5β-Dihydrosteroids: Formation and Properties. International Journal of Molecular Sciences, 25(16), 8857. https://doi.org/10.3390/ijms25168857







