Human Primary Lens Epithelial Cultures on Basal Laminas Studied by Synchrotron-Based FTIR Microspectroscopy for Understanding Posterior Capsular Opacification
Abstract
:1. Introduction
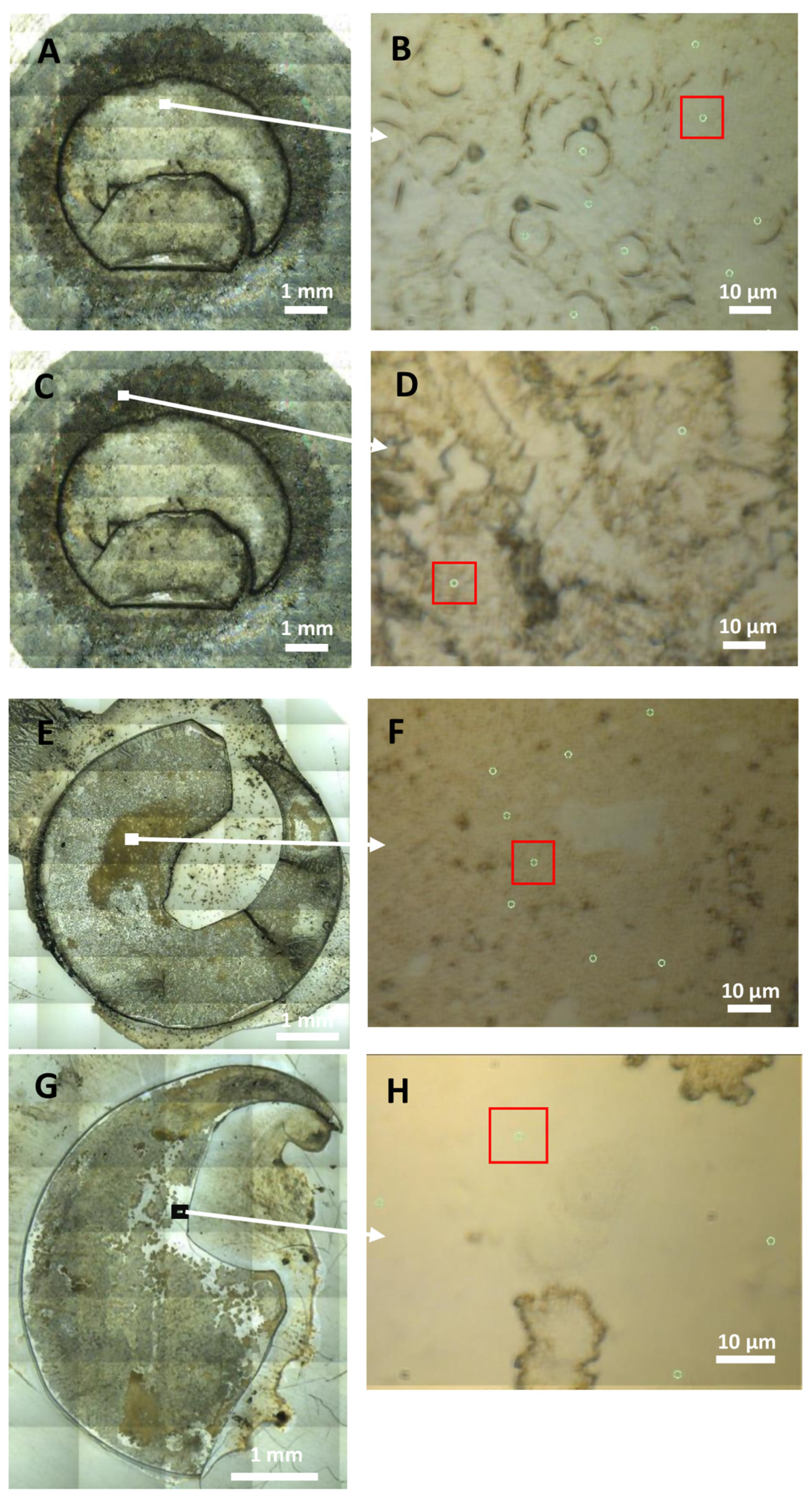
2. Results
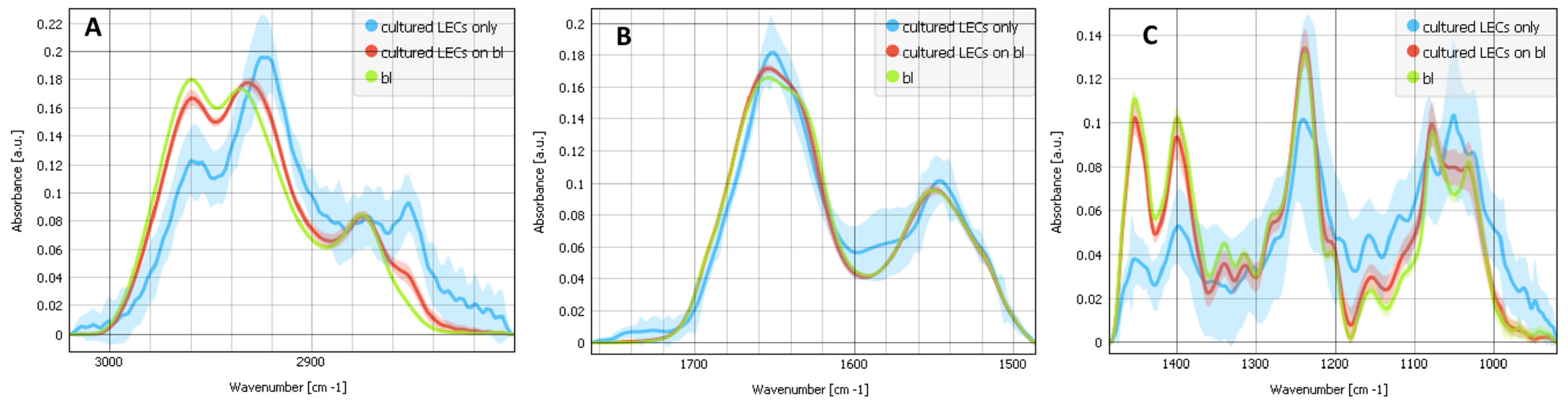
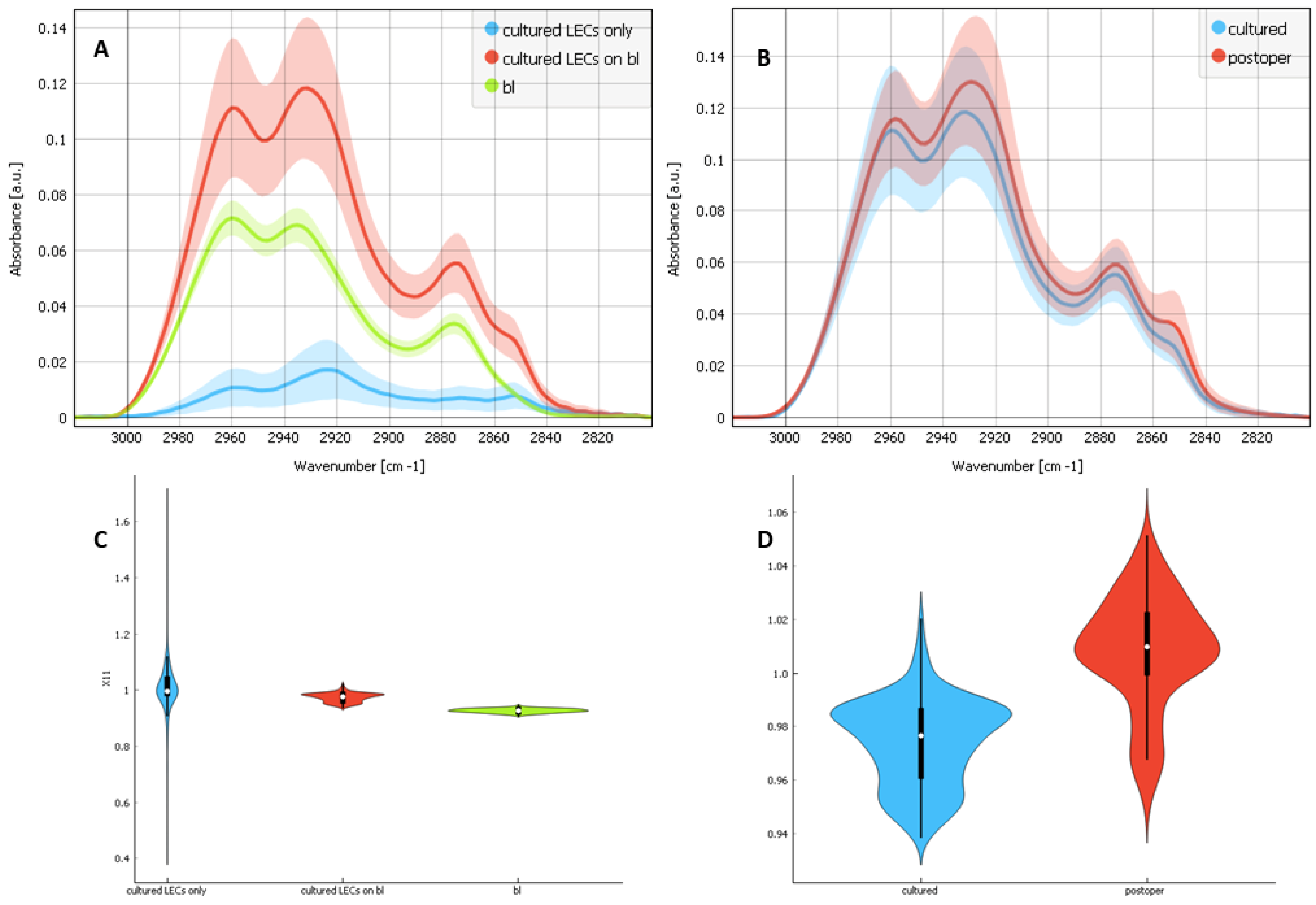
2.1. Differences between the Composites of Cultured LECs on Bls, Cultured LECs Only and Bls Only
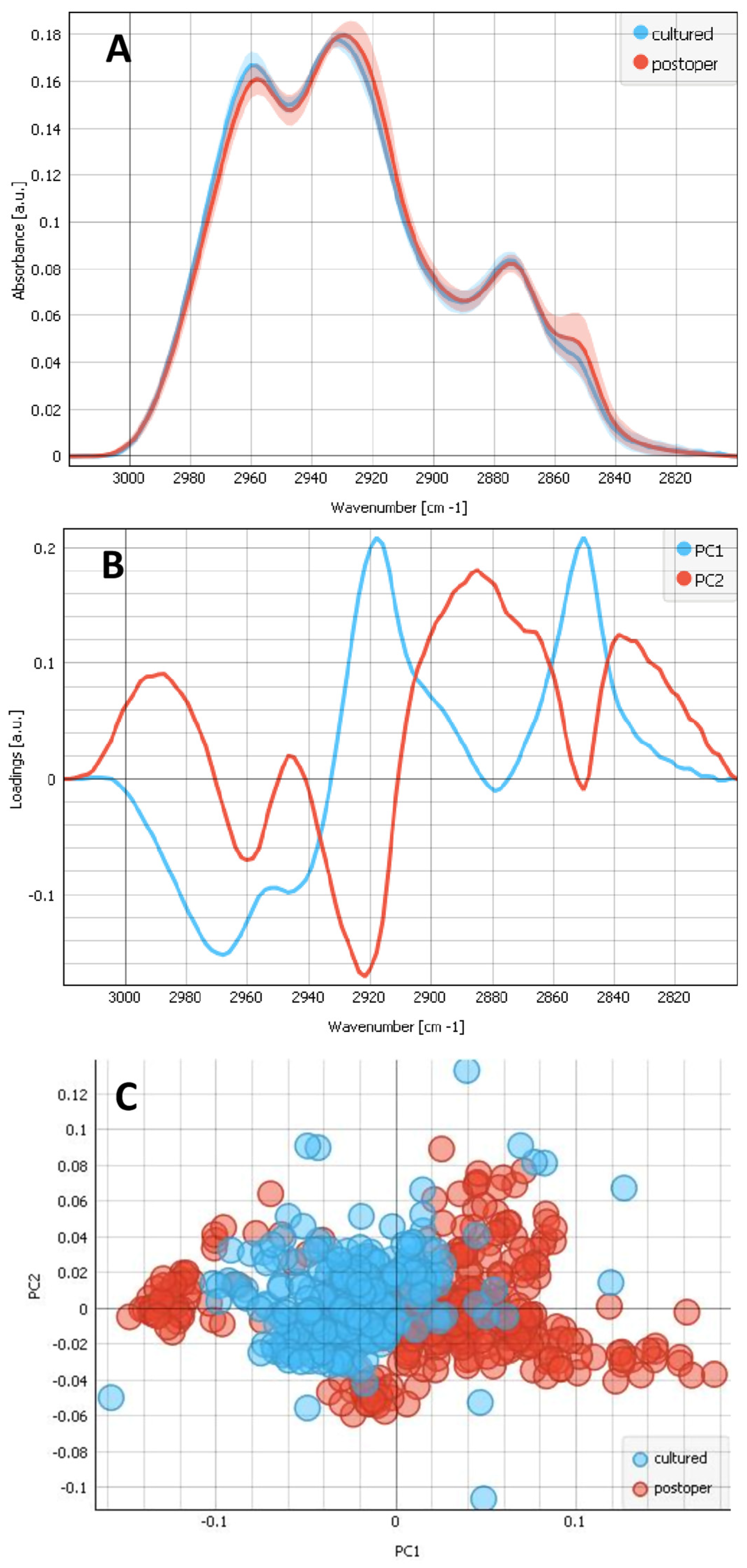
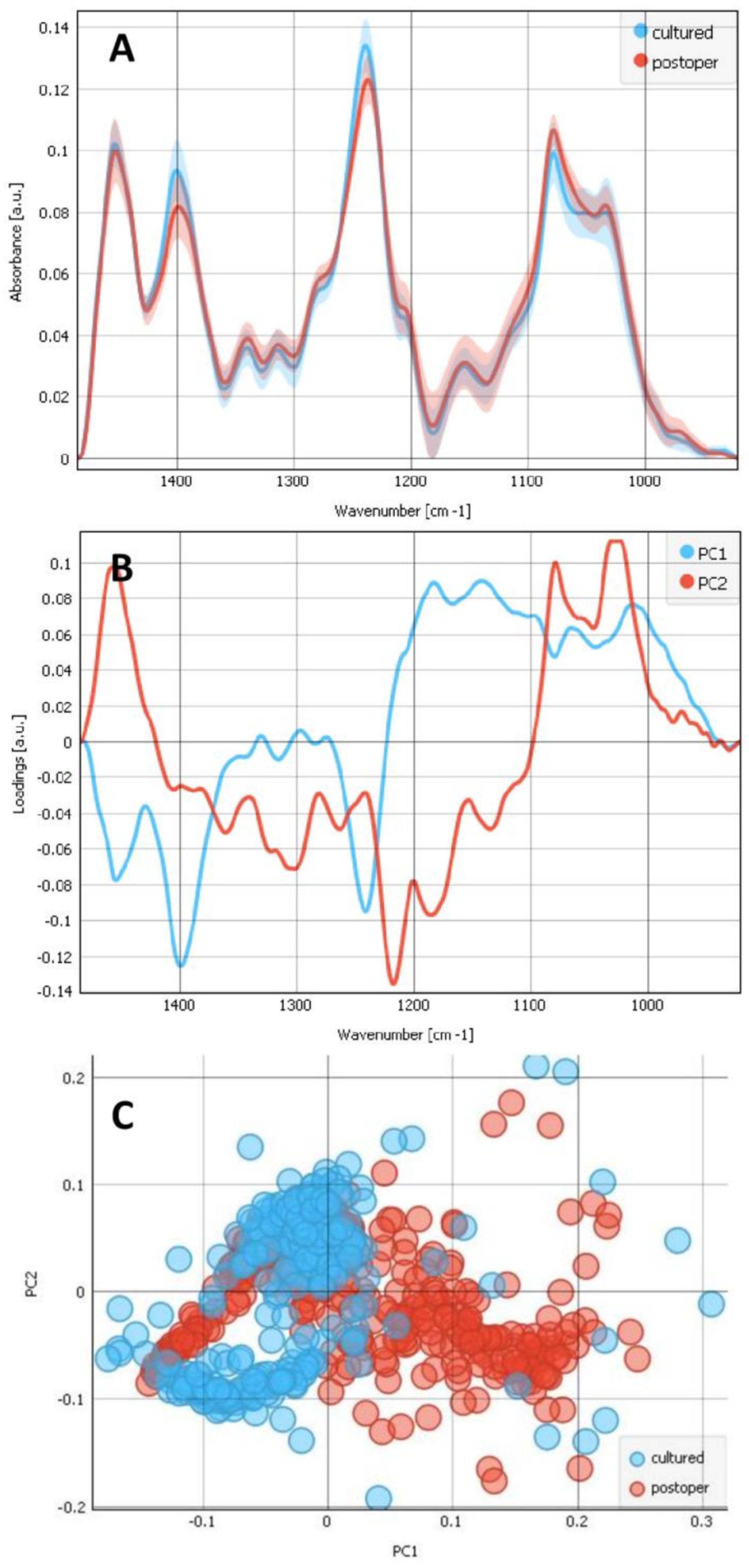
2.2. Differences between the Composites of Cultured and Postoperative LECs on Bls
2.3. Contributions of the Different Components
3. Discussion
4. Materials and Methods
4.1. Tissue Collection and Processing
4.2. Primary LECs Cultures
4.3. Postoperative Bl with LECs
4.4. SR-FTIR Microspectroscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, D.; Veis, A. Structure of bovine anterior lens capsule basement membrane collagen molecules from electron microscopy. Biopolymers 1979, 18, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Danysh, B.P.; Duncan, M.K. The lens capsule. Exp. Eye Res. 2009, 88, 151–164. [Google Scholar] [CrossRef]
- Balaram, M.; Tung, W.H.; Kuszak, J.R.; Ayaki, M.; Shinohara, T.; Chylack, L.T., Jr. Noncontact specular microscopy of human lens epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 474–481. [Google Scholar] [PubMed]
- Konopińska, J.; Młynarczyk, M.; Dmuchowska, D.A.; Obuchowska, I. Posterior Capsule Opacification: A Review of Experimental Studies. J. Clin. Med. 2021, 10, 2847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, B.E.; Klein, R.; Lee, K.E. Incidence of age-related cataract over a 10-year interval: The Beaver Dam Eye Study. Ophthalmology 2002, 109, 2052–2057. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Dana, M.R.; Christen, W.G.; Glynn, R.J. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology 1998, 105, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- González-Martín-Moro, J.; González-López, J.J.; Gómez-Sanz, F.; Zarallo-Gallardo, J.; Cobo-Soriano, R. Posterior capsule opacification, capsular distension syndrome, and anterior capsular phimosis: A retrospective cohort study. Arch. Soc. Esp. Oftalmol. 2015, 90, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Vasavada, A. Cataract and surgery for cataract. BMJ 2006, 333, 128–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, W.H. Mechanotransduction in cells. Cell Biol. Int. 2012, 36, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.; Wang, L.; Neilson, G.J.; Wormstone, I.M. Lens cell survival after exposure to stress in the closed capsular bag. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2701–2707. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 347–376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Huang, W.; Sang, F.; Li, J.; Ren, Y.; Huang, H.; Wang, M.; Li, K.; Zhang, J.; et al. Extracellular HSP90 promotes differentiation of lens epithelial cells to fiber cells by activating LRP1-YAP-PROX1 axis. FASEB J. 2023, 37, e22783. [Google Scholar] [CrossRef] [PubMed]
- Wernecke, L.; Keckeis, S.; Reichhart, N.; Strauß, O.; Salchow, D.J. Epithelial-Mesenchymal Transdifferentiation in Pediatric Lens Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5785–5794, Erratum in Investig. Ophthalmol. Vis. Sci. 2019, 60, 460. [Google Scholar] [CrossRef] [PubMed]
- Wormstone, I.M.; Wang, L.; Liu, C.S. Posterior capsule opacification. Exp. Eye Res. 2009, 88, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Ohmi, S.; Ooshima, A.; Kimura, M.; Ohkawa, K.; Okada, Y.; Tanaka, S.; Ohnishi, Y.; Yamanaka, A. Collagen synthesis in lens epithelial cells on capsules containing intraocular lens implants in rabbits: Immunolocalization of prolyl 4-hydroxylase. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Nishi, O.; Nishi, K.; Fujiwara, T.; Shirasawa, E. Types of collagen synthesised by the lens epithelial cells of human cataracts. Br. J. Ophthalmol. 1995, 79, 939–943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooksley, G.; Lacey, J.; Dymond, M.K.; Sandeman, S. Factors Affecting Posterior Capsule Opacification in the Development of Intraocular Lens Materials. Pharmaceutics 2021, 13, 860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nibourg, L.M.; Gelens, E.; Kuijer, R.; Hooymans, J.M.; van Kooten, T.G.; Koopmans, S.A. Prevention of posterior capsular opacification. Exp. Eye Res. 2015, 136, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Shekhar, H.; Sharma, N.; Titiyal, J.S.; Vajpayee, R.B. Posterior capsular opacification: A review. Indian J. Ophthalmol. 2013, 61, 371–376. [Google Scholar] [CrossRef]
- Wormstone, I.M.; Eldred, J.A. Experimental models for posterior capsule opacification research. Exp. Eye Res. 2016, 142, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Andjelić, S.; Drašlar, K.; Lumi, X.; Yan, X.; Graw, J.; Facskó, A.; Hawlina, M.; Petrovski, G. Morphological and proliferative studies on ex vivo cultured human anterior lens epithelial cells—Relevance to capsular opacification. Acta Ophthalmol. 2015, 93, e499–e506. [Google Scholar] [CrossRef] [PubMed]
- Andjelic, S.; Drašlar, K.; Hvala, A.; Lopic, N.; Strancar, J.; Hawlina, M. Anterior lens epithelial cells attachment to the basal lamina. Acta Ophthalmol. 2016, 94, e183–e188. [Google Scholar] [CrossRef] [PubMed]
- Andjelic, S.; Drašlar, K.; Hvala, A.; Hawlina, M. Structural Characteristics of the Lens in Presenile Cataract. Front. Med. 2021, 8, 802275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andjelic, S.; Drašlar, K.; Hvala, A.; Hawlina, M. Anterior lens epithelium in cataract patients with retinitis pigmentosa—Scanning and transmission electron microscopy study. Acta Ophthalmol. 2017, 95, e212–e220. [Google Scholar] [CrossRef] [PubMed]
- Andjelic, S.; Drašlar, K.; Hvala, A.; Hawlina, M. Anterior lens epithelium in intumescent white cataracts—Scanning and transmission electron microscopy study. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Hawlina, M.; Stunf, S.; Hvala, A. Ultrastructure of anterior lens capsule of intumescent white cataract. Acta Ophthalmol. 2011, 89, e367–e370. [Google Scholar] [CrossRef] [PubMed]
- Stunf, S.; Hvala, A.; Vidovič Valentinčič, N.; Kraut, A.; Hawlina, M. Ultrastructure of the anterior lens capsule and epithelium in cataracts associated with uveitis. Ophthalmic Res. 2012, 48, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Dučić, T.; Hawlina, M.; Andjelic, S. Synchrotron based FTIR microspectroscopy of protein aggregation and lipids peroxidation changes in human cataractous lens epithelial cells. Sci. Rep. 2020, 10, 15489. [Google Scholar] [CrossRef]
- Liu, K.Z.; Dixon, I.M.; Mantsch, H.H. Distribution of collagen deposition in cardiomyopathic hamster hearts determined by infrared microscopy. Cardiovasc. Pathol. 1999, 8, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Petibois, C.; Gouspillou, G.; Wehbe, K.; Delage, J.P.; Déléris, G. Analysis of type I and IV collagens by FT-IR spectroscopy and imaging for a molecular investigation of skeletal muscle connective tissue. Anal. Bioanal. Chem. 2006, 386, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sanad, W.; Miller, L.M.; Voigt, A.; Klingel, K.; Kandolf, R.; Stangl, K.; Baumann, G. Infrared imaging of compositional changes in inflammatory cardiomyopathy. Vib. Spectrosc. 2005, 38, 217–222. [Google Scholar] [CrossRef]
- Firtina, Z.; Danysh, B.P.; Bai, X.; Gould, D.B.; Kobayashi, T.; Duncan, M.K. Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. J. Biol. Chem. 2009, 284, 35872–35884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hogan, M.J.; Alvarado, J.A.; Weddell, J.E. Histology of the Human Eye; WB Saunders Co.: Philadelphia, PA, USA, 1971. [Google Scholar]
- Emin, G.; Islam, S.T.; King, R.E.; Fowler, V.M.; Cheng, C.; Parreno, J. Whole Mount Imaging to Visualize and Quantify Peripheral Lens Structure, Cell Morphology, and Organization. J. Vis. Exp. 2024, 203. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gordon, P.; Hao, C.; Huangfu, J.; Fan, E.; Zhang, X.; Yan, H.; Fan, X. Aged Lens Epithelial Cells Suppress Proliferation and Epithelial-Mesenchymal Transition-Relevance for Posterior Capsule Opacification. Cells 2022, 11, 2001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riaza, T.; Zeeshana, R.; Zarifa, F.; Ilyasa, K.; Muhammada, N.; Safia, S.Z.; Rahima, A.; Rizvib, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Nagata, M.; Matsushima, H.; Senoo, T. Effect of surface-modified intraocular lenses on long-term postoperative inhibition of posterior capsule opacification. Heliyon 2024, 10, e33006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Wang, J.; Hong, Y.; Han, Y.; Liang, L.; Yang, Y.; Wu, Z.; Lin, Q. Photo-controllable drug releasing bulk polyacrylic intraocular lens material for safer posterior capsular opacification prevention. J. Control. Release 2024, 366, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Huang, W.L.; Yang, Y.; Kuan, C.H.; Tseng, C.L.; Wang, T.W. Zwitterionic modified and freeze-thaw reinforced foldable hydrogel as intraocular lens for posterior capsule opacification prevention. Biomaterials 2024, 309, 122593. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Qin, C.; Duo, L.; Fei, F.; Jia, Q.; Chen, H.; Lin, Q. Polydopamine based photothermal/photodynamic synchronous coating modified intraocular lens for efficient and safer posterior capsule opacification prevention. Biomater. Adv. 2024, 158, 213792. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Overmass, M.; Fan, J.; Hodge, C.; Sutton, G.; Lovicu, F.J.; You, J. Application of Collagen I and IV in Bioengineering Transparent Ocular Tissues. Front. Surg. 2021, 8, 639500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andjelic, S.; Lumi, X.; Veréb, Z.; Josifovska, N.; Facskó, A.; Hawlina, M.; Petrovski, G. A simple method for establishing adherent ex vivo explant cultures from human eye pathologies for use in subsequent calcium imaging and inflammatory studies. J. Immunol. Res. 2014, 2014, 232659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousef, I.; Ribó, L.; Crisol, A.; Šics, I.; Ellis, G.; Ducic, T.; Kreuzer, M.; Benseny-Cases, N.; Quispe, M.; Dumas, P.; et al. MIRAS: The Infrared Synchrotron Radiation Beamline at ALBA. Synchrotron Radiat. News 2017, 30, 4–6. [Google Scholar] [CrossRef]
- Miller, L.M.; Dumas, P. Chemical imaging of biological tissue with synchrotron infrared light. Biochim. Biophys. Acta Biomembr. 2006, 1758, 846–857. [Google Scholar] [CrossRef]
- Toplak, M.; Birarda, G.; Read, S.; Sandt, C.; Rosendahl, S.M.; Vaccari, L.; Demšar, J.; Borondics, F. Infrared Orange: Connecting Hyperspectral Data with Machine Learning. Synchrotron Radiat. News 2017, 30, 40–45. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andjelic, S.; Hawlina, M. Human Primary Lens Epithelial Cultures on Basal Laminas Studied by Synchrotron-Based FTIR Microspectroscopy for Understanding Posterior Capsular Opacification. Int. J. Mol. Sci. 2024, 25, 8858. https://doi.org/10.3390/ijms25168858
Andjelic S, Hawlina M. Human Primary Lens Epithelial Cultures on Basal Laminas Studied by Synchrotron-Based FTIR Microspectroscopy for Understanding Posterior Capsular Opacification. International Journal of Molecular Sciences. 2024; 25(16):8858. https://doi.org/10.3390/ijms25168858
Chicago/Turabian StyleAndjelic, Sofija, and Marko Hawlina. 2024. "Human Primary Lens Epithelial Cultures on Basal Laminas Studied by Synchrotron-Based FTIR Microspectroscopy for Understanding Posterior Capsular Opacification" International Journal of Molecular Sciences 25, no. 16: 8858. https://doi.org/10.3390/ijms25168858





