The Impact of High-Fructose Diet and Co-Sensitization to House Dust Mites and Ragweed Pollen on the Modulation of Airway Reactivity and Serum Biomarkers in Rats
Abstract
:1. Introduction
2. Results
2.1. The Impact of Dietary Factors and Allergic Responses on Serum Biomarkers
2.1.1. Measurement of Anthropometric Parameters
2.1.2. Serum Lipidic and Glucose Profiles in Sensitized Rats
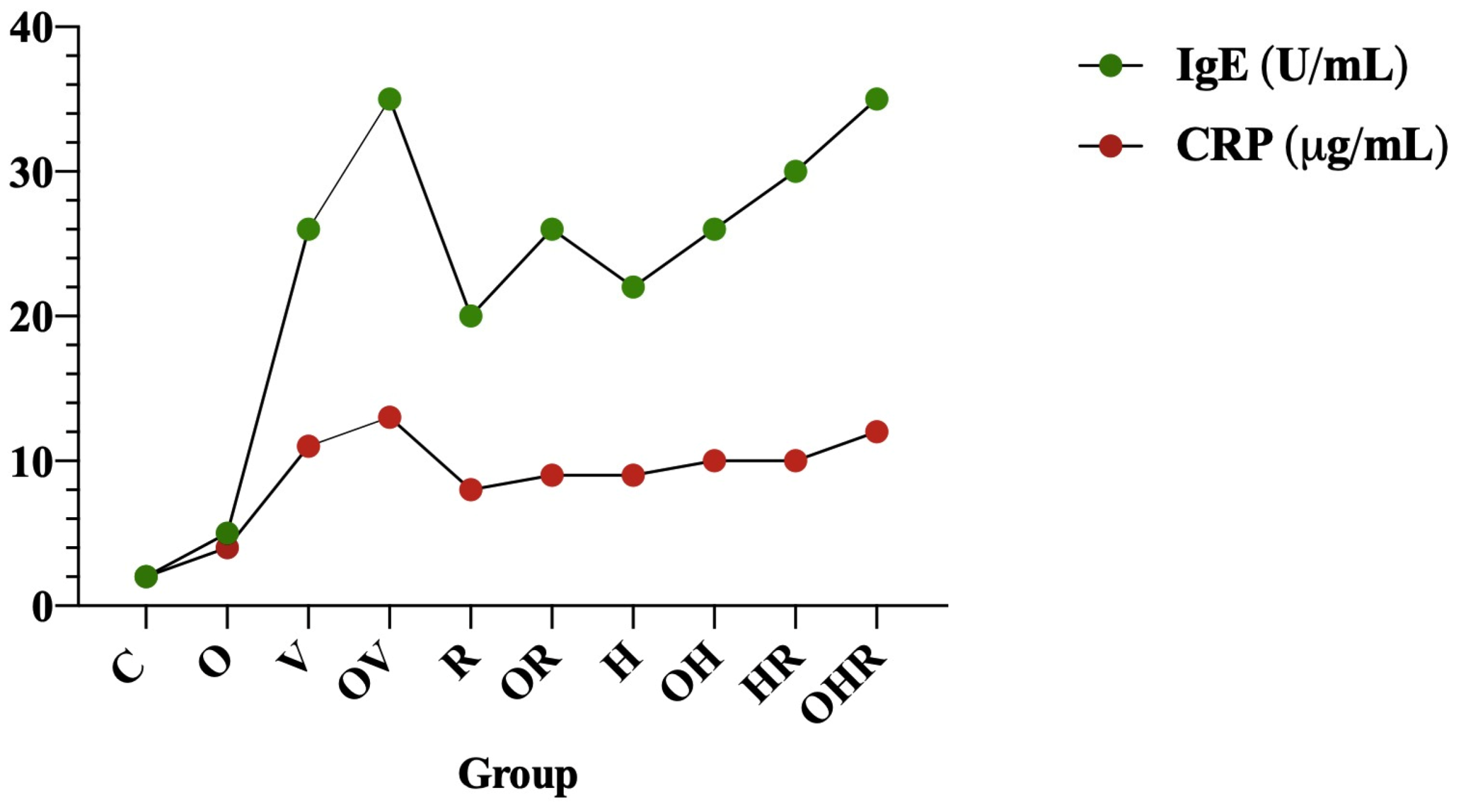
2.1.3. Modulation of Serum C-Reactive Protein and Serum Total IgE Antibody Levels Following Allergen Sensitization
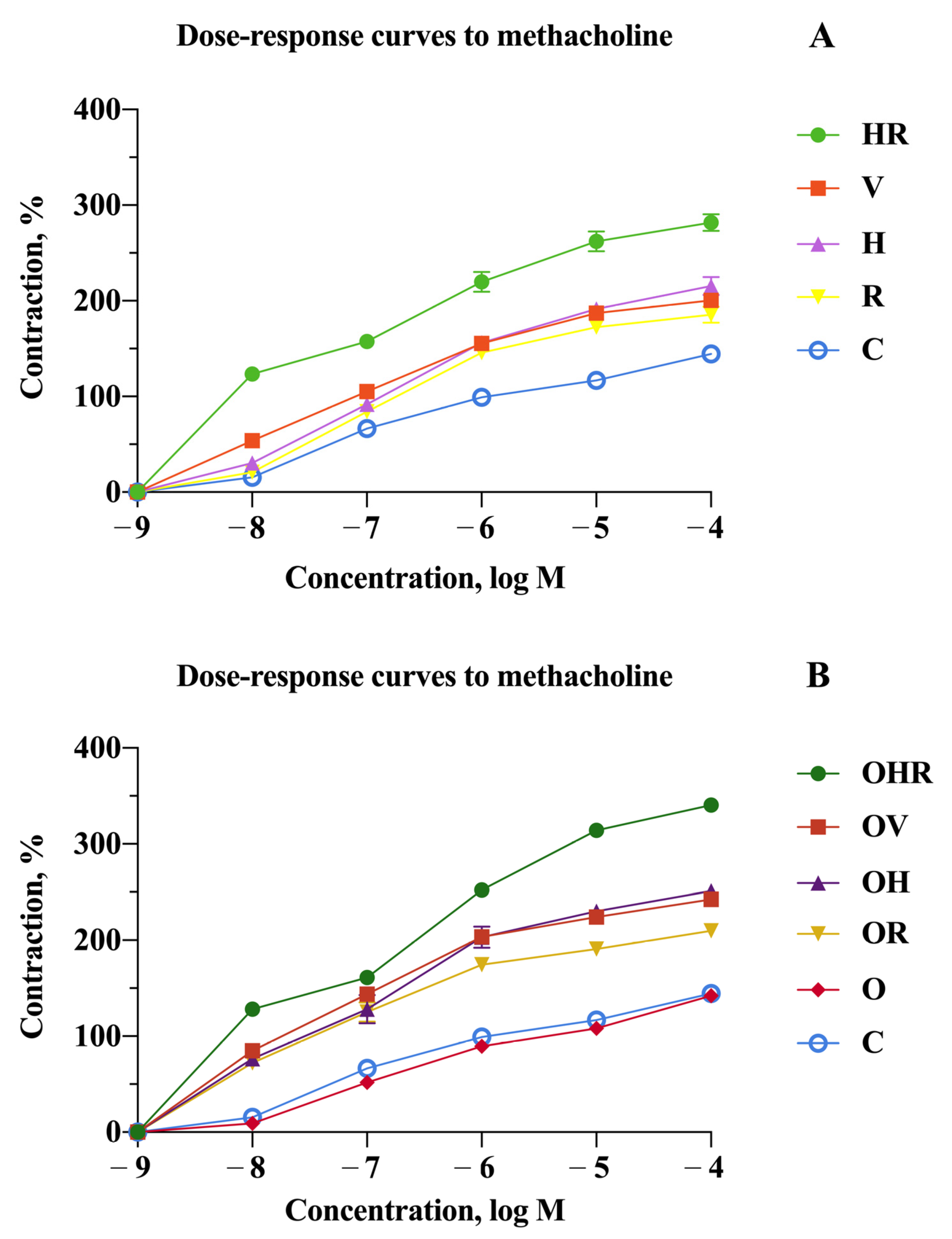
2.2. Revealing the Impact of High-Fructose Diet and Co-Sensitization in Contrast to Monosensitization to Allergenic Extracts in Rats
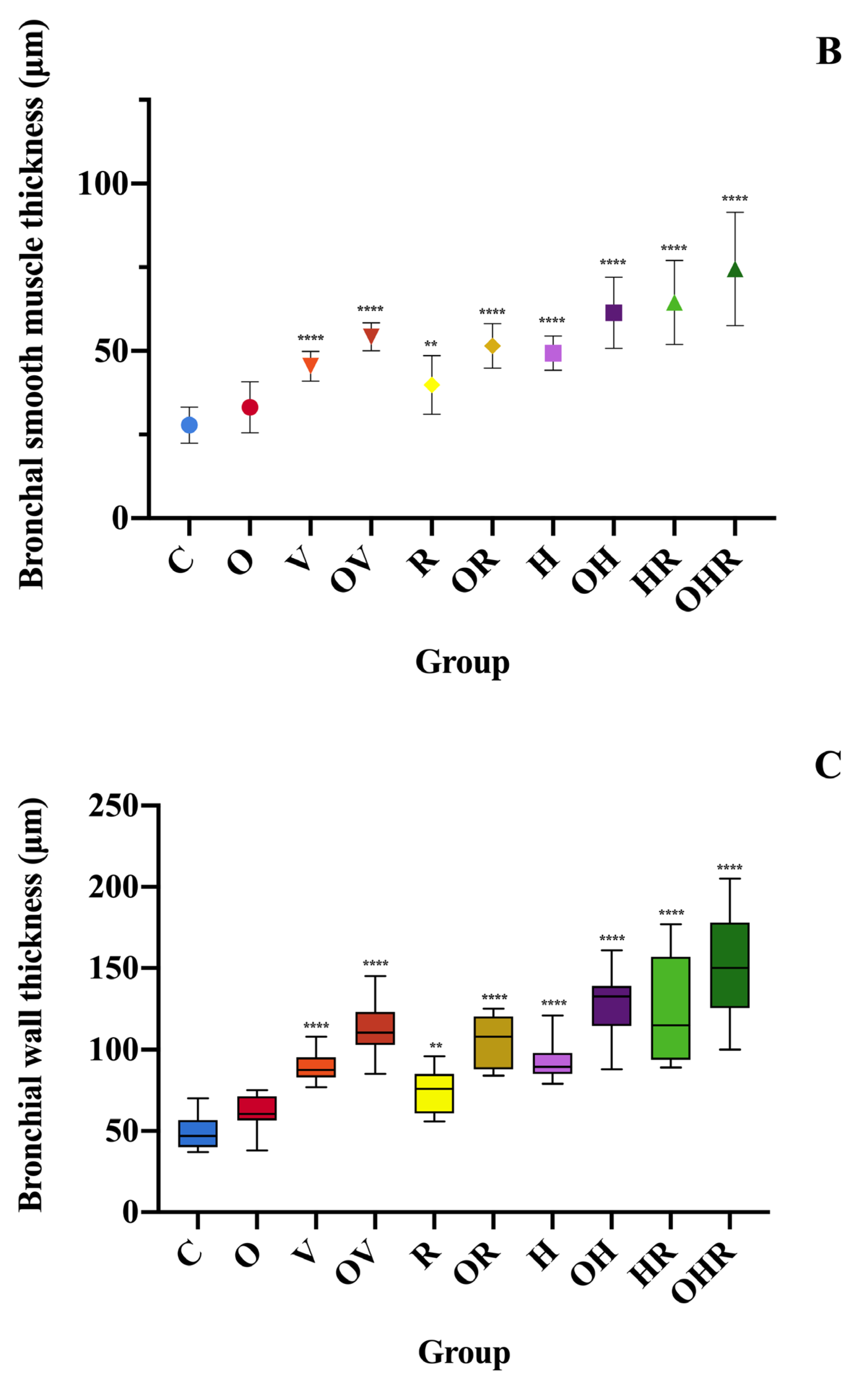
2.3. Morphologic Analysis and Characterization of Inflammatory Cell Infiltration, Bronchial Smooth Muscle Hypertrophy, and Bronchial Wall Thickness
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Allergens
4.3. Animals
4.4. Quantification of Metabolic Parameters and Serum Inflammation Markers
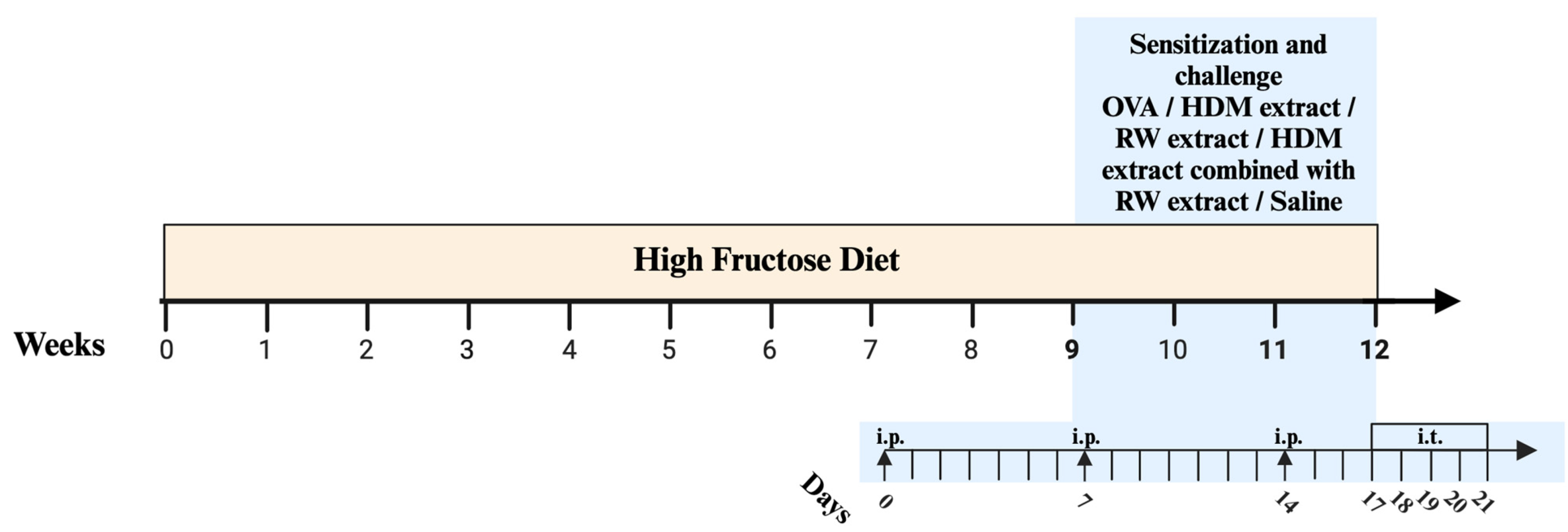
4.5. Sensitization and Challenge Protocol
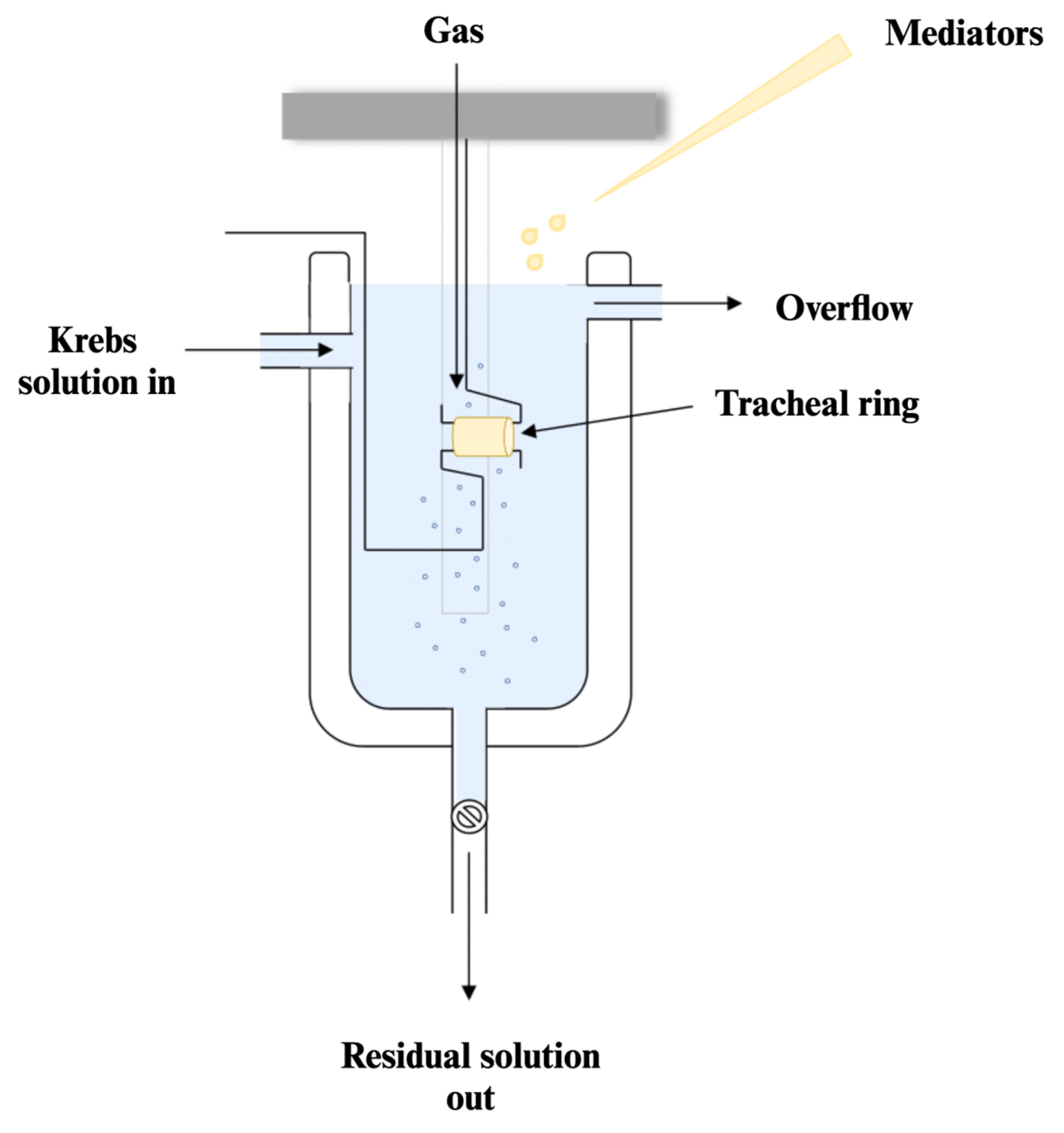
4.6. Tracheal Ring Preparation and Its Responsiveness Assessment
4.7. Histological and Morphometric Analysis of the Airways
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Category | Nutrient | UM | Quantity |
|---|---|---|---|
| General | Digestible energy | kJ/g | 16.0 |
| Fat | g/kg | 50.0 | |
| Crude protein | g/kg | 180.0 | |
| Arginine | g/kg | 4.3 | |
| Asparagine | g/kg | 4.0 | |
| Glutamic acid | g/kg | 40.0 | |
| Histidine | g/kg | 2.8 | |
| Isoleucine | g/kg | 6.2 | |
| Leucine | g/kg | 10.7 | |
| Lysine | g/kg | 9.2 | |
| Methionine + Cystine | g/kg | 9.8 | |
| Phenylalanine + Tyrosine | g/kg | 10.2 | |
| Threonine | g/kg | 6.2 | |
| Tryptophan | g/kg | 2.0 | |
| Valine | g/kg | 7.4 | |
| Minerals | Calcium | g/kg | 5.0 |
| Magnesium | g/kg | 0.5 | |
| Chlorine | g/kg | 0.5 | |
| Phosphorus | g/kg | 3.0 | |
| Potassium | g/kg | 3.6 | |
| Sodium | g/kg | 0.5 | |
| Copper | g/kg | 5.0 | |
| Iodine | g/kg | 0.15 | |
| Iron | g/kg | 35.0 | |
| Selenium | g/kg | 0.15 | |
| Vitamins | Thiamine (B1) | mg/kg | 4.0 |
| Riboflavin (B2) | mg/kg | 3.0 | |
| Pyridoxine (B6) | mg/kg | 6.0 | |
| Cyanocobalamin (B12) | mg/kg | 50.0 | |
| Menadione (K) | mg/kg | 1.0 | |
| Retinol (A) | mg/kg | 1.2 | |
| Cholecalciferol (D3) | mg/kg | 25.0 | |
| Tocopherol (E) | mg/kg | 27.0 | |
| Choline | mg/kg | 750.0 |
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2023. Available online: https://Ginasthma.Org/Reports/ (accessed on 7 May 2024).
- Woodrow, J.S.; Sheats, M.K.; Cooper, B.; Bayless, R. Asthma: The Use of Animal Models and Their Translational Utility. Cells 2023, 12, 1091. [Google Scholar] [CrossRef]
- Thakur, V.R.; Khuman, V.; Beladiya, J.V.; Chaudagar, K.K.; Mehta, A.A. An Experimental Model of Asthma in Rats Using Ovalbumin and Lipopolysaccharide Allergens. Heliyon 2019, 5, e02864. [Google Scholar] [CrossRef]
- Chen, K.-W.; Marusciac, L.; Tamas, P.T.; Valenta, R.; Panaitescu, C. Ragweed Pollen Allergy: Burden, Characteristics, and Management of an Imported Allergen Source in Europe. Int. Arch. Allergy Immunol. 2018, 176, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Russjan, E. The Role of Peptides in Asthma–Obesity Phenotype. Int. J. Mol. Sci. 2024, 25, 3213. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Hsu, J.-W.Y.; Zeiger, R.S.; Chen, W.; Dorenbaum, A.; Chipps, B.E.; Haselkorn, T. Phenotypes Determined by Cluster Analysis in Severe or Difficult-to-Treat Asthma. J. Allergy Clin. Immunol. 2014, 133, 1549–1556. [Google Scholar] [CrossRef]
- Spasovska, M.; Kadifkova Panovska, T. Adiponectin as Anti-Inflammatory Marker in Developmen of Allergic Asthma. Maced. Pharm. Bull. 2023, 68, 75–81. [Google Scholar] [CrossRef]
- Sood, A.; Shore, S.A. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. J. Allergy 2013, 2013, 785835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, Y.; Zhang, H.; Zhong, W.; Zhang, J. Association of Asthma Diagnosis with Leptin and Adiponectin: A Systematic Review and Meta-Analysis. J. Investig. Med. 2017, 65, 57–64. [Google Scholar] [CrossRef]
- Serafino-Agrusa, L. Asthma and Metabolic Syndrome: Current Knowledge and Future Perspectives. World J. Clin. Cases 2015, 3, 285. [Google Scholar] [CrossRef]
- Gruchała-Niedoszytko, M.; Małgorzewicz, S.; Niedoszytko, M.; Gnacińska, M.; Jassem, E. The Influence of Obesity on Inflammation and Clinical Symptoms in Asthma. Adv. Med. Sci. 2013, 58, 15–21. [Google Scholar] [CrossRef]
- Menegati, L.M.; De Oliveira, E.E.; Oliveira, B.D.C.; Macedo, G.C.; De Castro E Silva, F.M. Asthma, Obesity, and Microbiota: A Complex Immunological Interaction. Immunol. Lett. 2023, 255, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Taylor, A.E.; Thuesen, B.H.; Jacobsen, R.K.; Friedrich, N.; Møllehave, L.T.; Hansen, S.; Larsen, S.C.; Völker, U.; Nauck, M.; et al. Estimating the Causal Effect of Body Mass Index on Hay Fever, Asthma and Lung Function Using Mendelian Randomization. Allergy 2018, 73, 153–164. [Google Scholar] [CrossRef]
- Beuther, D.A.; Sutherland, E.R. Overweight, Obesity, and Incident Asthma: A Meta-Analysis of Prospective Epidemiologic Studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Akkoyunlu, M.; Rabin, R.L. Macrophages—Common Culprit in Obesity and Asthma. Allergy 2018, 73, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ren, Y.; Li, M.; Zhao, X.; Kong, L.; Kang, J. Association between Lipid Profile and the Prevalence of Asthma: A Meta-Analysis and Systemic Review. Curr. Med. Res. Opin. 2018, 34, 423–433. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Tucker, K.L. Excess Free Fructose, High-Fructose Corn Syrup and Adult Asthma: The Framingham Offspring Cohort. Br. J. Nutr. 2018, 119, 1157–1167. [Google Scholar] [CrossRef]
- DeChristopher, L.R. 40 Years of Adding More Fructose to High Fructose Corn Syrup than Is Safe, through the Lens of Malabsorption and Altered Gut Health–Gateways to Chronic Disease. Nutr. J. 2024, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Ebert, K.; Witt, H. Fructose Malabsorption. Mol. Cell. Pediatr. 2016, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Kari, F.W.; Selgrade, M.K.; Gilmour, M.I. Attenuated Allergic Responses to House Dust Mite Antigen in Feed-Restricted Rats. Environ. Health Perspect. 2000, 108, 1125–1131. [Google Scholar] [CrossRef]
- Brnoliakova, Z.; Knezl, V.; Sotnikova, R.; Gasparova, Z. Metabolic Syndrome in Hypertriglyceridemic Rats: Effects of Antioxidants. Physiol. Res. 2023, 72, S31–S35. [Google Scholar] [CrossRef]
- Fujita, M.; Ueki, S.; Ito, W.; Chiba, T.; Takeda, M.; Saito, N.; Kayaba, H.; Chihara, J. C-Reactive Protein Levels in the Serum of Asthmatic Patients. Ann. Allergy Asthma Immunol. 2007, 99, 48–53. [Google Scholar] [CrossRef]
- Yasuda, Y.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Group 2 Innate Lymphoid Cells and the House Dust Mite-Induced Asthma Mouse Model. Cells 2020, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Maltby, S.; Tay, H.L.; Yang, M.; Foster, P.S. Mouse Models of Severe Asthma: U Nderstanding the Mechanisms of Steroid Resistance, Tissue Remodelling and Disease Exacerbation. Respirology 2017, 22, 874–885. [Google Scholar] [CrossRef]
- Jacquet, A. The Role of Innate Immunity Activation in House Dust Mite Allergy. Trends Mol. Med. 2011, 17, 604–611. [Google Scholar] [CrossRef]
- Buzan, M.; Zbîrcea, L.; Gattinger, P.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K. Complex IgE Sensitization Patterns in Ragweed Allergic Patients: Implications for Diagnosis and Specific Immunotherapy. Clin. Transl. Allergy 2022, 12, e12179. [Google Scholar] [CrossRef] [PubMed]
- Cellat, M.; Kuzu, M.; İşler, C.T.; Etyemez, M.; Dikmen, N.; Uyar, A.; Gökçek, İ.; Türk, E.; Güvenç, M. Tyrosol Improves Ovalbumin (OVA)-Induced Asthma in Rat Model through Prevention of Airway Inflammation. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Aliyeva, M.; Daphtary, N.; Martin, R.A.; Poynter, M.E.; Kostin, S.F.; Van Der Velden, J.L.; Hyman, A.M.; Stevenson, C.S.; Phillips, J.E.; et al. Antigen-Induced Mast Cell Expansion and Bronchoconstriction in a Mouse Model of Asthma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L196–L206. [Google Scholar] [CrossRef]
- Pérez-Corredor, P.A.; Gutiérrez-Vargas, J.A.; Ciro-Ramírez, L.; Balcazar, N.; Cardona-Gómez, G.P. High Fructose Diet-Induced Obesity Worsens Post-Ischemic Brain Injury in the Hippocampus of Female Rats. Nutr. Neurosci. 2022, 25, 122–136. [Google Scholar] [CrossRef]
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.-Y.; Johnson, R.J.; Scarpace, P.J. Fructose-Induced Leptin Resistance Exacerbates Weight Gain in Response to Subsequent High-Fat Feeding. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1370–R1375. [Google Scholar] [CrossRef]
- Benkhaled, A.; Réggami, Y.; Boudjelal, A.; Senator, A.; Bouriche, H.; Demirtaş, I.; Kheniche, A.; Benyettou, H.; Larabi, N.; Ruberto, G. Chemical Characterisation, Hypoglycaemic and Renoprotective Effects of Aqueous Leaf Extract of Limoniastrum guyonianum on Fructose-Induced Metabolic Syndrome in Rats. Arch. Physiol. Biochem. 2022, 128, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, E.; Kim, M.; Kim, I. Acute Exposure to Fructose Impairs Endothelium-Dependent Relaxation via Oxidative Stress in Isolated Rat Aortic Rings. J. Vasc. Res. 2020, 57, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, Y.A.; Elyamany, M.F.; Al-Shorbagy, M.Y.; Badary, O.A. Effects of TNF-α Antagonist Infliximab on Fructose-Induced Metabolic Syndrome in Rats. Hum. Exp. Toxicol. 2021, 40, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Andrade, S.; Silva, C.; Rodrigues, I.; Guardão, L.; Guimarães, J.T.; Keating, E.; Martel, F. Chronic Consumption of the Dietary Polyphenol Chrysin Attenuates Metabolic Disease in Fructose-Fed Rats. Eur. J. Nutr. 2020, 59, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Ikutani, M.; Aminuddin, A.; Takikawa, A.; Tsuneyama, K.; Mahmood, A.; Goda, N.; Nagai, Y.; Takatsu, K.; et al. Adipose Tissue Hypoxia Induces Inflammatory M1 Polarity of Macrophages in an HIF-1α-Dependent and HIF-1α-Independent Manner in Obese Mice. Diabetologia 2013, 56, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Zbîrcea, L.-E.; Buzan, M.-R.; Grijincu, M.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Relationship between IgE Levels Specific for Ragweed Pollen Extract, Amb a 1 and Cross-Reactive Allergen Molecules. Int. J. Mol. Sci. 2023, 24, 4040. [Google Scholar] [CrossRef]
- Baena, M.; Sangüesa, G.; Dávalos, A.; Latasa, M.-J.; Sala-Vila, A.; Sánchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but Not Glucose, Impairs Insulin Signaling in the Three Major Insulin-Sensitive Tissues. Sci. Rep. 2016, 6, 26149. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-W.; Ren, L.-F.; Zhou, X.; Han, D.-W. A High-Fructose Diet Induces Hippocampal Insulin Resistance and Exacerbates Memory Deficits in Male Sprague-Dawley Rats. Nutr. Neurosci. 2015, 18, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Vinding, R.K.; Stokholm, J.; Chawes, B.L.K.; Bisgaard, H. Blood Lipid Levels Associate with Childhood Asthma, Airway Obstruction, Bronchial Hyperresponsiveness, and Aeroallergen Sensitization. J. Allergy Clin. Immunol. 2016, 137, 68–74.e4. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tung, K.Y.; Tsai, C.H.; Su, M.W.; Wang, P.C.; Chen, C.H.; Lee, Y.L. Lipid Profiles in Children with and without Asthma: Interaction of Asthma and Obesity on Hyperlipidemia. Diabetes Metab. Syndr. Clin. Res. Rev. 2013, 7, 20–25. [Google Scholar] [CrossRef]
- Georgescu, M.; Drăghici, G.A.; Oancea, E.-F.; Dehelean, C.A.; Şoica, C.; Vlăduţ, N.-V.; Nica, D.V. Effects of Cadmium Sulfate on the Brown Garden Snail Cornu Aspersum: Implications for DNA Methylation. Toxics 2021, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Gurgone, D.; McShane, L.; McSharry, C.; Guzik, T.J.; Maffia, P. Cytokines at the Interplay Between Asthma and Atherosclerosis? Front. Pharmacol. 2020, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- van Zelst, C.M.; de Boer, G.M.; Türk, Y.; van Huisstede, A.; In’t Veen, J.C.C.M.; Birnie, E. Association between Elevated Serum Triglycerides and Asthma in Patients with Obesity: An Explorative Study. Allergy Asthma Proc. 2021, 42, E71–E76. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Kaveh, M.; Shakeri, F.; Mohammadian Roshan, N.; Rezaee, R. Alpha-Linolenic Acid Ameliorates Bronchial Asthma Features in Ovalbumin-Sensitized Rats. J. Pharm. Pharmacol. 2019, 71, 1089–1099. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, H.; Zhang, J.; Yu, C.; Liu, C.; Sun, H.; Wu, Y.; Wang, Y.; Lin, X. Antiasthmatic Activity of Quercetin Glycosides in Neonatal Asthmatic Rats. 3 Biotech 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Berkani, L.M.; Gharnaout; Djidjik, R. Les Cellules De L’immunité Innée De Type 2 (ILC2): Les Nouveaux Acteurs Dans Les Maladies Allergiques. Rev. Alger. D’allergol. 2017, 2, 9–14. [Google Scholar]
- Hammad, H.; Chieppa, M.; Perros, F.; Willart, M.A.; Germain, R.N.; Lambrecht, B.N. House Dust Mite Allergen Induces Asthma via Toll-like Receptor 4 Triggering of Airway Structural Cells. Nat. Med. 2009, 15, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Buzan, M.-R.; Grijincu, M.; Zbîrcea, L.-E.; Haidar, L.; Tamaș, T.-P.; Cotarcă, M.-D.; Tănasie, G.; Weber, M.; Babaev, E.; Stolz, F.; et al. Insect Cell-Expressed Major Ragweed Allergen Amb a 1.01 Exhibits Similar Allergenic Properties to Its Natural Counterpart from Common Ragweed Pollen. Int. J. Mol. Sci. 2024, 25, 5175. [Google Scholar] [CrossRef] [PubMed]
- De Alba, J.; Raemdonck, K.; Dekkak, A.; Collins, M.; Wong, S.; Nials, A.T.; Knowles, R.G.; Belvisi, M.G.; Birrell, M.A. House Dust Mite Induces Direct Airway Inflammation in Vivo: Implications for Future Disease Therapy? Eur. Respir. J. 2010, 35, 1377–1387. [Google Scholar] [CrossRef]
- Haidar, L.; Tamas, T.-P.; Stolz, F.; Petrisor Patrascu, R.F.; Chen, K.-W.; Panaitescu, C. Symptom Patterns and Comparison of Diagnostic Methods in Ragweed Pollen Allergy. Exp. Ther. Med. 2021, 21, 525. [Google Scholar] [CrossRef]
- Li, D.-Q.; Zhang, L.; Pflugfelder, S.C.; De Paiva, C.S.; Zhang, X.; Zhao, G.; Zheng, X.; Su, Z.; Qu, Y. Short Ragweed Pollen Triggers Allergic Inflammation through Toll-like Receptor 4–Dependent Thymic Stromal Lymphopoietin/OX40 Ligand/OX40 Signaling Pathways. J. Allergy Clin. Immunol. 2011, 128, 1318–1325.e2. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, K.; Itazawa, T.; Boldogh, I.; Sur, S. MD2 Facilitates Pollen and Cat Dander-Induced Innate and Allergic Airway Inflammation. J. Allergy Clin. Immunol. 2016, 137, AB69. [Google Scholar] [CrossRef]
- Thomas, W.R. Hierarchy and Molecular Properties of House Dust Mite Allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Divanovic, S.; Visintin, A.; Blanchard, C.; Hegde, R.S.; Madan, R.; Thorne, P.S.; Wills-Karp, M.; Gioannini, T.L.; Weiss, J.P.; et al. Allergenicity Resulting from Functional Mimicry of a Toll-like Receptor Complex Protein. Nature 2009, 457, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Aki, T.; Isobe, T.; Matsuoka, A.; Hayashi, T.; Ono, K.; Kawamoto, S. Der f 35: An MD -2-like House Dust Mite Allergen That Cross-reacts with Der f 2 and Pso o 2. Allergy 2017, 72, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.; Calder, P. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Grijincu, M.; Huțu, I.; Weber, M.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K.-W. Physicochemical and Immunological Characterization of Amb a 12, a Novel Ragweed (Ambrosia artemisiifolia) Pollen Allergen. Mol. Immunol. 2023, 157, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Mincham, K.; Scott, N.; Lauzon-Joset, J.-F.; Leffler, J.; Stumbles, P.; Holt, P.; Strickland, D. Early Life Ovalbumin Sensitization and Aerosol Challenge for the Induction of Allergic Airway Inflammation in a BALB/c Murine Model. Bio Protoc. 2019, 9, e3181. [Google Scholar] [CrossRef] [PubMed]
- Aun, M.; Bonamichi-Santos, R.; Arantes-Costa, F.M.; Kalil, J.; Giavina-Bianchi, P. Animal Models of Asthma: Utility and Limitations. J. Asthma Allergy 2017, 10, 293–301. [Google Scholar] [CrossRef]
- Sakai, K.; Yokoyama, A.; Kohno, N.; Hiwada, K. Effect of Different Sensitizing Doses of Antigen in a Murine Model of Atopic Asthma. Clin. Exp. Immunol. 2001, 118, 9–15. [Google Scholar] [CrossRef]
- Farhadi, N.; Ulrich, K.; Lamb, D.; Allen, S.; Douglas, G.; Whybrow, L.; Mori, H.; Janies, J.; Parker, N.; Adcock, J.; et al. Development of House Dust Mite Allergen-Induced Airway Inflammation Model in Brown Norway Rats; Modulation by Roflumilast and Fluticasone. In C30. Inflammatory and Immunological Models of Asthma; ATS: San Diego, CA, USA, 2009; p. A4221. [Google Scholar] [CrossRef]
- Daubeuf, F.; Reber, L.; Frossard, N. Allergen Sensitization and Challenge to Ovalbumin. Bio Protoc. 2013, 3, e644. [Google Scholar] [CrossRef]
- Goplen, N.; Karim, M.Z.; Liang, Q.; Gorska, M.M.; Rozario, S.; Guo, L.; Alam, R. Combined Sensitization of Mice to Extracts of Dust Mite, Ragweed, and Aspergillus Species Breaks through Tolerance and Establishes Chronic Features of Asthma. J. Allergy Clin. Immunol. 2009, 123, 925–932.e11. [Google Scholar] [CrossRef] [PubMed]
- Nials, A.T.; Uddin, S. Mouse Models of Allergic Asthma: Acute and Chronic Allergen Challenge. Dis. Models Mech. 2008, 1, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, B.; Tykocki, N.R.; Watts, S.W.; Cobbett, P.J. Measurement of Smooth Muscle Function in the Isolated Tissue Bath-Applications to Pharmacology Research. J. Vis. Exp. 2015, 95, 52324. [Google Scholar] [CrossRef]
- Upchurch, W.J.; Iaizzo, P.A. In Vitro Contractile Studies within Isolated Tissue Baths: Translational Research from Visible Heart® Laboratories. Exp. Biol. Med. 2022, 247, 584–597. [Google Scholar] [CrossRef]




| Group | 0 Weeks | 6 Weeks | 12 Weeks |
|---|---|---|---|
| C | 309.50 ± 16.80 | 325.00 ±14.27 | 343.17 ± 14.92 |
| O | 311.83 ±11.82 | 362.17 ± 17.12 **** | 405.83 ± 13.96 **** |
| V | 310.17 ± 17.27 | 332.33 ± 14.19 | 331.33 ± 15.91 |
| OV | 306.83 ± 12.73 | 354.00 ± 15.82 **** | 378.83 ± 17.09 **** |
| R | 315.83 ± 18.27 | 330.83 ± 19.25 | 326.33 ± 20.76 |
| OR | 312.67 ±15.91 | 354.33 ± 14.75 *** | 393.17 ± 12.32 **** |
| H | 306.50 ± 15.73 | 319.67 ± 17.5 | 323.17 ± 19.73 |
| OH | 311.67 ± 18.68 | 359.67 ± 13.88 **** | 380.83 ± 20.46 **** |
| HR | 319.50 ±12.79 | 337.33 ± 16.16 | 310.17 ± 12.40 |
| OHR | 308.83 ± 18.59 | 350.67 ± 17.19 *** | 363.17 ± 14.20 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimbru, R.-I.; Zimbru, E.-L.; Ordodi, V.-L.; Bojin, F.-M.; Crîsnic, D.; Grijincu, M.; Mirica, S.-N.; Tănasie, G.; Georgescu, M.; Huțu, I.; et al. The Impact of High-Fructose Diet and Co-Sensitization to House Dust Mites and Ragweed Pollen on the Modulation of Airway Reactivity and Serum Biomarkers in Rats. Int. J. Mol. Sci. 2024, 25, 8868. https://doi.org/10.3390/ijms25168868
Zimbru R-I, Zimbru E-L, Ordodi V-L, Bojin F-M, Crîsnic D, Grijincu M, Mirica S-N, Tănasie G, Georgescu M, Huțu I, et al. The Impact of High-Fructose Diet and Co-Sensitization to House Dust Mites and Ragweed Pollen on the Modulation of Airway Reactivity and Serum Biomarkers in Rats. International Journal of Molecular Sciences. 2024; 25(16):8868. https://doi.org/10.3390/ijms25168868
Chicago/Turabian StyleZimbru, Răzvan-Ionuț, Elena-Larisa Zimbru, Valentin-Laurențiu Ordodi, Florina-Maria Bojin, Daniela Crîsnic, Manuela Grijincu, Silvia-Nicoleta Mirica, Gabriela Tănasie, Marius Georgescu, Ioan Huțu, and et al. 2024. "The Impact of High-Fructose Diet and Co-Sensitization to House Dust Mites and Ragweed Pollen on the Modulation of Airway Reactivity and Serum Biomarkers in Rats" International Journal of Molecular Sciences 25, no. 16: 8868. https://doi.org/10.3390/ijms25168868






