Structural and Mechanistic Insights into a Novel Monooxygenase for Poly(acrylic acid) Biodegradation
Abstract
:1. Introduction
2. Results and Discussion
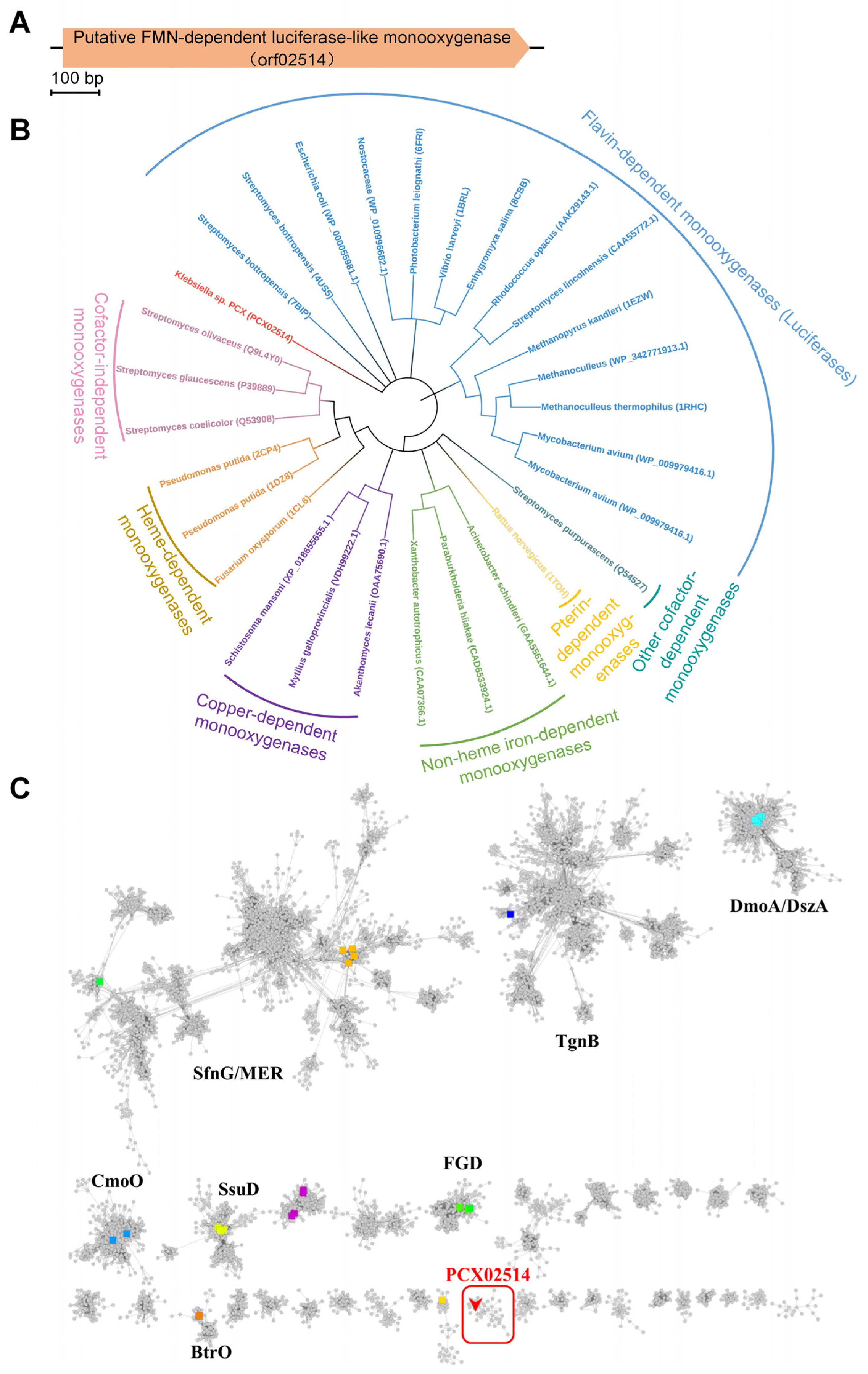
2.1. Bioinformatic Analyses of Monooxygenase PCX02514 with PAA Degradation Ability
2.2. Biochemical Analyses of Monooxygenase PCX02514
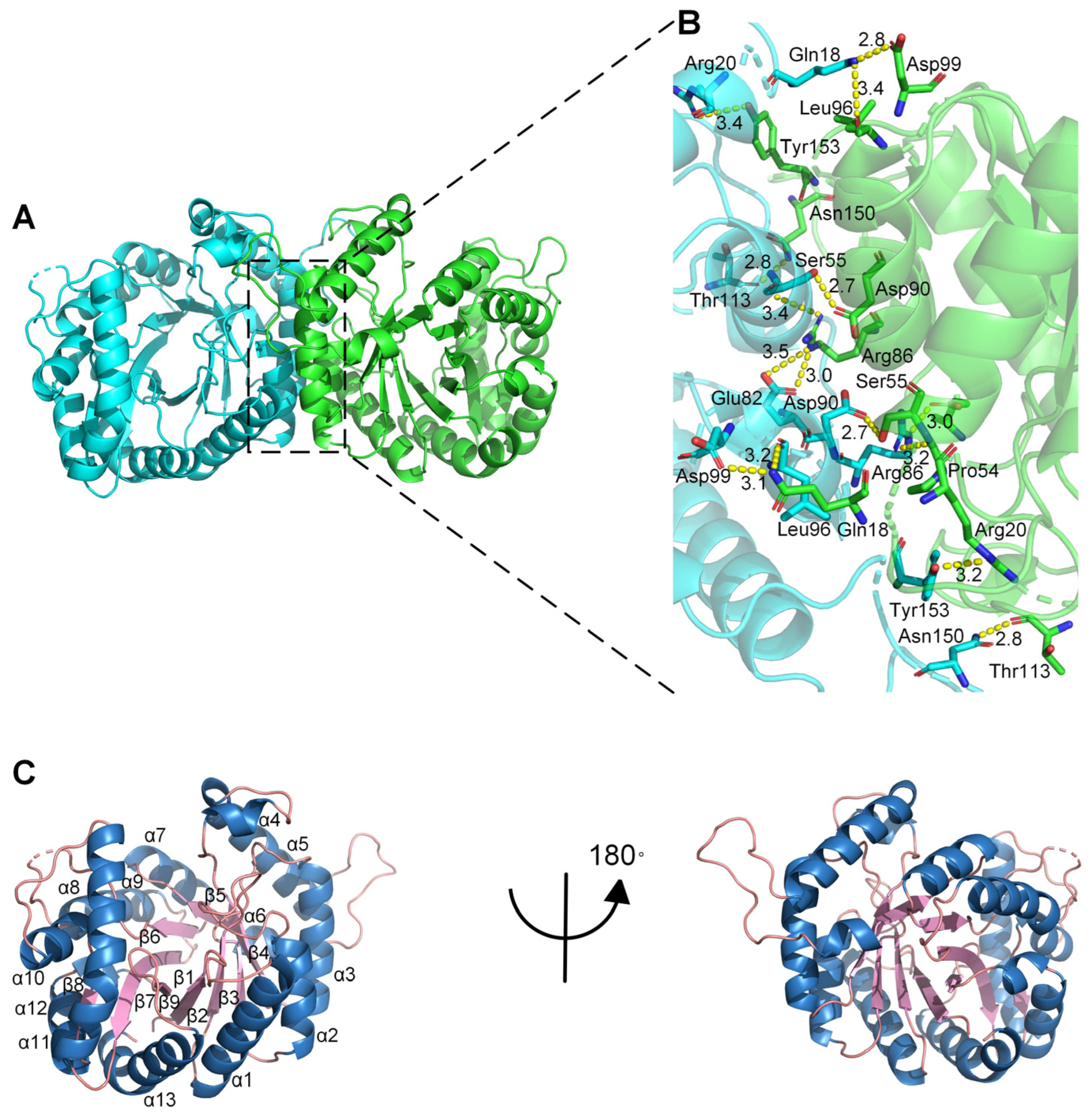
2.3. Crystal Structure of Monooxygenase PCX02514
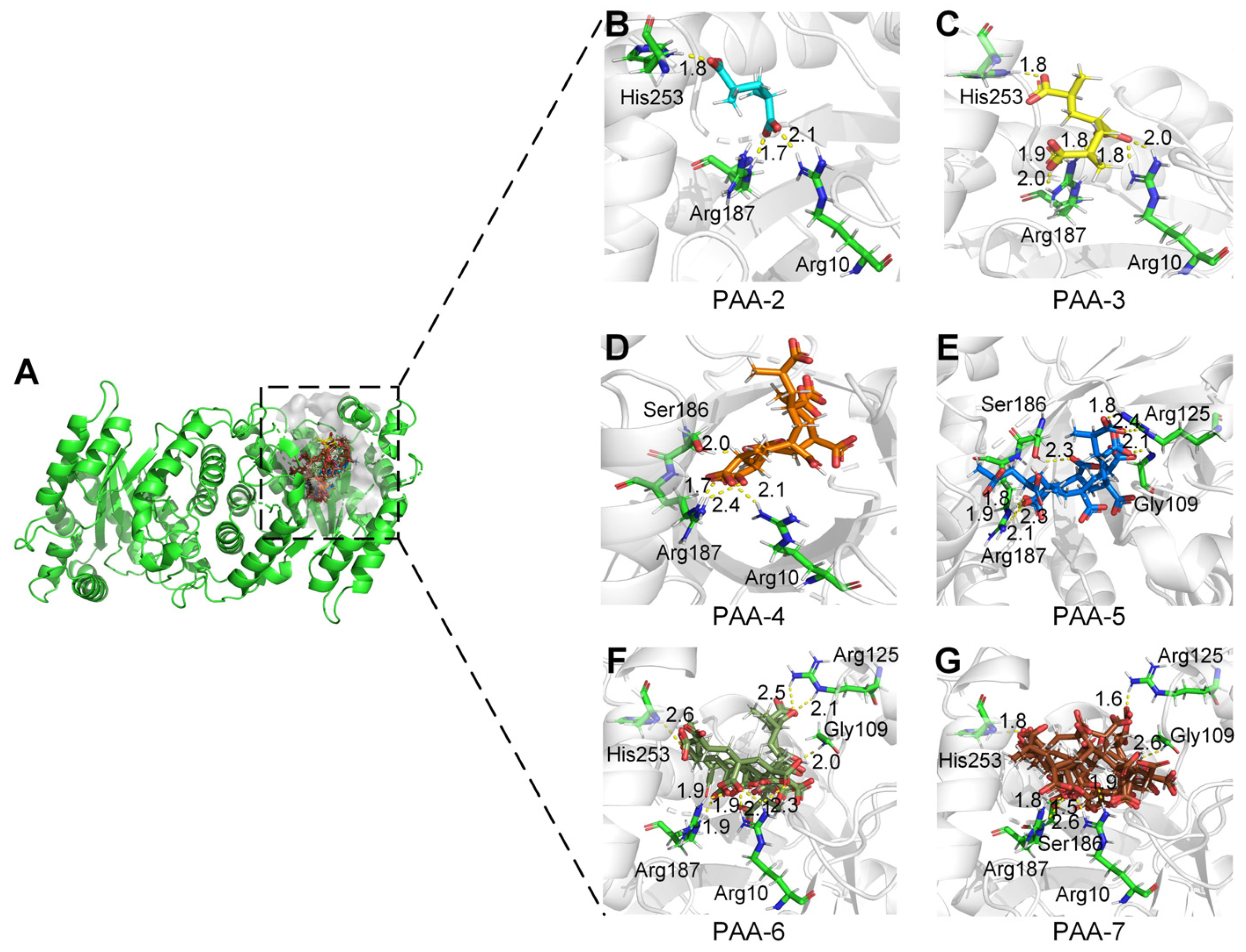
2.4. Active Sites of Monooxygenase PCX02514 Binding with Substrates
3. Materials and Methods
3.1. Bacterial Strain, Culture Medium, and Chemicals
3.2. Bioinformatic Analyses
3.3. Cloning, Expression, and Purification
3.4. HPLC–MS Analysis
3.5. Gel Permeation Chromatography Analysis
3.6. Enzyme Kinetic Analysis
3.7. Isothermal Titration Calorimetry
3.8. Crystallization, Data Collection, and Structure Determination
3.9. Molecular Docking of Monooxygenase PCX02514 and Substrates
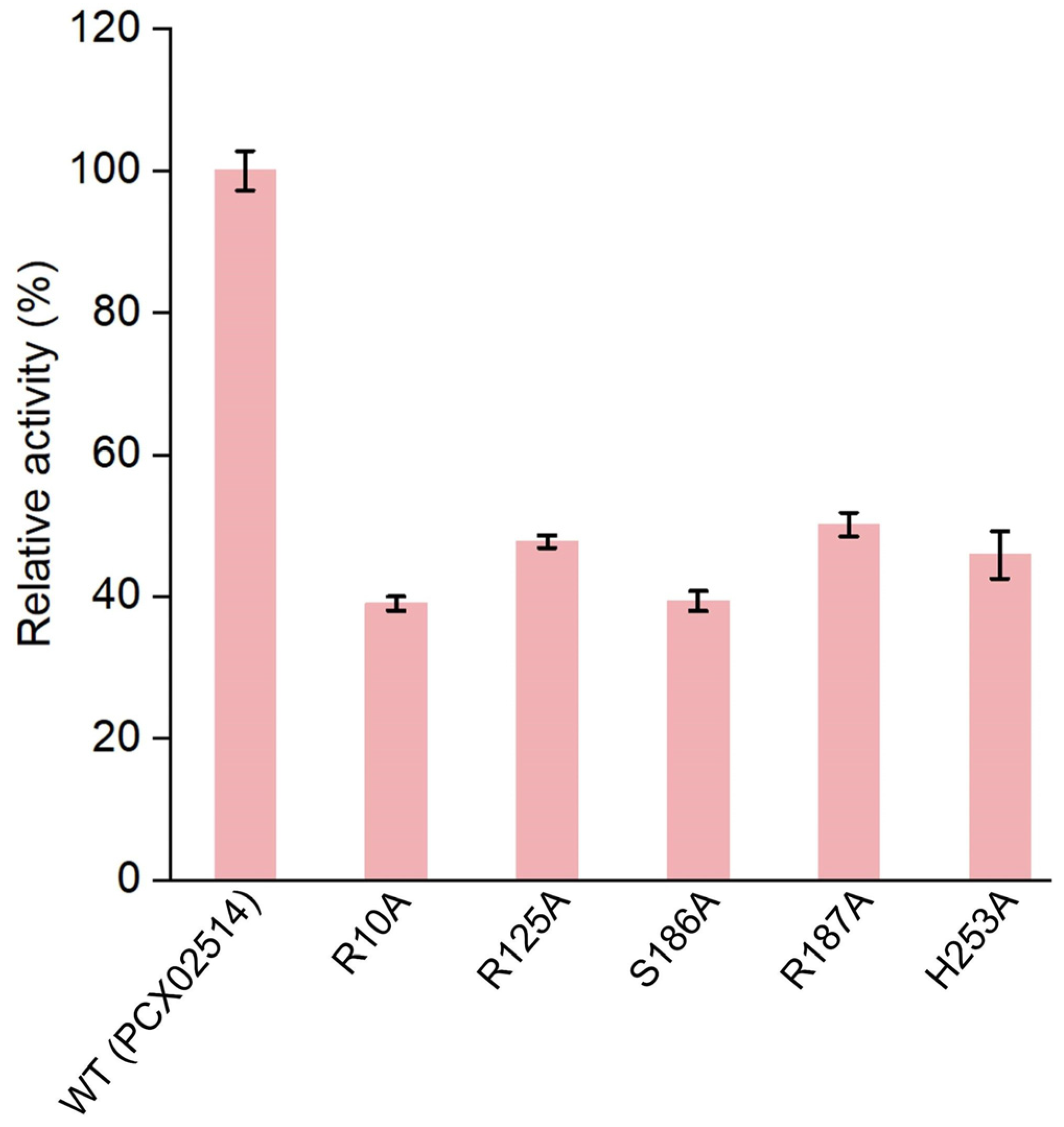
3.10. Site-Directed Mutagenesis and Enzyme Activity Measurement
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godwin Uranta, K.; Rezaei-Gomari, S.; Russell, P.; Hamad, F. Studying the effectiveness of polyacrylamide (PAM) application in hydrocarbon reservoirs at different operational conditions. Energies 2018, 11, 2201. [Google Scholar] [CrossRef]
- Kay-Shoemake, J.L.; Watwood, M.E.; Sojka, R.E.; Lentz, R.D. Soil amidase activity in polyacrylamide-treated soils and potential activity toward common amide-containing pesticides. Biol. Fertil. Soils 2000, 31, 183–186. [Google Scholar] [CrossRef]
- Gaytán, I.; Burelo, M.; Loza-Tavera, H. Current status on the biodegradability of acrylic polymers: Microorganisms, enzymes and metabolic pathways involved. Appl. Microbiol. Biotechnol. 2021, 105, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Ma, L.; Hu, T.; Liu, Y.; Liu, J.; Wang, Y.; Wang, P.; Zhou, J.; Chen, M.; Yang, B.; Li, L. Combination of biochar and immobilized bacteria accelerates polyacrylamide biodegradation in soil by both bio-augmentation and bio-stimulation strategies. J. Hazard. Mater. 2021, 405, 124086. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.B.; Daraboina, N.; Madras, G. Oxidative and photooxidative degradation of poly(acrylic acid). Polym. Degrad. Stab. 2009, 94, 1238–1244. [Google Scholar] [CrossRef]
- Nuss, P.; Gardner, K.H. Attributional life cycle assessment (ALCA) of polyitaconic acid production from northeast US softwood biomass. Int. J. Life Cycle Assess. 2012, 18, 603–612. [Google Scholar] [CrossRef]
- Oksińska, M.P.; Magnucka, E.G.; Lejcuś, K.; Jakubiak-Marcinkowska, A.; Ronka, S.; Trochimczuk, A.W.; Pietr, S.J. Colonization and biodegradation of the cross-linked potassium polyacrylate component of water absorbing geocomposite by soil microorganisms. Appl. Soil Ecol. 2019, 133, 114–123. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Performance and microbial characteristics of bioaugmentation systems for polyacrylamide degradation. J. Polym. Environ. 2010, 19, 125–132. [Google Scholar] [CrossRef]
- Matsuoka, H.; Ishimura, F.; Takeda, T.; Hikuma, M. Isolation of polyacrylamide-degrading microorganisms from soil. Biotechnol. Bioprocess Eng. 2002, 7, 327–330. [Google Scholar] [CrossRef]
- Bao, M.; Chen, Q.; Li, Y.; Jiang, G. Biodegradation of partially hydrolyzed polyacrylamide by bacteria isolated from production water after polymer flooding in an oil field. J. Hazard. Mater. 2010, 184, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.J.; Abed, R.M.M. Biodegradation of polyacrylamide and its derivatives. Environ. Process. 2017, 4, 463–476. [Google Scholar] [CrossRef]
- Cha, M.; Chambliss, G.H. Cloning and sequence analysis of the heat-stable acrylamidase from a newly isolated thermophilic bacterium, Geobacillus thermoglucosidasius AUT-01. Biodegradation 2013, 24, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Lin, K.; Cai, W. Microbial degradation of polyacrylamide by aerobic granules. Environ. Technol. 2012, 33, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Nyyssölä, A.; Ahlgren, J. Microbial degradation of polyacrylamide and the deamination product polyacrylate. Int. Biodeterior. Biodegrad. 2019, 139, 24–33. [Google Scholar] [CrossRef]
- Torres Pazmiño, D.E.; Winkler, M.; Glieder, A.; Fraaije, M.W. Monooxygenases as biocatalysts: Classification, mechanistic aspects and biotechnological applications. J. Biotechnol. 2010, 146, 9–24. [Google Scholar] [CrossRef] [PubMed]
- van Berkel, W.J.H.; Kamerbeek, N.M.; Fraaije, M.W. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 2006, 124, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Yang, W.; Xu, F.; Wang, W.; Feng, L.; Bartlam, M.; Wang, L.; Rao, Z. Crystal structure of long-chain alkane monooxygenase (LadA) in complex with coenzyme FMN: Unveiling the long-chain alkane hydroxylase. J. Mol. Biol. 2008, 376, 453–465. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Lutz-Wahl, S.; Schmid, R.D. Microbial P450 enzymes in biotechnology. Appl. Microbiol. Biotechnol. 2004, 64, 317–325. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, L.; Liang, J.; Li, L.; Ma, L. Screening and degradation characteristics of a polyacrylamide degrading bacterium. Biotechnol. Bull. 2019, 35, 178–183. [Google Scholar]
- Li, C.; Hu, H.; Liu, J.; Yang, S.; Mu, B. Research progress in biodegradation of polyacrylamide. Oilfield Chem. 2016, 33, 557–563+570. [Google Scholar]
- Fisher, A.J.; Thompson, T.B.; Thoden, J.B.; Baldwin, T.O.; Rayment, I. The 1.5-Å resolution crystal structure of bacterial luciferase in low salt conditions. J. Biol. Chem. 1996, 271, 21956–21968. [Google Scholar] [CrossRef]
- Gaytán, I.; Sánchez-Reyes, A.; Burelo, M.; Vargas-Suárez, M.; Liachko, I.; Press, M.; Sullivan, S.; Cruz-Gómez, M.J.; Loza-Tavera, H. Degradation of recalcitrant polyurethane and xenobiotic additives by a selected landfill microbial community and its biodegradative potential revealed by proximity ligation-based metagenomic analysis. Front. Microbiol. 2020, 10, 2986. [Google Scholar] [CrossRef]
- Mai, T.; Hu, G.; Chen, C. Visualizing and clustering protein similarity networks: Sequences, structures, and functions. J. Proteome Res. 2016, 15, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F. Biodegradation of polyethers and polyacrylate. Biodegrad. Plast. Polym. 1994, 12, 24–38. [Google Scholar]
- Gancet, C.; Pirri, R.; Dalens, J.M.; Boutevin, B.; Guyot, B.; Loubat, C.; Le Petit, J.; Farnet, A.M.; Tagger, S. Methodology in studying improvement of polyacrylates biodegradability. Macromol. Symp. 2011, 144, 211–217. [Google Scholar] [CrossRef]
- Stroobants, K.; Saadallah, D.; Bruylants, G.; Parac-Vogt, T.N. Thermodynamic study of the interaction between hen egg white lysozyme and Ce(IV)-Keggin polyoxotungstate as artificial protease. Phys. Chem. Chem. Phys. 2014, 16, 21778–21787. [Google Scholar] [CrossRef] [PubMed]
- Banner, D.W.; Bloomer, A.C.; Petsko, G.A.; Phillips, D.C.; Pogson, C.I.; Wilson, I.A.; Corran, P.H.; Furth, A.J.; Milman, J.D.; Offord, R.E.; et al. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 Å resolution: Using amino acid sequence data. Nature 1975, 255, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Warkentin, E.; Grabarse, W.; Sordel, M.; Wicke, M.; Thauer, R.K.; Ermler, U. Structure of coenzyme F420 dependent methylenetetrahydromethanopterin reductase from two methanogenic archaea. J. Mol. Biol. 2000, 300, 935–950. [Google Scholar] [CrossRef]
- Eichhorn, E.; Davey, C.A.; Sargent, D.F.; Leisinger, T.; Richmond, T.J. Crystal structure of Escherichia coli alkanesulfonate monooxygenase SsuD. J. Mol. Biol. 2002, 324, 457–468. [Google Scholar] [CrossRef]
- Alali, A.; Zhang, L.; Li, J.; Zuo, C.; Wassouf, D.; Yan, X.; Schwarzer, P.; Günther, S.; Einsle, O.; Bechthold, A. Biosynthesis of the tricyclic aromatic type Ⅱ polyketide rishirilide: New potential third ring oxygenation after three cyclization steps. Mol. Biotechnol. 2021, 63, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.J.; Raushel, F.M.; Baldwin, T.O.; Rayment, I. Three-dimensional structure of bacterial luciferase from Vibrio harveyi at 2.4 Å resolution. Biochemistry 1995, 34, 6581–6586. [Google Scholar] [CrossRef] [PubMed]
- Aufhammer, S.W.; Warkentin, E.; Berk, H.; Shima, S.; Thauer, R.K.; Ermler, U. Coenzyme binding in F420-dependent secondary alcohol dehydrogenase, a member of the bacterial luciferase family. Structure 2004, 12, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Pflüger, T.; Loesgen, S.; Asmus, K.; Brötz, E.; Paululat, T.; Zeeck, A.; Andrade, S.; Bechthold, A. Insights into the bioactivity of mensacarcin and epoxide formation by MsnO8. Chembiochem A Eur. J. Chem. Biol. 2014, 15, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kursula, P.; Ferroni, F.M.; Tolmie, C.; Smit, M.S.; Opperman, D.J. Structural and catalytic characterization of a fungal Baeyer-Villiger monooxygenase. PLoS ONE 2016, 11, e0160186. [Google Scholar]
- Oberg, N.; Zallot, R.; Gerlt, J.A. EFI-EST, EFI-GNT, and EFI-CGFP: Enzyme function initiative (EFI) web resource for genomic enzymology tools. J. Mol. Biol. 2023, 435, 168018. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Cymborowski, M.; Otwinowski, Z.; Chruszcz, M. HKL-3000: The integration of data reduction and structure solution-from diffraction images to an initial model in minutes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 859–866. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Kunstleve, R.W.; Afonine, P.V.; Zwart, P.H.; Terwilliger, T.C.; Moriarty, N.W.; Urzhumtsev, A.; Adams, P.D. Phenix refine developments. Acta Crystallogr. Sect. A Found. Adv. 2007, 63, s80. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]

| KM (μM) | Vm (μM s−1) | kcat (s−1) | kcat/KM (s−1 M−1) |
|---|---|---|---|
| 158.9 ± 16.4 | 0.5376 ± 0.1284 | 0.00214 ± 0.000511 | 13.47 |
| N (Sites) | KD (×10−9 M) | ΔH (kcal mol−1) | ΔS (cal mol−1 K−1) | ΔG (kcal mol−1) | −TΔS (kcal mol−1) |
|---|---|---|---|---|---|
| 0.034 ± 0.0027 | 239 ± 15.7 | −32.9 ± 5.42 | −0.08 ± 0.013 | −9.04 ± 1.63 | 23.9 ± 3.79 |
| Protein | PCX02514 |
|---|---|
| PDB accession | 8K74 |
| Data collection | |
| Beamline | BL18U1 |
| Wavelength (Å) | 0.9870 |
| Space group | P 1 21 1 |
| Cell dimensions | |
| a, b, c (Å) | 73.23, 59.61, 79.72 |
| α, β, γ (°) | 90.00, 98.84, 90.00 |
| Resolution (Å) | 41.54–1.97(2.02–1.97) a |
| No. observed reflections | 44,451 (2618) |
| No. unique reflections | 44,419 (2618) |
| Rmerge (%) | 10.6 (68.0) |
| I/σI | 8.2 (3.15) |
| Completeness (%) | 92.7 (78.3) |
| Redundancy | 1.0 (1.0) |
| CC1/2 (%) | 0.0 |
| Refinement | |
| Resolution (Å) | 41.54–1.97 |
| Rwork/Rfree | 0.183/0.222 |
| No. atoms | 5201 |
| Protein | 4932 |
| Ligand/ion | 0 |
| Water | 269 |
| B-factors | 24.20 |
| Protein | 25.79 |
| Ligand/ion | 0 |
| Water | 27.76 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.007 |
| Bond angles (°) | 0.942 |
| Ramachandran plot (%) | |
| Favored | 97.83 |
| Allowed | 1.86 |
| Disallowed | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, R.; Zhao, J.; Li, X.; Dong, S.; Ma, D. Structural and Mechanistic Insights into a Novel Monooxygenase for Poly(acrylic acid) Biodegradation. Int. J. Mol. Sci. 2024, 25, 8871. https://doi.org/10.3390/ijms25168871
Feng R, Zhao J, Li X, Dong S, Ma D. Structural and Mechanistic Insights into a Novel Monooxygenase for Poly(acrylic acid) Biodegradation. International Journal of Molecular Sciences. 2024; 25(16):8871. https://doi.org/10.3390/ijms25168871
Chicago/Turabian StyleFeng, Rui, Juyi Zhao, Xiaochen Li, Sijun Dong, and Dan Ma. 2024. "Structural and Mechanistic Insights into a Novel Monooxygenase for Poly(acrylic acid) Biodegradation" International Journal of Molecular Sciences 25, no. 16: 8871. https://doi.org/10.3390/ijms25168871






