The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes
Abstract
1. Introduction
2. Production of Oxygen Radicals in Peroxisomes
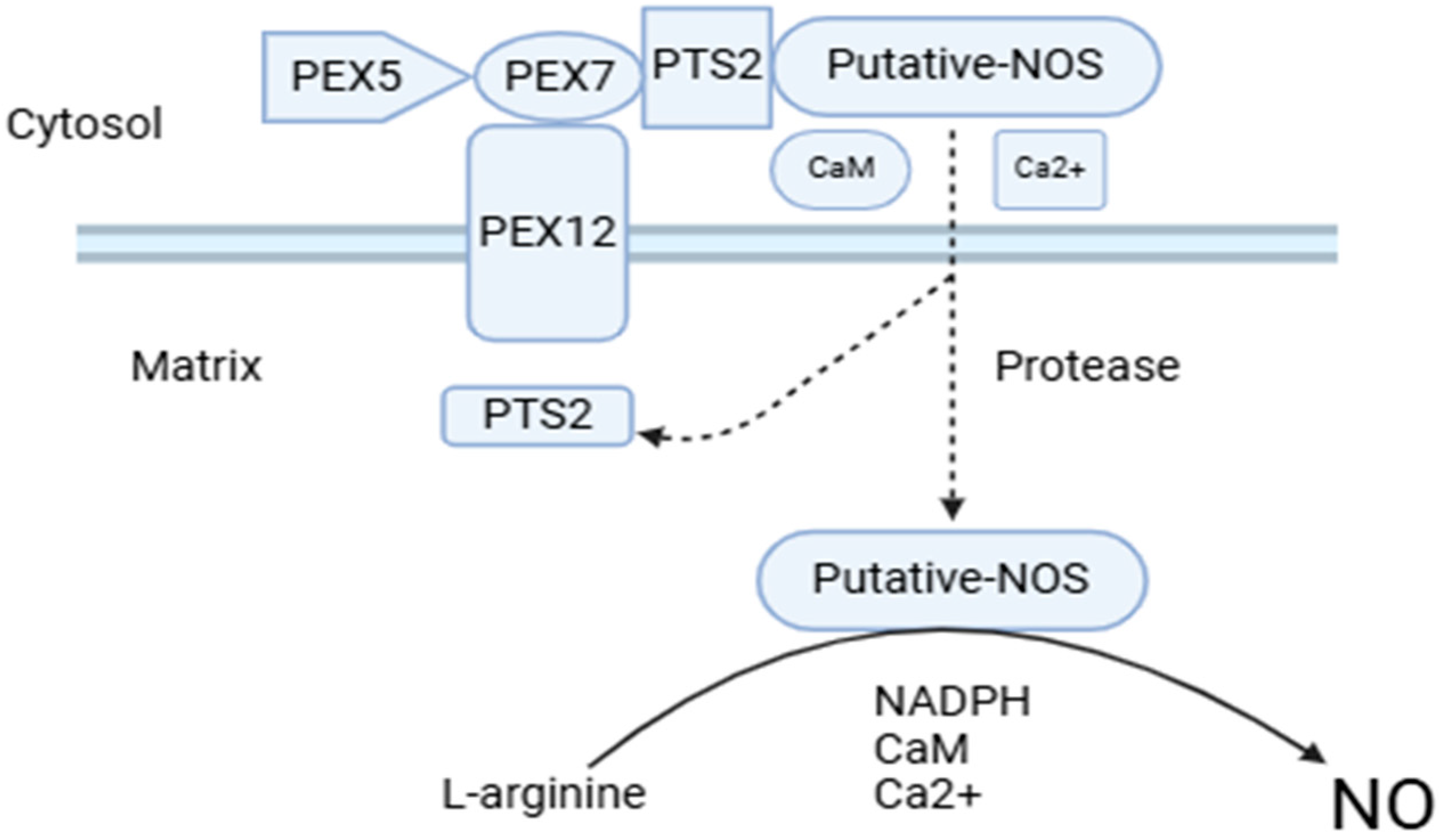
3. Peroxisomes Serve as Generators of Not Only ROS but Also NOS, Which Function as Signaling Molecules
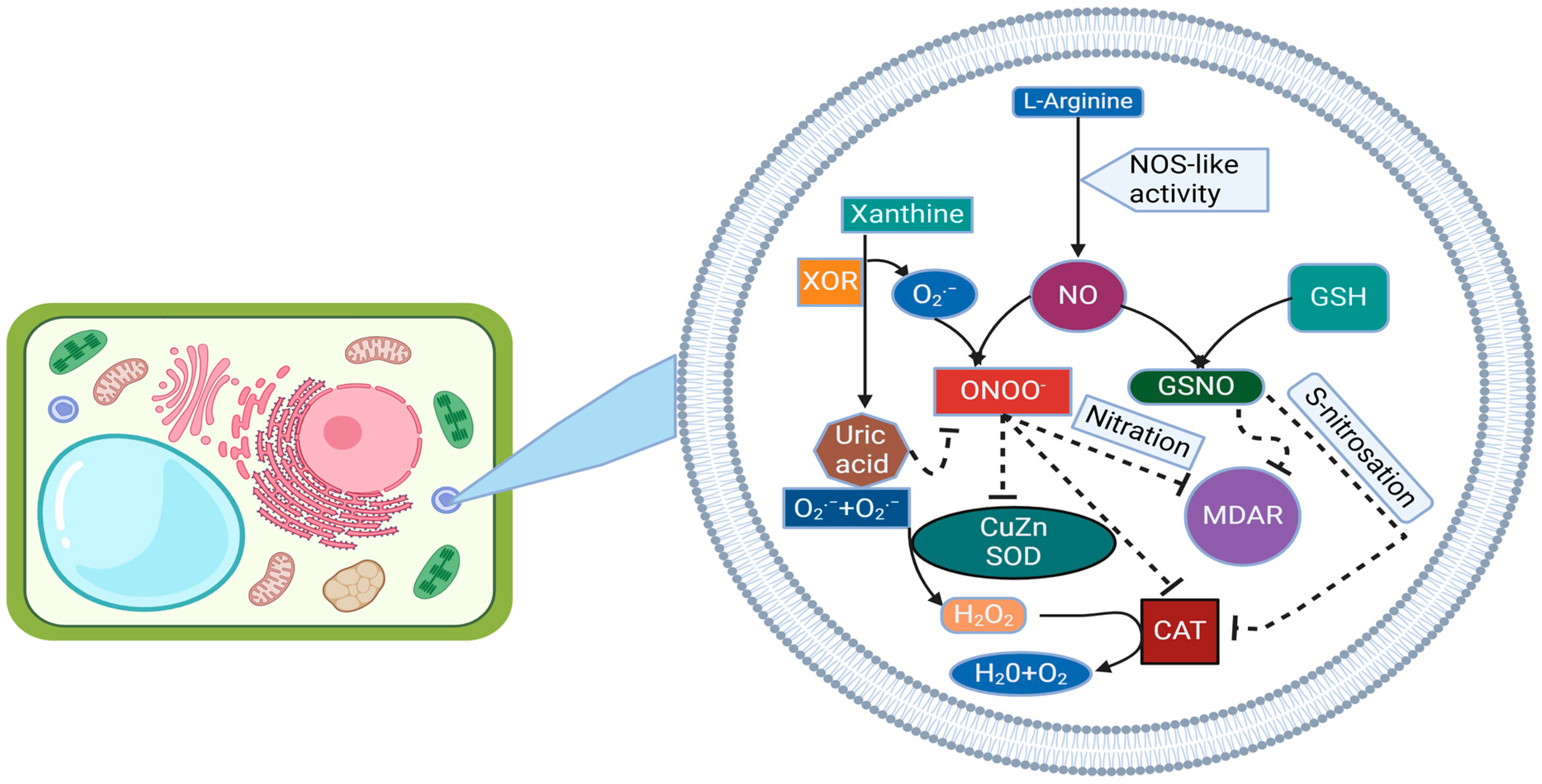
4. Nitric Oxide-Induced S-Nitrosylation, Tyrosine Nitration, Transnitrosylation, and Denitrosylation Processes
4.1. Catalase
4.2. S-Nitrosation of Catalase
4.3. Tyrosine Nitration and Metabolism of RO
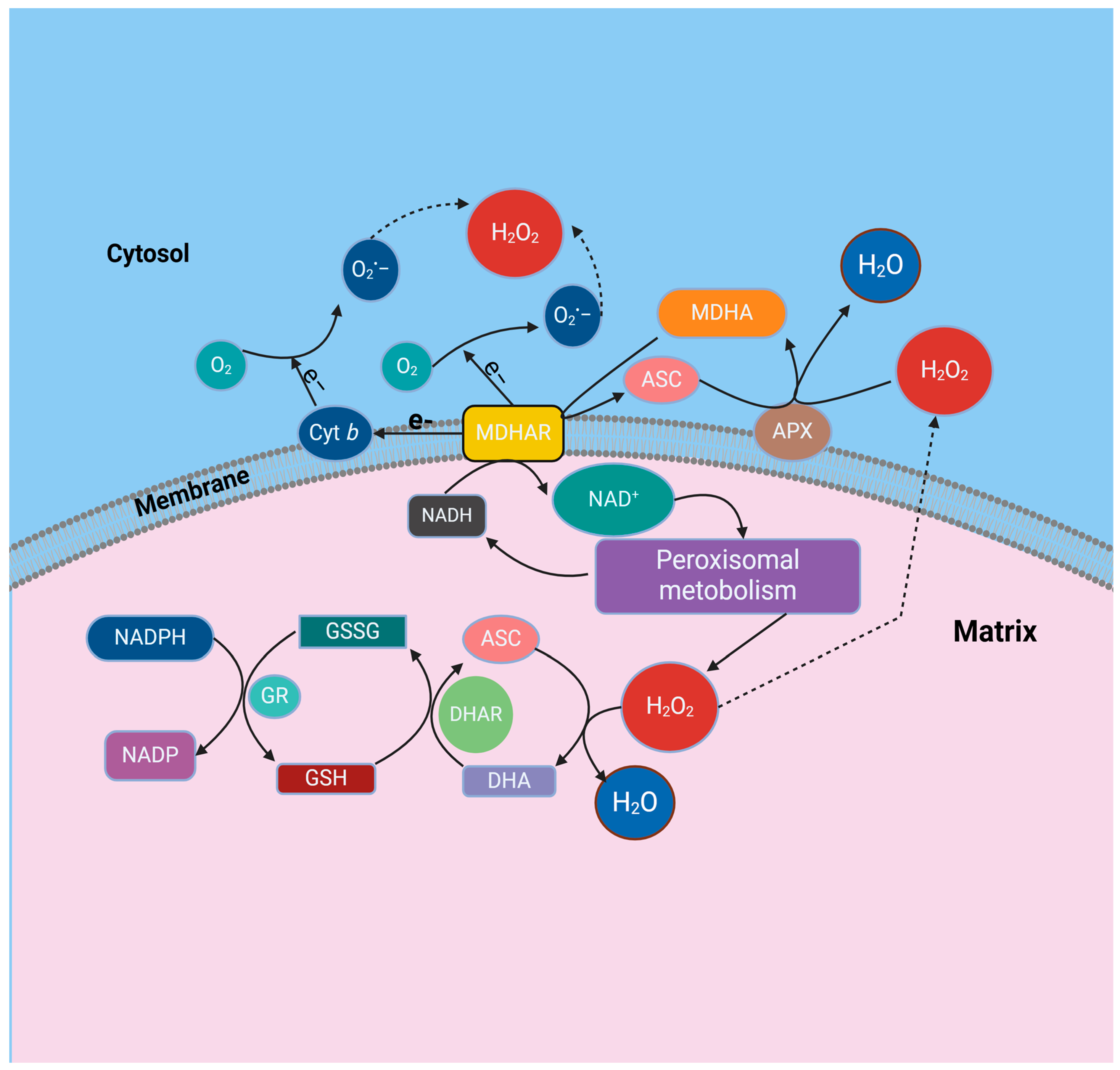
4.4. Monodehydroascorbate Reductase
4.5. SOD Emerges as a Significant Protein Warranting Further Exploration as a Target of PTMs Mediated by NO
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production; Hasanuzzaman, M., Fujita, M., Teixeira Filho, M.C.M., Nogueira, T.A.R., Galindo, F.S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When bad guys become good ones: The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, V.; Khare, T. Differential growth and yield responses of salt-tolerant and susceptible rice cultivars to individual (Na+ and Cl−) and additive stress effects of NaCl. Acta Physiol. Plant. 2016, 38, 170. [Google Scholar] [CrossRef]
- Corpas, F.J.; del Río, L.A.; Palma, J.M. Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J. Integr. Plant Biol. 2019, 61, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.B.; Valderrama, R.; Corpas, F.J. Immunolocalization of S-nitrosoglutathione, S-nitrosoglutathione reductase and tyrosine nitration in pea leaf organelles. Acta Physiol. Plant. 2013, 35, 2635–2640. [Google Scholar] [CrossRef]
- Sun, A. The EPR Method for Detecting Nitric Oxide in Plant Senescence. Methods Mol. Biol. 2018, 1744, 119–124. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Collado-Arenal, A.M.; Romero-Puertas, M.C. Deciphering peroxisomal reactive species interactome and redox signalling networks. Free Radic. Biol. Med. 2023, 197, 58–70. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.S.; Kelly, C.L.; Mordaka, P.M.; Heap, J.T. Review of NAD(P)H-dependent oxidoreductases: Properties, engineering and application. Biochim. Biophys. Acta-Proteins Proteom. 2018, 1866, 327–347. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.-M.; Nam, W. Catalytic recycling of NAD(P)H. J. Inorg. Biochem. 2019, 199, 110777. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Bortolotti, M.; Polito, L.; Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Lespagnol, E.; Tagougui, S.; Fernandez, B.O.; Zerimech, F.; Matran, R.; Maboudou, P.; Berthoin, S.; Descat, A.; Kim, I.; Pawlak-Chaouch, M.; et al. Circulating biomarkers of nitric oxide bioactivity and impaired muscle vasoreactivity to exercise in adults with uncomplicated type 1 diabetes. Diabetologia 2021, 64, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Camp, O.G.; Bai, D.; Awonuga, A.; Goud, P.T.; Abu-Soud, H.M. Hypochlorous acid facilitates inducible nitric oxide synthase subunit dissociation: The link between heme destruction, disturbance of the zinc-tetrathiolate center, and the prevention by melatonin. Nitric Oxide 2022, 124, 32–38. [Google Scholar] [CrossRef] [PubMed]
- López-Huertas, E.; Sandalio, L.M.; Gómez, M.; Del Ríd, L.A. Superoxide radical generation in peroxisomal mimbranes: Evidence for the participation of the 18-kDa integral membrane polypeptide. Free Radic. Res. 1997, 26, 497–506. [Google Scholar] [CrossRef] [PubMed]
- de Bont, L.; Mu, X.; Wei, B.; Han, Y. Abiotic stress-triggered oxidative challenges: Where does H2S act? J. Genet. Genom. 2022, 49, 748–755. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Airaki, M.; Sánchez-Moreno, L.; Leterrier, M.; Barroso, J.B.; Palma, J.M.; Corpas, F.J. Detection and Quantification of S-Nitrosoglutathione (GSNO) in Pepper (Capsicum annuum L.) Plant Organs by LC-ES/MS. Plant Cell Physiol. 2011, 52, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, M.L.; Feng, P.; Rapoport, T.A. Towards solving the mystery of peroxisomal matrix protein import. Trends Cell Biol. 2024, 34, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Terrón-Camero, L.C.; Rodríguez-Serrano, M.; Sandalio, L.M.; Romero-Puertas, M.C. Nitric oxide is essential for cadmium-induced peroxule formation and peroxisome proliferation. Plant Cell Environ. 2020, 43, 2492–2507. [Google Scholar] [CrossRef]
- Mohn, M.A.; Thaqi, B.; Fischer-Schrader, K. Isoform-specific NO synthesis by Arabidopsis thaliana nitrate reductase. Plants 2019, 8, 67. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Peroxisomal plant nitric oxide synthase (NOS) protein is imported by peroxisomal targeting signal type 2 (PTS2) in a process that depends on the cytosolic receptor PEX7 and calmodulin. FEBS Lett. 2014, 588, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.P.; Wyatt, S.E. Nitric oxide, gravity response, and a unified schematic of plant signaling. Plant Sci. 2022, 314, 111105. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Gow, A.J.; Doherty, M.K. S-Nitrosoglutathione as a biological signaling molecule: The role of S-nitrosylation and S-transnitrosation. Free. Radic. Biol. Med. 2007, 42, 1524–1534. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kolbert, Z.; Durner, J.; Lindermayr, C.; Corpas, F.J.; Brouquisse, R.; Barroso, J.B.; Umbreen, S.; Palma, J.M.; Hancock, J.T.; et al. Regulating the regulator: Nitric oxide control of post-translational modifications. New Phytol. 2020, 227, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, L.; Zuo, J. Protein S-nitrosylation in plants: Current progresses and challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Igamberdiev, A.U.; Palma, J.M. Advances in Nitric Oxide Signalling and Metabolism in Plants. Int. J. Mol. Sci. 2023, 24, 6397. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.; Doulias, P.-T.; Tenopoulou, M.; Raju, K.; Ischiropoulos, H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J. Biol. Chem. 2013, 288, 26473–26479. [Google Scholar] [CrossRef] [PubMed]
- Khator, K.; Parihar, S.; Jasik, J.; Shekhawat, G.S. Nitric oxide in plants: An insight on redox activity and responses toward abiotic stress signaling. Plant Signal. Behav. 2024, 19, 2298053. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Oh, C.-K.; Zhang, X.; Tannenbaum, S.R.; Lipton, S.A. Protein Transnitrosylation Signaling Networks Contribute to Inflammaging and Neurodegenerative Disorders. Antioxid. Redox Signal. 2021, 35, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation: Determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef]
- Chen, L.; Wu, R.; Feng, J.; Feng, T.; Wang, C.; Hu, J.; Zhan, N.; Li, Y.; Ma, X.; Ren, B.; et al. Transnitrosylation mediated by the non-canonical catalase ROG1 regulates nitric oxide signaling in plants. Dev. Cell 2020, 53, 444–457.e445. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. Hidden networks of aberrant protein transnitrosylation contribute to synapse loss in Alzheimer’s disease. Free Radic Biol Med. 2022, 193 Pt 1, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.M.; Bizzozero, O.A. Intracellular glutathione mediates the denitrosylation of protein nitrosothiols in the rat spinal cord. J. Neurosci. Res. 2009, 87, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; Powrie, F.; Puri, R.N.; Meister, A. Glutathione monoethyl ester: Preparation, uptake by tissues, and conversion to glutathione. Arch. Biochem. Biophys. 1985, 239, 538–548. [Google Scholar] [CrossRef]
- Smith, R.J.; Johnson, L.T. Role of Glutathione in the Stability of Nitrosothiols: Insights from Recent Findings on NPSH and Residual SNO Levels. J. Biochem. Res. 2023, 45, 1234–1245. [Google Scholar]
- Forrester, M.T.; Foster, M.W.; Stamler, J.S. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J. Biol. Chem. 2007, 282, 13977–13983. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, A.; Banerjee, D.; Billiar, T.R.; Sengupta, R. Understanding the role of S-nitrosylation/nitrosative stress in inflammation and the role of cellular denitrosylases in inflammation modulation: Implications in health and diseases. Free Radic. Biol. Med. 2021, 172, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Wang, L.; Wong, C.C.; Scott, F.L.; Eckelman, B.P.; Han, X.; Tzitzilonis, C.; Meng, F.; Gu, Z.; Holland, E.A.; et al. Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol. Cell 2010, 39, 184–195. [Google Scholar] [CrossRef]
- Li, H.; Wan, A.; Xu, G.; Ye, D. Small changes huge impact: The role of thioredoxin 1 in the regulation of apoptosis by S-nitrosylation. Acta Biochim. Biophys. Sin. 2013, 45, 153–161. [Google Scholar] [CrossRef]
- Ye, H.; Wu, J.; Liang, Z.; Zhang, Y.; Huang, Z. Protein S-Nitrosation: Biochemistry, Identification, Molecular Mechanisms, and Therapeutic Applications. J. Med. Chem. 2022, 65, 5902–5925. [Google Scholar] [CrossRef]
- Kneeshaw, S.; Gelineau, S.; Tada, Y.; Loake, G.J.; Spoel, S.H. Selective Protein Denitrosylation Activity of Thioredoxin-h5 Modulates Plant Immunity. Mol. Cell 2014, 56, 153–162. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Front. Plant Sci. 2017, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, M.; Chen, T.; Zhang, L.; Fan, L.; Zhang, W.; Wei, B.; Li, S.; Xuan, W.; Noctor, G.; et al. Glutathione-dependent denitrosation of GSNOR1 promotes oxidative signalling downstream of H2O2. Plant Cell Environ. 2020, 43, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; López-Jaramillo, J.; Padilla, M.N.; Carreras, A.; Corpas, F.J.; Barroso, J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gong, B.; Jin, Z.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J. Plant Physiol. 2015, 186–187, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, C.; Chen, Q.; Chen, H.; Ren, B.; Li, X.; Zuo, J. S-nitrosylation of phosphotransfer proteins represses cytokinin signaling. Nat. Commun. 2013, 4, 1529. [Google Scholar] [CrossRef]
- Fares, A.; Nespoulous, C.; Rossignol, M.; Peltier, J.B. Simultaneous identification and quantification of nitrosylation sites by combination of biotin switch and ICAT labeling. Plant Proteom. 2014, 1072, 609–620. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Rahim, W.; Khan, M.; Lee, D.S.; Lee, G.M.; Al Azzawi, T.N.I.; Hussain, A.; Kil Kim, C.; Yun, B.W. Phytohormonal Regulation through Protein S-Nitrosylation under Stress. Front. Plant Sci. 2022, 13, 865542. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Leterrier, M.; Begara-Morales, J.C.; Valderrama, R.; Chaki, M.; López-Jaramillo, J.; Luque, F.; Palma, J.M.; Padilla, M.N.; Sánchez-Calvo, B.; et al. Inhibition of peroxisomal hydroxypyruvate reductase (HPR1) by tyrosine nitration. Biochim. Biophys. Acta 2013, 1830, 4981–4989. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M. Hydrogen sulfide: A novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J. Integr. Plant Biol. 2019, 61, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Klein, C. Stability of dehydrogenases III. malate dehydrogenases. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 1982, 707, 133–141. [Google Scholar] [CrossRef]
- Arora, D.; Jain, P.; Singh, N.; Kaur, H.; Bhatla, S.C. Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic. Res. 2016, 50, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Ischiropoulos, H.; Zhu, L.; Chen, J.; Tsai, M.; Martin, J.C.; Smith, C.D.; Beckman, J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992, 298, 431–437. [Google Scholar] [CrossRef]
- Sánchez-Pérez, G.F.; García, M.A. Effects of S-nitrosation and tyrosine nitration on the activity of ascorbate-related enzymes in plants. J. Exp. Bot. 2009, 60, 2497–2509. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissues. Methods Enzymol. 1995, 297, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Mhamdi, A.; Chaouch, S.; Noctor, G. Regulation of basal and oxidative stress-triggered jasmonic acid-related gene ex-pression by glutathione. Plant Cell Environ. 2013, 36, 1135–1146. [Google Scholar] [CrossRef]
- Kim, Y.; Jang, H.H. The Role of Peroxiredoxin family in cancer signaling. J. Cancer Prev. 2019, 24, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. Bicentennial of catalase research, 1818–2018. Orvosi Hetil. 2018, 159, 959–964. [Google Scholar] [CrossRef]
- Khan, T.A.; Yusuf, M.; Fariduddin, Q. Fariduddin Hydrogen peroxide in regulation of plant metabolism: Signalling and its effect under abiotic stress. Photosynthetica 2018, 56, 1237–1248. [Google Scholar] [CrossRef]
- Wolhuter, K.; Eaton, P. How widespread is stable protein S-nitrosylation as an end-effector of protein regulation? Free Radic. Biol. Med. 2017, 109, 156–166. [Google Scholar] [CrossRef]
- Gupta, K.J.; Hancock, J.T.; Petrivalsky, M.; Kolbert, Z.; Lindermayr, C.; Durner, J.; Barroso, J.B.; Palma, J.M.; Brouquisse, R.; Wendehenne, D.; et al. Recommendations on terminology and experimental best practice associated with plant nitric oxide research. New Phytol. 2020, 225, 1828–1834. [Google Scholar] [CrossRef]
- Feelisch, M.; Noack, E.A. The pharmacology of nitrosothiols: The biology of S-nitrosothiols. J. Pharmacol. Exp. Ther. 2000, 292, 293–301. [Google Scholar]
- Gómez-Moreno, C.; Cadenas, E. Redox modulation of catalases by reactive nitrogen and sulfur species in plants: Mechanisms and physiological implications. Antioxidants 2021, 10, 1265. [Google Scholar] [CrossRef]
- Gong, B.; Yan, Y.; Zhang, L.; Cheng, F.; Liu, Z.; Shi, Q. Unravelling GSNOR-Mediated S-Nitrosylation and Multiple Developmental Programs in Tomato Plants. Plant Cell Physiol. 2019, 60, 2523–2537. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Hu, J.; Huang, X.; Chen, L.; Sun, X.; Lu, C.; Zhang, L.; Wang, Y.; Zuo, J. Site-specific nitrosoproteomic identification of endogenously S-nitrosylated proteins in arabidopsis. Plant Physiol. 2015, 167, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Frungillo, L.; Skelly, M.J.; Loake, G.J.; Spoel, S.H.; Salgado, I. S-nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nat. Commun. 2014, 5, 5401. [Google Scholar] [CrossRef]
- Gong, B.; Shi, Q. Identifying S-nitrosylated proteins and unraveling S-nitrosoglutathione reductase-modulated sodic alkaline stress tolerance in Solanum lycopersicum L. Plant Physiol. Biochem. 2019, 142, 84–93. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Mata-Pérez, C.; Valderrama, R.; Padilla, M.N.; López-Jaramillo, J.; Luque, F.; Corpas, F.J.; Barroso, J.B. Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J. Exp. Bot. 2015, 66, 5983–5996. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takahashi, R.; Hamada, A.; Terai, Y.; Ogawa, T.; Sawa, Y.; Ishikawa, T.; Maruta, T. Distribution and Functions of Monodehydroascorbate Reductases in Plants: Comprehensive Reverse Genetic Analysis of Arabidopsis thaliana Enzymes. Antioxidants 2021, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.M.; Lin, W.R.; Kao, Y.T.; Hsu, Y.T.; Yech, C.H.; Hong, C.Y.; Kao, C.H. Identification and characterization of novel chloro-plast/mitochondria co-localized glutathione reductase 3 involved in salt stress response in rice. Plant Mol. Biol. 2013, 83, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Romero-Puertas, M.C.; Corpas, F.J.; Sandalio, L.M.; Leterrier, M.; Rodriguez-Serrano, M.; Rio, L.A.; Palma, J.M. Glutathione reductase from pea leaves: Response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol. 2006, 170, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Lindermayr, C. Nitric oxide-dependent posttranslational modification in plants: An update. Int. J. Mol. Sci. 2012, 13, 15193–15208. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Corpas, F.J.; Barroso, J.B.; Sandalio, L.M.; del Río, L.A. Peroxisomal monodehydroascorbate reductase. genomic clone characterization and functional analysis under environmental stress conditions. Plant Physiol. 2005, 138, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Kang, Y.N.; Shigemizu, H.; Morishita, N.; Yoon, H.J.; Saito, K.; Asada, K.; Mikami, B. Crystallization and preliminary crys-tallographic analysis of monodehydroascorbate radical reductase from cucumber. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 1498–1499. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Radi, R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003, 25, 295–311. [Google Scholar] [CrossRef]
- Wang, X.; Hargrove, M.S. Nitric oxide in plants: The roles of ascorbate and hemoglobin. PLoS ONE 2013, 8, e82611. [Google Scholar] [CrossRef]
- Decros, G.; Dussarrat, T.; Baldet, P.; Cassan, C.; Cabasson, C.; Dieuaide-Noubhani, M.; Destailleur, A.; Flandin, A.; Prigent, S.; Mori, K.; et al. Enzyme-based kinetic modelling of ASC–GSH cycle during tomato fruit development reveals the importance of reducing power and ROS availability. New Phytol. 2023, 240, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol.-Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Murphy, M.P. Targeting the peroxisomal Cu,Zn superoxide dismutase (SOD) in Arabidopsis: Characterization and modification by reactive nitrogen species. J. Exp. Bot. 2014, 65, 4455–4467. [Google Scholar] [CrossRef]

| Peroxisomal Enzyme | Function | Effect on Activity | Effect on Activity | References |
|---|---|---|---|---|
| Hydroxypyruvate reductase (HPR) | Photorespiration | S-nitrosation/Tyr nitration | Inhibition/Inhibition | [51] |
| Glycolate oxidase (GOX) | Photorespiration | S-nitrosation/Tyr nitration | Inhibition/Inhibition | [52] |
| Malate dehydrogenase (MDH) | Fatty acid β-oxidation | S-nitrosation/Tyr nitration | Inhibition/Inhibition | [53] |
| Catalase (CAT) | H2O2 decomposition | S-nitrosation/Tyr nitration | Inhibition/Inhibition | [54] |
| CuZn superoxide dismutase (CSD3) | O2•− dismutation | Tyr nitration | Inhibition/Inhibition | [55] |
| Monodehydroascorbate reductase (MDAR) | Ascorbate glutathione cycle | S-nitrosation/Tyr nitration | Inhibition/Inhibition | [56,57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergashev, U.; Yu, M.; Luo, L.; Tang, J.; Han, Y. The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes. Int. J. Mol. Sci. 2024, 25, 8873. https://doi.org/10.3390/ijms25168873
Ergashev U, Yu M, Luo L, Tang J, Han Y. The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes. International Journal of Molecular Sciences. 2024; 25(16):8873. https://doi.org/10.3390/ijms25168873
Chicago/Turabian StyleErgashev, Ulugbek, Mei Yu, Long Luo, Jie Tang, and Yi Han. 2024. "The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes" International Journal of Molecular Sciences 25, no. 16: 8873. https://doi.org/10.3390/ijms25168873
APA StyleErgashev, U., Yu, M., Luo, L., Tang, J., & Han, Y. (2024). The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes. International Journal of Molecular Sciences, 25(16), 8873. https://doi.org/10.3390/ijms25168873







