Nucleic Acid Armor: Fortifying RNA Therapeutics through Delivery and Targeting Innovations for Immunotherapy
Abstract
1. Introduction
2. RNA-Based Immunotherapy
3. RNA Delivery Systems
3.1. Delivery Vehicles
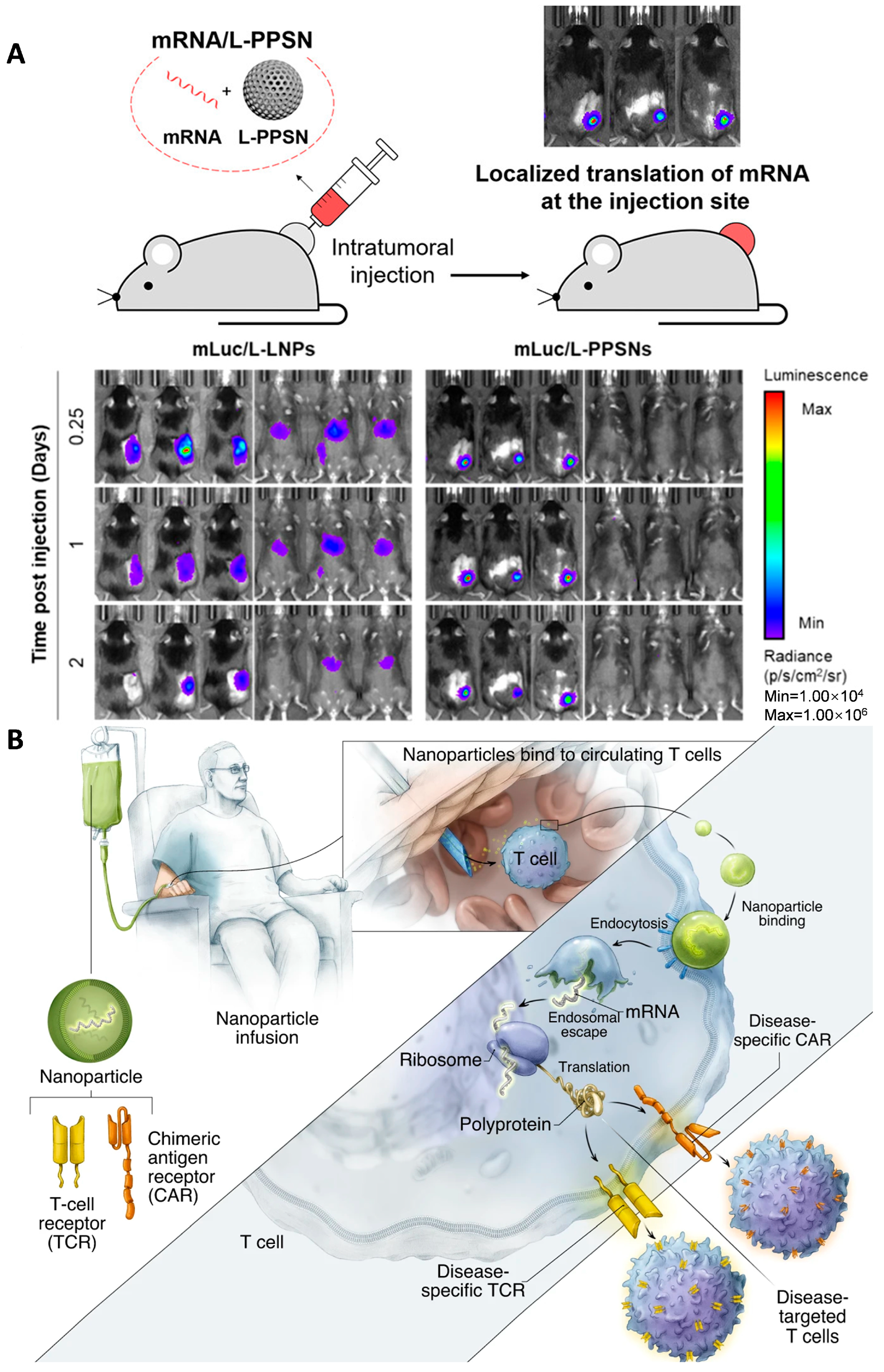
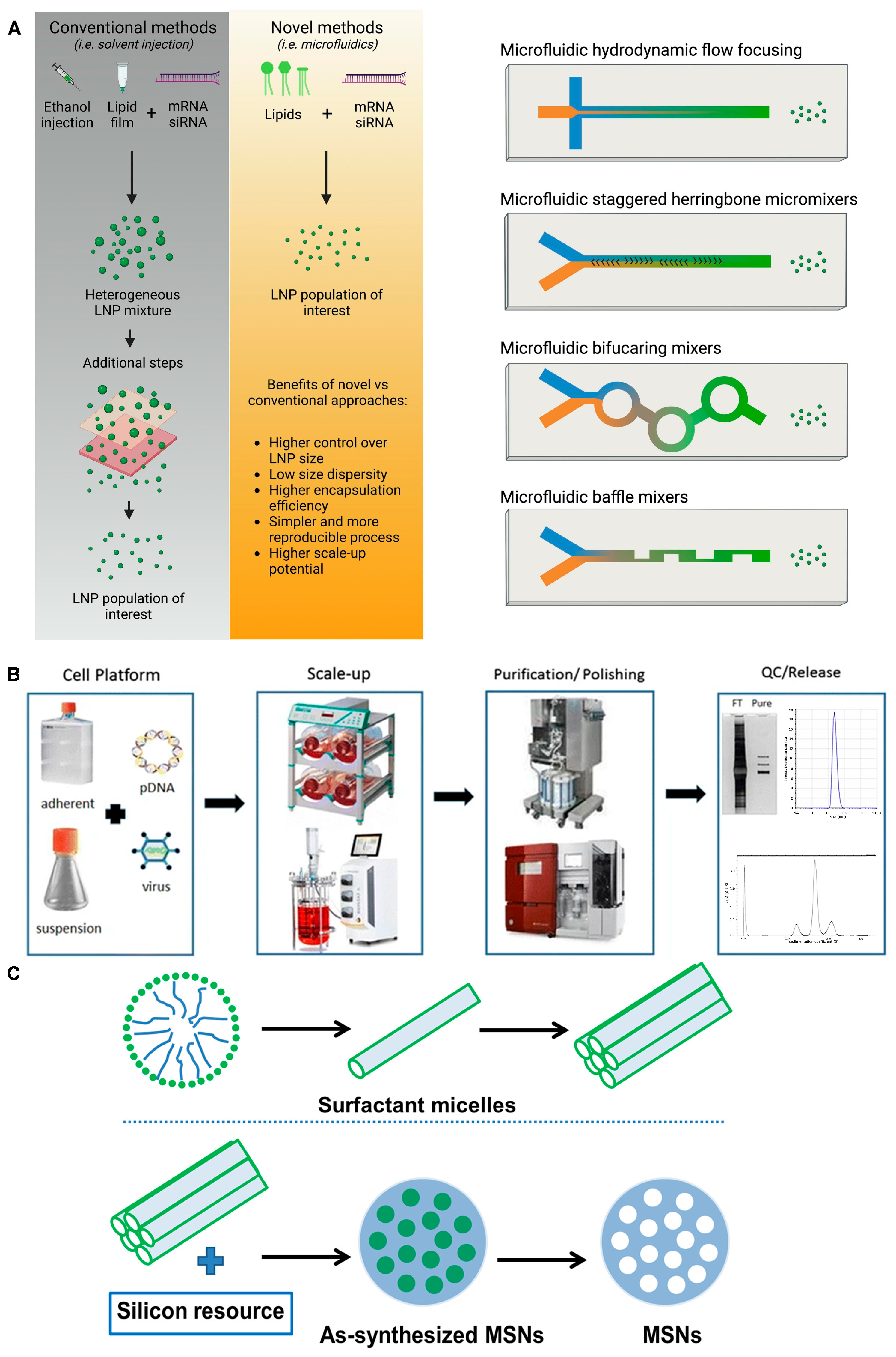
3.2. Lipid Nanoparticles (LNPs) and Liposomes
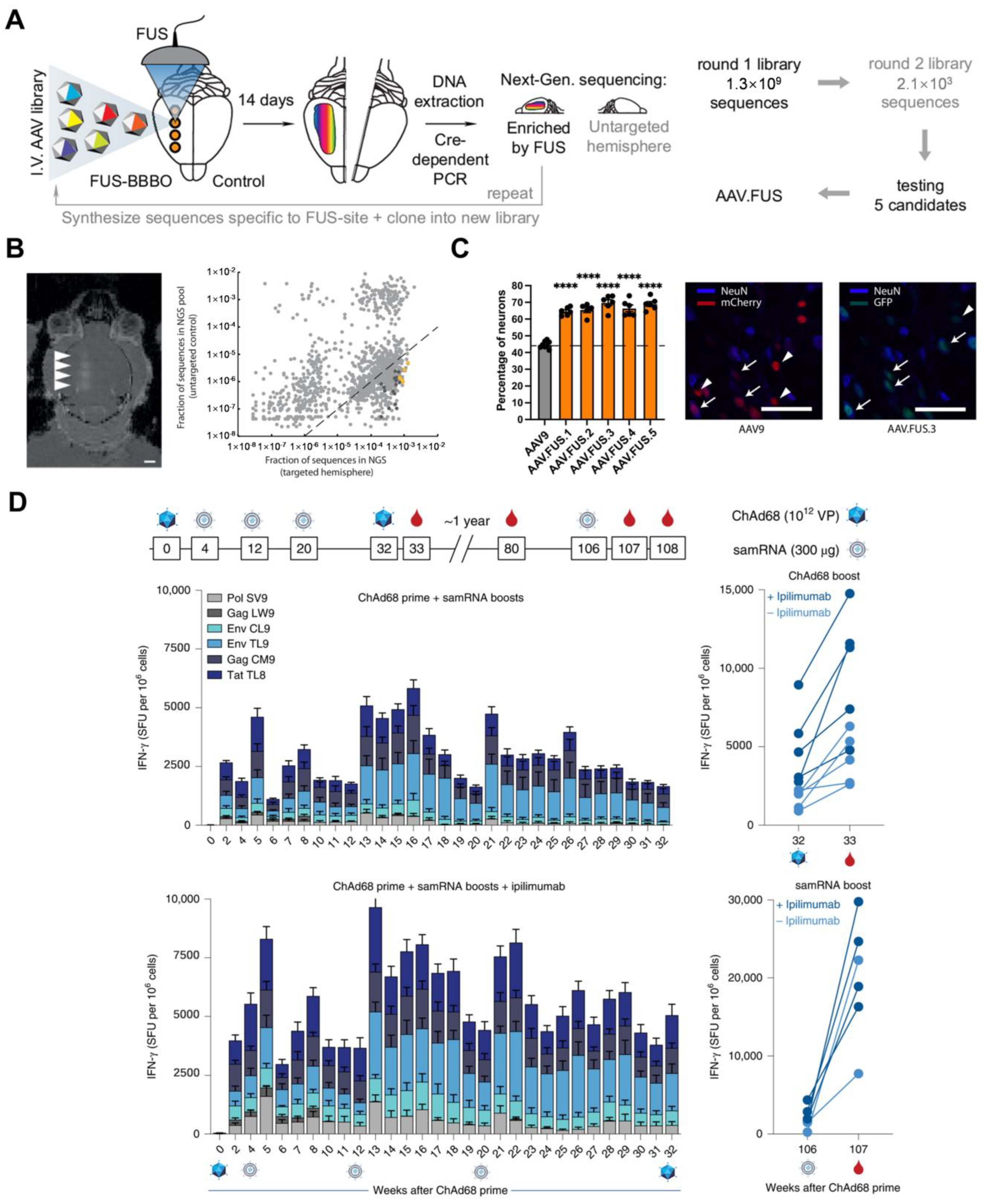
3.3. Viral Vectors
3.4. Inorganic/Polymer Nanomaterial-Based RNA Delivery Vehicles
3.5. Chemical Modification


4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| ADCs | antibody-Drug Conjugates |
| ADME | absorption, distribution, metabolism, and excretion |
| AOCs | antibody-oligonucleotide conjugates |
| ASGPR | sialoglycoprotein receptor |
| ASO | antisense oligonucleotide |
| BCR | B-cell receptor |
| CAR | chimeric antigen receptors |
| ChAd68 | chimpanzee adenovirus |
| CLAN | cationic lipid-assisted nanoparticles |
| ctDNA | circulating tumor DNA |
| CTL | cytotoxic T lymphocytes |
| DBCO | dibenzoazacyclooctyne |
| DC | dendritic cell |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| GalNAc | N-acetylgalactosamine |
| hATTR | hereditary transthyretin amyloidosis |
| HCC | hepatocellular carcinoma |
| HIV | human immunodeficiency virus |
| HO1 | heme oxygenase-1 |
| ICD | immunogenic cell death |
| ICIs | immune checkpoint inhibitors |
| IL-12 | interleukin-12 |
| LNPs | Lipid Nanoparticles |
| L-PPSN | polyethylenimine-modified porous silica nanoparticle |
| MARV | Marburg virus |
| MDSCs | Myeloid-derived suppressor cells |
| mRNA | messenger RNA |
| MSNs/MSNPs | mesoporous silica nanoparticles |
| MSS-CRC | microsatellite-stable colorectal cancer |
| PCL | poly(ε-caprolactone) |
| PD-L1 | programmed cell death protein 1 |
| PEG | polyethylene glycol |
| PEI | polyethyleneimine |
| PGs | poly(glycerols) |
| PHEMA | poly(2-hydroxyethyl methacrylate) |
| PHPMA | poly(hydroxypropyl methacrylate) |
| PLGA | poly(lactic-co-glycolic acid) |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| PMO | morpholine oligonucleotide |
| POX | poly(oxazolines) |
| samRNA | self-amplifying RNA |
| siRNA | small interference RNA |
| SIV | simian immunodeficiency virus |
| SSNs | spiked silica nanoparticle |
| TAMs | tumor-associated macrophages |
| TCRs | T cell receptors |
| TDLN | tumor draining lymph node |
| TfR1 | transferrin receptor 1 |
| TLR | Toll-like receptors |
| TME | tumor microenvironment |
| TTR | transthyretin |
| VLP | virus-like particle |
References
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef]
- Weide, B.; Carralot, J.P.; Reese, A.; Scheel, B.; Eigentler, T.K.; Hoerr, I.; Rammensee, H.G.; Garbe, C.; Pascolo, S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J. Immunother. 2008, 31, 180–188. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71–110. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.S.; Peer, D. RNA nanomedicines: The next generation drugs? Curr. Opin. Biotechnol. 2016, 39, 28–34. [Google Scholar] [CrossRef]
- Sparmann, A.; Vogel, J. RNA-based medicine: From molecular mechanisms to therapy. EMBO J. 2023, 42, e114760. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Yu, A.M.; Choi, Y.H.; Tu, M.J. RNA Drugs and RNA Targets for Small Molecules: Principles, Progress, and Challenges. Pharmacol. Rev. 2020, 72, 862–898. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; Simard, M.J. Argonaute proteins: Key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef]
- Alshaer, W.; Zureigat, H.; Al, K.A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Iavarone, C.; O’Hagan, D.T.; Yu, D.; Delahaye, N.F.; Ulmer, J.B. Mechanism of action of mRNA-based vaccines. Expert Rev. Vaccines 2017, 16, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Hu, Q. Advances in saRNA Vaccine Research against Emerging/Re-Emerging Viruses. Vaccines 2023, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wei, J.; Zhang, L.; Hou, X.; Tan, J.; Yuan, Q.; Tan, W. Aptamer-Protein Interactions: From Regulation to Biomolecular Detection. Chem. Rev. 2023, 123, 12471–12506. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.W.; Colton, M.D.; Mitra, S.S.; Messersmith, W.A. Innate Immune Checkpoint Inhibitors: The Next Breakthrough in Medical Oncology? Mol. Cancer Ther. 2021, 20, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Buss, N.A.; Henderson, S.J.; McFarlane, M.; Shenton, J.M.; de Haan, L. Monoclonal antibody therapeutics: History and future. Curr. Opin. Pharmacol. 2012, 12, 615–622. [Google Scholar] [CrossRef] [PubMed]
- de Assis, L.H.; Fassi, D.E.; Hutchings, M. Bispecific antibody therapies. Hematology 2023, 2023, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Galisteo, A.; Alvarez-Vallina, L.; Sanz, L. Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies. J. Hematol. Oncol. 2023, 16, 83. [Google Scholar] [CrossRef]
- Szabo, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef]
- Zhang, P.; Narayanan, E.; Liu, Q.; Tsybovsky, Y.; Boswell, K.; Ding, S.; Hu, Z.; Follmann, D.; Lin, Y.; Miao, H.; et al. A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat. Med. 2021, 27, 2234–2245. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- El, M.S.; Garbayo, E.; Amundarain, A.; Pascual-Gil, S.; Carrasco-Leon, A.; Prosper, F.; Agirre, X.; Blanco-Prieto, M.J. Lipid nanoparticles for siRNA delivery in cancer treatment. J. Control Release 2023, 361, 130–146. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.R.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. Biodrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Luo, Z.; Huang, Z.; Sun, F.; Guo, F.; Wang, Y.; Kao, S.; Yang, G.; Huang, J.; Li, J.; Zhao, S.; et al. The clinical effects of inclisiran, a first-in-class LDL-C lowering siRNA therapy, on the LDL-C levels in Chinese patients with hypercholesterolemia. J. Clin. Lipidol. 2023, 17, 392–400. [Google Scholar] [CrossRef]
- Ray, K.K.; Stoekenbroek, R.M.; Kallend, D.; Nishikido, T.; Leiter, L.A.; Landmesser, U.; Wright, R.S.; Wijngaard, P.; Kastelein, J. Effect of 1 or 2 Doses of Inclisiran on Low-Density Lipoprotein Cholesterol Levels: One-Year Follow-up of the ORION-1 Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 1067–1075. [Google Scholar] [CrossRef]
- Artiga, A.; Serrano-Sevilla, I.; De Matteis, L.; Mitchell, S.G.; de la Fuente, J.M. Current status and future perspectives of gold nanoparticle vectors for siRNA delivery. J. Mater. Chem. B 2019, 7, 876–896. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Singh, S.K.; Luisi, D.L.; Pak, R.H. Antibody-Drug Conjugates: Design, Formulation and Physicochemical Stability. Pharm. Res. 2015, 32, 3541–3571. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Toussaint, F.; Djemaa, S.B.; Laloy, J.; Pendeville, H.; Evrard, B.; Jerome, C.; Lechanteur, A.; Mottet, D.; Debuigne, A.; et al. Poly(vinyl pyrrolidone) derivatives as PEG alternatives for stealth, non-toxic and less immunogenic siRNA-containing lipoplex delivery. J. Control. Release 2023, 361, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Toussaint, F.; Ben, D.S.; Maquoi, E.; Pendeville, H.; Evrard, B.; Jerome, C.; Leblond, C.J.; Lechanteur, A.; Mottet, D.; et al. Poly(N-methyl-N-vinylacetamide): A Strong Alternative to PEG for Lipid-Based Nanocarriers Delivering siRNA. Adv. Healthc. Mater. 2024, 13, e2302712. [Google Scholar] [CrossRef]
- Ertl, H. T Cell-Mediated Immune Responses to AAV and AAV Vectors. Front. Immunol. 2021, 12, 666666. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, G.; Zheng, H.; Chen, X. Rigid nanoparticle-based delivery of anti-cancer siRNA: Challenges and opportunities. Biotechnol. Adv. 2014, 32, 831–843. [Google Scholar] [CrossRef]
- Yadav, D.N.; Ali, M.S.; Thanekar, A.M.; Pogu, S.V.; Rengan, A.K. Recent Advancements in the Design of Nanodelivery Systems of siRNA for Cancer Therapy. Mol. Pharm. 2022, 19, 4506–4526. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guan, N.; Liu, F. T7 peptide-decorated exosome-based nanocarrier system for delivery of Galectin-9 siRNA to stimulate macrophage repolarization in glioblastoma. J. Neurooncol. 2023, 162, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Nunvarova, S.M.J. Synthesis of amphiphilic copolymers based on dendritic polyethylene grafted by polyhydroxyethylmethacrylate and polyhydroxypropylmethacrylate and their use for construction of nanoparticles. Eur. Polym. J. 2019, 115, 193–200. [Google Scholar] [CrossRef]
- Patil, S.D.; Rhodes, D.G.; Burgess, D.J. Anionic liposomal delivery system for DNA transfection. AAPS J. 2004, 6, e29. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Y. siRNA modification and delivery for drug development. Trends Mol. Med. 2022, 28, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Hald, A.C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Z.; Zhao, W.; Zhang, X.; Momin, N.; Zhang, C.; Wittrup, K.D.; Dong, Y.; Irvine, D.J.; Weiss, R. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 2020, 1, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.B.; Ramishetti, S.; Goldsmith, M.; Diesendruck, Y.; Hazan-Halevy, I.; Chatterjee, S.; Somu, N.G.; Ezra, A.; Peer, D. Dual-Targeted Lipid Nanotherapeutic Boost for Chemo-Immunotherapy of Cancer. Adv. Mater. 2022, 34, e2106350. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.K.; Yin, B.; Lam, C.; Huang, Y.; Yan, J.; Tan, Z.; Wong, S. The Interplay Between Epigenetic Regulation and CD8+ T Cell Differentiation/Exhaustion for T Cell Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 783227. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Q.; Lam, C.; Li, C.; Yang, C.; Zhong, Z.; Zhang, R.; Yan, J.; Chen, J.; Yin, B.; et al. An Aggregation-Induced Emission-Based Dual Emitting Nanoprobe for Detecting Intracellular pH and Unravelling Metabolic Variations in Differentiating Lymphocytes. ACS Nano 2024, 18, 15935–15949. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Ali, Z.S.; Fatima, F.; Ali, Z.S.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: Challenges and strategies. J. Nanobiotechnol. 2023, 21, 381. [Google Scholar] [CrossRef]
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef]
- Goyal, S.; Tisdale, J.; Schmidt, M.; Kanter, J.; Jaroscak, J.; Whitney, D.; Bitter, H.; Gregory, P.D.; Parsons, G.; Foos, M.; et al. Acute Myeloid Leukemia Case after Gene Therapy for Sickle Cell Disease. N. Engl. J. Med. 2022, 386, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Hutson, T.H.; Foster, E.; Moon, L.D.; Yanez-Munoz, R.J. Lentiviral vector-mediated RNA silencing in the central nervous system. Hum. Gene Ther. Methods 2014, 25, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Harb, M.; Heath, J.E.; Trippett, J.S.; Shapiro, M.G.; Szablowski, J.O. Engineering viral vectors for acoustically targeted gene delivery. Nat. Commun. 2024, 15, 4924. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huo, S.; Hardie, J.; Liang, X.J.; Rotello, V.M. Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expert Opin. Drug Deliv. 2016, 13, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic Polymers as Transfection Reagents for Nucleic Acid Delivery. Pharmaceutics 2023, 15, 1502. [Google Scholar] [CrossRef]
- Oladele, J.O. Utilization of Chitosan for Gene Delivery. In Integration of Biomaterials for Gene Therapy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 223–246. [Google Scholar]
- Lombardo, R.; Ruponen, M.; Rautio, J.; Lampinen, R.; Kanninen, K.M.; Koivisto, A.M.; Penttilä, E.; Löppönen, H.; Demartis, S.; Giunchedi, P.; et al. A technological comparison of freeze-dried poly-ε-caprolactone (PCL) and poly (lactic-co-glycolic acid) (PLGA) nanoparticles loaded with clozapine for nose-to-brain delivery. J. Drug Deliv. Sci. Technol. 2024, 93, 105419. [Google Scholar] [CrossRef]
- Fu, J.; Han, W.; Zhang, X.; Sun, Y.; Bhadane, R.; Wei, B.; Li, L.; Yu, L.; Yang, J.; Rosenholm, J.M.; et al. Silica Nanoparticles with Virus-Mimetic Spikes Enable Efficient siRNA Delivery In Vitro and In Vivo. Research 2022, 2022, 0014. [Google Scholar] [CrossRef]
- Shin, H.; Kang, S.; Won, C.; Min, D.H. Enhanced Local Delivery of Engineered IL-2 mRNA by Porous Silica Nanoparticles to Promote Effective Antitumor Immunity. ACS Nano 2023, 17, 17554–17567. [Google Scholar] [CrossRef]
- Tan, F.; Du, Z.; Zhong, J.; Wu, Y.; Mou, J.; Zhao, F.; Liu, Y.; Chen, J.; Liang, Z.; Zhou, Y.; et al. Application of Nanomedicine in Tumor Targeting Inflammatory Pathway. Curr. Med. Chem. 2024. [CrossRef]
- Hiltensperger, M.; Krackhardt, A.M. Current and future concepts for the generation and application of genetically engineered CAR-T and TCR-T cells. Front. Immunol. 2023, 14, 1121030. [Google Scholar] [CrossRef]
- Parayath, N.N.; Stephan, S.B.; Koehne, A.L.; Nelson, P.S.; Stephan, M.T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 2020, 11, 6080. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Jiang, J.; Wu, M.; Lin, P. Applications and challenges of CRISPR-Cas gene-editing to disease treatment in clinics. Precis. Clin. Med. 2021, 4, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Xu, C.F.; Li, H.J.; Cao, Z.T.; Liu, J.; Wang, J.L.; Du, X.J.; Yang, X.Z.; Gu, Z.; Wang, J. Macrophage-Specific In Vivo Gene Editing Using Cationic Lipid-Assisted Polymeric Nanoparticles. ACS Nano 2018, 12, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, D.; Schomaker, M.; Kalies, S.; Schieck, M.; Carlson, R.; Murua, E.H.; Ripken, T.; Meyer, H.; Heisterkamp, A. Gold nanoparticle mediated laser transfection for efficient siRNA mediated gene knock down. PLoS ONE 2013, 8, e58604. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Kim, C.S.; Ahn, Y.C.; Chen, Z.; Kwon, Y.J. Combined multimodal optical imaging and targeted gene silencing using stimuli-transforming nanotheragnostics. J. Am. Chem. Soc. 2010, 132, 8316–8324. [Google Scholar] [CrossRef]
- An, G. Pharmacokinetics and Pharmacodynamics of GalNAc-Conjugated siRNAs. J. Clin. Pharmacol. 2024, 64, 45–57. [Google Scholar] [CrossRef]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid. Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Samuel, E.; Watford, M.; Egolum, U.O.; Ombengi, D.N.; Ling, H.; Cates, D.W. Inclisiran: A First-in-Class siRNA Therapy for Lowering Low-Density Lipoprotein Cholesterol. Ann. Pharmacother. 2023, 57, 317–324. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Wang, J.; Tan, M.; Wang, Y.; Liu, X.; Lin, A. Advances in modification and delivery of nucleic acid drugs. Zhejiang Da Xue Xue Bao Yi Xue Ban 2023, 52, 417–428. [Google Scholar] [CrossRef]
- Willoughby, J.; Chan, A.; Sehgal, A.; Butler, J.S.; Nair, J.K.; Racie, T.; Shulga-Morskaya, S.; Nguyen, T.; Qian, K.; Yucius, K.; et al. Evaluation of GalNAc-siRNA Conjugate Activity in Pre-clinical Animal Models with Reduced Asialoglycoprotein Receptor Expression. Mol. Ther. 2018, 26, 105–114. [Google Scholar] [CrossRef]
- Kurreck, J. Antisense technologies. Improvement through novel chemical modifications. Eur. J. Biochem. 2003, 270, 1628–1644. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Holland, R.; Wood, M.; Pasetka, C.; Palmer, L.; Samaridou, E.; McClintock, K.; Borisevich, V.; Geisbert, T.W.; Cross, R.W.; et al. Combination treatment of mannose and GalNAc conjugated small interfering RNA protects against lethal Marburg virus infection. Mol. Ther. 2023, 31, 269–281. [Google Scholar] [CrossRef]
- Alla, D.; Paruchuri, S.; Tiwari, A.; Alla, S.; Pillai, R.T.; Bandakadi, S.; Pradeep, A.; Shah, D.J.; Sabiroglu, M.; Chavda, S.; et al. The mortality, modes of infection, diagnostic tests, and treatments of Marburg virus disease: A systematic review. Health Sci. Rep. 2023, 6, e1545. [Google Scholar] [CrossRef]
- Koziolkiewicz, M.; Wojcik, M.; Kobylanska, A.; Karwowski, B.; Rebowska, B.; Guga, P.; Stec, W.J. Stability of stereoregular oligo(nucleoside phosphorothioate)s in human plasma: Diastereoselectivity of plasma 3′-exonuclease. Antisense Nucleic Acid. Drug Dev. 1997, 7, 43–48. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Piotrowicz, M.; Gapinska, M.; Trzybinski, D.; Wozniak, K.; Golding, T.M.; Stringer, T.; Smith, G.S.; Czerwieniec, R.; Kowalski, K. Chemistry of glycol nucleic acid (GNA): Synthesis, photophysical characterization and insight into the biological activity of phenanthrenyl GNA constituents. Bioorg. Chem. 2022, 125, 105847. [Google Scholar] [CrossRef] [PubMed]
- Malecova, B.; Burke, R.S.; Cochran, M.; Hood, M.D.; Johns, R.; Kovach, P.R.; Doppalapudi, V.R.; Erdogan, G.; Arias, J.D.; Darimont, B.; et al. Targeted tissue delivery of RNA therapeutics using antibody-oligonucleotide conjugates (AOCs). Nucleic Acids Res. 2023, 51, 5901–5910. [Google Scholar] [CrossRef]
- Jin, Y.; Schladetsch, M.A.; Huang, X.; Balunas, M.J.; Wiemer, A.J. Stepping forward in antibody-drug conjugate development. Pharmacol. Ther. 2022, 229, 107917. [Google Scholar] [CrossRef]
- Song, E.; Zhu, P.; Lee, S.K.; Chowdhury, D.; Kussman, S.; Dykxhoorn, D.M.; Feng, Y.; Palliser, D.; Weiner, D.B.; Shankar, P.; et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005, 23, 709–717. [Google Scholar] [CrossRef]
- Mullard, A. Antibody-oligonucleotide conjugates enter the clinic. Nat. Rev. Drug Discov. 2022, 21, 6–8. [Google Scholar] [CrossRef]



| Name | Principle | Advantages | limitation | Reference |
|---|---|---|---|---|
| GalNAc | GalNAc binds highly specifically to the ASGPR receptor, allowing rapid targeting of the liver and entry into cells in the form of endocytosis. | Low off-target effect, low immunogenicity, improved stability, strong targeting, long duration of efficacy, no cytotoxicity, high efficacy. | Targeting only the liver has many limitations, and the targeting of normal liver cells is stronger than that of diseased liver cells | [25,26] |
| Thiophosphoric acid skeleton modification, methoxyl modification, fluoro modification | By changing the structure of the RNA, the stability of the RNA is increased while ensuring biological affinity, so that it can have more time to reach the target site with blood circulation. | Enhancing the stability of RNA, not easily degraded, and enhancing the half-life of RNA. | It affects the overall chemical properties of nucleic acids and even limits their function, producing different isomers and changing the function. | [27] |
| GNA | RNA oligomers containing GNA residues have been shown to form double strands with DNA and RNA to enhance stability while increasing their targeting. | Improving the thermal stability of RNA, reducing off-target effects, and easy chemical synthesis. It has significant hybridization characteristics, improving the therapeutic index of RNA. | It does not exist in nature so there may be certain safety issues. | [28] |
| Antibody (AOC) | Through the binding of monoclonal antibodies corresponding to the receptors on the surface of the target cells, RNA is rapidly delivered to the target site through the targeting of the antibody. | Strong targeting, low-toxicity side effects, small size, high stability, high efficacy. | The pharmacokinetics are complex, the efficacy is short, and the monoclonal antibody is degraded after endocytosis. | [29] |
| Lipid Nanoparticles (LNPs) and Liposomes | RNA is encapsulated by LNPs to avoid disease clearance while enhancing its stability, enabling targeted delivery through altered permeability in the tumor microenvironment or modification on the lipid surface. | Strongly lipophilic, enhancing RNA stability, difficult immune clearance, easy to enter the cell, simple preparation, easy metabolism. | The addition of PEG resulted in cytotoxicity, serious off-target effects, short storage time, and harsh transport conditions. | [30] |
| Viral Vector | RNA is placed into an AAV vector to mimic the process of virus invasion into cells, and cytolysis is achieved by binding the viral protein to the receptor on the surface of the target cell | Strong targeting, enhanced RNA stability, no cytotoxicity, and strong self-stability. | Easily cleared by the immune system and will lead to drug resistance, leading to reduced efficacy. | [31,32,33] |
| Delivery Carrier Utilizing Inorganic Materials | RNA is stored in the pores of inorganic materials to form a special solid–liquid interface, and the stability of RNA in solid media is greatly increased. Specific functional groups are added to the surface of inorganic materials to achieve targeted delivery. | Enhanced RNA stability, difficult immune clearance, flexible design size and shape, easy preparation, preparation process pollution is small, low cost. | There are certain off-target effects, security problems, and low targeting. | [34,35] |
| Quantum Dot | The delivery of quantum dots is still in the theoretical stage, and the target is achieved by depositing RNA in quantum dots to achieve point delivery of RNA. | Strong stability, scalable application to diagnosis, and good efficacy. | The load is limited and PEG has a certain toxicity. | [36] |
| Polymer nanocarriers | Enhancement of RNA stability is achieved by means of multimers, and targeting is achieved by modifying specific structures. | High thermodynamic and kinetic stability, simple and scalable synthesis, diverse structure, and high transfection rate. | Endosomal escape disorder, metabolic problems, and potential toxicity after carrier delivery. | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Jiang, B.; Wang, Z.; Li, Y.; Cheung, J.C.W.; Yin, B.; Wong, S.H.D. Nucleic Acid Armor: Fortifying RNA Therapeutics through Delivery and Targeting Innovations for Immunotherapy. Int. J. Mol. Sci. 2024, 25, 8888. https://doi.org/10.3390/ijms25168888
Jiang Y, Jiang B, Wang Z, Li Y, Cheung JCW, Yin B, Wong SHD. Nucleic Acid Armor: Fortifying RNA Therapeutics through Delivery and Targeting Innovations for Immunotherapy. International Journal of Molecular Sciences. 2024; 25(16):8888. https://doi.org/10.3390/ijms25168888
Chicago/Turabian StyleJiang, Yi, Bolong Jiang, Zhenru Wang, Yuxi Li, James Chung Wai Cheung, Bohan Yin, and Siu Hong Dexter Wong. 2024. "Nucleic Acid Armor: Fortifying RNA Therapeutics through Delivery and Targeting Innovations for Immunotherapy" International Journal of Molecular Sciences 25, no. 16: 8888. https://doi.org/10.3390/ijms25168888
APA StyleJiang, Y., Jiang, B., Wang, Z., Li, Y., Cheung, J. C. W., Yin, B., & Wong, S. H. D. (2024). Nucleic Acid Armor: Fortifying RNA Therapeutics through Delivery and Targeting Innovations for Immunotherapy. International Journal of Molecular Sciences, 25(16), 8888. https://doi.org/10.3390/ijms25168888








