Dried Blood Spot Metabolome Features of Ischemic–Hypoxic Encephalopathy: A Neonatal Rat Model
Abstract
:1. Introduction
2. Results
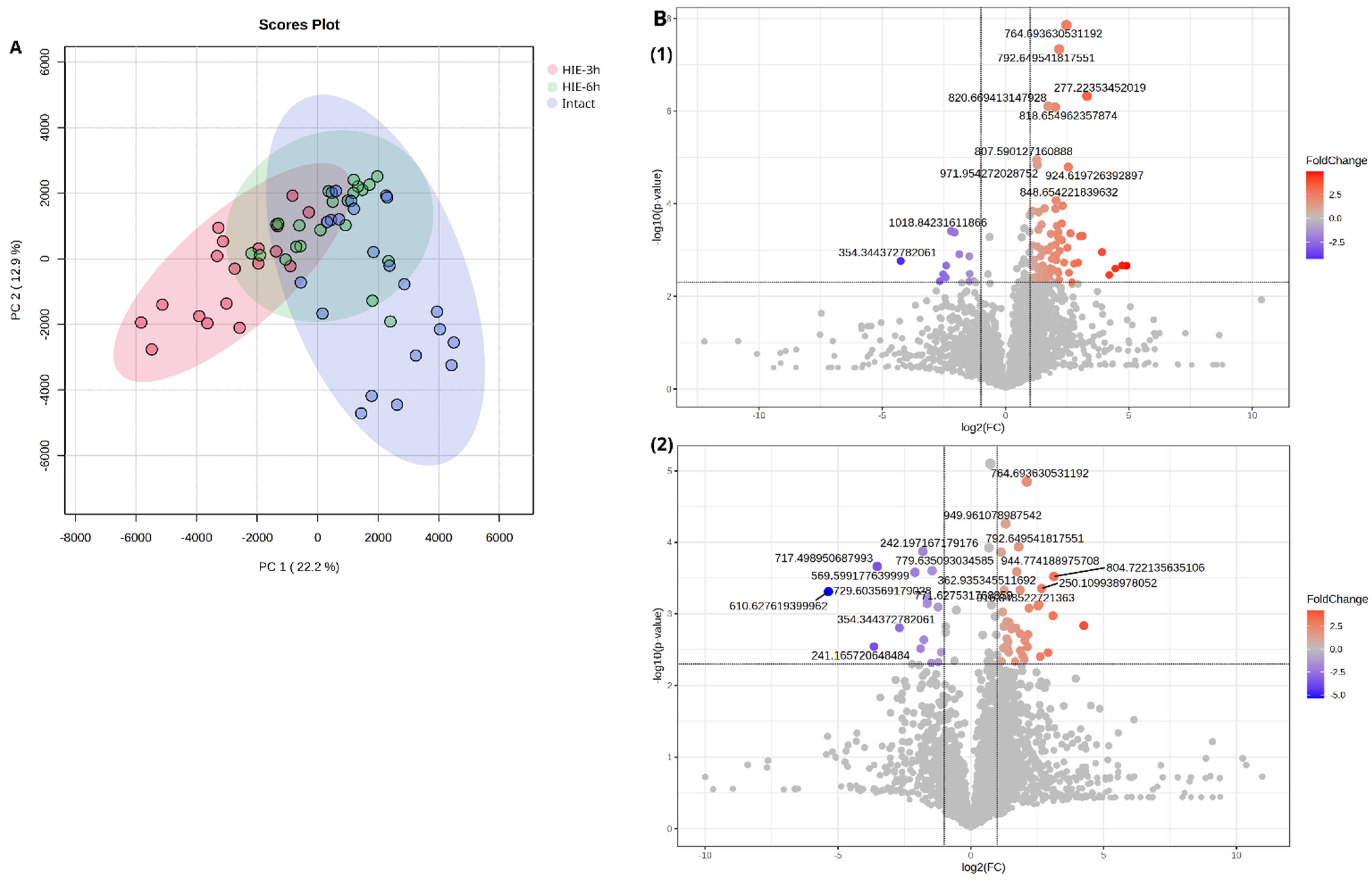
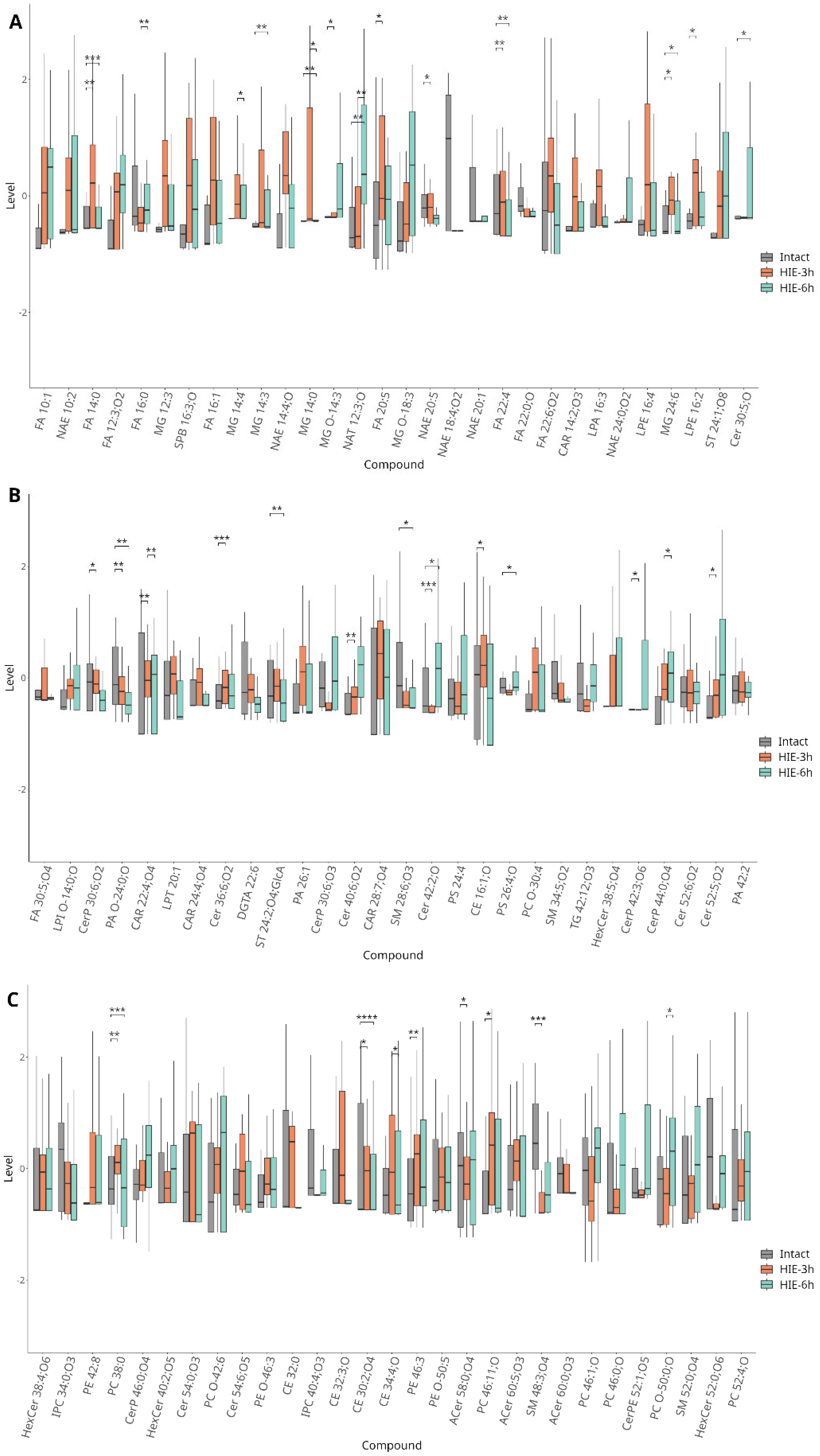
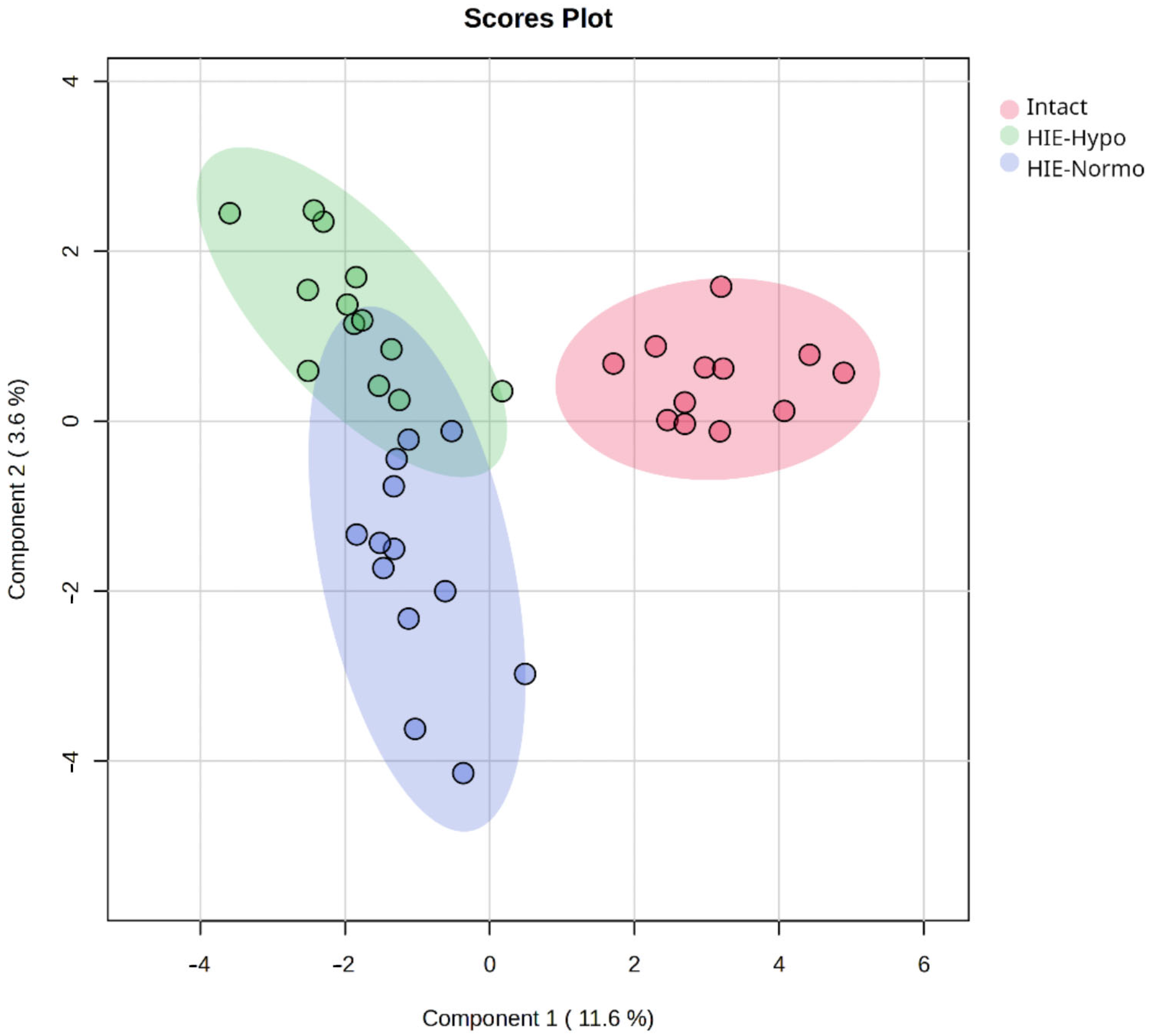
2.1. Time-Related Features of Metabolome DBS Caused by Hypoxia–Ischemia
- 3 h after HIE
- 6 h after HIE
2.2. Therapeutic Effect of Hypothermia in Hypoxic–Ischemic Injury
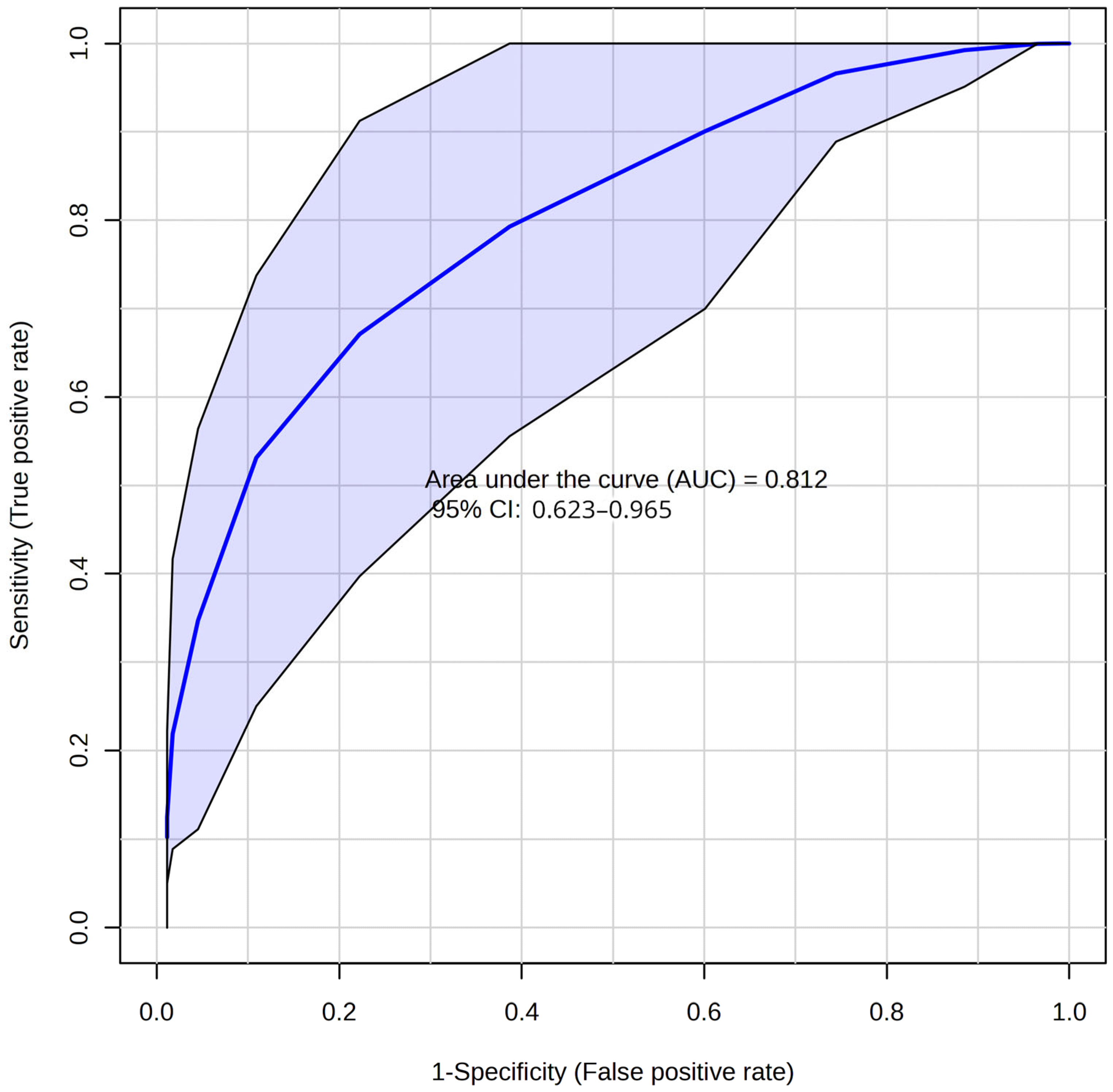
2.3. Development of the Diagnostic Model for HIE
3. Discussion
4. Materials and Methods
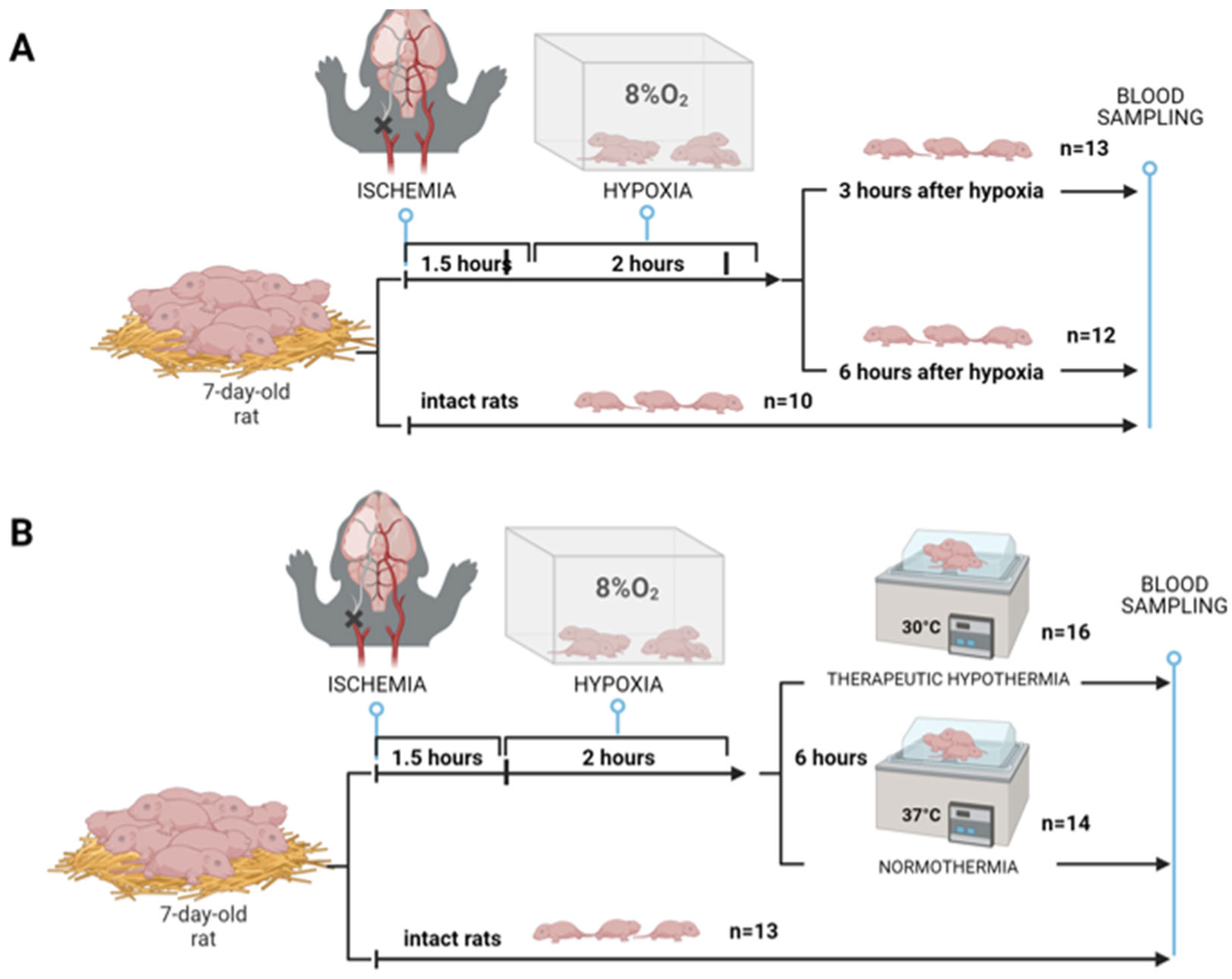
4.1. Rice–Vannucci HIE Rat Model
4.2. HIE with Sampling at Different Times
4.3. Modeling of Therapeutic Hypothermia
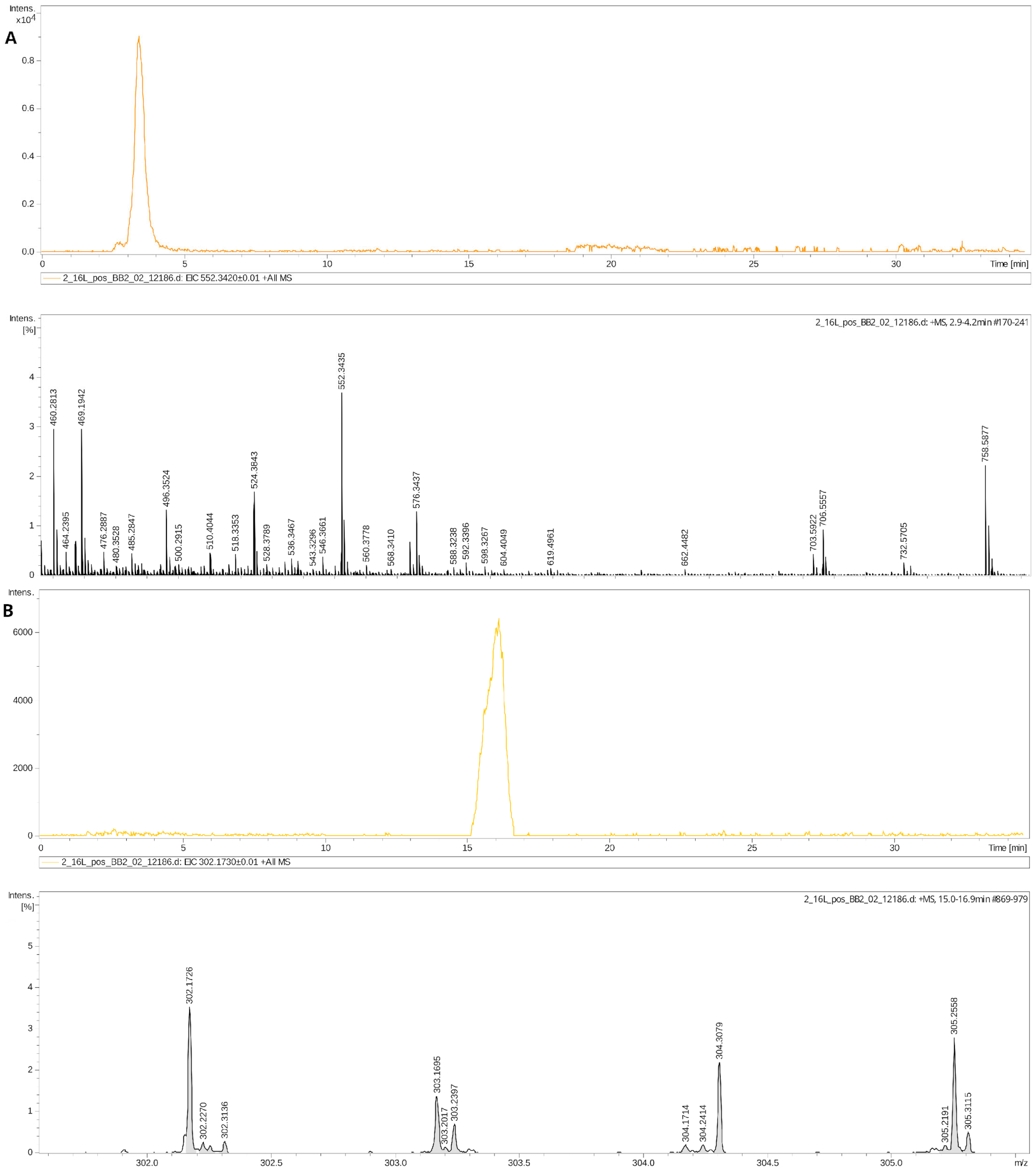
4.4. DBS Metabolome Analysis (HPLC-MS)
5. Conclusions
Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pin, T.W.; Eldridge, B.; Galea, M.P. A review of developmental outcomes of term infants with post-asphyxia neonatal encephalopathy. Eur. J. Paediatr. Neurol. 2009, 13, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, I.; Coelho, M.; Rosado, M.; Cardoso, C.M.P.; Dinis, A.; Duarte, C.B.; Grãos, M.; Manadas, B. Biomarkers of hypoxic-ischemic encephalopathy: A systematic review. World J. Pediatr. 2023, 19, 505–548. [Google Scholar] [CrossRef]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Abate, B.B.; Bimerew, M.; Gebremichael, B.; Mengesha Kassie, A.; Kassaw, M.; Gebremeskel, T.; Bayih, W.A. Effects of therapeutic hypothermia on death among asphyxiated neonates with hypoxic-ischemic encephalopathy: A systematic review and meta-analysis of randomized control trials. PLoS ONE 2021, 16, e0247229. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhang, Z.Y.; Fan, B.; Li, G.Y. Neuroprotection by Therapeutic Hypothermia. Front. Neurosci. 2019, 13, 586. [Google Scholar] [CrossRef]
- Korf, J.M.; McCullough, L.D.; Caretti, V. A narrative review on treatment strategies for neonatal hypoxic ischemic encephalopathy. Transl. Pediatr. 2023, 12, 1552–1571. [Google Scholar] [CrossRef] [PubMed]
- Ovcjak, A.; Pontello, R.; Miller, S.P.; Sun, H.S.; Feng, Z.P. Hypothermia combined with neuroprotective adjuvants shortens the duration of hospitalization in infants with hypoxic ischemic encephalopathy: Meta-analysis. Front. Pharmacol. 2023, 13, 1037131. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Laptook, A.R.; Robertson, N.J.; Barks, J.D.; Thoresen, M.; Wassink, G.; Bennet, L. Therapeutic hypothermia translates from ancient history in to practice. Pediatr. Res. 2017, 81, 202–209. [Google Scholar] [CrossRef]
- Walas, W.; Wilińska, M.; Bekiesińska-Figatowska, M.; Halaba, Z.; Śmigiel, R. Methods for assessing the severity of perinatal asphyxia and early prognostic tools in neonates with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia. Adv. Clin. Exp. Med. 2020, 29, 1011–1016. [Google Scholar] [CrossRef]
- She, H.Q.; Sun, Y.F.; Chen, L.; Xiao, Q.X.; Luo, B.Y.; Zhou, H.S.; Zhou, D.; Chang, Q.Y.; Xiong, L.L. Current analysis of hypoxic-ischemic encephalopathy research issues and future treatment modalities. Front. Neurosci. 2023, 17, 1136500. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Pellegrini-Giampietro, D.E.; Facchinetti, F. Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines 2022, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Popa-Wagner, A.; Kleinschnitz, C.; Doeppner, T.R. Animal models of ischemic stroke and their impact on drug discovery. Expert Opin. Drug Discov. 2019, 14, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, Y.; Eldarov, C.; Starodubtseva, N.; Goryunov, K.; Chagovets, V.; Ionov, O.; Plotnikov, E.; Silachev, D. Identification of Metabolomic Signatures for Ischemic Hypoxic Encephalopathy Using a Neonatal Rat Model. Children 2023, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Starodubtseva, N.L.; Eldarov, C.M.; Kirtbaya, A.R.; Balashova, E.N.; Gryzunova, A.S.; Ionov, O.V.; Zubkov, V.V.; Silachev, D.N. Recent Advances in Diagnostics of Neonatal Hypoxic Ischemic Encephalopathy. Bull. Russ. State Med. Univ. 2022, 4, 5–16. [Google Scholar] [CrossRef]
- Shevtsova, Y.; Starodubtseva, N.; Tokareva, A.; Goryunov, K.; Sadekova, A.; Vedikhina, I.; Ivanetz, T.; Ionov, O.; Frankevich, V.; Plotnikov, E.; et al. Metabolite Biomarkers for Early Ischemic–Hypoxic Encephalopathy: An Experimental Study Using the NeoBase 2 MSMS Kit in a Rat Model. Int. J. Mol. Sci. 2024, 25, 2035. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Solberg, R.; Sánchez-Illana, Á.; Pankratov, L.; Parra-Llorca, A.; Quintás, G.; Saugstad, O.D.; Vento, M. Plasma metabolite score correlates with Hypoxia time in a newly born piglet model for asphyxia. Redox Biol. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.A.; Cascio, M.A.; Ferrand, A.; Shevell, M.; Racine, E. The complexity of physicians’ understanding and management of prognostic uncertainty in neonatal hypoxic-ischemic encephalopathy. J. Perinatol. 2019, 39, 278–285. [Google Scholar] [CrossRef]
- Natarajan, N.; Pardo, A.C. Challenges in neurologic prognostication after neonatal brain injury. Semin. Perinatol. 2017, 41, 117–123. [Google Scholar] [CrossRef]
- Mooney, C.; O’Boyle, D.; Finder, M.; Hallberg, B.; Walsh, B.H.; Henshall, D.C.; Boylan, G.B.; Murray, D.M. Predictive modelling of hypoxic ischaemic encephalopathy risk following perinatal asphyxia. Heliyon 2021, 7, e07411. [Google Scholar] [CrossRef]
- Nanavati, T.; Seemaladinne, N.; Regier, M.; Yossuck, P.; Pergami, P. Can We Predict Functional Outcome in Neonates with Hypoxic Ischemic Encephalopathy by the Combination of Neuroimaging and Electroencephalography? Pediatr. Neonatol. 2015, 56, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Shellhaas, R.A.; Kushwaha, J.S.; Plegue, M.A.; Selewski, D.T.; Barks, J.D.E. An Evaluation of Cerebral and Systemic Predictors of 18-Month Outcomes for Neonates with Hypoxic Ischemic Encephalopathy. J. Child Neurol. 2015, 30, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Şimşek, A.; Tansuǧ, N.; Sezer, R.G.; Özkol, M.; Başpinar, P.; Tekgül, H. Prediction of neurodevelopmental outcome in term neonates with hypoxic-ischemic encephalopathy. Eur. J. Paediatr. Neurol. 2013, 17, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Du Plessis, A. Neurodiagnostic techniques in neonatal critical care. Curr. Neurol. Neurosci. Rep. 2012, 12, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, P.; Zhou, W.; Xia, S.; Zhou, W.; Zhou, X.; Cheng, X.; Shi, Y.; Lin, Z.; Song, D.; et al. Neonatal hypoxic-ischemic encephalopathy diagnosis and treatment: A National Survey in China. BMC Pediatr. 2021, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Fanos, V.; Noto, A.; Xanthos, T.; Lussu, M.; Murgia, F.; Barberini, L.; Finco, G.; D’Aloja, E.; Papalois, A.; Iacovidou, N.; et al. Metabolomics network characterization of resuscitation after normocapnic hypoxia in a newborn piglet model supports the hypothesis that room air is better. BioMed Res. Int. 2014, 2014, 731620. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Connor, J.R.; Mauger, D.T.; Palmer, C.; Smith, M.B.; Towfighi, J.; Vannucci, S.J. Rat model of perinatal hypoxic-ischemic brain damage. J. Neurosci. Res. 1999, 55, 158–163. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Vannucci, S.J. Perinatal hypoxic-ischemic brain damage: Evolution of an animal model. Dev. Neurosci. 2005, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Escobar, J.; Vento, M.; Escrig, R.; Quintás, G.; Sastre, J.; Saugstad, O.D.; Solberg, R. Metabolic adaptation and neuroprotection differ in the retina and choroid in a piglet model of acute postnatal hypoxia. Pediatr. Res. 2014, 76, 127–134. [Google Scholar] [CrossRef]
- Liu, J.; Sheldon, R.A.; Segal, M.R.; Kelly, M.J.S.; Pelton, J.G.; Ferriero, D.M.; James, T.L.; Litt, L. 1H nuclear magnetic resonance brain metabolomics in neonatal mice after hypoxia-ischemia distinguished normothermic recovery from mild hypothermia recoveries. Pediatr. Res. 2013, 74, 170–179. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Back, S.A. The Vannucci Model of Hypoxic-Ischemic Injury in the Neonatal Rodent: 40 years Later. Dev. Neurosci. 2022, 44, 186–193. [Google Scholar] [CrossRef]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia-Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Koulman, A.; Prentice, P.; Wong, M.C.Y.; Matthews, L.; Bond, N.J.; Eiden, M.; Griffin, J.L.; Dunger, D.B. The development and validation of a fast and robust dried blood spot based lipid profiling method to study infant metabolism. Metabolomics 2014, 10, 1018–1025. [Google Scholar] [CrossRef]
- McCandless, S.E.; Wright, E.J. Mandatory newborn screening in the United States: History, current status, and existential challenges. Birth Defects Res. 2020, 112, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Nordfalk, F.; Ekstrøm, C.T. Newborn dried blood spot samples in Denmark: The hidden figures of secondary use and research participation. Eur. J. Hum. Genet. 2019, 27, 203–210. [Google Scholar] [CrossRef]
- Solevåg, A.L.; Zykova, S.N.; Thorsby, P.M.; Schmölzer, G.M. Metabolomics to Diagnose Oxidative Stress in Perinatal Asphyxia: Towards a Non-Invasive Approach. Antioxidants 2021, 10, 1753. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-R. Erratum to: Screening newborns for metabolic disorders based on targeted metabolomics using tandem mass spectrometry. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 238. [Google Scholar] [CrossRef]
- Lee, W.L.A.; Michael-Titus, A.T.; Shah, D.K. Hypoxic-Ischaemic Encephalopathy and the Blood-Brain Barrier in Neonates. Dev. Neurosci. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Reinke, S.N.; Walsh, B.H.; Boylan, G.B.; Sykes, B.D.; Kenny, L.C.; Murray, D.M.; Broadhurst, D.I. 1H NMR derived metabolomic profile of neonatal asphyxia in umbilical cord serum: Implications for hypoxic ischemic encephalopathy. J. Proteome Res. 2013, 12, 4230–4239. [Google Scholar] [CrossRef]
- Solberg, R.; Kuligowski, J.; Pankratov, L.; Escobar, J.; Quintás, G.; Lliso, I.; Sánchez-Illana, Á.; Saugstad, O.D.; Vento, M. Changes of the plasma metabolome of newly born piglets subjected to postnatal hypoxia and resuscitation with air. Pediatr. Res. 2016, 80, 284–292. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Makarewicz, D.; Stańczak-Mrozek, K.I.; Grieb, P. CDP-choline (citicoline) attenuates brain damage in a rat model of birth asphyxia. Acta Neurobiol. Exp. 2008, 68, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Penry, J.T.; Manore, M.M. Choline: An important micronutrient for maximal endurance-exercise performance? Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Greco, P.; Nencini, G.; Piva, I.; Scioscia, M.; Volta, C.A.; Spadaro, S.; Neri, M.; Bonaccorsi, G.; Greco, F.; Cocco, I.; et al. Pathophysiology of hypoxic-ischemic encephalopathy: A review of the past and a view on the future. Acta Neurol. Belg. 2020, 120, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Rink, C.; Khanna, S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid. Redox Signal. 2011, 14, 1889–1903. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Hatcher, J.F. Phospholipase A2, reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008, 41, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, H.; Imaizumi, S.; Yoshimoto, T.; Yamamoto, H.; Motomiya, M. Changes of Polyphosphoinositides, Lysophospholipid, and Free Fatty Acids in Transient Cerebral Ischemia of Rat Brain. Mol. Chem. Neuropathol. 1990, 12, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; David, S. Lysophosphatidylcholine Induces Rapid Recruitment and Activation of Macrophages in the Adult Mouse Spinal Cord. Glia 2000, 30, 92–104. [Google Scholar] [CrossRef]
- Schilling, T.; Lehmann, F.; Rückert, B.; Eder, C.; Lechmann, F.; Rückert, B.; Eder, C. Physiological mechanisms of lysophosphatidylcholine-induced de-ramification of murine microglia. J. Physiol. 2004, 557, 105–120. [Google Scholar] [CrossRef]
- Gürsoy-Ozdemir, Y.; Can, A.; Dalkara, T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke 2004, 35, 1449–1453. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of inflammation in brain: A matter of fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef]
- Muralikrishna Adibhatla, R.; Hatcher, J.F. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic. Biol. Med. 2006, 40, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Bazinet, R.P. β-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 33–40. [Google Scholar] [CrossRef]
- Inceoglu, B.; Jinks, S.L.; Ulu, A.; Hegedus, C.M.; Georgi, K.; Schmelzer, K.R.; Wagner, K.; Jones, P.D.; Morisseau, C.; Hammock, B.D. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 18901–18906. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, C.; Gong, W.; Li, Y.; Edin, M.L.; Zeldin, D.C.; Wang, D.W. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J. Pharmacol. Exp. Ther. 2011, 339, 451–463. [Google Scholar] [CrossRef]
- Solberg, R.; Enot, D.; Deigner, H.P.; Koal, T.; Scholl-Bürgi, S.; Saugstad, O.D.; Keller, M. Metabolomic analyses of plasma reveals new insights into asphyxia and resuscitation in pigs. PLoS ONE 2010, 5, e9606. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.H.; Broadhurst, D.I.; Mandal, R.; Wishart, D.S.; Boylan, G.B.; Kenny, L.C.; Murray, D.M. The metabolomic profile of umbilical cord blood in neonatal hypoxic ischaemic encephalopathy. PLoS ONE 2012, 7, e50520. [Google Scholar] [CrossRef]
- Rauchová, H.; Vokurková, M.; Koudelová, J. Hypoxia-induced lipid peroxidation in the brain during postnatal ontogenesis. Physiol. Res. 2012, 61 (Suppl. 1), S89–S101. [Google Scholar] [CrossRef]
- Berger, C.; Schmid, P.C.; Schabitz, W.R.; Wolf, M.; Schwab, S.; Schmidt, H.H.O. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J. Neurochem. 2004, 88, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cordaro, M.; Cuzzocrea, S. Roles of fatty acid ethanolamides (FAE) in traumatic and ischemic brain injury. Pharmacol. Res. 2014, 86, 26–31. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Heinemann, A.; Hudz, Y.A.; Thomas, D.; Sturm, E.M. Stearoylethanolamide interferes with retrograde endocannabinoid signalling and supports the blood-brain barrier integrity under acute systemic inflammation. Biochem. Pharmacol. 2020, 174, 113783. [Google Scholar] [CrossRef]
- Mencarelli, C.; Martinez-Martinez, P. Ceramide function in the brain: When a slight tilt is enough. Cell. Mol. Life Sci. 2013, 70, 181–203. [Google Scholar] [CrossRef]
- Car, H.; Zendzian-Piotrowska, M.; Fiedorowicz, A.; Prokopiuk, S.; Sadowska, A.; Kurek, K. [The role of ceramides in selected brain pathologies: Ischemia/hypoxia, Alzheimer disease]. Postep. Hig. Med. Dosw. 2012, 66, 295–303. [Google Scholar] [CrossRef]
- Pant, D.C.; Aguilera-Albesa, S.; Pujol, A. Ceramide signalling in inherited and multifactorial brain metabolic diseases. Neurobiol. Dis. 2020, 143, 105014. [Google Scholar] [CrossRef]
- Lee, T.H.; Cheng, C.N.; Chao, H.C.; Lee, C.H.; Kuo, C.H.; Tang, S.C.; Jeng, J.S. Plasma ceramides are associated with outcomes in acute ischemic stroke patients. J. Formos. Med. Assoc. 2022, 121, 43–50. [Google Scholar] [CrossRef]
- Chao, H.C.; Lee, T.H.; Chiang, C.S.; Yang, S.Y.; Kuo, C.H.; Tang, S.C. Sphingolipidomics Investigation of the Temporal Dynamics after Ischemic Brain Injury. J. Proteome Res. 2019, 18, 3470–3478. [Google Scholar] [CrossRef]
- Lee, T.H.; Cheng, C.N.; Lee, C.W.; Kuo, C.H.; Tang, S.C.; Jeng, J.S. Investigating sphingolipids as biomarkers for the outcomes of acute ischemic stroke patients receiving endovascular treatment. J. Formos. Med. Assoc. 2023, 122, 19–28. [Google Scholar] [CrossRef]
- Gunn, A.J.; Gunn, T.R.; Gunning, M.I.; Williams, C.E.; Gluckman, P.D. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 1998, 102, 1098–1106. [Google Scholar] [CrossRef]
- Lemyre, B.; Chau, V. Hypothermia for newborns with hypoxic-ischemic encephalopathy. Paediatr. Child Health 2018, 23, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Longini, M.; Giglio, S.; Perrone, S.; Vivi, A.; Tassini, M.; Fanos, V.; Sarafidis, K.; Buonocore, G. Proton nuclear magnetic resonance spectroscopy of urine samples in preterm asphyctic newborn: A metabolomic approach. Clin. Chim. Acta 2015, 444, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.M. Biomarkers in neonatal hypoxic-ischemic encephalopathy-Review of the literature to date and future directions for research. Handb. Clin. Neurol. 2019, 162, 281–293. [Google Scholar] [PubMed]
- Johnston, M.V. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005, 15, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Pietz, J.; Guttenberg, N.; Gluck, L. Hypoxanthine: A marker for asphyxia. Obstet. Gynecol. 1988, 72, 762–766. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldarov, C.; Starodubtseva, N.; Shevtsova, Y.; Goryunov, K.; Ionov, O.; Frankevich, V.; Plotnikov, E.; Sukhikh, G.; Zorov, D.; Silachev, D. Dried Blood Spot Metabolome Features of Ischemic–Hypoxic Encephalopathy: A Neonatal Rat Model. Int. J. Mol. Sci. 2024, 25, 8903. https://doi.org/10.3390/ijms25168903
Eldarov C, Starodubtseva N, Shevtsova Y, Goryunov K, Ionov O, Frankevich V, Plotnikov E, Sukhikh G, Zorov D, Silachev D. Dried Blood Spot Metabolome Features of Ischemic–Hypoxic Encephalopathy: A Neonatal Rat Model. International Journal of Molecular Sciences. 2024; 25(16):8903. https://doi.org/10.3390/ijms25168903
Chicago/Turabian StyleEldarov, Chupalav, Natalia Starodubtseva, Yulia Shevtsova, Kirill Goryunov, Oleg Ionov, Vladimir Frankevich, Egor Plotnikov, Gennady Sukhikh, Dmitry Zorov, and Denis Silachev. 2024. "Dried Blood Spot Metabolome Features of Ischemic–Hypoxic Encephalopathy: A Neonatal Rat Model" International Journal of Molecular Sciences 25, no. 16: 8903. https://doi.org/10.3390/ijms25168903









