Molecular Alterations Associated with Histologically Overt Stromal Response in Patients with Prostate Cancer
Abstract
:1. Introduction
2. Results
2.1. Study Population and Gene Expression Analysis
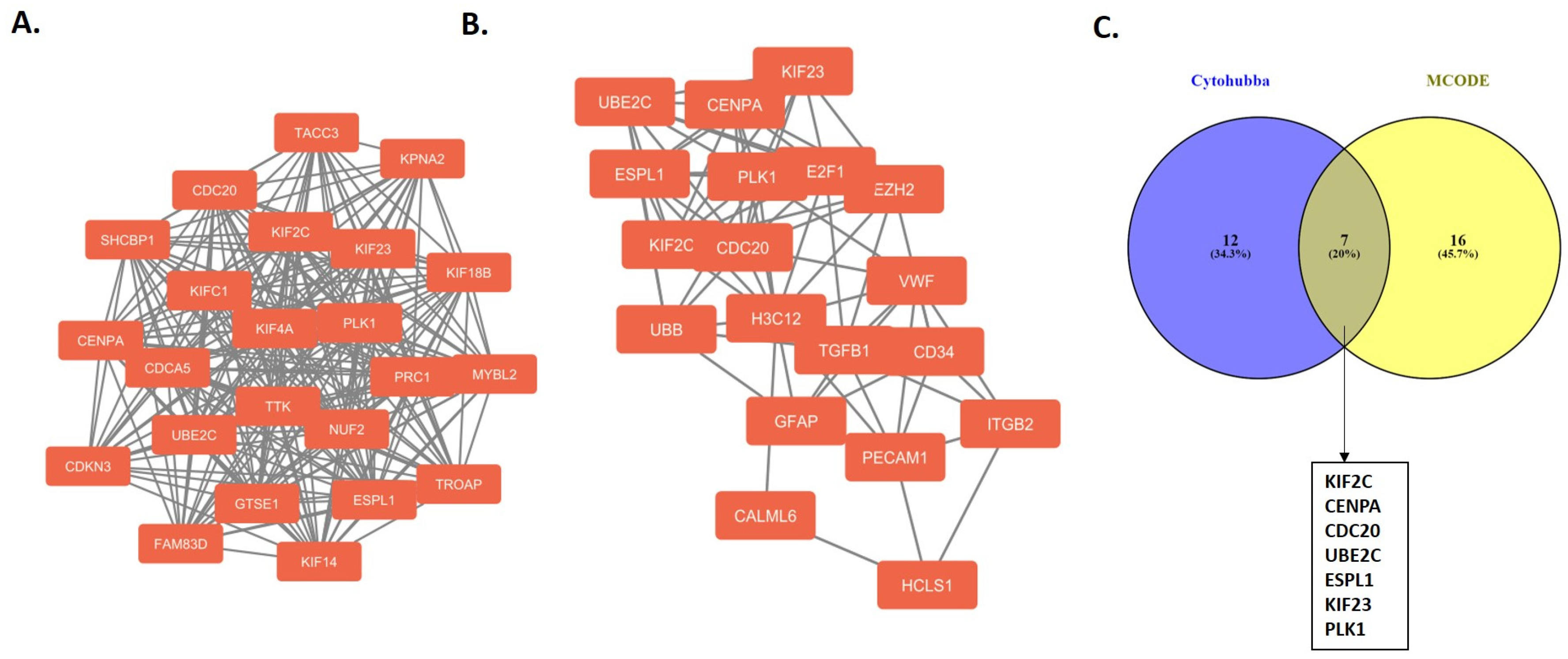
2.2. Protein–Protein Interaction Network and Hub Genes
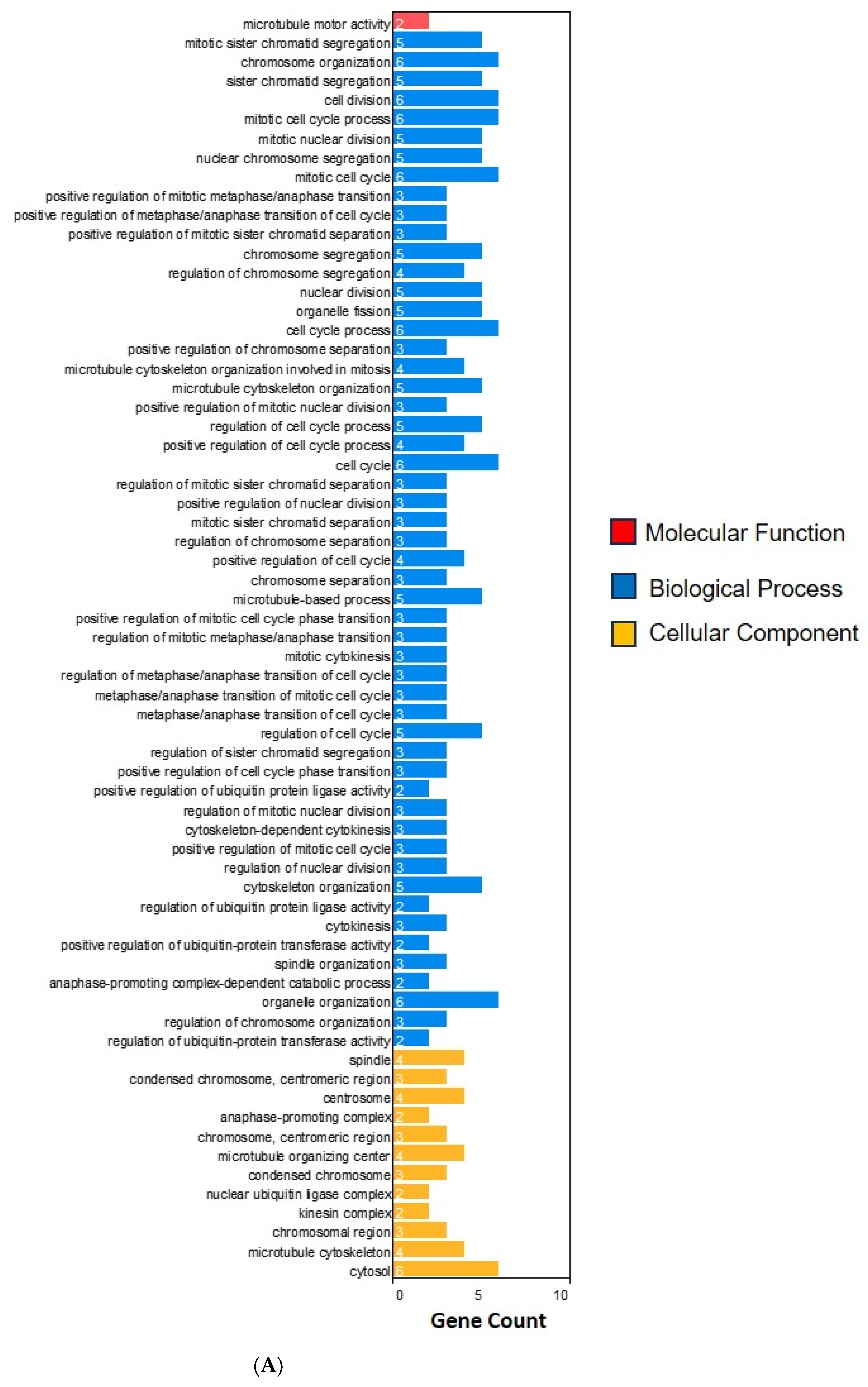
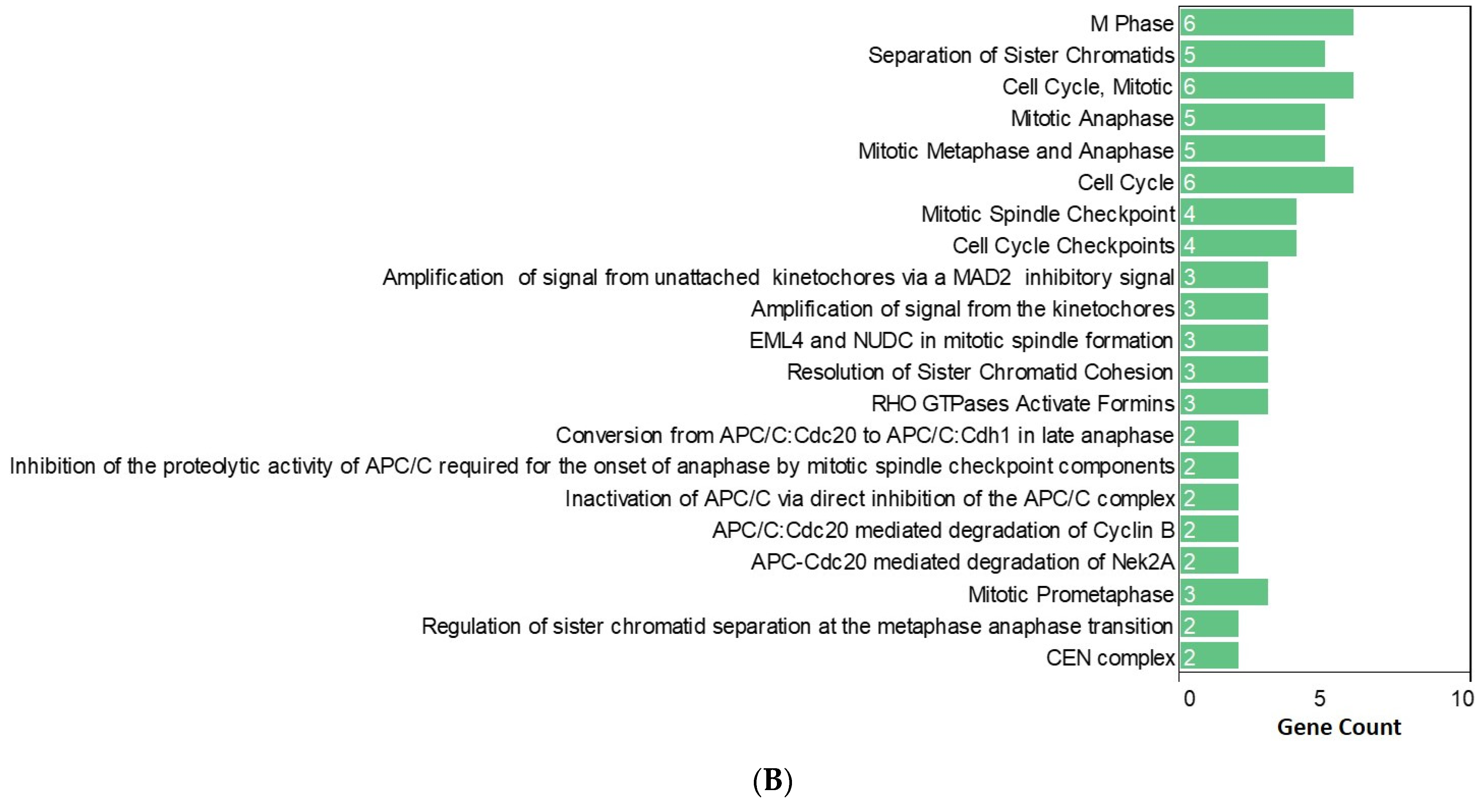
2.3. Functional Enrichment Analysis
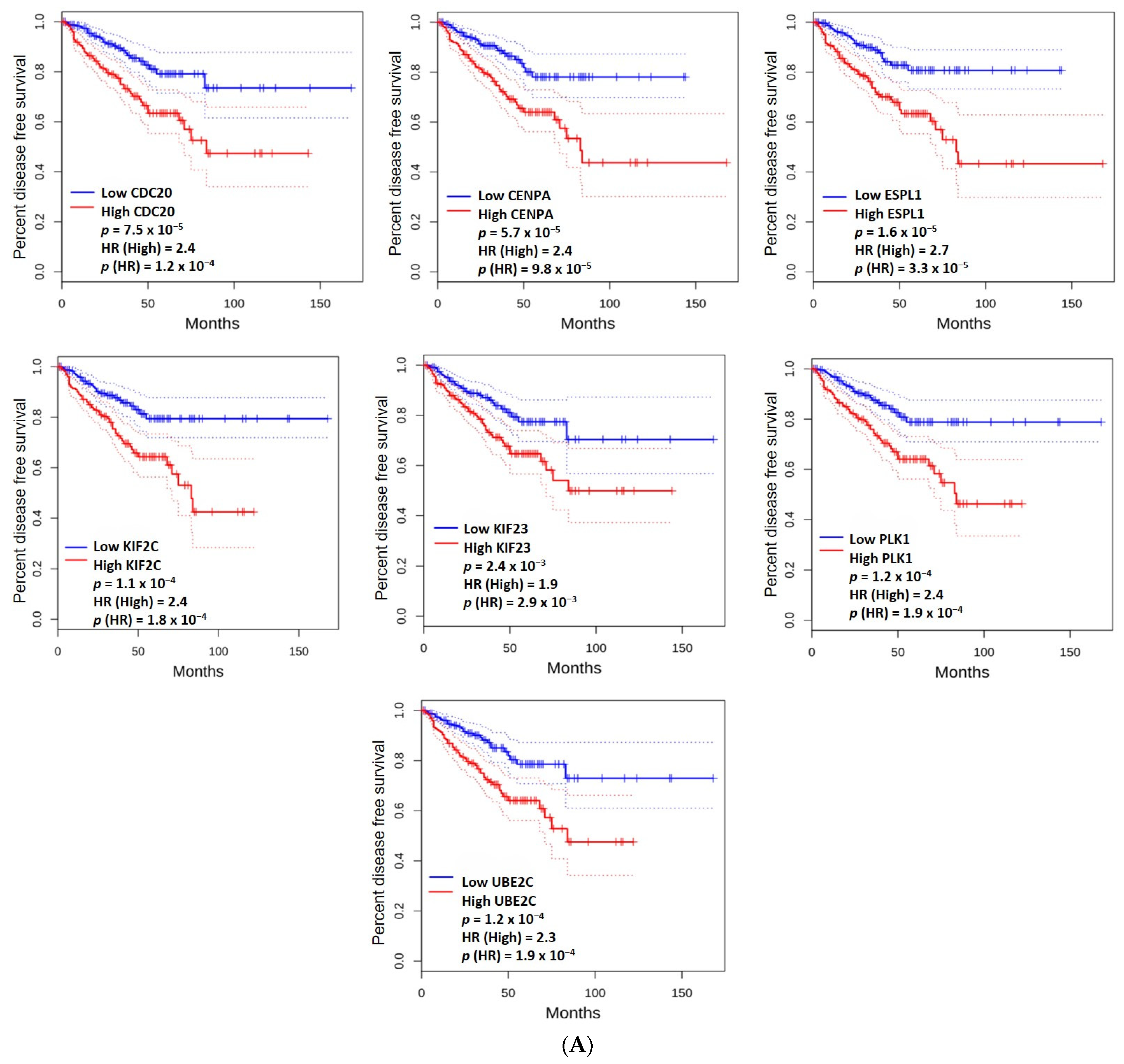
2.4. The Prognostic Value of the Hub Genes
3. Discussion
4. Materials and Methods
4.1. Study Population and Pathological Assessment
4.2. Gene Expression Analysis
4.3. Construction of Protein–Protein Interaction Network and Identification of Hub Genes
4.4. Functional Enrichment Analysis
4.5. The Prognostic Value of the Hub Genes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Cancer Genome Atlas Research Network. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, T.; Singh, P.; Jairajpuri, D.S.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Dohare, R.; Hassan, M.I. Differential Gene Expression and Weighted Correlation Network Dynamics in High-Throughput Datasets of Prostate Cancer. Front. Oncol. 2022, 12, 881246. [Google Scholar] [CrossRef] [PubMed]
- Shoag, J.; Barbieri, C.E. Clinical variability and molecular heterogeneity in prostate cancer. Asian J. Androl. 2016, 18, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Ettinger, S.L.; Qu, S.; Xue, H.; Nabavi, N.; Choi, S.Y.C.; Bell, R.H.; Mo, F.; Haegert, A.M.; Gout, P.W.; et al. Metabolic heterogeneity signature of primary treatment-naïve prostate cancer. Oncotarget 2017, 8, 25928–25941. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.; Ittmann, M.; Wheeler, T.; Sayeeduddin, M.; Cubilla, A.; Rowley, D.; Bu, P.; Ding, Y.; Gao, Y.; Lee, M.; et al. Moving Beyond Gleason Scoring. Arch. Pathol. Lab. Med. 2019, 143, 565–570. [Google Scholar] [CrossRef] [PubMed]
- De Vivar, A.D.; Sayeeduddin, M.; Rowley, D.; Cubilla, A.; Miles, B.; Kadmon, D.; Ayala, G. Histologic features of stromogenic carcinoma of the prostate (carcinomas with reactive stroma grade 3). Hum. Pathol. 2017, 63, 202–211. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.K.; Wei, W.; Hawley, S.; Auman, H.; Newcomb, L.F.; Boyer, H.D.; Fazli, L.; Simko, J.; Hurtado-Coll, A.; Troyer, D.A.; et al. Histologic Grading of Prostatic Adenocarcinoma Can Be Further Optimized: Analysis of the Relative Prognostic Strength of Individual Architectural Patterns in 1275 Patients from the Canary Retrospective Cohort. Am. J. Surg. Pathol. 2016, 40, 1439–1456. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Tuxhorn, J.A.; Wheeler, T.M.; Frolov, A.; Scardino, P.T.; Ohori, M.; Wheeler, M.; Spitler, J.; Rowley, D.R. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 4792–4801. [Google Scholar] [PubMed]
- Sayan, M.; Tuac, Y.; Kucukcolak, S.; Rowan, M.D.; Pratt, G.K.; Aktan, C.; Tjio, E.; Akbulut, D.; Moningi, S.; Leeman, J.E.; et al. Histologically Overt Stromal Response and the Risk of Progression after Radical Prostatectomy for Prostate Cancer. Cancers 2024, 16, 1871. [Google Scholar] [CrossRef]
- Orimo, A.; Weinberg, R.A. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 2006, 5, 1597–1601. [Google Scholar] [CrossRef]
- Frisbie, L.; Buckanovich, R.J.; Coffman, L. Carcinoma-Associated Mesenchymal Stem/Stromal Cells: Architects of the Pro-tumorigenic Tumor Microenvironment. Stem Cells 2022, 40, 705–715. [Google Scholar] [CrossRef]
- Wright, K.; Ly, T.; Kriet, M.; Czirok, A.; Thomas, S.M. Cancer-Associated Fibroblasts: Master Tumor Microenvironment Modifiers. Cancers 2023, 15, 1899. [Google Scholar] [CrossRef] [PubMed]
- Miyai, Y.; Esaki, N.; Takahashi, M.; Enomoto, A. Cancer-associated fibroblasts that restrain cancer progression: Hypotheses and perspectives. Cancer Sci. 2020, 111, 1047–1057. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Marozzi, M.; Parnigoni, A.; Negri, A.; Viola, M.; Vigetti, D.; Passi, A.; Karousou, E.; Rizzi, F. Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 8102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Shlyakhtina, Y.; Moran, K.L.; Portal, M.M. Genetic and Non-Genetic Mechanisms Underlying Cancer Evolution. Cancers 2021, 13, 1380. [Google Scholar] [CrossRef]
- Pernot, S.; Evrard, S.; Khatib, A.M. The Give-and-Take Interaction between the Tumor Microenvironment and Immune Cells Regulating Tumor Progression and Repression. Front. Immunol. 2022, 13, 850856. [Google Scholar] [CrossRef] [PubMed]
- Viner-Breuer, R.; Yilmaz, A.; Benvenisty, N.; Goldberg, M. The essentiality landscape of cell cycle related genes in human pluripotent and cancer cells. Cell Div. 2019, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Hu, P.; Xu, L.; Qiu, H. KIF2C is a Biomarker Correlated with Prognosis and Immunosuppressive Microenvironment in Human Tumors. Front. Genet. 2022, 13, 891408. [Google Scholar] [CrossRef]
- An, L.; Zhang, J.; Feng, D.; Zhao, Y.; Ouyang, W.; Shi, R.; Zhou, X.; Yu, Z.; Wei, S.; Min, J.; et al. KIF2C Is a Novel Prognostic Biomarker and Correlated with Immune Infiltration in Endometrial Cancer. Stem Cells Int. 2021, 2021, 1434856. [Google Scholar] [CrossRef]
- Liu, S.; Ye, Z.; Xue, V.W.; Sun, Q.; Li, H.; Lu, D. KIF2C is a prognostic biomarker associated with immune cell infiltration in breast cancer. BMC Cancer 2023, 23, 307. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Liu, P.; Li, Y.; Hu, Q.; Li, H.; Pang, X.-y.; Wu, H. Multi-omics analysis of kinesin family member 2C in human tumors: Novel prognostic biomarker and tumor microenvironment regulator. Am. J. Cancer Res. 2022, 12, 4954–4976. [Google Scholar]
- Mahlke, M.A.; Nechemia-Arbely, Y. Guarding the Genome: CENP-A-Chromatin in Health and Cancer. Genes 2020, 11, 810. [Google Scholar] [CrossRef]
- Aldwaik, R.K.; Shian, D.; Thapa, R.; Vasudevan, S.; Ashqar, M.A.-A.; Reich, E.; Kravchenko-Balasha, N.; Klutstein, M. Overexpressed kinetochore genes are used by cancer cells as genome destabilizers and transformation catalysts. Transl. Oncol. 2023, 34, 101703. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dimitrov, S.; Hamiche, A.; Van Dyck, E. Centromeric and ectopic assembly of CENP-A chromatin in health and cancer: Old marks and new tracks. Nucleic Acids Res. 2018, 47, 1051–1069. [Google Scholar] [CrossRef]
- Wu, F.; Sun, Y.; Chen, J.; Li, H.; Yao, K.; Liu, Y.; Liu, Q.; Lu, J. The Oncogenic Role of APC/C Activator Protein Cdc20 by an Integrated Pan-Cancer Analysis in Human Tumors. Front. Oncol. 2021, 11, 721797. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Ghelli Luserna di Rorà, A.; Napolitano, R.; Soverini, S.; Martinelli, G.; Simonetti, G. CDC20 in and out of mitosis: A prognostic factor and therapeutic target in hematological malignancies. J. Exp. Clin. Cancer Res. 2022, 41, 159. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Powell, C.; Yao, M.; Wu, J.; Dong, Q. Ubiquitin-conjugating enzyme E2C: A potential cancer biomarker. Int. J. Biochem. Cell Biol. 2014, 47, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, H.; Cowell, J.K. Ubiquitin-conjugating enzyme UBE2C: Molecular biology, role in tumorigenesis, and potential as a biomarker. Tumor Biol. 2012, 33, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kariri, Y.A.; Toss, M.S.; Alsaleem, M.A.; Elsharawy, K.A.; Joseph, C.; Mongan, N.P.; Green, A.R.; Rakha, E.A. Ubiquitin-conjugating enzyme 2C (UBE2C) is a poor prognostic biomarker in invasive breast cancer. Breast Cancer Res. Treat. 2022, 192, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Pati, D. Biology and insights into the role of cohesin protease separase in human malignancies. Biol. Rev. 2017, 92, 2070–2083. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, Y.M. The role of kinesin family proteins in tumorigenesis and progression. Cancer 2010, 116, 5150–5160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Q.; Wang, X. PLK1, A Potential Target for Cancer Therapy. Transl. Oncol. 2016, 10, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wen, D. The Emerging Role of Polo-Like Kinase 1 in Epithelial-Mesenchymal Transition and Tumor Metastasis. Cancers 2017, 9, 131. [Google Scholar] [CrossRef]
- Barron, D.A.; Rowley, D.R. The reactive stroma microenvironment and prostate cancer progression. Endocr. Relat. Cancer 2012, 19, R187–R204. [Google Scholar] [CrossRef]
- Greil, C.; Engelhardt, M.; Wäsch, R. The Role of the APC/C and Its Coactivators Cdh1 and Cdc20 in Cancer Development and Therapy. Front. Genet. 2022, 13, 941565. [Google Scholar] [CrossRef] [PubMed]
- Sansregret, L.; Patterson, J.O.; Dewhurst, S.; López-García, C.; Koch, A.; McGranahan, N.; Chao, W.C.H.; Barry, D.J.; Rowan, A.; Instrell, R.; et al. APC/C Dysfunction Limits Excessive Cancer Chromosomal Instability. Cancer Discov. 2017, 7, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-X.; Dai, P.-H.; Jiang, Y.-F.; Wang, Y.-Q.; Liu, W. A pan-cancer landscape of centromere proteins in tumorigenesis and anticancer drug sensitivity. Transl. Oncol. 2023, 31, 101658. [Google Scholar] [CrossRef] [PubMed]
- Régnier, V.; Vagnarelli, P.; Fukagawa, T.; Zerjal, T.; Burns, E.; Trouche, D.; Earnshaw, W.; Brown, W. CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol. Cell Biol. 2005, 25, 3967–3981. [Google Scholar] [CrossRef] [PubMed]
- Westhorpe, F.G.; Straight, A.F. Functions of the centromere and kinetochore in chromosome segregation. Curr. Opin. Cell Biol. 2013, 25, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.M.; Tuzova, M.N.; Cruikshank, W.W.; Center, D.M. Regulation of Cellular Processes by Interleukin-16 in Homeostasis and Cancer. J. Cell. Physiol. 2014, 229, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mathy, N.L.; Scheuer, W.; Lanzendörfer, M.; Honold, K.; Ambrosius, D.; Norley, S.; Kurth, R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 2000, 100, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nature Reviews Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Bollrath, J.; Greten, F.R. IKK/NF-kappaB and STAT3 pathways: Central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009, 10, 1314–1319. [Google Scholar] [CrossRef]
- Gao, P.; Niu, N.; Wei, T.; Tozawa, H.; Chen, X.; Zhang, C.; Zhang, J.; Wada, Y.; Kapron, C.M.; Liu, J. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 2017, 8, 69139–69161. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, I.; Frantzi, M.; Salcher, S.; Tymoszuk, P.; Martowicz, A.; Gomez-Gomez, E.; Blanca, A.; Lendinez Cano, G.; Latosinska, A.; Mischak, H.; et al. Prediction of Clinically Significant Prostate Cancer by a Specific Collagen-related Transcriptome, Proteome, and Urinome Signature. Eur. Urol. Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Tuac, Y.; Akgul, M.; Pratt, G.K.; Rowan, M.D.; Akbulut, D.; Kucukcolak, S.; Tjio, E.; Moningi, S.; Leeman, J.E.; et al. Prognostic Significance of the Cribriform Pattern in Prostate Cancer: Clinical Outcomes and Genomic Alterations. Cancers 2024, 16, 1248. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayan, M.; Tuac, Y.; Akgul, M.; Kucukcolak, S.; Tjio, E.; Akbulut, D.; Chen, L.W.; Yang, D.D.; Moningi, S.; Leeman, J.E.; et al. Molecular Alterations Associated with Histologically Overt Stromal Response in Patients with Prostate Cancer. Int. J. Mol. Sci. 2024, 25, 8913. https://doi.org/10.3390/ijms25168913
Sayan M, Tuac Y, Akgul M, Kucukcolak S, Tjio E, Akbulut D, Chen LW, Yang DD, Moningi S, Leeman JE, et al. Molecular Alterations Associated with Histologically Overt Stromal Response in Patients with Prostate Cancer. International Journal of Molecular Sciences. 2024; 25(16):8913. https://doi.org/10.3390/ijms25168913
Chicago/Turabian StyleSayan, Mutlay, Yetkin Tuac, Mahmut Akgul, Samet Kucukcolak, Elza Tjio, Dilara Akbulut, Luke W. Chen, David D. Yang, Shalini Moningi, Jonathan E. Leeman, and et al. 2024. "Molecular Alterations Associated with Histologically Overt Stromal Response in Patients with Prostate Cancer" International Journal of Molecular Sciences 25, no. 16: 8913. https://doi.org/10.3390/ijms25168913




