Citrus Flavanone Effects on the Nrf2-Keap1/GSK3/NF-κB/NLRP3 Regulation and Corticotroph-Stress Hormone Loop in the Old Pituitary
Abstract
1. Introduction
2. Results
2.1. Treatment with NAR and HES Affects Nrf2 Immunofluorescent Signal, Protein, and Gene Expression in the Old Pituitary
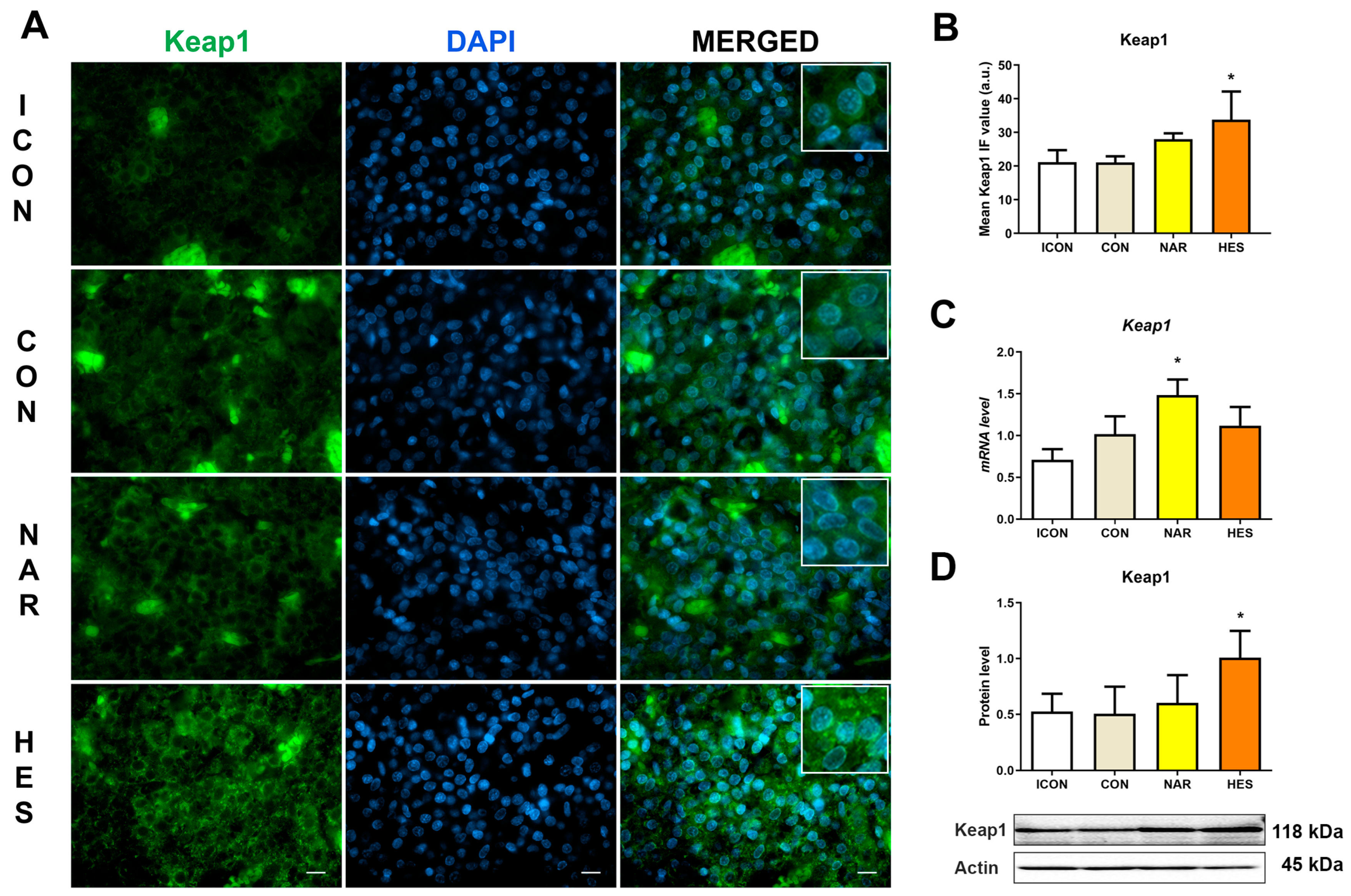
2.2. Treatment with NAR and HES Affects Keap1 Immunofluorescent Signal, Protein, and Gene Expression in the Old Pituitary
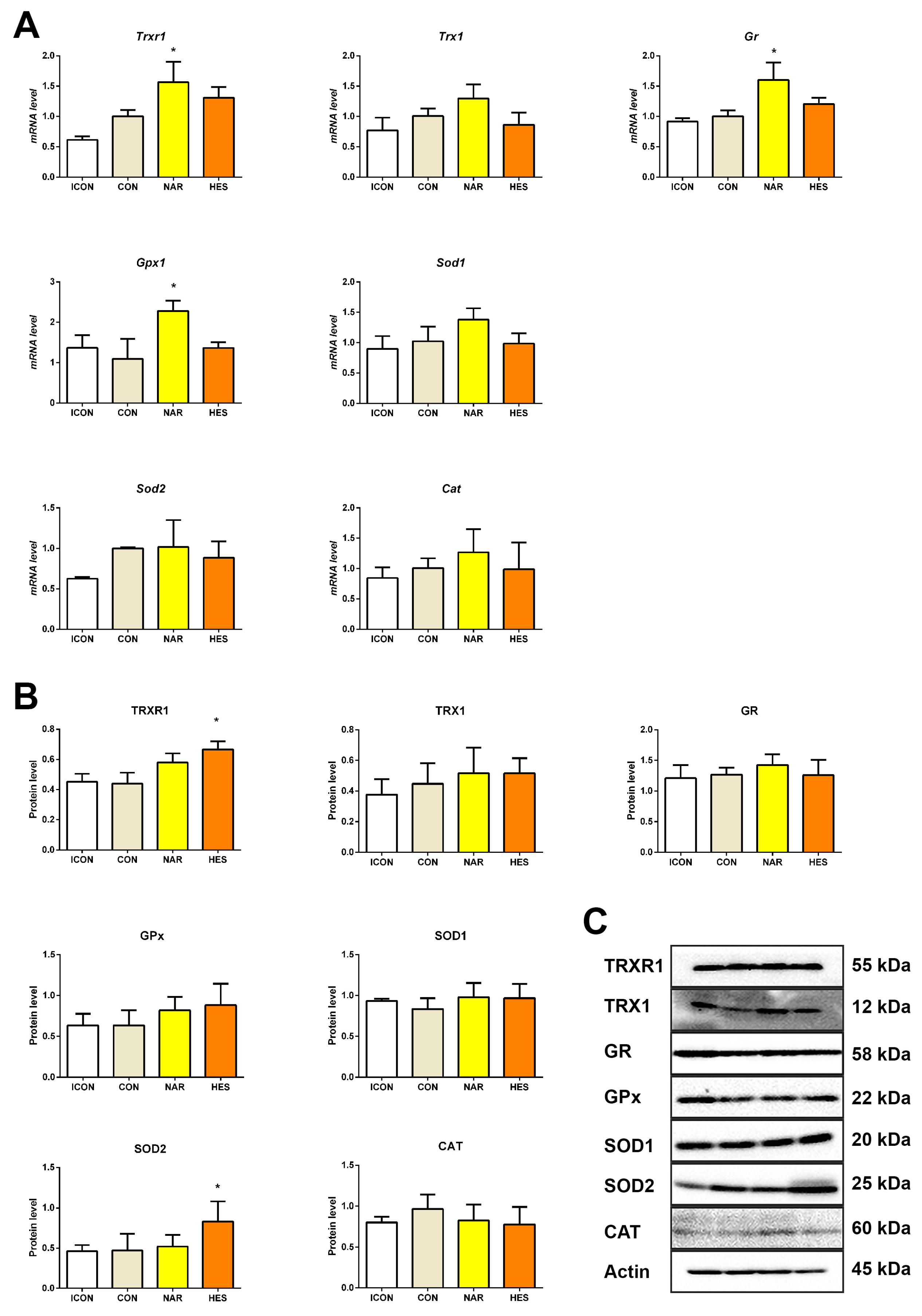
2.3. Treatment with NAR and HES Affects Antioxidant Enzyme Gene and Protein Expressions in the Old Pituitary
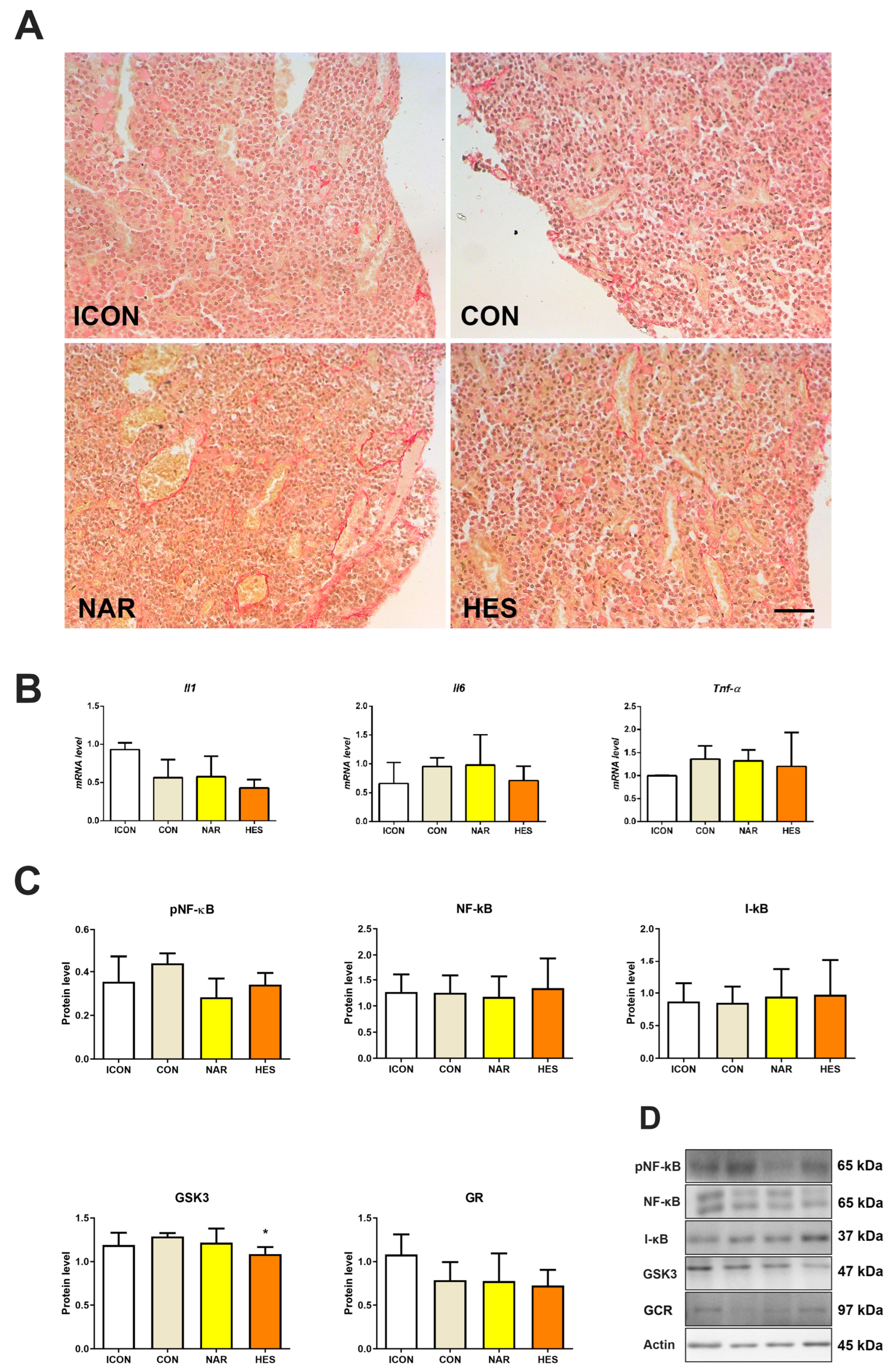
2.4. Treatment with NAR and HES Affects Collagen Abundance, While Inflammatory Markers Remain Unchanged in the Old Pituitary
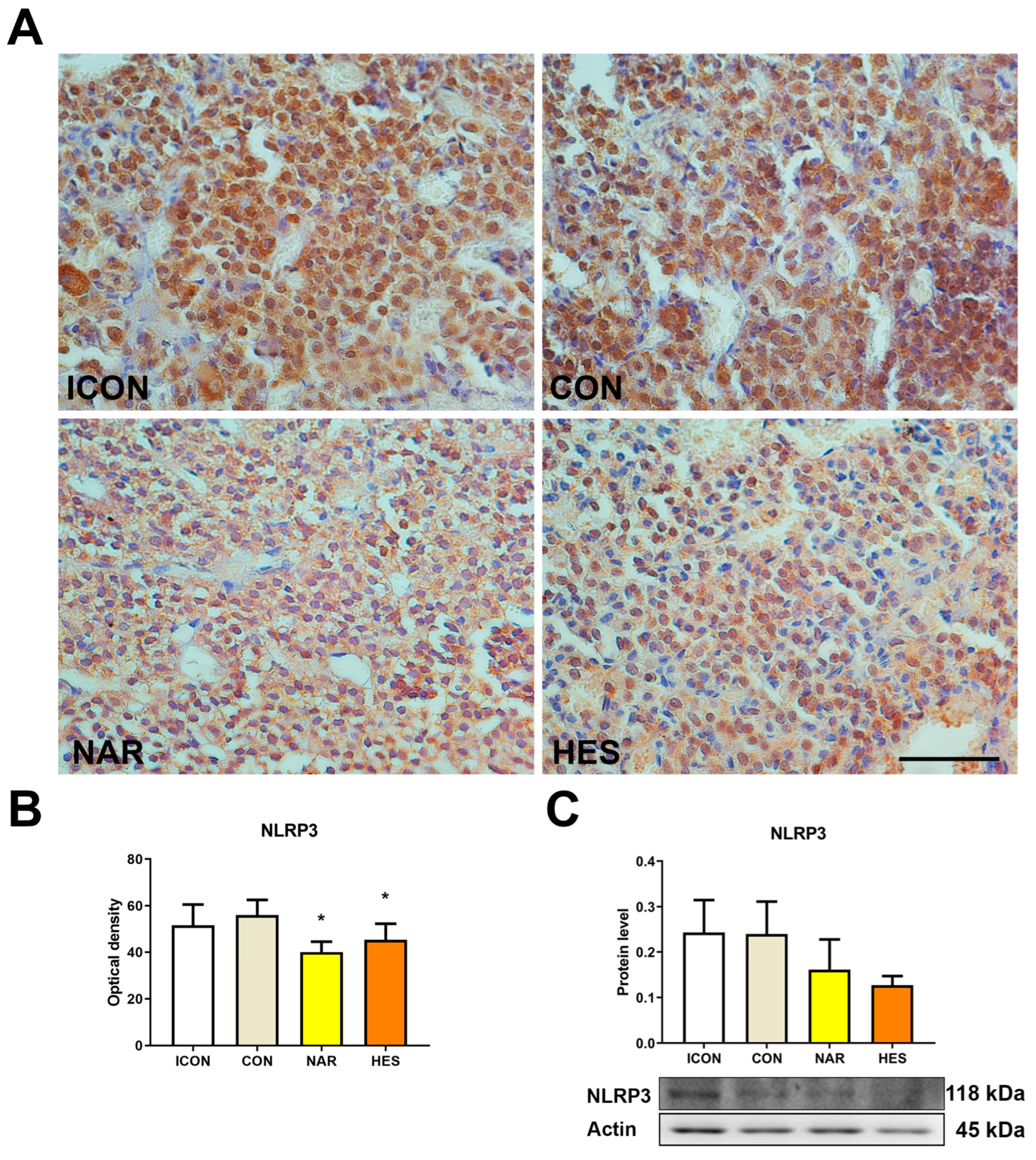
2.5. Treatment with NAR and HES Affects NLRP3 Expression in the Old Pituitary
2.6. Treatment with NAR and HES Do Not Affect ACTH Signal Intensity in the Old Pituitary
2.7. Treatment with NAR and HES Affects the Serum Level of Corticosterone but No ACTH Level in Old Rats
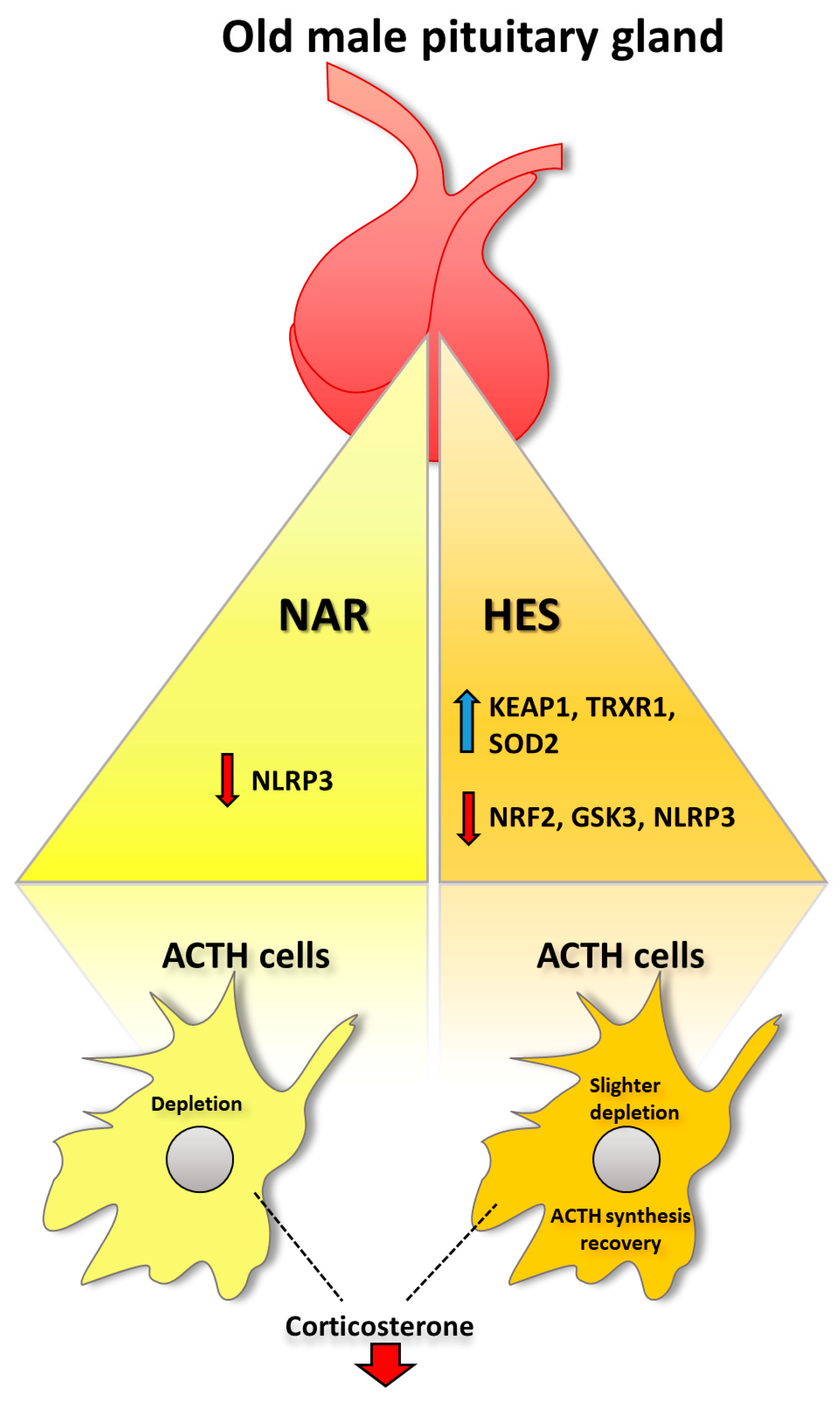
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Dosage Regimen
4.3. RNA Isolation, cDNA Transcription and Real-Time PCR
4.4. Protein Isolation for Western Blot
4.5. SDS Polyacrylamide Gel Electrophoresis and Western Blot
4.6. Histological Sample Preparation
4.7. Sirius Red Histochemical Staining
4.8. Immunohistochemical Staining of ACTH and NLRP3
4.9. Immunofluorescent Staining of Nrf2 and Keap1
4.10. Image Acquisition
4.11. Concentration of the ACTH and Corticosterone
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chahal, H.; Drake, W. The endocrine system and ageing. J. Pathol. 2007, 211, 173–180. [Google Scholar] [CrossRef]
- Ajdžanović, V.; Trifunović, S.; Miljić, D.; Šošić-Jurjević, B.; Filipović, B.; Miler, M.; Ristić, N.; Manojlović-Stojanoski, M.; Milošević, V. Somatopause, weaknesses of the therapeutic approaches and the cautious optimism based on experimental ageing studies with soy isoflavones. EXCLI J. 2018, 17, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Salvioli, S.; Franceschi, C. Oxidative stress and the ageing endocrine system. Nat. Rev. Endocrinol. 2013, 9, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Cravello, L.; Muzzoni, B.; Casarotti, D.; Paltro, M.; Solerte, S.; Fioravanti, M.; Cuzzoni, G.; Pontiggia, B.; Magri, F. Age-related changes of the hypothalamic-pituitary-adrenal axis: Pathophysiological correlates. Eur. J. Endocrinol. 2001, 144, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Brändli-Baiocco, A.; Balme, E.; Bruder, M.; Chandra, S.; Hellmann, J.; Hoenerhoff, M.J.; Kambara, T.; Landes, C.; Lenz, B.; Mense, M.; et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Endocrine System. J. Toxicol. Pathol. 2018, 31, 1S–95S. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, S.; Cano, M.; Machado, A.; Ayala, A. Effect of aging and oxidative stress on elongation factor-2 in hypothalamus and hypophysis. Mech. Ageing Dev. 2011, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Siqueira, I.; Fochesatto, C.; Da Silva Torres, I.L.; Dalmaz, C.; Netto, C.A. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005, 78, 271–278. [Google Scholar] [CrossRef]
- Nessi, A.C.; De Hoz, G.; Tanoira, C.; Guaraglia, E.; Consens, G. Pituitary physiological and ultrastructural changes during aging. Endocrine 1995, 3, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Stucker, S.; De Angelis, J.; Kusumbe, A.P. Heterogeneity and Dynamics of Vasculature in the Endocrine System During Aging and Disease. Front. Physiol. 2021, 12, 624928. [Google Scholar] [CrossRef]
- Wang, P.; Lo, M.; Medical, M.K.-J. Glucocorticoids and aging. J. Formos. Med. Assoc. 1997, 96, 792–801. [Google Scholar]
- Attia, M.A. Neoplastic and non-neoplastic lesions in the mammary gland, endocrine and genital organs in aging male and female Sprague-Dawley rats. Arch. Toxicol. 1996, 70, 461–473. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Mazzucchelli, R. Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers 2023, 15, 3037. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ma, W.; Afrashteh, A.; Sajadi, M.A.; Fakheri, H.; Valilo, M. The nuclear factor erythroid 2-related factor 2/p53 axis in breast cancer. Biochem. Med. 2023, 33, 030504. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Yousef, M.H.; Salama, M.; El-Fawal, H.A.N.; Abdelnaser, A. Selective GSK3β Inhibition Mediates an Nrf2-Independent Anti-inflammatory Microglial Response. Mol. Neurobiol. 2022, 59, 5591–5611. [Google Scholar] [CrossRef]
- Rana, A.K.; Singh, D. Targeting glycogen synthase kinase-3 for oxidative stress and neuroinflammation: Opportunities, challenges and future directions for cerebral stroke management. Neuropharmacology 2018, 139, 124–136. [Google Scholar] [CrossRef]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2007, 32, 577. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Oreščanin-Dušić, Z.; Milenković, D.; Konić-Ristić, A.; Blagojević, D.; Milošević, V.; Šošić-Jurjević, B. Citrus flavanones naringenin and hesperetin improve antioxidant status and membrane lipid compositions in the liver of old-aged Wistar rats. Exp. Gerontol. 2016, 84, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Milenkovic, D.; Jarić, I.; Šošić-Jurjević, B.; Milošević, V. Citrus Flavanones Upregulate Thyrotroph Sirt1 and Differently Affect Thyroid Nrf2 Expressions in Old-Aged Wistar Rats. J. Agric. Food Chem. 2020, 68, 8242–8254. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Milenkovic, D.; Cesar, T.; Filipović, M.R.; Milošević, V. Lemon extract reduces the hepatic oxidative stress and persulfidation levels by upregulating the Nrf2 and Trx1 expression in old rats. Biofactors 2024, 50, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Signorile, A.; Laura Ontario, M.; Castorina, S.; De Pasquale, C.; Eckert, G.; Calabrese, E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016, 94, 1588–1603. [Google Scholar] [CrossRef] [PubMed]
- Mercau, M.E.; Repetto, E.M.; Perez, M.N.; Calejman, C.M.; Puch, S.S.; Finkielstein, C.V.; Cymeryng, C.B. Moderate Exercise Prevents Functional Remodeling of the Anterior Pituitary Gland in Diet-Induced Insulin Resistance in Rats: Role of Oxidative Stress and Autophagy. Endocrinology 2016, 157, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Nudler, S.I.; Quinteros, F.A.; Miler, E.A.; Cabilla, J.P.; Ronchetti, S.A.; Duvilanski, B.H. Chromium VI administration induces oxidative stress in hypothalamus and anterior pituitary gland from male rats. Toxicol. Lett. 2009, 185, 187–192. [Google Scholar] [CrossRef]
- Ren, J.C.; Banan, A.; Keshavarzian, A.; Zhu, Q.; LaPaglia, N.; McNulty, J.; Emanuele, N.V.; Emanuele, M.A. Exposure to ethanol induces oxidative damage in the pituitary gland. Alcohol 2005, 35, 91–101. [Google Scholar] [CrossRef]
- Ajdžanović, V.Z.; TŠošič-Jurjević, B.; Filipović, B.; Trifunović, S.L.; Brkic, D.D.; Sekulić, M.I.; Milosević, V.L.J. Genistein affects the morphology of pituitary ACTH cells and decreases circulating levels of ACTH and corticosterone in middle-aged male rats. Biol. Res. 2009, 42, 13–23. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S.J. TrxR1 as a Potent Regulator of the Nrf2-Keap1 Response System. Antioxid. Redox Signal. 2015, 23, 823. [Google Scholar] [CrossRef] [PubMed]
- Larkin, S.; Ansorge, O. Development And Microscopic Anatomy of the Pituitary Gland. Endotext 2017, 1625, 206–217. [Google Scholar]
- Szabó, K.; Csányi, K. The vascular architecture of the developing pituitary-median eminence complex in the rat. Cell Tissue Res. 1982, 224, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Palde, P.B.; Carroll, K.S. A universal entropy-driven mechanism for thioredoxin-target recognition. Proc. Natl. Acad. Sci. USA 2015, 112, 7960–7965. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Vallur, P.G.; Phaëton, R.; Mythreye, K.; Hempel, N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876. [Google Scholar] [CrossRef]
- Li, H. Sirtuin 1 and oxidative stress. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 417–435. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Hsiao, Y.-P.; Lin, Y.-T.; Chen, C.; Lee, C.-M.; Liao, W.-C.; Tsou, S.-C.; Lin, H.-W.; Chang, Y.-Y.; Hsu, C.; et al. Quercetin Alleviates the Accumulation of Superoxide in Sodium Iodate-Induced Retinal Autophagy by Regulating Mitochondrial Reactive Oxygen Species Homeostasis through Enhanced Deacetyl-SOD2 via the Nrf2-PGC-1α-Sirt1 Pathway. Antioxidants 2021, 10, 1125. [Google Scholar] [CrossRef]
- Rijal, S.; Jang, S.H.; Cho, D.H.; Han, S.K. Hydrogen peroxide suppresses excitability of gonadotropin-releasing hormone neurons in adult mouse. Front. Endocrinol. 2022, 13, 939699. [Google Scholar] [CrossRef]
- Maixner, D.W.; Weng, H.-R. The Role of Glycogen Synthase Kinase 3 Beta in Neuroinflammation and Pain. J. Pharm. Pharmacol. 2013, 1, 001. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Li, J.; Di, L. jun Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med. Res. Rev. 2022, 42, 946. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhu, J.; Lei, S.; Dong, Y.; Li, L.; Jiang, B.; Tan, L.; Wu, J.; Yu, S.; et al. GSK-3β downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci. Rep. 2016, 6, 20196. [Google Scholar] [CrossRef]
- Sano, T.; Kovacs, K.T.; Scheithauer, B.W.; Young, W.F. Aging and the human pituitary gland. Mayo Clin. Proc. 1993, 68, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Vennekens, A.; Laporte, E.; Hermans, F.; Cox, B.; Modave, E.; Janiszewski, A.; Nys, C.; Kobayashi, H.; Malengier-Devlies, B.; Chappell, J.; et al. Interleukin-6 is an activator of pituitary stem cells upon local damage, a competence quenched in the aging gland. Proc. Natl. Acad. Sci. USA 2021, 118, e2100052118. [Google Scholar] [CrossRef]
- Mao, J.; Huang, H.; Liu, F.; Mai, Y.; Liao, X.; Qiu, B.; Mei, F.; Bao, Y. Activation of Age-Related Nuclear Factor-κB Signaling Pathway Leads to Chronic Inflammation and Pituitary Fibrosis. World Neurosurg. 2022, 157, e417–e423. [Google Scholar] [CrossRef]
- Escoter-Torres, L.; Greulich, F.; Quagliarini, F.; Wierer, M.; Uhlenhaut, N.H. Anti-inflammatory functions of the glucocorticoid receptor require DNA binding. Nucleic Acids Res. 2020, 48, 8393–8407. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A.S.; McCalman, M.T.; Moulos, P.; Francoijs, K.J.; Chatziioannou, A.; Kolisis, F.N.; Alexis, M.N.; Mitsiou, D.J.; Stunnenberg, H.G. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res. 2011, 21, 1404–1416. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol. 2007, 3, 389–406. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Anbalagan, S.; Gordon, L.; Blechman, J.; Matsuoka, R.L.; Rajamannar, P.; Wircer, E.; Biran, J.; Reuveny, A.; Leshkowitz, D.; Stainier, D.Y.R.; et al. Pituicyte Cues Regulate the Development of Permeable Neuro-Vascular Interfaces. Dev. Cell 2018, 47, 711–726.e5. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Latz, E.; Duewell, P. NLRP3 inflammasome activation in inflammaging. Semin. Immunol. 2018, 40, 61–73. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Ding, Q.Y.; Zhang, W.Y.; Chen, Y.G.; Wu, J.H.; Zhang, H.F.; Guo, X.L. Microglial NLRP3 inflammasome activation-mediated inflammation promotes prolactinoma development. Endocr. Relat. Cancer 2021, 28, 433–448. [Google Scholar] [CrossRef]
- Jing, S.; Wang, X.; Zhang, Z.; Cao, D.; Huang, K.; Wang, Y.; Liu, Z.; Su, S.; Wang, Q. Hesperetin attenuates cognitive dysfunction via SIRT6/NLRP3 pathway in scopolamine-induced mice. Metab. Brain Dis. 2023, 38, 2443–2456. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.Z.; He, X.M.; Li, D.D.; Wang, G.Q.; Li, J.J.; Zhang, F. Naringenin produces neuroprotection against LPS-induced dopamine neurotoxicity via the inhibition of microglial NLRP3 inflammasome activation. Front. Immunol. 2019, 10, 936. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J. Vitagenes, cellular stress response, and acetylcarnitine: Relevance to hormesis. Biofactors 2009, 35, 146–160. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Crupi, R.; Di Paola, R.; Ruggieri, M.; Bianchini, R.; Ontario, M.L.; Cuzzocrea, S.; Calabrese, E.J. Hormesis, cellular stress response and neuroinflammation in schizophrenia: Early onset versus late onset state. J. Neurosci. Res. 2017, 95, 1182–1193. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing 2018, 15, 8. [Google Scholar] [CrossRef]
- Aguilera, G.; Harwood, J.P.; Wilson, J.X.; Morell, J.; Brown, J.H.; Catt, K.J. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. ASBMB 1983, 258, 8039–8045. [Google Scholar] [CrossRef]
- Humphries, K.M.; Pennypacker, J.K.; Taylor, S.S. Redox Regulation of cAMP-dependent Protein Kinase Signaling. J. Biol. Chem. 2007, 282, 22072–22079. [Google Scholar] [CrossRef]
- Wilkinson, C.W. Human Glucocorticoid Feedback Inhibition Is Reduced in Older Individuals: Evening Study. J. Clin. Endocrinol. Metab. 2001, 86, 545–550. [Google Scholar] [CrossRef]
- Boscaro, M.; Paoletta, A.; Scarpa, E.; Barzon, L.; Fusaro, P.; Fallo, F.; Sonino, N. Age-Related Changes in Glucocorticoid Fast Feedback Inhibition of Adrenocorticotropin in Man. J. Clin. Endocrinol. Metab. 1998, 83, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Ajdžanović, V.; Miler, M.; Živanović, J.; Filipović, B.; Šošić-Jurjević, B.; Popovska-Perčinić, F.; Milošević, V. The adrenal cortex after estradiol or daidzein application in a rat model of the andropause: Structural and hormonal study. Ann. Anat. 2020, 230, 151487. [Google Scholar] [CrossRef]
- Ajdžanović, V.; Šošić-Jurjević, B.; Filipović, B.; Trifunović, S.; Manojlović-Stojanoski, M.; Sekulić, M.; Milošević, V. Genistein-Induced Histomorphometric and Hormone Secreting Changes in the Adrenal Cortex in Middle-Aged Rats. Exp. Biol. Med. 2009, 234, 148–156. [Google Scholar] [CrossRef]
- Yiallouris, A.; Tsioutis, C.; Agapidaki, E.; Zafeiri, M.; Agouridis, A.P.; Ntourakis, D.; Johnson, E.O. Adrenal aging and its implications on stress responsiveness in humans. Front. Endocrinol. 2019, 10, 435083. [Google Scholar] [CrossRef]
- Moffat, S.D.; An, Y.; Resnick, S.M.; Diamond, M.P.; Ferrucci, L. Longitudinal Change in Cortisol Levels Across the Adult Life Span. J. Gerontol. Ser. A 2020, 75, 394–400. [Google Scholar] [CrossRef]
- Noordam, R.; Jansen, S.W.M.; Akintola, A.A.; Oei, N.Y.L.; Maier, A.B.; Pijl, H.; Slagboom, P.E.; Westendorp, R.G.J.; van der Grond, J.; de Craen, A.J.M.; et al. Familial Longevity Is Marked by Lower Diurnal Salivary Cortisol Levels: The Leiden Longevity Study. PLoS ONE 2012, 7, e31166. [Google Scholar] [CrossRef]
- Noordam, R.; Gunn, D.A.; Tomlin, C.C.; Rozing, M.P.; Maier, A.B.; Slagboom, P.E.; Westendorp, R.G.J.; van Heemst, D.; De Craen, A.J.M. Cortisol serum levels in familial longevity and perceived age: The Leiden Longevity Study. Psychoneuroendocrinology 2012, 37, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Krøll, J. Correlations of plasma cortisol levels, chaperone expression and mammalian longevity: A review of published data. Biogerontology 2010, 11, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Bredehöft, J.; Perniss, A.; Fuchs, F.; Roth, J.; Rummel, C. Age dependent hypothalamic and pituitary responses to novel environment stress or lipopolysaccharide in rats. Front. Behav. Neurosci. 2018, 12, 343528. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miler, M.; Živanović, J.; Kovačević, S.; Vidović, N.; Djordjevic, A.; Filipović, B.; Ajdžanović, V. Citrus Flavanone Effects on the Nrf2-Keap1/GSK3/NF-κB/NLRP3 Regulation and Corticotroph-Stress Hormone Loop in the Old Pituitary. Int. J. Mol. Sci. 2024, 25, 8918. https://doi.org/10.3390/ijms25168918
Miler M, Živanović J, Kovačević S, Vidović N, Djordjevic A, Filipović B, Ajdžanović V. Citrus Flavanone Effects on the Nrf2-Keap1/GSK3/NF-κB/NLRP3 Regulation and Corticotroph-Stress Hormone Loop in the Old Pituitary. International Journal of Molecular Sciences. 2024; 25(16):8918. https://doi.org/10.3390/ijms25168918
Chicago/Turabian StyleMiler, Marko, Jasmina Živanović, Sanja Kovačević, Nevena Vidović, Ana Djordjevic, Branko Filipović, and Vladimir Ajdžanović. 2024. "Citrus Flavanone Effects on the Nrf2-Keap1/GSK3/NF-κB/NLRP3 Regulation and Corticotroph-Stress Hormone Loop in the Old Pituitary" International Journal of Molecular Sciences 25, no. 16: 8918. https://doi.org/10.3390/ijms25168918
APA StyleMiler, M., Živanović, J., Kovačević, S., Vidović, N., Djordjevic, A., Filipović, B., & Ajdžanović, V. (2024). Citrus Flavanone Effects on the Nrf2-Keap1/GSK3/NF-κB/NLRP3 Regulation and Corticotroph-Stress Hormone Loop in the Old Pituitary. International Journal of Molecular Sciences, 25(16), 8918. https://doi.org/10.3390/ijms25168918








