Prolactin Drives Iron Release from Macrophages and Uptake in Mammary Cancer Cells through CD44
Abstract
1. Introduction
2. Results
2.1. Prolactin Drives Iron Accumulation in Mammary Cancer Cells
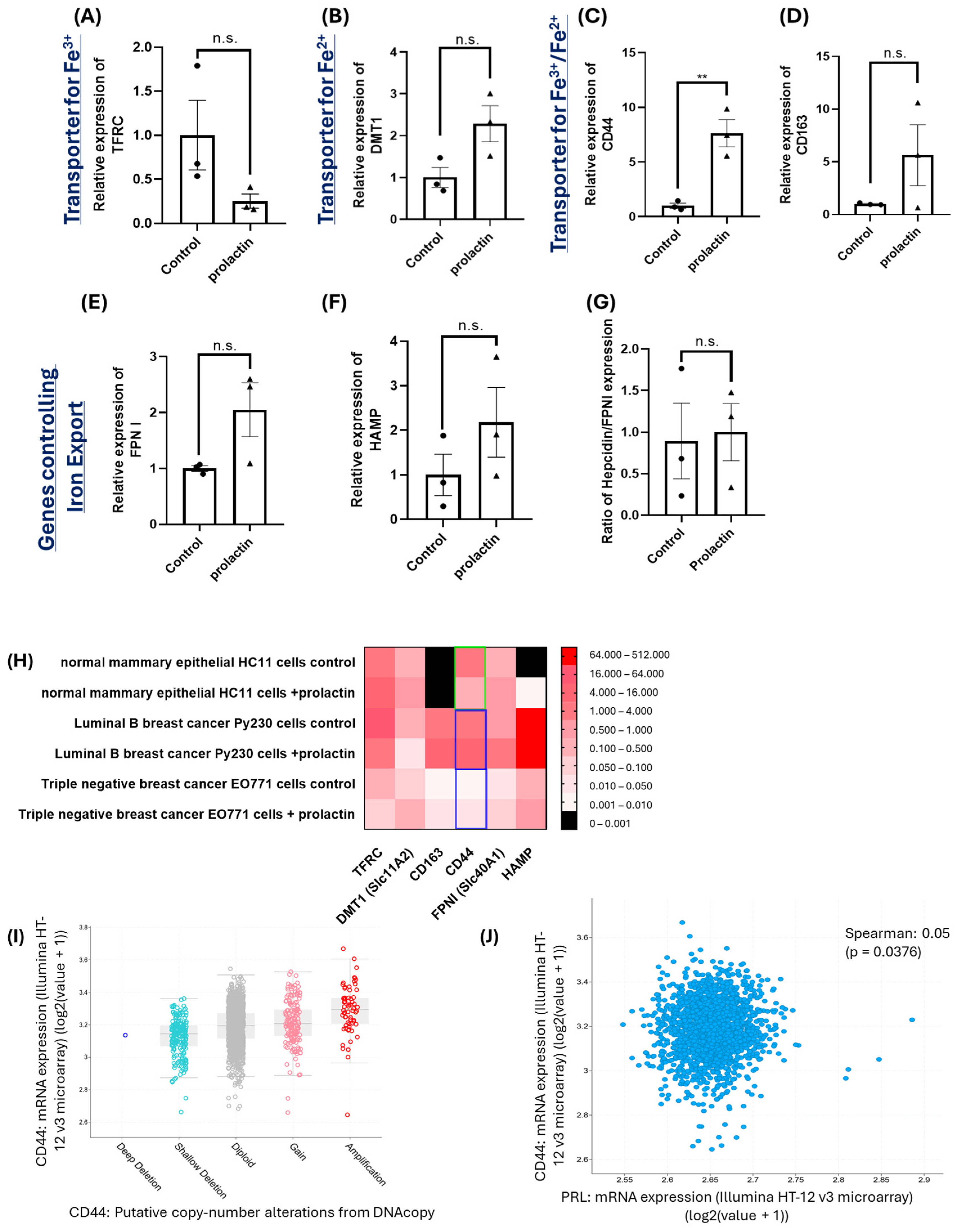
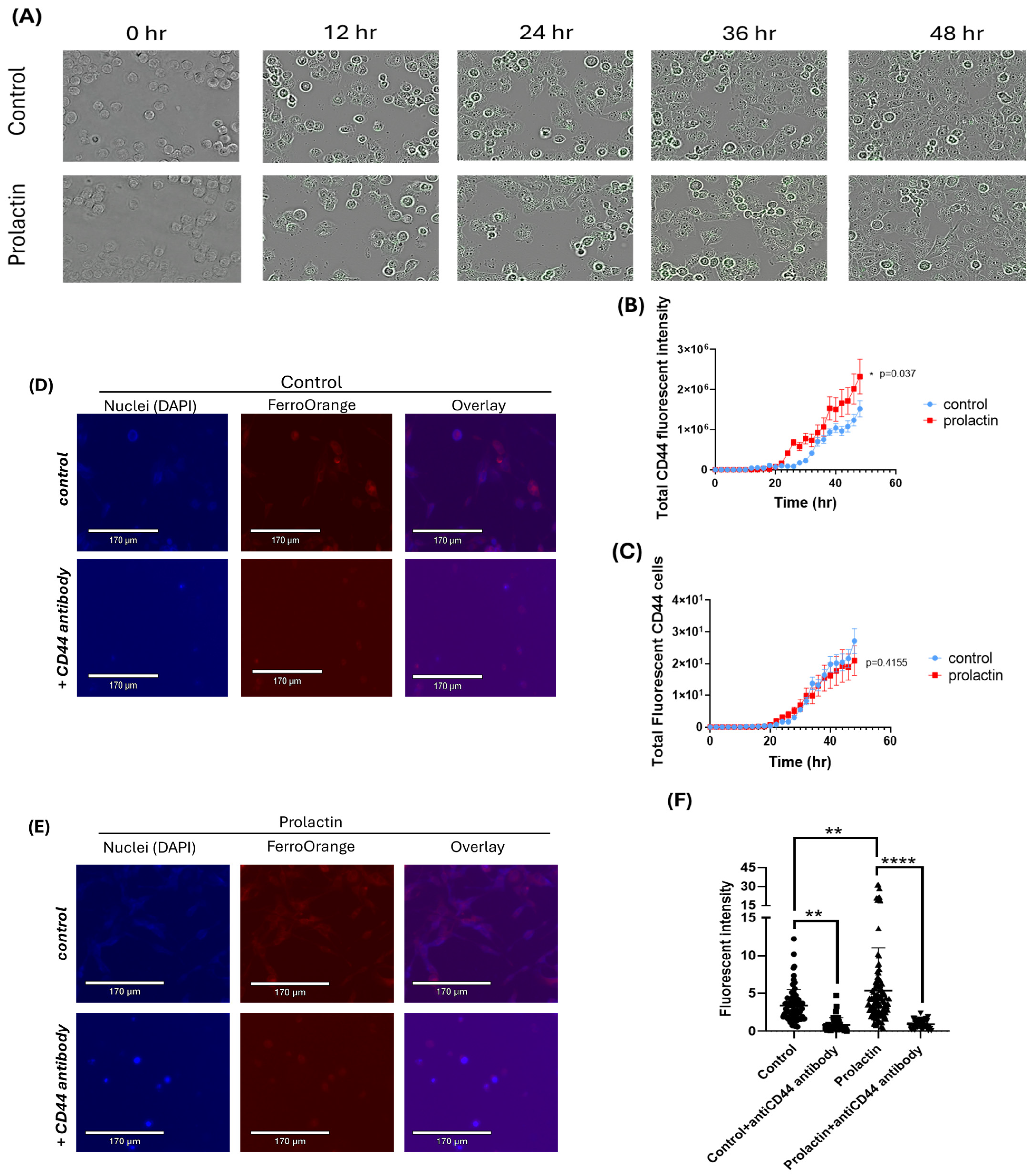
2.2. Upregulation of CD44 by Prolactin Stimulation May Contribute to Iron Accumulation in Mammary Cancer Cells
2.3. CD44 Is Essential for Prolactin-Driven Accumulation of the Labile Iron Pool
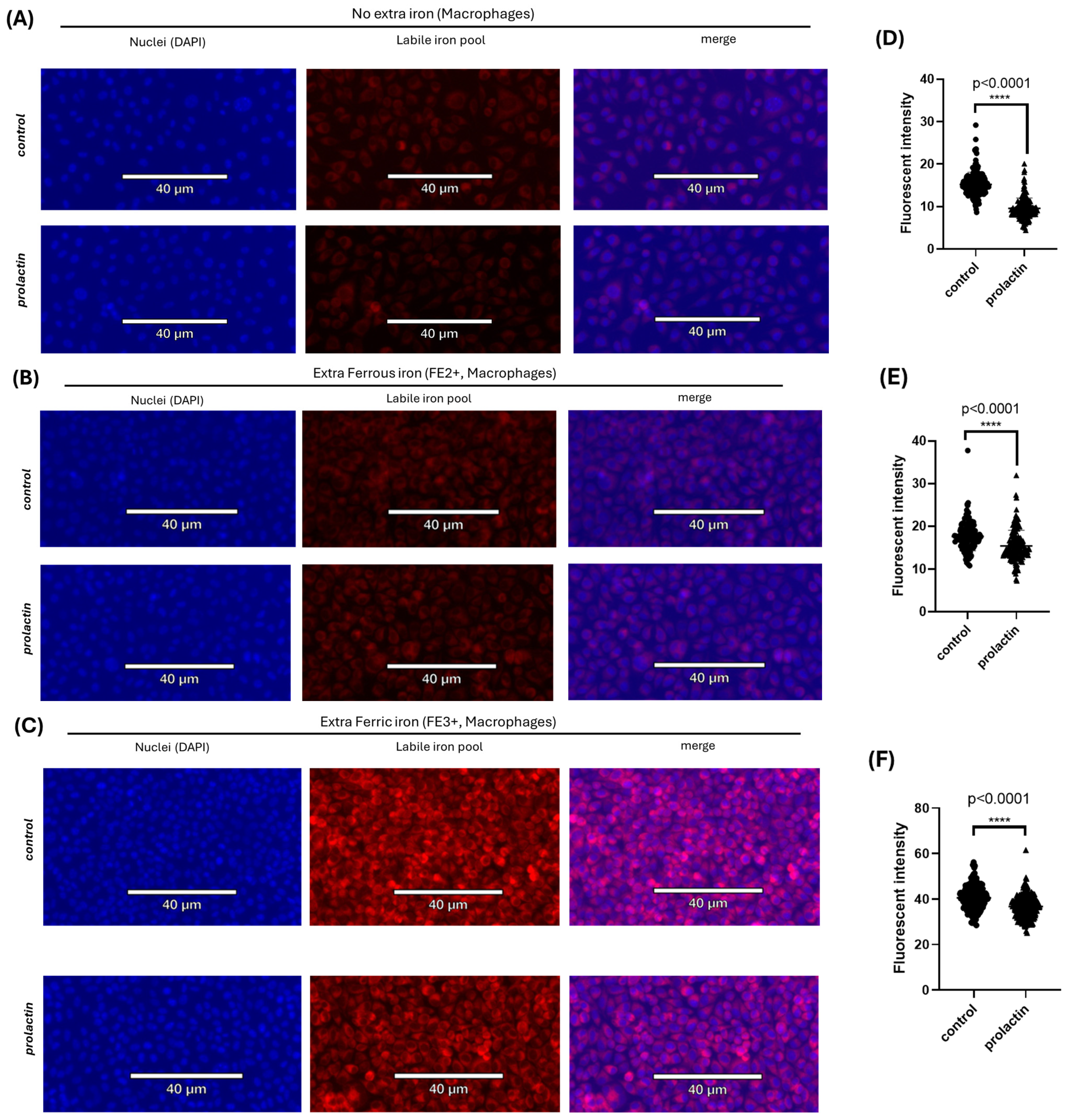
2.4. Increased Iron Export in Macrophages Is Stimulated by Prolactin
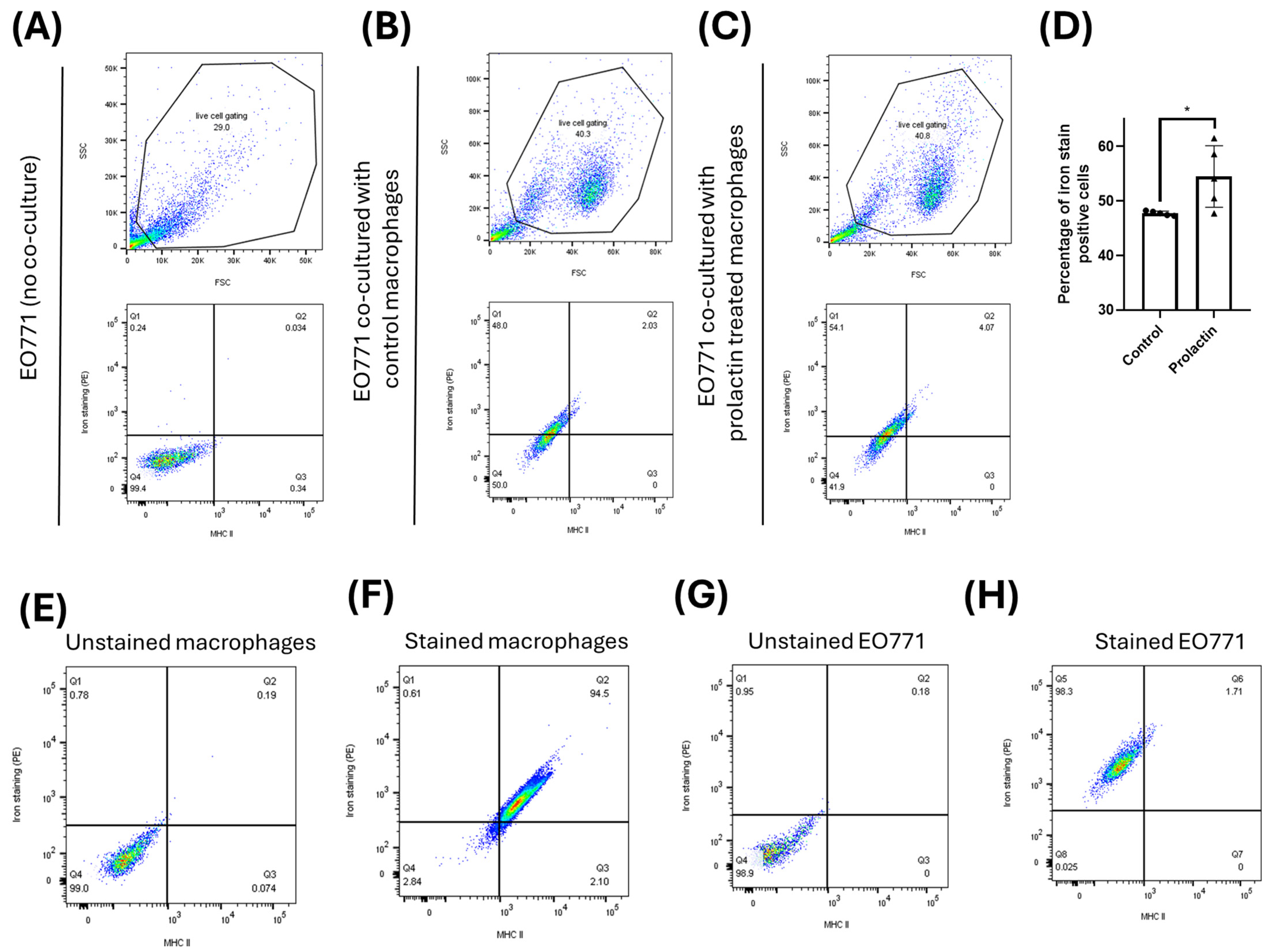
2.5. Macrophages Donate Ferrous Ions Directly from Their Labile Pool to Mammary Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Gene Expression Analyses in Breast Cancer Cell Lines, Macrophages, Normal Mammary Cell Line and Human Breast Cancer Patients
4.3. Labile Pool Iron Staining (FerroOrange Stain)
4.4. Determination of Iron Transfer between Macrophages and Mammary Cancer Cells
4.5. CD44 Expression Analysis by Cellcyte X
4.6. CD44 Neutralization Assay
4.7. Flow Cytometry
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganz, T. Systemic iron homeostasis. Physiol. Rev. 2013, 93, 1721–1741. [Google Scholar] [CrossRef] [PubMed]
- Das, B.K.; Wang, L.; Fujiwara, T.; Zhou, J.; Aykin-Burns, N.; Krager, K.J.; Lan, R.; Mackintosh, S.G.; Edmondson, R.; Jennings, M.L.; et al. Transferrin receptor 1-mediated iron uptake regulates bone mass in mice via osteoclast mitochondria and cytoskeleton. elife 2022, 11, e73539. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Happe, T.; Lu, Y.; Sawyer, A. Iron-sulphur cluster biogenesis via the SUF pathway. Metallomics 2018, 10, 1038–1052. [Google Scholar] [CrossRef]
- Travassos, L.H.; Vasconcellos, L.R.; Bozza, M.T.; Carneiro, L.A. Heme and iron induce protein aggregation. Autophagy 2017, 13, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Millett, E.S.; Efimov, I.; Basran, J.; Handa, S.; Mowat, C.G.; Raven, E.L. Heme-containing dioxygenases involved in tryptophan oxidation. Curr. Opin. Chem. Biol. 2012, 16, 60–66. [Google Scholar] [CrossRef]
- Yan, D.; Lin, Y.W.; Tan, X. Heme-containing enzymes and inhibitors for tryptophan metabolism. Metallomics 2017, 9, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 373, 485–486. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; Meuth, S.G.; Peixoto, C.A. The role of iron metabolism in the pathogenesis and treatment of multiple sclerosis. Front. Immunol. 2023, 14, 1137635. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Almutairi, M.; Moskovitz, J.; Xu, Y.G. Recent advances in iron homeostasis and regulation—A focus on epigenetic regulation and stroke. Free Radic. Res. 2021, 55, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Arima, Y.; Midorikawa, K.; Kobayashi, H.; Oikawa, S.; Zhao, W.; Zhang, Z.; Takeuchi, K.; Murata, M. Knockdown of TFRC suppressed the progression of nasopharyngeal carcinoma by downregulating the PI3K/Akt/mTOR pathway. Cancer Cell Int. 2023, 23, 185. [Google Scholar] [CrossRef]
- Ni, S.; Kuang, Y.; Yuan, Y.; Yu, B. Mitochondrion-mediated iron accumulation promotes carcinogenesis and Warburg effect through reactive oxygen species in osteosarcoma. Cancer Cell Int. 2020, 20, 399. [Google Scholar] [CrossRef]
- Kew, M.C. Hepatic iron overload and hepatocellular carcinoma. Cancer Lett. 2009, 286, 38–43. [Google Scholar] [PubMed]
- Marques, O.; da Silva, B.M.; Porto, G.; Lopes, C. Iron homeostasis in breast cancer. Cancer Lett. 2014, 347, 1–14. [Google Scholar] [PubMed]
- Khan, F.A.; Fisher, M.A.; Khakoo, R.A. Association of hemochromatosis with infectious diseases: Expanding spectrum. Int. J. Infect. Dis. 2007, 11, 482–487. [Google Scholar]
- Aglago, E.K.; Cross, A.J.; Riboli, E.; Fedirko, V.; Hughes, D.J.; Fournier, A.; Jakszyn, P.; Freisling, H.; Gunter, M.J.; Dahm, C.C.; et al. Dietary intake of total, heme and non-heme iron and the risk of colorectal cancer in a European prospective cohort study. Br. J. Cancer 2023, 128, 1529–1540. [Google Scholar]
- Igarashi, K.; Nishizawa, H.; Matsumoto, M. Iron in Cancer Progression: Does BACH1 Promote Metastasis by Altering Iron Homeostasis? Subcell Biochem. 2022, 100, 67–80. [Google Scholar] [PubMed]
- Rodriguez, R.; Schreiber, S.L.; Conrad, M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol. Cell 2022, 82, 728–740. [Google Scholar]
- Cosialls, E.; El Hage, R.; Dos Santos, L.; Gong, C.; Mehrpour, M.; Hamaï, A. Ferroptosis: Cancer Stem Cells Rely on Iron until “to Die for” It. Cells 2021, 10, 2981. [Google Scholar] [CrossRef] [PubMed]
- Salnikow, K. Role of iron in cancer. Semin. Cancer Biol. 2021, 76, 189–194. [Google Scholar] [CrossRef]
- Sanvisens, N.; Bañó, M.C.; Huang, M.; Puig, S. Regulation of ribonucleotide reductase in response to iron deficiency. Mol. Cell 2011, 44, 759–769. [Google Scholar] [CrossRef]
- Le, N.T.; Richardson, D.R. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim. Biophys. Acta 2002, 1603, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Noma, K.; Urano, S.; Watanabe, S.; Nishitani, S.; Tomono, Y.; Kimura, F.; Kagawa, S.; Shirakawa, Y.; Fujiwara, T. A novel synergistic effect of iron depletion on antiangiogenic cancer therapy. Int. J. Cancer 2013, 132, 2705–2713. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, X.; Liu, P.; Qiu, M.; Li, B.; Wen, Y.; Li, Y.; Wang, Q.; Wu, M.; Chen, Y.; et al. Engineering Alendronate-Composed Iron Nanochelator for Efficient Peritoneal Carcinomatosis Treatment. Adv. Sci. 2022, 9, e2203031. [Google Scholar] [CrossRef] [PubMed]
- Hoke, E.M.; Maylock, C.A.; Shacter, E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic. Biol. Med. 2005, 39, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.Y.; Kovacevic, Z.; V Menezes, S.; Kalinowski, D.S.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Novel thiosemicarbazones regulate the signal transducer and activator of transcription 3 (STAT3) pathway: Inhibition of constitutive and interleukin 6-induced activation by iron depletion. Mol. Pharmacol. 2015, 87, 543–560. [Google Scholar] [CrossRef]
- Bourseau-Guilmain, E.; Griveau, A.; Benoit, J.P.; Garcion, E. The importance of the stem cell marker prominin-1/CD133 in the uptake of transferrin and in iron metabolism in human colon cancer Caco-2 cells. PLoS ONE 2011, 6, e25515. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Sindikubwabo, F.; Cañeque, T.; Lafon, A.; Versini, A.; Lombard, B.; Loew, D.; Wu, T.D.; Ginestier, C.; Charafe-Jauffret, E.; et al. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat. Chem. 2020, 12, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S.; Correnti, M.; Gammella, E.; Raggi, C.; Invernizzi, P.; Cairo, G. Iron Metabolism in Liver Cancer Stem Cells. Front. Oncol. 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Chanvorachote, P.; Luanpitpong, S. Iron induces cancer stem cells and aggressive phenotypes in human lung cancer cells. Am. J. Physiol. Cell Physiol. 2016, 310, C728–C739. [Google Scholar] [CrossRef]
- Shan, Z.; Tang, W.; Shi, Z.; Shan, T. Ferroptosis: An Emerging Target for Bladder Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 8201–8214. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Voogt, J.L.; Lee, Y.; Yang, S.; Arbogast, L. Regulation of prolactin secretion during pregnancy and lactation. Prog. Brain Res. 2001, 133, 173–185. [Google Scholar] [PubMed]
- Mann, P.E.; Bridges, R.S. Lactogenic hormone regulation of maternal behavior. Prog. Brain Res. 2001, 133, 251–262. [Google Scholar] [PubMed]
- Muthuswamy, S.K. Autocrine prolactin: An emerging market for homegrown (prolactin) despite the imports. Genes. Dev. 2012, 26, 2253–2258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kong, X.; Wu, W.; Yuan, Y.; Pandey, V.; Wu, Z.; Lu, X.; Zhang, W.; Chen, Y.; Wu, M.; Zhang, M.; et al. Human growth hormone and human prolactin function as autocrine/paracrine promoters of progression of hepatocellular carcinoma. Oncotarget 2016, 7, 29465–29479. [Google Scholar] [CrossRef]
- Wu, Z.S.; Yang, K.; Wan, Y.; Qian, P.X.; Perry, J.K.; Chiesa, J.; Mertani, H.C.; Zhu, T.; Lobie, P.E. Tumor expression of human growth hormone and human prolactin predict a worse survival outcome in patients with mammary or endometrial carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, E1619–E1629. [Google Scholar] [CrossRef]
- Chen, K.H.E.; Ghosh, M.; Rivera, L.; Lin, S.; Kumar, A.; Seaminathan, S.; Lorenson, M.Y.; Walker, A.M. Prolactin enhances T regulatory cell promotion of breast cancer through the long form prolactin receptor. Transl. Oncol. 2021, 14, 101195. [Google Scholar] [CrossRef]
- Alkharusi, A.; Yu, S.; Landázuri, N.; Zadjali, F.; Davodi, B.; Nyström, T.; Gräslund, T.; Rahbar, A.; Norstedt, G. Stimulation of prolactin receptor induces STAT-5 phosphorylation and cellular invasion in glioblastoma multiforme. Oncotarget 2016, 7, 79572–79583. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Rugowski, D.E.; Shea, M.P.; Sullivan, R.; Moser, A.R.; Schuler, L.A. Prolactin synergizes with canonical Wnt signals to drive development of ER+ mammary tumors via activation of the Notch pathway. Cancer Lett. 2021, 503, 231–239. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Russo, A.; Dean, M.; Lantvit, D.D.; Endsley, M.; Burdette, J.E. Prolactin signaling drives tumorigenesis in human high grade serous ovarian cancer cells and in a spontaneous fallopian tube derived model. Cancer Lett. 2018, 433, 221–231. [Google Scholar] [CrossRef]
- Cuesta-Casanovas, L.; Delgado-Martínez, J.; Cornet-Masana, J.M.; Carbó, J.M.; Banús-Mulet, A.; Guijarro, F.; Esteve, J.; Risueño, R.M. Prolactin receptor signaling induces acquisition of chemoresistance and reduces clonogenicity in acute myeloid leukemia. Cancer Cell Int. 2023, 23, 97. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pulido, E.I.; Muñoz-Valle, J.F.; Del Toro-Arreola, S.; Jave-Suárez, L.F.; Bueno-Topete, M.R.; Estrada-Chávez, C.; Pereira-Suárez, A.L. High expression of prolactin receptor is associated with cell survival in cervical cancer cells. Cancer Cell Int. 2013, 13, 103. [Google Scholar] [CrossRef]
- Chen, K.H.; Walker, A.M. Prolactin inhibits a major tumor-suppressive function of wild type BRCA1. Cancer Lett. 2016, 375, 293–302. [Google Scholar] [CrossRef]
- Yonezawa, T.; Chen, K.H.; Ghosh, M.K.; Rivera, L.; Dill, R.; Ma, L.; Villa, P.A.; Kawaminami, M.; Walker, A.M. Anti-metastatic outcome of isoform-specific prolactin receptor targeting in breast cancer. Cancer Lett. 2015, 366, 84–92. [Google Scholar] [CrossRef]
- Chen, K.E.; Bustamante, K.; Nguyen, V.; Walker, A.M. Involvement of miR-106b in tumorigenic actions of both prolactin and estradiol. Oncotarget 2017, 8, 36368–36382. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Visintin, I.; Lai, Y.; Zhao, H.; Schwartz, P.; Rutherford, T.; Yue, L.; Bray-Ward, P.; Ward, D.C. Serum protein markers for early detection of ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 7677–7682. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.J.; Sutter, B.; Fehr, M.; Stute, P. Impact of body iron store on sexual function: A comprehensive review and pilot cohort study in midlife women. Arch. Gynecol. Obstet. 2019, 300, 469–480. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Xu, Z.; Dai, J.; Song, P.; Shi, J.; Hu, Y.; Hu, Z.; Nie, G.; Chang, Y.Z.; et al. Hepcidin levels in hyperprolactinemic women monitored by nanopore thin film based assay: Correlation with pregnancy-associated hormone prolactin. Nanomedicine 2015, 11, 871–878. [Google Scholar] [CrossRef]
- Felt, B.; Jimenez, E.; Smith, J.; Calatroni, A.; Kaciroti, N.; Wheatcroft, G.; Lozoff, B. Iron deficiency in infancy predicts altered serum prolactin response 10 years later. Pediatr. Res. 2006, 60, 513–517. [Google Scholar] [CrossRef]
- Lozoff, B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J. Nutr. 2011, 141, 740S–746S. [Google Scholar] [CrossRef] [PubMed]
- Faron-Górecka, A.; Latocha, K.; Pabian, P.; Kolasa, M.; Sobczyk-Krupiarz, I.; Dziedzicka-Wasylewska, M. The Involvement of Prolactin in Stress-Related Disorders. Int. J. Environ. Res. Public. Health 2023, 20, 3257. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Muneyyirci-Delale, O. Stress-Induced Hyperprolactinemia: Pathophysiology and Clinical Approach. Obstet. Gynecol. Int. 2018, 2018, 9253083. [Google Scholar] [CrossRef] [PubMed]
- Aranha, A.F.; Dos Anjos, L.G.; Turri, J.A.O.; Simões, R.S.; Maciel, G.A.R.; Baracat, E.C.; Soares-Júnior, J.M.; Carvalho, K.C. Impact of the prolactin levels in breast cancer: A systematic review and meta-analysis. Gynecol. Endocrinol. 2022, 38, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Clendenen, T.V.; Arslan, A.A.; Lokshin, A.E.; Liu, M.; Lundin, E.; Koenig, K.L.; Berrino, F.; Hallmans, G.; Idahl, A.; Krogh, V.; et al. Circulating prolactin levels and risk of epithelial ovarian cancer. Cancer Causes Control 2013, 24, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Levina, V.V.; Nolen, B.; Su, Y.; Godwin, A.K.; Fishman, D.; Liu, J.; Mor, G.; Maxwell, L.G.; Herberman, R.B.; Szczepanski, M.J.; et al. Biological significance of prolactin in gynecologic cancers. Cancer Res. 2009, 69, 5226–5233. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Villareal, L.B.; Liu, Z.; Haneef, M.; Falcon, D.M.; Martin, D.R.; Lee, H.J.; Dame, M.K.; Attili, D.; Chen, Y.; et al. Transferrin Receptor-Mediated Iron Uptake Promotes Colon Tumorigenesis. Adv. Sci. 2023, 10, e2207693. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, H.; Sadler, P.J. Transferrin as a metal ion mediator. Chem. Rev. 1999, 99, 2817–2842. [Google Scholar] [CrossRef] [PubMed]
- Knutson, M.D. Steap proteins: Implications for iron and copper metabolism. Nutr. Rev. 2007, 65, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, L.H.; Yuan, X.M. Macrophage hemoglobin scavenger receptor and ferritin accumulation in human atherosclerotic lesions. Ann. N. Y. Acad. Sci. 2004, 1030, 196–201. [Google Scholar] [CrossRef]
- Yanatori, I.; Richardson, D.R.; Imada, K.; Kishi, F. Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2. J. Biol. Chem. 2016, 291, 17303–17318. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef]
- Slusarczyk, P.; Mandal, P.K.; Zurawska, G.; Niklewicz, M.; Chouhan, K.; Mahadeva, R.; Jończy, A.; Macias, M.; Szybinska, A.; Cybulska-Lubak, M.; et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. elife 2023, 12, e79196. [Google Scholar] [CrossRef]
- Jahandideh, A.; Yarizadeh, M.; Noei-Khesht Masjedi, M.; Fatehnejad, M.; Jahandideh, R.; Soheili, R.; Eslami, Y.; Zokaei, M.; Ahmadvand, A.; Ghalamkarpour, N.; et al. Macrophage’s role in solid tumors: Two edges of a sword. Cancer Cell Int. 2023, 23, 150. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Jung, M.; Mertens, C.; Brüne, B. Macrophage iron homeostasis and polarization in the context of cancer. Immunobiology 2015, 220, 295–304. [Google Scholar] [CrossRef]
- Jung, M.; Weigert, A.; Mertens, C.; Rehwald, C.; Brüne, B. Iron Handling in Tumor-Associated Macrophages-Is There a New Role for Lipocalin-2? Front. Immunol. 2017, 8, 1171. [Google Scholar] [CrossRef]
- Ansell, S.M.; Vonderheide, R.H. Cellular composition of the tumor microenvironment. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2013. [Google Scholar]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Wenxuan, L.; Liu, L.; Zhang, L.; Qiu, Z.; Wu, Z.; Deng, W. Role of gonadally synthesized steroid hormones in the colorectal cancer microenvironment. Front. Oncol. 2023, 13, 1323826. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, J.; Zheng, Z.; Xiao, P.; Yu, X.; Wu, M.; Xue, L.; Xu, X.; Wang, X.; Guo, Y.; et al. Amino acids and their roles in tumor immunotherapy of breast cancer. J. Gene Med. 2023, 26, e3647. [Google Scholar] [CrossRef] [PubMed]
- Dagvadorj, A.; Collins, S.; Jomain, J.B.; Abdulghani, J.; Karras, J.; Zellweger, T.; Li, H.; Nurmi, M.; Alanen, K.; Mirtti, T.; et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology 2007, 148, 3089–3101. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N.; Liby, K.; McFarland, M.; Zinger, M. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol. Metab. 2002, 13, 245–250. [Google Scholar] [CrossRef]
- Barkey, R.J.; Ben-Shachar, D.; Amit, T.; Youdim, M.B. Increased hepatic and reduced prostatic prolactin (PRL) binding in iron deficiency and during neuroleptic treatment: Correlation with changes in serum PRL and testosterone. Eur. J. Pharmacol. 1985, 109, 193–200. [Google Scholar] [CrossRef]
- Barkey, R.J.; Amit, T.; Ben-Shachar, D.; Youdim, M.B. Characterization of the hepatic prolactin receptors induced by chronic iron deficiency and neuroleptics. Eur. J. Pharmacol. 1986, 122, 259–267. [Google Scholar] [CrossRef]
- Fujiwara, K.; Yatabe, M.; Tofrizal, A.; Jindatip, D.; Yashiro, T.; Nagai, R. Identification of M2 macrophages in anterior pituitary glands of normal rats and rats with estrogen-induced prolactinoma. Cell Tissue Res. 2017, 368, 371–378. [Google Scholar] [CrossRef]
- Mertens, C.; Mora, J.; Ören, B.; Grein, S.; Winslow, S.; Scholich, K.; Weigert, A.; Malmström, P.; Forsare, C.; Fernö, M.; et al. Macrophage-derived lipocalin-2 transports iron in the tumor microenvironment. Oncoimmunology 2018, 7, e1408751. [Google Scholar] [CrossRef]
- Simovich, M.J.; Conrad, M.E.; Umbreit, J.N.; Moore, E.G.; Hainsworth, L.N.; Smith, H.K. Cellular location of proteins related to iron absorption and transport. Am. J. Hematol. 2002, 69, 164–170. [Google Scholar] [CrossRef]
- Imoto, S.; Sawamura, T.; Shibuya, Y.; Kono, M.; Ohbuchi, A.; Suzuki, T.; Mizokoshi, Y.; Saigo, K. Labile iron, ROS, and cell death are prominently induced by haemin, but not by non-transferrin-bound iron. Transfus. Apher. Sci. 2022, 61, 103319. [Google Scholar] [CrossRef]
- Zöller, M. CD44, Hyaluronan, the Hematopoietic Stem Cell, and Leukemia-Initiating Cells. Front. Immunol. 2015, 6, 235. [Google Scholar]
- Mishra, M.N.; Chandavarkar, V.; Sharma, R.; Bhargava, D. Structure, function and role of CD44 in neoplasia. J. Oral. Maxillofac. Pathol. 2019, 23, 267–272. [Google Scholar] [CrossRef]
- Li, W.; Ma, H.; Zhang, J.; Zhu, L.; Wang, C.; Yang, Y. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 2017, 7, 13856. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Xie, K.; Wei, D. The Role of CD44 and Cancer Stem Cells. Methods Mol. Biol. 2018, 1692, 31–42. [Google Scholar]
- Oatley, M.; Bölükbası, Ö.; Svensson, V.; Shvartsman, M.; Ganter, K.; Zirngibl, K.; Pavlovich, P.V.; Milchevskaya, V.; Foteva, V.; Natarajan, K.N.; et al. Single-cell transcriptomics identifies CD44 as a marker and regulator of endothelial to haematopoietic transition. Nat. Commun. 2020, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Schumann, J.; Stanko, K.; Schliesser, U.; Appelt, C.; Sawitzki, B. Differences in CD44 Surface Expression Levels and Function Discriminates IL-17 and IFN-γ Producing Helper T Cells. PLoS ONE 2015, 10, e0132479. [Google Scholar]
- Rajarajan, A.; Bloor, B.K.; Desai, H.; Stokes, A.; Odell, E.W. Variant CD44 expression by human fibroblasts. Biomarkers 2008, 13, 307–318. [Google Scholar] [CrossRef]
- Protin, U.; Schweighoffer, T.; Jochum, W.; Hilberg, F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J. Immunol. 1999, 163, 4917–4923. [Google Scholar] [CrossRef]
- Witschen, P.M.; Chaffee, T.S.; Brady, N.J.; Huggins, D.N.; Knutson, T.P.; LaRue, R.S.; Munro, S.A.; Tiegs, L.; McCarthy, J.B.; Nelson, A.C.; et al. Tumor Cell Associated Hyaluronan-CD44 Signaling Promotes Pro-Tumor Inflammation in Breast Cancer. Cancers 2020, 12, 1325. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Liu, Y.N.; Tsai, M.F.; Wu, S.G.; Chang, T.H.; Shih, J.Y. CD44s and CD44v8-10 isoforms confer acquired resistance to osimertinib by activating the ErbB3/STAT3 signaling pathway. Life Sci. 2024, 336, 122345. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lowery, A.T.; Lin, S.; Walker, A.M.; Chen, K.E. Tumor cell-derived asymmetric dimethylarginine regulates macrophage functions and polarization. Cancer Cell Int. 2022, 22, 351. [Google Scholar] [CrossRef]
- Cain, T.J.; Smith, A.T. Ferric iron reductases and their contribution to unicellular ferrous iron uptake. J. Inorg. Biochem. 2021, 218, 111407. [Google Scholar] [CrossRef] [PubMed]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, A.; Koffi, Y.; Diab, M.; Le Guennec, D.; Rougé, S.; Aldekwer, S.; Goncalves-Mendes, N.; Talvas, J.; Farges, M.C.; Caldefie-Chezet, F.; et al. EO771, the first luminal B mammary cancer cell line from C57BL/6 mice. Cancer Cell Int. 2020, 20, 328. [Google Scholar] [CrossRef]
- Mehdi, A.; Attias, M.; Arakelian, A.; Piccirillo, C.A.; Szyf, M.; Rabbani, S.A. Co-Targeting Luminal B Breast Cancer with S-Adenosylmethionine and Immune Checkpoint Inhibitor Reduces Primary Tumor Growth and Progression, and Metastasis to Lungs and Bone. Cancers 2022, 15, 48. [Google Scholar] [CrossRef]
- Johnstone, C.N.; Smith, Y.E.; Cao, Y.; Burrows, A.D.; Cross, R.S.; Ling, X.; Redvers, R.P.; Doherty, J.P.; Eckhardt, B.L.; Natoli, A.L.; et al. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis. Model. Mech. 2015, 8, 237–251. [Google Scholar] [CrossRef]
- Contreras-Zárate, M.J.; Day, N.L.; Ormond, D.R.; Borges, V.F.; Tobet, S.; Gril, B.; Steeg, P.S.; Cittelly, D.M. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene 2019, 38, 4685–4699. [Google Scholar] [CrossRef]
- Bouri, S.; Martin, J. Investigation of iron deficiency anaemia. Clin. Med. 2018, 18, 242–244. [Google Scholar] [CrossRef]
- Mulani, J.; Murhar, B.; Jambhulkar, R.; Mishra, G. Serum Prolactin: A Clue to Breast Malignancy. Med. Lab. J. 2022, 16, 13–19. [Google Scholar]
- Sornapudi, T.R.; Nayak, R.; Guthikonda, P.K.; Pasupulati, A.K.; Kethavath, S.; Uppada, V.; Mondal, S.; Yellaboina, S.; Kurukuti, S. Comprehensive profiling of transcriptional networks specific for lactogenic differentiation of HC11 mammary epithelial stem-like cells. Sci. Rep. 2018, 8, 11777. [Google Scholar] [CrossRef]
- Pereira, B.; Chin, S.F.; Rueda, O.M.; Vollan, H.K.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.J.; et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef] [PubMed]
- Rueda, O.M.; Sammut, S.J.; Seoane, J.A.; Chin, S.F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]



| (No Extra Iron) | EO771 | Py230 |
|---|---|---|
| Control | 7.58 ± 6.158 | 15.96 ± 2.665 |
| Prolactin | 15.67 ± 7.43 | 19.48 ± 6.119 |
| No Extra Iron | Extra Ferrous Iron | Extra Ferric Iron | |
|---|---|---|---|
| Control | 15.23 ± 2.844 | 17.62 ± 3.289 | 40.59 ± 5.491 |
| Prolactin | 9.59 ± 2.435 | 15.47 ± 3.573 | 36.75 ± 4.889 |
| TBP | Forward 5′-GGGTATCTGCTGGCGGTTT-3′ Reverse 5′-TGAAATAGTGATGCTGGGCACT-3′ |
| TFRC | Forward 5′-TTCGCAGGCCAGTGCTAGG-3′ Reverse 5′-GAGTACCCCGACAGCCGTTC-3′ |
| DMT1 | Forward 5′-AAGATGCCAGACGATGGCG-3′ Reverse 5′-GAGTTGCTGTAGGCAGGGTT-3′ |
| CD163 | Forward 5′-CCTCTGCTGTCACTAACGCT-3′ Reverse 5′-CAGTTGTTTTCACCACCCGC-3′ |
| CD44 | Forward 5′-GAGCACCCCAGAAAGCTACA-3′ Reverse 5′-TGAGTGCACAGTTGAGGCAA-3′ |
| FPN I | Forward 5′-AGACAAAAAGAAGACCCCGTGA-3′ Reverse 5′-CACAACAGCCTTATGCCGAA-3′ |
| HAMP | Forward 5′-AGAGAGACACCAACTTCCCCA-3′ Reverse 5′-GCAACAGATACCACACTGGGA-3′ |
| NOS2 | Forward 5′-CAGGGAGAAAGCGCAAAACAT-3′ Reverse 5′-CATTCTGTGCTGTCCCAGTGA-3′ |
| TNFα | Forward 5′-TGGCCTCCCTCTCATCAGTTC-3′ Reverse 5′-CCATCTCATCCCATGCCTAACT-3′ |
| CD206 | Forward 5′-ACGAGCAGGTGCAGTTTACA-3′ Reverse 5′-ACATCCCATAAGCCACCTGC-3′ |
| Arg2 | Forward 5′-GTAATCCCCTCCCTGCCAATC-3′ Reverse 5′-TCAGGACGCAAGGAATTTGC-3′ |
| IL10 | Forward 5′-CCTGGGTGAGAAGCTGAAGAC-3′ Reverse 5′-TGTAGACACCTTGGTCTTGGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrell, R.; Pascuzzi, N.; Chen, Y.-L.; Kim, M.; Torres, M.; Gollahon, L.; Chen, K.-H.E. Prolactin Drives Iron Release from Macrophages and Uptake in Mammary Cancer Cells through CD44. Int. J. Mol. Sci. 2024, 25, 8941. https://doi.org/10.3390/ijms25168941
Farrell R, Pascuzzi N, Chen Y-L, Kim M, Torres M, Gollahon L, Chen K-HE. Prolactin Drives Iron Release from Macrophages and Uptake in Mammary Cancer Cells through CD44. International Journal of Molecular Sciences. 2024; 25(16):8941. https://doi.org/10.3390/ijms25168941
Chicago/Turabian StyleFarrell, Reagan, Nicholas Pascuzzi, Yi-Ling Chen, Mary Kim, Miguel Torres, Lauren Gollahon, and Kuan-Hui Ethan Chen. 2024. "Prolactin Drives Iron Release from Macrophages and Uptake in Mammary Cancer Cells through CD44" International Journal of Molecular Sciences 25, no. 16: 8941. https://doi.org/10.3390/ijms25168941
APA StyleFarrell, R., Pascuzzi, N., Chen, Y.-L., Kim, M., Torres, M., Gollahon, L., & Chen, K.-H. E. (2024). Prolactin Drives Iron Release from Macrophages and Uptake in Mammary Cancer Cells through CD44. International Journal of Molecular Sciences, 25(16), 8941. https://doi.org/10.3390/ijms25168941






