Dynamics of Actin Filaments Play an Important Role in Root Hair Growth under Low Potassium Stress in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
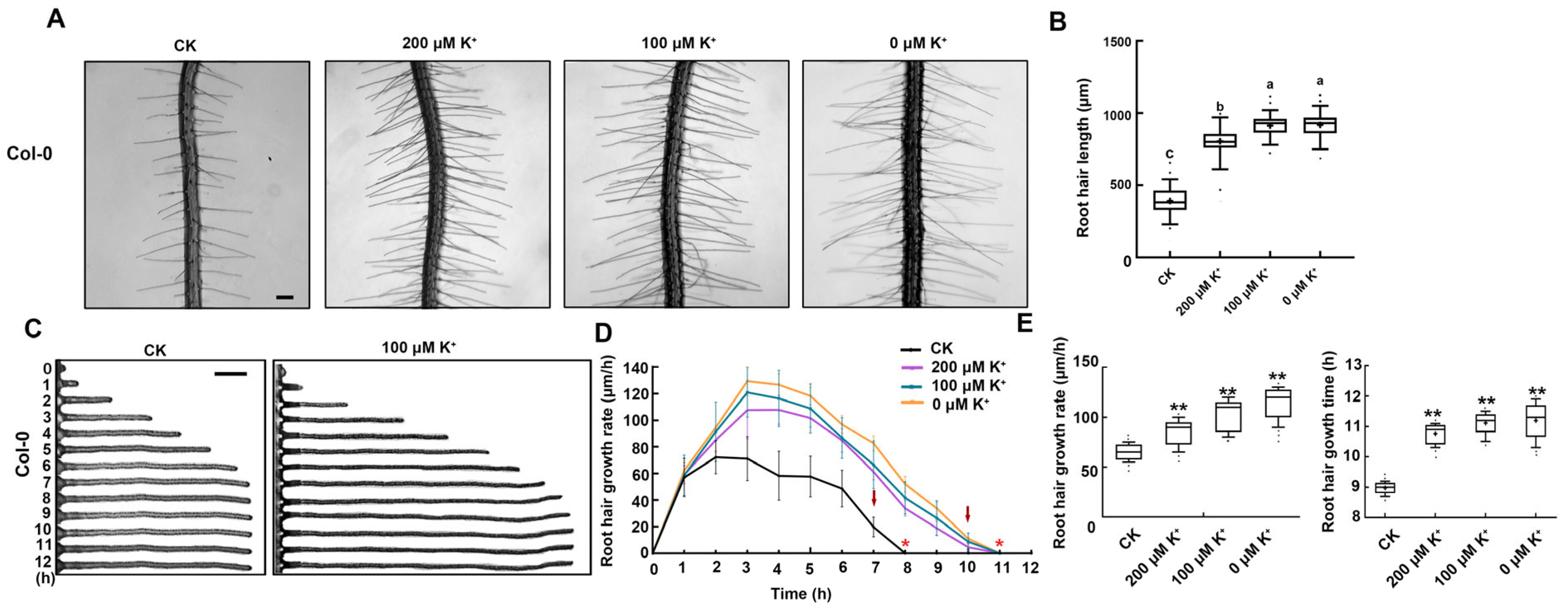
2.1. Low K+ Stress Prolongs Root Hair Growth Time and Improves Root Hair Growth Rate
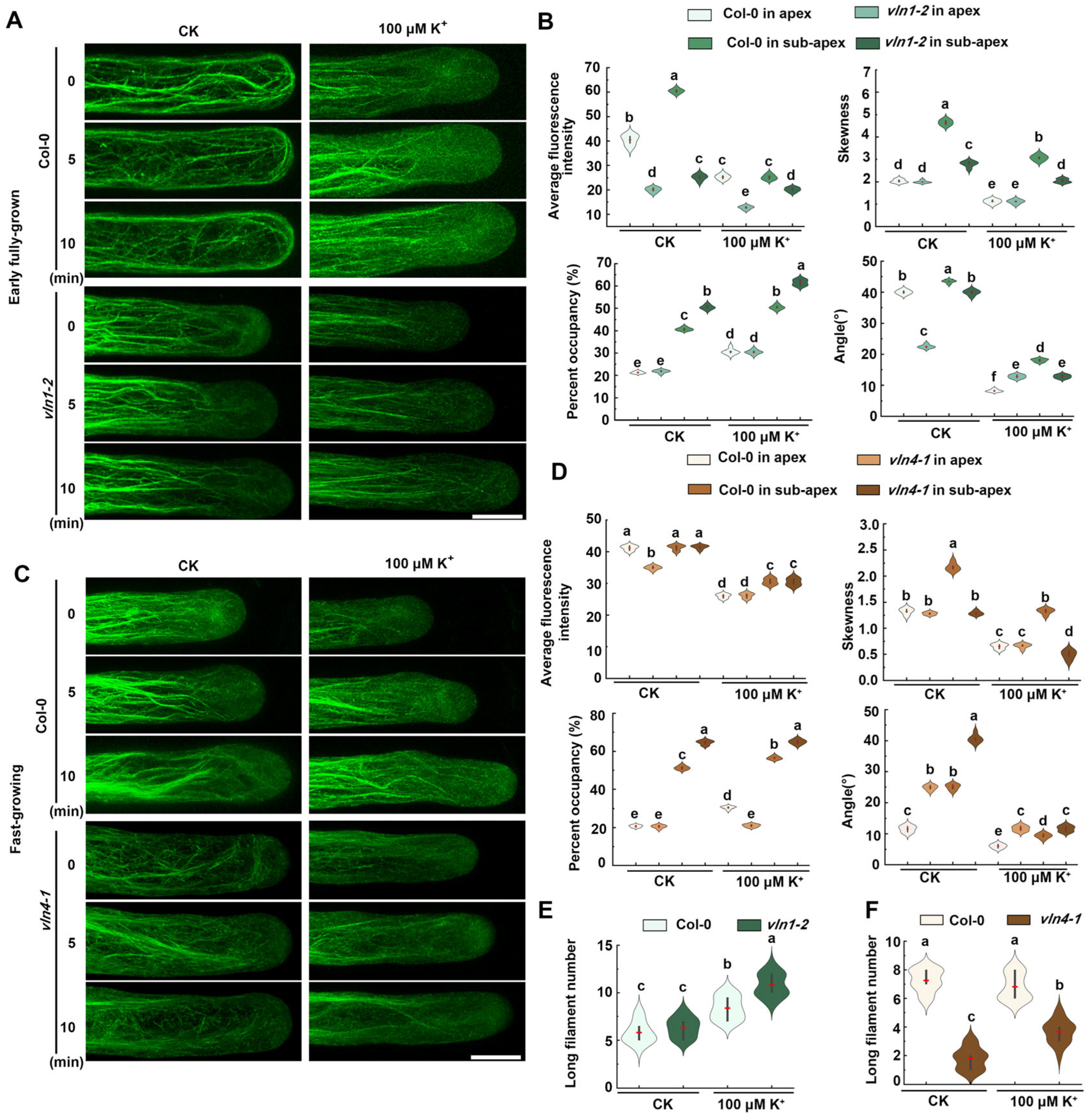
2.2. Dynamics of Actin Filaments Are Affected in Root Hair Growth under Low K+ Stress
2.3. The Expression of VLN1 and VLN4 in Root Hairs Is Affected under Low K+ Stress
2.4. VLN1 and VLN4 Regulate Root Hair Growth under Low K+ Stress
2.5. VLN1 and VLN4 Regulate Dynamics of Actin Filaments in Root Hair Growth under Low K+ Stress
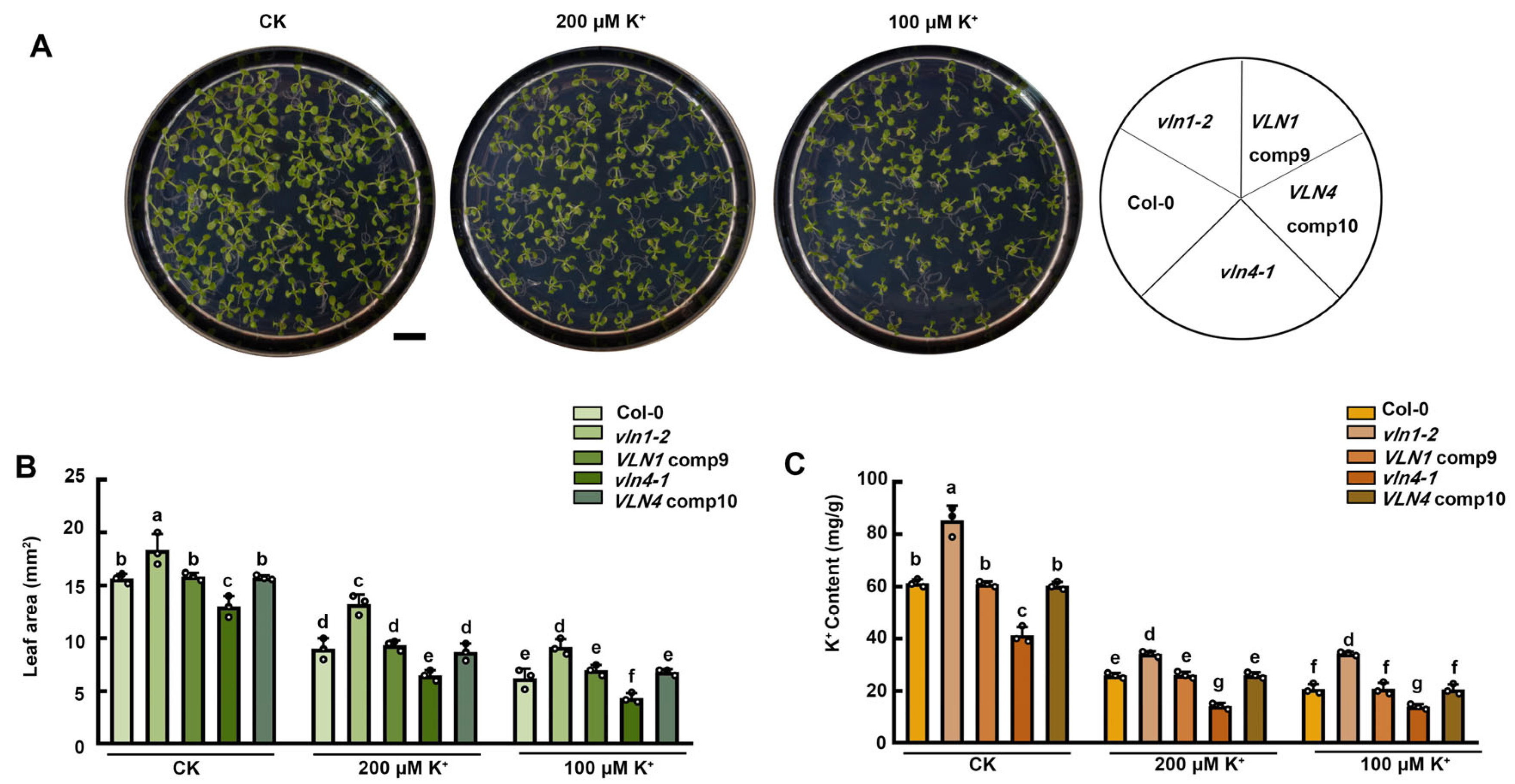
2.6. VLN1 and VLN4 Affect the Seedlings Growth under Low K+ Stress
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Growth Conditions and the Measurement of Growth Status
5.2. Plasmid Construction and Plant Transformation
5.3. Observation and Analysis of Root Hair Growth
5.4. Gene Expression and GUS Activity Analysis
5.5. Western Blot Assays
5.6. Quantitative Analysis of Actin Arrays
5.7. Time-Lapse Imaging of Signal Actin Filament Dynamics
5.8. Potassium Sensitivity Analysis
5.9. Potassium Content Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarkson, D.T.; Hanson, J.B. The mineral nutrition of higher plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Véry, A.A.; Sentenac, H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 2003, 54, 575–603. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms and physiological roles of K+ efflux from root cells. J. Plant Physiol. 2014, 171, 696–707. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Ward, J.M.; Gassmann, W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: Biophysical implications for K+ uptake. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 441–471. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, W.; Wang, Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 2012, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Cavell, A.C.; Dolan, L.; Roberts, K.; Grierson, C.S. Genetic interactions during root hair morpho-genesis in Arabidopsis. Plant Cell 2000, 12, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Li, M.; Liu, C.; Liu, X.; Cheng, J.; Wang, L.; Wang, J.; Lv, Y.; He, M.; Cheng, X.; et al. Actin depolymerizing factor ADF7 inhibits actin bundling protein VILLIN1 to regulate root hair formation in response to osmotic stress in Arabidopsis. PLoS Genet. 2022, 18, e1010338. [Google Scholar] [CrossRef]
- Ashley, M.K.; Grant, M.; Grabov, A. Plant responses to potassium deficiencies: A role for potassium transport proteins. J. Exp. Bot. 2006, 57, 425–436. [Google Scholar] [CrossRef]
- Gierth, M.; Maser, P. Potassium transporters in plants-involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007, 581, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Kim, M.J.; Ruzicka, D.; Shin, R.; Schachtman, D.P. The Arabidopsis AP2/ERF transcription factor RAP2.11 modulates plant response to low-potassium conditions. Mol. Plant 2012, 5, 1042–1057. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jiang, Q.; Wu, J.; An, L.; Zhou, Z.; Wong, C.; Wu, M.; Yu, H.; Gan, Y. Zinc finger protein 5 (ZFP5) associates with ethylene signaling to regulate the phosphate and potassium deficiency-induced root hair development in Arabidopsis. Plant Mol. Biol. 2020, 102, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, J.; Bi, S.; Li, M.; Wang, L.; Wang, L.; Li, T.; Zhang, X.; Gao, Y.; Zhu, L.; et al. GLABRA 2 regulates ETHYLENE OVERPRODUCER 1 accumulation during nutrient deficiency-induced root hair growth. Plant Physiol. 2024, 1995, 1906–1924. [Google Scholar] [CrossRef]
- Libault, M.; Brechenmacher, L.; Cheng, J.; Xu, D.; Stacey, G. Root hair systems biology. Trends Plant Sci. 2010, 15, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Du, F.; Zhang, Y.; He, T.; Ren, H. Control of the actin cytoskeleton in root hair development. Plant Sci. 2012, 187, 10–18. [Google Scholar] [CrossRef]
- Ketelaar, T. The actin cytoskeleton in root hairs: All is fine at the tip. Curr. Opin. Plant Biol. 2013, 16, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.K.; McKinney, E.C.; Meagher, R.B. A single vegetative actin isovariant overexpressed under the control of multiple regulatory sequences is sufficient for normal Arabidopsis development. Plant Cell 2009, 21, 701–718. [Google Scholar] [CrossRef]
- Klahre, U.; Friederich, E.; Kost, B.; Louvard, D.; Chua, N.H. Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis. Plant Physiol. 2000, 122, 35–48. [Google Scholar] [CrossRef]
- Ketelaar, T.; Faivre-Moskalenko, C.; Esseling, J.J.; de Ruijter, N.C.; Grierson, C.S.; Dogterom, M.; Emons, A.M.C. Positioning of nuclei in Arabidopsis root hairs: An actin-regulated process of tip growth. Plant Cell 2002, 14, 2941–2955. [Google Scholar] [CrossRef]
- Huang, S.; Robinson, R.C.; Gao, L.; Matsumoto, T.; Brunet, A.; Blanchoin, L.; Staiger, C.J. Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization. Plant Cell 2005, 17, 486–501. [Google Scholar] [CrossRef]
- Khurana, P.; Henty, J.L.; Huang, S.; Staiger, A.M.; Blanchoin, L.; Staiger, C.J. Arabidopsis VILLIN1 and VILLIN3 have overlapping and distinct activities in actin bundle formation and turnover. Plant Cell 2010, 22, 2727–2748. [Google Scholar] [CrossRef]
- Wang, X.; Bi, S.; Wang, L.; Li, H.; Gao, B.; Huang, S.; Qu, X.; Cheng, J.; Wang, S.; Liu, C.; et al. GLABRA2 regulates actin bundling protein VILLIN1 in root hair growth in response to osmotic stress. Plant Physiol. 2020, 184, 176–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Du, F.; Cao, L.; Dong, H.; Ren, H. Arabidopsis VILLIN4 is involved in root hair growth through regulating actin organization in a Ca2+-dependent manner. New Phytol. 2011, 190, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qu, X.; Bao, C.; Khurana, P.; Wang, Q.; Xie, Y.; Zheng, Y.; Chen, N.; Blanchoin, L.; Staiger, C.J.; et al. Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell 2010, 22, 2749–2767. [Google Scholar] [CrossRef]
- Bao, C.; Wang, J.; Zhang, R.; Zhang, B.; Zhang, H.; Zhou, Y.; Huang, S. Arabidopsis VILLIN2 and VILLIN3 act redundantly in sclerenchyma development via bundling of actin filament. Plant J. 2012, 71, 962–975. [Google Scholar] [CrossRef]
- van der Honing, H.S.; Kieft, H.; Emons, A.M.C.; Ketelaar, T. Arabidopsis VILLIN2 and VILLIN3 are required for the generation of thick actin filament bundles and for directional organ growth. Plant Physiol. 2012, 158, 1426–1438. [Google Scholar] [CrossRef]
- Kang, E.; Zheng, M.; Zhang, Y.; Yuan, M.; Yalovsky, S.; Zhu, L.; Fu, Y. The microtubule-associated protein MAP18 affects ROP2 GTPase activity during root hair growth. Plant Physiol. 2017, 174, 202–222. [Google Scholar] [CrossRef]
- Qian, D.; Li, T.; Zheng, C.; Niu, Y.; Niu, Y.; Li, C.; Wang, M.; Yang, Y.; An, L.; Xiang, Y. Actin-depolymerizing factors 8 and 11 promote root hair elongation at high pH. Plant Commun. 2024, 5, 100787. [Google Scholar] [CrossRef]
- Desbrosses, G.; Josefsson, C.; Rigas, S.; Hatzopoulos, P.; Dolan, L. AKT1 and TRH1 are required during root hair elongation in Arabidopsis. J. Exp. Bot. 2003, 54, 781–788. [Google Scholar] [CrossRef]
- Jung, J.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Miller, D.D.; De Ruijter, N.C.A.; Bisseling, T.; Emons, A.M.C. The role of actin in root hair morphogenesis: Studies with lipochito oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 1999, 17, 141–154. [Google Scholar] [CrossRef]
- Dos Remedios, C.G.; Chhabra, D.; Kekic, M.; Dedova, I.V.; Tsubakihara, M.; Berry, D.A.; Nosworthy, N.J. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol. Rev. 2003, 83, 433–473. [Google Scholar] [CrossRef]
- Thomas, C.; Tholl, S.; Moes, D.; Dieterle, M.; Papuga, J.; Moreau, F.; Steinmetz, A. Actin bundling in plants. Cell Motil. Cytoskel. 2009, 66, 940–957. [Google Scholar] [CrossRef]
- Liu, X.; Qin, T.; Ma, Q.; Sun, J.; Liu, Z.; Yuan, M.; Mao, T. Light-regulated hypocotyl elongation involves proteasome-dependent degradation of the microtubule regulatory protein WDL3 in Arabidopsis. Plant Cell 2013, 25, 1740–1755. [Google Scholar] [CrossRef]
- Li, J.; Henty-Ridilla, J.L.; Staiger, B.H.; Day, B.; Staiger, C.J. Capping protein integrates multiple MAMP signalling pathways to modulate actin dynamics during plant innateimmunity. Nat. Commun. 2015, 6, 7206. [Google Scholar] [CrossRef]
- Zou, M.; Ren, H.; Li, J. An auxin transport inhibitor targets villin-mediated actin dynamics to regulate polar auxin transport. Plant Physiol. 2019, 181, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Henty-Ridilla, J.L.; Huang, S.; Wang, X.; Blanchoin, L.; Staiger, C.J. Capping protein modulates the dynamic behavior of actin filaments in response to phosphatidic acid in Arabidopsis. Plant Cell 2012, 24, 3742–3754. [Google Scholar] [CrossRef]
- Wu, S.; Xie, Y.; Zhang, J.; Ren, Y.; Zhang, X.; Wang, J.; Gao, X.; Wu, F.; Sheng, P.; Wang, J.; et al. VLN2 regulates plant architecture by affecting microfilament dynamics and polar auxin transport in rice. Plant Cell 2015, 27, 2829–2845. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, E.; Yuan, M.; Fu, Y.; Zhu, L. PCaP2 regulates nuclear positioning in growing Arabidopsis thaliana root hairs by modulating filamentous actin organization. Plant Cell Rep. 2015, 34, 1317–1330. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liu, S.; Wang, J.; Cheng, X.; Diao, C.; Yan, D.; Gao, Y.; Wang, C. Dynamics of Actin Filaments Play an Important Role in Root Hair Growth under Low Potassium Stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 8950. https://doi.org/10.3390/ijms25168950
Li M, Liu S, Wang J, Cheng X, Diao C, Yan D, Gao Y, Wang C. Dynamics of Actin Filaments Play an Important Role in Root Hair Growth under Low Potassium Stress in Arabidopsis thaliana. International Journal of Molecular Sciences. 2024; 25(16):8950. https://doi.org/10.3390/ijms25168950
Chicago/Turabian StyleLi, Mingyang, Shihang Liu, Jinshu Wang, Xin Cheng, Chengxuan Diao, Dabo Yan, Yue Gao, and Che Wang. 2024. "Dynamics of Actin Filaments Play an Important Role in Root Hair Growth under Low Potassium Stress in Arabidopsis thaliana" International Journal of Molecular Sciences 25, no. 16: 8950. https://doi.org/10.3390/ijms25168950




