Role of Bone Metastases in Lung Neuroendocrine Neoplasms: Clinical Presentation, Treatment and Impact on Prognosis
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Study Cohort
3.2. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggeri, R.M.; Benevento, E.; De Cicco, F.; Fazzalari, B.; Guadagno, E.; Hasballa, I.; Tarsitano, M.G.; Isidori, A.M.; Colao, A.; Faggiano, A. Neuroendocrine Neoplasms in the Context of Inherited Tumor Syndromes: A Reappraisal Focused on Targeted Therapies. J. Endocrinol. Investig. 2023, 46, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Liccardi, A.; Minotta, R.; Cannavale, G.; Benevento, E.; Colao, A. Current Understanding of Pathogenetic Mechanisms in Neuroendocrine Neoplasms. Expert. Rev. Endocrinol. Metab. 2024, 19, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Marcos-Gragera, R.; Capdevila, J.; Buxó, M.; Ortiz, R.M.; Barretina, P.; Vilardell, L.; Brunet, J.; Beltran, M.; Izquierdo, À. Neuroendocrine Tumors: A Population-Based Study of Incidence and Survival in Girona Province, 1994–2004. Cancer Epidemiol. 2011, 35, e49–e54. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Alcala, N.; Leblay, N.; Gabriel, A.A.G.; Mangiante, L.; Hervas, D.; Giffon, T.; Sertier, A.S.; Ferrari, A.; Derks, J.; Ghantous, A.; et al. Integrative and Comparative Genomic Analyses Identify Clinically Relevant Pulmonary Carcinoid Groups and Unveil the Supra-Carcinoids. Nat. Commun. 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2021, 17, 362–387. [Google Scholar] [CrossRef]
- Rekhtman, N. Lung Neuroendocrine Neoplasms: Recent Progress and Persistent Challenges. Mod. Pathol. 2022, 35, 36–50. [Google Scholar] [CrossRef]
- Altieri, B.; Di Dato, C.; Martini, C.; Sciammarella, C.; Di Sarno, A.; Colao, A.; Faggiano, A.; Albertelli, M.; Ambrosetti, E.; Bianchi, A.; et al. Bone Metastases in Neuroendocrine Neoplasms: From Pathogenesis to Clinical Management. Cancers 2019, 11, 1332. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; O’Toole, D.; Falconi, M.; Gross, D.; Klöppel, G.; Sundin, A.; Ramage, J.; Öberg, K.; Wiedenmann, B.; Komminoth, P.; et al. ENETS Consensus Guidelines for the Management of Bone and Lung Metastases from Neuroendocrine Tumors. Neuroendocrinology 2010, 91, 341–350. [Google Scholar] [CrossRef]
- Robelin, P.; Hadoux, J.; Forestier, J.; Planchard, D.; Hervieu, V.; Berdelou, A.; Scoazec, J.Y.; Valette, P.J.; Leboulleux, S.; Ducreux, M.; et al. Characterization, Prognosis, and Treatment of Patients With Metastatic Lung Carcinoid Tumors. J. Thorac. Oncol. 2019, 14, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Gardner, N.; Kvols, L. Survival and Prognostic Factor Analysis of 146 Metastatic Neuroendocrine Tumors of the Mid-Gut. Neuroendocrinology 2009, 89, 471–476. [Google Scholar] [CrossRef]
- Peri, M.; Botteri, E.; Pisa, E.; De Marinis, F.; Ungaro, A.; Spada, F.; Grana, C.M.; Gasparri, R.; Spaggiari, S.; Romentz, N.; et al. A Single-Institution Retrospective Analysis of Metachronous and Synchronous Metastatic Bronchial Neuroendocrine Tumors. J. Thorac. Dis. 2018, 10, 3928–3939. [Google Scholar] [CrossRef]
- Modica, R.; Liccardi, A.; Minotta, R.; Cannavale, G.; Benevento, E.; Colao, A. Therapeutic Strategies for Patients with Neuroendocrine Neoplasms: Current Perspectives. Expert. Rev. Endocrinol. Metab. 2022, 17, 389–403. [Google Scholar] [CrossRef]
- Woodard, G.A.; Jones, K.D.; Jablons, D.M. Lung Cancer Staging and Prognosis. Cancer Treat. Res. 2016, 170, 47–75. [Google Scholar] [PubMed]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and Clarification: From the RECIST Committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- La Salvia, A.; Siciliani, A.; Rinzivillo, M.; Verrico, M.; Baldelli, R.; Puliani, G.; Modica, R.; Zanata, I.; Persano, I.; Fanciulli, G.; et al. Thyroid Transcription Factor-1 Expression in Lung Neuroendocrine Tumours: A Gender-Related Biomarker? Endocrine 2023, 83, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Di Dato, C.; Modica, R.; Bottiglieri, F.; Di Sarno, A.; Pittaway, J.F.H.; Martini, C.; Faggiano, A.; Colao, A. Bone Metabolism and Vitamin D Implication in Gastroenteropancreatic Neuroendocrine Tumors. Nutrients 2020, 12, 1021. [Google Scholar] [CrossRef]
- Garcia-Torralba, E.; Spada, F.; Lim, K.H.J.; Jacobs, T.; Barriuso, J.; Mansoor, W.; McNamara, M.G.; Hubner, R.A.; Manoharan, P.; Fazio, N.; et al. Knowns and Unknowns of Bone Metastases in Patients with Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Cancer Treat. Rev. 2021, 94, 102168. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The Epidemiology of Metastases in Neuroendocrine Tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Swiha, M.M.; Sutherland, D.E.K.; Sistani, G.; Khatami, A.; Abazid, R.M.; Mujoomdar, A.; Wiseman, D.P.; Romsa, J.G.; Reid, R.H.; Laidley, D.T. Survival Predictors of 177Lu-Dotatate Peptide Receptor Radionuclide Therapy (PRRT) in Patients with Progressive Well-Differentiated Neuroendocrine Tumors (NETS). J. Cancer Res. Clin. Oncol. 2021, 148, 225–236. [Google Scholar] [CrossRef]
- Lelièvre, M.; Triumbari, E.K.A.; Brixi, H.; Perrier, M.; Cadiot, G.; Deguelte, S.; Morland, D. Bone Metastases in Midgut Neuroendocrine Tumors: Imaging Characteristics, Distribution, and Risk Factors. Endocrine 2022, 78, 380–386. [Google Scholar] [CrossRef]
- Scopel, M.; De Carlo, E.; Bergamo, F.; Murgioni, S.; Carandina, R.; Cervino, A.R.; Burei, M.; Vianello, F.; Zagonel, V.; Fassan, M.; et al. Bone Metastases from Neuroendocrine Tumors: Clinical and Biological Considerations. Endocr. Connect. 2022, 11, e210568. [Google Scholar] [CrossRef]
- Simbolo, M.; Barbi, S.; Fassan, M.; Mafficini, A.; Ali, G.; Vicentini, C.; Sperandio, N.; Corbo, V.; Rusev, B.; Mastracci, L.; et al. Gene Expression Profiling of Lung Atypical Carcinoids and Large Cell Neuroendocrine Carcinomas Identifies Three Transcriptomic Subtypes with Specific Genomic Alterations. J. Thorac. Oncol. 2019, 14, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Capobianco, E.; McGaughey, V.; Seraphin, G.; Heckel, J.; Rieger, S.; Lisse, T.S. Vitamin D Inhibits Osteosarcoma by Reprogramming Nonsense-Mediated RNA Decay and SNAI2-Mediated Epithelial-to-Mesenchymal Transition. Front. Oncol. 2023, 13, 1188641. [Google Scholar] [CrossRef]
- La Salvia, A.; Persano, I.; Siciliani, A.; Verrico, M.; Bassi, M.; Modica, R.; Audisio, A.; Zanata, I.; Trabalza Marinucci, B.; Trevisi, E.; et al. Prognostic Significance of Laterality in Lung Neuroendocrine Tumors. Endocrine 2022, 76, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Liccardi, A.; Colao, A.; Modica, R. Gender Differences in Lung Neuroendocrine Tumors: A Single-Center Experience. Neuroendocrinology 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Sonzogni, A.; Harari, S.; Albini, A.; Bresaola, E.; Marchiò, C.; Massa, F.; Righi, L.; Gatti, G.; Papanikolaou, N.; et al. Classification of Pulmonary Neuroendocrine Tumors: New Insights. Transl. Lung Cancer Res. 2017, 6, 513–529. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Patients with L-NEN | Patients with L-NEN and BM |
|---|---|---|
| Number | 50 (100%) | 13 |
| Males: Females | 25:25 (50%; 50%) | 9:4 (69%; 31%) |
| Sporadic | 50 (100%) | 13 (100%) |
| Median age at diagnosis (years) | 55 (45–65) | 55 (50–65) |
| Smoking status: | ||
| Current smokers | 13 (26%) | 6 (46%) |
| Former smokers | 8 (16%) | 0 (0%) |
| Not smoker | 29 (58%) | 7 (54%) |
| Respiratory symptoms at diagnosis (cough; hemoptysis; dyspnea) | 21 (42%) | 6 (46%) |
| Bone pain at diagnosis | 4 (8%) | 1 (8%) |
| Diagnostic method of primary lesion or of BM: | ||
| CT | 30 (62%) | 2 (15%) |
| RX | 9 (18%) | 0 (0%) |
| MRI | 0 (0%) | 3 (23%) |
| Octreoscan | 1 (2%) | 0 (0%) |
| 68-Gallium-dotapeptide-PET | 0 (0%) | 5 (38%) |
| FDG-PET | 0 (0%) | 3 (23%) |
| Others | 9 (18%) | 0 (0%) |
| Primary lesion median size (mm) | 26 (15–40) | 39.5 (21.5–56.5) |
| Primary lesion position: | ||
| Right lung | 25 (50%) | 5 (38%) |
| Left lung | 18 (36%) | 4 (31%) |
| Bilateral | 7 (14%) | 4 (31%) |
| Histotype: | ||
| TC | 26 (52%) | 4 (31%) |
| AC | 18 (36%) | 7 (54%) |
| LCNEC | 4 (8%) | 1 (7%) |
| SCLC | 2 (4%) | 1 (7%) |
| Ki67 (median and range) | 5 (3–16) | 5 (3–31) |
| Functioning NEN | 7 (14%) | 1 (7%) |
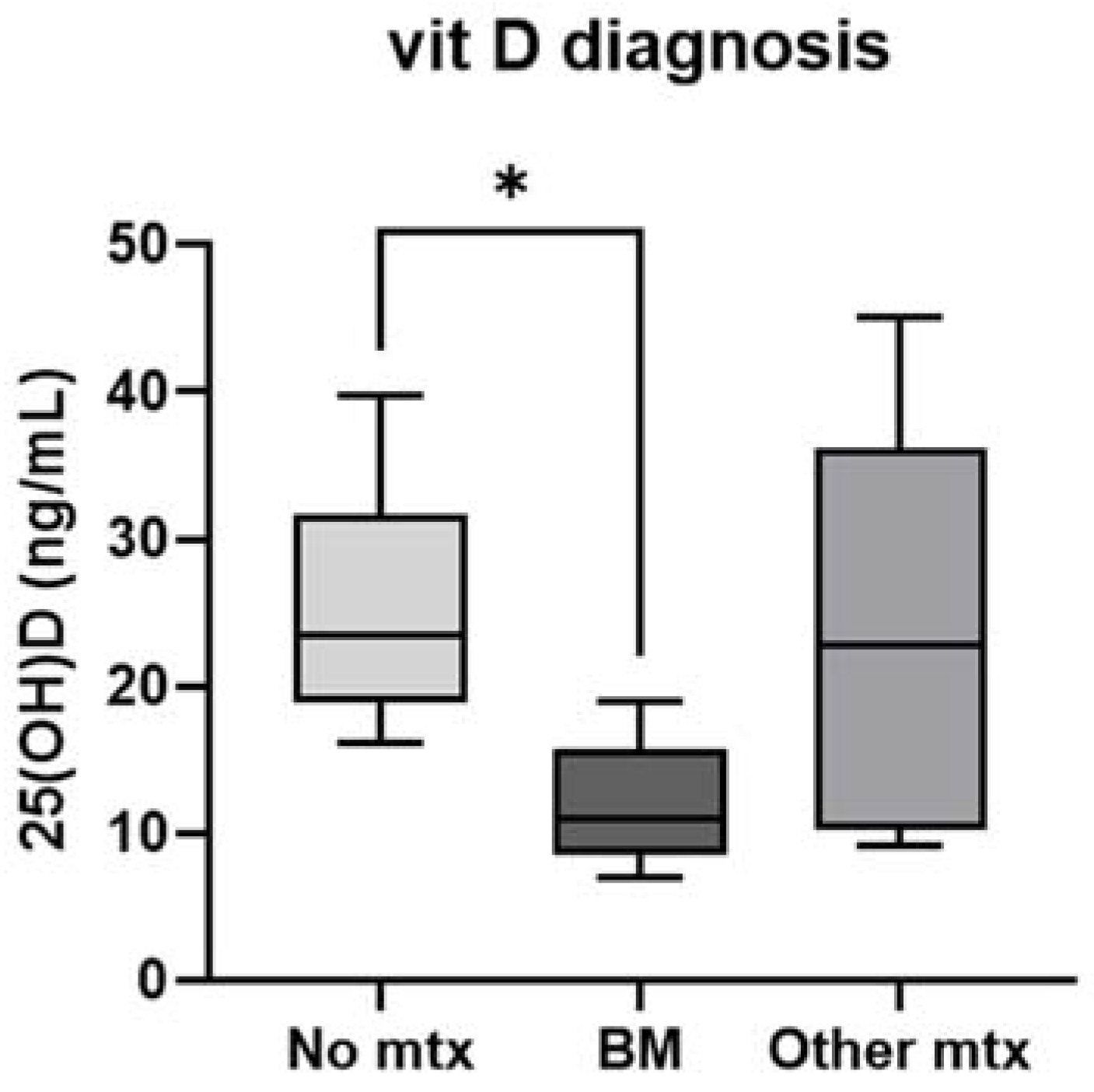
| Median 25OH vitamin D at diagnosis (ng/mL) | 21 (16.2–30.4) | 11 (8.5–15.7) |
| Stage at diagnosis | ||
| 1 | 18 (36%) | 2 (15%) |
| 2 | 11 (22%) | 1 (7%) |
| 3 | 4 (8%) | 1 (7%) |
| 4 | 17 (34%) | 9 (69%) |
| Metastatic site at diagnosis | ||
| Liver | 15 (30%) | 7 (54%) |
| Bone | 3 (6%) | 3 (23%) |
| Other sites | 2 (4%) | 5 (38%) |
| L-NEN treatment before BM: | ||
| Surgery | 29 (58%) | 5 (38%) |
| Endobronchial treatment | 1 (2%) | 0 (0%) |
| Somatostatin analogue (SSA) | 19 (38%) | 6 (46%) |
| Target therapy | 2 (4%) | 0 (0%) |
| Radioligand therapy (RLT) | 8 (16%) | 2 (15%) |
| Chemotherapy | 9 (18%) | 4 (31%) |
| Radiotherapy | 1 (2%) | 1 (7%) |
| Dead | 9 (18%) | 6 (46%) |
| Median follow-up (months) | 50 (19.25–121.25) | 47 (17–88) |
| BM characteristics | Patients with L-NEN and BM (13) | |
| Clinic: | ||
| Pain from bone metastases | 8 (61%) | |
| Fractures or compressions | 0 (0%) | |
| Hypercalcemia after metastases | 0 (0%) | |
| Localization: | ||
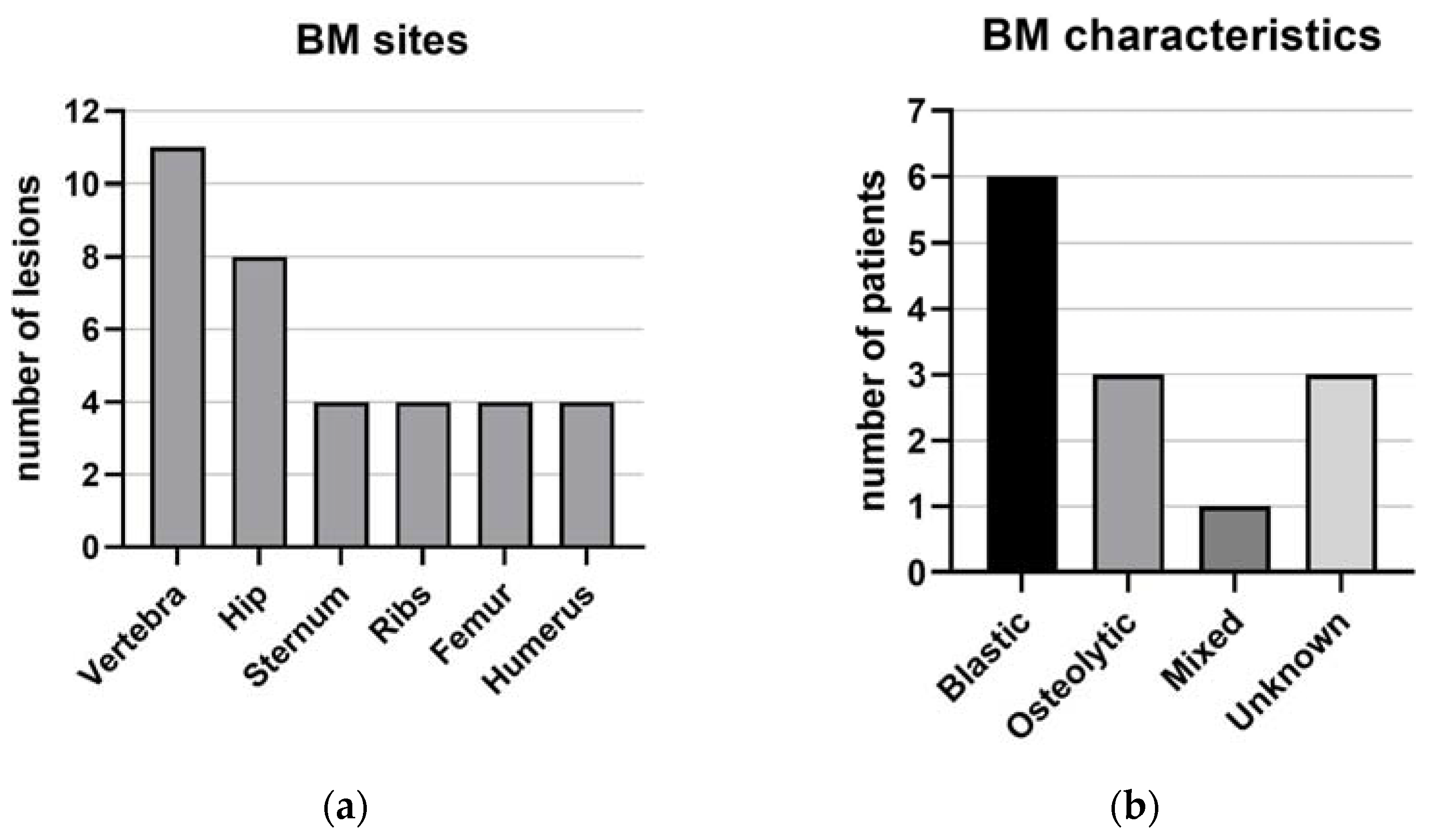
| Sternum | 4 (31%) | |
| Ribs | 4 (31%) | |
| Vertebrae | 11 (85%) | |
| Hip | 8 (61%) | |
| Femur | 4 (31%) | |
| Humerus | 4 (31%) | |
| Single bone metastases | 2 (15%) | |
| Multiple bone metastases | 11 (85%) | |
| Octreoscan/68-Gallium-Dotapeptide-PET: | ||
| Positive | 11 (85%) | |
| Negative | 1 (7%) | |
| Unknown | 1 (7%) | |
| Median SUV | 4.3 (4.15–43.15) | |
| FDG- PET: | ||
| Positive | 6 (46%) | |
| Negative | 4 (31%) | |
| Unknown | 3 (23%) | |
| Median SUV | 4.05 (3.6–4.7) | |
| BM classification: | ||
| Blastic bone lesions | 6 (46%) | |
| Osteolytic | 3 (23%) | |
| Mixed | 1 (7%) | |
| Unknown | 3 (23%) | |
| Treatment: | ||
| Zoledronic acid | 5 (38%) | |
| Denosumab | 3 (23%) | |
| Chronic use of opioid for pain therapy | 1 (7%) | |
| External radiotherapy | 1 (7%) | |
| Vertebroplasty | 0 (0%) | |
| Variables | No Mtx (n = 23) | BM (n = 13) | OtherMtx (n = 14) | p Value, x2 |
|---|---|---|---|---|
| Age, years | 46 (39–65) | 53 (47–67) | 59 (53–65) | 0.31 |
| Sex: | 0.27, 2.60 | |||
| M | 10 (43.5%) | 9 (69.2%) | 6 (42.9%) | |
| F | 13 (56.5%) | 4 (30.8%) | 8 (57.1%) | |
| BMI, Kg/m2 | 28.2 (23.7–31.3) | 27.3 (23.3–30.7) | 25.1 (21.2–28.5) | 0.26 |
| Smoking status: | 0.11, 7.40 | |||
| Never smoked | 17 (73.9%) | 4 (30.8%) | 6 (42.9%) | |
| Current smoker | 3 (13.0%) | 5 (38.5%) | 5 (35.7%) | |
| Ex-smoker | 3 (13.0%) | 4 (30.8%) | 3 (21.4%) | |
| 25(OH)D levels, ng/mL | 23.4 (18.8–31.6) | 11.0 (8.5–15.7) | 22.8 (10.3–36.2) | 0.02 |
| Vitamin D supplementation: | 0.38, 1.95 | |||
| Yes | 3 (13.0%) | 4 (30.8%) | 2 (14.3%) | |
| No | 20 (87.0%) | 9 (69.2%) | 12 (85.7%) | |
| Tumor size, mm | 17.0 (10.0–48.0) | 39.5 (21.5–56.5) | 24.5 (20.0–35.2) | 0.21 |
| Number of primary lung lesions: | 0.35, 2.09 | |||
| Single | 16 (69.6%) | 6 (46.2%) | 7 (53.8%) | |
| Multiple | 7 (30.4%) | 7 (53.8%) | 6 (46.2%) | |
| Ki67% | 4 (1–7) | 5 (3–31) | 11 (5–49) | 0.008 |
| Histology: | 0.03, 10.37 | |||
| Typical | 16 (69.6%) | 4 (30.8%) | 6 (42.9%) | |
| Atypical | 7 (30.4%) | 7 (53.8%) | 4 (28.6%) | |
| Carcinoma | 0 | 2 (15.4%) | 4 (28.6%) | |
| Tumor stage: | <0.001, 25.15 | |||
| I A–B | 16 (69.6%) | 2 (15.4%) | 2 (14.3%) | |
| II A–B | 5 (21.7%) | 1 (7.7%) | 3 (21.4%) | |
| III A | 2 (8.7%) | 1 (7.7%) | 1 (7.1%) | |
| IV | 0 | 9 (69.2%) | 8 (57.1%) |
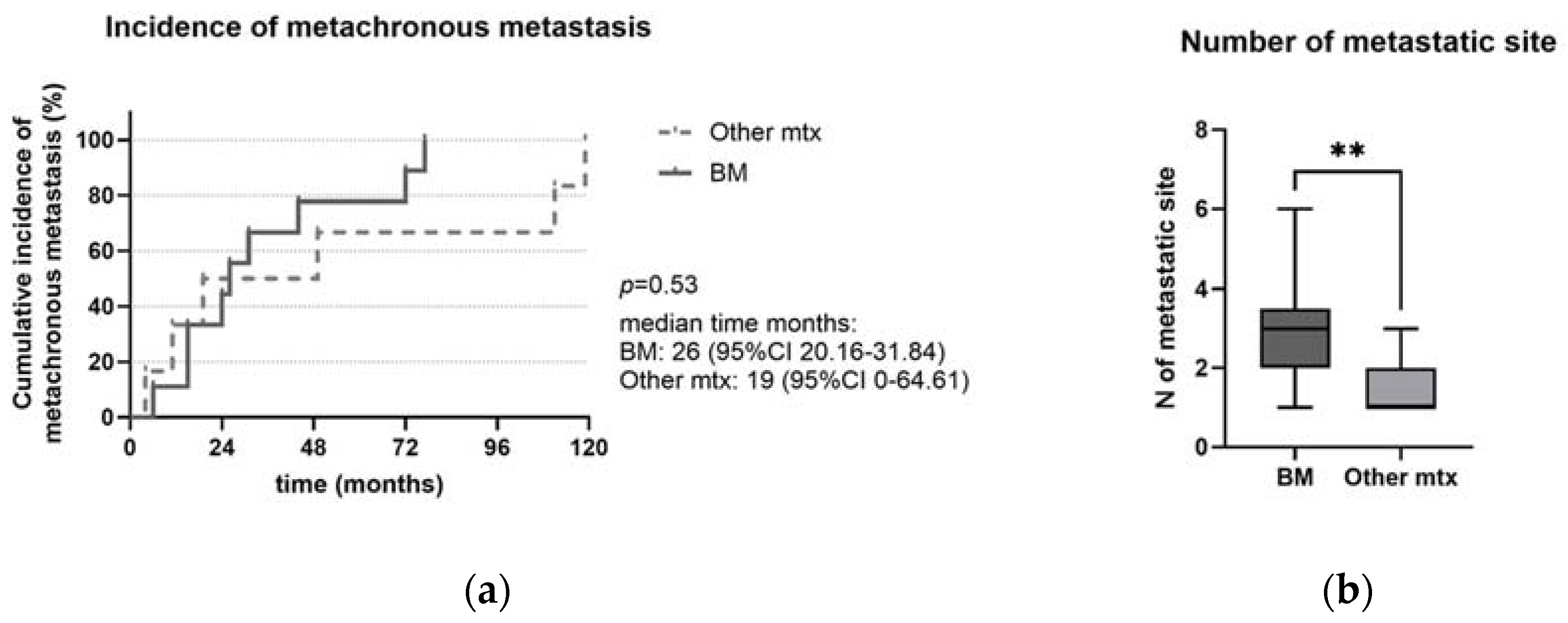
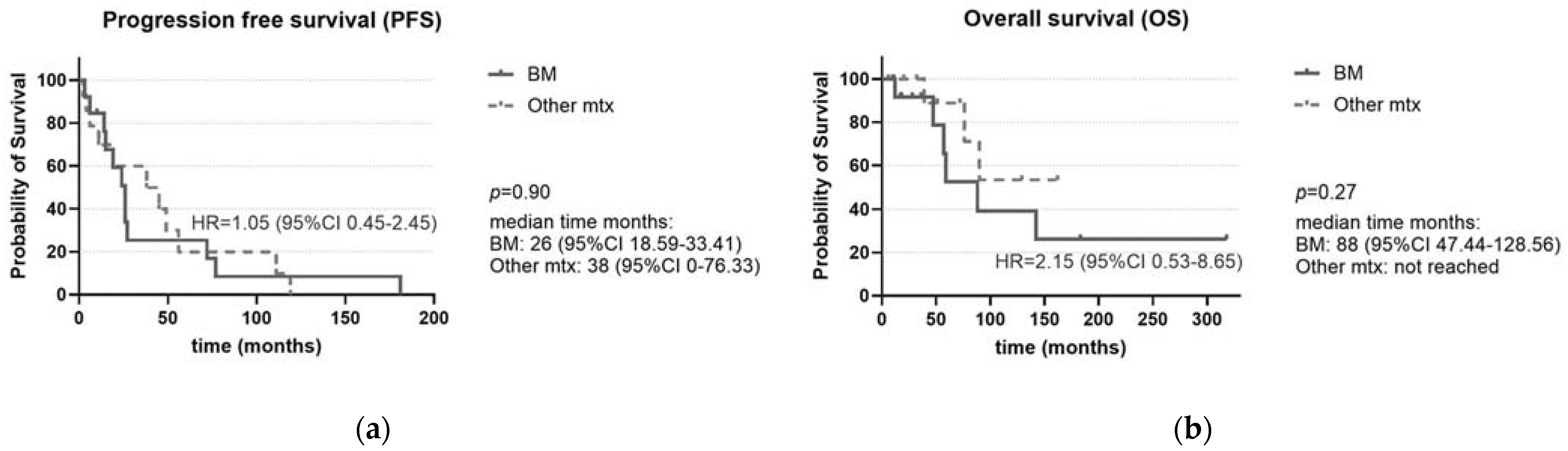
| (A) Progression-Free Survival (PFS) | |||||
|---|---|---|---|---|---|
| Variable | n Patients | Median Time (Months) | HR | 95% CI | p |
| |||||
| 14 | 38 | 1 | ||
| 13 | 26 | 1.05 | 0.45–2.45 | 0.90 |
| |||||
| 15 | 26 | 1 | ||
| 12 | 45 | 0.48 | 0.18–1.26 | 0.12 |
| |||||
| 10 | 38 | 1 | ||
| 11 | 26 | 1.30 | 0.52–3.25 | |
| 6 | 14 | 4.01 | 0.79–20.30 | 0.20 |
| |||||
| 10 | 27 | 1 | ||
| 17 | 26 | 2.12 | 0.82–5.49 | 0.11 |
| |||||
| 9 | 26 | 1 | ||
| 5 | 49 | 1.06 | 0.34–3.33 | |
| 10 | 14 | 1.40 | 0.50–3.95 | 0.80 |
| (B) Overall Survival (OS) | |||||
| Variable | nPatients | Median Time (Months) | HR | 95% CI | p |
| |||||
| 14 | 38 | 1 | ||
| 13 | 26 | 2.15 | 0.53–8.65 | 0.27 |
| |||||
| 15 | 76 | 1 | ||
| 12 | Not reached | 0.25 | 0.05–1.24 | 0.07 |
| |||||
| 10 | 90 | 1 | ||
| 11 | 59 | 2.28 | 0.54–9-69 | |
| 6 | Not reached | not evaluable | 0.02 | |
| |||||
| 10 | Not reached | 1 | ||
| 17 | 59 | 5.77 | 1.14–29.19 | 0.02 |
| |||||
| 9 | 88 | 1 | ||
| 5 | Not reached | 0.49 | 0.05–4.62 | |
| 10 | 59 | 2.06 | 0.40–10.49 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modica, R.; Benevento, E.; Altieri, B.; Minotta, R.; Liccardi, A.; Cannavale, G.; Di Iasi, G.; Colao, A. Role of Bone Metastases in Lung Neuroendocrine Neoplasms: Clinical Presentation, Treatment and Impact on Prognosis. Int. J. Mol. Sci. 2024, 25, 8957. https://doi.org/10.3390/ijms25168957
Modica R, Benevento E, Altieri B, Minotta R, Liccardi A, Cannavale G, Di Iasi G, Colao A. Role of Bone Metastases in Lung Neuroendocrine Neoplasms: Clinical Presentation, Treatment and Impact on Prognosis. International Journal of Molecular Sciences. 2024; 25(16):8957. https://doi.org/10.3390/ijms25168957
Chicago/Turabian StyleModica, Roberta, Elio Benevento, Barbara Altieri, Roberto Minotta, Alessia Liccardi, Giuseppe Cannavale, Gianfranco Di Iasi, and Annamaria Colao. 2024. "Role of Bone Metastases in Lung Neuroendocrine Neoplasms: Clinical Presentation, Treatment and Impact on Prognosis" International Journal of Molecular Sciences 25, no. 16: 8957. https://doi.org/10.3390/ijms25168957
APA StyleModica, R., Benevento, E., Altieri, B., Minotta, R., Liccardi, A., Cannavale, G., Di Iasi, G., & Colao, A. (2024). Role of Bone Metastases in Lung Neuroendocrine Neoplasms: Clinical Presentation, Treatment and Impact on Prognosis. International Journal of Molecular Sciences, 25(16), 8957. https://doi.org/10.3390/ijms25168957







