The Other Side of the Perfect Cup: Coffee-Derived Non-Polyphenols and Their Roles in Mitigating Factors Affecting the Pathogenesis of Type 2 Diabetes
Abstract
:1. Introduction
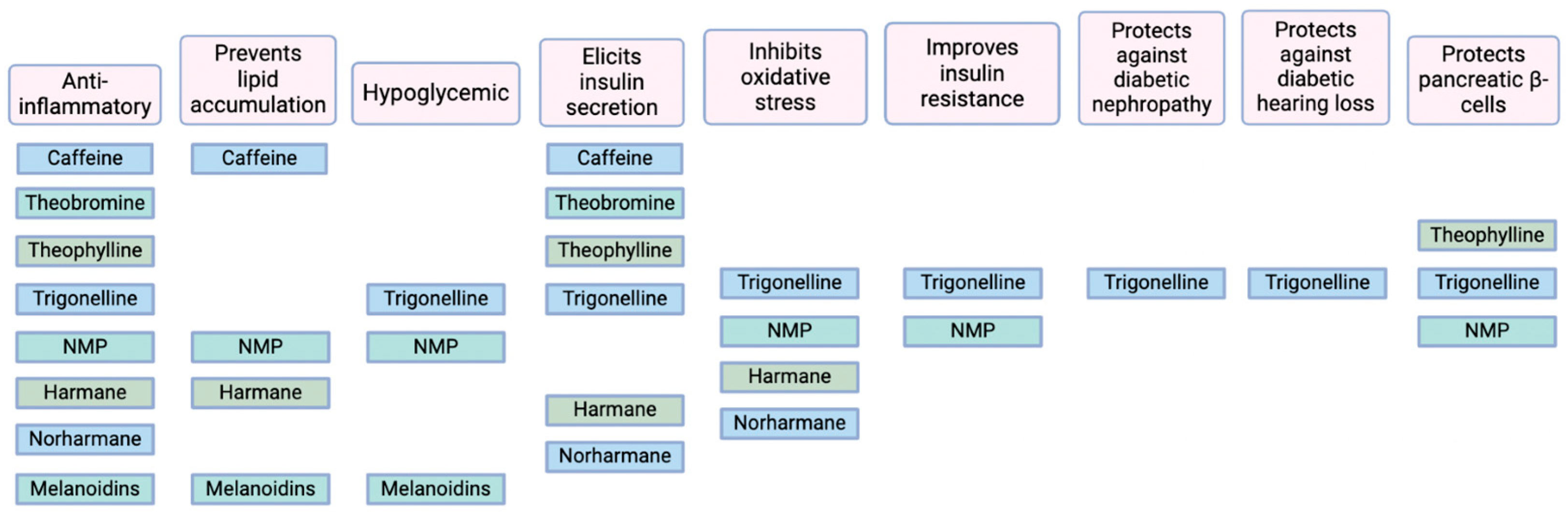
2. Components of T2D Pathogenesis That Are Affected by Coffee-Derived Non-Polyphenols
2.1. Inflammation and Obesity
2.2. Oxidative Stress
3. Purine Alkaloids
3.1. Overview
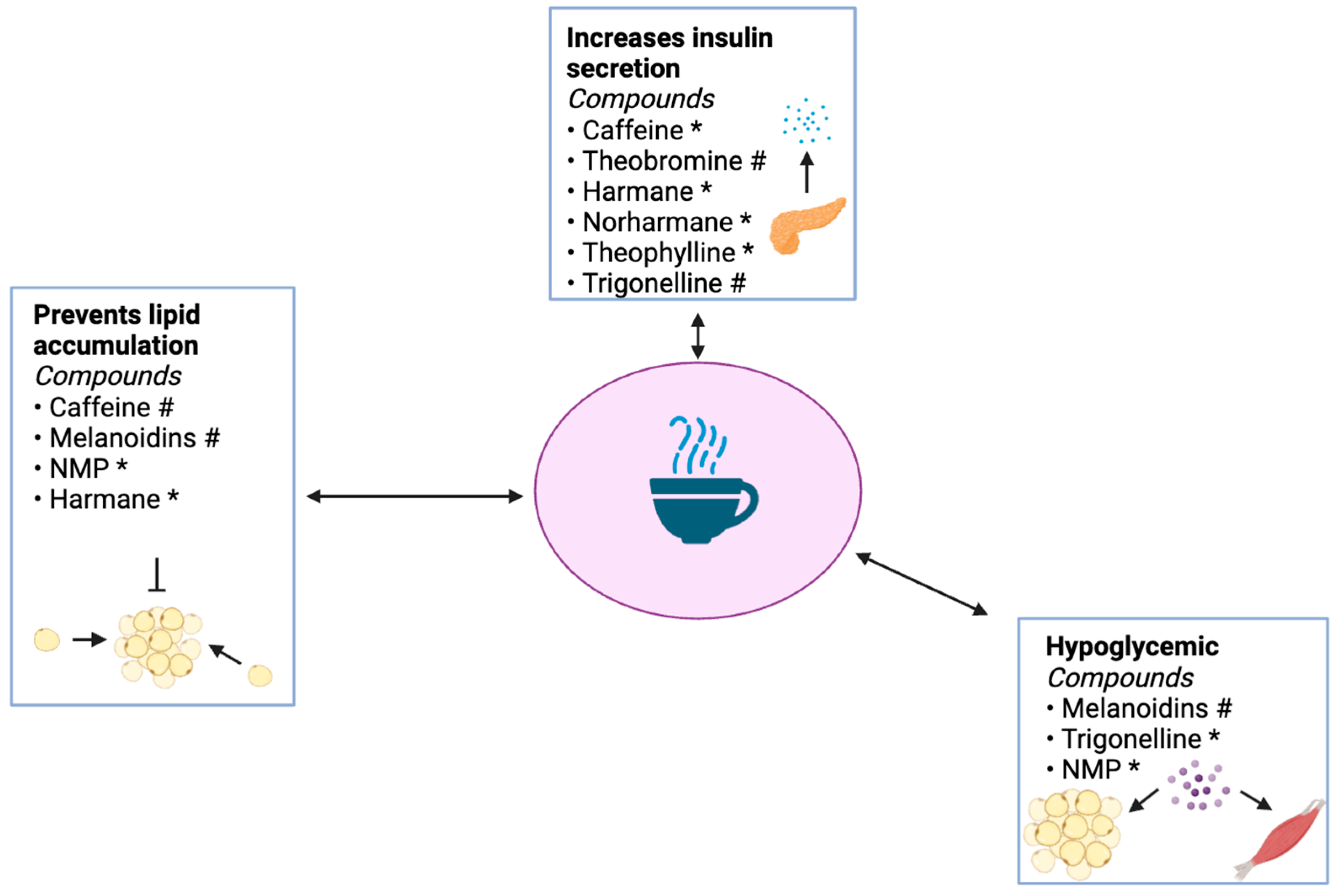
3.2. Caffeine and Its Metabolites
3.3. Absorption and Metabolism of Caffeine and Its Metabolites
3.4. Antidiabetic Effects of Caffeine
3.4.1. Effects of Theobromine on T2D Pathogenesis
3.4.2. Effects of Theophylline on T2D Pathogenesis
4. Pyridine Alkaloids
4.1. Overview
4.2. Absorption and Metabolism of Trigonelline and Its Degradation Product N-Methylpyridinium
4.3. Antidiabetic Effects of Trigonelline
Antidiabetic Effects of NMP
4.4. Harmane and Norharmane
4.4.1. Absorption and Metabolism of Harmane and Norharmane
4.4.2. Antidiabetic Effects of Harmane and Norharmane
5. Polysaccharides
5.1. Melanoidins
5.2. Absorption and Metabolism of Melanoidins
5.3. Antidiabetic Effects of Melanoidins
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diabetes Quick Facts|Basics|Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/about/index.html (accessed on 7 February 2024).
- Diabetes—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/diabetes (accessed on 7 February 2024).
- CDC Newsroom. Available online: https://www.cdc.gov/media/releases/2022/p1229-future-diabetes-surge.html (accessed on 7 February 2024).
- National Diabetes Statistics Report|Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 7 February 2024).
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Hudish, L.I.; Reusch, J.E.B.; Sussel, L. β Cell Dysfunction during Progression of Metabolic Syndrome to Type 2 Diabetes. J. Clin. Investig. 2019, 129, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Welsh, N.; Jonas, J.-C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of Pancreatic β-Cell Death in Type 1 and Type 2 Diabetes: Many Differences, Few Similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Diagnosis & Tests|ADA. Available online: https://diabetes.org/about-diabetes/diagnosis (accessed on 11 February 2024).
- Farmaki, P.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Savvanis, S.; Diamantis, E. Complications of the Type 2 Diabetes Mellitus. Curr. Cardiol. Rev. 2020, 16, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Lower Your Risk of Diabetes Complications|ADA. Available online: https://diabetes.org/about-diabetes/complications (accessed on 11 February 2024).
- Kolb, H.; Martin, S.; Kempf, K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef]
- Pereira, M.A.; Parker, E.D.; Folsom, A.R. Coffee Consumption and Risk of Type 2 Diabetes Mellitus: An 11-Year Prospective Study of 28 812 Postmenopausal Women. Arch. Intern. Med. 2006, 166, 1311–1316. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and Decaffeinated Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review and a Dose-Response Meta-Analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Zemdegs, J.C.; Theodoro, J.A.; Mota, J.F. Does Long-Term Coffee Intake Reduce Type 2 Diabetes Mellitus Risk? Diabetol. Metab. Syndr. 2009, 1, 6. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Chapple, B.; Woodfin, S.; Moore, W. The Perfect Cup? Coffee-Derived Polyphenols and Their Roles in Mitigating Factors Affecting Type 2 Diabetes Pathogenesis. Molecules 2024, 29, 751. [Google Scholar] [CrossRef]
- Antonietti, S.; Silva, A.M.; Simões, C.; Almeida, D.; Félix, L.M.; Papetti, A.; Nunes, F.M. Chemical Composition and Potential Biological Activity of Melanoidins From Instant Soluble Coffee and Instant Soluble Barley: A Comparative Study. Front. Nutr. 2022, 9, 825584. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a Potential Functional Food Ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Andrei, A.M.; Berbecaru-Iovan, A.; Din-Anghel, F.R.I.; Stănciulescu, C.E.; Berbecaru-Iovan, S.; Baniţă, I.M.; Pisoschi, C.G.; Andrei, A.M.; Berbecaru-Iovan, A.; Din-Anghel, F.R.I.; et al. Interplay between Hypoxia, Inflammation and Adipocyte Remodeling in the Metabolic Syndrome. In Hypoxia and Human Diseases; IntechOpen: London, UK, 2017; ISBN 978-953-51-2896-0. [Google Scholar]
- Corvera, S.; Gealekman, O. Adipose Tissue Angiogenesis: Impact on Obesity and Type-2 Diabetes. Biochim. Biophys. Acta 2014, 1842, 463–472. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose Tissue Macrophages as Potential Targets for Obesity and Metabolic Diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef] [PubMed]
- Surmi, B.K.; Hasty, A.H. Macrophage Infiltration into Adipose Tissue. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor Necrosis Factor Alpha Inhibits Signaling from the Insulin Receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Papavassiliou, A.G. Serine Phosphorylation of Insulin Receptor Substrate-1: A Novel Target for the Reversal of Insulin Resistance. Mol. Endocrinol. 2001, 15, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Su, C.-M.; Wang, L.; Yoo, D. Activation of NF-κB and Induction of Proinflammatory Cytokine Expressions Mediated by ORF7a Protein of SARS-CoV-2. Sci. Rep. 2021, 11, 13464. [Google Scholar] [CrossRef] [PubMed]
- Jilani, T.N.; Preuss, C.V.; Sharma, S. Theophylline. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kobashi, C.; Asamizu, S.; Ishiki, M.; Iwata, M.; Usui, I.; Yamazaki, K.; Tobe, K.; Kobayashi, M.; Urakaze, M. Inhibitory Effect of IL-8 on Insulin Action in Human Adipocytes via MAP Kinase Pathway. J. Inflamm. 2009, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Meniailo, M.E.; Malashchenko, V.V.; Shmarov, V.A.; Gazatova, N.D.; Melashchenko, O.B.; Goncharov, A.G.; Seledtsova, G.V.; Seledtsov, V.I. Interleukin-8 Favors pro-Inflammatory Activity of Human Monocytes/Macrophages. Int. Immunopharmacol. 2018, 56, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Pineda, W.D.; Parra-Rojas, I.; Rodríguez-Ruíz, H.A.; Illades-Aguiar, B.; Matia-García, I.; Garibay-Cerdenares, O.L. The Regulatory Role of Insulin in Energy Metabolism and Leukocyte Functions. J. Leukoc. Biol. 2022, 111, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of Monocyte Chemoattractant Protein-1 in Adipose Tissues Causes Macrophage Recruitment and Insulin Resistance*. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Imani, S.; Tao, L.; Deng, Y.; Cai, Y. Natural Killer Cells: Friend or Foe in Metabolic Diseases? Front. Immunol. 2021, 12, 614429. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Qi, Y.; Yi, H.; Mao, C.; Meng, Q.; Wang, H.; Zheng, C. The Roles of Adipose Tissue Macrophages in Human Disease. Front. Immunol. 2022, 13, 908749. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- McGillicuddy, F.C.; Chiquoine, E.H.; Hinkle, C.C.; Kim, R.J.; Shah, R.; Roche, H.M.; Smyth, E.M.; Reilly, M.P. Interferon γ Attenuates Insulin Signaling, Lipid Storage, and Differentiation in Human Adipocytes via Activation of the JAK/STAT Pathway. J. Biol. Chem. 2009, 284, 31936–31944. [Google Scholar] [CrossRef]
- Dong, R.; Xue, Z.; Fan, G.; Zhang, N.; Wang, C.; Li, G.; Da, Y. Pin1 Promotes NLRP3 Inflammasome Activation by Phosphorylation of P38 MAPK Pathway in Septic Shock. Front. Immunol. 2021, 12, 620238. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-Induced Beta Cell Production of IL-1beta Contributes to Glucotoxicity in Human Pancreatic Islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Yolcu, E.S.; Shirwan, H.; Askenasy, N. Fas/Fas-Ligand Interaction As a Mechanism of Immune Homeostasis and β-Cell Cytotoxicity: Enforcement Rather Than Neutralization for Treatment of Type 1 Diabetes. Front. Immunol. 2017, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.J.; Stadler, K.; Lu, D.; Gleason, E.; Han, A.; Donohoe, D.R.; Rogers, R.C.; Hermann, G.E.; Karlstad, M.D.; Collier, J.J. IL-1β Reciprocally Regulates Chemokine and Insulin Secretion in Pancreatic β-Cells via NF-κB. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E715–E726. [Google Scholar] [CrossRef] [PubMed]
- Mourad, N.I.; Nenquin, M.; Henquin, J.-C. cAMP-Mediated and Metabolic Amplification of Insulin Secretion Are Distinct Pathways Sharing Independence of β-Cell Microfilaments. Endocrinology 2012, 153, 4644–4654. [Google Scholar] [CrossRef] [PubMed]
- Pua, L.J.W.; Mai, C.-W.; Chung, F.F.-L.; Khoo, A.S.-B.; Leong, C.-O.; Lim, W.-M.; Hii, L.-W. Functional Roles of JNK and P38 MAPK Signaling in Nasopharyngeal Carcinoma. Int. J. Mol. Sci. 2022, 23, 1108. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kuma, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative Stress in the Pathophysiology of Type 2 Diabetes and Related Complications: Current Therapeutics Strategies and Future Perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of Oxidant Generation by Catalase. Ann. N. Y. Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Bakala, H.; Hamelin, M.; Mary, J.; Borot-Laloi, C.; Friguet, B. Catalase, a Target of Glycation Damage in Rat Liver Mitochondria with Aging. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Moritz, J.T.; Epstein, P.N. Overexpression of Catalase Provides Partial Protection to Transgenic Mouse Beta Cells. Free Radic. Biol. Med. 1999, 27, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Torii, S.; Saito, N.; Hosaka, M.; Takeuchi, T. Reactive Oxygen Species-Mediated Pancreatic β-Cell Death Is Regulated by Interactions between Stress-Activated Protein Kinases, P38 and c-Jun N-Terminal Kinase, and Mitogen-Activated Protein Kinase Phosphatases. Endocrinology 2008, 149, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Triantaphylidès, C.; Krischke, M.; Hoeberichts, F.A.; Ksas, B.; Gresser, G.; Havaux, M.; Van Breusegem, F.; Mueller, M.J. Singlet Oxygen Is the Major Reactive Oxygen Species Involved in Photooxidative Damage to Plants. Plant Physiol. 2008, 148, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. The Contribution of Singlet Oxygen to Insulin Resistance. Oxid. Med. Cell. Longev. 2017, 2017, 8765972. [Google Scholar] [CrossRef] [PubMed]
- Čolak, E.; Žorić, L. 6—Antioxidants and Age-Related Macular Degeneration. In Handbook of Nutrition, Diet, and the Eye, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 85–106. ISBN 978-0-12-815245-4. [Google Scholar]
- Madi, M.; Babu, S.; Kumari, S.; Shetty, S.; Achalli, S.; Madiyal, A.; Bhat, M. Status of Serum and Salivary Levels of Superoxide Dismutase in Type 2 Diabetes Mellitus with Oral Manifestations: A Case Control Study. Ethiop. J. Health Sci. 2016, 26, 523–532. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chuang, L.-M. The Role of Oxidative Stress in the Pathogenesis of Type 2 Diabetes: From Molecular Mechanism to Clinical Implication. Am. J. Transl. Res. 2010, 2, 316–331. [Google Scholar]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; McFarlane-Anderson, N.; Gordon-Strachan, G.M.; Wright-Pascoe, R.A.; Jahoor, F.; Boyne, M.S. Glutathione Metabolism in Type-2 Diabetes and Its Relationship with Microvascular Complications and Glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-L.; Hou, Y.-C. Chapter 12—Glutamine and Antioxidant Potential in Diabetes. In Diabetes: Oxidative Stress and Dietary Antioxidants; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 121–128. ISBN 978-0-12-405885-9. [Google Scholar]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.; Klyosova, E.; Polonikov, A. The Link between Type 2 Diabetes Mellitus and the Polymorphisms of Glutathione-Metabolizing Genes Suggests a New Hypothesis Explaining Disease Initiation and Progression. Life 2021, 11, 886. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological activities and toxicities of alkaloids on human health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar] [CrossRef]
- Kurek, J. Introductory Chapter: Alkaloids—Their Importance in Nature and for Human Life. In Alkaloids—Their Importance in Nature and Human Life; IntechOpen: London, UK, 2019; ISBN 978-1-78984-577-8. [Google Scholar]
- Ashihara, H. Biosynthetic Pathways of Purine and Pyridine Alkaloids in Coffee Plants. Nat. Prod. Commun. 2016, 11, 1047–1054. [Google Scholar] [CrossRef]
- Nuhu, A.A. Bioactive Micronutrients in Coffee: Recent Analytical Approaches for Characterization and Quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xue, W.; Liang, S.; Zhao, J.; Zhang, X. Acute Caffeine Ingestion Reduces Insulin Sensitivity in Healthy Subjects: A Systematic Review and Meta-Analysis. Nutr. J. 2016, 15, 103. [Google Scholar] [CrossRef]
- How Much Caffeine Is in Your Cup? Available online: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/caffeine/art-20049372 (accessed on 13 February 2024).
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of Various Factors on Caffeine Content in Coffee Brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef]
- Makiso, M.U.; Tola, Y.B.; Ogah, O.; Endale, F.L. Bioactive Compounds in Coffee and Their Role in Lowering the Risk of Major Public Health Consequences: A Review. Food Sci. Nutr. 2024, 12, 734–764. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K. Bioactive Phytocomponents and Their Analysis. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–328. ISBN 978-0-12-813374-3. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Coffee, Tea, Mate, Methylxanthines and Methylglyoxal; International Agency for Research on Cancer: Lyon, France, 1991. [Google Scholar]
- Gonzales-Yépez, K.A.; Vilela, J.L.; Reátegui, O. Determination of Caffeine, Theobromine, and Theophylline by HPLC-DAD in Beverages Commonly Consumed in Lima, Peru. Int. J. Food Sci. 2023, 2023, 4323645. [Google Scholar] [CrossRef] [PubMed]
- Júnior, P.C.G.; dos Santos, V.B.; Lopes, A.S.; de Souza, J.P.I.; Pina, J.R.S.; Chagas Júnior, G.C.A.; Marinho, P.S.B. Determination of Theobromine and Caffeine in Fermented and Unfermented Amazonian Cocoa (Theobroma cacao L.) Beans Using Square Wave Voltammetry after Chromatographic Separation. Food Control. 2020, 108, 106887. [Google Scholar] [CrossRef]
- Yang, Y.; Darwish, A.G.; El-Sharkawy, I.; Zhu, Q.; Sun, S.; Tan, J. Rapid Determination of the Roasting Degree of Cocoa Beans by Extreme Learning Machine (ELM)-Based Imaging Analysis. J. Agric. Food Res. 2022, 10, 100437. [Google Scholar] [CrossRef]
- Kumar, M.; Chatterjee, J.; Rani, D.; Kumar, R. Chapter 5—FDA Approved Five-Membered Ring Fused Pyrimidine-Based Derivatives and Their Biological Properties. In Fused Pyrimidine-Based Drug Discovery; Kumar, R., Vardanyan, R., Eds.; Heterocyclic Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 117–164. ISBN 978-0-443-18616-5. [Google Scholar]
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747. [Google Scholar] [CrossRef] [PubMed]
- Carrey, E.A.; Perrett, D.; Simmonds, H.A. Nucleic Acids, Purine, and Pyrimidine Nucleotides and Nucleosides: Physiology, Toxicology, and Dietary Sources. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 189–196. ISBN 978-0-12-384885-7. [Google Scholar]
- Jeszka-Skowron, M.; Frankowski, R.; Zgoła-Grześkowiak, A. Comparison of Methylxantines, Trigonelline, Nicotinic Acid and Nicotinamide Contents in Brews of Green and Processed Arabica and Robusta Coffee Beans—Influence of Steaming, Decaffeination and Roasting Processes on Coffee Beans. LWT 2020, 125, 109344. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Military Nutrition Research. Pharmacology of Caffeine. In Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- dePaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Lee, S.; Min, J.; Min, K. Caffeine and Caffeine Metabolites in Relation to Insulin Resistance and Beta Cell Function in U.S. Adults. Nutrients 2020, 12, 1783. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Jayasooriya, R.G.P.T.; Dilshara, M.G.; Choi, Y.H.; Jeong, Y.-K.; Kim, N.D.; Kim, G.-Y. Caffeine Suppresses Lipopolysaccharide-Stimulated BV2 Microglial Cells by Suppressing Akt-Mediated NF-κB Activation and ERK Phosphorylation. Food Chem. Toxicol. 2012, 50, 4270–4276. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Vargas-Pozada, E.E.; Ramos-Tovar, E.; Rodriguez-Callejas, J.D.; Cardoso-Lezama, I.; Galindo-Gómez, S.; Talamás-Lara, D.; Vásquez-Garzón, V.R.; Arellanes-Robledo, J.; Tsutsumi, V.; Villa-Treviño, S.; et al. Caffeine Inhibits NLRP3 Inflammasome Activation by Downregulating TLR4/MAPK/NF-κB Signaling Pathway in an Experimental NASH Model. Int. J. Mol. Sci. 2022, 23, 9954. [Google Scholar] [CrossRef]
- Karcz-Kubicha, M.; Antoniou, K.; Terasmaa, A.; Quarta, D.; Solinas, M.; Justinova, Z.; Pezzola, A.; Reggio, R.; Müller, C.E.; Fuxe, K.; et al. Involvement of Adenosine A1 and A2A Receptors in the Motor Effects of Caffeine after Its Acute and Chronic Administration. Neuropsychopharmacology 2003, 28, 1281–1291. [Google Scholar] [CrossRef]
- Pasquini, S.; Contri, C.; Borea, P.A.; Vincenzi, F.; Varani, K. Adenosine and Inflammation: Here, There and Everywhere. Int. J. Mol. Sci. 2021, 22, 7685. [Google Scholar] [CrossRef]
- Moon, S.-M.; Joo, M.-J.; Lee, Y.-S.; Kim, M.-G. Effects of Coffee Consumption on Insulin Resistance and Sensitivity: A Meta-Analysis. Nutrients 2021, 13, 3976. [Google Scholar] [CrossRef]
- Reis, C.E.G.; Dórea, J.G.; da Costa, T.H.M. Effects of Coffee Consumption on Glucose Metabolism: A Systematic Review of Clinical Trials. J. Tradit. Complement. Med. 2018, 9, 184–191. [Google Scholar] [CrossRef]
- Sanni, O.; Terre’Blanche, G. Dual A1 and A2A Adenosine Receptor Antagonists, Methoxy Substituted 2-Benzylidene-1-Indanone, Suppresses Intestinal Postprandial Glucose and Attenuates Hyperglycaemia in Fructose-Streptozotocin Diabetic Rats. BMC Endocr. Disord. 2023, 23, 97. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Ravid, K. Adenosine, Adenosine Receptors and Their Role in Glucose Homeostasis and Lipid Metabolism. J. Cell. Physiol. 2013, 228, 1703–1712. [Google Scholar] [CrossRef]
- Lee, S.; Hudson, R.; Kilpatrick, K.; Graham, T.E.; Ross, R. Caffeine Ingestion Is Associated with Reductions in Glucose Uptake Independent of Obesity and Type 2 Diabetes before and after Exercise Training. Diabetes Care 2005, 28, 566–572. [Google Scholar] [CrossRef]
- Rathmell, J.C.; Fox, C.J.; Plas, D.R.; Hammerman, P.S.; Cinalli, R.M.; Thompson, C.B. Akt-Directed Glucose Metabolism Can Prevent Bax Conformation Change and Promote Growth Factor-Independent Survival. Mol. Cell. Biol. 2003, 23, 7315–7328. [Google Scholar] [CrossRef]
- Ide, T.; Shimano, H.; Yahagi, N.; Matsuzaka, T.; Nakakuki, M.; Yamamoto, T.; Nakagawa, Y.; Takahashi, A.; Suzuki, H.; Sone, H.; et al. SREBPs Suppress IRS-2-Mediated Insulin Signaling in the Liver. Nat. Cell Biol. 2004, 6, 351–357. [Google Scholar] [CrossRef]
- Alperet, D.J.; Rebello, S.A.; Khoo, E.Y.-H.; Tay, Z.; Seah, S.S.-Y.; Tai, B.-C.; Tai, E.-S.; Emady-Azar, S.; Chou, C.J.; Darimont, C.; et al. The Effect of Coffee Consumption on Insulin Sensitivity and Other Biological Risk Factors for Type 2 Diabetes: A Randomized Placebo-Controlled Trial. Am. J. Clin. Nutr. 2020, 111, 448–458. [Google Scholar] [CrossRef]
- Ohnaka, K.; Ikeda, M.; Maki, T.; Okada, T.; Shimazoe, T.; Adachi, M.; Nomura, M.; Takayanagi, R.; Kono, S. Effects of 16-Week Consumption of Caffeinated and Decaffeinated Instant Coffee on Glucose Metabolism in a Randomized Controlled Trial. J. Nutr. Metab. 2012, 2012, e207426. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee Acutely Modifies Gastrointestinal Hormone Secretion and Glucose Tolerance in Humans: Glycemic Effects of Chlorogenic Acid and Caffeine2. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Yusni, Y.; Yusuf, H. The Acute Effects of Coffee Consumption on Blood Glucose and It’s Relationship with Serum Cortisol and Insulin in Females. Pharmacia 2022, 69, 903–910. [Google Scholar] [CrossRef]
- Marcus, G.M.; Rosenthal, D.G.; Nah, G.; Vittinghoff, E.; Fang, C.; Ogomori, K.; Joyce, S.; Yilmaz, D.; Yang, V.; Kessedjian, T.; et al. Acute Effects of Coffee Consumption on Health among Ambulatory Adults. N. Engl. J. Med. 2023, 388, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Maratos-Flier, E.; Flier, J.S. Reduced Adiposity and High-Fat Diet-Induced Adipose Inflammation in Mice Deficient for Phosphodiesterase 4B. Endocrinology 2009, 150, 3076–3082. [Google Scholar] [CrossRef]
- Gu, R.; Shi, Y.; Huang, W.; Lao, C.; Zou, Z.; Pan, S.; Huang, Z. Theobromine Mitigates IL-1β-Induced Oxidative Stress, Inflammatory Response, and Degradation of Type II Collagen in Human Chondrocytes. Int. Immunopharmacol. 2020, 82, 106226. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Zonfrillo, M.; Villivà, C.; Bonmassar, E.; Ravagnan, G. Inflammatory Microenvironment and Adipogenic Differentiation in Obesity: The Inhibitory Effect of Theobromine in a Model of Human Obesity In Vitro. Mediat. Inflamm. 2019, 2019, 1515621. [Google Scholar] [CrossRef]
- Smolders, L.; Mensink, R.P.; Boekschoten, M.V.; de Ridder, R.J.J.; Plat, J. Theobromine Does Not Affect Postprandial Lipid Metabolism and Duodenal Gene Expression, but Has Unfavorable Effects on Postprandial Glucose and Insulin Responses in Humans. Clin. Nutr. 2018, 37, 719–727. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Oñatibia-Astibia, A.; Franco, R. The Relevance of Theobromine for the Beneficial Effects of Cocoa Consumption. Front. Pharmacol. 2015, 6, 126866. [Google Scholar] [CrossRef]
- Cosio, B.G.; Tsaprouni, L.; Ito, K.; Jazrawi, E.; Adcock, I.M.; Barnes, P.J. Theophylline Restores Histone Deacetylase Activity and Steroid Responses in COPD Macrophages. J. Exp. Med. 2004, 200, 689–695. [Google Scholar] [CrossRef]
- Sugiura, H.; Kawabata, H.; Ichikawa, T.; Koarai, A.; Yanagisawa, S.; Kikuchi, T.; Minakata, Y.; Matsunaga, K.; Nakanishi, M.; Hirano, T.; et al. Inhibitory Effects of Theophylline on the Peroxynitrite-Augmented Release of Matrix Metalloproteinases by Lung Fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L764–L774. [Google Scholar] [CrossRef]
- Esnault, S.; Kelly, E.A.; Johnson, S.H.; DeLain, L.P.; Haedt, M.J.; Noll, A.L.; Sandbo, N.; Jarjour, N.N. Matrix Metalloproteinase-9-Dependent Release of IL-1β by Human Eosinophils. Mediat. Inflamm. 2019, 2019, e7479107. [Google Scholar] [CrossRef] [PubMed]
- Zariffard, M.R.; Anastos, K.; French, A.L.; Munyazesa, E.; Cohen, M.; Landay, A.L.; Spear, G.T. Cleavage/Alteration of Interleukin-8 by Matrix Metalloproteinase-9 in the Female Lower Genital Tract. PLoS ONE 2015, 10, e0116911. [Google Scholar] [CrossRef]
- Groot Nibbelink, M.; Marchioli, G.; Moroni, L.; Karperien, M.; Van Apeldoorn, A. A Protocol to Enhance INS1E and MIN6 Functionality—The Use of Theophylline. Int. J. Mol. Sci. 2016, 17, 1532. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Komatsu, M.; Schermerhorn, T.; Aizawa, T.; Kaneko, T.; Nagai, M.; Sharp, G.W.; Hashizume, K. cAMP Enhances Insulin Secretion by an Action on the ATP-Sensitive K+ Channel-Independent Pathway of Glucose Signaling in Rat Pancreatic Islets. Diabetes 1999, 48, 1006–1012. [Google Scholar] [CrossRef]
- Rabinovitch, A.; Sumoski, W.L. Theophylline Protects against Diabetes in BB Rats and Potentiates Cyclosporine Protection. Diabetologia 1990, 33, 506–508. [Google Scholar] [CrossRef]
- Habtemariam, S. Chapter 17—The Chemical and Pharmacological Basis of Fenugreek (Trigonella foenum-graecum L.) as Potential Therapy for Type 2 Diabetes and Associated Diseases. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; Habtemariam, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 579–637. ISBN 978-0-08-102922-0. [Google Scholar]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Grebby, S.; Fisk, I.D. Non-Destructive Analysis of Sucrose, Caffeine and Trigonelline on Single Green Coffee Beans by Hyperspectral Imaging. Food Res. Int. 2018, 106, 193–203. [Google Scholar] [CrossRef]
- Damani, L.A.; Case, D.E. 1.09—Metabolism of Heterocycles. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1984; pp. 223–246. ISBN 978-0-08-096519-2. [Google Scholar]
- Shimizu, S.; Watanabe, N.; Kataoka, T.; Shoji, T.; Abe, N.; Morishita, S.; Ichimura, H. Pyridine and Pyridine Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 978-3-527-30673-2. [Google Scholar]
- Kotyczka, C.; Boettler, U.; Lang, R.; Stiebitz, H.; Bytof, G.; Lantz, I.; Hofmann, T.; Marko, D.; Somoza, V. Dark Roast Coffee Is More Effective than Light Roast Coffee in Reducing Body Weight, and in Restoring Red Blood Cell Vitamin E and Glutathione Concentrations in Healthy Volunteers. Mol. Nutr. Food Res. 2011, 55, 1582–1586. [Google Scholar] [CrossRef]
- Brandl, B.; Czech, C.; Wudy, S.I.; Beusch, A.; Hauner, H.; Skurk, T.; Lang, R. Validation of N-Methylpyridinium as a Feasible Biomarker for Roasted Coffee Intake. Beverages 2024, 10, 12. [Google Scholar] [CrossRef]
- Lang, R.; Wahl, A.; Skurk, T.; Yagar, E.F.; Schmiech, L.; Eggers, R.; Hauner, H.; Hofmann, T. Development of a Hydrophilic Liquid Interaction Chromatography−High-Performance Liquid Chromatography−Tandem Mass Spectrometry Based Stable Isotope Dilution Analysis and Pharmacokinetic Studies on Bioactive Pyridines in Human Plasma and Urine after Coffee Consumption. Anal. Chem. 2010, 82, 1486–1497. [Google Scholar] [CrossRef]
- Farid, M.M.; Yang, X.; Kuboyama, T.; Tohda, C. Trigonelline Recovers Memory Function in Alzheimer’s Disease Model Mice: Evidence of Brain Penetration and Target Molecule. Sci. Rep. 2020, 10, 16424. [Google Scholar] [CrossRef]
- Lindenblad, G.E.; Kaihara, M.; Price, J.M. The Occurrence of N-Methyl-2-Pyridone-5-Carboxylic Acid and Its Glycine Conjugate in Normal Human Urine. J. Biol. Chem. 1956, 219, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, S.; Kawano, Y. Urinary Excretion of N1-Methyl-2-Pyridone-5-Carboxylic Acid and the Fate of Remaining of Trigonelline. Adv. Exp. Med. Biol. 1996, 398, 599–603. [Google Scholar] [CrossRef]
- Bresciani, L.; Tassotti, M.; Rosi, A.; Martini, D.; Antonini, M.; Dei Cas, A.; Bonadonna, R.; Brighenti, F.; Del Rio, D.; Mena, P. Absorption, Pharmacokinetics, and Urinary Excretion of Pyridines after Consumption of Coffee and Cocoa-Based Products Containing Coffee in a Repeated Dose, Crossover Human Intervention Study. Mol. Nutr. Food Res. 2020, 64, e2000489. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Mnafgui, K.; Amri, Z.; Aloulou, A.; Elfeki, A. Inhibition of Key Digestive Enzymes Related to Diabetes and Hyperlipidemia and Protection of Liver-Kidney Functions by Trigonelline in Diabetic Rats. Sci. Pharm. 2013, 81, 233–246. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. Int. J. Mol. Sci. 2024, 25, 3385. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Diwedi, V.; Bhardwaj, Y. In Vitro α -Amylase and α-Glucosidase Inhibitory Potential of Trigonella Foenum-Graecum Leaves Extract. Ayu 2013, 34, 109–112. [Google Scholar] [CrossRef]
- Riedel, A.; Hochkogler, C.M.; Lang, R.; Bytof, G.; Lantz, I.; Hofmann, T.; Somoza, V. N-Methylpyridinium, a Degradation Product of Trigonelline upon Coffee Roasting, Stimulates Respiratory Activity and Promotes Glucose Utilization in HepG2 Cells. Food Funct. 2014, 5, 454–462. [Google Scholar] [CrossRef]
- Subramanian, S.P.; Prasath, G.S. Antidiabetic and Antidyslipidemic Nature of Trigonelline, a Major Alkaloid of Fenugreek Seeds Studied in High-Fat-Fed and Low-Dose Streptozotocin-Induced Experimental Diabetic Rats. Biomed. Prev. Nutr. 2014, 4, 475–480. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, X.; Cao, Y.; Wang, X.; Lu, J.; Xie, L.; Liu, K.; Li, X. The Neuroprotective and Antidiabetic Effects of Trigonelline: A Review of Signaling Pathways and Molecular Mechanisms. Biochimie 2023, 206, 93–104. [Google Scholar] [CrossRef]
- Liu, L.; Du, X.; Zhang, Z.; Zhou, J. Trigonelline Inhibits Caspase 3 to Protect β Cells Apoptosis in Streptozotocin-Induced Type 1 Diabetic Mice. Eur. J. Pharmacol. 2018, 836, 115–121. [Google Scholar] [CrossRef]
- Tharaheswari, M.; Jayachandra Reddy, N.; Kumar, R.; Varshney, K.C.; Kannan, M.; Sudha Rani, S. Trigonelline and Diosgenin Attenuate ER Stress, Oxidative Stress-Mediated Damage in Pancreas and Enhance Adipose Tissue PPARγ Activity in Type 2 Diabetic Rats. Mol. Cell. Biochem. 2014, 396, 161–174. [Google Scholar] [CrossRef]
- Gong, M.; Guo, Y.; Dong, H.; Wu, W.; Wu, F.; Lu, F. Trigonelline Inhibits Tubular Epithelial-Mesenchymal Transformation in Diabetic Kidney Disease via Targeting Smad7. Biomed. Pharmacother. 2023, 168, 115747. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K. Transforming Growth Factor-β Signaling in Epithelial-Mesenchymal Transition and Progression of Cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 314–323. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, C.; Lou, Z.; Li, Q. Trigonelline Reduced Diabetic Nephropathy and Insulin Resistance in Type 2 Diabetic Rats through Peroxisome Proliferator-Activated Receptor-γ. Exp. Ther. Med. 2019, 18, 1331–1337. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Protective Roles of Trigonelline against Oxalate-Induced Epithelial-to-Mesenchymal Transition in Renal Tubular Epithelial Cells: An in Vitro Study. Food Chem. Toxicol. 2020, 135, 110915. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, R.; Rodriguez, I.; Nam, Y.H.; Hong, B.N.; Kang, T.H. Trigonelline Promotes Auditory Function through Nerve Growth Factor Signaling on Diabetic Animal Models. Phytomedicine 2017, 36, 128–136. [Google Scholar] [CrossRef]
- Chiazza, F.; Collino, M. Chapter 9—Peroxisome Proliferator-Activated Receptors (PPARs) in Glucose Control. In Molecular Nutrition and Diabetes; Mauricio, D., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 105–114. ISBN 978-0-12-801585-8. [Google Scholar]
- Bakuradze, T.; Parra, G.A.M.; Riedel, A.; Somoza, V.; Lang, R.; Dieminger, N.; Hofmann, T.; Winkler, S.; Hassmann, U.; Marko, D.; et al. Four-Week Coffee Consumption Affects Energy Intake, Satiety Regulation, Body Fat, and Protects DNA Integrity. Food Res. Int. 2014, 63, 420–427. [Google Scholar] [CrossRef]
- Quarta, S.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Damiano, F.; Siculella, L.; Wabitsch, M.; Verri, T.; Favari, C.; et al. Coffee Bioactive N-Methylpyridinium Attenuates Tumor Necrosis Factor (TNF)-α-Mediated Insulin Resistance and Inflammation in Human Adipocytes. Biomolecules 2021, 11, 1545. [Google Scholar] [CrossRef]
- Kukula-Koch, W.A.; Widelski, J. Chapter 9—Alkaloids. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 163–198. ISBN 978-0-12-802104-0. [Google Scholar]
- Thatikayala, M.; Wadhwa, P.; Kaur, P.; Singh, P.K.; Yadav, A.; Kaushik, M.; Sahu, S.K. Beta-Carboline as a Promising Heterocyclic Nucleus: Synthetic Aspects, Pharmacological Potential and Structure Activity Relationship. Eur. J. Med. Chem. Rep. 2022, 6, 100096. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Shi, L.; Li, Y. Bioactive β-Carbolines Harman and Norharman in Sesame Seed Oils in China. Molecules 2022, 27, 402. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Casal, S.; Oliveira, B.P.P. Factors Influencing the Norharman and Harman Contents in Espresso Coffee. J. Agric. Food Chem. 2007, 55, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Louis, E.D.; Zheng, W. Toxicokinetics of Tremorogenic Natural Products, Harmane and Harmine, in Male Sprague-Dawley Rats. J. Toxicol. Environ. Health A 2001, 64, 645–660. [Google Scholar] [CrossRef]
- Herraiz, T.; Guillén, H.; Arán, V.J. Oxidative Metabolism of the Bioactive and Naturally Occurring β-Carboline Alkaloids, Norharman and Harman, by Human Cytochrome P450 Enzymes. Chem. Res. Toxicol. 2008, 21, 2172–2180. [Google Scholar] [CrossRef]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-Carbolines in Food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef]
- Behl, T.; Gupta, A.; Albratty, M.; Najmi, A.; Meraya, A.M.; Alhazmi, H.A.; Anwer, M.K.; Bhatia, S.; Bungau, S.G. Alkaloidal Phytoconstituents for Diabetes Management: Exploring the Unrevealed Potential. Molecules 2022, 27, 5851. [Google Scholar] [CrossRef]
- Cooper, E.J.; Hudson, A.L.; Parker, C.A.; Morgan, N.G. Effects of the β-Carbolines, Harmane and Pinoline, on Insulin Secretion from Isolated Human Islets of Langerhans. Eur. J. Pharmacol. 2003, 482, 189–196. [Google Scholar] [CrossRef]
- Moura, D.J.; Richter, M.F.; Boeira, J.M.; Pêgas Henriques, J.A.; Saffi, J. Antioxidant Properties of Beta-Carboline Alkaloids Are Related to Their Antimutagenic and Antigenotoxic Activities. Mutagenesis 2007, 22, 293–302. [Google Scholar] [CrossRef]
- Pari, K.; Sundari, C.S.; Chandani, S.; Balasubramanian, D. β-Carbolines That Accumulate in Human Tissues May Serve a Protective Role against Oxidative Stress*. J. Biol. Chem. 2000, 275, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. β-Carboline Alkaloids in Soy Sauce and Inhibition of Monoamine Oxidase (MAO). Molecules 2023, 28, 2723. [Google Scholar] [CrossRef]
- Berlowitz, I.; Egger, K.; Cumming, P. Monoamine Oxidase Inhibition by Plant-Derived β-Carbolines; Implications for the Psychopharmacology of Tobacco and Ayahuasca. Front. Pharmacol. 2022, 13, 886408. [Google Scholar] [CrossRef]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, S.; Soubhye, J.; Aldib, I.; Bournine, L.; Nguyen, A.T.; Vanhaeverbeek, M.; Rousseau, A.; Boudjeltia, K.Z.; Sarakbi, A.; Kauffmann, J.M.; et al. Inhibition of Myeloperoxidase Activity by the Alkaloids of Peganum harmala L. (Zygophyllaceae). J. Ethnopharmacol. 2014, 154, 361–369. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Wu, J.; Liu, W.; Li, D.; Xu, J. Harmane Ameliorates Obesity Though Inhibiting Lipid Accumulation and Inducing Adipocyte Browning. RSC Adv. 2020, 10, 4397–4403. [Google Scholar] [CrossRef]
- Shen, S.-H.; Singh, S.P.; Raffaele, M.; Waldman, M.; Hochhauser, E.; Ospino, J.; Arad, M.; Peterson, S.J. Adipocyte-Specific Expression of PGC1α Promotes Adipocyte Browning and Alleviates Obesity-Induced Metabolic Dysfunction in an HO-1-Dependent Fashion. Antioxidants 2022, 11, 1147. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Pastoriza, S. Chapter 20—Melanoidins in Coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 183–188. ISBN 978-0-12-409517-5. [Google Scholar]
- Feng, J.; Berton-Carabin, C.C.; Guyot, S.; Gacel, A.; Fogliano, V.; Schroën, K. Coffee Melanoidins as Emulsion Stabilizers. Food Hydrocoll. 2023, 139, 108522. [Google Scholar] [CrossRef]
- Tagliazucchi, D. Melanoidins from Coffee and Lipid Peroxidation. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 859–867. ISBN 978-0-12-409517-5. [Google Scholar]
- Barak, S.; Mudgil, D. Locust Bean Gum: Processing, Properties and Food Applications—A Review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Ito, K.; Fukuoka, K.; Nishigaki, N.; Hara, K.; Yoshimi, Y.; Kuki, H.; Takahashi, D.; Tsumuraya, Y.; Kotake, T. Structural Features Conserved in Subclass of Type II Arabinogalactan. Plant Biotechnol. 2020, 37, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Rodríguez Casas, A.; Del Castillo, M.D. Interest of Coffee Melanoidins as Sustainable Healthier Food Ingredients. Front. Nutr. 2021, 8, 730343. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Ángel Rufián-Henares, J. Bioactivity of Food Melanoidins Is Mediated by Gut Microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef]
- Sharma, J.K.; Sihmar, M.; Santal, A.R.; Prager, L.; Carbonero, F.; Singh, N.P. Barley Melanoidins: Key Dietary Compounds With Potential Health Benefits. Front. Nutr. 2021, 8, 708194. [Google Scholar] [CrossRef]
- Walker, J.M.; Mennella, I.; Ferracane, R.; Tagliamonte, S.; Holik, A.-K.; Hölz, K.; Somoza, M.M.; Somoza, V.; Fogliano, V.; Vitaglione, P. Melanoidins from Coffee and Bread Differently Influence Energy Intake: A Randomized Controlled Trial of Food Intake and Gut-Brain Axis Response. J. Funct. Foods 2020, 72, 104063. [Google Scholar] [CrossRef]
- Radosevich, P.M.; Lacy, D.B.; Brown, L.L.; Williams, P.E.; Abumrad, N.N. Central Effects of Beta-Endorphins on Glucose Homeostasis in the Conscious Dog. Am. J. Physiol. 1989, 256, E322–E330. [Google Scholar] [CrossRef]
- Ouedraogo, R.; Näslund, E.; Kirchgessner, A.L. Glucose Regulates the Release of Orexin-a from the Endocrine Pancreas. Diabetes 2003, 52, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Juhász, A.E.; Greff, D.; Teutsch, B.; Gede, N.; Hegyi, P.; Horváth, E.M.; Deák, P.Á.; Nyirády, P.; Ács, N.; Juhász, R. Galactomannans Are the Most Effective Soluble Dietary Fibers in Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Am. J. Clin. Nutr. 2023, 117, 266–277. [Google Scholar] [CrossRef]
- Gong, J.; Fang, K.; Dong, H.; Wang, D.; Hu, M.; Lu, F. Effect of Fenugreek on Hyperglycaemia and Hyperlipidemia in Diabetes and Prediabetes: A Meta-Analysis. J. Ethnopharmacol. 2016, 194, 260–268. [Google Scholar] [CrossRef]
- Trask, L.E.; Chaidarun, S.S.; Platt, D.; Parkin, C.G. Treatment with Novel Galactomannan Derivative Reduces 2-Hour Postprandial Glucose Excursions in Individuals with Type 2 Diabetes Treated with Oral Medications and/or Insulin. J. Diabetes Sci. Technol. 2014, 8, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee Reduces Liver Damage in a Rat Model of Steatohepatitis: The Underlying Mechanisms and the Role of Polyphenols and Melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, X.; Fotina, H.; Fotina, T. Anti-Inflammatory Effects of Chlorogenic Acid from Taraxacum Officinale on LTA-Stimulated Bovine Mammary Epithelial Cells via the TLR2/NF-κB Pathway. PLoS ONE 2023, 18, e0282343. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.L.; Ohm, J.E.; Rosenberger, T.A. Acetate Reduces PGE2 Release and Modulates Phospholipase and Cyclooxygenase Levels in Neuroglia Stimulated with Lipopolysaccharide. Lipids 2013, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, M.; Shibata, M.A.; Sano, M.; Takesada, Y.; Tamano, S.; Ito, N.; Shirai, T. Decrease of Prostaglandin E2 and 5-Bromo-2′-Deoxyuridine Labeling but Not Prostate Tumor Development by Indomethacin Treatment of Rats given 3,2′-Dimethyl-4-Aminobiphenyl and Testosterone Propionate. Jpn. J. Cancer Res. 1997, 88, 350–355. [Google Scholar] [CrossRef] [PubMed]
- De Marco, L.M.; Fischer, S.; Henle, T. High Molecular Weight Coffee Melanoidins Are Inhibitors for Matrix Metalloproteases. J. Agric. Food Chem. 2011, 59, 11417–11423. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Mach, F.; Libby, P. Generation of Biologically Active IL-1β by Matrix Metalloproteinases: A Novel Caspase-1-Independent Pathway of IL-1β Processing. J. Immunol. 1998, 161, 3340–3346. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramerth, A.; Chapple, B.; Winter, J.; Moore, W. The Other Side of the Perfect Cup: Coffee-Derived Non-Polyphenols and Their Roles in Mitigating Factors Affecting the Pathogenesis of Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 8966. https://doi.org/10.3390/ijms25168966
Ramerth A, Chapple B, Winter J, Moore W. The Other Side of the Perfect Cup: Coffee-Derived Non-Polyphenols and Their Roles in Mitigating Factors Affecting the Pathogenesis of Type 2 Diabetes. International Journal of Molecular Sciences. 2024; 25(16):8966. https://doi.org/10.3390/ijms25168966
Chicago/Turabian StyleRamerth, Alexis, Brooke Chapple, Jeremiah Winter, and William Moore. 2024. "The Other Side of the Perfect Cup: Coffee-Derived Non-Polyphenols and Their Roles in Mitigating Factors Affecting the Pathogenesis of Type 2 Diabetes" International Journal of Molecular Sciences 25, no. 16: 8966. https://doi.org/10.3390/ijms25168966



