Establishment and Maintenance of Heat-Stress Memory in Plants
Abstract
:1. Introduction
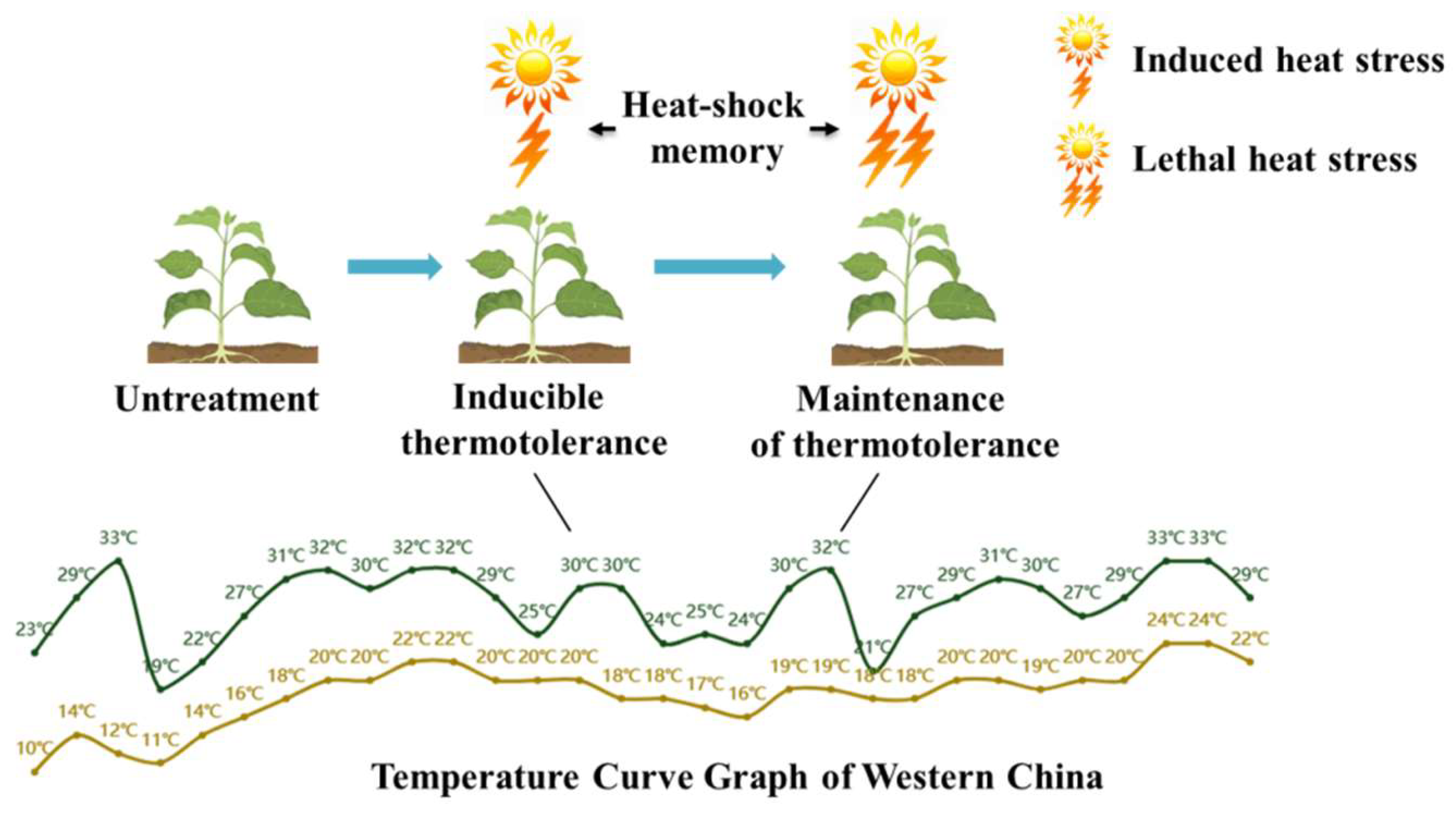
1.1. Heat-Stress Memory: An Important Strategy for Plant Adaptation to the Environment
1.2. Mechanism of High-Temperature Stress Memory in Plants
| Gene | Annotation | Description | References |
|---|---|---|---|
| Heat-shock proteins and heat-shock factors | |||
| HSA32 | Non-canonical heat-shock protein | [52] | |
| HSFA2 | Heat-shock transcription factor | Reduces H3K4me3 at HSP22 | [14,50] |
| HSP22/HSP17.6C | Small heat-shock proteins | Decrease or increase in chaperone function | [53] |
| HSFA1 | [54] | ||
| HSP21/HSP22.0/HSP18.2 | Small heat-shock proteins | Decrease or increase in chaperone function | [55] |
| HSP101 | Heat-shock protein | Decrease in chaperone function | [21] |
| HSFA2/HSFA3 | Heat-shock factors | Accessible chromatin environment, and heat-stress-induced enrichment of H3K4me3 | [25,26] |
| HSP101 | Heat-shock protein | HSP101 positively regulates HSA32 levels | [56] |
| Chromatin remodeler and nucleosome occupancy | |||
| CHR11/CHR17 | SWI2/SNF2 chromatin remodelers | Increases nucleosome occupancy at HSA32 | [31] |
| BRM | SWI2/SNF2 chromatin remodeler | Increases nucleosome occupancy at HSA32 | [31] |
| FGT1 | Ortholog of Strawberry notch | Interacts with chromatin remodelers of the SWI/SNF and ISWI families | [31] |
| Histone acetyltransferases/deacetylase in thermotolerance | |||
| FGT2 | Type 2C protein phosphatase | Changes in lipid homeostasis | [57] |
| PLDA2 | Phospholipase D α2 | Changes in lipid homeostasis | [57] |
| ROF1 | Peptidyl prolyl cis/trans isomerase, A member of the FKBP family | HsfA2-regulated expression of small HSP genes is greatly reduced in rof1 | [58] |
| HDA9-PWR-ABI4 | Proteins complex | Promotes drought tolerance through deacetylation | [30,33] |
| FtsH6 | FtsH metalloprotease | Promotes HSP21 accumulation | [55] |
| Histone methylation/demethylation in thermotolerance | |||
| TFB5, CHMP1B, RSZ22, CHLM, RZ1A, HDT2 | Pinus proteins | Long-term heat-stress splicing memory | [59] |
| NBR1 | A receptor for selective autophagy during recovery from HS | NBR1 interacts with HSP90.1 and ROF1 and mediates their degradation by autophagy, | [60] |
| CSN5A | CSN5A subunit of the COP9 signalosome | resetting transcriptional memory genes (APX2 and HSP22) and H3K4me3 following recurrent heat-stress | [61] |
| ATX1 | H3K4 methyltransferase | Regulates H3K4me3 levels at the promoters of HS recovery genes | [62] |
| JMJs | H3K27me3 demethylases | High H3K27me3 at HSP22/HSP17.6C | [63] |
| SDG25, ATX1 | Histone H3K4 methyltransferases | Decrease histone H3K4me3 levels and increase DNA cytosine methylation | [64] |
| CAS | Calcium sensing receptor (CAS) protein | cas mutants display enhanced biomass and reduced degradation of the small heat-shock protein HSP 17.6 | [65] |
| Non-coding RNA | |||
| miR156/SPL | MicroRNA/Transcription factors | Decrease or increase SPL mRNA levels | [66] |
| AGO1 | RNA slicer | High miR156 levels | [66] |
| DCL1 | Dicer | High miR156 levels | [66] |
| Autophagy in thermopriming | |||
| BRU1 | Tetratricopeptide-repeat (TPR) and leucine-rich repeat (LRR) protein interactor | The bru1 mutant shows strong induction of HSA32 during recovery | [67] |
| HLP1 | Ortholog of human Hikeshi | Accumulation of 3K4me3 at thermomemory-associated loci | [68] |
| TOR | Target of rapamycin | TOR promotes long-term accumulation of H3K4me3 on thermomemory-associated gene promoters | [62] |
| FORGETTER2 | Type 2C protein phosphatase (PP2C) of the D-clade | FGT2 interacts with phospholipase D α2 (PLDα2) | [57] |
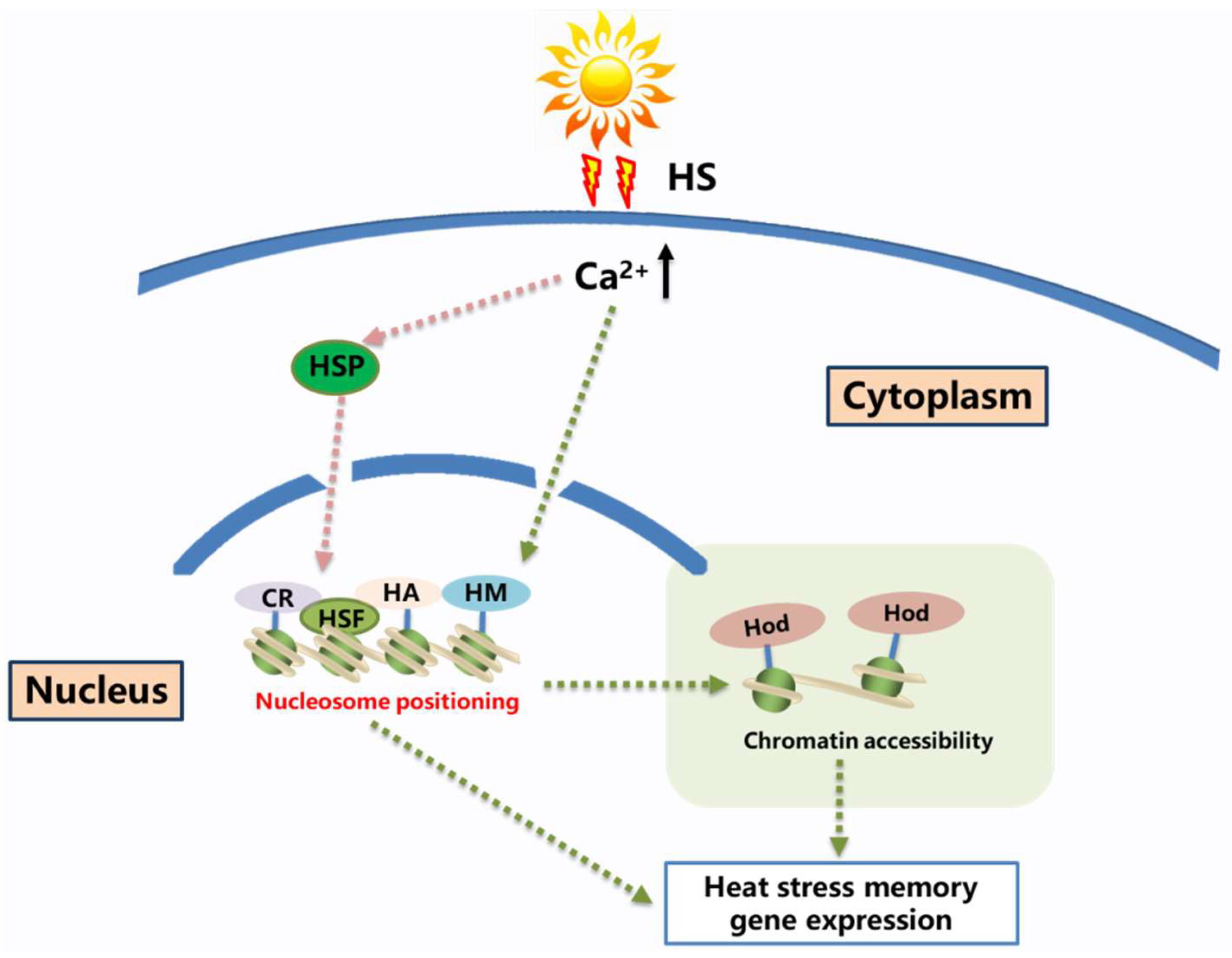
2. Establishment of Heat-Stress Memory: Calcium May Be a Key Signal
2.1. The Relationship between Heat Shock Signal Transduction and Heat Shock Memory
2.2. The Relationship between Ca2+ and Heat-Stress Memory
2.3. Ca2+ and Histone Modifications
2.4. Transcription Factors, Heat Shock Factors and Heat-Stress Memory
3. Maintenance of Heat-Stress Memory: Chromatin Accessibility Is a Key Structural Basis
3.1. Chromatin Accessibility: A Structural Foundation of Stress Memory
3.2. Nucleosome Positioning and Heat-Stress Memory
3.3. Enhanced Chromatin Accessibility through Histone Acetylation Modification
3.4. Histone Methylation and Heat-Stress
4. Challenges and Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nishio, H.; Kawakatsu, T.; Yamaguchi, N. Beyond heat waves: Unlocking epigenetic heat stress memory in Arabidopsis. Plant Physiol. 2024, 194, 1934–1951. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; De Lima, C.F.F.; De Smet, I. The Heat is On: How Crop Growth, Development and Yield Respond to High Temperature. J. Exp. Bot. 2021, 72, 7359–7373. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Martre, P.; Zhao, Z.; Ewert, F.; Maiorano, A.; Rotter, R.P.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. The uncertainty of crop yield projections is reduced by improved temperature response functions. Nat. Plants 2017, 3, 17102. [Google Scholar] [CrossRef] [PubMed]
- Baurle, I. Plant Heat Adaptation: Priming in response to heat stress. F1000Research 2016, 5, 694. [Google Scholar] [CrossRef]
- Hilker, M.; Schmulling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Hatakeyama, Y.; Onda, Y.; Nonami, H.; Nakashima, T.; Erra-Balsells, R.; Morita, S.; Hiraoka, K.; Tanaka, F.; Nakano, H. Multiple strategies for heat adaptation in the rice endosperm. J. Exp. Bot. 2018, 70, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef]
- Suss, K.H.; Yordanov, I.T. Biosynthetic cause of in vivo acquired thermotolerance of photosynthetic light reactions and metabolic responses of chloroplasts to heat stress. Plant Physiol. 1986, 81, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Mach, J. Calcium channels and acquired thermotolerance: Here comes the sun and it’s all right. Plant Cell 2012, 24, 3167. [Google Scholar] [CrossRef]
- Vidya, S.M.; Kumar, H.S.V.; Bhatt, R.M.; Laxman, R.H.; Ravishankar, K.V. Transcriptional profiling and genes involved in acquired thermotolerance in Banana: A non-model crop. Sci. Rep. 2018, 8, 10683. [Google Scholar] [CrossRef]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schafer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatmehr, M.; Thirumalaikumar, V.P.; Kamranfar, I.; Marmagne, A.; Masclaux-Daubresse, C.; Balazadeh, S. A regulatory role of autophagy for resetting the memory of heat stress in plants. Plant Cell Environ. 2019, 42, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Lamke, J.; Brzezinka, K.; Altmann, S.; Baurle, I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 2016, 35, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Avramova, Z. Transcriptional ‘memory’ of a stress: Transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. Cell Mol. Biol. 2015, 83, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Lamke, J.; Brzezinka, K.; Baurle, I. HSFA2 orchestrates transcriptional dynamics after heat stress in Arabidopsis thaliana. Transcription 2016, 7, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Serrano, N.; Gao, G.; Atia, M.; Mokhtar, M.; Woo, Y.H.; Bazin, J.; Veluchamy, A.; Benhamed, M.; Crespi, M.; et al. Thermopriming triggers splicing memory in Arabidopsis. J. Exp. Bot. 2018, 69, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Arico, D.; Legris, M.; Castro, L.; Garcia, C.F.; Laino, A.; Casal, J.J.; Mazzella, M.A. Neighbour signals perceived by phytochrome B increase thermotolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; Cimini, S.; De Gara, L. ROS and redox balance as multifaceted players of cross-tolerance: Epigenetic and retrograde control of gene expression. J. Exp. Bot. 2018, 69, 3373–3391. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatmehr, M.; Thirumalaikumar, V.P.; Kamranfar, I.; Schulz, K.; Mueller-Roeber, B.; Sampathkumar, A.; Balazadeh, S. Autophagy complements metalloprotease FtsH6 in degrading plastid heat shock protein HSP21 during heat stress recovery. J. Exp. Bot. 2021, 72, 7498–7513. [Google Scholar] [CrossRef]
- Wu, T.Y.; Juan, Y.T.; Hsu, Y.H.; Wu, S.H.; Liao, H.T.; Fung, R.W.; Charng, Y.Y. Interplay between heat shock proteins HSP101 and HSA32 prolongs heat acclimation memory posttranscriptionally in Arabidopsis. Plant Physiol. 2013, 161, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, F.; Basha, E.; Fowler, M.E.; Kim, M.; Bordowitz, J.; Katiyar-Agarwal, S.; Vierling, E. Class I and II Small Heat Shock Proteins Together with HSP101 Protect Protein Translation Factors during Heat Stress. Plant Physiol. 2016, 172, 1221–1236. [Google Scholar] [PubMed]
- Gurley, W.B. HSP101: A key component for the acquisition of thermotolerance in plants. Plant Cell 2000, 12, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, K.; Saad, M.M.; Sheikh, A.; Mariappan, K.; Al-Mahmoudi, H.; Abdulhakim, F.; Eida, A.A.; Jalal, R.; Masmoudi, K.; Hirt, H. Root endophyte induced plant thermotolerance by constitutive chromatin modification at heat stress memory gene loci. EMBO Rep. 2021, 22, e51049. [Google Scholar] [CrossRef] [PubMed]
- Kappel, C.; Friedrich, T.; Oberkofler, V.; Jiang, L.; Crawford, T.; Lenhard, M.; Baurle, I. Genomic and epigenomic determinants of heat stress-induced transcriptional memory in Arabidopsis. Genome Biol. 2023, 24, 129. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Oberkofler, V.; Trindade, I.; Altmann, S.; Brzezinka, K.; Lamke, J.; Gorka, M.; Kappel, C.; Sokolowska, E.; Skirycz, A.; et al. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat. Commun. 2021, 12, 3426. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Zhang, Z.; Mullasseri, S.; Kalendar, R.; Ahmad, Z.; Sharma, A.; Liu, G.; Zhou, M.; Wei, Q. Epigenetic stress memory: A new approach to study cold and heat stress responses in plants. Front. Plant Sci. 2022, 13, 1075279. [Google Scholar] [CrossRef]
- Vineis, P.; Chatziioannou, A.; Cunliffe, V.T.; Flanagan, J.M.; Hanson, M.; Kirsch-Volders, M.; Kyrtopoulos, S. Epigenetic memory in response to environmental stressors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Perrella, G.; Baurle, I.; van Zanten, M. Epigenetic regulation of thermomorphogenesis and heat stress tolerance. New Phytol. 2022, 234, 1144–1160. [Google Scholar] [CrossRef]
- Malik, S.; Zhao, D. Epigenetic Regulation of Heat Stress in Plant Male Reproduction. Front. Plant Sci. 2022, 13, 826473. [Google Scholar] [CrossRef] [PubMed]
- Brzezinka, K.; Altmann, S.; Czesnick, H.; Nicolas, P.; Gorka, M.; Benke, E.; Kabelitz, T.; Jahne, F.; Graf, A.; Kappel, C.; et al. Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. eLife 2016, 5, e17061. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Seki, M. Histone Modifications Form Epigenetic Regulatory Networks to Regulate Abiotic Stress Response. Plant Physiol. 2020, 182, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Yun, D.J. Chromatin remodeling complex HDA9-PWR-ABI4 epigenetically regulates drought stress response in plants. Plant Signal. Behav. 2020, 15, 1803568. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Z. Small DNA Methylation, Big Player in Plant Abiotic Stress Responses and Memory. Front. Plant Sci. 2020, 11, 595603. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Z.; Liu, J.; Wang, Y.Y.; Deng, X. DNA methylation-mediated modulation of rapid desiccation tolerance acquisition and dehydration stress memory in the resurrection plant Boea hygrometrica. PLoS Genet. 2021, 17, e1009549. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Nayyar, H.; Jha, R.; Khurshid, M.; Zhou, M.; Mantri, N.; Siddique, K.H.M. Long non-coding RNAs: Emerging players regulating plant abiotic stress response and adaptation. BMC Plant Biol. 2020, 20, 466. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, F.; Zhou, B. The Characters of Non-Coding RNAs and Their Biological Roles in Plant Development and Abiotic Stress Response. Int. J. Mol. Sci. 2022, 23, 4124. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ci, D.; Tian, M.; Zhang, D. Stable methylation of a non-coding RNA gene regulates gene expression in response to abiotic stress in Populus simonii. J. Exp. Bot. 2016, 67, 1477–1492. [Google Scholar] [CrossRef]
- Rodriguez-Granados, N.Y.; Ramirez-Prado, J.S.; Veluchamy, A.; Latrasse, D.; Raynaud, C.; Crespi, M.; Ariel, F.; Benhamed, M. Put your 3D glasses on: Plant chromatin is on show. J. Exp. Bot. 2016, 67, 3205–3221. [Google Scholar] [CrossRef]
- Pandey, G.; Sharma, N.; Sahu, P.P.; Prasad, M. Chromatin-Based Epigenetic Regulation of Plant Abiotic Stress Response. Curr. Genom. 2016, 17, 490–498. [Google Scholar] [CrossRef]
- Iwasaki, M.; Paszkowski, J. Identification of genes preventing transgenerational transmission of stress-induced epigenetic states. Proc. Natl. Acad. Sci. USA 2014, 111, 8547–8552. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, R.P.; Misra, H.S.; Saini, A. Heat-stress priming and alternative splicing-linked memory. J. Exp. Bot. 2018, 69, 2431–2434. [Google Scholar] [CrossRef] [PubMed]
- Murgia, I.; Giacometti, S.; Balestrazzi, A.; Paparella, S.; Pagliano, C.; Morandini, P. Analysis of the transgenerational iron deficiency stress memory in Arabidopsis thaliana plants. Front. Plant Sci. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Migicovsky, Z.; Yao, Y.; Kovalchuk, I. Transgenerational phenotypic and epigenetic changes in response to heat stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e27971. [Google Scholar] [CrossRef]
- Shilo, S.; Melamed-Bessudo, C.; Dorone, Y.; Barkai, N.; Levy, A.A. DNA Crossover Motifs Associated with Epigenetic Modifications Delineate Open Chromatin Regions in Arabidopsis. Plant Cell 2015, 27, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, L.; Narsai, R.; De Clercq, I.; Whelan, J.; Berkowitz, O. Mitochondrial signalling is critical for acclimation and adaptation to flooding in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2020, 103, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.W., 3rd; Heckathorn, S.A. Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol. 2001, 126, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ugurlar, F.; Adamakis, I.S. Epigenetic Modifications of Hormonal Signaling Pathways in Plant Drought Response and Tolerance for Sustainable Food Security. Int. J. Mol. Sci. 2024, 25, 8229. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Boopathi, T.; Paramasivan, M. A status-quo review on CRISPR-Cas9 gene editing applications in tomato. Int. J. Biol. Macromol. 2021, 190, 120–129. [Google Scholar] [CrossRef]
- Sedaghatmehr, M.; Stuwe, B.; Mueller-Roeber, B.; Balazadeh, S. Heat shock factor HSFA2 fine-tunes resetting of thermomemory via plastidic metalloprotease FtsH6. J. Exp. Bot. 2022, 73, 6394–6404. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Chae, S.; Oh, N.I.; Nguyen, N.H.; Cheong, J.J. Recurrent Drought Conditions Enhance the Induction of Drought Stress Memory Genes in Glycine max L. Front. Genet. 2020, 11, 576086. [Google Scholar] [CrossRef]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Hsu, F.C.; Ko, S.S. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol. 2006, 140, 1297–1305. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Liu, Y.; Wu, Y.; Xie, Q. The sHSP22 Heat Shock Protein Requires the ABI1 Protein Phosphatase to Modulate Polar Auxin Transport and Downstream Responses. Plant Physiol. 2018, 176, 2406–2425. [Google Scholar] [CrossRef]
- Huang, Y.; An, J.; Sircar, S.; Bergis, C.; Lopes, C.D.; He, X.; Da Costa, B.; Tan, F.Q.; Bazin, J.; Antunez-Sanchez, J.; et al. HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer-promoter interactions. Nat. Commun. 2023, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatmehr, M.; Mueller-Roeber, B.; Balazadeh, S. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat. Commun. 2016, 7, 12439. [Google Scholar] [CrossRef]
- Lin, M.Y.; Chai, K.H.; Ko, S.S.; Kuang, L.Y.; Lur, H.S.; Charng, Y.Y. A positive feedback loop between HEAT SHOCK PROTEIN101 and HEAT STRESS-ASSOCIATED 32-KD PROTEIN modulates long-term acquired thermotolerance illustrating diverse heat stress responses in rice varieties. Plant Physiol. 2014, 164, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Urrea Castellanos, R.; Friedrich, T.; Petrovic, N.; Altmann, S.; Brzezinka, K.; Gorka, M.; Graf, A.; Baurle, I. FORGETTER2 protein phosphatase and phospholipase D modulate heat stress memory in Arabidopsis. Plant J. Cell Mol. Biol. 2020, 104, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Meiri, D.; Breiman, A. Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. Cell Mol. Biol. 2009, 59, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Roces, V.; Lamelas, L.; Valledor, L.; Carbo, M.; Canal, M.J.; Meijon, M. Integrative analysis in Pinus revealed long-term heat stress splicing memory. Plant J. Cell Mol. Biol. 2022, 112, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, V.P.; Gorka, M.; Schulz, K.; Masclaux-Daubresse, C.; Sampathkumar, A.; Skirycz, A.; Vierstra, R.D.; Balazadeh, S. Selective autophagy regulates heat stress memory in Arabidopsis by NBR1-mediated targeting of HSP90.1 and ROF1. Autophagy 2021, 17, 2184–2199. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dhanapal, S.; Finkelshtein, A.; Chamovitz, D.A. CSN5A Subunit of COP9 Signalosome Is Required for Resetting Transcriptional Stress Memory after Recurrent Heat Stress in Arabidopsis. Biomolecules 2021, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, M.; Jamsheer, K.M.; Laxmi, A. A glucose-target of rapamycin signaling axis integrates environmental history of heat stress through maintenance of transcription-associated epigenetic memory in Arabidopsis. J. Exp. Bot. 2022, 73, 7083–7102. [Google Scholar] [CrossRef]
- Yamaguchi, N. Removal of H3K27me3 by JMJ Proteins Controls Plant Development and Environmental Responses in Arabidopsis. Front. Plant Sci. 2021, 12, 687416. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.T.; Zhang, L.L.; Han, J.J.; Zhou, M.; Liu, J.X. Histone H3K4 methyltransferases SDG25 and ATX1 maintain heat-stress gene expression during recovery in Arabidopsis. Plant J. Cell Mol. Biol. 2021, 105, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Sukiran, N.A.; Jacobs, B.; Knight, M.R. Chloroplast calcium signalling regulates thermomemory. J. Plant Physiol. 2021, 264, 153470. [Google Scholar] [CrossRef] [PubMed]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.R.; Baurle, I. Arabidopsis miR156 Regulates Tolerance to Recurring Environmental Stress through SPL Transcription Factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Brzezinka, K.; Altmann, S.; Baurle, I. BRUSHY1/TONSOKU/MGOUN3 is required for heat stress memory. Plant Cell Environ. 2018, 42, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Banday, Z.Z.; Shukla, B.N.; Laxmi, A. Glucose-regulated HLP1 acts as a key molecule in governing thermomemory. Plant Physiol. 2019, 180, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Han, X.; Wu, J.; Zheng, S.; Shang, Z.; Sun, D.; Zhou, R.; Li, B. A heat-activated calcium-permeable channel--Arabidopsis cyclic nucleotide-gated ion channel 6--is involved in heat shock responses. Plant J. Cell Mol. Biol. 2012, 70, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Z.; Liu, Y.L.; Li, B.; Shang, Z.L.; Zhou, R.G.; Sun, D.Y. Phosphoinositide-specific phospholipase C9 is involved in the thermotolerance of Arabidopsis. Plant J. Cell Mol. Biol. 2012, 69, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Song, N.; Zheng, M.; Liu, X.; Liu, Z.; Xing, J.; Ma, J.; Guo, W.; Yao, Y.; Peng, H.; et al. Histone acetyltransferase GCN5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J. Cell Mol. Biol. 2015, 84, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Yang, Y.; Feng, H.; Pan, Z.; Shen, W.H.; Zhu, Y.; Dong, A. Histone chaperone ASF1 is involved in gene transcription activation in response to heat stress in Arabidopsis thaliana. Plant Cell Environ. 2014, 37, 2128–2138. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, Y.; Peng, Y.; Jia, Y.; Yan, Y.; Dong, X.; Li, H.; Dong, J.; Li, J.; Gong, Z.; et al. Cold-Induced CBF-PIF3 Interaction Enhances Freezing Tolerance by Stabilizing the phyB Thermosensor in Arabidopsis. Mol. Plant 2020, 13, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Shao, Y.J.; Ding, L.; Wang, M.J.; Davis, S.J.; Liu, J.X. XBAT31 regulates thermoresponsive hypocotyl growth through mediating degradation of the thermosensor ELF3 in Arabidopsis. Sci. Adv. 2021, 7, eabf4427. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Kodama, Y. Temperature Sensing in Plants: On the Dawn of Molecular Thermosensor Research. Plant Cell Physiol. 2022, 63, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.L.; Zhang, G.B.; Lv, X.F.; Guan, Y.; Yi, H.Y.; Gong, J.M. Arabidopsis histone methylase CAU1/PRMT5/SKB1 acts as an epigenetic suppressor of the calcium signaling gene CAS to mediate stomatal closure in response to extracellular calcium. Plant Cell 2013, 25, 2878–2891. [Google Scholar] [CrossRef]
- Wang, Y.; Sherrard, A.; Zhao, B.; Melak, M.; Trautwein, J.; Kleinschnitz, E.M.; Tsopoulidis, N.; Fackler, O.T.; Schwan, C.; Grosse, R. GPCR-induced calcium transients trigger nuclear actin assembly for chromatin dynamics. Nat. Commun. 2019, 10, 5271. [Google Scholar] [CrossRef]
- Lai, D.; Wan, M.; Wu, J.; Preston-Hurlburt, P.; Kushwaha, R.; Grundstrom, T.; Imbalzano, A.N.; Chi, T. Induction of TLR4-target genes entails calcium/calmodulin-dependent regulation of chromatin remodeling. Proc. Natl. Acad. Sci. USA 2009, 106, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nguyen, H.; Geng, C.; Hinman, M.N.; Luo, G.; Lou, H. Calcium-mediated histone modifications regulate alternative splicing in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2014, 111, E4920–E4928. [Google Scholar] [CrossRef]
- Harper, C.V.; McNamara, A.V.; Spiller, D.G.; Charnock, J.C.; White, M.R.H.; Davis, J.R.E. Calcium dynamics and chromatin remodelling underlie heterogeneity in prolactin transcription. J. Mol. Endocrinol. 2021, 66, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.P., Jr.; Augustine, R.; Schulman, H. Calcium-dependent phosphorylation of histone H3 in butyrate-treated HeLa cells. Nature 1980, 287, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Siegel, R.S.; Valerio, G.; Brandt, B.; Schroeder, J.I. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann. Bot. 2012, 109, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Finka, A.; Cuendet, A.F.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Liu, Y.L.; Li, B.; Zhou, R.G.; Sun, D.Y.; Zheng, S.Z. Arabidopsis thaliana phosphoinositide-specific phospholipase C isoform 3 (AtPLC3) and AtPLC9 have an additive effect on thermotolerance. Plant Cell Physiol. 2014, 55, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, M.; Baev, V.; Apostolova, E.; Gospodinova, N.; Sablok, G.; Gozmanova, M.; Yahubyan, G. High-temperature effect on genes engaged in DNA methylation and affected by DNA methylation in Arabidopsis. Plant Physiol. Biochem. PPB 2015, 87, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Nisar, N.; Roberts, A.C.; Murray, K.D.; Borevitz, J.O.; Pogson, B.J. A chromatin modifying enzyme, SDG8, is involved in morphological, gene expression, and epigenetic responses to mechanical stimulation. Front. Plant Sci. 2014, 5, 533. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Gao, F.; Li, G.L.; Han, J.L.; Liu, D.L.; Sun, D.Y.; Zhou, R.G. The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2008, 55, 760–773. [Google Scholar] [CrossRef]
- Pajoro, A.; Madrigal, P.; Muino, J.M.; Matus, J.T.; Jin, J.; Mecchia, M.A.; Debernardi, J.M.; Palatnik, J.F.; Balazadeh, S.; Arif, M.; et al. Dynamics of chromatin accessibility and gene regulation by MADS-domain transcription factors in flower development. Genome Biol. 2014, 15, R41. [Google Scholar] [CrossRef] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, L.; Gu, X.; Deng, X.; Qiu, Q.; Li, Q.; Zhang, Y.; Wang, M.; Deng, Y.; Wang, E.; et al. An H3K27me3 demethylase-HSFA2 regulatory loop orchestrates transgenerational thermomemory in Arabidopsis. Cell Res. 2019, 29, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Shahnejat-Bushehri, S.; Mueller-Roeber, B.; Balazadeh, S. Arabidopsis NAC transcription factor JUNGBRUNNEN1 affects thermomemory-associated genes and enhances heat stress tolerance in primed and unprimed conditions. Plant Signal. Behav. 2012, 7, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, Q.; Ji, C.; Hu, G.; Zhang, P.; Wang, Y.; Yang, L.; Gu, X. Reorganization of the 3D chromatin architecture of rice genomes during heat stress. BMC Biol. 2021, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Potok, M.E.; Zhong, Z.; Picard, C.L.; Liu, Q.; Do, T.; Jacobsen, C.E.; Sakr, O.; Naranbaatar, B.; Thilakaratne, R.; Khnkoyan, Z.; et al. The role of ATXR6 expression in modulating genome stability and transposable element repression in Arabidopsis. Proc. Natl. Acad. Sci. USA 2022, 119, e2115570119. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, P.; Ji, T.; Zheng, L.; Shen, C.; Ran, S.; Liu, J.; Zhao, Y.; Niu, Y.; Wang, T.; et al. The histone methyltransferase SUVR2 promotes DSB repair via chromatin remodeling and liquid-liquid phase separation. Mol. Plant 2022, 15, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzso, E.; Renziehausen, T.; Frings, S.; Frohn, S.; von Bongartz, K.; Igisch, C.P.; Mann, J.; Hager, L.; Macholl, J.; Leisse, D.; et al. Endoplasmic reticulum-bound ANAC013 factor is cleaved by RHOMBOID-LIKE 2 during the initial response to hypoxia in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2023, 120, e2221308120. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, L.; Busoms, S.; Tolra, R.; Poschenrieder, C. Transcriptomics Reveals Fast Changes in Salicylate and Jasmonate Signaling Pathways in Shoots of Carbonate-Tolerant Arabidopsis thaliana under Bicarbonate Exposure. Int. J. Mol. Sci. 2021, 22, 1226. [Google Scholar] [CrossRef] [PubMed]
- Zha, P.; Jing, Y.; Xu, G.; Lin, R. PICKLE chromatin-remodeling factor controls thermosensory hypocotyl growth of Arabidopsis. Plant Cell Environ. 2017, 40, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Park, J.H.; Lee, S.; To, T.K.; Kim, J.M.; Seki, M.; Park, C.M. The cold signaling attenuator HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE1 activates FLOWERING LOCUS C transcription via chromatin remodeling under short-term cold stress in Arabidopsis. Plant Cell 2013, 25, 4378–4390. [Google Scholar] [CrossRef] [PubMed]
- Waidmann, S.; Petutschnig, E.; Rozhon, W.; Molnar, G.; Popova, O.; Mechtler, K.; Jonak, C. GSK3-mediated phosphorylation of DEK3 regulates chromatin accessibility and stress tolerance in Arabidopsis. FEBS J. 2022, 289, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Moyano, T.C.; Zhang, T.; Gras, D.E.; Herrera, F.J.; Araus, V.; O’Brien, J.A.; Carrillo, L.; Medina, J.; Vicente-Carbajosa, J.; et al. Local Changes in Chromatin Accessibility and Transcriptional Networks Underlying the Nitrate Response in Arabidopsis Roots. Mol. Plant 2019, 12, 1545–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Feng, S.; Duttke, S.H.; Potok, M.E.; Zhang, Y.; Gallego-Bartolome, J.; Liu, W.; Jacobsen, S.E. DNA methylation-linked chromatin accessibility affects genomic architecture in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2023347118. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Seddon, A.E.; Tsai, Z.T.; Major, I.T.; Floer, M.; Howe, G.A.; Shiu, S.H. Determinants of nucleosome positioning and their influence on plant gene expression. Genome Res. 2015, 25, 1182–1195. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Jakada, B.H.; Cao, S.; Qin, Y. SWR1 Chromatin Remodeling Complex: A Key Transcriptional Regulator in Plants. Cells 2019, 8, 1621. [Google Scholar] [CrossRef]
- Torres, E.S.; Deal, R.B. The histone variant H2A.Z and chromatin remodeler BRAHMA act coordinately and antagonistically to regulate transcription and nucleosome dynamics in Arabidopsis. Plant J. Cell Mol. Biol. 2019, 99, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, Y.; Qin, Y.; Chen, D.; Zhu, T.; Peng, K.; Wang, H.; Tang, N.; Li, X.; Wang, Y.; et al. A lamin-like protein OsNMCP1 regulates drought resistance and root growth through chromatin accessibility modulation by interacting with a chromatin remodeller OsSWI3C in rice. New Phytol. 2020, 227, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Huang, T.; Irish, V.F. Do Epigenetic Timers Control Petal Development? Front. Plant Sci. 2021, 12, 709360. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, R.K.; Bertolini, E.; Shamimuzzaman, M.; Vera, D.L.; Lung, P.Y.; Rice, B.R.; Zhang, J.; Brown, P.J.; Lipka, A.E.; Bass, H.W.; et al. The regulatory landscape of early maize inflorescence development. Genome Biol. 2020, 21, 165. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- McGowan, M.T.; Zhang, Z.; Ficklin, S.P. Chromosomal characteristics of salt stress heritable gene expression in the rice genome. BMC Genom. Data 2021, 22, 17. [Google Scholar] [CrossRef]
- Huang, R.; Shu, S.; Liu, M.; Wang, C.; Jiang, B.; Jiang, J.; Yang, C.; Zhang, S. Nuclear Prohibitin3 Maintains Genome Integrity and Cell Proliferation in the Root Meristem through Minichromosome Maintenance 2. Plant Physiol. 2019, 179, 1669–1691. [Google Scholar] [CrossRef]
- Skirycz, A.; Claeys, H.; De Bodt, S.; Oikawa, A.; Shinoda, S.; Andriankaja, M.; Maleux, K.; Eloy, N.B.; Coppens, F.; Yoo, S.D.; et al. Pause-and-stop: The effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 2011, 23, 1876–1888. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.L.; Shen, W.; Matus, R.; Kakkar, M.; Jones, C.; Dolan, D.; Grellscheid, S.; Yang, X.; Zhang, N.; Mozaffari-Jovin, S.; et al. MERISTEM-DEFECTIVE regulates the balance between stemness and differentiation in the root meristem through RNA splicing control. Development 2023, 150, dev201476. [Google Scholar] [CrossRef] [PubMed]
- Goodman, H.L.; Kroon, J.T.M.; Tome, D.F.A.; Hamilton, J.M.U.; Alqarni, A.O.; Chivasa, S. Extracellular ATP targets Arabidopsis RIBONUCLEASE 1 to suppress mycotoxin stress-induced cell death. New Phytol. 2022, 235, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N. Heat memory in plants: Histone modifications, nucleosome positioning and miRNA accumulation alter heat memory gene expression. Genes. Genet. Syst. 2021, 96, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Zofall, M.; Balachandran, V.; Thillainadesan, G.; Sugiyama, T.; Wheeler, D.; Zhou, M.; Grewal, S.I. SNF2 Family Protein Fft3 Suppresses Nucleosome Turnover to Promote Epigenetic Inheritance and Proper Replication. Mol. Cell 2017, 66, 50–62.e56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, B.; Deng, J.; Pang, S.; Tang, H. Histone acetylation promotes long-lasting defense responses and longevity following early life heat stress. PLoS Genet. 2019, 15, e1008122. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Bai, J.; Liu, X.; Zhang, H.; Bao, J.; Zhao, W.; Hou, Y.; Deng, X.; Yang, C.; Guo, L.; et al. HISTONE DEACETYLASE 9 transduces heat signal in plant cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2206846119. [Google Scholar] [CrossRef] [PubMed]
- Lambing, C.; Tock, A.J.; Topp, S.D.; Choi, K.; Kuo, P.C.; Zhao, X.; Osman, K.; Higgins, J.D.; Franklin, F.C.H.; Henderson, I.R. Interacting Genomic Landscapes of REC8-Cohesin, Chromatin, and Meiotic Recombination in Arabidopsis. Plant Cell 2020, 32, 1218–1239. [Google Scholar] [CrossRef] [PubMed]
- Boros, J.; Arnoult, N.; Stroobant, V.; Collet, J.F.; Decottignies, A. Polycomb repressive complex 2 and H3K27me3 cooperate with H3K9 methylation to maintain heterochromatin protein 1alpha at chromatin. Mol. Cell. Biol. 2014, 34, 3662–3674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Wu, S.; Yu, C.; Wang, X.; Wang, Y.; Peng, Z.; Gao, Y.; Li, R.; Shen, Y.; et al. Coronatine Enhances Chilling Tolerance of Tomato Plants by Inducing Chilling-Related Epigenetic Adaptations and Transcriptional Reprogramming. Int. J. Mol. Sci. 2022, 23, 10049. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, W.; Marand, A.P.; Zhu, B.; Buell, C.R.; Jiang, J. Cold stress induces enhanced chromatin accessibility and bivalent histone modifications H3K4me3 and H3K27me3 of active genes in potato. Genome Biol. 2019, 20, 123. [Google Scholar] [CrossRef]
- Feng, X.J.; Li, J.R.; Qi, S.L.; Lin, Q.F.; Jin, J.B.; Hua, X.J. Light affects salt stress-induced transcriptional memory of P5CS1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, E8335–E8343. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Luo, J.H.; Cui, Z.H.; Xue, M.; Wang, L.; Zhang, X.Y.; Pawlowski, W.P.; He, Y. ATX3, ATX4, and ATX5 encode putative H3K4 methyltransferases and are critical for plant development. Plant Physiol. 2017, 174, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular mechanisms of plant tolerance to heat stress: Current landscape and future perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef] [PubMed]
- Desvoyes, B.; Vergara, Z.; Sequeira-Mendes, J.; Madeira, S.; Gutierrez, C. A Rapid and Efficient ChIP Protocol to Profile Chromatin Binding Proteins and Epigenetic Modifications in Arabidopsis. Methods Mol. Biol. 2018, 1675, 71–82. [Google Scholar]
- Buenrostro, J.D.; Wu, B.; Chang, H.Y.; Greenleaf, W.J. ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr. Protoc. Mol. Biol. 2015, 109, 21.29.1–21.29.9. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Sun, K.; Ma, T.; Jiang, H.; Hahn, M.; Ma, Z.; Jiao, C.; Yin, Y. SUMOylation regulates low-temperature survival and oxidative DNA damage tolerance in Botrytis cinerea. New Phytol. 2023, 238, 817–834. [Google Scholar] [CrossRef]
- Cui, L.H.; Min, H.J.; Yu, S.G.; Byun, M.Y.; Oh, T.R.; Lee, A.; Yang, H.W.; Kim, W.T. OsATL38 mediates mono-ubiquitination of the 14-3-3 protein OsGF14d and negatively regulates the cold stress response in rice. J. Exp. Bot. 2022, 73, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhu, T.; Yang, T.; Yang, Z.; Ren, A.; Shi, L.; Zhu, J.; Yu, H.; Zhao, M. Nitric oxide regulates ganoderic acid biosynthesis by the S-nitrosylation of aconitase under heat stress in Ganoderma lucidum. Environ. Microbiol. 2021, 23, 682–695. [Google Scholar] [CrossRef]
- Pinto, D.; Page, V.; Fisher, R.P.; Tanny, J.C. New connections between ubiquitylation and methylation in the co-transcriptional histone modification network. Curr. Genet. 2021, 67, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Miao, Y.; Xu, L.; Zhang, Y.; Yuan, C.; Wang, J.; Zhuang, T.; Lin, X.; Jiang, L.; Wang, N.; et al. Transgenerational memory of gene expression changes induced by heavy metal stress in rice (Oryza sativa L.). BMC Plant Biol. 2019, 19, 282. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.P.; Kubisch, A.; Ouborg, N.J.; Pagel, J.; Schmid, K.J.; Vergeer, P.; Lampei, C. Transgenerational effects of mild heat in Arabidopsis thaliana show strong genotype specificity that is explained by climate at origin. New Phytol. 2017, 215, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, K.; Katano, K.; Yunose, M.; Suzuki, N. Memory of 5-min heat stress in Arabidopsis thaliana. Plant Signal. Behav. 2020, 15, 1778919. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xin, C.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Heat Priming Induces Trans-generational Tolerance to High Temperature Stress in Wheat. Front. Plant Sci. 2016, 7, 501. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.A.; Rolando, J.L.; Yactayo, W.; Monneveux, P.; Mares, V.; Quiroz, R. Improving potato drought tolerance through the induction of long-term water stress memory. Plant Sci. 2015, 238, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Q.; Sun, M.; Zhang, T.; Li, H.; Chen, B.; Xu, K.; Gao, G.; Li, F.; Yan, G.; et al. Global DNA methylation variations after short-term heat shock treatment in cultured microspores of Brassica napus cv. Topas. Sci. Rep. 2016, 6, 38401. [Google Scholar] [CrossRef] [PubMed]
- Burgos, A.; Szymanski, J.; Seiwert, B.; Degenkolbe, T.; Hannah, M.A.; Giavalisco, P.; Willmitzer, L. Analysis of short-term changes in the Arabidopsis thaliana glycerolipidome in response to temperature and light. Plant J. Cell Mol. Biol. 2011, 66, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Bilichak, A.; Kovalchuk, I. Transgenerational response to stress in plants and its application for breeding. J. Exp. Bot. 2016, 67, 2081–2092. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Zhao, W.; Liu, Z.; Geng, Z.; Li, Q.; Liu, B.; Li, B.; Bai, J. Establishment and Maintenance of Heat-Stress Memory in Plants. Int. J. Mol. Sci. 2024, 25, 8976. https://doi.org/10.3390/ijms25168976
Zheng S, Zhao W, Liu Z, Geng Z, Li Q, Liu B, Li B, Bai J. Establishment and Maintenance of Heat-Stress Memory in Plants. International Journal of Molecular Sciences. 2024; 25(16):8976. https://doi.org/10.3390/ijms25168976
Chicago/Turabian StyleZheng, Shuzhi, Weishuang Zhao, Zimeng Liu, Ziyue Geng, Qiang Li, Binhui Liu, Bing Li, and Jiaoteng Bai. 2024. "Establishment and Maintenance of Heat-Stress Memory in Plants" International Journal of Molecular Sciences 25, no. 16: 8976. https://doi.org/10.3390/ijms25168976





