Impact of Eccentric Exercise Interventions with Small and Large Ranges of Motion on Rat Skeletal Muscle Tissue and Muscle Force Production
Abstract
1. Introduction
2. Results
2.1. Number of EBD+ Fibers and Localization of Dystrophin
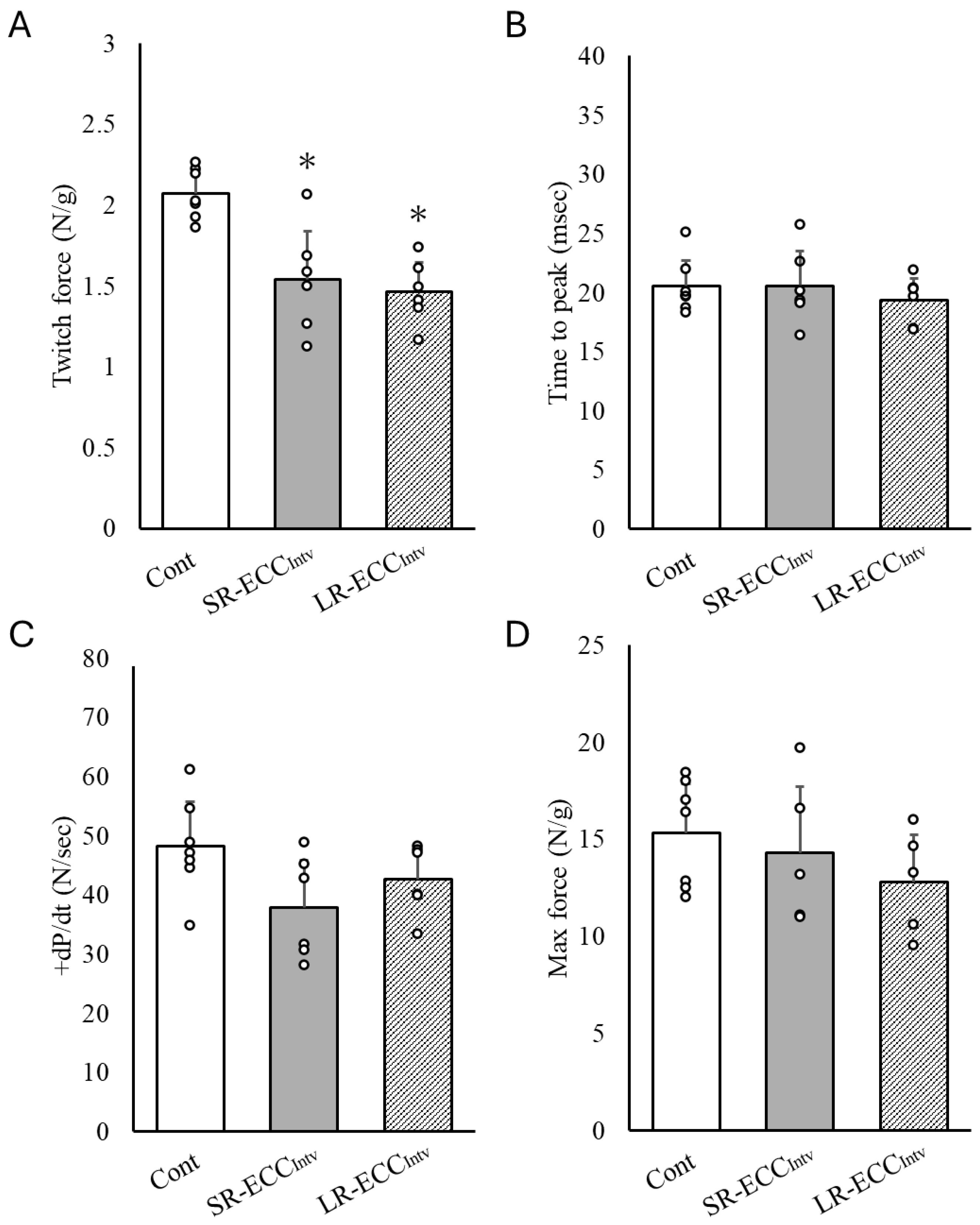
2.2. Muscle Contraction Force
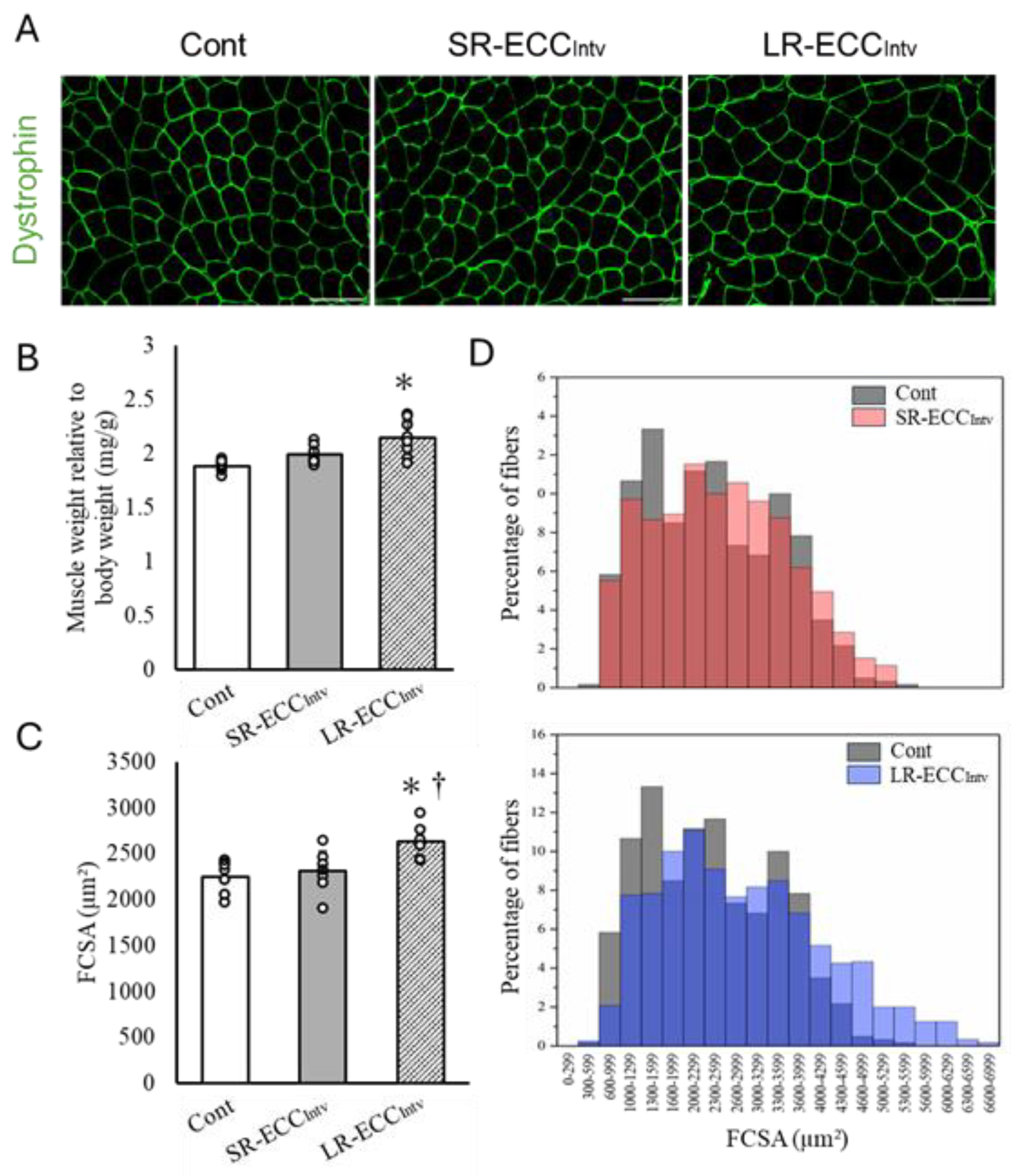
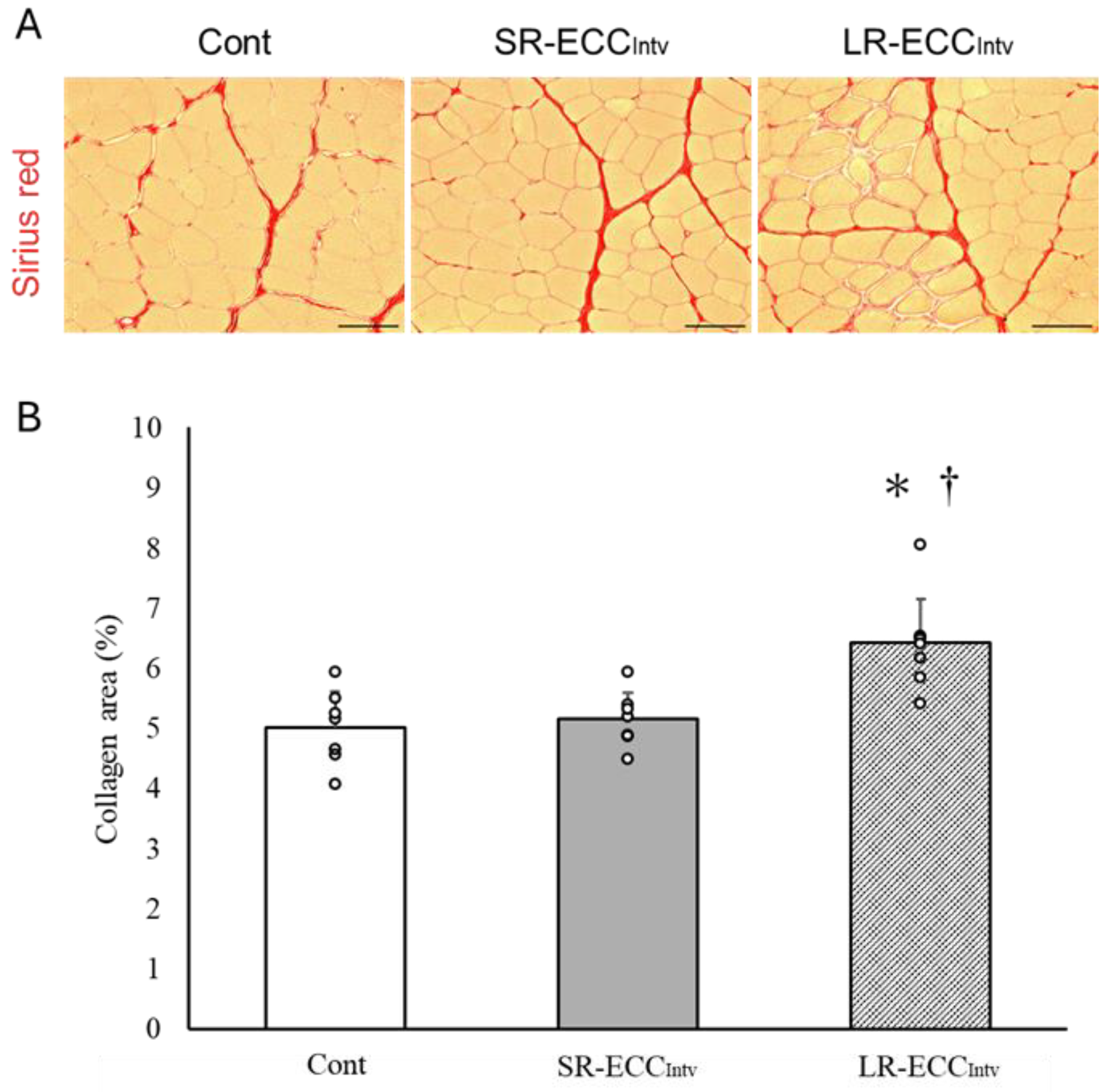
2.3. Muscle Weight, FCSA, and Connective Tissue
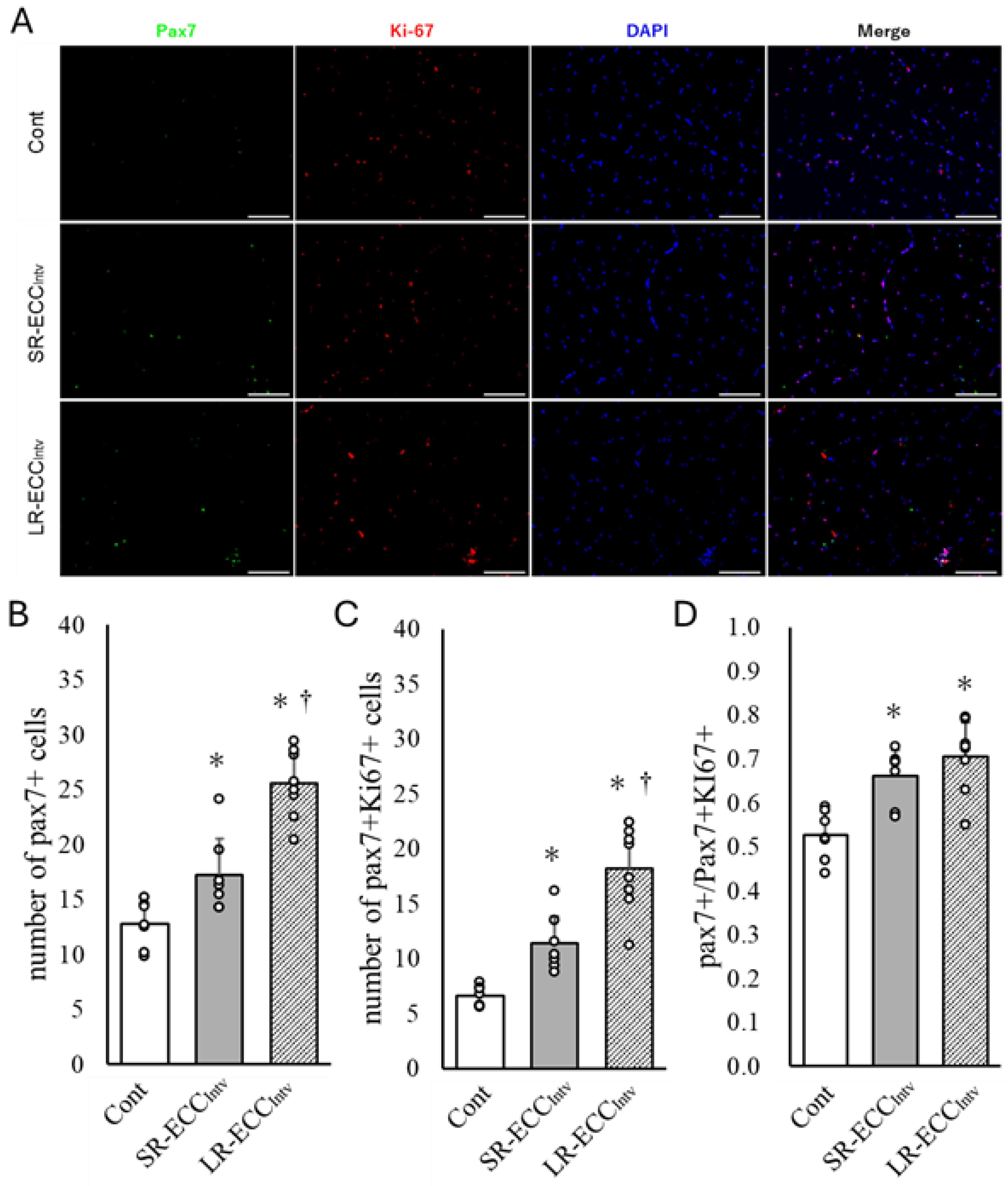
2.4. Increase in Satellite Cell Number and Satellite Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.1.1. Experiment 1
4.1.2. Experiment 2
4.2. ECC Procedures Induced by Electrical Stimulation
4.3. EBD Injection
4.4. Muscle Contraction Force Measurement
4.5. Tissue Preparation
4.6. Histological Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LaStayo, P.; Marcus, R.; Dibble, L.; Frajacomo, F.; Lindstedt, S. Eccentric exercise in rehabilitation: Safety, feasibility, and application. J. Appl. Physiol. 2014, 116, 1426–1434. [Google Scholar] [CrossRef]
- Roberts, M.D.; McCarthy, J.J.; Hornberger, T.A.; Phillips, S.M.; Mackey, A.L.; Nader, G.A.; Boppart, M.D.; Kavazis, A.N.; Reidy, P.T.; Ogasawara, R.; et al. Mechanisms of mechanical overload-induced skeletal muscle hypertrophy: Current understanding and future directions. Physiol. Rev. 2023, 103, 2679–2757. [Google Scholar] [CrossRef] [PubMed]
- Herzog, W. Mechanisms of enhanced force production in lengthening (eccentric) muscle contractions. J. Appl. Physiol. 2014, 116, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.C.; Monroy, J.A.; Uyeno, T.E.; Yeo, S.H.; Pai, D.K.; Lindstedt, S.L. Is titin a ‘winding filament’? A new twist on muscle contraction. Proc. Biol. Sci. 2012, 279, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Guharay, F.; Sachs, F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J. Physiol. 1984, 352, 685–701. [Google Scholar] [CrossRef]
- Spangenburg, E.E.; McBride, T.A. Inhibition of stretch-activated channels during eccentric muscle contraction attenuates p70S6K activation. J. Appl. Physiol. 2006, 100, 129–135. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.P. Recent advances in the understanding of the repeated bout effect: The protective effect against muscle damage from a single bout of eccentric exercise. Scand. J. Med. Sci. Sports 2003, 13, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Aldayel, A.; Jubeau, M.; Chen, T.C. Muscle damage induced by electrical stimulation. Eur. J. Appl. Physiol. 2011, 111, 2427–2437. [Google Scholar] [CrossRef]
- Brooks, S.V.; Zerba, E.; Faulkner, J.A. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J. Physiol. 1995, 488 Pt 2, 459–469. [Google Scholar] [CrossRef]
- Fochi, A.G.; Damas, F.; Berton, R.; Alvarez, I.; Miquelini, M.; Salvini, T.F.; Libardi, C.A. Greater eccentric exercise-induced muscle damage by large versus small range of motion with the same end-point. Biol. Sport 2016, 33, 285–289. [Google Scholar] [CrossRef]
- Hayashi, K.; Katanosaka, K.; Abe, M.; Yamanaka, A.; Nosaka, K.; Mizumura, K.; Taguchi, T. Muscular mechanical hyperalgesia after lengthening contractions in rats depends on stretch velocity and range of motion. Eur. J. Pain 2017, 21, 125–139. [Google Scholar] [CrossRef]
- Talbot, J.A.; Morgan, D.L. The effects of stretch parameters on eccentric exercise-induced damage to toad skeletal muscle. J. Muscle Res. Cell Motil. 1998, 19, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Snijders, T.; McKay, B.R.; Parise, G.; Verdijk, L.B.; Tarnopolsky, M.A.; Gibala, M.J.; Van Loon, L.J. Eccentric exercise increases satellite cell content in type II muscle fibers. Med. Sci. Sports Exerc. 2013, 45, 230–237. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Olson, T.; Welling, T.; Groscost, L.; Parcell, A.C. Satellite cell activity is differentially affected by contraction mode in human muscle following a work-matched bout of exercise. Front. Physiol. 2014, 5, 485. [Google Scholar] [CrossRef] [PubMed]
- Karimi Majd, S.; Gholami, M.; Bazgir, B. PAX7 and MyoD Proteins Expression in Response to Eccentric and Concentric Resistance Exercise in Active Young Men. Cell J. 2023, 25, 135–142. [Google Scholar] [CrossRef]
- Fukada, S.I.; Higashimoto, T.; Kaneshige, A. Differences in muscle satellite cell dynamics during muscle hypertrophy and regeneration. Skelet. Muscle 2022, 12, 17. [Google Scholar] [CrossRef]
- Boppart, M.D.; Mahmassani, Z.S. Integrin signaling: Linking mechanical stimulation to skeletal muscle hypertrophy. Am. J. Physiol. Cell Physiol. 2019, 317, C629–C641. [Google Scholar] [CrossRef]
- Baroni, B.M.; Pompermayer, M.G.; Cini, A.; Peruzzolo, A.S.; Radaelli, R.; Brusco, C.M.; Pinto, R.S. Full Range of Motion Induces Greater Muscle Damage Than Partial Range of Motion in Elbow Flexion Exercise With Free Weights. J. Strength Cond. Res. 2017, 31, 2223–2230. [Google Scholar] [CrossRef]
- Váczi, M.; Costa, A.; Rácz, L.; Tihanyi, J. Effects of consecutive eccentric training at different range of motion on muscle damage and recovery. Acta Physiol. Hung. 2009, 96, 459–468. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef]
- McHugh, M.P.; Connolly, D.A.; Eston, R.G.; Gleim, G.W. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 1999, 27, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Whitehead, N.P.; Yeung, E.W. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: Role of ionic changes. J. Physiol. 2005, 567, 723–735. [Google Scholar] [CrossRef]
- Hamer, P.W.; McGeachie, J.M.; Davies, M.J.; Grounds, M.D. Evans Blue Dye as an in vivo marker of myofibre damage: Optimising parameters for detecting initial myofibre membrane permeability. J. Anat. 2002, 200, 69–79. [Google Scholar] [CrossRef]
- Duncan, C.J. Role of intracellular calcium in promoting muscle damage: A strategy for controlling the dystrophic condition. Experientia 1978, 34, 1531–1535. [Google Scholar] [CrossRef]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. Excitation-contraction uncoupling: Major role in contraction-induced muscle injury. Exerc. Sport Sci. Rev. 2001, 29, 82–87. [Google Scholar] [CrossRef]
- Balnave, C.D.; Allen, D.G. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J. Physiol. 1995, 488 Pt 1, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ruell, P.A.; Ghoddusi, M.; Kee, A.; Hardeman, E.C.; Hoffman, K.M.; Thompson, M.W. Ultrastructural changes and sarcoplasmic reticulum Ca2+ regulation in red vastus muscle following eccentric exercise in the rat. Exp. Physiol. 2007, 92, 437–447. [Google Scholar] [CrossRef]
- Yasuda, T.; Sakamoto, K.; Nosaka, K.; Wada, M.; Katsuta, S. Loss of sarcoplasmic reticulum membrane integrity after eccentric contractions. Acta Physiol. Scand. 1997, 161, 581–582. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Wackerhage, H.; De Souza, E. Inter-set stretch: A potential time-efficient strategy for enhancing skeletal muscle adaptations. Front. Sports Act. Living 2022, 4, 1035190. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Mula, J.; Miyazaki, M.; Erfani, R.; Garrison, K.; Farooqui, A.B.; Srikuea, R.; Lawson, B.A.; Grimes, B.; Keller, C.; et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 2011, 138, 3657–3666. [Google Scholar] [CrossRef]
- Fry, C.S.; Lee, J.D.; Jackson, J.R.; Kirby, T.J.; Stasko, S.A.; Liu, H.; Dupont-Versteegden, E.E.; McCarthy, J.J.; Peterson, C.A. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014, 28, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.A. Skeletal muscle fibrosis: An overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Hayao, K.; Tamaki, H.; Nakagawa, K.; Tamakoshi, K.; Takahashi, H.; Yotani, K.; Ogita, F.; Yamamoto, N.; Onishi, H. Effects of Streptomycin Administration on Increases in Skeletal Muscle Fiber Permeability and Size Following Eccentric Muscle Contractions. Anat. Rec. 2018, 301, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Hayao, K.; Tamaki, H.; Tamakoshi, K.; Takahashi, H.; Onishi, H. Myofiber Permeability and Force Production of Rat Muscles Following Eccentric Contractions: The Repeated Bout Effect Depends on the Interval. J. Biomed. Sci. Eng. 2020, 13, 275–289. [Google Scholar] [CrossRef]
- Wang, X.D.; Kawano, F.; Matsuoka, Y.; Fukunaga, K.; Terada, M.; Sudoh, M.; Ishihara, A.; Ohira, Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol. 2006, 290, C981–C989. [Google Scholar] [CrossRef]
- Tamaki, H.; Tomori, K.; Yotani, K.; Ogita, F.; Sugawara, K.; Kirimto, H.; Onishi, H.; Yamamoto, N.; Kasuga, N. Electrical stimulation of denervated rat skeletal muscle retards trabecular bone loss in early stages of disuse musculoskeletal atrophy. J. Musculoskelet. Neuronal Interact. 2014, 14, 220–228. [Google Scholar]
- Tamaki, H.; Yotani, K.; Ogita, F.; Hayao, K.; Kirimto, H.; Onishi, H.; Kasuga, N.; Yamamoto, N. Low-Frequency Electrical Stimulation of Denervated Skeletal Muscle Retards Muscle and Trabecular Bone Loss in Aged Rats. Int. J. Med. Sci. 2019, 16, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Yotani, K.; Ogita, F.; Sugawara, K.; Kirimto, H.; Onishi, H.; Kasuga, N.; Yamamoto, N. Effect of electrical stimulation-induced muscle force and streptomycin treatment on muscle and trabecular bone mass in early-stage disuse musculoskeletal atrophy. J. Musculoskelet. Neuronal Interact. 2015, 15, 270–278. [Google Scholar]
- Ceglia, L.; Niramitmahapanya, S.; Price, L.L.; Harris, S.S.; Fielding, R.A.; Dawson-Hughes, B. An evaluation of the reliability of muscle fiber cross-sectional area and fiber number measurements in rat skeletal muscle. Biol. Proced. Online 2013, 15, 6. [Google Scholar] [CrossRef]

| Parameters | SR-ECC | LR-ECC |
|---|---|---|
| Range of motion (°) | 5 | 100 |
| Angular velocity (°/s) | 100 | 100 |
| Repetitions per exercise (reps/exercise) | 20 | 1 |
| Number of exercises (exercises/set) | 10 | 10 |
| Sets (sets) | 8 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oga, R.; Nakagawa, K.; Chen, Y.-C.; Nita, Y.; Tamaki, H. Impact of Eccentric Exercise Interventions with Small and Large Ranges of Motion on Rat Skeletal Muscle Tissue and Muscle Force Production. Int. J. Mol. Sci. 2024, 25, 8978. https://doi.org/10.3390/ijms25168978
Oga R, Nakagawa K, Chen Y-C, Nita Y, Tamaki H. Impact of Eccentric Exercise Interventions with Small and Large Ranges of Motion on Rat Skeletal Muscle Tissue and Muscle Force Production. International Journal of Molecular Sciences. 2024; 25(16):8978. https://doi.org/10.3390/ijms25168978
Chicago/Turabian StyleOga, Ryoya, Koki Nakagawa, Yi-Chen Chen, Yoshihiro Nita, and Hiroyuki Tamaki. 2024. "Impact of Eccentric Exercise Interventions with Small and Large Ranges of Motion on Rat Skeletal Muscle Tissue and Muscle Force Production" International Journal of Molecular Sciences 25, no. 16: 8978. https://doi.org/10.3390/ijms25168978
APA StyleOga, R., Nakagawa, K., Chen, Y.-C., Nita, Y., & Tamaki, H. (2024). Impact of Eccentric Exercise Interventions with Small and Large Ranges of Motion on Rat Skeletal Muscle Tissue and Muscle Force Production. International Journal of Molecular Sciences, 25(16), 8978. https://doi.org/10.3390/ijms25168978






