Abstract
Lupus Nephritis (LN) still represents one of the most severe complications of Systemic Lupus Erythematosus (SLE) and a major risk factor for morbidity and mortality. However, over the last few years, several studies have paved the way for a deeper understanding of its pathogenetic mechanisms and more targeted treatments. This review aims to provide a comprehensive update on progress on several key aspects in this setting: pathogenetic mechanisms of LN, including new insight into the role of autoantibodies, complement, vitamin D deficiency, and interaction between infiltrating immune cells and kidney resident ones; the evolving role of renal biopsy and biomarkers, which may integrate information from renal histology; newly approved drugs such as voclosporin (VOC) and belimumab (BEL), allowing a more articulate strategy for induction therapy, and other promising phase III-immunosuppressive (IS) agents in the pipeline. Several adjunctive treatments aimed at reducing cardiovascular risk and progression of chronic renal damage, such as antiproteinuric agents, represent an important complement to IS therapy. Furthermore, non-pharmacological measures concerning general lifestyle and diet should also be adopted when managing LN. Integrating these therapeutic areas requires an effort towards a holistic and multidisciplinary approach. At the same time, the availability of an increasingly wider armamentarium may translate into improvements in patient’s renal outcomes over the next decades.
1. Introduction
Lupus Nephritis (LN) represents one of the most severe and frequent complications of Systemic Lupus Erythematosus (SLE) and a major risk factor for morbidity and mortality, potentially leading to end-stage renal disease (ESRD) [1].
Etiopathogenetic mechanisms are complex and involve multiple inflammatory pathways and cell types, far exceeding immune complex (IC) deposition. Significant progress has been made over the last years in understanding the role of innate immunity cells, such as neutrophils, monocytes and dendritic cells (DC) [2] and the interaction between them and kidney resident cells [3]. At the same time, the increasing availability of new serum and urinary biomarkers is changing the role of renal biopsy as a diagnostic and prognostic tool [4]; newly approved drugs, such as voclosporin (VOC) and belimumab (BEL), are allowing a more articulate strategy for induction therapy and are even redefining traditional views of induction and maintenance treatment; several other promising phase III-IS agents are in the pipeline are likely to expand the therapeutic landscape in the near future [5]. This review aims to provide a comprehensive update on recent progress on each of these key aspects of LN, with a focus on papers published over the last 4 years (January 2020–June 2024). A combination of Medical Subject Headings (MeSH) and keywords related to SLE, LN, pathogenesis, new therapies, and biomarkers were employed and references of relevant articles were also checked.
2. Epidemiology
SLE affects people of all races, genders, and ages. However, it is more commonly observed in high-income nations, particularly among women in their thirties to forties, with a female-to-male ratio of around 9:1. The worldwide incidence of SLE is estimated to be 5.14 per 100,000 person-years and the prevalence would be 43.7 per 100,000 individuals, affecting a total of 3.41 million people. However, epidemiological data on SLE are missing in 79.8% of nations [6]. Overall more than 10% of kidney biopsies lead to a diagnosis of LN, which affects around 40% of SLE patients [7,8,9], representing the most frequent secondary glomerular disease [10,11,12,13]. In around one-third of patients, it is the presenting feature leading to the diagnosis of SLE [14].
The prevalence of SLE and the chances of developing LN vary considerably between different regions of the world, socioeconomic status, and ethnicities [15]. A greater prevalence of LN is documented among individuals of Hispanic, African, and Asian descent when compared to Caucasians [16,17,18,19]. The prevalence of LN within a total number of diagnoses in renal biopsy registries of different countries is outlined in Table 1.

Table 1.
Prevalence of Lupus Nephritis (LN) in renal biopsy registries from different countries.
The overall incidence of LN seems to be higher in women than men, with a male-to-female ratio of approximately 1:5 [13,18,30]. Women had incidence rates up to 10 times greater than men in the 30–39 year age group, which decreased to levels comparable to those of men after the age of 60, according to a Danish Registry study [31].
Despite progress, LN prognosis remains rather unpredictable and around 10–30% of patients progress to ESRD within the first ten years of the disease [13].
Patients of Hispanic and African descent are characterized by increased disease activity and relapse rates, more rapid progression to chronic kidney disease (CKD) and early mortality [13,16,32], whereas male sex and increased creatinine levels at diagnosis are independent prognostic elements for CKD in Caucasians with LN.
The incidence of ESRD in wealthy nations experienced a significant decline from the 1970s to the mid-1990s and it has since stabilized [33], whereas poverty is a significant risk factor for progression of LN, regardless of race or ethnicity [34].
3. Pathogenesis of Lupus Nephritis (LN)
The pathogenesis of LN is complex and multi-factorial. It involves a variety of extra- and intra-renal pathogenic mechanisms, resulting from genetic predisposition as well as environmental and hormonal factors [35]. We will focus on the most important progress in understanding LN pathogenetic mechanisms.
3.1. Genetics and Epigenetics
Over 100 susceptibility loci in the human genome are linked to SLE and LN. Genetic variants are involved in loss of tolerance against nuclear autoantigens, abnormal lymphocyte and complement function and kidney damage. Genetic variants contribute to the racial disparities and clinical heterogeneity of SLE and LN [36].
The strongest LN genetic association relates to the major histocompatibility complex (MHC) region, with an increased risk due to amplified tissue inflammation (HLA-DR2 and HLA-DR15); on the other hand, different HLA alleles may exert a protective effect (HLA-DR4 and HLA-DR11) [36]. An association between the burden of SLE risk loci and the risk of LN was found in two large multi-ethnic cohorts of 1237 SLE patients. Genetic risk score effects of HLA and non-HLA loci increased in magnitude when analysis was restricted to proliferative classes [37]. Zhang et al. screened a Chinese cohort of 1886 patients with LN by whole-exome sequencing and identified only a small fraction of patients with pathogenic gene variants, primarily related to NF-kB, type I interferon (IFN-I), PI3K/AKT, JAK/STAT, RAS/MAPK and complement pathways [38].
A genome-wide association study of LN in a Han Chinese population found some promising candidates associated with LN, especially polymorphisms related to Transforming Growth Factor-β (TGF β) pathways [39].
Yavuz et al. recently showed that variants in the Mer-tyrosine kinase (MERTK) gene modulate the risk of developing ESRD in SLE [40]. Interestingly, MERTK is a member of the Tyro3/Axl/Mer receptor kinase family and the main receptor for apoptotic cells on macrophages, playing a key role in the regulation of efferocytosis [41].
In addition to this complex scenario of modulating polymorphisms, mendelian monogenic defects are involved in causing early-onset LN in children, young adults, male patients, and familial cases. These determine defects in the clearance of apoptotic cells or ICs, interferonopathies, JAK-STAT-TLRopathies, and T and B cell dysregulation [42].
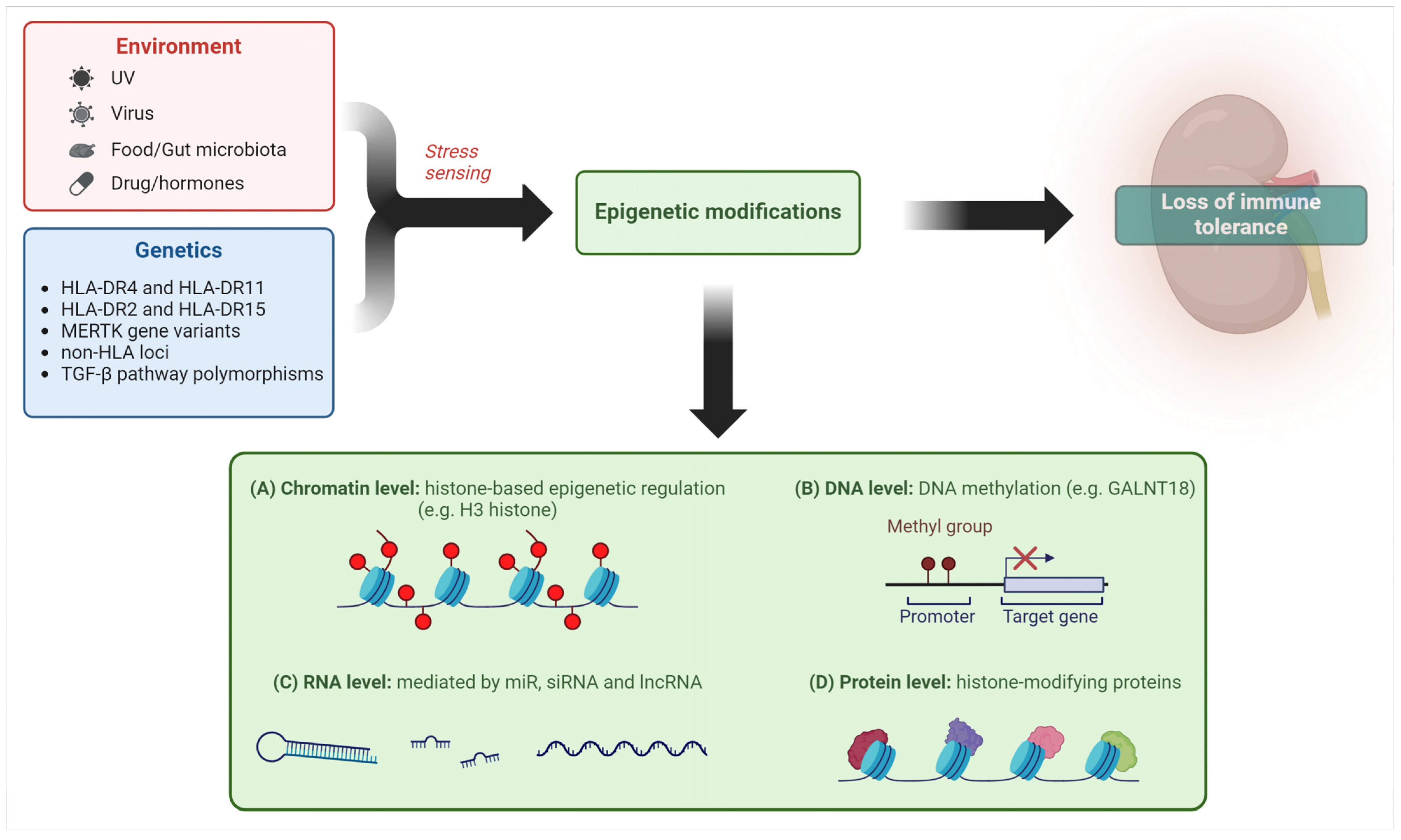
Mechanisms of epigenetic regulation of gene expression have also been investigated in LN, such as DNA methylation/acetylation and histone and non-histone protein modifications [43], as shown in Figure 1.
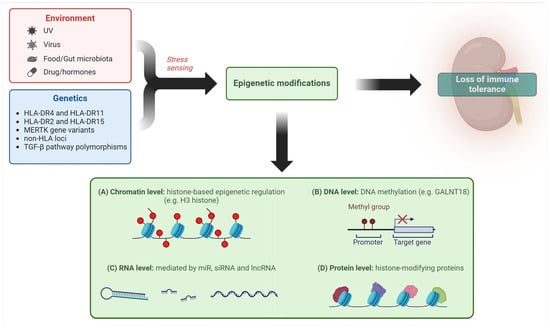
Figure 1.
Different epigenetic mechanisms mediate the impact of environmental factors in predisposing to loss of tolerance in LN (created with BioRender.com). Legend. miR—microRNA; siRNA—small interfering RNA; lncRNA—long non-coding RNA.
Coit et al. demonstrated that demethylation of a CpG site of the GALNT18 gene in neutrophils was relevant to LN in a 4-year longitudinal analysis [44] and Wu proved that an inhibitor of enhancer of zest homologue 2, an enzymatic subunit of a complex which promotes transcriptional silencing by methylating H3 histone, can mitigate LN in a mouse model [45].
The role of microRNAs (miR) in LN pathogenesis has also been increasingly recognized [46]. Significant progress has been made in defining the actions of non-coding RNA (nc-RNA) as epigenetic regulators of gene expression through mRNA targeting. This family includes long non-coding RNA (lncRNAs), characterized by more extended length and containing circular RNA (circRNA), and miRs. Both lncRNAs and circRNA can increase or blunt the generally inhibitory effect of miRNA. Dysfunction of this complex regulatory system is observed in SLE and LN at different levels, for example in renal resident cells and urinary exosomes, and results in abnormal cell proliferation, inflammation, and fibrosis [47].
3.2. Environmental Factors
SLE pathogenesis is probably the result of complex interactions between ethnic, genetic, epigenetic, hormonal and environmental factors, which trigger an immunological disorder. However, only a small number of studies have examined whether environmental exposure interacts with genetic factors, despite the fact that a variety of exogenous triggers, including physical and chemical factors, have been proposed to influence the risk of SLE and LN [48]. We will briefly analyze the most significant ones.
The role of ultraviolet beams (UVB) in modulating the expression of disease is well known [48]. Interestingly, UVB appears to stimulate neutrophil migration also to the kidney in an interleukin (IL)-17A-dependent manner, creating a “kidney-skin” axis [49].
Air pollution has been recently drawing attention. Bai et al. demonstrated that the risk of LN in SLE patients may be increased by short-term exposure to nitrogen dioxide (NO2) and fine particulate matter (PM)2.5, but underlying pathogenic mechanisms are still obscure [50].
The role of viral infections in triggering SLE and modulating the risk of LN is well known [51], including recent reports of de novo LN following SARS-CoV-2 infection [52].
An association between dysbiosis and microbial antigens with LN has been emerging. Bacterial metabolites mimicking autoantigens could be a promising target for disease management [53].
Gut microbiota actually appears to modulate renal inflammation, as a “leaky gut” allows pathogenic bacteria to enter the bloodstream and stimulates the formation of antibodies and ICs which deposit in the kidney in experimental models [54].
The role of food in modulating inflammation is an expanding area of research. Supplementation of vitamin D or E and omega-3 fatty acid have been associated with improvement of inflammatory markers and endothelial function in SLE [55] and curcumin appears to exert interesting anti-inflammatory and antioxidant effects [56] along with an anti-proteinuric effect in LN [57]. These data deserve further study but suggest the potential impact of food antigens on autoimmunity.
3.3. Immunological Mechanisms
Dysregulation of a wide range of immune system elements characterizes SLE and LN. We will focus on some key aspects of intrarenal immunological mechanisms: the role of pathogenic autoantibodies and the interaction between infiltrating immune cells and resident cells.
3.3.1. Role of Autoantibodies
The role of autoantibodies and IC production in kidney inflammation is well known [58]. Stimulation of autoantibodies requires a source of extracellular DNA in an immunologically accessible form, such as extracellular DNA emerging from dead and dying cells due to abnormal apoptosis [59]. Autoantibodies directed against nuclear and cellular antigens lead to the formation of ICs, which accumulate in both glomeruli and interstitium, activating complement and recruiting immune cells [60].
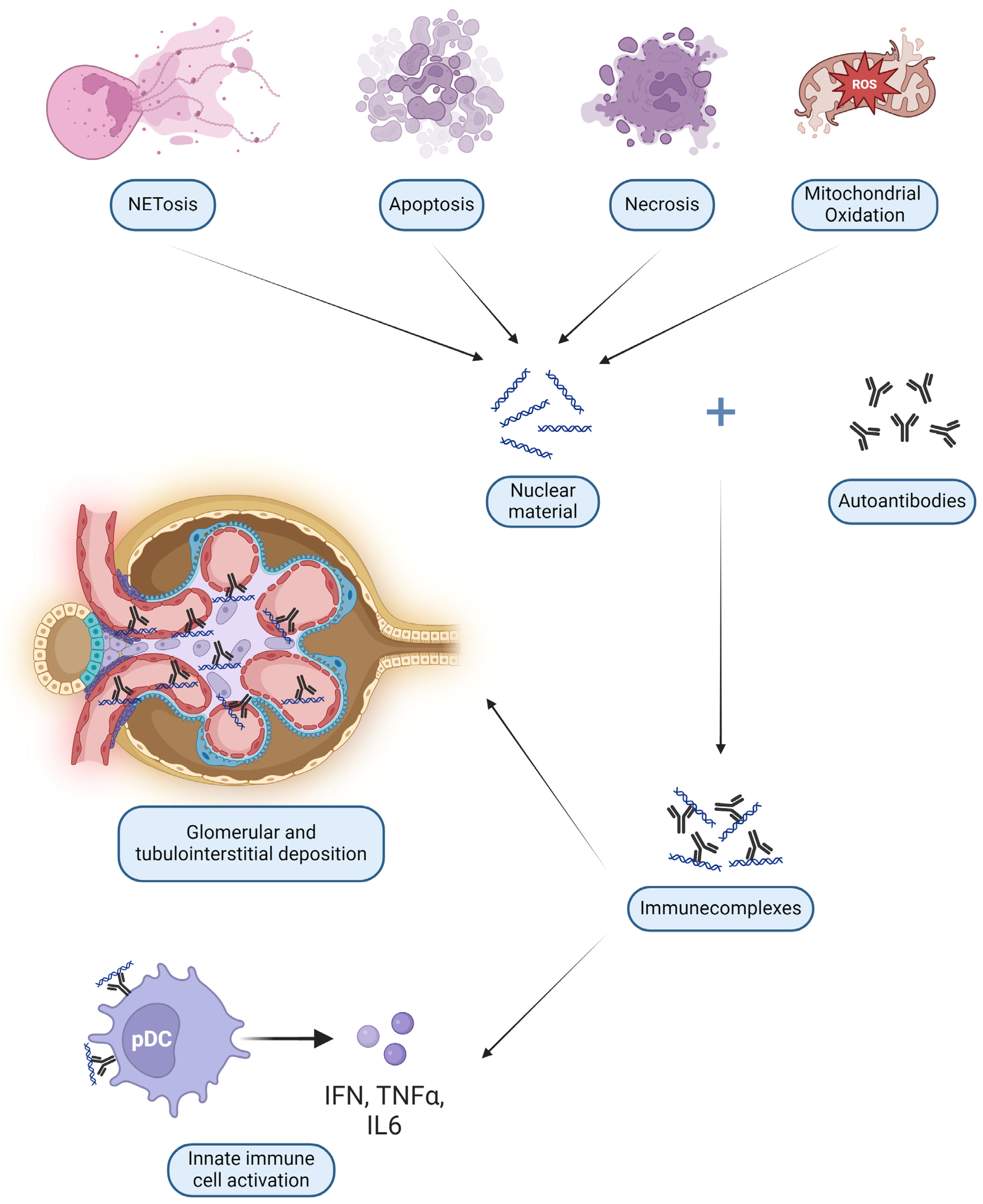
Anti-DNA antibodies are probably the most studied type of autoantibodies. After binding DNA they form ICs with a dual pathogenetic role: glomerular deposition and uptake into innate immune cells. The latter process determines the interaction of nucleic acids with internal sensors, with consequent cell activation and release of cytokines [61]. These DNA and RNA ICs resemble viral particles and elicit the same viral nucleic acid recognition receptors such as Toll-like receptors (TLR) on all antigen-presenting cells (APC), especially B lymphocytes and DC [62]. Plasmacytoid DC (pDC) can then start a broad pseudo-antiviral response, releasing IFN-I and Tumor Necrosis Factor (TNF) α [63], as shown in Figure 2 and detailed in Section 3.3.4.
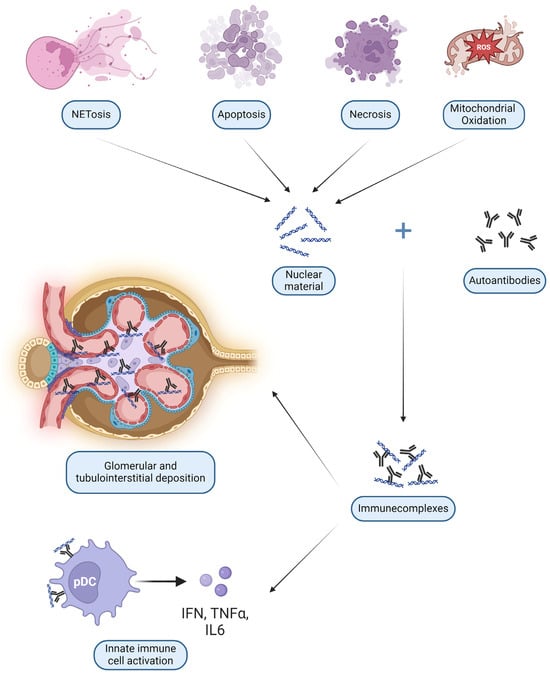
Figure 2.
Main mechanisms of damage by nephritogenic immune complexes (IC): deposition in glomeruli and interstitium and direct activation of innate immunity cells such as plasmacytoid dendritic cells (pDC) (created with BioRender.com).
The specific pathogenetic meaning of traditional types of autoantibodies has been critically reviewed in a recent paper. These Authors underline the weak link between anti-double stranded DNA (anti-dsDNA) and renal pathology and argue that circulating levels of anti-enolase 1 (anti-ENO1) and anti-histone 2 (anti-H2) IgG2 better characterize patients with LN vs. those with non-renal SLE and decrease after IS therapy [60]. Another recently identified autoantibody is represented by anti-superoxide dismutase 2 (SOD2), a ‘second wave antibody’ which may interfere with a protective anti-oxidant mechanism in a late phase of inflammation [64].
Of interest, glycosylation of autoantibodies can modulate their cytotoxic potential in opposite ways, as sialylation reduces [65], whereas fucosylation increases their cytotoxicity [66].
Different autoantibodies co-existing with anti-dsDNA may also contribute to the pathogenesis of LN, including anti-C1q, anti-nucleosome, anti-α actinin, and anticardiolipin (aCL) [60]. Some autoantibody classes such as anti-dsDNA are highly heterogeneous, with multiple target antigens only partly known [67]. Furthermore, considerable cross-reactions exist. For example, α-actinin cross-reacts with anti-dsDNA antibodies and characterizes a subset of anti-dsDNA with high avidity. Interactions with both resident and infiltrating cells are equally complex, as different autoantibodies can trigger a wide range of responses. For example, aCL antibodies can induce mesangial cell (MC) apoptosis [68], whereas anti-dsDNA can stimulate neutrophil extracellular traps (NET) release by neutrophils and increase their immunogenicity [69].
The role of new autoantibodies such as anti-modified/monomeric C-reactive protein (CRP) [70] and anti-NET antibodies (ANETA) [71] is being defined.
3.3.2. The Complex Role of Complement
Complement plays a dual role in LN pathogenesis. Effective clearance of ICs by early complement components protects against the development of LN, as shown by the consequences of genetic defects in complement; however, its uncontrolled activation promotes kidney damage in SLE [72].
While classical pathway activation by ICs was initially regarded as the main contributor to LN pathogenesis, increasing evidence indicates that also alternative and lectin pathways are involved [73].
Excessive complement consumption is a hallmark of SLE. Reduction in plasma levels of C3 and C4 complement fractions indirectly reflects IC formation and immunological activity. C3 reduction, but not C4, correlates with renal flares. These are probably caused by C3 activation in the kidney, promoting inflammation and exposing C3b epitopes with consequent production of anti-complement antibodies, suggesting an important role of alternative pathway [72].
Recent studies have highlighted the role of an elevation of circulating complement split products, C3dg, iC3b, and C4d; interestingly, C3dg/C3 and iC3b/C3 ratios correlate with active SLE and C4d are higher in patients with LN [74].
Cell-bound complement activation products also seem to play a pathogenetic role. For example, erythrocyte-bound C4d levels better correlate with disease activity than low plasma complement levels and are elevated in LN. Moreover, C4d deposition in renal peritubular capillaries predicts a worse prognosis [75].
A better understanding of the contribution of complement pathways to LN pathogenesis may pave the way for novel therapies targeting this system and clinical trials with several anti-complement molecules are ongoing. This approach, however, is complicated by the complement’s dual role, providing protection on the one hand and mediating tissue damage on the other [72].
3.3.3. Vitamin D Deficiency
Vitamin D deficiency (serum 25-hydroxyvitamin D3 < 15 ng/mL) is common in SLE due to a variety of reasons: reduced sun exposure to avoid photosensitivity, possible interference by glucocorticoids (GC) and hydroxychloroquine (HCQ), presence of autoantibodies against it. Vitamin D has complex antiproliferative and immunomodulating functions and low levels correlate with consumption of C3 and C4 complement fractions and higher SLEDAI [76].
In a recent study, serum 25-hydroxyvitamin D3 levels in patients with initial-onset childhood SLE negatively correlated with T helper (Th)-17 and related cytokines and positively with T regulatory (Treg) subset [77].
Interestingly, patients with LN have lower serum levels than patients with LES without renal involvement and renal tissue expression of vitamin D receptors is reduced and negatively related to the activity index of LN [78]. Expression of vitamin D-synthetizing enzyme 1α-hydroxylase in peripheral blood mononuclear cells is also significantly reduced in SLE patients, especially if LN is present, and negatively correlates to SLEDAI [79].
A growing body of evidence suggests that vitamin D regulates the renin-angiotensin-aldosterone system, inhibits renal inflammation, preserves the expression of nephrin and podocin and protects podocytes damaged by autoantibodies from aberrant autophagy in LN [80].
The activated vitamin D form, calcitriol, can potentiate the antiproteinuric effect of angiotensin 2 inhibitors in LN and other types of GN, also exerting actions on podocytes through the vitamin D receptor [81].
Overall vitamin D deficiency appears to be involved both in the mechanism of immune dysregulation of SLE and in the pathogenesis of LN. Furthermore, it seems to exert a broader nephroprotective effect, as demonstrated also in other nephropathies [81].
3.3.4. Role of Infiltrating Immune Cells
The role of infiltrating immune cells in LN has been the focus of intense research over the last 5 years and will be analyzed separately.
Neutrophil
Neutrophils have been emerging as key players in SLE pathogenesis. SLE is characterized by deregulation of hematopoiesis, with inflammatory priming and myeloid skewing of bone marrow-derived hematopoietic stem and progenitor cells (HSPC). Emergency granulopoiesis and extramedullary hemopoiesis (EMH) are amplified and result in an increased release of neutrophils from bone marrow in order to meet the demand for inflamed tissues. EMH develops in the spleen and in the kidneys themselves and correlates with LN severity. Neutrophils produced in these sites are likely involved in endothelial and renal damage [82].
In addition to a strong granulocytic molecular signature in peripheral blood, patients with SLE are characterized by augmented NETosis, a specific form of cellular death in which NETs containing nuclear DNA, chromatin and highly immunogenic and proinflammatory cytoplasmatic proteins are released (Figure 2) [83]. While few intact neutrophils are present in renal biopsy, the persistence of tissue factor and IL 17A-bearing NETs has been demonstrated in proliferative forms of LN and is likely to mediate thrombo-inflammation and fibrosis even in the absence of these cells [84]. Of note, increased autophagy appears to be essential for NETosis and is reduced by HCQ [85].
Deficiency in either DNAase I or C1q, both necessary for NETs degradation and clearance, promotes persistent autoimmune stimulation by nuclear autoantigens [86].
Determination of baseline neutrophil to lymphocyte ratio, of circulating NETs remnants and of ANETA have been proposed as biomarkers to predict activity and outcomes in LN [87].
Monocyte/Macrophage
Monocytes and macrophages are important players in the pathogenesis of LN.
Infiltration of kidneys with these cells is associated with more severe disease and an increased risk of evolution towards ESRD [88].
Bulk transcriptome data identified differentially expressed genes and activation of IFN-I signaling in correlation with the abundance of infiltrating macrophages, both in glomeruli and in tubule-interstitium [89]. Macrophages involved in LN are characterized by a phenotypic change, from inflammatory patrolling monocytes to phagocytic and then to APCs capable of secreting complement components [90]. This evolution results in dysfunctional phagocytosis and an impaired removal of apoptotic cells, aggravating renal damage [91].
High expression of the Sphingosine-1-phosphate (S1P) receptor (S1PR1) and activation of the S1P/S1PR1 axis promotes macrophage accumulation and polarization towards the M1 pro-inflammatory phenotype through NLRP3 inflammasome [92].
Tissue Moesin deficiency has been associated with lupus-like nephritis, with an accumulation of chemokine (C-X-C motif) ligand (CXCL)13-producing patrolling monocytes and macrophages due to reduced migration to S1P [93]. Lymphangiogenesis, which appears to occur in LN but not in normal kidneys, may facilitate the infiltration of LN-specific monocytes [94].
Consistent with the important role played by monocytes, urinary levels of Monocyte chemoattractant protein 1 (MCP-1), which promotes monocyte migration to the kidney, are higher in individuals with active LN and correlate with early activity of LN, facilitating identification of “silent” LN [95].
Lymphocyte
A selective accumulation of T and B cells occurs in LN, with peculiar features. T follicular helper (TFH) and Th cells are an important pathogenic subset of CD4+ T cells in SLE. An increased TFH/Treg ratio can be observed in the peripheral blood of patients with active LN, especially in classes III and IV, and immunohistochemistry has revealed TFH1 cell infiltration in kidneys with LN [96]. A specific subset of infiltrating C-C chemokine receptor (CCR)4+ TFH lymphocytes seems to be involved in LN pathogenesis and T cell receptor repertoire appears to be relatively restricted, suggesting an oligoclonal expression of intrarenal lymphocytes [97]. Pathogenic TFH cell accumulation and function have been recently shown to depend on programmed death ligand (PD-L)1 and IL-4 in basophils, which can induce a transcriptional program leading to TFH2 cell differentiation [98].
The SLAM-associated protein (SAP) regulates TFH and Th function by binding to the co-stimulatory signaling lymphocyte activation molecule family (SLAMF) receptors that mediate interactions between T and B cells. SAP and SLAMF play a key role in Th-dependent B cell maturation into autoantibody-producing plasma cells (PC) in SLE. SAP expression is increased in LN in infiltrating T cells, including the TFH-like CD4+ and effector CD8+ T cells Furthermore the frequency of SAP+Th in circulation appears to correlate with disease activity and the presence of LN [99].
Another important T cell subset is represented by double-negative T cells, which are expanded in active SLE [100] and associated with high IL-17 levels, supporting production of autoantibodies [101].
On the other hand, Sm-specific Tregs (Sm-Tregs) have been shown to suppress disease. An HLA-DR15 restricted immunodominant Sm-T cell epitope suppresses Sm-specific pro-inflammatory responses in vitro and disease progression in a humanized mouse model of LN [102].
Progress in characterizing B lymphocytes has also been made. Memory B cells show a mitochondrial dysfunction in SLE. Reduced expression of an oxidative phosphorylation-regulating gene, Peroxiredoxin 6, has been found in SLE B cells and causes an upregulated mitochondrial respiration and increased antibody production [103]. Interestingly, the oxidative phosphorylation inhibitor IM156 inhibits activated B cells by regulating mitochondrial membrane potential and also blunts LN in the NZB/W F1 mice [104].
Abnormalities in lipid metabolism and mammalian target of rapamycin (mTOR) signaling also seem to play a role in the deranged immunometabolism observed in SLE [105].
Tertiary lymphoid structures (TLS), clusters of immune cells which organize in nonlymphoid tissue within renal interstitium, play an important role in the pathogenesis of LN and correlate with tubulointerstitial inflammation, higher immunological activity and clinical severity. This process is triggered by the local production of chemokines such as CXCL13 and results in an increased local production of IFN-I and also anti-dsDNA, anti-Sm, and anti-RNP. Of interest, the resolution of TLS occurs after inhibiting B-cell activating factor (BAFF) in animal models [106]. The role of tubular epithelial cells (TEC) in stimulating the formation of TLS is described in Section 3.4.1.
Dendritic Cell (DC)
Plasmacytoid DCs (pDCs) are the master producers of IFN-I in response to ICs and play a key role in TLR-mediated development of renal inflammation, autoimmunity, and fibrosis [107].
A novel population of inflammatory DC (infDC) appears to be differentially expressed in the LN kidney. These cells strongly express Fc receptor γ-chain, especially infiltrate periglomerular regions, and are adjacent to intrarenal CD3+ T cells, suggesting an interaction with these cells [108].
A strong interaction with CD4+ T cells is also typical of another recently characterized DC subset, CD163+DC (DC3s), which is enriched in LN and correlates with severity. DC3s contribute to intrarenal T cell expansion, effector T cell activation and polarization towards the Th1/Th17 phenotype. Of interest, injured proximal TECs may play a role in triggering DC3 recruitment within LN kidneys [109].
The autophagy-lysosome pathway has been identified as a potential mechanism involved in DC dysregulated maturation which is typical of SLE. TLR 9 seems to be involved in the activation of autophagy and lysosome acidification through the TRAF6-cGAS-STING pathway, making this a potential therapeutic target to modulate DC function [110].
Mitophagy dysfunction is another critical aspect in the development of LN and a novel mitophagy inducer, UMI-77, mitigated histological damage in the murine model of LN by inhibiting DC proinflammatory phenotypes. This drug also restored mitochondrial function in myeloid cells from patients with LN in vitro [111].
A recent study has demonstrated that IgA autoantibodies against a major SLE autoantigen, Sm ribonucleoproteins, play a role in IC-mediated activation of pDCs, which express the IgA-specific Fc receptor, FcαR. IgA1 autoantibodies appear to synergize with IgG in RNA-containing ICs in eliciting a robust IFNα response by pDCs [112].
Finally, recent evidence suggests that autotaxin, an enzyme that catalyzes the production of lysophosphatidic acid in the extracellular space, is produced by pDCs and correlates with levels of IFN-I. Autotaxin is increased in serum and urine of patients with LN and may represent an important biomarker [113].
3.4. Role of Kidney Resident Cells
Autoimmunity alone cannot lead to kidney damage without the essential contribution of resident cells, which are prone to significant functional changes due to chronic inflammation [114]. Several studies have expanded evidence of the important role of kidney resident cells in the pathogenesis of LN. The TECs, glomerular endothelial cell (GEC), MCs, podocyte, and parietal epithelial cell are exposed to ICs and inflammatory cytokines and each cell type actively contributes to inflammatory milieu in LN [115].
Endoplasmic reticulum stress has recently emerged as a shared mechanism of damage of renal resident cells in LN. For example, it can induce podocyte apoptosis, promote the secretion of inflammatory mediators by MCs, expression of adhesion molecules in GECs and apoptosis of TECs [116].
We will briefly analyze the most recent evidence on the role of each cell type.
3.4.1. Tubular Epithelial Cells (TEC)
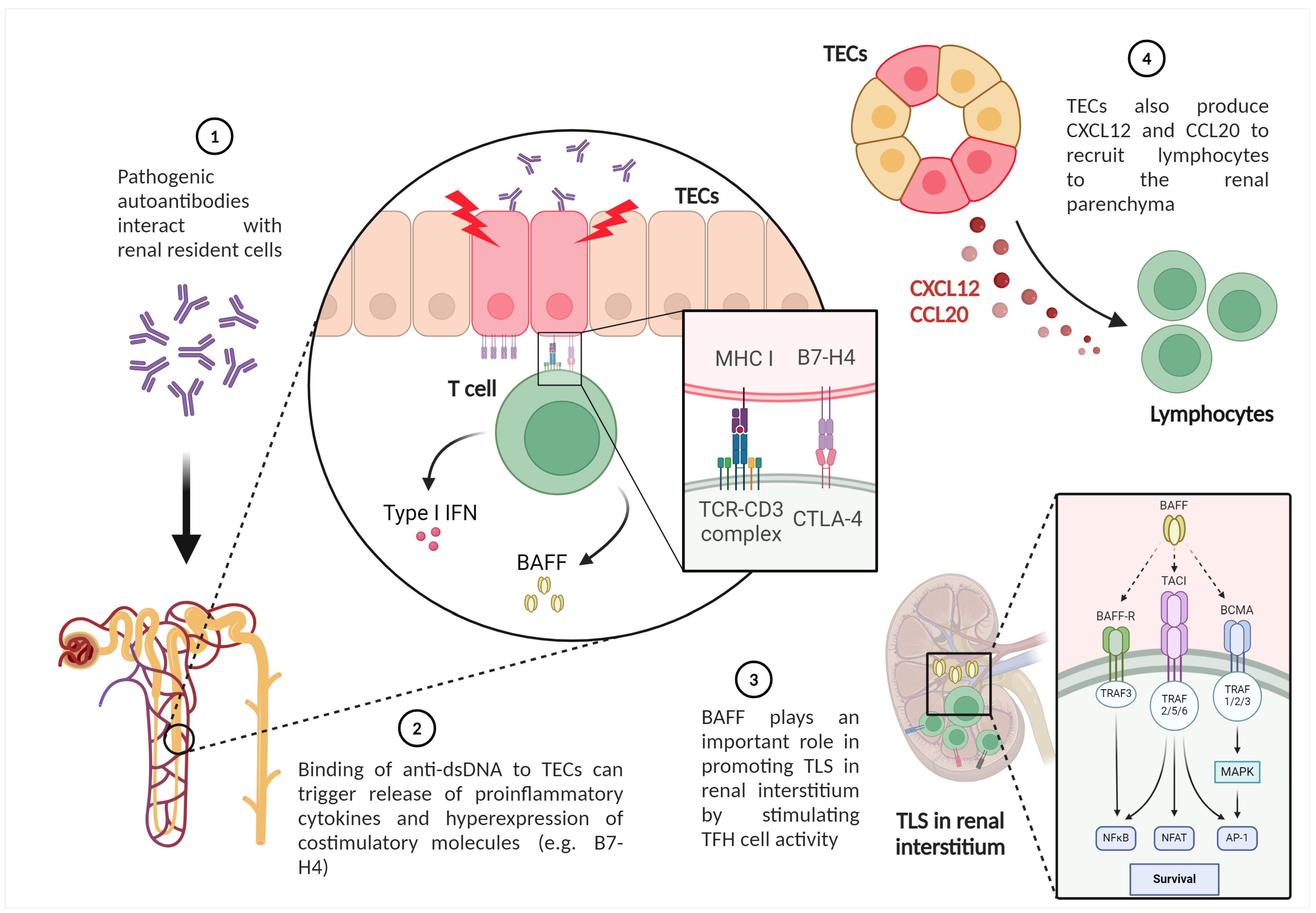
Tubulointerstitial lesions have been recognized as an important component in the pathology of LN. TECs modulate interstitial milieu, promoting T cell infiltration and TLS formation. Loss of TEC integrity has been associated with intrarenal activation of adaptive immunity through several mechanisms, as illustrated in Figure 3. The binding of anti-dsDNA to TECs can directly trigger the release of proinflammatory cytokines. Furthermore, TECs express the costimulatory molecule B7-H4, which can activate T cells and secrete IFN-I and BAFF [117]. Even an autocrine loop of BAFF with its receptors on TEC has been demonstrated. This mediator plays a crucial role in promoting TLS in LN by stimulating TFH activity [115]. In addition, TECs also produce CXCL-12 and C-C motif ligand (CCL)20 to recruit lymphocytes to the renal parenchyma, along with several pro-inflammatory and pro-fibrotic factors [118].
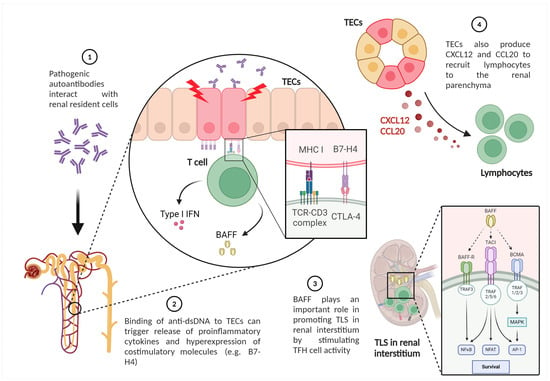
Figure 3.
Activated or damaged renal tubular epithelial cells exert important immunological effects in LN (created with BioRender.com). Legend. TECs—tubular epithelial cells; BAFF—B cell activating factor; MHC—major histocompatibility complex; TLS—Tertiary lymphoid structures; TCR—T cell receptor; IFN—interferon.
3.4.2. Podocyte
As observed for TEC, also podocytes can be directly damaged by autoantibodies in SLE and are involved in the immunological process. Injured podocytes activate innate immunity through the expression of TLRs and also trigger T cells through upregulated MHC and costimulatory molecules (CD80/CD86), acting as APC. Podocytes also contribute to crescent formation, together with parietal epithelial cells [119,120].
3.4.3. Mesangial Cell (MC)
MCs respond early to IC deposition in LN. Anti-dsDNA antibodies, serum, or plasma from patients with LN have been shown to activate multiple signaling pathways such as JAK/STAT/SOCS, PI3K/AKT, and MAPK, inducing proliferation, expression of proinflammatory cytokines and profibrotic factors [121].
Also infiltrating inflammatory cells can stimulate MCs hyperproliferation and production of extracellular matrix, which contribute to glomerular fibrosis. Macrophages modulate the proliferation of MCs by interacting through the CXCL 12/dipeptidyl peptidase 4 axis. Of interest, treatment with linagliptin inhibited MC proliferation and reduced urinary protein levels in LN mice [122].
Renal deposition of Pentraxin 3 correlates with proteinuria and inflammation in LN and facilitates MC proliferation through the MAPK/ERK1/2 signaling pathway. Protopanaxadiol can effectively inhibit the abnormal proliferation of MCs and improve proteinuria by inhibiting this pathway [123].
MCs also exert phagocytosis, APC function and proinflammatory effects, aberrantly participating to a renal-resident immune response which triggers a second wave of mesangial damage after the initial phase of IC deposition [124].
3.4.4. Glomerular Endothelial Cell (GEC)
Deposition of ICs within the subendothelial area appears to affect GEC functions, including activation of apoptosis, inhibition of angiogenesis [125], and dysregulation of the coagulation/fibrinolysis system [126]. Increased expression of TLR3 on GEC mediates the production of IFN-I and consequently of IFN-stimulated genes. Among these, IFN-stimulated exonuclease gene (ISG 20), coding for an antiviral effector protein, was intensely expressed in biopsy specimens from proliferative LN and regulated CX3CL1 production, promoting glomerular inflammation [127].
Renal endothelial-podocyte crosstalk, an important aspect of LN, is mediated by activation of the mTOR signaling pathway. Glomerular activation of the mTOR pathway was significantly increased in patients with endocapillary hypercellularity and podocyte damage [128].
4. The Role of Renal Biopsy and of New Biomarkers in the Management of LN
Despite progress in the discovery of diagnostic and prognostic biomarkers, renal biopsy remains essential in clinical practice to diagnose LN, monitor its activity, and provide prognostic elements [129].
4.1. Renal Biopsy
4.1.1. Renal Biopsy as a Diagnostic Tool
Renal biopsy remains an essential diagnostic tool to classify renal lesions and provide information about activity or chronicity, all crucial aspects to guide therapy. A revised renal classification of the International Society of Nephrology/Renal Pathology Society classification, published in 2018, eliminated some class subdivisions of previous versions (class IV segmental and global) and proposed the modified National Institutes of Health chronicity index to define the degree of activity in all classes [130]. A large study validated this new classification in a Chinese population in 2020, showing that fibrous crescents, tubular atrophy/interstitial fibrosis and this chronicity index reliably predicted a composite renal outcome [131].
Despite such improvements, however, four important histological patterns are not included in this classification: glomerular crescents, lupus podocytopathy, tubulointerstitial lesions, and thrombotic microangiopathy (TMA). These peculiar lesions and their underlying pathogenesis deserve further study and may require specific therapies as compared to “traditional” classes [132]. The presence of TMA, for example, can often be resistant to first-line IS therapy and might benefit from plasma exchange or anti-complement agents [133]. A wider classification of LN has been proposed on this basis [132].
Another aspect is the high burden of premature arteriosclerotic renal lesions which has been demonstrated in a recent study, suggesting that these develop two decades earlier in LN patients compared to their healthy peers and are overlooked by pathologists in half of cases [134].
A recent study has underlined that the assessment of interstitial inflammation in the entire cortical parenchyma (and not only in unscarred areas) allows for the identification of patients at risk for CKD progression in LN, in contrast to the current classification of interstitial inflammation [135].
Finally, inter-rater reliability remains a limit of current classification. The inclusion of molecular classifiers into histologic analysis has been proposed to improve diagnostic precision and identify more precisely kidney biopsy phenotypes and therapeutic targets. For example, an abundance of transcripts related to glomerular fibronectin, secreted phosphoprotein-1, and galectin-3 appears to correlate with disease activity and response to treatment [136]. Identifying different genetic landscapes of LN based on patterns defined by the expression of sets of “hub genes” is an evolving perspective, which will likely change the role of traditional analysis of renal biopsy, as discussed in Section 8 [137,138,139].
4.1.2. Renal Biopsy as a Prognostic Tool
Currently, only serum creatinine and proteinuria can predict long-term evolution in LN. For example, achieving a reduction of proteinuria below 0.5 g/d with preserved renal function—complete remission (CR)—has prognostic value and a cut-off level of 0.7 g/d at month 12 has been associated with stable renal function after 7 years [140]. However, this parameter has limited specificity: proteinuria can express either residual active inflammation or onset/evolution of chronic lesions and histological activity may persist despite reduction of proteinuria [141]. This makes interpretation difficult especially in cases of partial renal response (PRR), expressed by a reduction of 50% in proteinuria to sub-nephrotic levels.
Potential significant dissociation between clinical and histological findings makes renal biopsy still pivotal in managing LN, both for initial diagnosis and as a tool for response assessment (repeat or per-protocol biopsies). The prognostic value of tubulo-interstitial inflammation on 10-year risk of ESRD and of crescents on mortality has been recently confirmed by a real-world Chinese study [142] and similar results have been reported in other ethnicities [143]. Ideal timing for per-protocol repeat renal biopsy is debated [144]. An early approach (6 months after diagnosis) has been proposed to verify the response to induction, as earlier detection of poor prognostic signs in patients without clinical deterioration might improve outcomes [145]; alternatively, a renal biopsy could be performed after 1–2 years to assess treatment efficacy and modulate the duration of maintenance therapy [146].
The “ReBIOLUP” randomized study is currently evaluating the role of per-protocol repeat biopsies in guiding therapy, especially at the initial phase (incident LN), to prevent residual inflammation and long-term nephron loss [147]. Renal biopsy remains essential for the concept of “treat to target” in LN, as CR can only be reliably defined through histology.
Findings at the second per-cause biopsy, but not at the first one, provided histological predictors of long-term risk of ESRD in persistently active or relapsing LN in a large Italian study [148,149].
4.2. Potential Biomarkers
There is an unmet need for non-invasive biomarkers of disease activity to inform treatment responses and guide therapy.
Several biomarkers hold promise towards optimization of LN management, with the use of integrated omics and sets of biomarkers which could, in the future, limit the need for renal biopsies [150]. The main ones available are summarized in Table 2.
Urinary biomarkers are especially interesting as they may better reflect the compartmentalized renal response in LN, unlike serum studies, which are generally less kidney-specific [4]. Increasingly widespread use of cutting-edge omic technologies is leading to the discovery of many novel potential biomarkers for LN [151].

Table 2.
Main serum and urinary candidate biomarkers for LN.
Table 2.
Main serum and urinary candidate biomarkers for LN.
| Biomarker | Biological Fluid | Associations | References |
|---|---|---|---|
| u-Gal-3BP | Urine | Histological disease activity | [152,153,154] |
| MCP-1 | Urine | Clinical severity | [155,156] |
| TWEAK | Urine | Diagnosis | [157] |
| CAM | Urine | Clinical severity | [158,159,160] |
| miR146a miR135b | Urine | Histological disease activity; Response to therapy | [161,162,163] |
| Axl | Serum | Diagnosis; Clinical severity; Response to therapy; Prognosis | [164,165,166] |
| HE4 | Serum | Diagnosis | [167,168] |
| IGFBP-2 | Serum | Diagnosis; Clinical severity; Organ damage | [169] |
| miR | Serum | Diagnosis | [170,171] |
| IL-17 and IL-18 | Serum | Clinical severity and histological activity | [172,173,174] |
| sTNFRII | Serum | Clinical severity and histological activity Organ damage | [175,176] |
| Adipokines | Serum | Clinical severity; Atherosclerotic organ damage and insulin-resistance | [177,178,179,180] |
| BAFF and APRIL | Serum | Clinical severity and histological activity; Response to therapy | [181,182,183,184,185,186,187] |
| Syndecan-1 | Serum | Clinical severity and histological (tubulointerstitial) activity | [188] |
Legend. u-Gal-3BP—Urinary galectin 3 Binding protein; MCP-1—Monocyte chemoattractant protein 1; TWEAK—TNF-like weak inducer of apoptosis; CAM—cell adhesion molecule; MiR—MicroRNA; HE4—Human epididymis protein 4; IGFBP2—Insulin-like growth factor-binding protein 2; BAFF—B cell activating factor; APRIL—a proliferation inducing ligand.
4.2.1. Urinary Biomarkers
Urinary Galectin 3 Binding Protein
This β-galactosidase-binding lectin involved in apoptosis, inflammation, and fibrosis, is often released into biological fluids, including urine, from the surface of injured and inflammatory cells. It appears to be a diagnostic or prognostic biomarker for several autoimmune disorders and kidney disease at their early stages [152]. In the setting of SLE, it can discriminate against patients with active LN from active non-renal and inactive patients. Furthermore, it correlates with histological activity, with higher levels detected in proliferative (class III/IV) and membranous LN than in mesangial (class II) form [153]. This profile makes it a potential surrogate biomarker of renal biopsy [154].
Monocyte Chemoattractant Protein-1 (MCP-1)
Also referred to as CCL2, it is a key mediator of innate immunity involved in kidney disease-related inflammation and its expression directly correlates with the severity of nephropathy. MCP-1 can induce migration and infiltration of lymphocytes, NK cells, and monocytes [155]. Of interest, CCR2, the receptor of MCP-1, is highly expressed on the surface of peripheral γδT cells, which accumulate in renal tissue in LN [156].
TNF-like Weak Inducer of Apoptosis (TWEAK)
TWEAK is an important member of the TNF superfamily. Upon binding to its receptor, fibroblast growth factor-inducible 14 (Fn14), it regulates inflammatory and fibrotic processes. Dysregulation of the TWEAK/Fn14 axis probably plays a significant role in LN [157].
Cell Adhesion Molecules (CAM)
Several urinary CAMs released from cell membranes could serve as biomarkers, such as vascular CAM1 (VCAM-1), activated leukocyte CAM (ALCAM, or CD 166), kidney injury molecule 1 (KIM1), neutrophil gelatinase-associated lipocalin (NGAL) and soluble CD163 receptor (sCD163) shed by M2 macrophages. Overall elevated urinary levels of these CAMs can reliably distinguish renal from non-renal SLE and active from inactive LN.
ALCAM binds to the CD6 receptor on T lymphocytes, resulting in activation and recruitment of these cells into renal tissue. Levels above threshold value of 270 ng/mg can identify active LN and are positively correlated with SLEDAI and negatively with complement C3 and C4 fractions [158]. An ELISA assay for urinary ALCAM may represent a convenient tool for early detection of LN and of renal flares, even facilitating home-monitoring of LN activity [159].
NGAL has shown the best performance in predicting clinical response 6 month after induction therapy among these CAMs. Furthermore, NGAL urinary levels are especially high in class IV LN; in this setting they positively correlate with anti-ds-DNA and proteinuria and negatively correlate with serum albumin and C3 fraction level [160].
MicroRNA (miRs)
A recent meta-analysis identified 4 urinary miRs isolated in extracellular vesicles (EV) related to several pathways involved in LN [161]. Among these, miR146a baseline levels appear to be associated with albuminuria and renal flares [162], whereas miR 135b would identify responders to IS therapy [163].
4.2.2. Serum Biomarkers
Axl
Axl is an important tyrosine kinase receptor found in myeloid cells, with a role in immune innate system regulation and in the clearance of apoptotic cells [164]. Elevated sAxl levels are found in LN and correlate with renal activity. Furthermore, worse chronicity scores are associated with elevated post-treatment sAxl levels. These features make Axl a potential biomarker to monitor renal response to IS therapy and the progression of renal damage in LN [165]. Persistently high sAxl levels after treatment completion may suggest the need for intensified treatment [166].
Human Epididymis Protein 4 (HE4)
HE4 is commonly used as a tumor marker, especially for ovarian cancer, and elevated serum levels can also be found in CKD [167]. Increased serum HE4 levels have been recently found to be independently linked to a higher risk of developing LN. However, renal dysfunction might reduce HE4 clearance, complicating the interpretation of elevated serum HE4 levels in this setting [168].
Insulin-like Growth Factor-Binding Protein 2 (IGFBP2)
IGFBP-2 is a member of the IGFBPs family, with a potential role as a biomarker for several malignant tumors [189]. Patients with LN are characterized by elevated levels of serum IGFBP-2 compared to CKD patients and healthy controls, making it a potential diagnostic biomarker reflecting renal and global immunological activity. Serum IGFBP-2 can also correlate with serological (anti-dsDNA antibody titers and complement levels) and renal parameters (serum creatinine and urine protein-to-creatinine ratio), in addition to the renal chronicity index [169].
miR-21
Inhibition of miR-21 in T and B cells may improve multiple organ damage in SLE, suggesting a pathogenetic role [170]. A more intense expression of miR-21 in active LN than in LN-absent and inactive LN patients makes it an interesting tool. However, there was no significant correlation between miR expression and LN pathological classes [171].
IL-17 and IL 18
Some cytokines are especially associated with LN and could represent biomarkers. Circulating IL-17 levels are higher in patients with active LN than in patients with inactive LN or controls. IL-17-producing cells are present in glomeruli from LN patients and are associated with complement activation and increased immunoglobulin deposition. IS therapy appears to reduce IL-17 levels in active LN and a significant correlation exists between LN exacerbations, elevated serum levels of IL-17 and IL-23, and SLEDAI [172]. Another important cytokine is IL-18, which plays a major pathogenetic role in LN by promoting cytokine imbalance towards Th1-type immune response [173]. Interestingly, heightened levels of IL-18 and its binding protein (IL-18 BP) have been found in both serum and glomeruli of patients with active LN [174].
Soluble TNF Receptor 2 (sTNFR2)
Soluble TNF receptor 2 (sTNFR2) is mainly expressed on Treg cells and plays an important role in regulating apoptosis and proliferation of thymocytes and cytotoxic T-cells. sTNFR2 levels are higher in SLE patients than in HC and they correlate with disease activity and evolution, decreasing after IS treatment [165]. In another recent study sTNFR2 levels correlated with chronic index scores in renal biopsies and long-term eGFR deterioration [175]. Overall sTNFR2 may represent a biomarker of therapy response, LN activity, and prognosis [176].
Adipokines
LN patients are characterized by endothelial dysfunction and premature atherosclerosis and proinflammatory adipokines are involved in this process and may represent biomarkers.
Serum but not urine resistin has been correlated with SLE disease activity, insulin resistance [177], and renal dysfunction in LN [178].
Adiponectin, leptin, and visfatin levels, along with Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) index, were higher whereas brachial artery flow-mediated vasodilatation was lower in LN cases than in SLE without renal involvement in a recent study [179].
Higher levels of adiponectin and leptin were confirmed to be higher in SLE patients and positively correlated with SLEDAI and LN, as well as with greater BMI and CRP levels [180].
B Lymphocyte Activating Factor (BAFF) and “a Proliferation-Inducing Ligand” (APRIL)
BAFF is a B cell survival factor which supports autoreactive B cells and is strongly involved in SLE pathogenesis. In addition to autoimmunity, it is also involved in adipogenesis, atherosclerosis, and neuroinflammation [181].
BAFF is overexpressed in SLE, a significant correlation between serum BAFF levels and disease activity has been demonstrated [182] and renal tissue expression of BAFF and its receptors is associated with class IV proliferative LN [183].
Serum BAFF and IFN-I have been proposed as biomarkers to stratify patients; high BAFF identifies those with LN and high IFN-I marks those with blood and skin manifestations. Levels of these two pivotal cytokines would guide therapy with appropriate biologics [184].
The TNF superfamily member “a proliferation-inducing ligand” (APRIL) plays a late role in humoral immunity at the level of antibody-producing PCs and is considered a target to dampen autoantibody production [185]. High serum levels of APRIL characterize severe proliferative LN with specific lesions such as endocapillary proliferation, neutrophil infiltration, and fibrinoid necrosis.
Interestingly, both BAFF and APRIL levels are associated with response to IS therapy, although in a different way. Low baseline BAFF levels (<1.5 ng/mL) seem to predict treatment response in LN, especially in proliferative forms, whereas high APRIL levels (>4 ng/mL) strongly predict treatment failure. However, only APRIL levels decrease after induction treatment in responders and its intrarenal mRNA levels would be associated with resistance to treatment, proteinuria, and histological activity [186,187]. These pivotal mediators may help predict response to IS therapy and identification of resistant cases.
Syndecan-1 and Other Glycocalyx Components
Glycocalyx is a gel-like layer at the interface between endothelial cells and the bloodstream, composed of proteoglycans and glycosaminoglycans, glycoproteins, and plasma proteins on the luminal side [190]. This structure can be damaged by multiple noxae including inflammation, which determines the shedding of glycocalyx components. As shedding is an early process associated with endothelial activation and damage, some of these circulating molecules act as “danger-associated molecular patterns” and have been proposed as biomarkers of cardiovascular disease and renal damage in LN. Sydecan-1 is probably the most promising of these molecules, as increased levels are observed in active LN compared to remission and they correlate with anti-dsDNA titer, SLEDAI-2 K, proteinuria, serum creatinine, and severity of interstitial inflammation [188].
Circulating levels of hyaluronan and thrombomodulin have also been associated with LN and represent potential biomarkers [191].
5. Immunosuppressive (IS) Therapies in LN
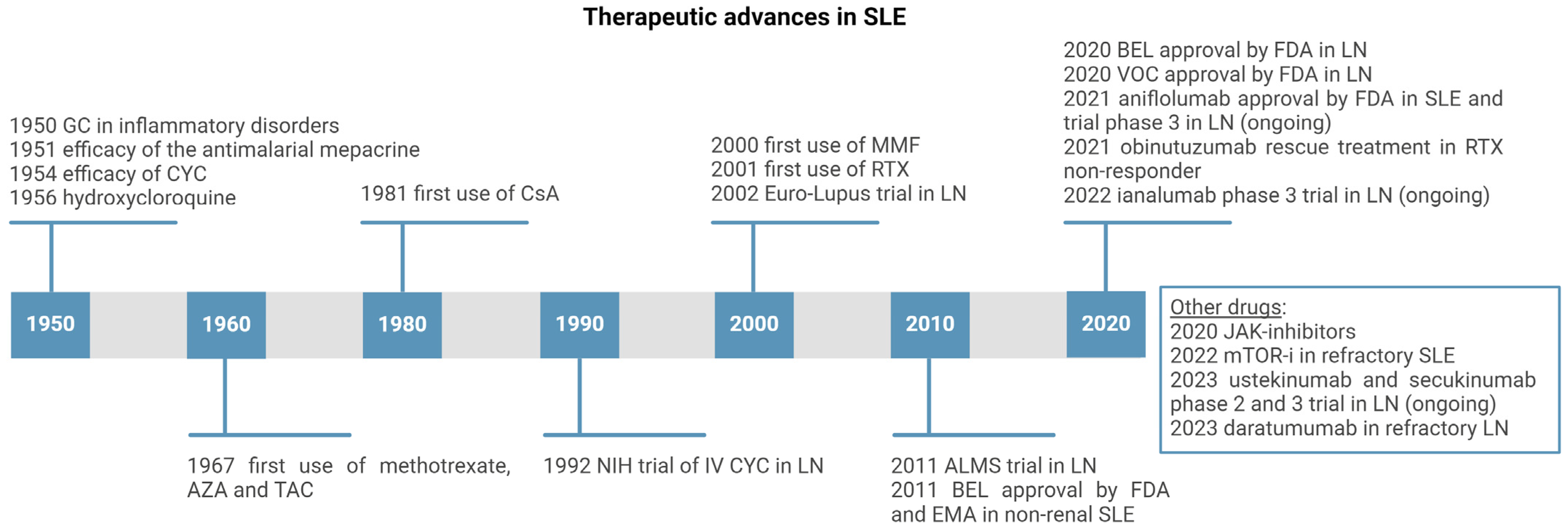
The therapeutic landscape of LN has been rapidly changing over the last few years (Figure 4). We will first analyze conventional IS therapies, which still represent the backbone of IS therapy, along with RTX, and then focus on new FDA-approved drugs, BEL and VOC, and other drugs under investigation in phase II-III trials.
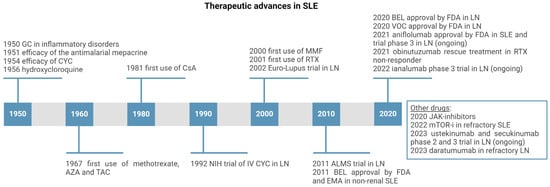
Figure 4.
Timeline of the development of main immunosuppressants employed in treatment of SLE and LN (created with BioRender.com). Legend. GC—glucocorticoid; CYC—cyclophosphamide; CsA—Cyclosporin A; AZA—Azathioprine; TAC—Tacrolimus; MMF—mycophenolate mofetil; RTX—Rituximab; BEL—Belimumab; VOC—voclosporin; JAK—Janus Kinase; mTOR-i—mammalian target of rapamycin inhibitor.
5.1. Conventional Is Therapies
The standard of care of LN has been based on high-dose GCs combined with either cyclophosphamide (CYC) or mycophenolate mofetil (MMF) for induction treatment and on low-dose GC coupled with either MMF or azathioprine (AZA) for maintenance therapy.
We will review the most recent studies on conventional IS therapies for the induction and maintenance phase. Rituximab (RTX), which has been mainly used as rescue therapy for resistant or relapsing forms of LN, will be separately analyzed.
5.1.1. Induction Therapy
A systematic meta-analysis was recently performed on the control arms of 50 RCTs, in which LN was treated with GCs in combination with mycophenolic acid (MPA) analogs or CYC. A dose-response gradient was observed between induction steroid dose and all outcomes at six months. A higher steroid dose was associated with better renal outcomes including CR, but at the price of increased infections and mortality [192]. In a post-hoc analysis of a retrospective multicenter Egyptian study, an increase in mortality was shown with every gram increase in cumulative methylprednisolone (MP), especially in doses exceeding 2.75–3.25 g [193]. Interestingly, the addition of low-dose steroid pulses (MP 125 mg) to each fortnightly dose of 500 mg of CYC within Eurolupus Nephritis Trial (ELNT) protocol appears to enhance response and reduce the need for oral GC in LN of class III, IV and V [194]. This evidence reinforces the concept of using steroid pulses at the lowest possible dose as induction [195].
CYC still remains one of the cornerstones of traditional induction therapy and it is usually administered according to ELNT protocol [196]. Long-term effectiveness in the preservation of renal function [197] and an acceptable profile of side effects [198] are its strengths. Furthermore, it is still the preferred agent in special scenarios of SLE with life-threatening extrarenal manifestation [199] (e.g., central nervous system or pulmonary involvement) and in rapidly progressive LN [200].
Even though MMF was shown to be effective as an alternative induction therapy to CYC, actual superiority of MMF in African Americans and Hispanics has been recently confirmed [201]. Furthermore, a meta-analysis of 18 trials on a Chinese population also showed that MMF was significantly more effective than CYC induction in proliferative LN, with a more favorable profile of side effects [202]. Interestingly, differential effects of CYC and MMF on B cell subsets may partly account for improved response to MMF in high-risk ethnicities. MMF appears to be associated with an earlier reduction of circulating plasmablasts and PCs compared with CYC, whereas CYC would determine preferential depletion of less mature B cells (naïve and pre-switched memory B cells) compared with MMF [203]. An important common limit of CYC and MMF is that neither drug had significant effects on class-switched memory B cells, which are typically resting during active LN but can reactivate and trigger disease relapse in the maintenance phase, even in patients who responded to induction [204].
Another class of drugs which can be employed as induction therapy is represented by calcineurin-inhibitors (CNI). Both tacrolimus (TAC) and cyclosporine A (CsA) have long been used in LN but TAC has been the preferred agent over the last few years [205].
Several RCTs have demonstrated non-inferiority of TAC to MMF or CYC for induction therapy of LN and a low-dose combination of TAC and MMF (“multi-target therapy”) has even been shown to outperform CYC pulses in inducing LN remission in Chinese patients. Furthermore, TAC can be an alternative option for SLE patients who are intolerant or refractory to conventional IS therapy due to its different safety profile and mechanism of action [206]. The availability of VOC is now allowing another type of multitarget therapy (VOC + MMF) (reviewed in Section 5.2.2).
A recent meta-analysis (6 RCTs, including 1.437 patients) showed that different types of multitarget therapies such as VOC + MMF and TAC + MMF achieved a higher CR rate than monotherapy with MMF or CYC, but at the price of numerically higher cases of infections including pneumonia [207].
The concept of multitarget therapy with VOC and also BEL will be further discussed in Section 5.2.
5.1.2. Maintenance Therapy
Accumulating evidence has demonstrated MMF maintenance is associated with a lower risk of disease relapse compared with AZA, making it the drug of choice in this setting [208].
Two important studies published in the last few years have focused on the duration of maintenance therapy with MMF.
A recent RCT, “Weaning of immunosuppressive therapy in LN” (WIN study), compared a protocol of withdrawal of IS therapy after 2–3 years of maintenance therapy versus a standard one of longer duration in proliferative LN, demonstrating that the former was associated with more frequent severe flares at 24 months [209].
In a recent multicenter, open-label, randomized trial conducted in the USA, maintenance with MMF was withdrawn in stable patients (SLEDAI < 4) after 2 years. The increase in clinical disease reactivation by 60 weeks of randomization with MMF withdrawal was 7%, whereas infections were more frequent in the MMF maintenance group. The Authors concluded that MMF withdrawal was not significantly inferior to drug maintenance [210].
Overall optimal duration of maintenance therapy remains uncertain, although the availability of stronger induction protocols (i.e., “triple therapy” with either BEL or VOC) appears to allow a much more rapid steroid tapering and potentially also a global maintenance therapy of reduced intensity [192].
5.1.3. The Role of Rituximab (RTX)
Even if formal supporting evidence is lacking, RTX continues to be used off-label in LN for resistant or relapsing forms and as a steroid-sparing agent, based on a considerable amount of anecdotal clinical data, with a success rate of around 50–70% in proliferative classes [211]. However, current guidelines do not indicate it as a first-line induction drug [212], mainly as a result of a landmark LUNAR study, which did not prove its efficacy as an induction add-on therapy on top of MMF and GC [213]. A likely cause of inadequate response to RTX in this study was incomplete B cell depletion, which might correlate with an inability to reduce tubulointerstitial lymphoid aggregates [214]. A recent real-world Japanese study has underlined that RTX efficacy is heavily patient-driven [215] and that it can trigger production of antibodies which blunt its action [216].
Despite these limits, RTX remains an important biological agent in the management of LN and also its role as an induction agent is still a matter of debate. A recent Bayesian network meta-analysis on Chinese populations (1566 patients from 19 studies) comparing RTX, TAC MMF, and CYC as induction therapies concluded that RTX and TAC were the most effective drugs in order to achieve CR in LN, with RTX determining the highest risk of infection and TAC the lowest one [217].
Obinutuzumab, a new humanized and more potent anti-CD20 monoclonal antibody, may be effective in RTX non-responders and will be discussed in Section 5.3.2.
5.2. New FDA-Approved Drugs
Conventional regimens described in the previous section are generally effective and with an acceptable profile of toxicity. However, a significant proportion of patients experience renal or extra-renal flares (30–40%) and rates of long-term progression towards ESRD have remained unchanged over the last decades (10–30%). This has prompted a search for new treatments, which have exponentially grown especially over the last few years. The main recent phase III trials concerning LN therapy are outlined in Table 3.

Table 3.
Main recent phase III trials in LN therapy.
Since 2020 two drugs were approved by the FDA for induction, BEL and VOC, which can each be added to MMF/MPA and GC, leading to two new IS schemes of “triple therapy”. This multi-target approach adds to traditional “dual therapy” protocols (CYC and steroid; MMF/MPA and steroids) and is paving the way for a more individualized, tailored induction and also for a new vision of induction [224]. Starting with a double therapy and subsequently switching to a triple one if the response is inadequate (“step-up approach”) or, on the contrary, starting with triple therapy and then decreasing it to a double one (“step-down approach”) represent interesting new algorithms requiring further investigations [224,225]. This evolution is leading to a change from the traditional sequential “induction-maintenance” scheme to the concept of continuous, combined triple therapy in selected patients [226]. We will analyze BEL and VOC, which have made this progress possible.
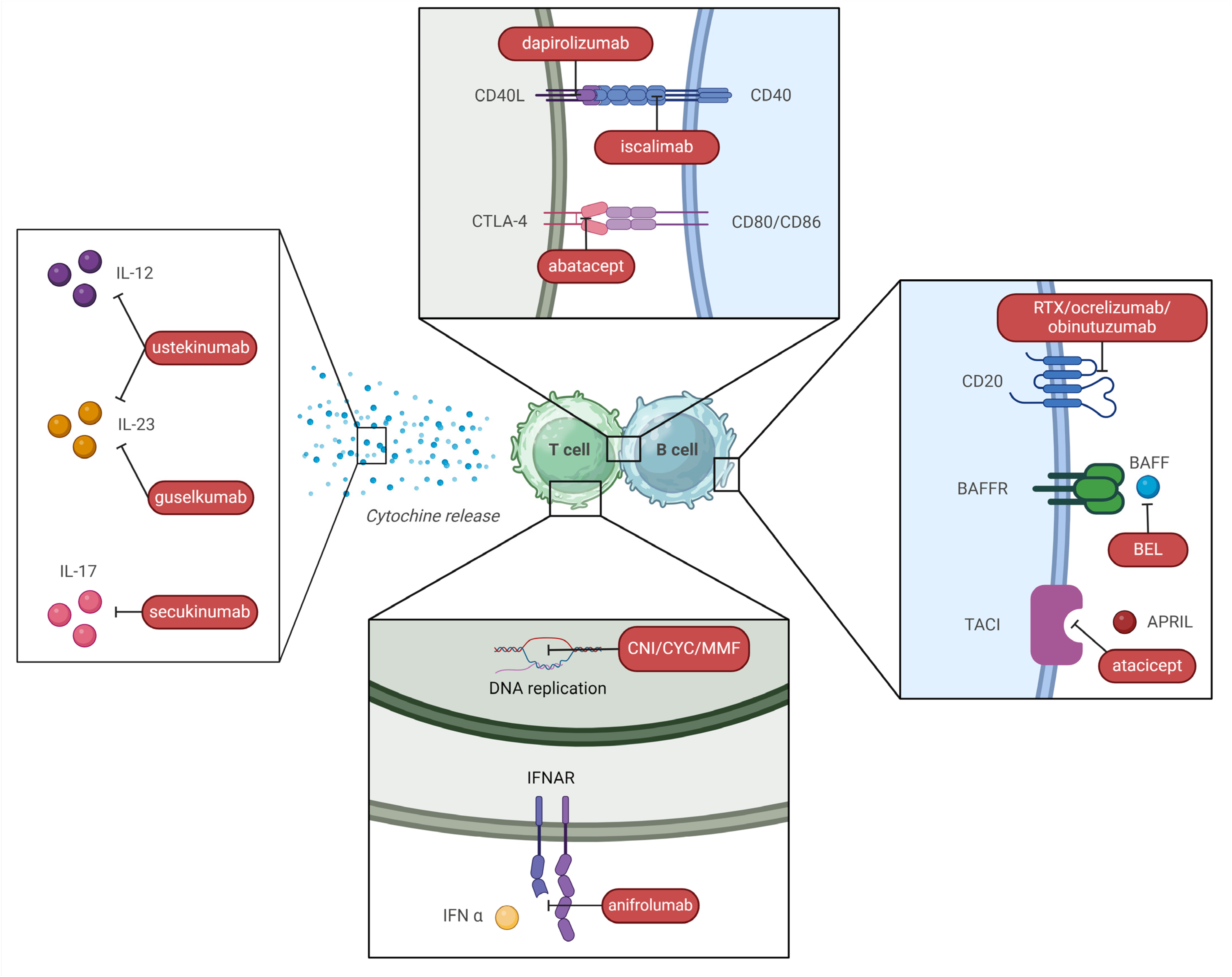
An overview of different mechanisms of action of these drugs and other immunosuppressants is outlined in Figure 5.
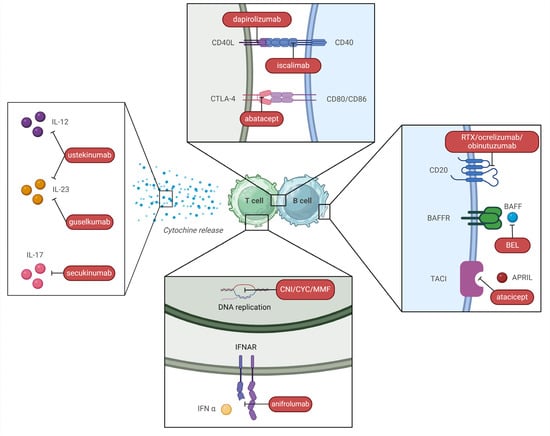
Figure 5.
Different mechanisms of action of immunosuppressants used in SLE treatment (created with BioRender.com). Legend. CNI—calcineurin inhibitor; CYC—cyclophosphamide; MMF—mofetil mycophenolate; BAFF—B cell activating factor; APRIL—a proliferation-inducing ligand.
5.2.1. Belimumab (BEL)
BEL is an anti-BAFF monoclonal antibody (Figure 5). After an initial FDA approval for active non-renal SLE in 2011, the drug was approved as an induction treatment for active LN in 2020, in addition to either MMF or CYP (ELNT) with steroid, based on the results of the BLISS-LN study [218]. This RCT evaluated 448 patients with class III, IV, and V LN at 2 years. Primary efficacy renal response was defined as a GFR of at least 60 mL/min or a GFR level above 20% of baseline level and a urinary protein-to-creatinine ratio < 700 mg/mg. Of note, steroid therapy had to be reduced to prednisone (PDN) 10 mg or less by week 24. This primary endpoint was met in 43% of patients with BEL arms vs. 32% of the placebo. Furthermore, a lower risk of renal events or death was observed in treated patients [227]. Overall current evidence suggests BEL can be preferred within an induction triple therapy in severe forms of LN with important renal function impairment (lack of nephrotoxicity) or in patients with a history of renal (or extra-renal) flares, in which prevention of further flares is essential to preserve renal function; on the contrary, BEL is probably less effective in LN with important proteinuria (>3 g/d), in which VOC seems to be a better alternative [224,228].
An open-label extension of the BLISS-LN study has actually demonstrated that upfront addition of BEL to MMF or CYP as induction therapy may improve renal outcomes as compared with BEL sequential introduction, reinforcing its role as therapy ab initio [229].
Other possible indications are the need to spare GCs [230] or the need to reduce the dose of MMF/MPA due to intolerance or side effects [231]. A recent Spanish study indicates that BEL reduces healthcare resource utilization, including hospital admissions, probably stabilizing disease activity [232].
BEL can also be employed within a sequential RTX-BEL scheme. In the BEAT LUPUS study, this approach significantly suppressed B-cell repopulation, reduced anti-dsDNA, and prevented flares caused by the post-RTX surge in BAFF levels in refractory SLE [219].
Finally, the “SynBioSe 2” multicenter phase III clinical trial (Synergetic B-cell Immunomodulation in SLE) is testing the effectiveness of association BEL + RTX combined with standard-of-care (SOC) as induction treatment, followed by BEL and steroid as maintenance treatment [233].
5.2.2. Voclosporin (VOC)
The use of CNI, CsA, and TAC with MMF has long been proposed as multi-target therapy (Figure 5) in several studies performed mainly on Chinese populations [234]. VOC is a new generation CNI with several advantages compared to CsA and TAC: it is better absorbed and has a more consistent pharmacokinetic-pharmacodynamic relationship due to enhanced calcineurin binding and reduced metabolite load, making therapeutic drug monitoring unnecessary [235]. Furthermore, it does not determine metabolic side effects such as dyslipidemia and new-onset diabetes, and it is not nephrotoxic, although it can cause a transient decrease in GFR and arterial hypertension [236,237]. However, a direct head-to-head trial of VOC versus CsA or TAC has never been performed [238]. Phase III AURORA 1 trial (2021), which determined drug approval, was conducted in patients with class III, IV, or V LN and showed that the association of VOC and MMF met the primary endpoint of CRR in 41% of treated patients vs. 23% of the placebo arm. CRR included urine protein/creatinine ratio ≤ 500 mg/g and ≥GFR 60 mL/min and no rescue treatment. Among secondary endpoints, VOC determined a much faster reduction of proteinuria, which was lowered to ≤500 mg/d in less than 6 months. Of note, steroid tapering was remarkably rapid in the treatment arm, reaching PDN 2.5 mg/day by week 16 [220]. Phase III AURORA 2 extension trial showed stable renal function after 3 years of therapy [221] and no evidence of CNI nephrotoxicity at renal biopsy [236].
Due to this profile, VOC is revolutionizing protocols to treat LN, paving the way to new possibilities beyond background therapy with MMF and steroids. Apart from triple induction with either BEL or VOC on top of MMF/MPA and GC, as suggested by KDIGO guidelines [239], VOC-BEL sequential protocols and VOC and BEL combination therapy (four-drug induction) have been recently proposed but both deserve further study [240].
5.3. Drugs in Phase III Clinical Trials
5.3.1. Anifrolumab
Anifrolumab, an anti-type 1 IFN receptor antibody (Figure 5), also showed promising results in the treatment of LN. After the “Treatment of Uncontrolled Lupus via the IFN Pathway” (TULIP) 2 trial, it was approved for SLE by the FDA in 2021 [241], whereas TULIP LN assessed its use as an induction therapy for LN (in addition to MMF and steroid) in 2022 [222]. Although the introduction of Anifrolumab did not result in a statistically significant superiority, numerical improvements as compared to placebo were reported for many secondary outcomes [242]. Of note, a post-hoc analysis showed that sustained GC tapering was possible in a significantly higher percentage of patients under anifrolumab (51% vs. 32%) with a PDN dose of 7.5 mg/d through week 52 [243]. The second-year extension of the TULIP LN trial confirmed superior renal response at week 104 with an intensified regimen [222]. A phase III trial is evaluating the association of Anifrolumab and MMF in class III or IV LN (ClinicalTrials.gov Identifier: NCT05138133).
5.3.2. Obinutuzumab
This is a new humanized anti-CD20 monoclonal antibody [223,244] (Figure 5) which was initially employed as rescue therapy for RTX non-responders, with promising results in case series [245]. The NOBILITY clinical trial has shown that obinutuzumab improves renal outcomes when added to a MMF and GC, an effect maintained at 104 weeks. Profound and rapid B cell depletion appears to account for it [223]. A post-hoc analysis of the NOBILITY trial [244] has demonstrated superior preservation of kidney function and prevention of flares as compared to SOC in proliferative forms of LN. Furthermore, CRR was achieved in a higher proportion of patients at week 76 (38% vs. 16% p < 0.01), despite receiving a lower steroid dose (PDN ≤ 7.5 mg or less). Of note, this difference lost statistical significance at week 76, but the trend was maintained (p = 0.06).
5.3.3. Ianalumab
Ianalumab is a human monoclonal antibody with a dual mechanism of action, based on binding to BAFF receptor and elimination of B cells expressing it with a mechanism of antibody-dependent cellular cytotoxicity. This monoclonal antibody was effective in reducing immunological activity in primary Sjogren syndrome [246]. A phase III trial including class III, IV, and V of LN is underway to assess Ianalumab and MMF as an induction therapy and will be completed by 2030 (ClinicalTrials.gov Identifier: NCT05126277).
5.3.4. Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2i)
This class of drugs is being increasingly employed in CKD and proteinuria, in which they represent an effective complement to renin-angiotensin inhibitors [247]. Furthermore, they are being investigated in several glomerulonephritis (GN) such as IgA nephropathy. Some evidence suggests that Sodium-glucose Cotransporter-2 inhibitors (SGLT2i) can alleviate podocyte damage in LN [248]. In a few, small trials they blunted proteinuria and had a moderate effect on blood pressure and BMI, without affecting immunological activity [249]. A recent USA multicenter cohort study investigated 1775 matched pairs of SGLT2i users and non-users within a SLE population and found that the former had a significantly lower risk of developing LN and of progressing to ESRD, in addition to reduced risk of heart failure and all-cause mortality [250]. SGLT2i are therefore starting to gain a role as nephro-cardioprotective drugs, in association with renin-angiotensin system inhibitors, even in the high-risk population of SLE patients, which were previously excluded from major trials due to concerns for risk of urinary infections [249].
5.4. Other Drugs
Several other drugs are being investigated in phase II and III trials and have provided promising results in LN, which deserve further confirmation.
5.4.1. Ustekinumab and Secukinumab
IL 17/23 axis and CD3+ CD4− CD8− double negative Th17 cells have been increasingly recognized as key players in LN pathogenesis [251].
Ustekinumab, an IL 12 and IL 23 inhibitor, met the primary endpoint in a phase II trial [252] but results were not confirmed in a phase III trial [253].
Secukinumab, an anti-IL17 antibody, has been employed in cases of refractory LN [254] and is currently being assessed in phase III trials (ClinicalTrials.gov Identifier: NCT04181762) (Figure 5).
5.4.2. Inhibitors of Mammalian Target of Rapamycin (mTOR)
Inhibitors of mammalian target of rapamycin (mTOR) complex activation plays a pivotal role in Treg cell dysfunction and decreased Treg/T helper ratio, a hallmark of SLE [255]. Two inhibitors of the mTOR signaling pathway, rapamycin and everolimus, have been employed as add-on treatments in small studies with promising results [256], especially in refractory SLE [257].
Sirolimus is being investigated in a phase III trial for LN (ClinicalTrials.gov Identifier: NCT04582136).
5.4.3. Janus Kinases (JAK) Inhibitors
Janus Kinases (JAK) inhibitors have been investigated in SLE in recent years with mixed results. JAK and signal transducer and activation of transcription (STAT) pathways mediate downstream effects of receptors for multiple chemokines and cytokines including IFN-I, making JAK-STAT signaling blockade an attractive approach in SLE [258]. In a preclinical RCT on MRL/MpJ-Faslpr mice, Baricitinib, an oral selective inhibitor of JAK 1 and 2, did not affect proteinuria, GFR, and histological lesions but improved lymphadenopathy and serological activity [259].
Baricitinib was studied in two large multi-center phase-3 RCTs in 2023, in which it was employed in addition to a background therapy of MMF and GC. The drug met the primary endpoint (higher proportion of patients reaching an SLE Responder Index -4 response at week 52) but not key secondary ones, including steroid tapering, in SLE-BRAVE-I study [260]; furthermore, results were not replicated in SLE-BRAVE-II [261].
Tyrosine kinase 2 (TYK2), a member of the JAK kinase family of intracellular signaling molecules, is another target of interest [262]. Deucravacitinib, a small molecule which inhibits TYK2, yielded greater response rates than placebo in a phase II trial of SLE, including Lupus Low Disease Activity State and cutaneous and articular manifestations [263]. Ongoing phase III trials of baricitinib and deucravacitinib may provide more conclusive evidence in the upcoming years.
5.4.4. Daratumumab
Daratumumab, an anti-CD38 monoclonal antibody, has been recently proposed as a monotherapy of refractory LN in a case study on six patients. In 5 out of 6 patients, the mean disease activity significantly decreased at 12 months after treatment, along with anti-dsDNA, IFNγ levels, proteinuria, and serum creatinine [264]. Other case reports have recently suggested the effectiveness of this drug in refractory LN [265] and anti-phospholipid syndrome (APS) [266].
6. Adjunctive Therapies beyond Immunosuppression
Management of LN also includes an adjuvant therapy which aims at treating frequent comorbidities of these patients, with a holistic approach. Cardiovascular disease, CKD, and infections currently remain the leading causes of mortality in SLE [267] and a recent systematic review and meta-analysis showed that patients with LN have a significantly higher risk of hypertension, dyslipidemia, and diabetes mellitus compared with those without it [268]. Therefore, control of key cardiovascular risk factors and introduction of nephro-cardioprotective drugs, such as ACE inhibitors or sartans, is recommended by all guidelines, including the most recent ones [205]. Management of drug-related side effects, such as GC-induced osteoporosis or obesity is equally important. We will briefly analyze the main therapies in this setting.
6.1. Control of Blood Pressure
This is a crucial aspect, which has an impact on both cardiovascular mortality and progression of CKD. Blood pressure values should be kept ≤120/80 mmHg, adapting this target according to patient tolerance. If a CKD is present, systolic blood pressure should be kept <120 mmHg [205]. As patients with LN frequently have nocturnal hypertension and reduced blood pressure dipping, 24-hour ambulatory blood pressure monitoring should be considered [269]. Non-dipper profile is associated with CKD progression and cardiovascular events [270]. Non-pharmacological measures in this setting include a low-sodium diet, physical activity (target of at least 150 min per week), and maintenance of ideal weight. Patient education plays an important role in helping to achieve these goals [271], as discussed in Section 7.3.
6.2. Control of Proteinuria
Reduction or prevention of proteinuria is in general a key measure to prevent or delay CKD progression. ACE inhibitors or sartans are first-line drugs not only to treat hypertension and prevent cardiovascular damage but also in case of isolated proteinuria [205,272]. SGLT2i may provide further nephro-cardioprotection in LN through their antiproteinuric effects, in addition to background therapy with ACE inhibitors or sartans, but their role in LN must still be defined [249], as previously discussed. Finerenone, a non-steroidal mineralocorticoid receptor antagonist, has also shown promising results in the treatment of persistent proteinuria despite therapy with ACE inhibitors or sartans in diabetic nephropathy and is being investigated in a phase III trial in CKD of non-diabetic aetiology [273].
6.3. Control of Dyslipidemia
Control of dyslipidemia through diet and lipid-lowering drugs is a key aspect and the target of LDL cholesterol reduction should be differentiated considering the degree of risk. According to the recommendations of the European Society of Cardiology [274], it should be <100 mg/dL for LN patients with normal renal function (moderate risk), <70 mg/dL in patients with CKD (high risk), and <50 mg/dL for those with clinical history or documented evidence of atherosclerotic cardiovascular disease (very high risk).
6.4. Prevention of Thromboembolism
Primary prophylaxis of CV events with low-dose aspirin (75–100 mg/d) may be considered on an individual basis in SLE patients with LN, whose cardiovascular risk is markedly higher than age and sex-matched healthy population and also compared to SLE without renal involvement; however, robust evidence supporting this approach is lacking [268]. Two settings which deserve special attention are presence of antiphosfolipid antibodies (aPL) and nephrotic syndrome. Primary prophylaxis with low-dose aspirin is recommended in the presence of aPL characterized by a high-risk profile and may be considered also in lower risk aPL patterns. In general warfarin is the gold-standard drug for secondary prophylaxis in case of previous events defining APS [275]. A more articulate therapeutic algorithm has been recently proposed by EULAR guidelines [276]. A recent systematic review showed that direct-acting oral anticoagulants rivaroxaban and apixaban were less effective than vitamin K antagonists in preventing thrombosis in patients with APS or positive for two or three different aPL types [277]. Another high-risk situation is nephrotic syndrome due to membranous LN, which requires starting prophylaxis with low molecular weight heparin or vitamin K antagonists in patients with an albumin concentration < 2.5 g/dL and associated risk factors (such as proteinuria >10 g/day), and continuing it until serum albumin reaches 3.0 g/dL [239]. Finally, a recent study has underlined the antiplatelet effect of HCQ by a total thrombus-formation analysis system [278], reinforcing the concept of a possible additive effect in association with low-dose aspirin [279].
6.5. Prevention of GC-Induced Osteoporosis
Incidence of fractures due to osteopenia ranges from 30% to 50% among patients on GC for more than three months [280]. Prevention of this complication includes minimization of GC cumulative dose [281] and correction of other modifiable risk factors, such as smoking, alcohol consumption and sedentarism [282]. An adequate amount of dietary calcium (1 g/day) and maintenance of satisfactory levels of vitamin D (>30 ng/mL) are important in this setting. Very high-risk patients (e.g., use of PDN equivalent ≥30 mg/day for more than 1 month; densitometric evidence of osteoporosis, age older than 40) should be treated with drugs including oral bisphosphonates, denosumab, teriparatide, and romosozumab [281].
6.6. Prevention of Infections
Infections are an important cause of mortality and morbidity in SLE patients, who are often treated with lifelong IS therapy [283]. A recent review has analyzed the topic of vaccines and antimicrobial prophylaxis in immunocompromised patients with kidney diseases [284]. Recommended or suggested vaccinations and prophylactic treatments to prevent reactivation of different infectious agents in SLE are outlined in Table 4. Vaccine responses are generally impaired, especially after the recent administration of B-cell depleting agents and high-dose MMF.

Table 4.
Vaccinations and prophylactic treatments in SLE [283,284].
7. The Role of Non-Pharmacological Management
Beyond IS therapy and adjuvant therapies, the concept of wider, holistic and multidisciplinary management of SLE patients should be emphasized. Many non-pharmacological interventions are an essential complement to traditional therapy and may have an impact also on kidney disease.
7.1. General Lifestyle Measures
Recently published EULAR recommendations for non-pharmacological management of SLE encompass a wide range of tailored measures aimed at improving health-related quality of life and enhancing disease awareness and self-management [271]. Among the main objectives are cessation of smoking habit, avoidance of cold exposure to prevent Raynaud’s phenomenon, photoprotection to prevent flares, physical exercise to progressively increase aerobic capacity and potentially reduce fatigue, and psychosocial interventions to mitigate anxiety and depressive symptoms.
7.2. Dietary and Nutritional Aspects
Information and support about diet is another crucial aspect. A patient-centered nutrition counseling appears to impact weight control, intake of sodium, and percentage of calories from fat and saturated fat. It also favors an increase in fruits and vegetables, helping to achieve a high-fiber, low-cholesterol diet [285]. A recent systematic review has confirmed that a low-fat intake and Mediterranean diet may reduce cardiovascular risk, but large interventional studies are needed [286]. Diet appears to exert its beneficial effects also by modulating autoimmunity through gut microbiota (as discussed in Section 3.2) and probably also has a direct impact on renal inflammation [53]. Interestingly, a high-fat diet determined increases in germinal center B cells, TFH in the spleen, more severe renal lesions and proteinuria in MRL/lpr mice [287].
A low-protein diet could be considered in CKD as early as stage III (GFR < 60 mL/min) to delay the progression of chronic kidney damage and a controlled sodium intake is an important aid in managing arterial hypertension, which is very frequent in proliferative forms of LN [288]. Interestingly, dietary fibers can increase gut bacterial diversity and reduce inflammation in rheumatoid arthritis and other autoimmune disorders [289]. Supplementation of vitamin D, vitamin E, and omega-3 fatty acids appears to be effective in reducing inflammatory markers, potentially improving endothelial function [55]. Correcting vitamin D deficiency, which is common in SLE, may help mitigate disease severity and reduce steroid dose through its immunomodulatory effects on innate and adaptive immunity. Direct supplementation of active vitamin 1,25-dihydroxyvitamin D3 (calcitriol) should be considered if CKD is present [290].
Finally, significant deficiency in several micronutrient intakes has been reported in SLE patients and may require supplementation, but further studies are needed to clarify this aspect [291].
7.3. Patient Education and Support
The need for long-term treatment with side effects and the burden of chronic disease itself can significantly reduce adherence to medical advice and therapy, especially in adolescents and early adults, making communication a crucial aspect [292]. Behavioral and psychosocial interventions can have an impact on fatigue, anxiety, and depression, thus improving quality of life and compliance [293]. The coexistence of renal involvement makes these aspects even more relevant. Patients should be informed about the prognosis of their nephropathy and warned about the risks of taking nephrotoxic drugs such as non-steroidal anti-inflammatory agents [294] and over-the-counter medications [295]. Adequate counseling about contraception and pregnancy should be offered, emphasizing the importance of starting pregnancy in a quiescent phase and estimating additional risks derived from renal disease, especially if CKD or severe hypertension are present [296]. All these issues can be dealt with more easily with a high-quality patient-doctor relationship, based on mutual trust and shared decision-making [297]. This might also help reduce disparities in SLE survival rates due to race, ethnicity, and social disadvantage, which are still reported in many countries [13].
8. Current Limitations and New Perspectives
Although the prognosis of LN has improved over the last few decades, it remains an important cause of morbidity and mortality in SLE. Around 70% of patients do not achieve CR after a 6-month standard induction treatment and 10–30% of them still progress to ESRD within 10 years of diagnosis, with an associated burden of increased cardiovascular and infectious risk [298]. Prevention of relapses and preservation of renal function remain a challenge, as well as minimization of drug toxicity, primarily due to long-term GC therapy that compromises the prognosis of LN, with increasing risk of infections and deaths [192,193,299].
VOC and BEL improved clinical results when used early as induction agents on top of standard therapy [300] and allowed a reduction in the cumulative dose of GC which may significantly reduce long-term side effects. Still, also VOC and BEL are not devoid from Adverse Events (AEs) and limitations. In particular, VOC can worsen hypertension and reduce GFR, limiting its use in CKD, and long-term histological data on nephrotoxicity are lacking [220,221]. BEL is generally well tolerated, but an increased rate of psychiatric AEs such as insomnia, anxiety, and depression-related symptoms was observed [218].
In addition to these aspects, there is an unmet need for biomarkers to identify high-risk patients who require a stronger induction therapy with a triple-drug regimen to achieve CR and distinguish them from an important proportion who may be instead overtreated with this approach [257]. Other current limitations are the lack of definite criteria for GC-reducing protocols, duration of maintenance therapy, and safe withdrawal of IS drugs in quiescent disease; better management of these aspects would likely help reduce the burden of long-term drug AEs [239].
Other biologic agents such as new generation anti-CD20 (obinutuzumab), IFN-I antagonists (anifrolumab), and RTX-BEL sequential therapy or combination hold promise in improving the efficacy-to-toxicity balance in LN treatment and in offering new tools to manage refractory and relapsing forms, which represent a therapeutic challenge due to lack of evidence-based therapies [301]. Both drugs show promising AE profiles, considering that obinutuzumab and anifrolumab were not associated with increases in serious AEs, infections, or deaths [222,223,244].
In addition to these drugs, several new developments are paving the way for potential major progress in LN management. A new frontier is represented by engineered T cells which express chimeric auto-antibody receptors (DNA-CAART) and can selectively target B cells expressing anti-dsDNA autoantibodies. This therapy has been assessed also in an organoid model of LN in vitro, showing anti-apoptotic and anti-inflammatory effects which resulted in improvements in morphology [302]. Another expanding area of research is that of therapeutic manipulation of different ncRNAs, which could inhibit the expression of crucial genes involved in LN, as summarized in Table 5. This intervention could modulate multiple pathways and interfere with key immunological and inflammatory mechanisms of kidney damage [46].

Table 5.
Potential therapeutic application of ncRNA in LN.
An interesting area of research connected with the previous one concerns extracellular vesicles (EVs), which may play multiple pathogenic roles in SLE [308]. They can carry autoantigens or complement factors, promote IC deposition on the glomerular basement membrane, and trigger inflammatory responses and coagulation cascade [309]. Targeting surface molecules on EVs has been proposed to “kill the messenger” and thus blunt autoantigenic fueling of ICs and stimulation of adaptive immune cells [310]. EVs also carry a variety of bioactive molecules and genetic material, including miRs and lncRNAs, and transfer them to target cells mediating deleterious effects. For example, miR-181d-5p, which is increased within EVs derived from M0 macrophages in LN, can target human MC stimulating proliferation and pyroptosis by binding to BCL-2. Of interest, these effects were reversed when levels of miR-181d-5p in EVs were reduced, confirming the therapeutic potential of miRNA antagonism [311]. On the other hand, EVs released from mesenchymal stromal cells (MSCs) show immunosuppressive and anti-inflammatory properties and have been successfully employed in several autoimmune disorders and GNs, including SLE and LN [312]. The beneficial actions of EVs are largely mediated by the transfer of miRs and lncRNAs which target cells and modulate sets of critical genes [313]. Nanomedicine techniques are allowing interesting manipulations of EVs. Engineered EVs presenting CD40 on their membrane can disrupt the CD40/CD40 ligand (CD40L) costimulatory axis through a blockade of CD40L on CD4+ T cells and appear to blunt the production of antibodies from B cells and restrain the generation of germinal centers in MRL/lpr mice. Furthermore, MMF can be also encapsulated within EV to inhibit the activity of lymphocytes and DCs [314]. Cationic liposomes modified with a cell-penetrating peptide were able to carry a small interfering RNA (siRNA) which suppressed B cell proliferation through the TLR4 signaling pathway, resulting in reduced proteinuria and serum anti-dsDNA titer [315].
All these novel tools, however, would require an accurate selection of candidate patients to maximize their effectiveness. One of the obstacles that still remains is the etiopathogenetic heterogeneity of LN and its need for a better disease classification. Response to anti-CD20 monoclonal antibodies, for example, can be highly variable and predictive factors to guide this therapy are lacking [316]. Molecular characterization, gene-signature fingerprints, and omic panels might improve patient stratification and facilitate individualized treatment with targeted drugs in the near future [317]. Artificial intelligence and machine learning techniques have been providing new tools for the prediction of renal flare risk, which may be useful for clinical decision-making [318]. Furthermore, they are allowing important progress in defining the genetic landscape of LN. In a recent study, four hub genes (out of 270 differentially expressed genes) involved in immune cell infiltration were identified, namely CD53, TGFBI, MS4A6A, and HERC6. They are especially expressed in macrophages and correlate with histological class, renal function, and proteinuria [137]. Another recent study identified four hub genes (STAT1, PRODH, TXN2, and SETX), associated with oxidative stress related to LN and most correlated with activated B and CD8 T cells [138]. Transcript analysis of serial LN kidney biopsies demonstrated the evolution of gene expression in the kidney according to clinical response to therapy. This molecular landscape differentiating responders from non-responders may help guide LN treatment on the basis of the actual pathogenesis of kidney injury [139].
Overall, this progress may lead to a molecular diagnosis of LN based on genetic fingerprints and specific pathway activation. The wealth of information generated by “omic” approaches, integrated with artificial intelligence and machine learning tools, is likely to lead to the discovery of many new non-invasive biomarkers, which could replace traditional ones and potentially even renal biopsy [319].
9. Conclusions
Significant progress has been made in unraveling pathogenetic mechanisms of LN over the last years, with new insight into mechanisms triggering the production of autoantibodies and interactions between infiltrating immune cells and kidney resident cells. This has been accompanied by a progressive increase in the number of candidate serum and urine biomarkers, which may integrate information from renal biopsy and change its role in the near future. Also IS therapy has been significantly expanding, with new approved drugs, such as BEL and VOC, allowing a stronger induction and the possibility of modulating it according to clinical features. Many other drugs in the pipeline may become part of LN therapy over the next years.
This renaissance in LN management is raising hopes that currently unmet clinical needs will be satisfied more easily in the near future. Achieving a higher rate of CR, preventing flares and progression of LN, reducing drug toxicity and personalizing treatment may have an impact on long-term outcomes of this challenging disease.
Author Contributions
A.R. contributed to paragraphs on pathophysiological mechanisms of LN; E.L.P. revised Tables and organized References; F.T. wrote the paragraph on the role of genetics in LN; B.B. and V.Z. contributed to paragraphs on biomarkers; G.M. built images; A.F. and A.G.M. critically revised the text; M.Q. designed and revised the Review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data employed for conducting this review are available upon request to the following e-mails: marco.quaglia@med.uniupo.it.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| aCL | anticardiolipin antibody |
| ALCAM | activated leukocyte CAM |
| APC | antigen presenting cell |
| aPL | anti-phospholipid |
| APS | anti-phospholipid syndrome |
| APRIL | a proliferation-inducing ligand |
| BAFF | B cell activating factor for TNF family |
| BEL | belimumab |
| CAM | cell-adhesion molecule |
| circRNA | circular RNA |
| CKD | chronic kidney disease |
| CYC | cyclophosphamide |
| CNI | calcineurin inhibitor |
| CR | complete remission |
| CRR | complete renal remission |
| DC | dendritic cell |
| DEG | differentially expressed genes |
| ELNT | Euro Lupus Nephritis Trial |
| ESRD | end stage renal disease |
| EV | extracellular vesicle |
| Fn14 | fibroblast growth factor-inducible 14 |
| GC | glucocorticoid |
| GEC | glomerular endothelial cell |
| GN | glomerulonephritis |
| HCQ | hydroxychloroquine |
| HE4 | human epididymis protein 4 |
| IC | immune complex |
| IFN | interferon |
| IFN-I | type I interferon |
| IL | interleukin |
| IS | immunosuppressive |
| JAK | Janus Kinase |
| KIM1 | kidney injury molecule 1 |
| LNA | locked nucleic acid |
| lncRNA | long non-coding RNA |
| MC | mesangial cell |
| MCP-1 | monocyte chemoattractant protein1 |
| MERTK | MER tyrosine kinase |
| MHC | major histocompatibility complex |
| miR | microRNA |
| MMF | mycophenolate mofetil |
| MN | Monocyte neutrophil |
| MP | methylprednisolone |
| MPA | mycophenolic acid |
| mTOR | mammalian target of rapamycin |
| ncRNA | non-coding RNA |
| NET | neutrophil extracellular trap |
| NGAL | neutrophil gelatinase associated lipocalin |
| NK | natural killer |
| pDC | plasmacytoid dendritic cell |
| PDN | prednisone |
| PR | partial remission |
| RNP | ribonucleoprotein |
| RTX | rituximab |
| SGLT2i | sodium-glucose cotransporter-2 inhibitor |
| SLAMF | signaling lymphocyte activation molecule family |
| SLEDAI | systemic lupus erythematosus disease activity index |
| Sm | Smith |
| SOC | standard of care |
| STAT | signal transducer and activation of transcription |
| TEC | tubular epithelia cell |
| TFH | T follicular helper |
| TGFβ | transforming growth factor β |
| TMA | thrombotic microangiopathy |
| TNF | tumor necrosis factor |
| Th | T helper lymphocyte |
| Treg | T regulatory lymphocyte |
| TWEAK | TNF-like weak inducer of apoptosis |
| SAP | SLAM associated protein |
| SLAM | costimulatory lymphocyte associated molecule |
| SLE | systemic lupus erythematosus |
| VCAM-1 | vascular CAM1 |
| VOC | voclosporin |
References
- Hoi, A.; Igel, T.; Mok, C.C.; Arnaud, L. Systemic Lupus Erythematosus. Lancet 2024, 403, 2326–2338. [Google Scholar] [CrossRef]
- Echavarria, R.; Cardona-Muñoz, E.G.; Ortiz-Lazareno, P.; Andrade-Sierra, J.; Gómez-Hermosillo, L.F.; Casillas-Moreno, J.; Campos-Bayardo, T.I.; Román-Rojas, D.; García-Sánchez, A.; Miranda-Díaz, A.G. The Role of the Oxidative State and Innate Immunity Mediated by TLR7 and TLR9 in Lupus Nephritis. Int. J. Mol. Sci. 2023, 24, 15234. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Li, K.-J.; Shen, C.-Y.; Lu, C.-H.; Lee, H.-T.; Wu, T.-H.; Ng, Y.-Y.; Tsao, Y.-P.; Hsieh, S.-C.; Yu, C.-L. Decipher the Immunopathological Mechanisms and Set Up Potential Therapeutic Strategies for Patients with Lupus Nephritis. Int. J. Mol. Sci. 2023, 24, 10066. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.H.; Shafqat, A.; Ahmad, O.; Nadri, J.; AlKattan, K.; Yaqinuddin, A. Urinary Biomarkers for Lupus Nephritis: A Systems Biology Approach. J. Clin. Med. 2024, 13, 2339. [Google Scholar] [CrossRef]
- Tsoi, A.; Nikolopoulos, D.; Parodis, I. Advances in the Pharmacological Management of Systemic Lupus Erythematosus. Expert Opin. Pharmacother. 2024, 25, 705–716. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yao, X.; Huang, Y.; Lu, Q. Global Epidemiology of Systemic Lupus Erythematosus: A Comprehensive Systematic Analysis and Modelling Study. Ann. Rheum. Dis. 2023, 82, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-S.; Liu, Z.-H. Epidemiologic Data of Renal Diseases from a Single Unit in China: Analysis Based on 13,519 Renal Biopsies. Kidney Int. 2004, 66, 920–923. [Google Scholar] [CrossRef]
- Parichatikanond, P.; Chawanasuntorapoj, R.; Shayakul, C.; Choensuchon, B.; Vasuvattakul, S.; Vareesangthip, K.; Chanchairujira, T.; Sritippayawan, S.; Vongwiwatana, A.; Premasathian, N.; et al. An Analysis of 3,555 Cases of Renal Biopsy in Thailand. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2006, 89 (Suppl. S2), S106–S111. [Google Scholar]
- Kurnatowska, I.; Jędrzejka, D.; Małyska, A.; Wągrowska-Danilewicz, M.; Danilewicz, M.; Nowicki, M. Trends in the Incidence of Biopsy-Proven Glomerular Diseases in the Adult Population in Central Poland in the Years 1990–2010. Kidney Blood Press. Res. 2012, 35, 254–258. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Rovin, B.H. Epidemiology and Management of Lupus Nephritis. In Dubois’ Lupus Erythematosus and Related Syndromes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 727–744. ISBN 978-0-323-47927-1. [Google Scholar]
- Shin, J.I.; Li, H.; Park, S.; Yang, J.W.; Lee, K.H.; Jo, Y.; Park, S.; Oh, J.; Kim, H.; An, H.J.; et al. Induction and Maintenance Treatment of Lupus Nephritis: A Comprehensive Review of Meta-Analyses. J. Clin. Med. 2022, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.; Kalunian, K.; Eaddy, M.; Ogbonnaya, A.; Farrelly, E.; Turowski, E.; Birardi, V.; Solomons, N.; Randhawa, S.; Mina-Osorio, P. Real-World Treatment Utilization and Economic Implications of Lupus Nephritis Disease Activity in the United States. J. Manag. Care Spec. Pharm. 2023, 29, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Pryor, K.P.; Barbhaiya, M.; Costenbader, K.H.; Feldman, C.H. Disparities in Lupus and Lupus Nephritis Care and Outcomes Among US Medicaid Beneficiaries. Rheum. Dis. Clin. N. Am. 2021, 47, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Izmirly, P.M.; Wan, I.; Sahl, S.; Buyon, J.P.; Belmont, H.M.; Salmon, J.E.; Askanase, A.; Bathon, J.M.; Geraldino-Pardilla, L.; Ali, Y.; et al. The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol. 2017, 69, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Hoover, P.J.; Costenbader, K.H. Insights into the Epidemiology and Management of Lupus Nephritis from the US Rheumatologist’s Perspective. Kidney Int. 2016, 90, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Burgos, P.I.; McGwin, G.; Pons-Estel, G.J.; Reveille, J.D.; Alarcon, G.S.; Vila, L.M. US Patients of Hispanic and African Ancestry Develop Lupus Nephritis Early in the Disease Course: Data from LUMINA, a Multiethnic US Cohort (LUMINA LXXIV). Ann. Rheum. Dis. 2011, 70, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Pons-Estel, B.A.; Catoggio, L.J.; Cardiel, M.H.; Soriano, E.R.; Gentiletti, S.; Villa, A.R.; Abadi, I.; Caeiro, F.; Alvarellos, A.; Alarcón-Segovia, D. The GLADEL Multinational Latin American Prospective Inception Cohort of 1,214 Patients with Systemic Lupus Erythematosus: Ethnic and Disease Heterogeneity among “Hispanics”. Medicine 2004, 83, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Li, Y.; Ye, L.; Deng, Z.; Li, L.; Feng, Y.; Liu, W.; Liu, H. Geographical Distribution, a Risk Factor for the Incidence of Lupus Nephritis in China. BMC Nephrol. 2014, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Piga, M.; Floris, A.; Cappellazzo, G.; Chessa, E.; Congia, M.; Mathieu, A.; Cauli, A. Failure to Achieve Lupus Low Disease Activity State (LLDAS) Six Months after Diagnosis Is Associated with Early Damage Accrual in Caucasian Patients with Systemic Lupus Erythematosus. Arthritis Res. Ther. 2017, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Bobart, S.A.; Portalatin, G.; Sawaf, H.; Shettigar, S.; Carrion-Rodriguez, A.; Liang, H.; Herlitz, L.; Gebreselassie, S.K. The Cleveland Clinic Kidney Biopsy Epidemiological Project. Kidney360 2022, 3, 2077. [Google Scholar] [CrossRef]
- The Norwegian Renal Registry Annual Report 2022; Norsk Nyreregister: Oslo, Norway, 2022.
- Laurens, W.; Deleersnijder, D.; Dendooven, A.; Lerut, E.; De Vriese, A.S.; Dejagere, T.; Helbert, M.; Hellemans, R.; Koshy, P.; Maes, B.; et al. Epidemiology of Native Kidney Disease in Flanders: Results from the FCGG Kidney Biopsy Registry. Clin. Kidney J. 2022, 15, 1361–1372. [Google Scholar] [CrossRef]
- Trends of Renal Diseases in Germany: Review of a Regional Renal Biopsy Database from 1990 to 2013—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6885677/ (accessed on 29 July 2024).
- López-Gómez, J.M.; Rivera, F. Spanish Registry of Glomerulonephritis 2020 Revisited: Past, Current Data and New Challenges. Nefrol. Engl. Ed. 2020, 40, 371–383. [Google Scholar] [CrossRef]
- Hu, R.; Quan, S.; Wang, Y.; Zhou, Y.; Zhang, Y.; Liu, L.; Zhou, X.J.; Xing, G. Spectrum of Biopsy Proven Renal Diseases in Central China: A 10-Year Retrospective Study Based on 34,630 Cases. Sci. Rep. 2020, 10, 10994. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Imaizumi, T.; Hamada, R.; Ishikura, K.; Kosugi, T.; Narita, I.; Sugiyama, H.; Shimizu, A.; Yokoyama, H.; Sato, H.; et al. Renal Pathology in Adult and Paediatric Population of Japan: Review of the Japan Renal Biopsy Registry Database from 2007 to 2017. J. Nephrol. 2023, 36, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Agarwal, S.K.; Singh, G.; Bhowmik, D.; Mahajan, S.; Dinda, A.; Bagchi, S. Spectrum of Biopsy-Proven Renal Disease in Northern India: A Single-Centre Study. Nephrology 2020, 25, 55–62. [Google Scholar] [CrossRef]
- Barrera-Herrera, L.E.; Panqueva, R.D.P.L.; Flórez Vargas, A.A.; Andrade Pérez, R.E. The Spectrum of Glomerular Disease between the Years 2003 and 2015 in Columbia: A Review of 12,613 Cases. Rev. Esp. Patol. 2017, 50, 3–7. [Google Scholar] [CrossRef]
- Lemrabott, A.T.O.; Faye, M.; Dial, M.C.; Faye, M.; Cissé, A.; Cissé, M.M.; Fall, K.; Kane, Y.; Mbengue, M.; Ba, B.; et al. SUN-427 senegal renal biopsy registry: Indications and histopathological patterns based on 1559 native renal biopsies. Kidney Int. Rep. 2020, 5, S373. [Google Scholar] [CrossRef][Green Version]
- Hermansen, M.-L.F.; Lindhardsen, J.; Torp-Pedersen, C.; Faurschou, M.; Jacobsen, S. Incidence of Systemic Lupus Erythematosus and Lupus Nephritis in Denmark: A Nationwide Cohort Study. J. Rheumatol. 2016, 43, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. A Systematic Review and Meta-Analysis of Prevalence of Biopsy-Proven Lupus Nephritis. Arch. Rheumatol. 2018, 33, 17–25. [Google Scholar] [CrossRef]
- Seligman, V.A.; Lum, R.F.; Olson, J.L.; Li, H.; Criswell, L.A. Demographic Differences in the Development of Lupus Nephritis: A Retrospective Analysis. Am. J. Med. 2002, 112, 726–729. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Dasgupta, A.; Ward, M.M. Risk of End-Stage Renal Disease in Patients with Lupus Nephritis, 1971–2015: A Systematic Review and Bayesian Meta-Analysis. Arthritis Rheumatol. 2016, 68, 1432–1441. [Google Scholar] [CrossRef]
- Barr, R.G. Prognosis in Proliferative Lupus Nephritis: The Role of Socio-Economic Status and Race/Ethnicity. Nephrol. Dial. Transplant. 2003, 18, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Obrișcă, B.; Sorohan, B.; Tuță, L.; Ismail, G. Advances in Lupus Nephritis Pathogenesis: From Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 3766. [Google Scholar] [CrossRef]
- Parikh, S.V.; Almaani, S.; Brodsky, S.; Rovin, B.H. Update on Lupus Nephritis: Core Curriculum 2020. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 76, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Webber, D.; Cao, J.; Dominguez, D.; Gladman, D.D.; Levy, D.M.; Ng, L.; Paterson, A.D.; Touma, Z.; Urowitz, M.B.; Wither, J.E.; et al. Association of Systemic Lupus Erythematosus (SLE) Genetic Susceptibility Loci with Lupus Nephritis in Childhood-Onset and Adult-Onset SLE. Rheumatology 2020, 59, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Han, X.; Jin, Y.; Chen, X.; Gong, C.; Peng, J.; Wang, Y.; Luo, X.; Yang, Z.; Zhang, Y.; et al. Pathogenic Gene Spectrum and Clinical Implication in Chinese Patients with Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2023, 18, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zheng, X.; Liu, X.; Sheng, Y.; Liu, L.; Wen, L.; Shang, S.; Deng, Y.; Ouyang, Q.; Sun, X.; et al. Genome-Wide Association Study of SNP- and Gene-Based Approaches to Identify Susceptibility Candidates for Lupus Nephritis in the Han Chinese Population. Front. Immunol. 2022, 13, 908851. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Pucholt, P.; Sandling, J.K.; Bianchi, M.; Leonard, D.; Bolin, K.; Imgenberg-Kreuz, J.; Eloranta, M.-L.; Kozyrev, S.V.; Lanata, C.M.; et al. Mer-Tyrosine Kinase: A Novel Susceptibility Gene for SLE Related End-Stage Renal Disease. Lupus Sci. Med. 2022, 9, e000752. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Quaglia, M.; Nerviani, A.; Mauro, D.; Lewis, M.; Goegan, F.; Gibbin, A.; Pagani, S.; Salmi, L.; Molinari, L.; et al. Increased Plasma Levels of Gas6 and Its Soluble Tyrosine Kinase Receptors Mer and Axl Are Associated with Immunological Activity and Severity of Lupus Nephritis. Clin. Exp. Rheumatol. 2021, 39, 132–138. [Google Scholar] [CrossRef]
- Tusseau, M.; Khaldi-Plassart, S.; Cognard, J.; Viel, S.; Khoryati, L.; Benezech, S.; Mathieu, A.-L.; Rieux-Laucat, F.; Bader-Meunier, B.; Belot, A. Mendelian Causes of Autoimmunity: The Lupus Phenotype. J. Clin. Immunol. 2024, 44, 99. [Google Scholar] [CrossRef]
- Mei, X.; Jin, H.; Zhao, M.; Lu, Q. Association of Immune-Related Genetic and Epigenetic Alterations with Lupus Nephritis. Kidney Dis. 2022, 8, 286–296. [Google Scholar] [CrossRef]
- Coit, P.; Ortiz-Fernandez, L.; Lewis, E.E.; McCune, W.J.; Maksimowicz-McKinnon, K.; Sawalha, A.H. A Longitudinal and Transancestral Analysis of DNA Methylation Patterns and Disease Activity in Lupus Patients. JCI Insight 2020, 5, e143654. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jiang, X.; Qi, C.; Zhang, C.; Qu, B.; Shen, N. EZH2 Inhibition Interferes with the Activation of Type I Interferon Signaling Pathway and Ameliorates Lupus Nephritis in NZB/NZW F1 Mice. Front. Immunol. 2021, 12, 653989. [Google Scholar] [CrossRef] [PubMed]
- So, B.Y.F.; Yap, D.Y.H.; Chan, T.M. MicroRNAs in Lupus Nephritis-Role in Disease Pathogenesis and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 10737. [Google Scholar] [CrossRef]
- Xu, N.; Liu, J.; Li, X. Lupus Nephritis: The Regulatory Interplay between Epigenetic and MicroRNAs. Front. Physiol. 2022, 13, 925416. [Google Scholar] [CrossRef]
- Cardelli, C.; Zucchi, D.; Elefante, E.; Signorini, V.; Menchini, M.; Stagnaro, C.; Mosca, M.; Tani, C. Environment and Systemic Lupus Erythematosus. Clin. Exp. Rheumatol. 2024, 42, 1104–1114. [Google Scholar] [CrossRef]
- Skopelja-Gardner, S.; Tai, J.; Sun, X.; Tanaka, L.; Kuchenbecker, J.A.; Snyder, J.M.; Kubes, P.; Mustelin, T.; Elkon, K.B. Acute Skin Exposure to Ultraviolet Light Triggers Neutrophil-Mediated Kidney Inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019097118. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, L.; Li, T.; Liu, C.; Zuo, X.; Liu, Y.; Hu, S.; Sun, L.; Zhang, M.; Lin, J.; et al. Acute Effects of Air Pollution on Lupus Nephritis in Patients with Systemic Lupus Erythematosus: A Multicenter Panel Study in China. Environ. Res. 2021, 195, 110875. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, M.; Merlotti, G.; De Andrea, M.; Borgogna, C.; Cantaluppi, V. Viral Infections and Systemic Lupus Erythematosus: New Players in an Old Story. Viruses 2021, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C.; Chu, C.S.; Tse, S.M. De Novo Lupus Nephritis after SARS-CoV-2 Infection. Lupus 2023, 32, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Monticolo, M.; Mucha, K.; Foroncewicz, B. Lupus Nephritis and Dysbiosis. Biomedicines 2023, 11, 1165. [Google Scholar] [CrossRef]
- Mohd, R.; Chin, S.-F.; Shaharir, S.S.; Cham, Q.S. Involvement of Gut Microbiota in SLE and Lupus Nephritis. Biomedicines 2023, 11, 653. [Google Scholar] [CrossRef]
- Jiao, H.; Acar, G.; Robinson, G.A.; Ciurtin, C.; Jury, E.C.; Kalea, A.Z. Diet and Systemic Lupus Erythematosus (SLE): From Supplementation to Intervention. Int. J. Environ. Res. Public. Health 2022, 19, 11895. [Google Scholar] [CrossRef]
- Ramessar, N.; Borad, A.; Schlesinger, N. The Impact of Curcumin Supplementation on Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review. Lupus 2023, 32, 644–657. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Zhou, M.; Li, M.; Li, M.; Tan, H. Curcumin Attenuates Murine Lupus via Inhibiting NLRP3 Inflammasome. Int. Immunopharmacol. 2019, 69, 213–216. [Google Scholar] [CrossRef]
- Gatto, M.; Iaccarino, L.; Ghirardello, A.; Punzi, L.; Doria, A. Clinical and Pathologic Considerations of the Qualitative and Quantitative Aspects of Lupus Nephritogenic Autoantibodies: A Comprehensive Review. J. Autoimmun. 2016, 69, 1–11. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Unique Interplay Between Antinuclear Antibodies and Nuclear Molecules in the Pathogenesis of Systemic Lupus Erythematosus. Arthritis Rheumatol. 2024, art.42863. [Google Scholar] [CrossRef]
- Bruschi, M.; Angeletti, A.; Prunotto, M.; Meroni, P.L.; Ghiggeri, G.M.; Moroni, G.; Sinico, R.A.; Franceschini, F.; Fredi, M.; Vaglio, A.; et al. A Critical View on Autoantibodies in Lupus Nephritis: Concrete Knowledge Based on Evidence. Autoimmun. Rev. 2024, 23, 103535. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Saxena, R.; Zhao, M.-H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus Nephritis. Nat. Rev. Dis. Primer 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Devarapu, S.K.; Anders, H.-J. Toll-like Receptors in Lupus Nephritis. J. Biomed. Sci. 2018, 25, 35. [Google Scholar] [CrossRef]
- Van Eyndhoven, L.C.; Chouri, E.; Matos, C.I.; Pandit, A.; Radstake, T.R.D.J.; Broen, J.C.A.; Singh, A.; Tel, J. Unraveling IFN-I Response Dynamics and TNF Crosstalk in the Pathophysiology of Systemic Lupus Erythematosus. Front. Immunol. 2024, 15, 1322814. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, A.; Bruschi, M.; Kajana, X.; Spinelli, S.; Verrina, E.; Lugani, F.; Caridi, G.; Murtas, C.; Candiano, G.; Prunotto, M.; et al. Mechanisms Limiting Renal Tissue Protection and Repair in Glomerulonephritis. Int. J. Mol. Sci. 2023, 24, 8318. [Google Scholar] [CrossRef] [PubMed]
- Liou, L.; Chen, C.; Chiang, W.; Chen, M. De-Sialylated and Sialylated IgG Anti-dsDNA Antibodies Respectively Worsen and Mitigate Experimental Mouse Lupus Proteinuria and Possible Mechanisms. Int. Immunopharmacol. 2022, 109, 108837. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, Z.; Zhang, R.; You, Y.; Guo, Z.; Huang, J.; Wang, F.; Sun, Y.; Liu, H.; Cheng, X.; et al. Fucosylation of Anti-dsDNA IgG1 Correlates with Disease Activity of Treatment-Naïve Systemic Lupus Erythematosus Patients. eBioMedicine 2022, 77, 103883. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Wojciak-Stothard, B.; Ruseva, M.M.; Cook, H.T.; Kelleher, P.; Pickering, M.C.; Mongkolsapaya, J.; Screaton, G.R.; Xu, X.-N. Autoantibody-Dependent Amplification of Inflammation in SLE. Cell Death Dis. 2020, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ren, Z.; Li, J.; Zou, Z. Anticardiolipin Antibody Plays a More Important Role Than Anti-Β2-Glycoprotein I Antibody in Activating Complement in Patients with Lupus Nephritis. Int. J. Gen. Med. 2024, 17, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T. An Emerging Role for Anti-DNA Antibodies in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 16499. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tan, Y.; Zhao, M. The Role of Anti-mCRP Autoantibodies in Lupus Nephritis. Kidney Dis. 2023, 9, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Wang, Z.; Bao, H.; Di, C.; Xia, C.; Zhang, X.; Liu, Y. Antibodies against Neutrophil Extracellular Traps (NETs) Potentiate Clinical Performance of Anti-Double-Stranded DNA Antibodies in Systemic Lupus Erythematosus. Clin. Immunol. 2023, 249, 109297. [Google Scholar] [CrossRef] [PubMed]
- Li, N.L.; Birmingham, D.J.; Rovin, B.H. Expanding the Role of Complement Therapies: The Case for Lupus Nephritis. J. Clin. Med. 2021, 10, 626. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, R.; Belmont, H.M. The Role of Anticomplement Therapy in Lupus Nephritis. Transl. Res. 2022, 245, 1–17. [Google Scholar] [CrossRef]
- Weinstein, A.; Alexander, R.V.; Zack, D.J. A Review of Complement Activation in SLE. Curr. Rheumatol. Rep. 2021, 23, 16. [Google Scholar] [CrossRef]
- Qin, S.; Wang, X.; Wang, J.; Wu, H. Complement C4d as a Biomarker for Systemic Lupus Erythematosus and Lupus Nephritis. Lupus 2024, 33, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M.; Shoenfeld, Y. Vitamin D and Autoimmune Rheumatic Diseases. Biomolecules 2023, 13, 709. [Google Scholar] [CrossRef]
- Jiang, L.-J.; Rong, Z.-H.; Zhang, H.-F. The Changes of Treg and Th17 Cells Relate to Serum 25(OH)D in Patients with Initial-Onset Childhood Systemic Lupus Erythematosus. Front. Pediatr. 2023, 11, 1228112. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, S.; Liu, J.S.; Gui, M.; Zhang, H. Expression of Vitamin D Receptor in Renal Tissue of Lupus Nephritis and Its Association with Renal Injury Activity. Lupus 2019, 28, 290–294. [Google Scholar] [CrossRef]
- Luo, M.; Lıu, J.; Yuan, Y.; Chen, Y.; Yuan, G. The Role of Vitamin D-Synthesizing Enzyme CYP27B1 in Systemic Lupus Erythematosus. Turk. J. Med. Sci. 2022, 52, 984–989. [Google Scholar] [CrossRef]
- Yu, Q.; Qiao, Y.; Liu, D.; Liu, F.; Gao, C.; Duan, J.; Liang, L.; Di, X.; Yuan, Y.; Gao, Y.; et al. Vitamin D Protects Podocytes from Autoantibodies Induced Injury in Lupus Nephritis by Reducing Aberrant Autophagy. Arthritis Res. Ther. 2019, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Siligato, R.; Amatruda, M.; Conti, G.; Santoro, D. Vitamin D and Glomerulonephritis. Med. Kaunas Lith. 2021, 57, 186. [Google Scholar] [CrossRef] [PubMed]
- Zervopoulou, E.; Grigoriou, M.; Doumas, S.A.; Yiannakou, D.; Pavlidis, P.; Gasparoni, G.; Walter, J.; Filia, A.; Gakiopoulou, H.; Banos, A.; et al. Enhanced Medullary and Extramedullary Granulopoiesis Sustain the Inflammatory Response in Lupus Nephritis. Lupus Sci. Med. 2024, 11, e001110. [Google Scholar] [CrossRef]
- Juha, M.; Molnár, A.; Jakus, Z.; Ledó, N. NETosis: An Emerging Therapeutic Target in Renal Diseases. Front. Immunol. 2023, 14, 1253667. [Google Scholar] [CrossRef]
- Frangou, E.; Chrysanthopoulou, A.; Mitsios, A.; Kambas, K.; Arelaki, S.; Angelidou, I.; Arampatzioglou, A.; Gakiopoulou, H.; Bertsias, G.K.; Verginis, P.; et al. REDD1/Autophagy Pathway Promotes Thromboinflammation and Fibrosis in Human Systemic Lupus Erythematosus (SLE) through NETs Decorated with Tissue Factor (TF) and Interleukin-17A (IL-17A). Ann. Rheum. Dis. 2019, 78, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Cheng, Y.; Liu, W.-J.; Liu, K.; Wang, Y.; Xu, F.; Wang, D.-M.; Yang, Y. Effects of Neutrophil Fate on Inflammation. Inflamm. Res. 2023, 72, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, A.; Volpi, S.; Bruschi, M.; Lugani, F.; Vaglio, A.; Prunotto, M.; Gattorno, M.; Schena, F.; Verrina, E.; Ravelli, A.; et al. Neutrophil Extracellular Traps-DNase Balance and Autoimmunity. Cells 2021, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Whittall-Garcia, L.P.; Naderinabi, F.; Gladman, D.D.; Urowitz, M.; Touma, Z.; Konvalinka, A.; Wither, J. Circulating Neutrophil Extracellular Trap Remnants as a Biomarker to Predict Outcomes in Lupus Nephritis. Lupus Sci. Med. 2024, 11, e001038. [Google Scholar] [CrossRef]
- Davidson, A. Renal Mononuclear Phagocytes in Lupus Nephritis. ACR Open Rheumatol. 2021, 3, 442–450. [Google Scholar] [CrossRef]
- Kwant, L.E.; Vegting, Y.; Tsang-a-Sjoe, M.W.P.; Kwakernaak, A.J.; Vogt, L.; Voskuyl, A.E.; Van Vollenhoven, R.F.; De Winther, M.P.J.; Bemelman, F.J.; Anders, H.-J.; et al. Macrophages in Lupus Nephritis: Exploring a Potential New Therapeutic Avenue. Autoimmun. Rev. 2022, 21, 103211. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Shen, H.; Zhang, Y.; Liu, C.; Li, S.; Yao, J.; Jin, Z.; Yu, H. Integrative Analysis of Single-Cell and Bulk Transcriptome Data Reveal the Significant Role of Macrophages in Lupus Nephritis. Arthritis Res. Ther. 2024, 26, 84. [Google Scholar] [CrossRef] [PubMed]
- Játiva, S.; Torrico, S.; Calle, P.; Poch, E.; Muñoz, A.; García, M.; Larque, A.B.; Salido, M.T.T.; Hotter, G. The Phagocytosis Dysfunction in Lupus Nephritis Is Related to Monocyte/Macrophage CPT1a. Immunol. Lett. 2024, 266, 106841. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chang, S.; Wang, J.; Chen, J.; Xu, H.; Huang, T.; Wang, J.; Kang, J.; Fan, W.; Wang, Y. S1P/S1PR1 Axis Promotes Macrophage M1 Polarization through NLRP3 Inflammasome Activation in Lupus Nephritis. Mol. Immunol. 2023, 160, 55–66. [Google Scholar] [CrossRef]
- Ichioka, S.; Satooka, H.; Maruo, Y.; Hirata, T. Moesin Deficiency Leads to Lupus-like Nephritis with Accumulation of CXCL13-Producing Patrolling Monocytes. Biochem. Biophys. Res. Commun. 2024, 712–713, 149943. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, X.; Xu, R.; Ji, Y.; Liu, J.; Liu, J.; Zhuang, Q.; Zhang, H. Immune Landscape and the Key Role of APOE+ Monocytes of Lupus Nephritis under the Single-cell and Spatial Transcriptional Vista. Clin. Transl. Med. 2023, 13, e1237. [Google Scholar] [CrossRef]
- Gouda, W.; Abd Elaziz Alsaid, A.; Abbas, A.S.; Abdel-Aziz, T.M.; Shoaeir, M.Z.; Abd Elazem, A.A.S.; Sayed, M.H. Silent Lupus Nephritis: Renal Histopathological Profile and Early Detection with Urinary Monocyte Chemotactic Protein 1. Open Access Rheumatol. Res. Rev. 2022, 14, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Kanda, Y.; Nakayamada, S.; Kubo, S.; Yamagata, K.; Nawata, A.; Tanaka, H.; Kosaka, S.; Kanda, R.; Yu, S.; Fujita, Y.; et al. Modifying T Cell Phenotypes Using TYK2 Inhibitor and Its Implications for the Treatment of Systemic Lupus Erythematosus. RMD Open 2024, 10, e003991. [Google Scholar] [CrossRef] [PubMed]
- The Accelerating Medicines Partnership in SLE Network; Arazi, A.; Rao, D.A.; Berthier, C.C.; Davidson, A.; Liu, Y.; Hoover, P.J.; Chicoine, A.; Eisenhaure, T.M.; Jonsson, A.H.; et al. The Immune Cell Landscape in Kidneys of Patients with Lupus Nephritis. Nat. Immunol. 2019, 20, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Tchen, J.; Simon, Q.; Chapart, L.; Thaminy, M.K.; Vibhushan, S.; Saveanu, L.; Lamri, Y.; Saidoune, F.; Pacreau, E.; Pellefigues, C.; et al. PD-L1- and IL-4-Expressing Basophils Promote Pathogenic Accumulation of T Follicular Helper Cells in Lupus. Nat. Commun. 2024, 15, 3389. [Google Scholar] [CrossRef] [PubMed]
- Gartshteyn, Y.; Geraldino-Pardilla, L.; Khalili, L.; Bukhari, S.; Lerrer, S.; Winchester, R.J.; Askanase, A.D.; Mor, A. SAP-Expressing T Peripheral Helper Cells Identify Systemic Lupus Erythematosus Patients with Lupus Nephritis. Front. Immunol. 2024, 15, 1327437. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, D.; Dossybayeva, K.; Kozhakhmetov, S.; Rozenson, R.; Assylbekova, M. Double-Negative T (DNT) Cells in Patients with Systemic Lupus Erythematosus. Biomedicines 2024, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huo, H.; Zhang, Y.; Tao, J.; Yang, J.; Rong, X.; Yang, Y. Th17 Cells: A New Target in Kidney Disease Research. Int. Rev. Immunol. 2024, 1–17. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Cheong, R.M.Y.; Lo, C.; Chang, J.; Ng, B.H.; Ting, Y.T.; Monk, J.A.; Loh, K.L.; Broury, A.; Tay, E.S.V.; et al. Smith-Specific Regulatory T Cells Halt the Progression of Lupus Nephritis. Nat. Commun. 2024, 15, 899. [Google Scholar] [CrossRef]
- Takeshima, Y.; Iwasaki, Y.; Nakano, M.; Narushima, Y.; Ota, M.; Nagafuchi, Y.; Sumitomo, S.; Okamura, T.; Elkon, K.; Ishigaki, K.; et al. Immune Cell Multiomics Analysis Reveals Contribution of Oxidative Phosphorylation to B-Cell Functions and Organ Damage of Lupus. Ann. Rheum. Dis. 2022, 81, 845–853. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, E.J.; Lee, L.E.; Kim, J.Y.; Cho, Y.; Kim, H.; Kim, J.; Jang, S.H.; Son, J.; Cheong, J.-H.; et al. The Oxidative Phosphorylation Inhibitor IM156 Suppresses B-Cell Activation by Regulating Mitochondrial Membrane Potential and Contributes to the Mitigation of Systemic Lupus Erythematosus. Kidney Int. 2023, 103, 343–356. [Google Scholar] [CrossRef]
- Robinson, G.A.; Wilkinson, M.G.L.; Wincup, C. The Role of Immunometabolism in the Pathogenesis of Systemic Lupus Erythematosus. Front. Immunol. 2022, 12, 806560. [Google Scholar] [CrossRef]
- Wang, M.; Rajkumar, S.; Lai, Y.; Liu, X.; He, J.; Ishikawa, T.; Nallapothula, D.; Singh, R.R. Tertiary Lymphoid Structures as Local Perpetuators of Organ-Specific Immune Injury: Implication for Lupus Nephritis. Front. Immunol. 2023, 14, 1204777. [Google Scholar] [CrossRef]
- Ah Kioon, M.D.; Laurent, P.; Chaudhary, V.; Du, Y.; Crow, M.K.; Barrat, F.J. Modulation of Plasmacytoid Dendritic Cells Response in Inflammation and Autoimmunity. Immunol. Rev. 2024, 323, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.V.; Malvar, A.; Shapiro, J.; Turman, J.M.; Song, H.; Alberton, V.; Lococo, B.; Mejia-Vilet, J.M.; Madhavan, S.; Zhang, J.; et al. A Novel Inflammatory Dendritic Cell That Is Abundant and Contiguous to T Cells in the Kidneys of Patients with Lupus Nephritis. Front. Immunol. 2021, 12, 621039. [Google Scholar] [CrossRef]
- Chen, W.; Jin, B.; Cheng, C.; Peng, H.; Zhang, X.; Tan, W.; Tang, R.; Lian, X.; Diao, H.; Luo, N.; et al. Single-Cell Profiling Reveals Kidney CD163+ Dendritic Cell Participation in Human Lupus Nephritis. Ann. Rheum. Dis. 2024, 83, 608–623. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, F.; Zhan, Y.; Wang, R.; Mo, B.; Lin, S. TLR9 Regulates the Autophagy–Lysosome Pathway to Promote Dendritic Cell Maturation and Activation by Activating the TRAF6-cGAS-STING Pathway. Kaohsiung J. Med. Sci. 2023, 39, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, M.; Ma, Y.; Lan, L.; Jiang, X.; Cen, X.; Guo, G.; Zhou, Q.; Yuan, M.; Chen, J.; et al. Novel Mitophagy Inducer Alleviates Lupus Nephritis by Reducing Myeloid Cell Activation and Autoantigen Presentation. Kidney Int. 2024, 105, 759–774. [Google Scholar] [CrossRef]
- Waterman, H.R.; Dufort, M.J.; Posso, S.E.; Ni, M.; Li, L.Z.; Zhu, C.; Raj, P.; Smith, K.D.; Buckner, J.H.; Hamerman, J.A. Lupus IgA1 Autoantibodies Synergize with IgG to Enhance pDC Responses to RNA-Containing Immune Complexes. bioRxiv 2023. [Google Scholar]
- Tsuchida, Y.; Shoda, H.; Sawada, T.; Fujio, K. Role of Autotaxin in Systemic Lupus Erythematosus. Front. Med. 2023, 10, 1166343. [Google Scholar] [CrossRef]
- Chang, A.; Clark, M.R.; Ko, K. Cellular Aspects of the Pathogenesis of Lupus Nephritis. Curr. Opin. Rheumatol. 2021, 33, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Jamaly, S.; Rakaee, M.; Abdi, R.; Tsokos, G.C.; Fenton, K.A. Interplay of Immune and Kidney Resident Cells in the Formation of Tertiary Lymphoid Structures in Lupus Nephritis. Autoimmun. Rev. 2021, 20, 102980. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Huang, L.-F.; Huang, X.-R.; Wu, D.; Chen, X.-C.; Tang, J.-X.; An, N.; Liu, H.-F.; Yang, C. Endoplasmic Reticulum Stress in Systemic Lupus Erythematosus and Lupus Nephritis: Potential Therapeutic Target. J. Immunol. Res. 2023, 2023, 7625817. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Healy, H.; Kassianos, A.J. The Emerging Role of Renal Tubular Epithelial Cells in the Immunological Pathophysiology of Lupus Nephritis. Front. Immunol. 2020, 11, 578952. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Li, H.; Tsokos, G.C. Pathogenesis of Lupus Nephritis: The Contribution of Immune and Kidney Resident Cells. Curr. Opin. Rheumatol. 2023, 35, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, K. The Role of Podocytes in Lupus Nephritis: Insights and Implications. Clin. Immunol. 2024, 262, 110180. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wen, X.; Peng, X.; Zhao, M.; Mi, L.; Lei, J.; Xu, K. Immune Podocytes in the Immune Microenvironment of Lupus Nephritis (Review). Mol. Med. Rep. 2023, 28, 204. [Google Scholar] [CrossRef]
- Nowling, T.K. Mesangial Cells in Lupus Nephritis. Curr. Rheumatol. Rep. 2021, 23, 83. [Google Scholar] [CrossRef]
- Li, W.; Yao, C.; Guo, H.; Ni, X.; Zhu, R.; Wang, Y.; Yu, B.; Feng, X.; Gu, Z.; Da, Z. Macrophages Communicate with Mesangial Cells through the CXCL12/DPP4 Axis in Lupus Nephritis Pathogenesis. Cell Death Dis. 2024, 15, 344. [Google Scholar] [CrossRef]
- Li, Z.; Gan, H.; Ji, K.; Yang, M.; Pan, T.; Meng, X.; Liu, T.; Wang, Z.; Gong, B.; Liu, K.; et al. Protopanaxadiol Improves Lupus Nephritis by Regulating the PTX3/MAPK/ERK1/2 Pathway. J. Nat. Med. 2024, 78, 474–487. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, Y.; Hu, W.; Wang, C.; Wen, Z. Mesangial Cell: A Hub in Lupus Nephritis. Front. Immunol. 2022, 13, 1063497. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Law, H.K.W. Immune Complexes Impaired Glomerular Endothelial Cell Functions in Lupus Nephritis. Int. J. Mol. Sci. 2019, 20, 5281. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Aizawa, T.; Imaizumi, T.; Tsugawa, K.; Kawaguchi, S.; Seya, K.; Terui, K.; Tanaka, H. Effect of Sera from Lupus Patients on the Glomerular Endothelial Fibrinolysis System. Pediatr. Int. 2022, 64, e15099. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Sato, R.; Imaizumi, T.; Fujita, M.; Aizawa, T.; Tsugawa, K.; Mattinzoli, D.; Kawaguchi, S.; Seya, K.; Terui, K.; et al. Expression of Interferon-Stimulated Gene 20 (ISG20), an Antiviral Effector Protein, in Glomerular Endothelial Cells: Possible Involvement of ISG20 in Lupus Nephritis. Ren. Fail. 2023, 45, 2224890. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, Z.; Yuan, M.; Li, L.; Tan, Y.; Qu, Z.; Chen, M.; Yu, F. Glomerular mTORC1 Activation Was Associated with Podocytes to Endothelial Cells Communication in Lupus Nephritis. Lupus Sci. Med. 2023, 10, e000896. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Almaraz, E.; Gutiérrez-Solís, E.; Rabadán, E.; Rodríguez, P.; Carmona, L.; Morales, E.; Galindo, M. Something New about Prognostic Factors for Lupus Nephritis? A Systematic Review. Lupus 2021, 30, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society Classification for Lupus Nephritis: Clarification of Definitions, and Modified National Institutes of Health Activity and Chronicity Indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wang, H.; Yu, X.-J.; Tan, Y.; Yu, F.; Wang, S.-X.; Haas, M.; Glassock, R.J.; Zhao, M.-H. A Validation of the 2018 Revision of International Society of Nephrology/Renal Pathology Society Classification for Lupus Nephritis: A Cohort Study from China. Am. J. Nephrol. 2020, 51, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Haas, M.; Glassock, R.; Zhao, M.-H. Redefining Lupus Nephritis: Clinical Implications of Pathophysiologic Subtypes. Nat. Rev. Nephrol. 2017, 13, 483–495. [Google Scholar] [CrossRef]
- Zhang, B.; Xing, G. Thrombotic Microangiopathy Mediates Poor Prognosis among Lupus Nephritis via Complement Lectin and Alternative Pathway Activation. Front. Immunol. 2022, 13, 1081942. [Google Scholar] [CrossRef]
- Garg, S.; Bartels, C.M.; Hansen, K.E.; Zhong, W.; Huang, Y.; Semanik, M.G.; Smith, M.; Panzer, S.E. High Burden of Premature Arteriosclerosis on Renal Biopsy Results in Incident Lupus Nephritis. Arthritis Care Res. 2021, 73, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.D.; Wang, S.; Schwartz, D.; Mowrey, W.B.; Broder, A.; Goilav, B. Total Cortical Interstitial Inflammation Predicts Chronic Kidney Disease Progression in Patients with Lupus Nephritis. Nephrol. Dial. Transplant. 2023, 38, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Almaani, S.; Prokopec, S.D.; Zhang, J.; Yu, L.; Avila-Casado, C.; Wither, J.; Scholey, J.W.; Alberton, V.; Malvar, A.; Parikh, S.V.; et al. Rethinking Lupus Nephritis Classification on a Molecular Level. J. Clin. Med. 2019, 8, 1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, D.; Pei, G.; Zeng, R.; Yao, Y. Identification of Driver Genes in Lupus Nephritis Based on Comprehensive Bioinformatics and Machine Learning. Front. Immunol. 2023, 14, 1288699. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhuang, Y.; Yan, X.; He, X.; Qiu, Q.; Liu, W.; Zhang, Y. Machine Learning-Based Identification of Novel Hub Genes Associated with Oxidative Stress in Lupus Nephritis: Implications for Diagnosis and Therapeutic Targets. Lupus Sci. Med. 2024, 11, e001126. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.V.; Malvar, A.; Song, H.; Shapiro, J.; Mejia-Vilet, J.M.; Ayoub, I.; Almaani, S.; Madhavan, S.; Alberton, V.; Besso, C.; et al. Molecular Profiling of Kidney Compartments from Serial Biopsies Differentiate Treatment Responders from Non-Responders in Lupus Nephritis. Kidney Int. 2022, 102, 845–865. [Google Scholar] [CrossRef] [PubMed]
- Tamirou, F.; Lauwerys, B.R.; Dall’Era, M.; Mackay, M.; Rovin, B.; Cervera, R.; Houssiau, F.A. A Proteinuria Cut-off Level of 0.7 g/Day after 12 Months of Treatment Best Predicts Long-Term Renal Outcome in Lupus Nephritis: Data from the MAINTAIN Nephritis Trial. Lupus Sci. Med. 2015, 2, e000123. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Vilet, J.M.; Malvar, A.; Arazi, A.; Rovin, B.H. The Lupus Nephritis Management Renaissance. Kidney Int. 2022, 101, 242–255. [Google Scholar] [CrossRef]
- Liao, Y.-W.; Chen, Y.-M.; Hsieh, T.-Y.; Hung, W.-T.; Hsu, C.-Y.; Wen, M.-C.; Chen, Y.-H.; Huang, W.-N. Renal Histopathology Associated with Kidney Failure and Mortality in Patients With Lupus Nephritis: A Long-Term Real-World Data Study. J. Rheumatol. 2023, 50, 1127–1135. [Google Scholar] [CrossRef]
- Rodelo, J.; Aguirre, L.; Ortegón, K.; Ustáriz, J.; Calderon, L.; Taborda, A.; Arias, L.F.; González, L.A. Predicting Kidney Outcomes among Latin American Patients with Lupus Nephritis: The Prognostic Value of Interstitial Fibrosis and Tubular Atrophy and Tubulointerstitial Inflammation. Lupus 2023, 32, 411–423. [Google Scholar] [CrossRef]
- Morales, E.; Trujillo, H.; Bada, T.; Alonso, M.; Gutiérrez, E.; Rodríguez, E.; Gutiérrez, E.; Galindo, M.; Praga, M. What Is the Value of Repeat Kidney Biopsies in Patients with Lupus Nephritis? Lupus 2021, 30, 25–34. [Google Scholar] [CrossRef]
- Arriens, C.; Chen, S.; Karp, D.R.; Saxena, R.; Sambandam, K.; Chakravarty, E.; James, J.A.; Merrill, J.T. Prognostic Significance of Repeat Biopsy in Lupus Nephritis: Histopathologic Worsening and a Short Time between Biopsies Is Associated with Significantly Increased Risk for End Stage Renal Disease and Death. Clin. Immunol. 2017, 185, 3–9. [Google Scholar] [CrossRef]
- Parodis, I.; Moroni, G.; Calatroni, M.; Bellis, E.; Gatto, M. Is Per-Protocol Kidney Biopsy Required in Lupus Nephritis? Autoimmun. Rev. 2024, 23, 103422. [Google Scholar] [CrossRef]
- Parodis, I.; Tamirou, F.; Houssiau, F.A. Treat-to-Target in Lupus Nephritis. What Is the Role of the Repeat Kidney Biopsy? Arch. Immunol. Ther. Exp. 2022, 70, 8. [Google Scholar] [CrossRef]
- Gatto, M.; Radice, F.; Saccon, F.; Calatroni, M.; Frontini, G.; Trezzi, B.; Zen, M.; Ghirardello, A.; Tamborini, F.; Binda, V.; et al. Clinical and Histological Findings at Second but Not at First Kidney Biopsy Predict End-Stage Kidney Disease in a Large Multicentric Cohort of Patients with Active Lupus Nephritis. Lupus Sci. Med. 2022, 9, e000689. [Google Scholar] [CrossRef] [PubMed]
- Koopman, J.J.E.; Rennke, H.G.; Leatherwood, C.; Speyer, C.B.; D’Silva, K.; McMahon, G.M.; Waikar, S.S.; Costenbader, K.H. Renal Deposits of Complement Factors as Predictors of End-Stage Renal Disease and Death in Patients with Lupus Nephritis. Rheumatology 2020, 59, 3751–3758. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Li, Y.; Herlitz, L.; Mohan, C.; Putterman, C. Novel Biomarker Discovery through Comprehensive Proteomic Analysis of Lupus Mouse Serum. J. Autoimmun. 2024, 142, 103134. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Hasparyk, U.G.; Lopes, M.P.; De Barros, J.L.V.M.; Simões, E.; Silva, A.C. Novel Biomarkers for Lupus Nephritis in the “OMICS” Era. Curr. Med. Chem. 2021, 28, 6011–6044. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Faustini, F.; Idborg, H.; Fuzzi, E.; Larsson, A.; Lie, W.-R.; Pötzsch, S.; Okitsu, S.L.; Svenungsson, E.; Gunnarsson, I. Urine Galectin-3 Binding Protein Reflects Nephritis Activity in Systemic Lupus Erythematosus. Lupus 2023, 32, 252–262. [Google Scholar] [CrossRef]
- Ding, H.; Shen, Y.; Lin, C.; Qin, L.; He, S.; Dai, M.; Okitsu, S.L.; DeMartino, J.A.; Guo, Q.; Shen, N. Urinary Galectin-3 Binding Protein (G3BP) as a Biomarker for Disease Activity and Renal Pathology Characteristics in Lupus Nephritis. Arthritis Res. Ther. 2022, 24, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, K.; Xiang, Y.; Ma, B.; Li, H.; Li, Y.; Shi, Y.; Li, S.; Bai, Y. Role of MCP-1 as an Inflammatory Biomarker in Nephropathy. Front. Immunol. 2024, 14, 1303076. [Google Scholar] [CrossRef]
- Deng, T.; Lei, F.; Wang, Z.; Wang, Y.; Li, G.; Zhu, Y.; Du, B.; Xi, X. MCP-1/CCR2 Axis Is Involved in the Regulation of <γδT Cells in Lupus Nephritis. Scand. J. Immunol. 2023, 98, e13305. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, H.; Jing, H.; Tan, X.; Zhou, X.; Ma, Y. Emerging Role of TWEAK-Fn14 Axis in Lupus, a Disease Related to Autoimmunity and Fibrosis. Int. J. Rheum. Dis. 2022, 25, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.S.; Abdel Moneam, S.M.; Hashaad, N.I.; Yousef, E.M.; Abd El-Hassib, D.M. Clinico-Serological Associations of Urinary Activated Leukocyte Cell Adhesion Molecule in Systemic Lupus Erythematosus and Lupus Nephritis. Clin. Rheumatol. 2024, 43, 1015–1021. [Google Scholar] [CrossRef]
- Lei, R.; Thai, N.; Li, Y.; Petri, M.; Mohan, C. Analytical Validation of Urine ALCAM ELISA as a Test for Lupus Nephritis. Expert Rev. Mol. Diagn. 2023, 23, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Rabrenović, V.; Petrović, M.; Rabrenović, M. Comparison Urine Neutrophil Gelatinase—Associated Lipocalin with Standard Parameters in Monitoring Activity Lupus Nephritis: Class IV. J. Med. Biochem. 2023, 42, 78–85. [Google Scholar] [CrossRef]
- Roointan, A.; Gholaminejad, A.; Shojaie, B.; Hudkins, K.L.; Gheisari, Y. Candidate MicroRNA Biomarkers in Lupus Nephritis: A Meta-Analysis of Profiling Studies in Kidney, Blood and Urine Samples. Mol. Diagn. Ther. 2023, 27, 141–158. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solis-Salguero, M.A.; Chaves, F.J.; Redon, J.; Forner, M.J.; Cortes, R. Urinary Exosomal miR-146a as a Marker of Albuminuria, Activity Changes and Disease Flares in Lupus Nephritis. J. Nephrol. 2021, 34, 1157–1167. [Google Scholar] [CrossRef]
- Garcia-Vives, E.; Solé, C.; Moliné, T.; Vidal, M.; Agraz, I.; Ordi-Ros, J.; Cortés-Hernández, J. The Urinary Exosomal miRNA Expression Profile Is Predictive of Clinical Response in Lupus Nephritis. Int. J. Mol. Sci. 2020, 21, 1372. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM Receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Mok, C.C.; Ding, H.H.; Kharboutli, M.; Mohan, C. Axl, Ferritin, Insulin-Like Growth Factor Binding Protein 2, and Tumor Necrosis Factor Receptor Type II as Biomarkers in Systemic Lupus Erythematosus. Arthritis Care Res. 2016, 68, 1303–1309. [Google Scholar] [CrossRef]
- Parodis, I.; Ding, H.; Zickert, A.; Cosson, G.; Fathima, M.; Grönwall, C.; Mohan, C.; Gunnarsson, I. Serum Axl Predicts Histology-Based Response to Induction Therapy and Long-Term Renal Outcome in Lupus Nephritis. PLoS ONE 2019, 14, e0212068. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, Q.; Li, X.; Qin, Y. Potential Association between Elevated Serum Human Epididymis Protein 4 and Renal Fibrosis: A Systemic Review and Meta-Analysis. Medicine 2017, 96, e7824. [Google Scholar] [CrossRef]
- Ren, Y.; Xie, J.; Lin, F.; Luo, W.; Zhang, Z.; Mao, P.; Zhong, R.; Liang, Y.; Yang, Z. Serum Human Epididymis Protein 4 Is a Predictor for Developing Nephritis in Patients with Systemic Lupus Erythematosus: A Prospective Cohort Study. Int. Immunopharmacol. 2018, 60, 189–193. [Google Scholar] [CrossRef]
- Ding, H.; Kharboutli, M.; Saxena, R.; Wu, T. Insulin-like Growth Factor Binding Protein-2 as a Novel Biomarker for Disease Activity and Renal Pathology Changes in Lupus Nephritis. Clin. Exp. Immunol. 2016, 184, 11–18. [Google Scholar] [CrossRef]
- Garchow, B.; Kiriakidou, M. MicroRNA-21 Deficiency Protects from Lupus-like Autoimmunity in the Chronic Graft-versus-Host Disease Model of Systemic Lupus Erythematosus. Clin. Immunol. 2016, 162, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Khoshmirsafa, M.; Kianmehr, N.; Falak, R.; Mowla, S.J.; Seif, F.; Mirzaei, B.; Valizadeh, M.; Shekarabi, M. Elevated Expression of miR-21 and miR-155 in Peripheral Blood Mononuclear Cells as Potential Biomarkers for Lupus Nephritis. Int. J. Rheum. Dis. 2019, 22, 458–467. [Google Scholar] [CrossRef]
- Dedong, H.; Feiyan, Z.; Jie, S.; Xiaowei, L.; Shaoyang, W. Analysis of Interleukin-17 and Interleukin-23 for Estimating Disease Activity and Predicting the Response to Treatment in Active Lupus Nephritis Patients. Immunol. Lett. 2019, 210, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bao, C.; Hu, D. Elevated Interleukin-18 and Skewed Th1:Th2 Immune Response in Lupus Nephritis. Rheumatol. Int. 2012, 32, 223–229. [Google Scholar] [CrossRef]
- Maravillas-Montero, J.L.; Reyes-Huerta, R.F. Update on Novel Blood-Based Biomarkers for Lupus Nephritis beyond Diagnostic Approaches. Rev. Investig. Clín. 2022, 74, 9461. [Google Scholar] [CrossRef] [PubMed]
- Parodis, I.; Ding, H.; Zickert, A.; Arnaud, L.; Larsson, A.; Svenungsson, E.; Mohan, C.; Gunnarsson, I. Serum Soluble Tumour Necrosis Factor Receptor-2 (sTNFR2) as a Biomarker of Kidney Tissue Damage and Long-Term Renal Outcome in Lupus Nephritis. Scand. J. Rheumatol. 2017, 46, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Davas, E.M.; Tsirogianni, A.; Kappou, I.; Karamitsos, D.; Economidou, I.; Dantis, P.C. Serum IL-6, TNFα, P55 srTNFα, P75 srTNFα, srIL-2α Levels and Disease Acitivity in Systemic Lupus Erythematosus. Clin. Rheumatol. 1999, 18, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Tsai, T.-Y.; Huang, Y.-C. Insulin Resistance and Serum Levels of Adipokines in Patients with Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Lupus 2020, 29, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.; Ye, Y.; Han, J.; Arriens, C.; Saxena, R.; Li, Q.-Z.; Mohan, C.; Wu, T. Resistin as a Potential Marker of Renal Disease in Lupus Nephritis. Clin. Exp. Immunol. 2015, 179, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.Y.; Abdullah, H.; Abdallah, M.F.H.; Fayed, A. Impact of Adipokines in Brachial Artery Flow-Mediated Dilatation in Lupus Nephritis. Saudi J. Kidney Dis. Transplant. 2022, 33, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.M.; Abdel Azeem, M.E.; Mohamed, R.A.; Kamel, M.M.; Abdel Aleem (Abdelaleem), E.A. High Serum Leptin and Adiponectin Levels as Biomarkers of Disease Progression in Egyptian Patients with Active Systemic Lupus Erythematosus. Int. J. Immunopathol. Pharmacol. 2023, 37, 039463202311549. [Google Scholar] [CrossRef]
- Möckel, T.; Basta, F.; Weinmann-Menke, J.; Schwarting, A. B Cell Activating Factor (BAFF): Structure, Functions, Autoimmunity and Clinical Implications in Systemic Lupus Erythematosus (SLE). Autoimmun. Rev. 2021, 20, 102736. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Hasan Jokar, M.; Mehrnaz Aghili, S.; Mirfeizi, Z.; Mahmoudi, M.; Morovatdar, N.; Hashemzadeh, K. Association between Levels of Serum and Urinary B Cell-Activating Factor and Systemic Lupus Erythematosus Disease Activity. Arch. Rheumatol. 2023, 38, 429–440. [Google Scholar] [CrossRef]
- Marín-Rosales, M.; Palafox-Sánchez, C.A.; Franco-Topete, R.A.; Carrillo-Ballesteros, F.J.; Cruz, A.; Salazar-Camarena, D.C.; Muñoz-Valle, J.F.; Ramos-Solano, F. Renal Tissue Expression of BAFF and BAFF Receptors Is Associated with Proliferative Lupus Nephritis. J. Clin. Med. 2022, 12, 71. [Google Scholar] [CrossRef]
- Itotagawa, E.; Tomofuji, Y.; Kato, Y.; Konaka, H.; Tsujimoto, K.; Park, J.; Nagira, D.; Hirayama, T.; Jo, T.; Hirano, T.; et al. SLE Stratification Based on BAFF and IFN-I Bioactivity for Biologics and Implications of BAFF Produced by Glomeruli in Lupus Nephritis. Rheumatology 2023, 62, 1988–1997. [Google Scholar] [CrossRef]
- Baert, L.; Ahmed, M.C.; Manfroi, B.; Huard, B. The Number 13 of the Family: A Proliferation Inducing Ligand. Curr. Opin. Immunol. 2021, 71, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Parodis, I.; Zickert, A.; Sundelin, B.; Axelsson, M.; Gerhardsson, J.; Svenungsson, E.; Malmström, V.; Gunnarsson, I. Evaluation of B Lymphocyte Stimulator and a Proliferation Inducing Ligand as Candidate Biomarkers in Lupus Nephritis Based on Clinical and Histopathological Outcome Following Induction Therapy. Lupus Sci. Med. 2015, 2, e000061. [Google Scholar] [CrossRef] [PubMed]
- Treamtrakanpon, W.; Tantivitayakul, P.; Benjachat, T.; Somparn, P.; Kittikowit, W.; Eiam-ong, S.; Leelahavanichkul, A.; Hirankarn, N.; Avihingsanon, Y. APRIL, a Proliferation-Inducing Ligand, as a Potential Marker of Lupus Nephritis. Arthritis Res. Ther. 2012, 14, R252. [Google Scholar] [CrossRef]
- Kim, K.-J.; Kim, J.-Y.; Baek, I.-W.; Kim, W.-U.; Cho, C.-S. Elevated Serum Levels of Syndecan-1 Are Associated with Renal Involvement in Patients with Systemic Lupus Erythematosus. J. Rheumatol. 2015, 42, 202–209. [Google Scholar] [CrossRef]
- Russo, V.C.; Azar, W.J.; Yau, S.W.; Sabin, M.A.; Werther, G.A. IGFBP-2: The Dark Horse in Metabolism and Cancer. Cytokine Growth Factor Rev. 2015, 26, 329–346. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T.M. Endothelial Cell Activation and Glycocalyx Shedding—Potential as Biomarkers in Patients with Lupus Nephritis. Front. Immunol. 2023, 14, 1251876. [Google Scholar] [CrossRef]
- Yu, K.Y.C.; Yung, S.; Chau, M.K.M.; Tang, C.S.O.; Yap, D.Y.H.; Tang, A.H.N.; Ying, S.K.Y.; Lee, C.K.; Chan, T.M. Serum Syndecan-1, Hyaluronan and Thrombomodulin Levels in Patients with Lupus Nephritis. Rheumatology 2021, 60, 737–750. [Google Scholar] [CrossRef]
- Figueroa-Parra, G.; Cuéllar-Gutiérrez, M.C.; González-Treviño, M.; Sanchez-Rodriguez, A.; Flores-Gouyonnet, J.; Meade-Aguilar, J.A.; Prokop, L.J.; Murad, M.H.; Dall’Era, M.; Rovin, B.H.; et al. Impact of Glucocorticoid Dose on Complete Response, Serious Infections, and Mortality During the Initial Therapy of Lupus Nephritis: A Systematic Review and Meta-Analysis of the Control Arms of Randomized Controlled Trials. Arthritis Rheumatol. 2024, art.42920. [Google Scholar] [CrossRef]
- Sobhy, N.; Ezzat, Y.; Gamal, S.M.; Ghoniem, S.A.; Nasr, S.S.; Badran, S.; Soliman, A.; Fouad, N.A. Cumulative Pulse Methylprednisolone and Its Relation to Disease Activity, Damage and Mortality in Systemic Lupus Erythematosus Patients: A Post Hoc Analysis of COMOSLE-EGYPT Study. Clin. Rheumatol. 2024, 43, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Dueña-Bartolome, L.; Dunder, S.; Varona, J.; Gomez-Carballo, C.; Dominguez-Cainzos, J.; Rodrigo-Manjon, A.; Bueno, L.; Richez, C.; Duffau, P.; et al. Eurolupus Cyclophosphamide plus Repeated Pulses of Methyl-Prednisolone for the Induction Therapy of Class III, IV and V Lupus Nephritis. Autoimmun. Rev. 2021, 20, 102898. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Ruiz-Estevez, B.; Lazaro, E.; Ruiz-Arruza, I.; Duffau, P.; Martin-Cascon, M.; Richez, C.; Ugarte, A.; Blanco, P. Prolonged Remission in SLE Is Possible by Using Reduced Doses of Prednisone: An Observational Study from the Lupus-Cruces and Lupus-Bordeaux Inception Cohorts. Autoimmun. Rev. 2019, 18, 102359. [Google Scholar] [CrossRef] [PubMed]
- Houssiau, F.A.; Vasconcelos, C.; D’Cruz, D.; Sebastiani, G.D.; Garrido, E.D.R.; Danieli, M.G.; Abramovicz, D.; Blockmans, D.; Mathieu, A.; Direskeneli, H.; et al. Immunosuppressive Therapy in Lupus Nephritis: The Euro-Lupus Nephritis Trial, a Randomized Trial of Low-dose versus High-dose Intravenous Cyclophosphamide. Arthritis Rheum. 2002, 46, 2121–2131. [Google Scholar] [CrossRef]
- Houssiau, F.A.; Vasconcelos, C.; D’Cruz, D.; Sebastiani, G.D.; De Ramon Garrido, E.; Danieli, M.G.; Abramovicz, D.; Blockmans, D.; Cauli, A.; Direskeneli, H.; et al. The 10-Year Follow-up Data of the Euro-Lupus Nephritis Trial Comparing Low-Dose and High-Dose Intravenous Cyclophosphamide. Ann. Rheum. Dis. 2010, 69, 61–64. [Google Scholar] [CrossRef]
- Quan, X.; Chen, H.; Liang, S.; Yang, C.; Yao, C.; Xu, Y.; Liu, H.; An, N. Revisited Cyclophosphamide in the Treatment of Lupus Nephritis. BioMed Res. Int. 2022, 2022, 8345737. [Google Scholar] [CrossRef]
- Wenderfer, S.E.; Cooper, J.C. Do We Really Need Cyclophosphamide for Lupus Nephritis? Pediatr. Nephrol. 2024. [Google Scholar] [CrossRef]
- Uysal, C.; Ketenci Ertas, S.; Civan, M.; Akgun, H.; Kocyigit, I. Pauci-Immune Crescentic Glomerulonephritis Caused to Dilemma in a Patient with Suspected Systemic Lupus Erythematosus: A Case Report. CEN Case Rep. 2023, 13, 174–180. [Google Scholar] [CrossRef]
- Portalatin, G.M.; Gebreselassie, S.K.; Bobart, S.A. Lupus Nephritis—An Update on Disparities Affecting African Americans. J. Natl. Med. Assoc. 2022, 114, S34–S42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, M.; Han, X.; Yang, Y.; Yu, X. Mycophenolate Mofetil in the Treatment of Chinese Patients with Lupus Nephritis: A PRISMA-Compliant Meta-Analysis. Medicine 2020, 99, e21121. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.Y.H.; Chan, T.M. B Cell Abnormalities in Systemic Lupus Erythematosus and Lupus Nephritis—Role in Pathogenesis and Effect of Immunosuppressive Treatments. Int. J. Mol. Sci. 2019, 20, 6231. [Google Scholar] [CrossRef] [PubMed]
- Fassbinder, T.; Saunders, U.; Mickholz, E.; Jung, E.; Becker, H.; Schlüter, B.; Jacobi, A.M. Differential Effects of Cyclophosphamide and Mycophenolate Mofetil on Cellular and Serological Parameters in Patients with Systemic Lupus Erythematosus. Arthritis Res. Ther. 2015, 17, 92. [Google Scholar] [CrossRef]
- Reis-Neto, E.T.D.; Seguro, L.P.C.; Sato, E.I.; Borba, E.F.; Klumb, E.M.; Costallat, L.T.L.; Medeiros, M.M.D.C.; Bonfá, E.; Araújo, N.C.; Appenzeller, S.; et al. II Brazilian Society of Rheumatology Consensus for Lupus Nephritis Diagnosis and Treatment. Adv. Rheumatol. 2024, 64, 48. [Google Scholar] [CrossRef]
- Mok, C.C. Calcineurin Inhibitors in Systemic Lupus Erythematosus. Best Pract. Res. Clin. Rheumatol. 2017, 31, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Multitarget Therapy versus Monotherapy as Induction Treatment for Lupus Nephritis: A Meta-Analysis of Randomized Controlled Trials. Lupus 2022, 31, 1468–1476. [Google Scholar] [CrossRef]
- Dooley, M.A.; Jayne, D.; Ginzler, E.M.; Isenberg, D.; Olsen, N.J.; Wofsy, D.; Eitner, F.; Appel, G.B.; Contreras, G.; Lisk, L.; et al. Mycophenolate versus Azathioprine as Maintenance Therapy for Lupus Nephritis. N. Engl. J. Med. 2011, 365, 1886–1895. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Costedoat-Chalumeau, N.; Baumstarck, K.; Loundou, A.; Bouillet, L.; Burtey, S.; Caudwell, V.; Chiche, L.; Couzi, L.; Daniel, L.; et al. Weaning of Maintenance Immunosuppressive Therapy in Lupus Nephritis (WIN-Lupus): Results of a Multicentre Randomised Controlled Trial. Ann. Rheum. Dis. 2022, 81, 1420–1427. [Google Scholar] [CrossRef]
- Chakravarty, E.F.; Utset, T.; Kamen, D.L.; Contreras, G.; McCune, W.J.; Aranow, C.; Kalunian, K.; Massarotti, E.; Clowse, M.E.B.; Rovin, B.H.; et al. Mycophenolate Mofetil Withdrawal in Patients with Systemic Lupus Erythematosus: A Multicentre, Open-Label, Randomised Controlled Trial. Lancet Rheumatol. 2024, 6, e168–e177. [Google Scholar] [CrossRef] [PubMed]
- Contis, A.; Vanquaethem, H.; Truchetet, M.-E.; Couzi, L.; Rigothier, C.; Richez, C.; Lazaro, E.; Duffau, P. Analysis of the Effectiveness and Safety of Rituximab in Patients with Refractory Lupus Nephritis: A Chart Review. Clin. Rheumatol. 2016, 35, 517–522. [Google Scholar] [CrossRef]
- Avasare, R.; Drexler, Y.; Caster, D.J.; Mitrofanova, A.; Jefferson, J.A. Management of Lupus Nephritis: New Treatments and Updated Guidelines. Kidney360 2023, 4, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Furie, R.; Latinis, K.; Looney, R.J.; Fervenza, F.C.; Sanchez-Guerrero, J.; Maciuca, R.; Zhang, D.; Garg, J.P.; Brunetta, P.; et al. Efficacy and Safety of Rituximab in Patients with Active Proliferative Lupus Nephritis: The Lupus Nephritis Assessment with Rituximab Study. Arthritis Rheum. 2012, 64, 1215–1226. [Google Scholar] [CrossRef]
- Gomez Mendez, L.M.; Cascino, M.D.; Garg, J.; Katsumoto, T.R.; Brakeman, P.; Dall’Era, M.; Looney, R.J.; Rovin, B.; Dragone, L.; Brunetta, P. Peripheral Blood B Cell Depletion after Rituximab and Complete Response in Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2018, 13, 1502–1509. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakayamada, S.; Yamaoka, K.; Ohmura, K.; Yasuda, S. Rituximab in the Real-World Treatment of Lupus Nephritis: A Retrospective Cohort Study in Japan. Mod. Rheumatol. 2023, 33, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.C.; Castells, M.C. Rituximab Hypersensitivity and Desensitization. Ann. Allergy. Asthma. Immunol. 2019, 123, 11–15. [Google Scholar] [CrossRef]
- Li, K.; Yu, Y.; Gao, Y.; Zhao, F.; Liang, Z.; Gao, J. Comparative Effectiveness of Rituximab and Common Induction Therapies for Lupus Nephritis: A Systematic Review and Network Meta-Analysis. Front. Immunol. 2022, 13, 859380. [Google Scholar] [CrossRef] [PubMed]
- Plüß, M.; Piantoni, S.; Tampe, B.; Kim, A.H.J.; Korsten, P. Belimumab for Systemic Lupus Erythematosus—Focus on Lupus Nephritis. Hum. Vaccines Immunother. 2022, 18, 2072143. [Google Scholar] [CrossRef]
- Shipa, M.; Embleton-Thirsk, A.; Parvaz, M.; Santos, L.R.; Muller, P.; Chowdhury, K.; Isenberg, D.A.; Doré, C.J.; Gordon, C.; Ehrenstein, M.R.; et al. Effectiveness of Belimumab After Rituximab in Systemic Lupus Erythematosus: A Randomized Controlled Trial. Ann. Intern. Med. 2021, 174, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M.; Arriens, C.; Caster, D.J.; Romero-Diaz, J.; Gibson, K.; Kaplan, J.; Lisk, L.; Navarra, S.; et al. Efficacy and Safety of Voclosporin versus Placebo for Lupus Nephritis (AURORA 1): A Double-Blind, Randomised, Multicentre, Placebo-Controlled, Phase 3 Trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef]
- Saxena, A.; Ginzler, E.M.; Gibson, K.; Satirapoj, B.; Santillán, A.E.Z.; Levchenko, O.; Navarra, S.; Atsumi, T.; Yasuda, S.; Chavez-Perez, N.N.; et al. Safety and Efficacy of Long-Term Voclosporin Treatment for Lupus Nephritis in the Phase 3 AURORA 2 Clinical Trial. Arthritis Rheumatol. 2024, 76, 59–67. [Google Scholar] [CrossRef]
- Jayne, D.; Rovin, B.; Mysler, E.; Furie, R.; Houssiau, F.; Trasieva, T.; Knagenhjelm, J.; Schwetje, E.; Tang, W.; Tummala, R.; et al. Anifrolumab in Lupus Nephritis: Results from Second-Year Extension of a Randomised Phase II Trial. Lupus Sci. Med. 2023, 10, e000910. [Google Scholar] [CrossRef]
- Furie, R.A.; Aroca, G.; Cascino, M.D.; Garg, J.P.; Rovin, B.H.; Alvarez, A.; Fragoso-Loyo, H.; Zuta-Santillan, E.; Schindler, T.; Brunetta, P.; et al. B-Cell Depletion with Obinutuzumab for the Treatment of Proliferative Lupus Nephritis: A Randomised, Double-Blind, Placebo-Controlled Trial. Ann. Rheum. Dis. 2022, 81, 100–107. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Turner-Stokes, T.; Houssiau, F.; Rovin, B.H. Kidney Involvement in Systemic Lupus Erythematosus: From the Patient Assessment to a Tailored Treatment. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101925. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rivera, J.E.; García-Carro, C.; Ávila, A.I.; Espino, M.; Espinosa, M.; Fernández-Juárez, G.; Fulladosa, X.; Goicoechea, M.; Macía, M.; Morales, E.; et al. Diagnosis and Treatment of Lupus Nephritis: A Summary of the Consensus Document of the Spanish Group for the Study of Glomerular Diseases (GLOSEN). Clin. Kidney J. 2023, 16, 1384–1402. [Google Scholar] [CrossRef] [PubMed]
- Moriano, C.; Bellido-Pastrana, D.; San Román Gutiérrez, C.; Rodríguez, E. Evolution of Diagnosis and Treatment for Lupus Nephritis in Spain. Nefrol. Engl. Ed. 2023, 43, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Malvar, A.; Alberton, V.; Recalde, C.; Heguilen, R. Repeat Kidney Biopsy Findings of Lupus Nephritis Patients in Clinical Remission Treated with Mycophenolate Associated with Belimumab or Mycophenolate plus Standard of Care Therapy. A “Post-Hoc” Analysis of Participants in the BLISS-LN and Open Label Extension Study Belonging to a Single Center. Lupus 2023, 32, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Contreras, G.; Teng, Y.K.O.; Curtis, P.; Green, Y.; Okily, M.; Madan, A.; Roth, D.A. Safety and Efficacy of Belimumab in Patients with Lupus Nephritis: Open-Label Extension of BLISS-LN Study. Clin. J. Am. Soc. Nephrol. 2022, 17, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Worley, K.; Milligan, S.; Rubin, B. Steroid-Sparing Effect of Belimumab: Results from a Retrospective Observational Study of Real-World Data. Lupus Sci. Med. 2023, 10, e001024. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, D.P.E.; Basta, F.; Batani, V.; Afeltra, A. Belimumab and Low-Doses of Mycophenolate Mofetil as Induction Therapy of Class IV Lupus Nephritis: Case Series and Literature Review. BMC Nephrol. 2018, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Hernández, J.; Marras Fernández-Cid, C.; Andreu Sánchez, J.L.; Calvo Alén, J.; García-Aparicio, A.M.; Díez Álvarez, E.; Hidalgo Bermejo, F.J.; Coronell, C.; Perna, A.; Ordi Ros, J. Reduction of Disease Activity, Corticosteroids Use, and Healthcare Resource Utilisation in Patients with Systemic Lupus Erythematosus Treated with Belimumab in Clinical Practice Settings: OBSErve Spain Multicentre Study. Reumatol. Clín. Engl. Ed. 2023, 19, 312–318. [Google Scholar] [CrossRef]
- Van Schaik, M.; Arends, E.J.; Soonawala, D.; Van Ommen, E.; De Leeuw, K.; Limper, M.; Van Paassen, P.; Huizinga, T.W.J.; Toes, R.E.M.; Van Kooten, C.; et al. Efficacy of Belimumab Combined with Rituximab in Severe Systemic Lupus Erythematosus: Study Protocol for the Phase 3, Multicenter, Randomized, Open-Label Synbiose 2 Trial. Trials 2022, 23, 939. [Google Scholar] [CrossRef]
- Rafael-Vidal, C.; Altabás, I.; Pérez, N.; Mourino Rodríguez, C.; Pego-Reigosa, J.M.; Garcia, S. Calcineurin and Systemic Lupus Erythematosus: The Rationale for Using Calcineurin Inhibitors in the Treatment of Lupus Nephritis. Int. J. Mol. Sci. 2021, 22, 1263. [Google Scholar] [CrossRef]
- Abdel-Kahaar, E.; Keller, F. Clinical Pharmacokinetics and Pharmacodynamics of Voclosporin. Clin. Pharmacokinet. 2023, 62, 693–703. [Google Scholar] [CrossRef]
- Arriens, C.; Teng, Y.K.O.; Ginzler, E.M.; Parikh, S.V.; Askanase, A.D.; Saxena, A.; Gibson, K.; Caster, D.J.; Atsumi, T.; Lisk, L.; et al. Update on the Efficacy and Safety Profile of Voclosporin: An Integrated Analysis of Clinical Trials in Lupus Nephritis. Arthritis Care Res. 2023, 75, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Solomons, N.; Pendergraft, W.F.; Dooley, M.A.; Tumlin, J.; Romero-Diaz, J.; Lysenko, L.; Navarra, S.V.; Huizinga, R.B.; Adzerikho, I.; et al. A Randomized, Controlled Double-Blind Study Comparing the Efficacy and Safety of Dose-Ranging Voclosporin with Placebo in Achieving Remission in Patients with Active Lupus Nephritis. Kidney Int. 2019, 95, 219–231. [Google Scholar] [CrossRef]
- Van Gelder, T.; Lerma, E.; Engelke, K.; Huizinga, R.B. Voclosporin: A Novel Calcineurin Inhibitor for the Treatment of Lupus Nephritis. Expert Rev. Clin. Pharmacol. 2022, 15, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Ayoub, I.M.; Chan, T.M.; Liu, Z.-H.; Mejía-Vilet, J.M.; Floege, J. KDIGO 2024 Clinical Practice Guideline for the Management of LUPUS NEPHRITIS. Kidney Int. 2024, 105, S1–S69. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Shelke, V.; Lei, Y.; Gaikwad, A.B.; Anders, H.-J. Voclosporin: Unique Chemistry, Pharmacology and Toxicity Profile, and Possible Options for Implementation into the Management of Lupus Nephritis. Cells 2023, 12, 2440. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Rovin, B.; Mysler, E.F.; Furie, R.A.; Houssiau, F.A.; Trasieva, T.; Knagenhjelm, J.; Schwetje, E.; Chia, Y.L.; Tummala, R.; et al. Phase II Randomised Trial of Type I Interferon Inhibitor Anifrolumab in Patients with Active Lupus Nephritis. Ann. Rheum. Dis. 2022, 81, 496–506. [Google Scholar] [CrossRef]
- Bruce, I.N.; Van Vollenhoven, R.F.; Morand, E.F.; Furie, R.A.; Manzi, S.; White, W.B.; Abreu, G.; Tummala, R. Sustained Glucocorticoid Tapering in the Phase 3 Trials of Anifrolumab: A Post Hoc Analysis of the TULIP-1 and TULIP-2 Trials. Rheumatology 2023, 62, 1526–1534. [Google Scholar] [CrossRef]
- Rovin, B.H.; Furie, R.A.; Ross Terres, J.A.; Giang, S.; Schindler, T.; Turchetta, A.; Garg, J.P.; Pendergraft, W.F.; Malvar, A. Kidney Outcomes and Preservation of Kidney Function with Obinutuzumab in Patients With Lupus Nephritis: A Post Hoc Analysis of the NOBILITY Trial. Arthritis Rheumatol. 2024, 76, 247–254. [Google Scholar] [CrossRef]
- Arnold, J.; Dass, S.; Twigg, S.; Jones, C.H.; Rhodes, B.; Hewins, P.; Chakravorty, M.; Courtney, P.; Ehrenstein, M.; Md Yusof, M.Y.; et al. Efficacy and Safety of Obinutuzumab in Systemic Lupus Erythematosus Patients with Secondary Non-Response to Rituximab. Rheumatology 2022, 61, 4905–4909. [Google Scholar] [CrossRef]
- Bowman, S.J.; Fox, R.; Dörner, T.; Mariette, X.; Papas, A.; Grader-Beck, T.; Fisher, B.A.; Barcelos, F.; De Vita, S.; Schulze-Koops, H.; et al. Safety and Efficacy of Subcutaneous Ianalumab (VAY736) in Patients with Primary Sjögren’s Syndrome: A Randomised, Double-Blind, Placebo-Controlled, Phase 2b Dose-Finding Trial. Lancet 2022, 399, 161–171. [Google Scholar] [CrossRef]
- O’Hara, D.V.; Lam, C.S.P.; McMurray, J.J.V.; Yi, T.W.; Hocking, S.; Dawson, J.; Raichand, S.; Januszewski, A.S.; Jardine, M.J. Applications of SGLT2 Inhibitors beyond Glycaemic Control. Nat. Rev. Nephrol. 2024, 20, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, S.; He, Y.; Yan, L.; Lv, F.; Liang, Q.; Gan, Y.; Han, L.; Xu, H.; Li, Y.; et al. SGLT2 Inhibitors Alleviated Podocyte Damage in Lupus Nephritis by Decreasing Inflammation and Enhancing Autophagy. Ann. Rheum. Dis. 2023, 82, 1328–1340. [Google Scholar] [CrossRef]
- Wagner, B.R.; Rao, P.S. Sodium-Glucose Cotransporter 2 Inhibitors: Are They Ready for Prime Time in the Management of Lupus Nephritis? Curr. Opin. Rheumatol. 2024, 36, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-S.; Wang, S.-I.; Hsu, C.-C.; Hwu, C.-M.; Wei, J.C.-C. Sodium-Glucose Cotransporter-2 Inhibitors and Nephritis Among Patients with Systemic Lupus Erythematosus. JAMA Netw. Open 2024, 7, e2416578. [Google Scholar] [CrossRef] [PubMed]
- Petrić, M.; Radić, M. Is Th17-Targeted Therapy Effective in Systemic Lupus Erythematosus? Curr. Issues Mol. Biol. 2023, 45, 4331–4343. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenhoven, R.F.; Hahn, B.H.; Tsokos, G.C.; Wagner, C.L.; Lipsky, P.; Touma, Z.; Werth, V.P.; Gordon, R.M.; Zhou, B.; Hsu, B.; et al. Efficacy and Safety of Ustekinumab, an IL-12 and IL-23 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: Results of a Multicentre, Double-Blind, Phase 2, Randomised, Controlled Study. Lancet 2018, 392, 1330–1339. [Google Scholar] [CrossRef]
- Van Vollenhoven, R.F.; Kalunian, K.C.; Dörner, T.; Hahn, B.H.; Tanaka, Y.; Gordon, R.M.; Shu, C.; Fei, K.; Gao, S.; Seridi, L.; et al. Phase 3, Multicentre, Randomised, Placebo-Controlled Study Evaluating the Efficacy and Safety of Ustekinumab in Patients with Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2022, 81, 1556–1563. [Google Scholar] [CrossRef]
- Costa, R.; Antunes, P.; Salvador, P.; Oliveira, P.; Marinho, A. Secukinumab on Refractory Lupus Nephritis. Cureus 2021, 13, e17198. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wang, S.; Xie, J.; Cui, D. mTOR Signaling: A Pivotal Player in Treg Cell Dysfunction in Systemic Lupus Erythematosus. Clin. Immunol. 2022, 245, 109153. [Google Scholar] [CrossRef]
- Ding, M.; Jin, L.; Zhao, J.; Yang, L.; Cui, S.; Wang, X.; He, J.; Chang, F.; Shi, M.; Ma, J.; et al. Add-on Sirolimus for the Treatment of Mild or Moderate Systemic Lupus Erythematosus via T Lymphocyte Subsets Balance. Lupus Sci. Med. 2024, 11, e001072. [Google Scholar] [CrossRef]
- Banic, M.; Pavlisa, G.; Hecimovic, A.; Grzelja, J.; Anic, B.; Samarzija, M.; Jankovic Makek, M. Refractory Systemic Lupus Erythematosus with Chylous Effusion Successfully Treated with Sirolimus: A Case Report and Literature Review. Rheumatol. Int. 2023, 43, 1743–1749. [Google Scholar] [CrossRef]
- Garufi, C.; Mancuso, S.; Spinelli, F.R.; Truglia, S.; Ceccarelli, F.; Alessandri, C.; Conti, F. Janus Kinases Inhibitors for Treating Patients with Rhupus. Jt. Bone Spine 2020, 87, 673–674. [Google Scholar] [CrossRef]
- Lei, Y.; Sehnert, B.; Voll, R.E.; Jacobs-Cachá, C.; Soler, M.J.; Sanchez-Niño, M.D.; Ortiz, A.; Bülow, R.D.; Boor, P.; Anders, H.-J. A Multicenter Blinded Preclinical Randomized Controlled Trial on Jak1/2 Inhibition in MRL/MpJ-Fas Mice with Proliferative Lupus Nephritis Predicts Low Effect Size. Kidney Int. 2021, 99, 1331–1341. [Google Scholar] [CrossRef]
- Morand, E.F.; Vital, E.M.; Petri, M.; Van Vollenhoven, R.; Wallace, D.J.; Mosca, M.; Furie, R.A.; Silk, M.E.; Dickson, C.L.; Meszaros, G.; et al. Baricitinib for Systemic Lupus Erythematosus: A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial (SLE-BRAVE-I). Lancet 2023, 401, 1001–1010. [Google Scholar] [CrossRef]
- Petri, M.; Bruce, I.N.; Dörner, T.; Tanaka, Y.; Morand, E.F.; Kalunian, K.C.; Cardiel, M.H.; Silk, M.E.; Dickson, C.L.; Meszaros, G.; et al. Baricitinib for Systemic Lupus Erythematosus: A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial (SLE-BRAVE-II). Lancet 2023, 401, 1011–1019. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Parodis, I. Janus Kinase Inhibitors in Systemic Lupus Erythematosus: Implications for Tyrosine Kinase 2 Inhibition. Front. Med. 2023, 10, 1217147. [Google Scholar] [CrossRef] [PubMed]
- Morand, E.; Pike, M.; Merrill, J.T.; Van Vollenhoven, R.; Werth, V.P.; Hobar, C.; Delev, N.; Shah, V.; Sharkey, B.; Wegman, T.; et al. Deucravacitinib, a Tyrosine Kinase 2 Inhibitor, in Systemic Lupus Erythematosus: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2023, 75, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Roccatello, D.; Fenoglio, R.; Caniggia, I.; Kamgaing, J.; Naretto, C.; Cecchi, I.; Rubini, E.; Rossi, D.; De Simone, E.; Del Vecchio, G.; et al. Daratumumab Monotherapy for Refractory Lupus Nephritis. Nat. Med. 2023, 29, 2041–2047. [Google Scholar] [CrossRef]
- Alexander, T.; Ostendorf, L.; Biesen, R.; Schneider, U.; Burmester, G.R.; Hiepe, F. Sustained Responses after Anti-CD38 Treatment with Daratumumab in Two Patients with Refractory Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2023, 82, 1497–1499. [Google Scholar] [CrossRef]
- Pleguezuelo, D.E.; Díaz-Simón, R.; Cabrera-Marante, O.; Lalueza, A.; Paz-Artal, E.; Lumbreras, C.; Serrano Hernández, A. Case Report: Resetting the Humoral Immune Response by Targeting Plasma Cells with Daratumumab in Anti-Phospholipid Syndrome. Front. Immunol. 2021, 12, 667515. [Google Scholar] [CrossRef]
- Muñoz-Grajales, C.; Yilmaz, E.B.; Svenungsson, E.; Touma, Z. Systemic Lupus Erythematosus and Damage: What Has Changed over the Past 20 Years? Best Pract. Res. Clin. Rheumatol. 2023, 37, 101893. [Google Scholar] [CrossRef]
- Wong, C.Y.; Ma, B.M.Y.; Zhang, D.; Cheung, W.; Chan, T.M.; Yap, D.Y.H. Cardiovascular Risk Factors and Complications in Patients with Systemic Lupus Erythematosus with and without Nephritis: A Systematic Review and Meta-Analysis. Lupus Sci. Med. 2024, 11, e001152. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; López-Hernández, Y.J.; Trujeque-Matos, M.; Santander-Velez, J.I.; Cano-Verduzco, M.L.; Cruz, C.; Morales-Buenrostro, L.E. High Frequency of Nocturnal Hypertension in Lupus Nephritis: Should ABPM Be Implemented in Usual Practice? Clin. Rheumatol. 2020, 39, 1147–1155. [Google Scholar] [CrossRef]
- Borrelli, S.; Garofalo, C.; Gabbai, F.B.; Chiodini, P.; Signoriello, S.; Paoletti, E.; Ravera, M.; Bussalino, E.; Bellizzi, V.; Liberti, M.E.; et al. Dipping Status, Ambulatory Blood Pressure Control, Cardiovascular Disease, and Kidney Disease Progression: A Multicenter Cohort Study of CKD. Am. J. Kidney Dis. 2023, 81, 15–24.e1. [Google Scholar] [CrossRef]
- Parodis, I.; Girard-Guyonvarc’h, C.; Arnaud, L.; Distler, O.; Domján, A.; Van Den Ende, C.H.M.; Fligelstone, K.; Kocher, A.; Larosa, M.; Lau, M.; et al. EULAR Recommendations for the Non-Pharmacological Management of Systemic Lupus Erythematosus and Systemic Sclerosis. Ann. Rheum. Dis. 2023, ard-2023-224416. [Google Scholar] [CrossRef]
- Castro, M.; Ugolini-Lopes, M.; Borba, E.F.; Bonfá, E.; Seguro, L.P.C. Effectiveness of Renoprotective Approaches for Persistent Proteinuria in Lupus Nephritis: More than Just Immunosuppression. Lupus 2018, 27, 2215–2219. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Agarwal, R.; Bakris, G.L.; Cherney, D.Z.I.; Lam, C.S.P.; Neuen, B.L.; Sarafidis, P.A.; Tuttle, K.R.; Wanner, C.; Brinker, M.D.; et al. Design and Baseline Characteristics of the Finerenone, in Addition to Standard of Care, on the Progression of Kidney Disease in Patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) Randomized Trial. Nephrol. Dial. Transplant. 2024, gfae132. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice: Developed by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with Representatives of the European Society of Cardiology and 12 Medical Societies with the Special Contribution of the European Association of Preventive Cardiology (EAPC). Rev. Esp. Cardiol. Engl. Ed. 2022, 75, 429. [Google Scholar] [CrossRef]
- Venturelli, V.; Abrantes, A.M.; Rahman, A.; Isenberg, D.A. The Impact of Antiphospholipid Antibodies/Antiphospholipid Syndrome on Systemic Lupus Erythematosus. Rheumatology 2024, 63, SI72–SI85. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Girón-Ortega, J.A.; Girón-González, J.A. Direct-Acting Oral Anticoagulants in Antiphospholipid Syndrome: A Systematic Review. Med. Clin. 2023, 161, 65–77. [Google Scholar] [CrossRef]
- Hiraoka, D.; Ishizaki, J.; Yamanouchi, J.; Honda, T.; Niiya, T.; Horimoto, E.; Horie, K.; Yamasaki, H.; Matsumoto, T.; Suemori, K.; et al. Antiplatelet Effects of Hydroxychloroquine in Patients with Systemic Lupus Erythematosus Evaluated by the Total Thrombus-Formation Analysis System (T-TAS). Lupus Sci. Med. 2024, 11, e001223. [Google Scholar] [CrossRef]
- Fasano, S.; Pierro, L.; Pantano, I.; Iudici, M.; Valentini, G. Longterm Hydroxychloroquine Therapy and Low-Dose Aspirin May Have an Additive Effectiveness in the Primary Prevention of Cardiovascular Events in Patients with Systemic Lupus Erythematosus. J. Rheumatol. 2017, 44, 1032–1038. [Google Scholar] [CrossRef]
- Edens, C.; Robinson, A.B. Systemic Lupus Erythematosus, Bone Health, and Osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 422–431. [Google Scholar] [CrossRef]
- Rahman, A.; Haider, M.F. A Comprehensive Review on Glucocorticoids Induced Osteoporosis: A Medication Caused Disease. Steroids 2024, 207, 109440. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Fassio, A.; Rossini, M.; Caimmi, C.; Giollo, A.; Orsolini, G.; Viapiana, O.; Gatti, D. Osteoporosis in Rheumatic Diseases. Int. J. Mol. Sci. 2019, 20, 5867. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Singh, S. Systemic Lupus Erythematosus and Infections. Reumatismo 2020, 72, 154–169. [Google Scholar] [CrossRef]
- Windpessl, M.; Kostopoulou, M.; Conway, R.; Berke, I.; Bruchfeld, A.; Soler, M.J.; Sester, M.; Kronbichler, A. Preventing Infections in Immunocompromised Patients with Kidney Diseases: Vaccines and Antimicrobial Prophylaxis. Nephrol. Dial. Transplant. 2023, 38, ii40–ii49. [Google Scholar] [CrossRef] [PubMed]
- Everett, S.T.; Wolf, R.; Contento, I.; Haiduc, V.; Richey, M.; Erkan, D. Short-Term Patient-Centered Nutrition Counseling Impacts Weight and Nutrient Intake in Patients with Systemic Lupus Erythematosus. Lupus 2015, 24, 1321–1326. [Google Scholar] [CrossRef]
- Tsoi, A.; Gomez, A.; Boström, C.; Pezzella, D.; Chow, J.W.; Girard-Guyonvarc’h, C.; Stamm, T.; Arnaud, L.; Parodis, I. Efficacy of Lifestyle Interventions in the Management of Systemic Lupus Erythematosus: A Systematic Review of the Literature. Rheumatol. Int. 2024, 44, 765–778. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, J.; Shi, X.; Quinet, R.J.; Davis, W.; Zakem, J.; Keshavamurthy, C.; Patel, R.; Lobo, G.; Hellmers, L.; et al. Lupus Pathogenesis and Autoimmunity Are Exacerbated by High Fat Diet-Induced Obesity in MRL/Lpr Mice. Lupus Sci. Med. 2023, 10, e000898. [Google Scholar] [CrossRef]
- Torreggiani, M.; Wang, A.Y.-M.; Fois, A.; Piccoli, G.B. Personalized Low-Protein Diet Prescription in CKD Population: Merging Evidence from Randomized Trials With Observational Data. Semin. Nephrol. 2023, 43, 151402. [Google Scholar] [CrossRef] [PubMed]
- Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; Dort, S.; Calcagno, M.; Burgess, N.; Crosby, L.; Barnard, N.D. Nutrition Interventions in Rheumatoid Arthritis: The Potential Use of Plant-Based Diets. A Review. Front. Nutr. 2019, 6, 141. [Google Scholar] [CrossRef]
- Ho, L.-J.; Wu, C.-H.; Luo, S.-F.; Lai, J.-H. Vitamin D and Systemic Lupus Erythematosus: Causality and Association with Disease Activity and Therapeutics. Biochem. Pharmacol. 2024, 227, 116417. [Google Scholar] [CrossRef] [PubMed]
- Pocovi-Gerardino, G.; Correa-Rodríguez, M.; Callejas-Rubio, J.L.; Ríos-Fernández, R.; Ortego-Centeno, N.; Rueda-Medina, B. Dietary Intake and Nutritional Status in Patients with Systemic Lupus Erythematosus. Endocrinol. Diabetes Nutr. 2018, 65, 533–539. [Google Scholar] [CrossRef]
- Aberer, E. Epidemiologic, Socioeconomic and Psychosocial Aspects in Lupus Erythematosus. Lupus 2010, 19, 1118–1124. [Google Scholar] [CrossRef]
- Fangtham, M.; Kasturi, S.; Bannuru, R.R.; Nash, J.L.; Wang, C. Non-Pharmacologic Therapies for Systemic Lupus Erythematosus. Lupus 2019, 28, 703–712. [Google Scholar] [CrossRef]
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.D.F.; Silva Junior, G.B. da Pathophysiological Aspects of Nephropathy Caused by Non-Steroidal Anti-Inflammatory Drugs. J. Bras. Nefrol. 2019, 41, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Negm, W.A.; Abdelkader, A.M.; Alshehri, A.A.; El-Saber Batiha, G.; Osama, H. Most Common Over-the-Counter Medications and Effects on Patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1654–1666. [Google Scholar] [CrossRef]
- Sammaritano, L.R.; Bermas, B.L.; Chakravarty, E.E.; Chambers, C.; Clowse, M.E.B.; Lockshin, M.D.; Marder, W.; Guyatt, G.; Branch, D.W.; Buyon, J.; et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2020, 72, 461–488. [Google Scholar] [CrossRef]
- Georgopoulou, S.; Nel, L.; Sangle, S.R.; D’Cruz, D.P. Physician-Patient Interaction and Medication Adherence in Lupus Nephritis. Lupus 2020, 29, 1168–1178. [Google Scholar] [CrossRef]
- Gasparotto, M.; Gatto, M.; Binda, V.; Doria, A.; Moroni, G. Lupus Nephritis: Clinical Presentations and Outcomes in the 21st Century. Rheumatology 2020, 59, v39–v51. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, P.; Dang, X.; Zhang, X.; Mao, Y.; Chen, X. Lupus Nephritis: New Progress in Diagnosis and Treatment. J. Autoimmun. 2022, 132, 102871. [Google Scholar] [CrossRef]
- Xipell, M.; Lledó, G.M.; Egan, A.C.; Tamirou, F.; Del Castillo, C.S.; Rovira, J.; Gómez-Puerta, J.A.; García-Herrera, A.; Cervera, R.; Kronbichler, A.; et al. From Systemic Lupus Erythematosus to Lupus Nephritis: The Evolving Road to Targeted Therapies. Autoimmun. Rev. 2023, 22, 103404. [Google Scholar] [CrossRef]
- Mok, C.C.; Teng, Y.K.O.; Saxena, R.; Tanaka, Y. Treatment of Lupus Nephritis: Consensus, Evidence and Perspectives. Nat. Rev. Rheumatol. 2023, 19, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Solé, C.; Royo, M.; Sandoval, S.; Moliné, T.; Gabaldón, A.; Cortés-Hernández, J. Precise Targeting of Autoantigen-Specific B Cells in Lupus Nephritis with Chimeric Autoantibody Receptor T Cells. Int. J. Mol. Sci. 2024, 25, 4226. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, N.; Zhang, J.; Zhao, H.; Wang, X. miR-410 Suppresses the Expression of Interleukin-6 as Well as Renal Fibrosis in the Pathogenesis of Lupus Nephritis. Clin. Exp. Pharmacol. Physiol. 2016, 43, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Deng, Y. miR-125a-3p Decreases Levels of Interlukin-17 and Suppresses Renal Fibrosis via down-Regulating TGF-Β1 in Systemic Lupus Erythematosus Mediated Lupus Nephritic Mice. Am. J. Transl. Res. 2019, 11, 1843–1853. [Google Scholar] [PubMed]
- Wu, L.; Han, X.; Jiang, X.; Ding, H.; Qi, C.; Yin, Z.; Xiao, J.; Xiong, L.; Guo, Q.; Ye, Z.; et al. Downregulation of Renal Hsa-miR-127-3p Contributes to the Overactivation of Type I Interferon Signaling Pathway in the Kidney of Lupus Nephritis. Front. Immunol. 2021, 12, 747616. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; He, X.-J.; Zhang, W.; Chen, Y.-L.; Yang, J.; Xiang, W.; Ding, Y. MiR-145 Participates in the Development of Lupus Nephritis by Targeting CSF1 to Regulate the JAK/STAT Signaling Pathway. Cytokine 2022, 154, 155877. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Fu, J.; Chen, C.; Jiao, C.; Kong, W.; Zhang, Y.; Chang, Q.; Wang, Y.; Li, D.; Illei, G.G.; et al. LNA-Anti-miR-150 Ameliorated Kidney Injury of Lupus Nephritis by Inhibiting Renal Fibrosis and Macrophage Infiltration. Arthritis Res. Ther. 2019, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xie, L.; Qin, H.; Liu, X.; Chen, X.; Lv, F.; Wang, L.; Zhu, X.; Xu, J. The Role of Extracellular Vesicles in Systemic Lupus Erythematosus. Front. Cell Dev. Biol. 2022, 10, 835566. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, M.; Camussi, G.; Brizzi, M.F. Extracellular Vesicles Tune the Immune System in Renal Disease: A Focus on Systemic Lupus Erythematosus, Antiphospholipid Syndrome, Thrombotic Microangiopathy and ANCA-Vasculitis. Int. J. Mol. Sci. 2021, 22, 4194. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.T.; Rasmussen, N.S.; Heegaard, N.H.H.; Jacobsen, S. “Kill” the Messenger: Targeting of Cell-Derived Microparticles in Lupus Nephritis. Autoimmun. Rev. 2016, 15, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Chang, X.; Lin, Y.; Pang, X.; Luo, S.; Huang, H.; Qin, L.; Lan, Y.; Zeng, Y.; Wang, C. Effect of M0 Macrophage-Derived Exosome miR-181d-5p Targeting BCL-2 to Regulate NLRP3/Caspase-1/GSDMD Pathway on Human Renal Mesangial Cells Pyroptosis. Gene 2024, 908, 148289. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, M.; Merlotti, G.; Fornara, L.; Colombatto, A.; Cantaluppi, V. Extracellular Vesicles Released from Stem Cells as a New Therapeutic Strategy for Primary and Secondary Glomerulonephritis. Int. J. Mol. Sci. 2022, 23, 5760. [Google Scholar] [CrossRef]
- Liao, H.; Hsu, P. Immunomodulatory Effects of Extracellular Vesicles from Mesenchymal Stromal Cells: Implication for Therapeutic Approach in Autoimmune Diseases. Kaohsiung J. Med. Sci. 2024, 40, 520–529. [Google Scholar] [CrossRef]
- Fang, T.; Li, B.; Li, M.; Zhang, Y.; Jing, Z.; Li, Y.; Xue, T.; Zhang, Z.; Fang, W.; Lin, Z.; et al. Engineered Cell Membrane Vesicles Expressing CD40 Alleviate System Lupus Nephritis by Intervening B Cell Activation. Small Methods 2023, 7, 2200925. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Li, M.; Tao, J.; Xu, X.; Wang, Y.; Hu, Y. Therapeutic Effects of Cationic Liposomes on Lupus-Prone MRL/Lpr Mice Are Mediated via Inhibition of TLR4-Triggered B-Cell Activation. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102491. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, M.; Mendoza-Pinto, C.; Dyball, S.; Munguía-Realpozo, P.; Parker, B.; Bruce, I.N. Predictors and Prognostic Factors Influencing Outcomes of Anti-CD20 Monoclonal Antibodies in Systemic Lupus Erythematosus: A Systematic Review Update. Semin. Arthritis Rheum. 2024, 65, 152346. [Google Scholar] [CrossRef] [PubMed]
- Rekvig, O.P.; Thiyagarajan, D.; Pedersen, H.L.; Horvei, K.D.; Seredkina, N. Future Perspectives on Pathogenesis of Lupus Nephritis. Am. J. Pathol. 2016, 186, 2772–2782. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, S.; Chen, T.; Liang, D.; Yang, J.; Zeng, C.; Li, X.; Xie, G.; Liu, Z. Machine Learning for Prediction and Risk Stratification of Lupus Nephritis Renal Flare. Am. J. Nephrol. 2021, 52, 152–160. [Google Scholar] [CrossRef]
- Liu, T.; Yang, Y.; Zhou, Y.; Jiang, Y. Noninvasive Biomarkers for Lupus Nephritis. Lab. Med. 2024, lmae015. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).