Zinc and Its Impact on the Function of the Testicle and Epididymis
Abstract
1. Introduction
2. Zn as a Trace Element
3. Zn Transport
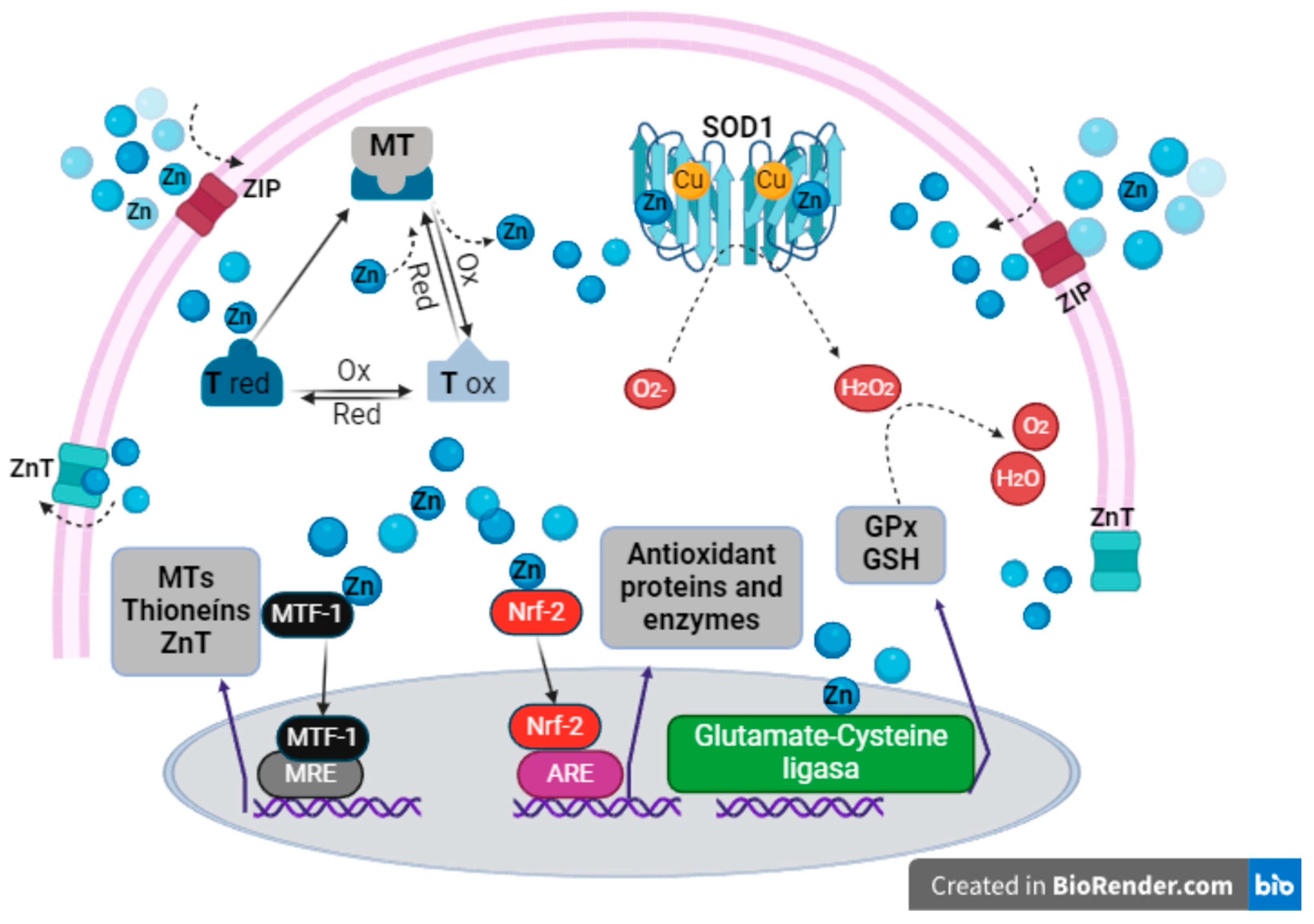
4. Zn as an Inert Antioxidant
5. The Relevance of Zn in the Testes during Spermatogenesis
6. The Relevance of Zn in the Epididymis and Sperm Maturation
7. Zn and Its Role in Sperm Quality
8. Zn in Other Organs of the Reproductive System
9. Conclusions and Future Research Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Phadke, M.; Packard, D.; Yadav, D.; Gorelick, F. Zinc: Roles in pancreatic physiology and disease. Pancreatology 2020, 20, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Harchegani, A.B. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Tinkov, A.A. Zinc. Adv. Food Nutr. Res. 2021, 96, 251–310. [Google Scholar] [CrossRef]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Nancy-Veenakumari, D.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef]
- Saper, R.B.; Rash, R. Zinc: An essential micronutrient. Am. Fam. Physician 2009, 79, 768–772. [Google Scholar]
- Maares, M.; Haase, H.A. Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Giachi, G.; Pallecchi, P.; Romualdi, A.; Ribechini, E.; Lucejko, J.J.; Colombini, M.P.; Mariotti-Lippi, M. Ingredients of a 2,000-y-old medicine revealed by chemical, mineralogical, and botanical investigations. Proc. Natl. Acad. Sci. USA 2013, 110, 1193–1196. [Google Scholar] [CrossRef]
- Kerns, K.; Zigo, M.; Sutovsky, P. Zinc: A Necessary Ion for Mammalian Sperm Fertilization Competency. Int. J. Mol. Sci. 2018, 19, 4097. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Impact on human health. Nutrition 2001, 17, 685–687. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- O’Dell, B.L. Role of zinc in plasma membrane function. J. Nutr. 2000, 130, 1432S–1436S. [Google Scholar] [CrossRef]
- Costello, L.C.; Fenselau, C.C.; Franklin, R.B. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J. Inorg. Biochem. 2011, 105, 589–599. [Google Scholar] [CrossRef]
- Hübner, C.; Haase, H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 2021, 41, 101916. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Bouron, A.; Oberwinkler, J. Contribution of calcium-conducting channels to the transport of zinc ions. Pflugers Arch. 2014, 466, 381–387. [Google Scholar] [CrossRef]
- Lu, Q.; Haragopal, H.; Slepchenko, K.G.; Stork, C.; Li, Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 35–43. [Google Scholar]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Hosios, A.M.; Vander-Heiden, M.G. The redox requirements of proliferating mammalian cells. J. Biol. Chem. 2018, 293, 7490–7498. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Kochańczyk, T.; Drozd, A.; Krężel, A. Relationship between the architecture of zinc coordination and zinc binding affinity in proteins--insights into zinc regulation. Metallomics 2015, 7, 244–257. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Passerini, A.; Andreini, C.; Menchetti, S.; Rosato, A.; Frasconi, P. Predicting zinc binding at the proteome level. BMC Bioinform. 2007, 8, 39. [Google Scholar] [CrossRef]
- Sirangelo, I.; Vella, F.M.; Irace, G.; Manco, G.; Iannuzzi, C. Glycation in Demetalated Superoxide Dismutase 1 Prevents Amyloid Aggregation and Produces Cytotoxic Ages Adducts. Front. Mol. Biosci. 2016, 3, 55. [Google Scholar] [CrossRef]
- Polykretis, P.; Cencetti, F.; Donati, C.; Luchinat, E.; Banci, L. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 2019, 21, 101102. [Google Scholar] [CrossRef]
- Marklund, S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984, 222, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta 2006, 1763, 747–758. [Google Scholar] [CrossRef]
- Sirangelo, I.; Iannuzzi, C. The Role of Metal Binding in the Amyotrophic Lateral Sclerosis-Related Aggregation of Copper-Zinc Superoxide Dismutase. Molecules 2017, 22, 1429. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cramaro, F.; Del-Conte, R.; Viezzoli, M.S. Solution structure of Apo Cu,Zn superoxide dismutase: Role of metal ions in protein folding. Biochemistry 2003, 42, 9543–9553. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Cantini, F.; Kozyreva, T.; Massagni, C.; Palumaa, P.; Rubino, J.T.; Zovo, K. Human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS). Proc. Natl. Acad. Sci. USA 2012, 109, 13555–13560. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Cousins, R.J.; Liuzzi, J.P.; Lichten, L.A. Mammalian Zinc Transport, Trafficking, and Signals. J. Biol. Chem. 2006, 281, 24085–24089. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef]
- Vallee, B.L.; Coleman, J.E.; Auld, D.S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc. Natl. Acad. Sci. USA 1991, 88, 999–1003. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Vallee, B.L. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc. Natl. Acad. Sci. USA 1998, 95, 3478–3482. [Google Scholar] [CrossRef]
- Dalton, T.; Palmiter, R.D.; Andrews, G.K. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994, 22, 5016–5023. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000, 59, 95–104. [Google Scholar] [CrossRef]
- Andrews, G.K. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals 2001, 14, 223–237. [Google Scholar] [CrossRef]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell. Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.N.; Chen, Y.; Cai, J.; Sternberg, P., Jr. Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: Implication for protection against oxidative stress. Invest. Ophthalmol. Vis. Sci. 2006, 47, 2709–2715. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Foster, M.; Samman, S. Zinc and redox signaling: Perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid. Redox Signal. 2010, 13, 1549–1573. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, D.; Guo, B.; Deng, W.; Chi, Z.H.; Cai, Y.; Wang, L.; Ma, J. Zinc transporter 5 and zinc transporter 7 induced by high glucose protects peritoneal mesothelial cells from undergoing apoptosis. Cell. Signal. 2013, 25, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Batchuluun, B.; Ho, L.; Zhu, D.; Prentice, K.J.; Bhattacharjee, A.; Zhang, M.; Pourasgari, F.; Hardy, A.B.; Taylor, K.M.; et al. Characterization of Zinc Influx Transporters (ZIPs) in Pancreatic β Cells: Roles in Regulating Cytosolic Zinc Homeostasis and Insulin Secretion. J. Biol. Chem. 2015, 290, 18757–18769. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef]
- Mancuso, F.; Arato, I.; Bellucci, C.; Lilli, C.; Eugeni, E.; Aglietti, M.C.; Stabile, A.M.; Pistilli, A.; Brancorsini, S.; Gaggia, F.; et al. Zinc restores functionality in porcine prepubertal Sertoli cells exposed to subtoxic cadmium concentration via regulating the Nrf2 signaling pathway. Front. Endocrinol. 2023, 14, 962519. [Google Scholar] [CrossRef]
- Ellis, R.E.; Stanfield, G.M. The regulation of spermatogenesis and sperm function in nematodes. Semin. Cell Dev. Biol. 2014, 29, 17–30. [Google Scholar] [CrossRef]
- Croxford, T.P.; McCormick, N.H.; Kelleher, S.L. Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J. Nutr. 2011, 141, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, H.; Zhai, X.; Dai, J.; Jiang, X.; Wang, G.; Li, W.; Cai, L. Effects of Zn deficiency, antioxidants, and low-dose radiation on diabetic oxidative damage and cell death in the testis. Toxicol. Mech. Methods 2013, 23, 42–47. [Google Scholar] [CrossRef]
- Razavi, S.; Khadivi, F.; Hashemi, F.; Bakhtiari, A. Effect of Zinc on Spermatogenesis and Sperm Chromatin Condensation in Bleomycin, Etoposide, Cisplatin Treated Rats. Cell J. 2019, 20, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bertelsmann, H.; Sieme, H.; Behne, D.; Kyriakopoulos, A. Is the distribution of selenium and zinc in the sublocations of spermatozoa regulated? Ann. New York Acad. Sci. 2007, 1095, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Bjorndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2009, 16, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L.; Kvist, U. A model for the importance of zinc in the dynamics of human sperm chromatin stabilization after ejaculation in relation to sperm DNA vulnerability. Syst. Biol. Reprod Med. 2011, 57, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Kudo, H.; Suzuki, S.; Nemoto, N.; Sassa, S.; Sakamoto, S. Down regulation by a low-zinc diet in gene expression of rat prostatic thymidylate synthase and thymidine kinase. Nutr. Metab. 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Beigi-Harchegani, A.; Dahan, H.; Tahmasbpour, E.; Bakhtiari-Kaboutaraki, H.; Shahriary, A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: The role of oxidative stress, inflammation and apoptosis. Hum Fertil. 2020, 23, 5–16. [Google Scholar] [CrossRef]
- Henkel, R.; Baldauf, C.; Schill, W.B. Resorption of the element zinc from spermatozoa by the epididymal epithelium. Reprod. Domest. Anim. 2003, 38, 97–101. [Google Scholar] [CrossRef]
- Teerds, K.J.; Huhtaniemi, I.T. Morphological and functional maturation of Leydig cells: From rodent models to primates. Hum. Reprod. Update 2015, 21, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, T.; Yang, B.; Chi, Z.; Wang, Z.Y.; Gu, H.F. A novel role for zinc transporter 8 in the facilitation of zinc accumulation and regulation of testosterone synthesis in Leydig cells of human and mouse testicles. Metabolism 2018, 88, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Abdelmelek, H.; Garrel, C.; Guiraud, P.; Douki, T.; Ravanat, J.L.; Favier, A.; Sakly, M.; Ben-Rhouma, K. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J. Reprod. Dev. 2008, 54, 129–134. [Google Scholar] [CrossRef]
- Kheradmand, F.; Nourmohammadi, I.; Modarressi, M.H.; Firoozrai, M.; Ahmadi-Faghih, M.A. Differential gene-expression of metallothionein 1M and 1G in response to zinc in sertoli TM4 cells. Iran. Biomed. J. 2010, 14, 9–15. [Google Scholar] [PubMed] [PubMed Central]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.T.; Di, Q.N.; Qian, L.L.; Lu, L.; Li, R.X.; Cao, W.X.; Xu, Q. Zinc supplement ameliorates phthalates-induced reproductive toxicity in male rats. Chemosphere 2020, 246, 125828. [Google Scholar] [CrossRef] [PubMed]
- Omu, A.E.; Al-Azemi, M.K.; Al-Maghrebi, M.; Mathew, C.T.; Omu, F.E.; Kehinde, E.O.; Anim, J.T.; Oriowo, M.A.; Memon, A. Molecular basis for the effects of zinc deficiency on spermatogenesis: An experimental study in the Sprague-dawley rat model. Indian J. Urol. 2015, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J.; et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Invest. 2009, 119, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef] [PubMed]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- Stoltenberg, M.; Ernst, E.; Andreasen, A.; Danscher, G. Histochemical localization of zinc ions in the epididymis of the rat. Histochem. J. 1996, 28, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Maldera, J.A.; Vasen, G.; Ernesto, J.I.; Weigel-Muñoz, M.; Cohen, D.J.; Cuasnicu, P.S. Evidence for the involvement of zinc in the association of CRISP1 with rat sperm during epididymal maturation. Biol. Reprod. 2011, 85, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Gadella, B.M.; Olrichs, N.K.; Kaloyanova, D.V.; Helms, J.B. The less conserved metal-binding site in human CRISP1 remains sensitive to zinc ions to permit protein oligomerization. Sci. Rep. 2021, 11, 5498. [Google Scholar] [CrossRef]
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R. Epididymosomes: Role of extracellular microvesicles in sperm maturation. Front. Biosci. 2016, 8, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. Review: The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar] [PubMed]
- Taylor, A.; Robson, A.; Houghton, B.C.; Jepson, C.A.; Ford, W.C.; Frayne, J. Epididymal specific, selenium-independent GPX5 protects cells from oxidative stress-induced lipid peroxidation and DNA mutation. Hum. Reprod. 2013, 28, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Noblanc, A.; Kocer, A.; Drevet, J.R. Protection post-testiculaire des gamètes mâles contre les dommages radicalaires: Le rôle de l’épididyme [Post-testicular protection of male gametes from oxidative damage. The role of the epididymis]. Med. Sci. 2012, 28, 519–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Li, Y.; Zhou, X.; Cao, Y.; Li, C. Preventive effects of supplemental dietary zinc on heat-induced damage in the epididymis of boars. J. Therm. Biol. 2017, 64, 58–66. [Google Scholar] [CrossRef]
- Vickram, A.S.; Raja, D.; Srinivas, M.S.; Kamini, A.R.; Jayaraman, G.; Sridharan, T.B. Prediction of Zn concentration in human seminal plasma of Normospermia samples by Artificial Neural Networks (ANN). J. Assist. Reprod. Genet. 2013, 30, 453–459. [Google Scholar] [CrossRef]
- Torabi, F.; Malekzadeh-Shafaroudi, M.; Rezaei, N. Combined protective effect of zinc oxide nanoparticles and melatonin on cyclophosphamide-induced toxicity in testicular histology and sperm parameters in adult Wistar rats. Int. J. Reprod. Biomed. 2017, 15, 403–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Anjum, M.R.; Madhu, P.; Reddy, K.P.; Reddy, P.S. The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats. Toxicol. Ind. Health 2017, 33, 265–276. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, J.; He, S. Zinc Protects against Heat Stress-Induced Apoptosis via the Inhibition of Endoplasmic Reticulum Stress in TM3 Leydig Cells. Biol. Trace Elem. Res. 2022, 200, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Wang, Y.; Yang, M.; Guo, M. Zinc Deficiency Promotes Testicular Cell Apoptosis in Mice. Biol. Trace Elem. Res. 2020, 195, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi-Nejad, S.; Golzari, Z.; Zangiabadian, M.; Salehi Amniyeh Khozani, A.A.; Ebrahimi, R.; Nejadghaderi, S.A.; Aletaha, A. The association between zinc and prostate cancer development: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0299398. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Durairajanayagam, D.; Halabi, J.; Peng, J.; Vazquez-Levin, M. Proteomics, oxidative stress and male infertility. Reprod. Biomed. Online 2014, 29, 32–58. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Huang, Y.L.; Pan, C.H.; Diawara, N. Role of low exposure to metals as male reproductive toxicants. Int. J. Environ. Health Res. 2015, 25, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Du-Plessis, S.S.; Gokul, S.; Agarwal, A. Semen hyperviscosity: Causes, consequences, and cures. Front. Biosci.-Elite 2013, 5, 224–231. [Google Scholar] [CrossRef]
- Abdul-Wahab, R.H.; Al-Daghistani, H.I.; Shquirat, W.D.; Abdel-Dayem, M.; Al-Swaifi, M. Sodium, Potassium, Calcium and Copper Levels in Seminal Plasma are Associated with Sperm Quality in Fertile and Infertile Men. Biochem. Pharmacol. 2014, 3, 141. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef]

| The Contributions of Previous Reviews | The Contribution of the Present Review |
|---|---|
| The biochemical aspects and mechanisms of Zn against oxidative stress [7]. | The properties and biology of Zn and its mechanisms of action. |
| Zn is an inert antioxidant, and it participates in oxidative stress [8]. | The interaction of Zn with proteins and enzymes, such as MTs, metalloenzymes, and gene regulatory proteins. |
| The deficiency in Zn in the testicle and seminal plasma and its role in male infertility [9]. | The importance of Zn in male fertility, from hormonal regulation by androgens and testicular and spermatogenic functions, as well as its importance in germ cells for differentiation into spermatozoa and its role in the epididymis. |
| The role of Zn in the hyperactivated motility of human spermatozoa [10]. | Zn participates in sperm survival and functionality, and it is necessary for successful fertilization. |
| The contribution of Zn to quality sperm parameters [11]. | Zn is a promoter of improvement in the quality of sperm parameters in male fertility. |
| The role of Zn in the testicle, prostate, and seminal vesicle and in sperm viability [12]. | The role of Zn and its regulation in the testicle and the epididymis for the optimization of sperm production and maturation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín de Jesús, S.; Vigueras-Villaseñor, R.M.; Cortés-Barberena, E.; Hernández-Rodriguez, J.; Montes, S.; Arrieta-Cruz, I.; Pérez-Aguirre, S.G.; Bonilla-Jaime, H.; Limón-Morales, O.; Arteaga-Silva, M. Zinc and Its Impact on the Function of the Testicle and Epididymis. Int. J. Mol. Sci. 2024, 25, 8991. https://doi.org/10.3390/ijms25168991
Marín de Jesús S, Vigueras-Villaseñor RM, Cortés-Barberena E, Hernández-Rodriguez J, Montes S, Arrieta-Cruz I, Pérez-Aguirre SG, Bonilla-Jaime H, Limón-Morales O, Arteaga-Silva M. Zinc and Its Impact on the Function of the Testicle and Epididymis. International Journal of Molecular Sciences. 2024; 25(16):8991. https://doi.org/10.3390/ijms25168991
Chicago/Turabian StyleMarín de Jesús, Sergio, Rosa María Vigueras-Villaseñor, Edith Cortés-Barberena, Joel Hernández-Rodriguez, Sergio Montes, Isabel Arrieta-Cruz, Sonia Guadalupe Pérez-Aguirre, Herlinda Bonilla-Jaime, Ofelia Limón-Morales, and Marcela Arteaga-Silva. 2024. "Zinc and Its Impact on the Function of the Testicle and Epididymis" International Journal of Molecular Sciences 25, no. 16: 8991. https://doi.org/10.3390/ijms25168991
APA StyleMarín de Jesús, S., Vigueras-Villaseñor, R. M., Cortés-Barberena, E., Hernández-Rodriguez, J., Montes, S., Arrieta-Cruz, I., Pérez-Aguirre, S. G., Bonilla-Jaime, H., Limón-Morales, O., & Arteaga-Silva, M. (2024). Zinc and Its Impact on the Function of the Testicle and Epididymis. International Journal of Molecular Sciences, 25(16), 8991. https://doi.org/10.3390/ijms25168991





