The Association between Telomere Length and Head and Neck Cancer Risk: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.1.1. Study Inclusion/Exclusion Criteria
2.1.2. Data Extraction
2.2. Statistical Analysis
2.3. Study Selection
3. Results
3.1. Study Characteristics
3.2. Results of Studies
3.2.1. Meta-Analysis
3.2.2. Subgroup Analysis
3.3. Publication Bias and Sensitivity Analysis
4. Discussion
- (a)
- (b)
- Significant heterogeneity was noted between the included studies. Although we conducted subgroup analyses in order to investigate the source of this heterogeneity, this particular problem may stem from factors that have not been accounted for in our analysis.
- (c)
- In some subgroups, the small number of studies and small sample size limited the ability to derive statistically significant results. Specifically, an important limitation was the lack of data to investigate the relationship of TL with HNC subtypes, with the exception of oral cancers.
- (d)
- While across all studies, the definitions of short and long TL were consistently based on the median telomere length, complete homogeneity was not achieved. In one study [40], the median of all subjects was used as the cutoff point and the necessary data were not provided for the derivation of the results using the median TL of controls as the cutoff, the cutoff used in the other studies.
- (e)
- The studies did not include enough data on TNM/UICC stage, p16 status, or HPV status for a meaningful analysis or subgroup meta-analysis to be performed. Future studies should include such critical data.
- (f)
- The studies included in our meta-analysis had widely heterogeneous approaches to reporting TL measurements (RTL—relative telomere length; age-adjusted relative LTL—leukocyte telomere length; normalized TCRa (NTCR); T/S median; etc.) and some did not include any direct measurements of TL. Therefore, no meaningful analysis could be conducted of the range/median/standard deviation of TL.
- (g)
- Finally, one other potential limitation could be the design of the included studies, as they generally followed a case–control pattern, which has been traditionally associated with an increased risk of bias, especially selection bias [60]. However, certain studies indicate that the results may be similarly robust [61].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef]
- Pulte, D.; Brenner, H. Changes in survival in head and neck cancers in the late 20th and early 21st century: A period analysis. Oncologist 2010, 15, 994–1001. [Google Scholar] [CrossRef]
- National Cancer Institute. Five-Year Survival Rates|SEER Training. 28 March 2024. Available online: https://www.training.seer.cancer.gov/head-neck/intro/survival.html (accessed on 15 January 2024).
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. The molecular structure of centromeres and telomeres. Annu. Rev. Biochem. 1984, 53, 163–194. [Google Scholar] [CrossRef]
- Moon, I.K.; Jarstfer, M.B. The human telomere and its relationship to human disease, therapy, and tissue engineering. Front. Biosci. 2007, 12, 4595–4620. [Google Scholar] [CrossRef]
- Baerlocher, G.M.; Mak, J.; Tien, T.; Lansdorp, P.M. Telomere length measurement by fluorescence in situ hybridization and flow cytometry: Tips and pitfalls. Cytometry 2002, 47, 89–99. [Google Scholar] [CrossRef]
- Kimura, M.; Stone, R.C.; Hunt, S.C.; Skurnick, J.; Lu, X.; Cao, X.; Harley, C.B.; Aviv, A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat. Protoc. 2010, 5, 1596–1607. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Bojesen, S.E. Telomeres and human health. J. Intern. Med. 2013, 274, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Sánchez, S.; Aubert, G.; Ripoll-Cladellas, A.; Henkelman, S.; Zhernakova, D.V.; Sinha, T.; Kurilshikov, A.; Cenit, M.C.; Jan Bonder, M.; Franke, L.; et al. Genetic, parental and lifestyle factors influence telomere length. Commun. Biol. 2022, 5, 565. [Google Scholar] [CrossRef]
- Cherkas, L.F. The Association Between Physical Activity in Leisure Time and Leukocyte Telomere Length. Arch. Intern. Med. 2008, 168, 154. [Google Scholar] [CrossRef]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, Y.; Huang, J.J.; Xia, S.S.; Yang, Y. Shorter leucocyte telomere length as a potential biomarker for nonalcoholic fatty liver disease-related advanced fibrosis in T2DM patients. Ann. Transl. Med. 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chen, A.F.; Wang, H.Z.; Xie, L.Y.; Sui, K.X.; Zhang, Q.Y. Association of shorter mean telomere length with large artery stiffness in patients with coronary heart disease. Aging Male 2011, 14, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chung, M.P.; Ley, B.; French, S.; Elicker, B.M.; Fiorentino, D.F.; Chung, L.S.; Boin, F.; Wolters, P.J. Peripheral blood leucocyte telomere length is associated with progression of interstitial lung disease in systemic sclerosis. Thorax 2021, 76, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef]
- Fan, H.-C.; Chang, F.-W.; Tsai, J.-D.; Lin, K.-M.; Chen, C.-M.; Lin, S.-Z.; Liu, C.-A.; Harn, H.-J. Telomeres and Cancer. Life 2021, 11, 1405. [Google Scholar] [CrossRef]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef]
- Blasco, M.A.; Lee, H.W.; Hande, M.P.; Samper, E.; Lansdorp, P.M.; DePinho, R.A.; Greider, C.W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997, 91, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; de Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell. Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, Z.; Wei, S.; Liu, Z.; Pooley, K.A.; Dunning, A.M.; Svenson, U.; Roos, G.; Hosgood, H.D., III; Shen, M.; et al. Shortened telomere length is associated with increased risk of cancer: A meta-analysis. PLoS ONE 2011, 6, e20466. [Google Scholar] [CrossRef]
- Zhu, X.; Han, W.; Xue, W.; Zou, Y.; Xie, C.; Du, J.; Jin, G. The association between telomere length and cancer risk in population studies. Sci. Rep. 2016, 6, 22243. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Yunesian, M.; Nabizadeh, R.; Mehdipour, P.; Aghaie, A. Is Leukocyte Telomere Length Related with Lung Cancer Risk?: A Meta-Analysis. Iran. Biomed. J. 2017, 21, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Aung, K.; Lai, P.K.; Mak, J.W. Association between telomere length and the risk of colorectal cancer: A meta-analysis of observational studies. BMC Cancer 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Benites-Zapata, V.A.; Ulloque-Badaracco, J.R.; Alarcón-Braga, E.A.; Fernández-Alonso, A.M.; López-Baena, M.T.; Pérez-López, F.R. Telomerase activity and telomere length in women with breast cancer or without malignancy: A systematic review and meta-analysis. Maturitas 2024, 180, 107882. [Google Scholar] [CrossRef]
- Caini, S.; Raimondi, S.; Johansson, H.; De Giorgi, V.; Zanna, I.; Palli, D.; Gandini, S. Telomere length and the risk of cutaneous melanoma and non-melanoma skin cancer: A review of the literature and meta-analysis. J. Dermatol. Sci. 2015, 80, 168–174. [Google Scholar] [CrossRef]
- Gao, M.; Zheng, G.; Li, Y.; Zhang, Y.; Hu, P.; Pan, Y. Telomere length in multiple cancer: Insight into recurrence risk from a meta-analysis. J. Gastroenterol. Hepatol. 2023, 38, 844–853. [Google Scholar] [CrossRef]
- Patel, M.M.; Parekh, L.J.; Jha, F.P.; Sainger, R.N.; Patel, J.B.; Patel, D.D.; Shah, P.M.; Patel, P.S. Clinical usefulness of telomerase activation and telomere length in head and neck cancer. Head Neck 2002, 24, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Sainger, R.N.; Telang, S.D.; Shukla, S.N.; Patel, P.S. Clinical significance of telomere length and associated proteins in oral cancer. Biomark Insights 2007, 2, 7–14. [Google Scholar] [CrossRef]
- Sainger, R.N.; Shah, F.D.; Telang, S.D.; Shah, P.M.; Patel, P.S. Telomere attrition and telomerase activity are associated with GSTM1 polymorphism in oral cancer. Cancer Biomark. 2009, 5, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhou, F.X.; Lei, H.; Yu, H.J.; Xie, C.H.; Zhou, Y.F.; Liu, S.Q. Increased expression of telomere-related proteins correlates with resistance to radiation in human laryngeal cancer cell lines. Oncol. Rep. 2009, 21, 1505–1509. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wu, X.; Amos, C.I.; Zhu, Y.; Zhao, H.; Grossman, B.H.; Shay, J.W.; Luo, S.; Hong, W.K.; Spitz, M.R. Telomere dysfunction: A potential cancer predisposition factor. J. Natl. Cancer Inst. 2003, 95, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Aida, J.; Izumo, T.; Shimomura, N.; Nakamura, K.I.; Ishikawa, N.; Matsuura, M.; Poon, S.S.; Fujiwara, M.; Sawabe, M.; Arai, T.; et al. Telomere lengths in the oral epithelia with and without carcinoma. Eur. J. Cancer 2010, 46, 430–438. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Wei, S.; Li, G.; Sturgis, E.M.; Wei, Q. Telomere length and TERT functional polymorphisms are not associated with risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2642–2645. [Google Scholar] [CrossRef]
- Zhang, Y.; Sturgis, E.M.; Dahlstrom, K.R.; Wen, J.; Liu, H.; Wei, Q.; Li, G.; Liu, Z. Telomere length in peripheral blood lymphocytes contributes to the development of hpv-associated oropharyngeal carcinoma. Cancer Res. 2013, 73, 5996–6003. [Google Scholar] [CrossRef]
- Bau, D.T.; Lippman, S.M.; Xu, E.; Gong, Y.; Lee, J.J.; Wu, X.; Gu, J. Short telomere lengths in peripheral blood leukocytes are associated with an increased risk of oral premalignant lesion and oral squamous cell carcinoma. Cancer 2013, 119, 4277–4283. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, C.; Miao, L.; Wang, L.; Xu, C.; Xue, W.; Du, J.; Yuan, H.; Dai, J.; Jin, G.; et al. Telomere length, genetic variants and risk of squamous cell carcinoma of the head and neck in Southeast Chinese. Sci. Rep. 2016, 6, 20675. [Google Scholar] [CrossRef]
- Alves-Paiva, R.M.; Gutierrez-Rodrigues, F.; Pereira-Martins, D.A.; Figueiredo, D.L.A.; Clé, D.V.; Conti-Freitas, L.C.; Mamede, R.C.M.; Calado, R.T. Short telomere length in peripheral blood leukocytes in head and neck cancer: Findings in a Brazilian cohort. Head Neck 2019, 41, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Giunco, S.; Rampazzo, E.; Brutti, M.; Spinato, G.; Menegaldo, A.; Stellin, M.; Mantovani, M.; Bandolin, L.; Rossi, M.; et al. TERT promoter hotspot mutations and their relationship with TERT levels and telomere erosion in patients with head and neck squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Vaiciulis, P.; Liutkeviciene, R.; Liutkevicius, V.; Vilkeviciute, A.; Gedvilaite, G.; Uloza, V. Association of Relative Leucocyte Telomere Length and Gene Single Nucleotide Polymorphisms (TERT, TRF1, TNKS2) in Laryngeal Squamous Cell Carcinoma. Cancer Genom. Proteom. 2020, 17, 431–439. [Google Scholar] [CrossRef]
- Friedrich, U.; Griese, E.; Schwab, M.; Fritz, P.; Thon, K.; Klotz, U. Telomere length in different tissues of elderly patients. Mech. Ageing Dev. 2000, 119, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369, eaaz6876. [Google Scholar] [CrossRef]
- Holesova, Z.; Krasnicanova, L.; Saade, R.; Pös, O.; Budis, J.; Gazdarica, J.; Repiska, V.; Szemes, T. Telomere length changes in cancer: Insights on carcinogenesis and potential for non-invasive diagnostic strategies. Genes 2023, 14, 715. [Google Scholar] [CrossRef]
- Ly, K.; Walker, C.; Berry, S.; Snell, R.; Marks, E.; Thayer, Z.; Atatoa-Carr, P.; Morton, S. Telomere length in early childhood is associated with sex and ethnicity. Sci. Rep. 2019, 9, 10359. [Google Scholar] [CrossRef]
- Thierry, A.D. Association between telomere length and neighborhood characteristics by race and region in US midlife and older adults. Health Place 2020, 62, 102272. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef]
- Kouka, M.; Hermanns, I.; Schlattmann, P.; Guntinas-Lichius, O. The Association between Patient’s Age and Head and Neck Cancer Treatment Decision-A Population-Based Diagnoses-Related Group-Based Nationwide Study in Germany. Cancers 2023, 15, 1780. [Google Scholar] [CrossRef]
- Vahl, J.M.; Wigand, M.C.; Denkinger, M.; Dallmeier, D.; Steiger, C.; Welke, C.; Kuhn, P.; Idel, C.; Doescher, J.; von Witzleben, A.; et al. Increasing Mean Age of Head and Neck Cancer Patients at a German Tertiary Referral Center. Cancers 2021, 13, 832. [Google Scholar] [CrossRef]
- Stoyanov, G.S.; Kitanova, M.; Dzhenkov, D.L.; Ghenev, P.; Sapundzhiev, N. Demographics of Head and Neck Cancer Patients: A Single Institution Experience. Cureus 2017, 9, e1418. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, A.; Taschler, B.; Ebmeier, K.P.; Smith, S.; Zhou, H.; Levey, D.F.; Codd, V.; Samani, N.J.; Gelernter, J.; Nichols, T.E. Alcohol consumption and telomere length: Mendelian randomization clarifies alcohol’s effects. Mol. Psychiatry 2022, 27, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef]
- Jethwa, A.R.; Khariwala, S.S. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Lin, L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS ONE 2018, 13, e0204056. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Rampazzo, E.; Perissinotto, E.; Piano, M.A.; Giunco, S.; Baboci, L.; Spinato, G.; Spinato, R.; Tirelli, G.; Da Mosto, M.C.; et al. Telomere shortening in mucosa surrounding the tumor: Biosensor of field cancerization and prognostic marker of mucosal failure in head and neck squamous cell carcinoma. Oral Oncol. 2015, 51, 500–507. [Google Scholar] [CrossRef]
- Lanza, A.; Ravaud, P.; Riveros, C.; Dechartres, A. Comparison of Estimates between Cohort and Case-Control Studies in Meta-Analyses of Therapeutic Interventions: A Meta-Epidemiological Study. PLoS ONE 2016, 11, e0154877. [Google Scholar] [CrossRef]
- Palumbo, S.A.; Robishaw, J.D.; Krasnoff, J.; Hennekens, C.H. Different biases in meta-analyses of case-control and cohort studies: An example from genomics and precision medicine. Ann. Epidemiol. 2021, 58, 38–41. [Google Scholar] [CrossRef]

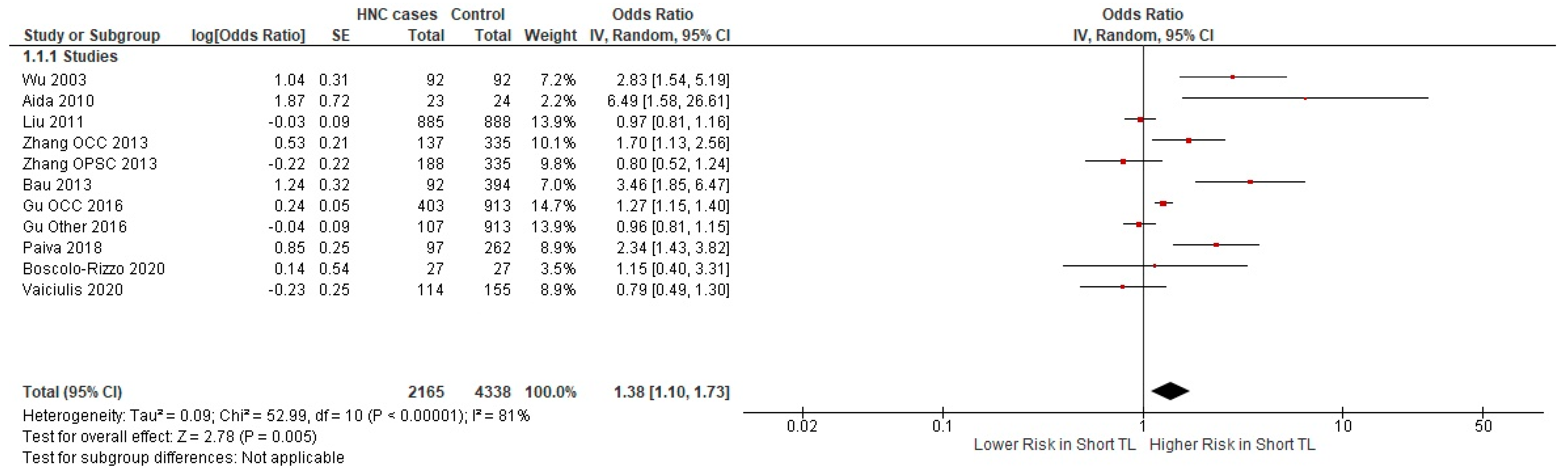
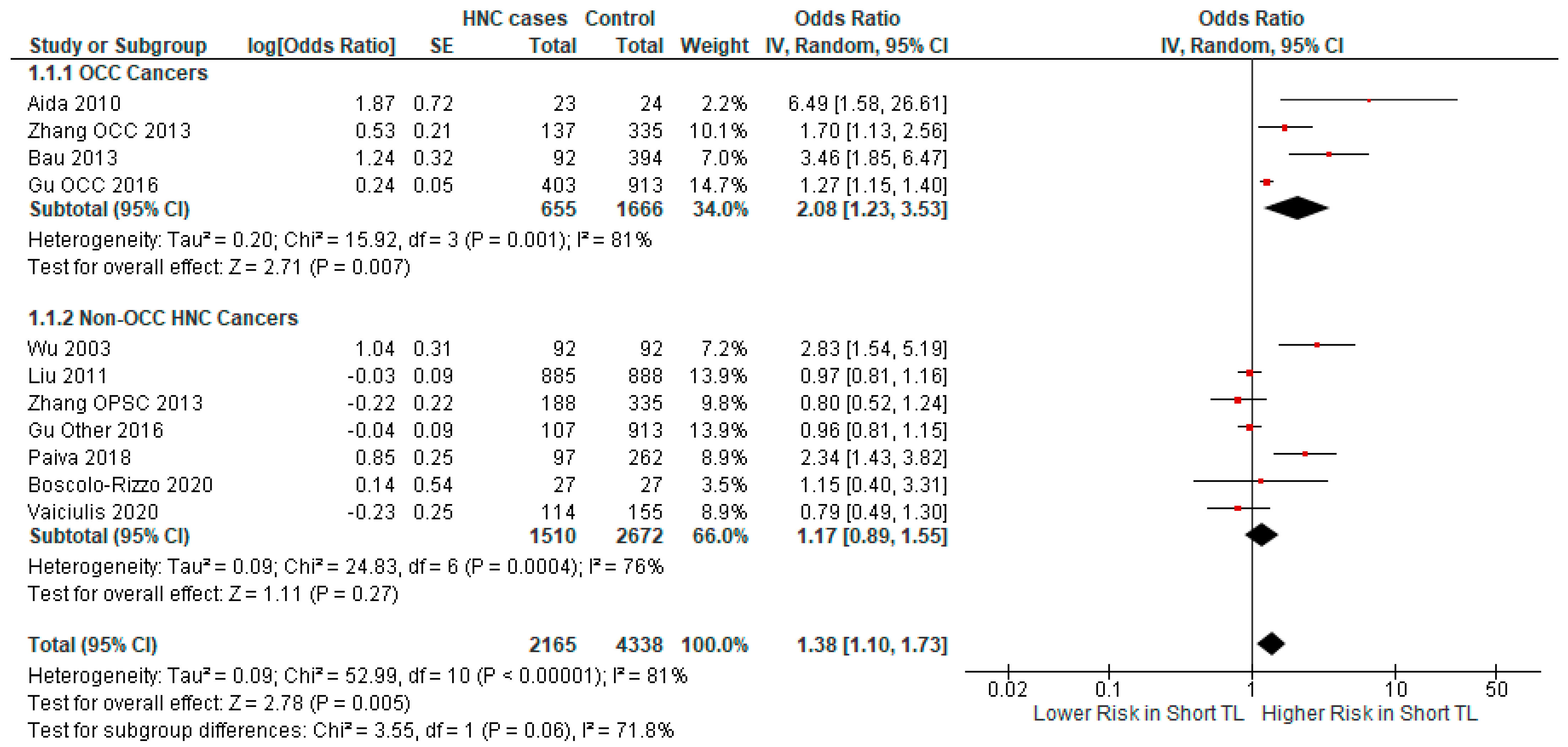
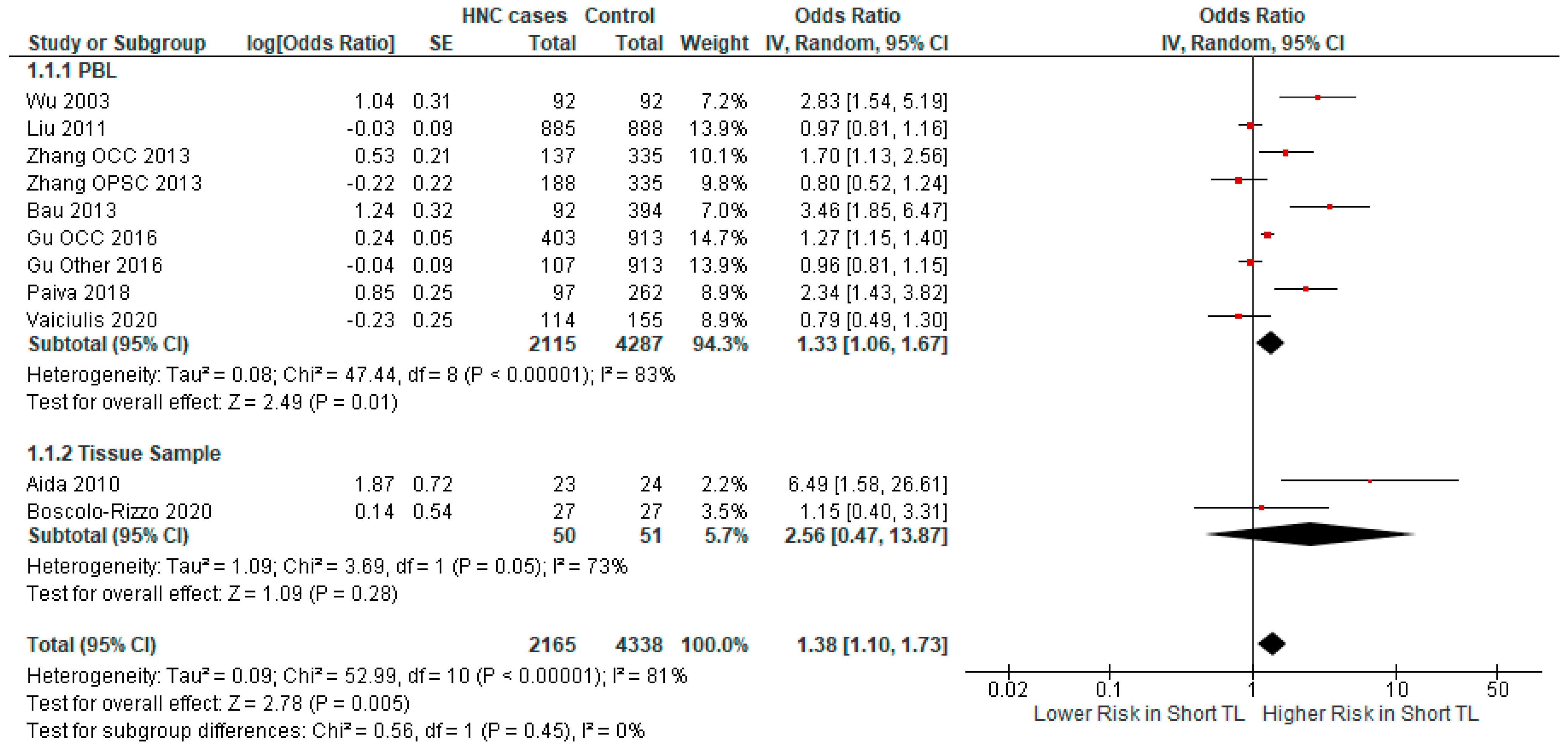
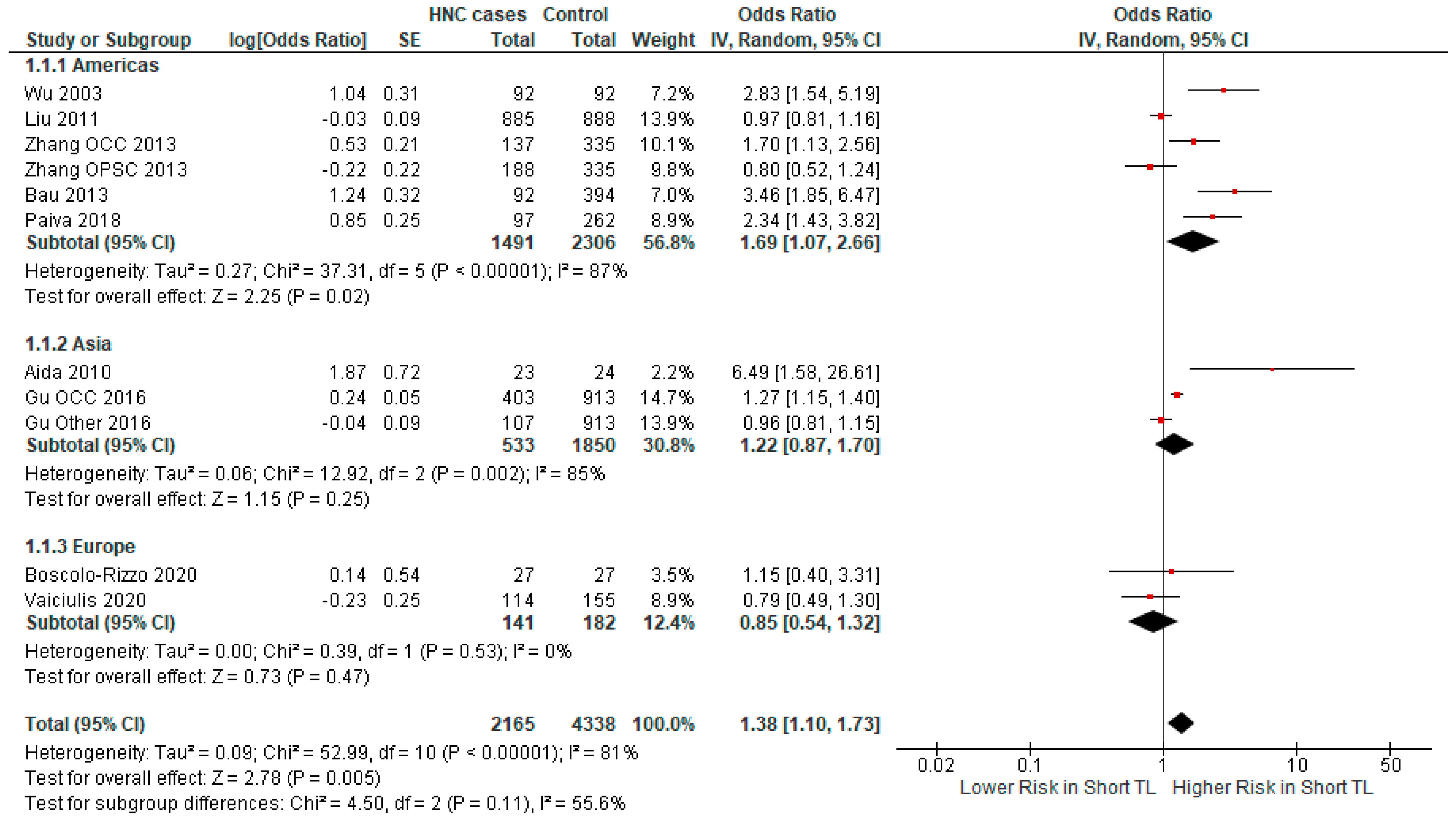
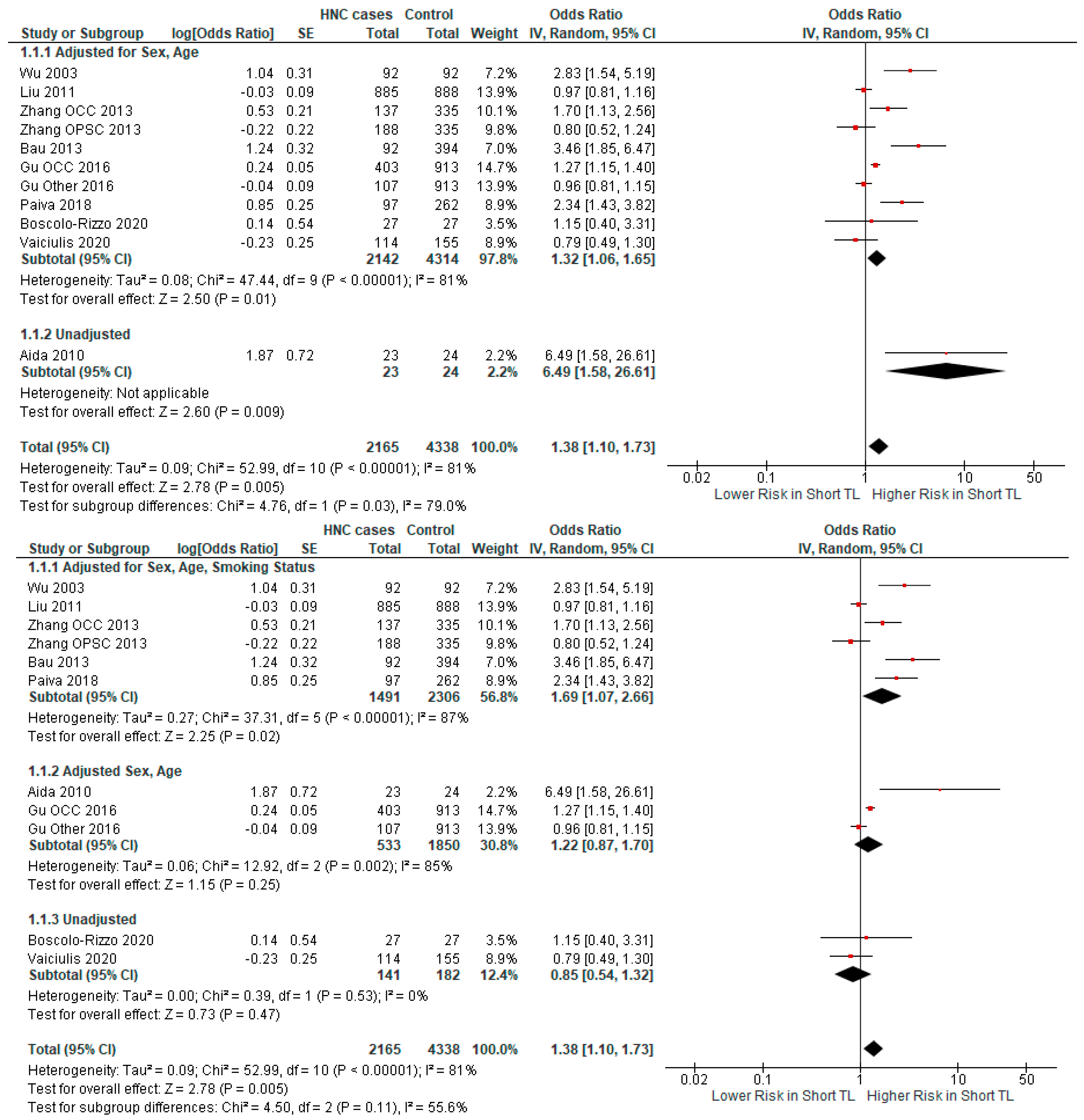
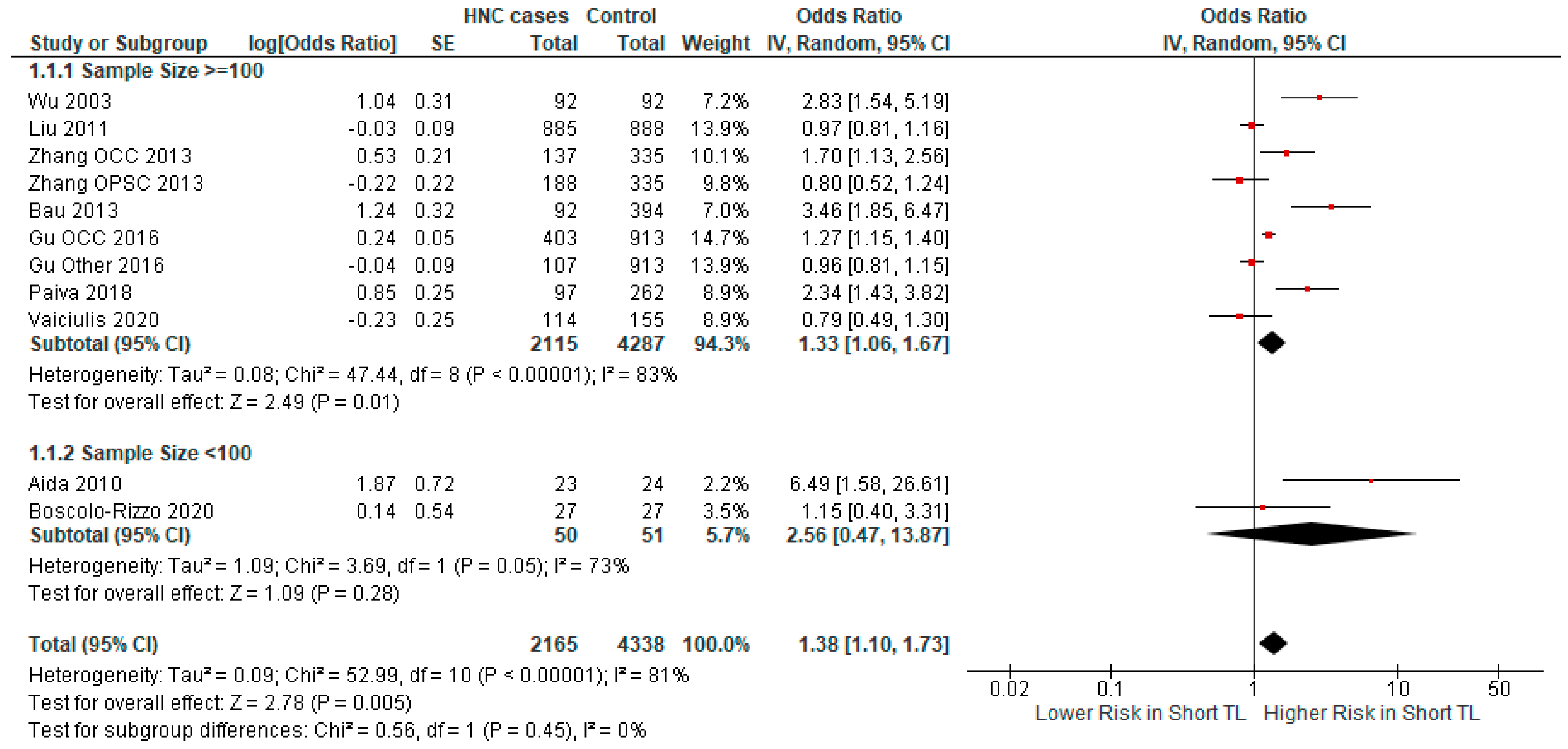
| Author | Year | Country | Cancer Type | Odds Ratio | Sample Size | Cases/ Controls |
|---|---|---|---|---|---|---|
| Wu [37] | 2003 | USA | Head and Neck | 2.83 | 184 | 92/92 |
| Aida [38] | 2010 | Japan | Oral—CIS | 6.5 | 49 | 25/24 |
| Liu [39] | 2011 | USA | Head and Neck | 0.97 | 1773 | 885/888 |
| Zhang [40] | 2013 | USA | OCC | 1.7 | 523 | 137/335 |
| Zhang [40] | 2013 | USA | OPC | 0.8 | 472 | 188/335 |
| Bau [41] | 2013 | USA | OSCC | 3.47 | 486 | 92/394 |
| Yayun Gu [42] | 2016 | China | Oral Cancer | 1.28 | 1316 | 403/913 |
| Yayun Gu [42] | 2016 | China | Other HNC sites | 0.96 | 1020 | 107/913 |
| Paiva [43] | 2018 | Brazil | Head and Neck | 2.34 | 359 | 97/262 |
| Boscolo-Rizzo [44] | 2020 | Italy | Head and Neck | 1.15 | 27 | 27/27 |
| Vaiciulis [45] | 2020 | Lithuania | Laryngeal | 0.79 | 269 | 114/155 |
| Author | Year | Study Design | DNA Source | Measurement Method | Mean Age | Gender (Percentage Male) | Adjusted |
|---|---|---|---|---|---|---|---|
| Wu [37] | 2003 | Retro | Leuko | Southern Blot | 57.6 cases, 57.4 controls | 96% cases, 96% controls | Age, sex, smoking |
| Aida [38] | 2010 | Retro | Tumor | Southern Blot | 63.3 cases, 55.4 controls | 75% cases, 74% controls | - |
| Liu [39] | 2011 | Retro | Leuko | Real-Time PCR | 56.8 cases, 55.4 controls | 74.8% cases, 74.4% controls | Age, sex, smoking, drinking |
| Zhang [40] | 2013 | Retro | Leuko | Real-Time PCR | - | 74.2% cases 1, 80.3% controls | Age, sex, smoking, drinking |
| Zhang [40] | 2013 | Retro | Leuko | Real-Time PCR | - | 74.2% cases 1, 80.3% controls | Age, sex, smoking, drinking |
| Bau [41] | 2013 | Retro | Leuko | Real-Time PCR | 57.22 cases, 58.30 controls | 59% cases, 57% controls | Age, sex, smoking, drinking |
| Yayun Gu [42] | 2016 | Retro | Leuko | Real-Time PCR | 61.29 cases 1, 59.78 controls | 70% cases 1, 74% controls | Age, sex, smoking, drinking |
| Yayun Gu [42] | 2016 | Retro | Leuko | Real-Time PCR | 61.29 cases 1, 59.78 controls | 70% cases 1, 74% controls | Age, sex, smoking, drinking |
| Paiva [43] | 2018 | Retro-Cohort | Leuko | Rreal-Time PCR | 55 cases | 89% cases | Age, sex |
| Boscolo-Rizzo [44] | 2020 | Retro | Tumor | Real-Time PCR | 66 cases | 73% cases | Age, sex, smoking, drinking |
| Vaiciulis [45] | 2020 | Retro | Leuko | Real-Time PCR | 62.8 cases, 62.5 controls | 96.3% cases, 96.7% controls | Age, sex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreikos, D.; Kyrodimos, E.; Kotsinas, A.; Chrysovergis, A.; Papacharalampous, G.X. The Association between Telomere Length and Head and Neck Cancer Risk: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 9000. https://doi.org/10.3390/ijms25169000
Andreikos D, Kyrodimos E, Kotsinas A, Chrysovergis A, Papacharalampous GX. The Association between Telomere Length and Head and Neck Cancer Risk: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(16):9000. https://doi.org/10.3390/ijms25169000
Chicago/Turabian StyleAndreikos, Dimitrios, Efthymios Kyrodimos, Athanassios Kotsinas, Aristeidis Chrysovergis, and Georgios X. Papacharalampous. 2024. "The Association between Telomere Length and Head and Neck Cancer Risk: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 25, no. 16: 9000. https://doi.org/10.3390/ijms25169000






