Effects of Sequential Induction Combining Thermal Treatment with Ultrasound or High Hydrostatic Pressure on the Physicochemical and Mechanical Properties of Pea Protein–Psyllium Hydrogels as Elderberry Extract Carriers
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Hydrogel Preparation
3.3. Color Parameters Measurements
3.4. Entrapment Efficiency (EE) Measurements
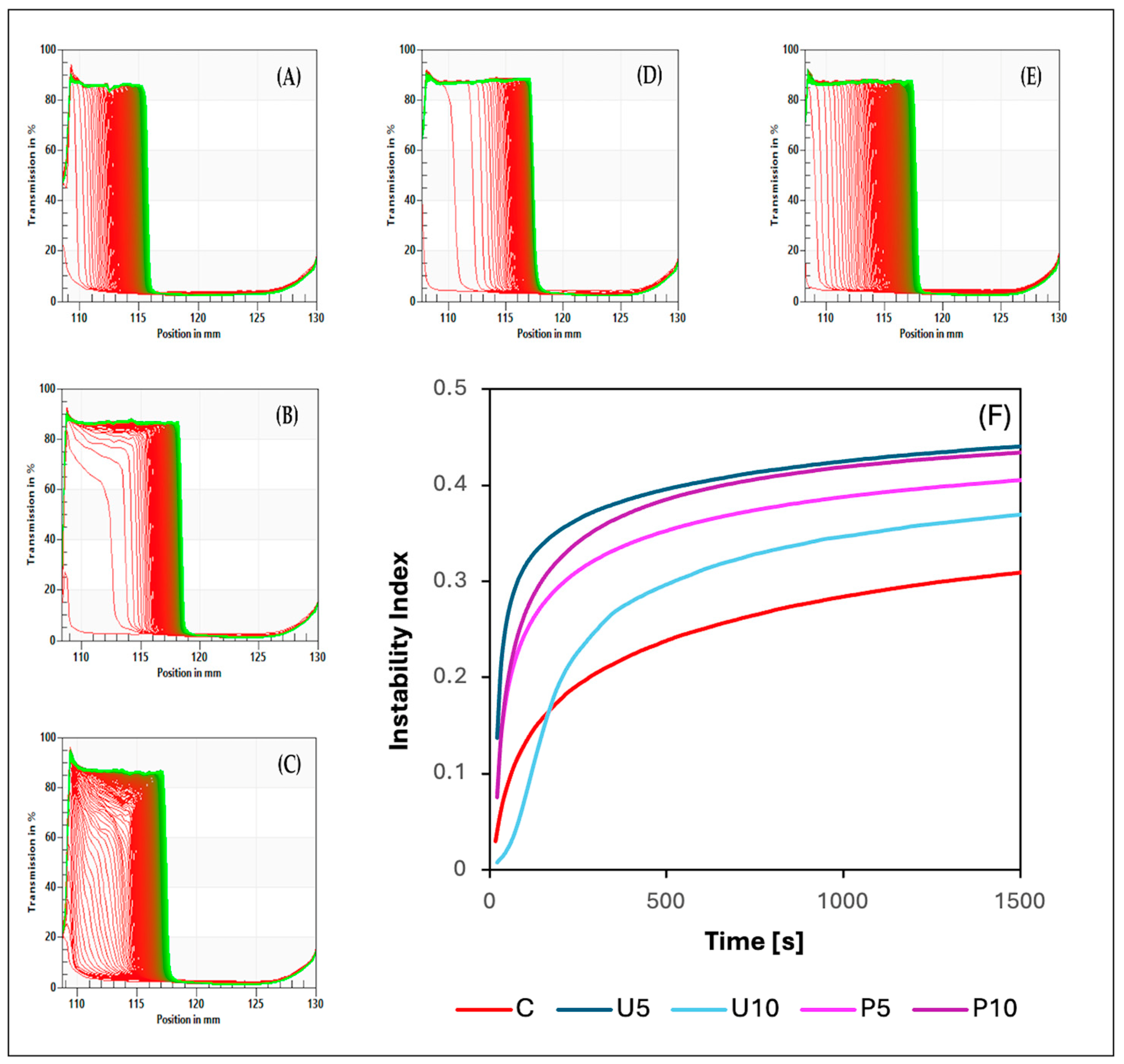
3.5. Physical Stability and Destabilization Behavior Measurements
3.6. Textural Measurements
3.7. Microrheological Measurements
3.8. Fourier Transform Infrared Spectroscopy Measurements
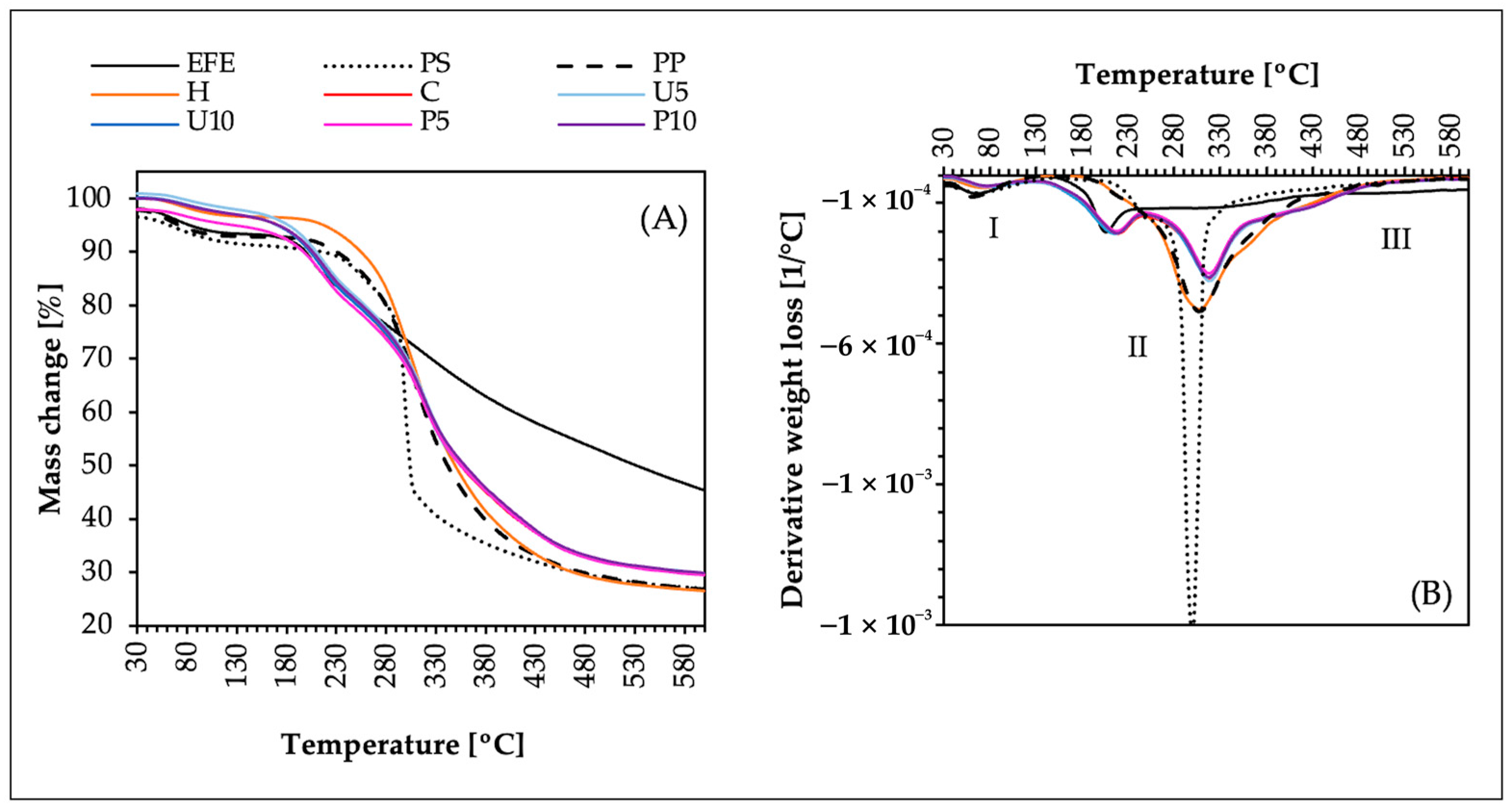
3.9. Thermal Degradation Measurements (TGA and DTG)
3.10. Microstructure Morphology—SEM Analysis
3.11. Chemical Analysis
3.11.1. Total Polyphenols Content (TPC)
3.11.2. Antioxidant Activity (AA)
3.11.3. Reducing Power (RP)
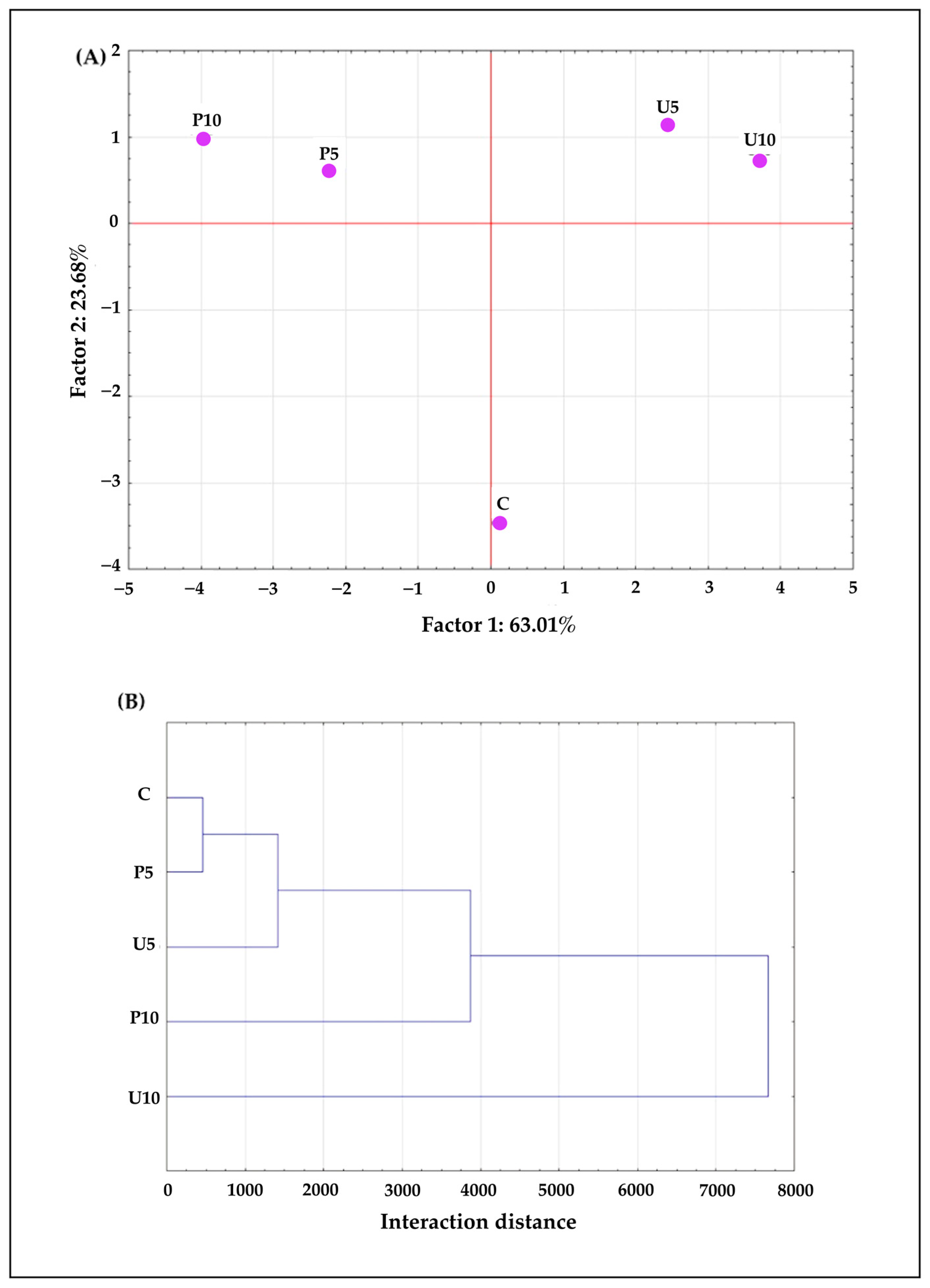
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-Thinking Functional Food Development through a Holistic Approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin Profile of Elderberry Juice: A Natural-Based Bioactive Colouring Ingredient with Potential Food Application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New Techniques and Challenges in Microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Chen, Y.; Belwal, T.; Xu, Y.; Ma, Q.; Li, D.; Li, L.; Xiao, H.; Luo, Z. Updated Insights into Anthocyanin Stability Behavior from Bases to Cases: Why and Why Not Anthocyanins Lose during Food Processing. Crit. Rev. Food Sci. Nutr. 2023, 63, 8639–8671. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry Anthocyanins: An Updated Review on Approaches to Enhancing Their Bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Hydrogels: Characteristics and Application as Delivery Systems of Phenolic and Aroma Compounds. Foods 2021, 10, 1252. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent Insights into Polysaccharide-Based Hydrogels and Their Potential Applications in Food Sector: A Review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, D.; Song, X.; Guo, M.; Wang, Z.; Geng, F.; Zhou, X.; Nie, S. Compound Hydrogels Derived from Gelatin and Gellan Gum Regulates the Release of Anthocyanins in Simulated Digestion. Food Hydrocoll. 2022, 127, 107487. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gerardi, C.; Giovinazzo, G.; Gorrasi, G. Encapsulation of Grape (Vitis vinifera L.) Pomace Polyphenols in Soybean Extract-Based Hydrogel Beads as Carriers of Polyphenols and PH-Monitoring Devices. Gels 2022, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N. Starch Based Hydrogel NPs Loaded by Anthocyanins Might Treat Glycogen Storage at Cardiomyopathy in Animal Fibrotic Model. Int. J. Biol. Macromol. 2021, 183, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ćorković, I.; Pichler, A.; Buljeta, I.; Šimunović, J.; Kopjar, M. Carboxymethylcellulose Hydrogels: Effect of Its Different Amount on Preservation of Tart Cherry Anthocyanins and Polyphenols. Curr. Plant Biol. 2021, 28, 100222. [Google Scholar] [CrossRef]
- Hilal, A.; Florowska, A.; Florowski, T.; Wroniak, M. A Comparative Evaluation of the Structural and Biomechanical Properties of Food-Grade Biopolymers as Potential Hydrogel Building Blocks. Biomedicines 2022, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Hilal, A.; Florowska, A.; Domian, E.; Wroniak, M. Binary Pea Protein–Psyllium Hydrogel: Insights into the Influence of PH and Ionic Strength on the Physical Stability and Mechanical Characteristics. Gels 2024, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Huang, W.; Guo, X.; Chen, L. Strong and Elastic Pea Protein Hydrogels Formed through PH-Shifting Method. Food Hydrocoll. 2021, 117, 106705. [Google Scholar] [CrossRef]
- Aniya; Cao, Y.; Liu, C.; Lu, S.; Fujii, Y.; Jin, J.; Xia, Q. Improved Stabilization and In Vitro Digestibility of Mulberry Anthocyanins by Double Emulsion with Pea Protein Isolate and Xanthan Gum. Foods 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Sun, J.; Pei, M.; Zhang, G.; Li, C.; Li, C.; Ma, X.; He, S.; Liu, L. Impact of Non-Covalent Bound Polyphenols on Conformational, Functional Properties and in Vitro Digestibility of Pea Protein. Food Chem. 2022, 383, 132623. [Google Scholar] [CrossRef]
- Fradinho, P.; Soares, R.; Niccolai, A.; Sousa, I.; Raymundo, A. Psyllium Husk Gel to Reinforce Structure of Gluten-Free Pasta? LWT 2020, 131, 109787. [Google Scholar] [CrossRef]
- Noguerol, A.T.; Marta Igual, M.; Pagán, M.J. Developing Psyllium Fibre Gel-Based Foods: Physicochemical, Nutritional, Optical and Mechanical Properties. Food Hydrocoll. 2022, 122, 107108. [Google Scholar] [CrossRef]
- Niu, Y.; Xia, Q.; Jung, W.; Yu, L. Polysaccharides-Protein Interaction of Psyllium and Whey Protein with Their Texture and Bile Acid Binding Activity. Int. J. Biol. Macromol. 2019, 126, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.S.; Poddar, S.; Varshney, N.; Sahi, A.K.; Vajanthri, K.Y.; Yadav, K.; Parmar, A.S.; Mahto, S.K. Printability Assessment of Psyllium Husk (Isabgol)/Gelatin Blends Using Rheological and Mechanical Properties. J. Biomater. Appl. 2021, 35, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dai, H.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Effect and Mechanism of Psyllium Husk (Plantago ovata) on Myofibrillar Protein Gelation. LWT 2021, 138, 110651. [Google Scholar] [CrossRef]
- Hilal, A.; Florowska, A.; Wroniak, M. Binary Hydrogels: Induction Methods and Recent Application Progress as Food Matrices for Bioactive Compounds Delivery—A Bibliometric Review. Gels 2023, 9, 68. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design Principles of Food Gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Klost, M.; Brzeski, C.; Drusch, S. Effect of Protein Aggregation on Rheological Properties of Pea Protein Gels. Food Hydrocoll. 2020, 108, 106036. [Google Scholar] [CrossRef]
- Florowska, A.; Florowski, T.; Kruszewski, B.; Janiszewska-Turak, E.; Bykowska, W.; Ksibi, N. Thermal and Modern, Non-Thermal Method Induction as a Factor of Modification of Inulin Hydrogel Properties. Foods 2023, 12, 4154. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, R.; Palmer, J.; Hemar, Y.; Yang, Z. Impact of High Hydrostatic Pressure on the Gelation Behavior and Microstructure of Quinoa Protein Isolate Dispersions. ACS Food Sci. Technol. 2021, 1, 2144–2151. [Google Scholar] [CrossRef]
- Zang, B.; Qiu, Z.; Zheng, Z.; Zhang, B.; Qiao, X. Quality Improvement of Garlic Paste by Whey Protein Isolate Combined with High Hydrostatic Pressure Treatment. Foods 2023, 12, 1500. [Google Scholar] [CrossRef]
- Naik, A.S.; Suryawanshi, D.; Kumar, M.; Waghmare, R. Ultrasonic Treatment: A Cohort Review on Bioactive Compounds, Allergens and Physico-Chemical Properties of Food. Curr. Res. Food Sci. 2021, 4, 470–477. [Google Scholar] [CrossRef]
- Khatkar, A.B.; Kaur, A.; Khatkar, S.K. Restructuring of Soy Protein Employing Ultrasound: Effect on Hydration, Gelation, Thermal, in-Vitro Protein Digestibility and Structural Attributes. LWT 2020, 132, 109781. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, L.; Zhao, F.; Yu, A.; Zhou, Y.; Wen, Q.; Wang, J.; Zheng, T.; Chen, P. Protein and Peptide-Based Nanotechnology for Enhancing Stability, Bioactivity, and Delivery of Anthocyanins. Adv. Healthc. Mater. 2023, 12, e2300473. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cheng, J.; Jiao, S.; Jing, P. Interaction of Mulberry Anthocyanins with Soybean Protein Isolate: Effect on the Stability of Anthocyanins and Protein in Vitro Digestion Characteristics. Int. J. Food Sci. Technol. 2022, 57, 2267–2276. [Google Scholar] [CrossRef]
- Montes, C.; Vicario, I.M.; Raymundo, M.; Fett, R.; Heredia, F.J. Application of Tristimulus Colorimetry to Optimize the Extraction of Anthocyanins from Jaboticaba (Myricia jaboticaba Berg.). Food Res. Int. 2005, 38, 983–988. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J.; Guo, X.; Lei, Y.; Yang, M. Effects of Ultrasonic Treatment on the Structure, Functional Properties of Chickpea Protein Isolate and Its Digestibility In Vitro. Foods 2022, 11, 880. [Google Scholar] [CrossRef]
- Cai, B.; Mazahreh, J.; Ma, Q.; Wang, F.; Hu, X. Ultrasound-Assisted Fabrication of Biopolymer Materials: A Review. Int. J. Biol. Macromol. 2022, 209, 1613–1628. [Google Scholar] [CrossRef]
- Mao, Y.; Li, X.; Qi, Q.; Wang, F.; Zhang, H.; Wu, Y.; Liu, J.; Zhao, C.; Xu, X. Riboflavin-Loaded Soy Protein Isolate Cold Gel Treated with Combination of High Intensity Ultrasound and High Hydrostatic Pressure: Gel Structure, Physicochemical Properties and Gastrointestinal Digestion Fate. Ultrason. Sonochem. 2024, 104, 106819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Wang, Y.S.; Chen, Y.; Luan, Q.Y.; Ding, M.Y.; Chen, H.H. Effects of Secondary Cross-Linking on the Physicochemical Properties of Sodium Alginate-Hydrogel and in Vitro Release of Anthocyanins. Int. J. Biol. Macromol. 2024, 276, 133926. [Google Scholar] [CrossRef]
- Florowska, A.; Hilal, A.; Florowski, T.; Wroniak, M. Addition of Selected Plant-Derived Proteins as Modifiers of Inulin Hydrogels Properties. Foods 2020, 9, 845. [Google Scholar] [CrossRef]
- Khalesi, H.; Lu, W.; Nishinari, K.; Fang, Y. New Insights into Food Hydrogels with Reinforced Mechanical Properties: A Review on Innovative Strategies. Adv. Colloid. Interface Sci. 2020, 285, 102278. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Chassenieux, C.; Nicolai, T. The Effect of Adding NaCl on Thermal Aggregation and Gelation of Soy Protein Isolate. Food Hydrocoll. 2017, 70, 88–95. [Google Scholar] [CrossRef]
- Su, J.; Cavaco-Paulo, A. Effect of Ultrasound on Protein Functionality. Ultrason. Sonochem. 2021, 76, 105653. [Google Scholar] [CrossRef] [PubMed]
- Inthavong, W.; Chassenieux, C.; Nicolai, T. Viscosity of Mixtures of Protein Aggregates with Different Sizes and Morphologies. Soft Matter 2019, 15, 4682–4688. [Google Scholar] [CrossRef] [PubMed]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Exploring the Interactions between Blackcurrant Polyphenols, Pectin and Wheat Biopolymers in Model Breads; A FTIR and HPLC Investigation. Food Chem. 2012, 131, 802–810. [Google Scholar] [CrossRef]
- Guo, N.; Song, M.; Liu, W.; Zhang, F.; Zhu, G. Preparation of an Elderberry Anthocyanin Film and Fresh-Keeping Effect of Its Application on Fresh Shrimps. PLoS ONE 2023, 18, e0290650. [Google Scholar] [CrossRef] [PubMed]
- Westfall, A.; Sigurdson, G.T.; Rodriguez-Saona, L.E.; Giusti, M.M. Ex Vivo and in Vivo Assessment of the Penetration of Topically Applied Anthocyanins Utilizing Atr-Ftir/Pls Regression Models and Hplc-Pda-Ms. Antioxidants 2020, 9, 486. [Google Scholar] [CrossRef]
- Waleed, M.; Saeed, F.; Afzaal, M.; Niaz, B.; Raza, M.A.; Hussain, M.; Tufail, T.; Rasheed, A.; Ateeq, H.; Al Jbawi, E. Structural and Nutritional Properties of Psyllium Husk Arabinoxylans with Special Reference to Their Antioxidant Potential. Int. J. Food Prop. 2022, 25, 2505–2513. [Google Scholar] [CrossRef]
- Ren, Y.; Yakubov, G.E.; Linter, B.R.; MacNaughtan, W.; Foster, T.J. Temperature Fractionation, Physicochemical and Rheological Analysis of Psyllium Seed Husk Heteroxylan. Food Hydrocoll. 2020, 104, 105737. [Google Scholar] [CrossRef]
- Moreno, H.M.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Borderías, A.J.; Tovar, C.A. Evaluation of Gels Made with Different Commercial Pea Protein Isolate: Rheological, Structural and Functional Properties. Food Hydrocoll. 2020, 99, 105375. [Google Scholar] [CrossRef]
- Ertugrul, U.; Namli, S.; Tas, O.; Kocadagli, T.; Gokmen, V.; Sumnu, S.G.; Oztop, M.H. Pea Protein Properties Are Altered Following Glycation by Microwave Heating. LWT 2021, 150, 111939. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešić, G.; Badanjak, M. Physical Properties of Ultrasound Treated Soy Proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Mo, X.; Zhou, N.; Yang, S.; Chen, K.; Ouyang, P. The Effect of Ultrasonication on Enzymatic Hydrolysis of Chitin to N-Acetyl Glucosamine via Sequential and Simultaneous Strategies. Process Biochem. 2020, 99, 265–269. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singha, P.; Singh, S.K. Comparison of the Effect of Hydrodynamic and Acoustic Cavitations on Functional, Rheological and Structural Properties of Egg White Proteins. Innov. Food Sci. Emerg. Technol. 2022, 82, 103166. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Wang, Y.; Li, Z.; Wang, Z.; Han, J. Effect of High Hydrostatic Pressure on Solubility and Conformation Changes of Soybean Protein Isolate Glycated with Flaxseed Gum. Food Chem. 2020, 333, 127530. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Zhou, X.; Zheng, Z.; Liang, Y.; Chen, S.; Zhang, S.; Li, Q. Investigating on the Interaction Behavior of Soy Protein Hydrolysates/β-Glucan/Ferulic Acid Ternary Complexes under High-Technology in the Food Processing: High Pressure Homogenization versus Microwave Treatment. Int. J. Biol. Macromol. 2020, 150, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Karaca, A.C.; Nowacka, M.; Mulla, M.Z.; Al-attar, H.; Rathnakumar, K.; Subasi, B.G.; Sehrawat, R.; Kheto, A.; Falsafi, S.R. How High Hydrostatic Pressure Treatment Modifies the Physicochemical and Nutritional Attributes of Polysaccharides? Food Hydrocoll. 2023, 137, 108375. [Google Scholar] [CrossRef]
- Kalak, T.; Dudczak-Hałabuda, J.; Tachibana, Y.; Cierpiszewski, R. Effective Use of Elderberry (Sambucus Nigra) Pomace in Biosorption Processes of Fe(III) Ions. Chemosphere 2020, 246, 125744. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, T.; Bildik, F.; Altay, F.; Ceylan, Z. Gelatin Nanofibers with Black Elderberry, Au Nanoparticles and SnO2 as Intelligent Packaging Layer Used for Monitoring Freshness of Hake Fish. Food Chem. 2024, 437, 137843. [Google Scholar] [CrossRef] [PubMed]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental Methods in Chemical Engineering: Thermogravimetric Analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of Ultrasound Assisted Emulsification in the Production of Pickering Emulsion Formulated with Chitosan Self-Assembled Particles: Stability, Macro, and Micro Rheological Properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Peyrano, F.; de Lamballerie, M.; Avanza, M.V.; Speroni, F. Gelation of Cowpea Proteins Induced by High Hydrostatic Pressure. Food Hydrocoll. 2021, 111, 106191. [Google Scholar] [CrossRef]
- Florowska, A.; Florowski, T.; Sokołowska, B.; Adamczak, L.; Szymańska, I. Effects of Pressure Level and Time Treatment of High Hydrostatic Pressure (HHP) on Inulin Gelation and Properties of Obtained Hydrogels. Foods 2021, 10, 2514. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Mu, R.J.; Gong, J.; Wang, Y.; Li, Y.; Ma, J.; Pang, J.; Wu, C. Effects of Konjac Glucomannan on the Structure, Properties, and Drug Release Characteristics of Agarose Hydrogels. Carbohydr. Polym. 2018, 190, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang, G.; Xiang, T.; Situ, W. Effect of Crosslinking Processing on the Chemical Structure and Biocompatibility of a Chitosan-Based Hydrogel. Food Chem. 2021, 354, 129476. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of Encapsulates and Their Relations with Encapsulation Efficiency and Controlled Release of Bioactive Constituents: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. 9—Technological Aspects and Stability of Polyphenols. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 295–323. ISBN 978-0-12-813572-3. [Google Scholar]
- Jordens, J.; Bamps, B.; Gielen, B.; Braeken, L.; Van Gerven, T. The Effects of Ultrasound on Micromixing. Ultrason. Sonochem. 2016, 32, 68–78. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, C.; Ma, Y.; Jia, M. Degradation Behavior of Polyphenols in Model Aqueous Extraction System Based on Mechanical and Sonochemical Effects Induced by Ultrasound. Sep. Purif. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, G.; Yu, Y.; Xu, Y.; Wu, J.; Zou, B.; Li, L. Low-Oxygen Pulping Combined with High Hydrostatic Pressure Improve the Stability of Blueberry Pulp Anthocyanins and Color during Storage. Food Control 2022, 138, 108991. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour Difference ∆E—A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of Nanoliposomal Carriers to Improve the Stability of Anthocyanins. LWT 2019, 109, 101–107. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.; Yue, X.Y.; Fu, R.; Liang, J.; Gao, X. Loading of Anthocyanins on Chitosan Nanoparticles Influences Anthocyanin Degradation in Gastrointestinal Fluids and Stability in a Beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; De Gaetano, F.; Ventura, C.A.; Fresta, M.; Paolino, D. The Rheolaser MasterTM and Kinexus Rotational Rheometer® to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel. Molecules 2020, 25, 1979. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Wiktor, A.; Kaveh, M.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Thermal and Non-Thermal Technologies on Kinetics and the Main Quality Parameters of Red Bell Pepper Dried with Convective and Microwave–Convective Methods. Molecules 2022, 27, 2164. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Athanassiou, A. Thermomechanical Plasticization of Fruits and Vegetables Processing Byproducts for the Preparation of Bioplastics. Adv. Sustain. Syst. 2023, 7, 2300179. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Yang, J.; Du, H.; Chai, N.; Sha, Z.; Geng, M.; Zhou, X.; He, C. Tannic Acid-Reinforced Methacrylated Chitosan/Methacrylated Silk Fibroin Hydrogels with Multifunctionality for Accelerating Wound Healing. Carbohydr. Polym. 2020, 247, 116689. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper. Molecules 2020, 25, 4287. [Google Scholar] [CrossRef]
- Świeca, M. Hydrogen Peroxide Treatment and the Phenylpropanoid Pathway Precursors Feeding Improve Phenolics and Antioxidant Capacity of Quinoa Sprouts via an Induction of L-Tyrosine and L-Phenylalanine Ammonia-Lyases Activities. J. Chem. 2016, 2016, 1936516. [Google Scholar] [CrossRef]


| Samples | Color Parameters | EE [%] | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h [°] | ΔE | ||
| C | 3.63 a ± 0.17 | 4.72 b ± 0.09 | −0.37 b ± 0.06 | 4.7 b ± 0.1 | 274 b ± 1 | - | 19 c ± 0 |
| U5 | 3.69 a ± 0.03 | 5.75 c ± 0.08 | −0.10 c ± 0.06 | 5.8 c ± 0.1 | 271 a ± 0 | 1.1 ± 0.1 | 3 a ± 0 |
| U10 | 3.66 a ± 0.03 | 5.89 c ± 0.04 | −0.07 c ± 0.03 | 5.9 c ± 0.0 | 271 a ± 0 | 1.2 ± 0.0 | 9 b ± 1 |
| P5 | 3.40 a ± 0.01 | 4.18 a ± 0.05 | −0.74 a ± 0.04 | 4.3 a ± 0.0 | 280 c ± 0 | 0.7 ± 0.0 | 20 d ± 0 |
| P10 | 3.44 a ± 0.14 | 4.19 a ± 0.09 | −0.81 a ± 0.05 | 4.3 a ± 0.1 | 281 c ± 1 | 0.7 ± 0.1 | 33 e ± 0 |
| Samples | Instability Index | Textural Parameters | Microrheological Parameters | |||
|---|---|---|---|---|---|---|
| Strength [N] | Adhesion [N] | Spreadability [N⋅s] | EI [nm−2] | SLB [-] | ||
| C | 0.35 a ± 0.03 | 0.17 b ± 0.00 | 0.08 d ± 0.00 | 3.27 ab ± 0.76 | 0.057 c ± 0.005 | 0.01 a ± 0.00 |
| U5 | 0.41 b ± 0.00 | 0.07 a ± 0.00 | 0.01 a ± 0.00 | 0.33 a ± 0.02 | 0.030 b ± 0.002 | 0.23 b ± 0.01 |
| U10 | 0.46 c ± 0.00 | 0.07 a ± 0.00 | 0.01 a ± 0.00 | 0.19 a ± 0.00 | 0.017 a ± 0.003 | 0.28 c ± 0.01 |
| P5 | 0.43 bc ± 0.01 | 0.19 c ± 0.00 | 0.04 c ± 0.00 | 4.71 b ± 0.91 | 0.016 a ± 0.003 | 0.33 d ± 0.01 |
| P10 | 0.45 c ± 0.01 | 0.20 d ± 0.00 | 0.03 b ± 0.00 | 8.79 c ± 0.22 | 0.026 ab ± 0.001 | 0.35 e ± 0.01 |
| Samples | TPC [mg Chlorogenic Acid/100 g d.m.] | ABTS [mg TE/g d.m.] | DPPH [mg TE/g d.m.] | RP [mg TE/g d.m.] |
|---|---|---|---|---|
| C | 18,403 b ± 221 | 4.8 b ± 0.1 | 6.8 b ± 0.0 | 0.87 b ± 0.03 |
| U5 | 16,987 b ± 1668 | 3.6 a ± 0.3 | 8.8 c ± 0.8 | 0.98 c ± 0.01 |
| U10 | 9317 a ± 1031 | 3.1 a ± 0.2 | 4.3 a ± 0.0 | 0.80 a ± 0.02 |
| P5 | 18,865 b ± 278 | 4.9 b ± 0.2 | 7.7 bc ± 0.6 | 0.89 b ± 0.01 |
| P10 | 22,741 c ± 1405 | 5.8 c ± 0.2 | 11.1 d ± 0.1 | 1.08 d ± 0.02 |
| Samples Code | Primary Induction | Secondary Induction | Secondary Induction Duration [min] |
|---|---|---|---|
| C | Heating at 80 °C for 30 min | - | - |
| U5 | Ultrasound treatment (25 kHz, 70 W, 100% pulse, 100% amplitude) | 5 | |
| U10 | 10 | ||
| P5 | High hydrostatic pressure (500 MPa) | 5 | |
| P10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilal, A.; Florowska, A.; Florowski, T.; Rybak, K.; Domian, E.; Szymański, M.; Wroniak, M. Effects of Sequential Induction Combining Thermal Treatment with Ultrasound or High Hydrostatic Pressure on the Physicochemical and Mechanical Properties of Pea Protein–Psyllium Hydrogels as Elderberry Extract Carriers. Int. J. Mol. Sci. 2024, 25, 9033. https://doi.org/10.3390/ijms25169033
Hilal A, Florowska A, Florowski T, Rybak K, Domian E, Szymański M, Wroniak M. Effects of Sequential Induction Combining Thermal Treatment with Ultrasound or High Hydrostatic Pressure on the Physicochemical and Mechanical Properties of Pea Protein–Psyllium Hydrogels as Elderberry Extract Carriers. International Journal of Molecular Sciences. 2024; 25(16):9033. https://doi.org/10.3390/ijms25169033
Chicago/Turabian StyleHilal, Adonis, Anna Florowska, Tomasz Florowski, Katarzyna Rybak, Ewa Domian, Marcin Szymański, and Małgorzata Wroniak. 2024. "Effects of Sequential Induction Combining Thermal Treatment with Ultrasound or High Hydrostatic Pressure on the Physicochemical and Mechanical Properties of Pea Protein–Psyllium Hydrogels as Elderberry Extract Carriers" International Journal of Molecular Sciences 25, no. 16: 9033. https://doi.org/10.3390/ijms25169033
APA StyleHilal, A., Florowska, A., Florowski, T., Rybak, K., Domian, E., Szymański, M., & Wroniak, M. (2024). Effects of Sequential Induction Combining Thermal Treatment with Ultrasound or High Hydrostatic Pressure on the Physicochemical and Mechanical Properties of Pea Protein–Psyllium Hydrogels as Elderberry Extract Carriers. International Journal of Molecular Sciences, 25(16), 9033. https://doi.org/10.3390/ijms25169033










