Brassicaceae Mustards: Phytochemical Constituents, Pharmacological Effects, and Mechanisms of Action against Human Disease
Abstract
:1. Introduction
2. Phytochemical Constituents of Mustards
2.1. Oil and Fatty Acids
2.2. Glucosinolates
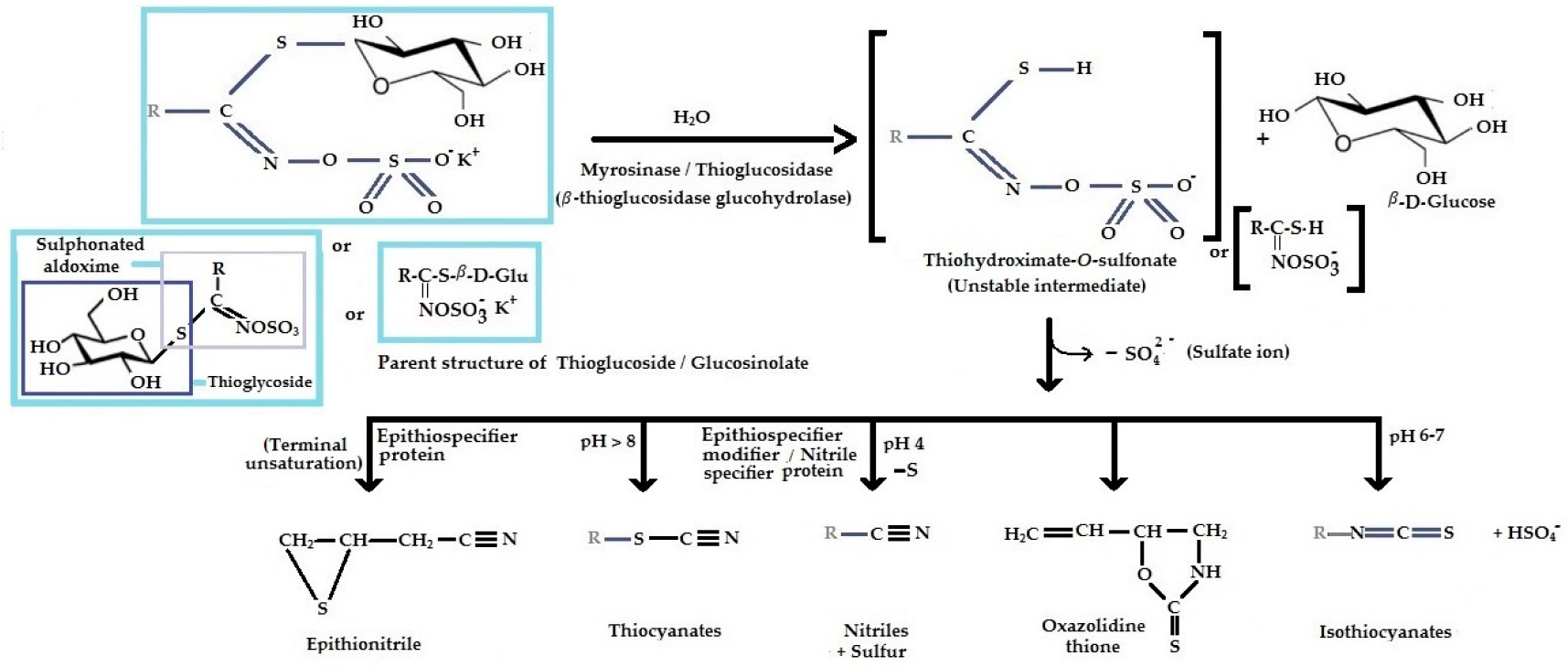 | |||
|---|---|---|---|
| Glucosinolate | In the Parent Structure of GSL Above, R = | Breakdown Product(s) | Structure of Breakdown Product(s) |
| Aliphatic GSL (Alkenyls derived from methionine) | |||
| Glucocapparin | CH3- | Methyl ITC. | CH3—N=C=S (Methyl ITC), |
| Sinigrin (Allyl-GSL or 2-propenyl-GSL or potassium myronate, modern name sinigroside, C10H16KNO9S2) | CH2=CH-CH2— | Allyl ITC (2-Propenyl-ITC), allyl cyanide, 1-cyano-2,3-epithiopropane, allyl thiocyanate and allyl nitrile. After further breakdown, allyl-ITC may produce highly toxic nitriles. | CH2=CH-CH2—N=C=S (Allyl ITC), KHSO4 (Potassium bisulphate), CH2=CH-CH2-C≡N (allyl cyanide), CH2=CH-CH2—S—C≡N (allyl thiocyanate), CH2=CH-CH2—C≡N (allyl nitrile),  1-cyano-2,3-epithio propane |
| Gluconapin (But-3-enyl GSL, C11H19NO9S2) | CH2=CH(CH2)2- (but-3-enyl or 3-butenyl) | Butyl ITC or 3-butenyl-ITC. | CH2=CH(CH2)2—N=C=S (Butyl ITC or 3-butenyl-ITC) |
| Glucobrassicanapin (pent-4-enyl GSL, C12H21NO9S2) | CH2=CH(CH2)3- (pent-4-enyl) | Pent-4-enyl ITC or 4-pentenyl ITC. | CH2=CH(CH2)3—N=C=S (Pent-4-enyl ITC or 4-pentenyl ITC) |
| Glucoiberverin, (3-methyl thio propyl GSL, C11H21NO9S3) | CH3-S-(CH2)3- [or CH3-S-CH2-CH2-CH2-] | Iberverin [(3-(methyl thio) propyl ITC, (C5H9NS2)] and iberverin nitrile [4-(methylthio) butane nitrile]. | CH3-S-CH2-CH2-CH2—N=C=S (Iberverin) |
| Glucoerucin, (4-methyl thio butyl GSL, C12H23NO9S3) | CH3-S-(CH2)4- [or CH3-S-CH2-CH2-CH2-CH2-] | Erucin [4-(methyl thio) butyl ITC or [isothio cyanato-4-(methyl thio)-butane] | CH3-S-CH2-CH2-CH2-CH2—N=C=S (Erucin) |
| Glucoberteroin (5-Methyl-thio-pentyl-GSL, C13H25NO9S3) | CH3-S-(CH2)5- | 5-Methyl-thio-pentyl-ITC. | CH3-S-(CH2)5—N=C=S (5-Methyl-thio-pentyl-ITC) |
| Glucoiberin (3-(methylsulfinyl) propyl GSL, C11H21NO10S3) | CH3-SO-(CH2)3— | Iberin (3-Methyl sulfinyl propyl ITC) or (1-isothiocyanato-3-(methylsulfinyl)-propane) which is the Structural analog of 4-(methyl sulfinyl) butyl isothiocyanate (sulforaphane). | CH3-SO-(CH2)3—N=C=S (Iberin) |
| Glucocheirolin (3-methyl-sulfonyl-propyl-GSL, C11H21NO11S3) | CH3-SO2-(CH2)3- | Cheirolin [3-(methyl sulfonyl) propyl ITC] or 1-ITC-3- (methyl sulphonyl) propane. | CH3-SO2-(CH2)3—N=C=S (Cheirolin) |
| Glucoraphanin (4-methyl sulfinyl butyl GSL, C12H23NO10S3) | CH3-SO-(CH2)4- | Sulforaphane [1-isothiocyanato-(4R)-(methyl sulfinyl) butane] or 4-methyl-sulfinyl-butyl-ITC. | CH3-SO-(CH2)4—N=C=S (Sulforaphane) |
| Glucoalyssin (5-methyl-sulfinyl-pentyl-GSL, C13H25NO10S3) | CH3-SO-(CH2)5- | 5-methyl-sulfinyl-pentyl ITC. | CH3-SO-(CH2)5—N=C=S (5-methyl-sulfinyl-pentyl ITC) |
| Progoitrin (2-hydroxy 3-butenyl-GSL, C11H19NO10S2) | CH2=CH-CH(OH)-CH2- | Goitrin [(2R)-2-hydroxy-3-butenyl-ITC or 5-ethenyl-1,3-oxazolidine-2-thione (C5H7NOS)], a cyclic thio-carbamate, unstable ITC and 3-hydroxy-4,5-epithio pentane nitrile. | CH2=CH-CH(OH)-CH2—N=C=S 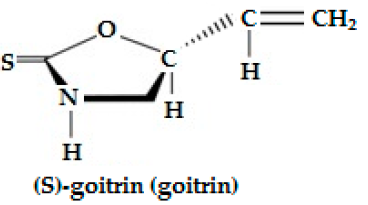 |
| Epiprogoitrin (2(S)-hydroxy-3-butenyl GSL, C11H19NO10S2) | CH2=CH-CHOH-CH2- | Epiprogoitrin does not break down to stable ITC, a further hydrolysis produces epigoitrin [(R)-5-vinyloxazolidine-2-thione] and finally goitrin. | CH2=CH-CH(OH)-CH2—N=C=S 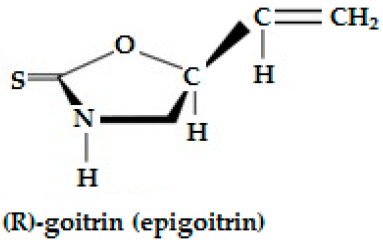 |
| Glucoalyssin (5-methyl-sulfinyl-pentyl-GSL, C13H25NO10S3) | CH3-SO-(CH2)5- | 5-methyl-sulfinyl-pentyl ITC. | CH3-SO-(CH2)5—N=C=S (5-methyl-sulfinyl-pentyl ITC) |
| Glucolepidiin (Ethyl GSL, C9H17NO9S2) | CH3-CH2- | Ethyl isothiocyanate. | CH3-CH2—N=C=S (Ethyl isothiocyanate). |
| 1-cyano-2-hydroxy-3-butene (or 2-hydoxybut-3-enyl cyanide, CH2=CH-CHOH-C≡N)- a Nitrile (R-C≡N) | - | 3-butenonitrile (2-methyl-3-butenenitrile), 4-methylthiobutanonitrile (4-methylsulfanylbutanenitrile oxide). | CH2=CH-CH2-C≡N (3-butenonitrile) CH3-S-CH2-CH2-C≡N=O (methylthiobutanonitrile) |
| Indolyl GSLs (Indolyl derivatives from tryptophan) | |||
| Glucobrassicin (3-indolyl methyl GSL, C16H20N2O9S2) | 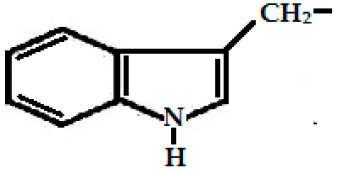 (3-indolyl methyl-) | Myrosinase breaks down glucobrassicin to 3-indoly-methyl-ITC (unstable) and simultaneously releases thiocynate anion (SCN-) to yield indole-3-carbinol (3-hydroxymethyl- indole). Indole-3-carbinole can combine with ascorbic acid to form Ascorbigen. | 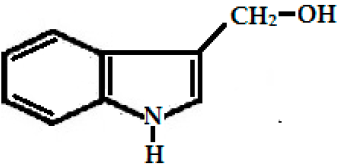 indole-3-carbinol |
| Napoleiferin (gluconapoleiferin) (2-hydroxy-4-pent enyl GSL, C6H9NOS); it is a natural homolog of goitrin. | R=CH2CH(OH)CH2CH=CH2- (2-hydroxy-4-pent enyl-) | Oxazolidine-2-thione. | 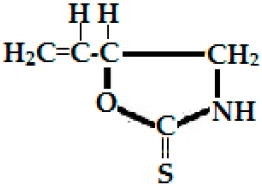 Oxazolidine-2-thione |
| Neoglucobrassicin (1-Methoxy-3-indolyl methyl GSL, C17H22N2O10S2) | Hydrolysis yields unsTable 1-methoxy-3-indolyl methyl ITC and thiocyanate ion (SCN−). | ||
| Aromatic GSL (Arylalkyls derivatives from phenylalanine) | |||
| Sinalbin (C30H42N2O15S2) (p-hydroxy benzyl GSL, sinapine glucosinalbate) or 4/p-hydroxybenzyl GSL or glucosinalbin), the choline ester of sinapic acid | p-OH-C6H4-CH2- | Allyl isothiocyanate, and on hydrolysis by myrosinase, produces unstable para hydroxy benzyl ITC. Para hydroxy benzyl ITC can further decomposes to para hydroxyl benzyl alcohol and thiocyanate ion (SCN−) and/or corresponding hydroxyl derivatives like di-(para hydroxyl benzyl disulphide). It also produces p-hydroxy benzyl amine known as the ‘white principles’. | CH2=CH-CH2—N=C=S (Allyl ITC), p-OH-C6H4-CH2—N=C=S (para hydroxy benzyl ITC), p-OH-C6H4-CH2—OH (para hydroxyl benzyl alcohol), HO—C6H4-CH2—NH2 [p-hydroxy benzyl amine (4-Hydroxybenzylamine)] |
| Gluconasturtiin (2-phenyl ethyl-GSL, C15H21NO9S2) | R=C6H5-CH2-CH2- | Phenethyl ITC (2-penyl-ethyl ITC). | C6H5-CH2-CH2-N=C=S (Phenethyl ITC) |
| Glucotropaeolin, Benzylglucosinolate (C14H19NO9S2) | R=C6H5-CH2- | Benzyl ITC, benzyl cyanide and benzyl thiocyanate. | C6H5-CH2-N=C=S (Benzyl ITC) |
| Glucosinolates (GSL) | Mustards | Biological Activity of GSLs and Their Metabolites (Referred from Table 4) | References |
|---|---|---|---|
| Glucosinolates (secondary metabolites) | |||
| Sinigrin, allyl glucosinolate (C10H16KNO9S2) | Brassica nigra (1% sinigrin), Sinapis alba, B. rapa var. campestris, B. carinata, B. juncea, Calepina irregularis, Alliaria petiolata, B. rapa, B. napus | Anti-cancer, anti-inflammatory, antibacterial, anti-fungal, antioxidant, and wound healing activity. Allyl-isothiocyanate is responsible for internal and external irritation and local vasodilation. It has been shown to prevent urinary bladder cancer and anti-proliferative activity against human prostate cancer cells. 1-cyano-2,3-epithiopropane has in vitro anti-cancer activity against human hepatocellular carcinoma. | [22,31,118,137,146,147,151,155,156,157,158,159,160,161,162,163,164] |
| Gluconapin, 3-Butenylglucosinolate, Butyl isothiocyanate (C11H19NO9S2) | B. rapa var. campestris, B. juncea, B. napus, Sinapis alba | Tumour inhibiting activity. | [22,147,160,165,166,167,168,169] |
| Glucocheirolin (C11H20NO11S3) | Calepina irregularis, Erysimum corinthium | Anti-proliferative activity on cancerous cells. Cheirolin improves cellular antioxidant defense and stress response mechanisms. | [31,34,146,155,170] |
| Glucoraphanin, 4-methylsulphinyl-butyl glucosinolate (C12H23NO10S3) | B. rapa var. campestris, B. nigra | Used in the treatment of neurodegenerative disorders as an antioxidant, gastric ulcers caused by Helicobacter pylori; against cancer like fibroblasts and malignant melanoma and employed to improve autism. Sulforaphane is a strong cancer chemopreventive agent both in vitro and in vivo and a potent monofunctional phase II enzyme inducer. | [22,24,30,38,155,171,172,173,174,175] |
| Progoitrin, (R)-2-hydroxybut-3-enylglucosinolate (C11H19NO10S2) | Brassica napus, B. rapa var. campestris, B. juncea | Thyroperoxidase enzyme inhibition and interference with the uptake and use of iodine by the thyroid gland. Anti-cancer effects. | [22,147,151,160,165,166,176] |
| Epiprogoitrin (C11H19NO10S2) | Brassica napus, B. olarecia | Nematicidal activity and insecticidal property as an effective fumigant. | [152,177,178] |
| Glucobrassicanapin, 4-pentenyl glucosinolate (C12H21NO99S2) | B. rapa var. campestris, B. napus, B. rapa | Anti-cancer activity | [22,147,165] |
| Napoleiferin (C6H9NOS) | Brassica rapa, B. oleracea, B. napus, B. rapa var. campestris, B. nigra, B. juncea, B. carinata. | Goiterogenic substance. | [167,179,180,181,182] |
| Nitriles R-C≡N | |||
| Erucin, 1-isothiocyanato-4-(methylthio)-butayl isothiocyanate (C6H11NS2) | Eruca sativa Mill. (rocket). | Erucin is a sulfone analog of sulforaphane. It is an enzymatic hydrolysis product of glucoerucin, responsible for the characteristic aroma of broccoli. Chemically, it is the reduced form of sulforaphane. It has antioxidant, neuroprotective, anti-fungal, and anti-inflammatory activities. It has strong cancer chemopreventive activity. It induces apoptosis in cancer cells, exerts antiproliferative effects, and causes cell cycle arrest in cancer cells. | [183,184,185,186] |
| Erucin nitrile (1-cyano-4-(methylthio)butane) may | Brassica oleracea var. italica (broccoli)-seeds, sprouts and root | Erucin nitrile could be formed in the anaerobic environment of the cecum or by hydrolysis of glucoerucin by reduction of sulforaphane nitrile. Erucin nitrile has potential activity as a phytoalexin, i.e., antimicrobial and antioxidant activities. | [183,186] |
| Sulforaphane nitrile, 5-(methylsulfinyl) pentanenitrile (C6H11NOS) | Brassica oleracea var. italica (broccoli) sprouts, Brassica oleracea var. botrytis (cauliflower) | Myrosinase cofactor epithiospecifier protein breaks down glucoraphanin to sulforaphane nitrile. Both sulforaphane and sulforaphane nitrile have chemoprotective, antioxidant, and anti-cancer effects, but sulforaphane nitrile is substantially less potent than sulforaphane. | [187,188] |
Epithionitrile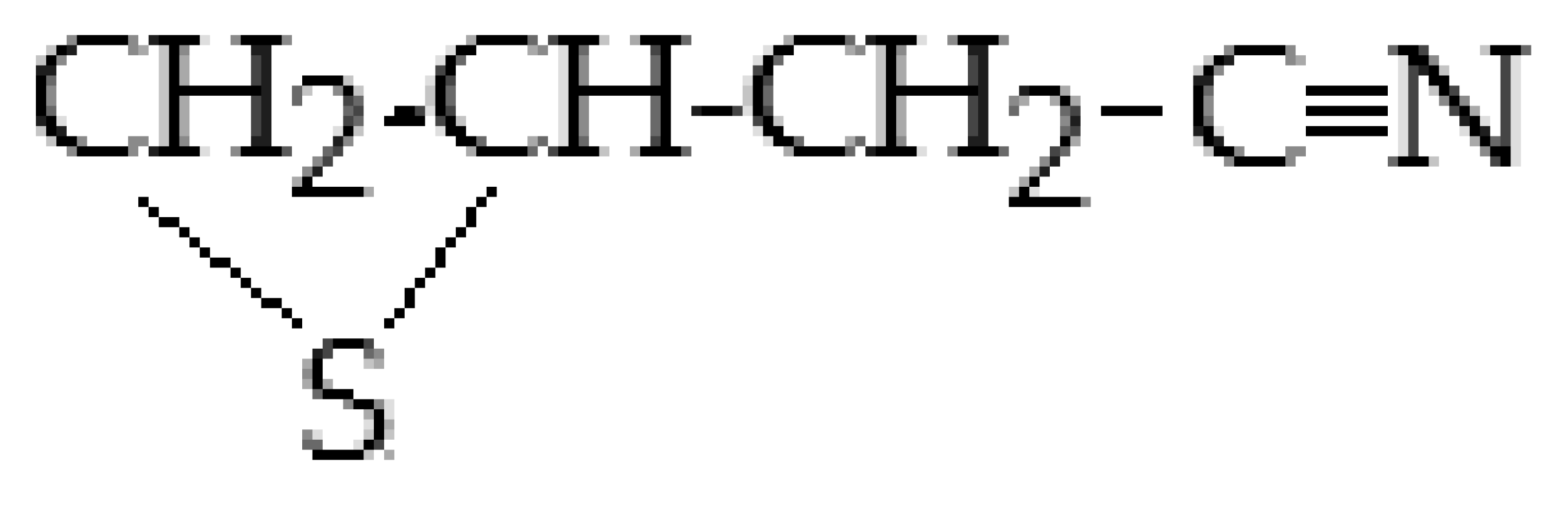 | |||
| Epithionitriles, major hydrolysis products | Brassica rapa, B. oleracea, B. napus, B. rapa var. campestris, B. nigra, B. juncea, B. carinata. | 3-butenonitrile has anti-cancer and antimicrobial activities. 4-methylthiobutanonitrile has a characteristic aroma. 1-cyano-2,3-epithiopropane, and 1-cyano-3,4-epithiobutane, 1-cyano-4,5-epithiopentane have cancer-preventative activities. | [189,190,191,192] |
| Glucoalyssin (C13H25NO10S3) | B. rapa var. campestris, B. napus, Degenia velebitica | The flavor component of cooked Brassicas. It is an antioxidant. | [163,193] |
| Gluconapoleiferin (C12H21NO10S2) | B. napus, B. rapa, B. rapa var. campestris, B. juncea, B. nigra | Tasteless, but hydrolytic, susceptibility to peptic digestion. A precursor to glucobrassicanapin biosynthesis. Thyroid gland function inhibitory effect. | [163,194] |
| Glucolepidiin (C9H17NO9S2) | Calepina irregularis | Antibacterial activity. | [31,146] |
| Thiocyanates R-S-C≡N | |||
| Glucobrassicin, the unsubstituted indole glucosinolate. (C16H20N2O9S2) | B. juncea, B. napus | Antioxidant, anti-inflammatory, and anti-cancer activities. It stimulates the bodily natural detoxifying enzymes. | [151,160,163,166,167,195,196,197] |
| Neoglucobrassicin (C17H22N2O10S2) | B. napus, S. officinale. | Antioxidant and anti-cancer activity. | [151,163,195] |
| Glucoiberin (C11H21NO10S3) | B. napus, B. incana, B. oleracea, Calepina irregularis, Erysimum corinthium, Iberis amara, Moringa oleifera | Anti-cancer effects | [22,31,34,146,147,151,165] |
| Glucoiberverin (C11H21NO9S3) | Calepina irregularis | Repellent and acts against insect herbivory | [31,146]. |
| Glucoerucin (C12H23NO9S3) | Calepina irregularis | Inhibition of cancerous cell proliferation. | [31,146,155]. |
| Sinalbin, P-Hydroxybenzyl Glucosinolate (C14H19NO10S2) | Sinapine is present in all the mustards in different amounts. Chiefly found in white mustard Sinapis alba (2.5% of seed weight) and also reported in B. rapa var. campestris, B. napus B. juncea and B. nigra | Antioxidant, anti-microbial, and anti-fungal activity. Sinapine and sinamic acid have antimicrobial, antioxidant, and anti-cancer properties. | [22,28,58,147,157,165,198,199,200] |
| Isothiocyanates R-N=C=S | |||
| Glucotropaeolin or benzyl glucosinolate, precursor of benzyl isothiocyanate (C14H19NO9S2) [C6H5-CH2-GSL] | Alliaria petiolate, B. juncea; B. nigra, Calepina irregularis, Lepidium sativum (garden criss), Sinapis alba. | Benzyl isothiocyanate or Glucotropaeolin has potential cancer-preventive activities, anti-tumorigenic (lung and esophagus in humans) activity, and works as an antimetastatic agent. It is also used as a food preservative because of its antimicrobial activity. | [31,146,155,160,166,169,201,202,203] |
| Gluconasturtiin or Phenethylglucosinolate, the precursor of phenethyl isothiocyanate (C15H21NO9S2) | B. napus, Nasturtium officinale (watercress) | Inhibits the development of tobacco-specific carcinogen-induced lung tumors. | [22,147,151,165,201,204] |
| Sulforaphane, 1-isothiocyanato-4-(methyl- sulfinyl) butane (C6H11NOS2). Sulforaphane is derived from glucoraphanin. | B. oleracea var. italica (broccoli) sprouts, kale, B. oleracea var. botrytis (cauliflower) or B. oleracea var. gongylodes (kohlrabi) | Anticarcinogenic activity by induction of cell cycle arrest and apoptosis in various human cancer cells; antioxidant, antiproliferative, anti-inflammatory, antimicrobial, anti-fungal, and antiviral activities. | [79,186,205,206,207] |
| Indole-3-carbinol, derived from glucobrassicin. (C9H9NO) | B. oleracea var. italica (broccoli), B. oleracea var. acephala (kale), Brassica oleracea var. capitata (cabbage) | Anti-cancer, antioxidant, and antiatherogenic activities. | |
2.3. Phenolics and Flavonoids
2.4. Proteins
2.5. Minor Chemical Compounds
3. Use of Mustard to Treat Ailments
3.1. Antimicrobial and Antiviral Activities
3.2. Antidiabetic Action
3.3. Hypolipidemic Effects
3.4. Cytotoxic and Anti-Cancer Activity
3.5. Broncho- and Vaso-Dilating Properties
3.6. Action on Cardiovascular System
3.7. Counter Irritation Action
3.8. Antiinflammatory and Analgesic Action
3.9. Antiarthritic Action
3.10. Diuretic Action
3.11. Anthelmintic Activity
3.12. Emetic Action
3.13. Laxative Action
3.14. Development of Body Muscle and Aphrodisiac Action
3.15. Treatment of Baldness
3.16. Effects on Blood Flow and Bodily Secretions
3.17. Reduction in Fat and Body Weight
3.18. Antidepressant Effects
3.19. Use in Adrenoleukodystrophy and Adrenomyeloneuropathy
4. Adverse Effects and Other Antinutritional Effects of Mustard
4.1. Cardiac Effects
4.2. Allergic Effects
4.3. Goitrogenic Effects
4.4. Hematological Effects
4.5. Neurological Effects
5. Possible Application in Pharmaceutical Manufacturing
6. Summary and Outlook
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef]
- Nesi, N.; Delourme, R.; Brégeon, M.; Falentin, C.; Renard, M. Genetic and molecular approaches to improve nutritional value of Brassica napus L. seed. Comptes Rendus Biol. 2008, 331, 763–771. [Google Scholar] [CrossRef]
- Chew, S.C. Cold pressed rapeseed (Brassica napus) oil. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–80. [Google Scholar]
- González-Pérez, S.; Arellano, J. Vegetable protein isolates. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Sawston, UK, 2009; pp. 1–27. [Google Scholar]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Byres, M.; Cox, P.; Delazar, A.; Jaspars, M.; Nahar, L.; Shoeb, M.; Sarker, S. Isolation, structure elucidation, and biological activity of flavone 6-C-glycosides from Alliaria petiolata. Chem. Nat. Compd. 2004, 40, 122–128. [Google Scholar] [CrossRef]
- Wiersema, J.H.; Leon, B. World Economic Plants: A Standard Reference; CRC Press: Melbourne, VIC, Australia, 2016. [Google Scholar]
- Agnihotri, A.; Prem, D.; Gupta, K. Biotechnology in quality improvement of oilseed Brassicas. In Plant Biotechnology and Molecular Markers; Springer: Berlin/Heidelberg, Germany, 2004; pp. 144–155. [Google Scholar]
- Bai, S.; Malik, A.; Seasotiya, L.; Bharti, P.; Dalal, S. In vitro antioxidant activity, total phenolic content and therapeutic potential of Brassica campestris L. (Brassicaceae) seed in inhibiting human pathogens. Int. J. Recent Adv. Pharm. Res. 2014, 4, 15–24. [Google Scholar]
- Kabir, S.M.R.; Rahman, M.; Khatun, A.; Saha, S.; Roy, A.; Rashid, A.H.M.A.; Hossain, S.J.; Mahmud, A. Total flavonoids content and reducing power assay of twelve common Bangladeshi leafy vegetables. PharmacologyOnline 2016, 2016, 6–14. [Google Scholar]
- Labana, K.S.; Banga, S.S.; Banga, S.K. Breeding Oilseed Brassicas; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lozano-Baena, M.-D.; Tasset, I.; Obregón-Cano, S.; de Haro-Bailon, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Antigenotoxicity and tumor growing inhibition by leafy Brassica carinata and sinigrin. Molecules 2015, 20, 15748. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Eskin, N.; Biliaderis, C. Chemical and physical properties of yellow mustard (Sinapis alba L.) mucilage. Food Chem. 1993, 46, 169–176. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Q.; Wen, C.; Hu, J.; Li, H.; Zhao, M.; Zhao, H. Mustard seed (Sinapis alba Linn) attenuates imiquimod-induced psoriasiform inflammation of BALB/c mice. J. Dermatol. 2013, 40, 543–552. [Google Scholar] [CrossRef]
- Hedrick, U. Sturtevant’s Edible Plants of the World; Dover: New York, NY, USA, 1972; 686p. [Google Scholar]
- Hill, F. How to Grow Microgreens: Nature’s Own Superfood; David Bateman: Auckland, New Zealand, 2011. [Google Scholar]
- Houbein, L. One Magic Square: Grow Your Own Food on One Square Metre; Wakefield Press: Adelaide, SA, Australia, 2008. [Google Scholar]
- Jham, G.N.; Moser, B.R.; Shah, S.N.; Holser, R.A.; Dhingra, O.D.; Vaughn, S.F.; Berhow, M.A.; Winkler-Moser, J.K.; Isbell, T.A.; Holloway, R.K. Wild Brazilian mustard (Brassica juncea L.) seed oil methyl esters as biodiesel fuel. J. Am. Oil Chem. Soc. 2009, 86, 917–926. [Google Scholar] [CrossRef]
- Salama, H.M.; Al Watban, A.A.; Al-Fughom, A.T. Effect of ultraviolet radiation on chlorophyll, carotenoid, protein and proline contents of some annual desert plants. Saudi J. Biol. Sci. 2011, 18, 79–86. [Google Scholar] [CrossRef]
- Li, L.F.; Olsen, K.M. Chapter Three—To Have and to Hold: Selection for Seed and Fruit Retention During Crop Domestication. In Current Topics in Developmental Biology; Orgogozo, V., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 119, pp. 63–109. [Google Scholar]
- Saeidnia, S.; Gohari, A.R. Importance of Brassica napus as a medicinal food plant. J. Med. Plants Res. 2012, 6, 2700–2703. [Google Scholar] [CrossRef]
- Sinha, S.; Jha, J.K.; Maiti, M.K.; Basu, A.; Mukhopadhyay, U.K.; Sen, S.K. Metabolic engineering of fatty acid biosynthesis in Indian mustard (Brassica juncea) improves nutritional quality of seed oil. Plant Biotechnol. Rep. 2007, 1, 185–197. [Google Scholar] [CrossRef]
- Thompson, L.U.; Liu, R.F.K.; Jones, J.D. Functional Properties and Food Applications of Rapeseed Protein Concentrate. J. Food Sci. 1982, 47, 1175–1180. [Google Scholar] [CrossRef]
- Greve, M.; Leyel, C.F. A Modern Herbal; Merchant Books: Dublin, Ireland, 1973. [Google Scholar]
- Kim, J.W.; Minamikawa, T. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra). Biosci. Biotechnol. Biochem. 1997, 61, 118–123. [Google Scholar]
- Oduor, A.M.; Lankau, R.A.; Strauss, S.Y.; Gómez, J.M. Introduced Brassica nigra populations exhibit greater growth and herbivore resistance but less tolerance than native populations in the native range. New Phytol. 2011, 191, 536–544. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2013; Volume 5, Fruits. [Google Scholar]
- Mailer, R.J.; McFadden, A.; Ayton, J.; Redden, B. Anti-nutritional components, fibre, sinapine and glucosinolate content, in Australian canola (Brassica napus L.) meal. J. Am. Oil Chem. Soc. 2008, 85, 937–944. [Google Scholar] [CrossRef]
- Rękas, A.; Wroniak, M.; Krygier, K. Effects of different roasting conditions on the nutritional value and oxidative stability of high-oleic and yellow-seeded Brassica napus oils. Grasas Aceites 2015, 66, e092. [Google Scholar] [CrossRef]
- Zareba, G.; Serradelf, N. Chemoprotective effects of broccoli and other Brassica vegetables. Drugs Future 2004, 29, 1097–1104. [Google Scholar] [CrossRef]
- Zekic, M.; Radonic, A.; Marijanovic, Z. Glucosinolate profiling of Calepina irregularis. Nat. Prod. Commun. 2016, 11, 1329–1332. [Google Scholar]
- Cojocariu, R.O.; Balmus, I.-M.; Lefter, R.; Hritcu, L.; Ababei, D.C.; Ciobica, A.; Copaci, S.; Mot, S.E.; Copolovici, L.; Copolovici, D.M. Camelina sativa Methanolic and Ethanolic Extract Potential in Alleviating Oxidative Stress, Memory Deficits, and Affective Impairments in Stress Exposure-Based Irritable Bowel Syndrome Mouse Models. Oxid. Med. Cell. Longev. 2020, 2020, 9510305. [Google Scholar] [CrossRef] [PubMed]
- Ghidoli, M.; Geuna, F.; De Benedetti, S.; Frazzini, S.; Landoni, M.; Cassani, E.; Scarafoni, A.; Rossi, L.; Pilu, S.R. Genetic study of Camelina sativa oilseed crop and selection of a new variety by the bulk method. Front. Plant Sci. 2024, 15, 1385332. [Google Scholar] [CrossRef]
- Huang, X.; Renwick, J.; Sachdev-Gupta, K. A chemical basis for differential acceptance of Erysimum cheiranthoides by two Pieris species. J. Chem. Ecol. 1993, 19, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Azimova, S.S.; Glushenkova, A.I.; Vinogradova, V.I. Lipids, Lipophilic Components and Essential Oils from Plant Sources; Springer: London, UK, 2011. [Google Scholar]
- Walsh, N.G.; Entwisle, T.J. Flora of Victoria: Dicotyledons: Winteraceae to Myrtaceae; Inkata Press: Melbourne, VIC, Australia, 1996; Volume 3. [Google Scholar]
- Angelo, R.; Boufford, D.E. Atlas of the flora of New England: Salicaceae to Brassicaceae. Phytoneuron 2011, 12, 1–16. [Google Scholar]
- Blažević, I.; Radonić, A.; Mastelić, J.; Zekić, M.; Skočibušić, M.; Maravić, A. Hedge mustard (Sisymbrium officinale): Chemical diversity of volatiles and their antimicrobial activity. Chem. Biodivers. 2010, 7, 2023–2034. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Vitalone, A.; Nicoletti, M.; Piccin, A.; Mazzanti, G. Pharmacological and phytochemical study on a Sisymbrium officinale Scop. extract. J. Ethnopharmacol. 2010, 127, 731–736. [Google Scholar] [CrossRef]
- Grieve, M. A Modern Herbal; Penguin Books: Melbourne, Australia, 1984. [Google Scholar]
- Lust, J. The Herb Book; Penguin Books: Melbourne, Austraslia, 1983. [Google Scholar]
- Buckley, R. Alien plants in central Australia. Bot. J. Linn. Soc. 1981, 82, 369–379. [Google Scholar] [CrossRef]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. PDR for Herbal Medicines; Medical Economics Company, Inc.: Montvale, NJ, USA, 2000. [Google Scholar]
- Awaad, A.S.; Al-Jaber, N.A. Antioxidant natural plant. RPMP Ethnomed. Source Mech. 2010, 27, 1–35. [Google Scholar]
- Azimova, S.S.; Glushenkova, A.I. Sisymbrium erysimoides (Desf.). In Lipids, Lipophilic Components and Essential Oils from Plant Sources; Azimova, S.S., Glushenkova, A.I., Eds.; Springer: London, UK, 2012; p. 274. [Google Scholar]
- Iranshahi, M. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Warwick, S.I. Brassicaceae in agriculture. In Genetics and Genomics of the Brassicaceae; Springer: Berlin/Heidelberg, Germany, 2011; pp. 33–65. [Google Scholar]
- Hemphill, J.; Hemphill, R. Book of Herbs and Spices; Omega: London, UK, 1984. [Google Scholar]
- Murdoch, R. Maori Healing and Herbal: New Zealand Ethnobotanical Sourcebook; Viking Sevenseas: Paraparaumu, New Zealand, 1994. [Google Scholar]
- Stewart, A. A review of Brassica species, cross-pollination and implications for pure seed production in New Zealand. Agron. N. Z. 2002, 32, 63–82. [Google Scholar]
- Webb, C.J.; Sykes, W.R.; Garnock-Jones, P.J. Flora of New Zealand. Vol. IV. Naturalised Pteridophytes, Gymnosperms, Dicotyledons; Botany Division DSIR: Christchurch, New Zealand, 1988.
- Wilson, H. ‘Alpine Plants—Highest of All’, Te Ara—The Encyclopedia of New Zealand. Available online: http://www.TeAra.govt.nz/en/alpine-plants/page-4 (accessed on 19 November 2019).
- Williams, P.M. Te Rongoa Maori: Maori Medicine; Reed Books: Birkenhead, UK, 1996; 79p, ISBN 790005107. [Google Scholar]
- Food Standards Australia New Zealand. Commonwealth of Australia, 2009. ISSN 1446-9685. Available online: https://www.foodstandards.gov.au/sites/default/files/food-standards-code/changes/gazette/Documents/Gazette%20Notice%20Amendment%20No%20112%20WEB%20VERSION.pdf (accessed on 2 August 2017).
- Rahman, S.; Parvez, A.K.; Islam, R.; Khan, M.H. Antibacterial activity of natural spices on multiple drug resistant Escherichia coli isolated from drinking water, Bangladesh. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Raymer, P.L. Canola: An emerging oilseed crop. Trends New Crops New Uses 2002, 1, 122–126. [Google Scholar]
- Rahman, M. Identification, Molecular and Proteomic Characterisation of Brassica rapa Seed Storage Proteins with Allergenic and Antimicrobial Potential. Ph.D. Thesis, Southern Cross University, Lismore, NSW, Australia, 2020. [Google Scholar]
- Khattab, R.; Rempel, C.; Suh, M.; Thiyam, U. Quality of Canola oil obtained by conventional and supercritical fluid extraction. Am. J. Anal. Chem. 2012, 3, 966–976. [Google Scholar] [CrossRef]
- Shahidi, F. Canola and Rapeseed: Production, Chemistry, Nutrition, and Processing Technology; Springer Science & Business Media: New York, NY, USA, 1990. [Google Scholar]
- Fiocchi, A.; Dahdah, L.; Riccardi, C.; Mazzina, O.; Fierro, V. Preacutionary labelling of cross-reactive foods: The case of rapeseed. Asthma Res. Pract. 2016, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Gabon, J.E. Individual glucosinolates in six Canola varieties. J. Food Qual. 1989, 11, 421–431. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D. Proteins of Brassicaceae oilseeds and their potential as a plant protein source. Crit. Rev. Food Sci. Nutr. 2011, 51, 635–677. [Google Scholar] [CrossRef]
- Velisek, J. The Chemistry of Food; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- He, Z.; Ji, R.; Havlickova, L.; Wang, L.; Li, Y.; Lee, H.T.; Song, J.; Koh, C.; Yang, J.; Zhang, M. Genome structural evolution in Brassica crops. Nat. Plants 2021, 7, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Qian, L.; Zheng, M.; Chen, L.; Chen, H.; Yang, L.; You, L.; Yang, B.; Yan, M.; Gu, Y. Genomic insights into the origin, domestication and diversification of Brassica juncea. Nat. Genet. 2021, 53, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, M.; Ritonga, F.N.; Wang, F.; Zhou, D.; Li, C.; Li, H.; Zhang, Y.; Gao, J. Metabolite Profiling and Comparative Metabolomics Analysis of Jiaozhou Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Planted in Different Areas. Front. Biosci. Landmark 2023, 28, 345. [Google Scholar]
- Liu, Y.; Rossi, M.; Liang, X.; Zhang, H.; Zou, L.; Ong, C.N. An integrated metabolomics study of glucosinolate metabolism in different Brassicaceae genera. Metabolites 2020, 10, 313. [Google Scholar] [CrossRef]
- Shu, J.; Ma, X.; Ma, H.; Huang, Q.; Zhang, Y.; Guan, M.; Guan, C. Transcriptomic, proteomic, metabolomic, and functional genomic approaches of Brassica napus L. during salt stress. PLoS ONE 2022, 17, e0262587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, C.; Chao, H.; Long, Y.; Wu, J.; Li, Z.; Ge, X.; Xia, H.; Yin, Y.; Batley, J. Integration of metabolome and transcriptome reveals flavonoid accumulation in the intergeneric hybrid between Brassica rapa and Raphanus sativus. Sci. Rep. 2019, 9, 18368. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Cheng, F.; Liu, B.; Hu, Z.; Chen, S.; Pental, D.; Ju, Y.; et al. The genome sequence of allopolyploid Brassica juncea and analysis of differential homoeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225. [Google Scholar] [CrossRef]
- Rahman, M.; Guo, Q.; Khartun, A.; Baten, A.; Mauleon, R.; Liu, L.; Barkla, B.J. Shotgun proteomics of Brassica rapa seed proteins identifies vicilin as a major seed storage protein in the mature seed. PLoS ONE 2021, 16, e0253384. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Baten, A.; Mauleon, R.; Borapatragohain, P.; Rahman, M.; Barkla, B. Brassica Rapa Subsp. Trilocularis R-o-18 (NCBI:txid1813537) Genome Sequencing and Assembly; National Center for Biotechnology Information (NCBI): Bethesda, MD, USA, 2021.
- Rahman, M.; Baten, A.; Mauleon, R.; King, G.J.; Liu, L.; Barkla, B.J. Identification, characterization and epitope mapping of proteins encoded by putative allergenic napin genes from Brassica rapa. Clin. Exp. Allergy 2020, 50, 848–868. [Google Scholar] [CrossRef]
- Von Der Haar, D.; Müller, K.; Bader-Mittermaier, S.; Eisner, P. Rapeseed proteins–Production methods and possible application ranges. OCL 2014, 21, D104. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Idrees, N.; Tabassum, B.; Sarah, R.; Hussain, M.K. Natural compound from genus brassica and their therapeutic activities. In Natural Bio-Active Compounds Volume 1: Production and Applications; Akhtar, M., Swamy, M., Sinniah, U., Eds.; Springer: Singapore, 2019; pp. 477–491. [Google Scholar]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Mann, S.K.; Khanna, N. Health promoting effects of phytochemicals from Brassicaceae: A review. Indian J. Pharm 2013, 1, 3. [Google Scholar] [CrossRef]
- Shankar, S.; Segaran, G.; Sundar, R.D.V.; Settu, S.; Sathiavelu, M. Brassicaceae—A classical review on its pharmacological activities. Int. J. Pharm. Sci. Rev. Res. 2019, 55, 107–113. [Google Scholar]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Bhat, M.; Gupta, M.; Banga, S.; Raheja, R.; Banga, S.; Rakow, G. Erucic acid heredity in Brassica juncea-some additional information. Plant Breed. 2002, 121, 456–458. [Google Scholar] [CrossRef]
- Burns, M.; Barnes, S.; Bowman, J.; Clarke, M.; Werner, C.; Kearsey, M. QTL analysis of an intervarietal set of substitution lines in Brassica napus: (i) Seed oil content and fatty acid composition. Heredity 2003, 90, 39–48. [Google Scholar] [CrossRef]
- George, L.; Abraham, V.; Suryavanshi, D.; Sipahimalani, A.; Srinivasan, V. Yield, oil content and fatty acid composition evaluated in androgenetic plants in Brassica juncea. Plant Breed. 1987, 98, 72–74. [Google Scholar] [CrossRef]
- Mutanen, M.; Freese, R.; Valsta, L.; Ahola, I.; Ahlström, A. Rapeseed oil and sunflower oil diets enhance platelet in vitro aggregation and thromboxane production in healthy men when compared with milk fat or habitual diets. Thromb. Haemost. 1992, 67, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil content and fatty acids composition in Brassica species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Valsta, L.M.; Jauhiainen, M.; Aro, A.; Katan, M.B.; Mutanen, M. Effects of a monounsaturated rapeseed oil and a polyunsaturated sunflower oil diet on lipoprotein levels in humans. Arterioscler. Thromb. Vasc. Biol. 1992, 12, 50–57. [Google Scholar] [CrossRef]
- Walsh, T.A.; Bevan, S.A.; Gachotte, D.J.; Larsen, C.M.; Moskal, W.A.; Merlo, P.A.O.; Sidorenko, L.V.; Hampton, R.E.; Stoltz, V.; Pareddy, D.; et al. Canola engineered with a microalgal polyketide synthase-like system produces oil enriched in docosahexaenoic acid. Nat. Biotechnol. 2016, 34, 881–887. [Google Scholar] [CrossRef]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R.; Patel, V.B. Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Linnemann, A.R.; Dijkstra, D.S. Toward sustainable production of protein-rich foods: Appraisal of eight crops for Western Europe. Part I. Analysis of the primary links of the production chain. Crit. Rev. Food Sci. Nutr. 2002, 42, 377–401. [Google Scholar] [CrossRef]
- Maršalkienė, N.; Sliesaravičius, A.; Karpavičienė, B.; Dastikaitė, A. Oil content and fatty acid composition of seeds of some Lithuanian wild crucifer species. Agron. Res. 2009, 7, 654–661. [Google Scholar]
- Singh, B.K.; Bala, M.; Rai, P.K. Fatty acid composition and seed meal characteristics of Brassica and allied genera. Natl. Acad. Sci. Lett. 2014, 37, 219–226. [Google Scholar] [CrossRef]
- Bretagnolle, F.; Matejicek, A.; Grégoire, S.; Reboud, X.; Gaba, S. Determination of fatty acids content, global antioxidant activity and energy value of weed seeds from agricultural fields in France. Weed Res. 2016, 56, 78–95. [Google Scholar] [CrossRef]
- Chen, B.; Heneen, W. Fatty acid composition of resynthesized Brassica napus L., B. campestris L. and B. alboglabra Bailey with special reference to the inheritance of erucic acid content. Heredity 1989, 63, 309–314. [Google Scholar] [CrossRef]
- Ostrikov, A.; Kleimenova, N.; Kopylov, M.; Bolgova, I. The study of the fatty acid composition of camelina oil obtained by cold pressing. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 042009. [Google Scholar] [CrossRef]
- Moser, B.R. Camelina (Camelina sativa L.) oil as a biofuels feedstock: Golden opportunity or false hope? Lipid Technol. 2010, 22, 270–273. [Google Scholar] [CrossRef]
- Riaz, R.; Ahmed, I.; Sizmaz, O.; Ahsan, U. Use of Camelina sativa and by-products in diets for dairy cows: A review. Animals 2022, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Goffman, F.D.; Thies, W.; Velasco, L. Chemotaxonomic value of tocopherols in Brassicaceae. Phytochemistry 1999, 50, 793–798. [Google Scholar] [CrossRef]
- Abbadi, A.; Leckband, G. Rapeseed breeding for oil content, quality, and sustainability. Eur. J. Lipid Sci. Technol. 2011, 113, 1198–1206. [Google Scholar] [CrossRef]
- Food Standard Australia New Zealand. Technical Report 21—Erucic Acid in Food: A Toxicological Review and Risk Assessment; Food Standard Australia New Zealand: Canberra, ACT, Australia, 2003.
- Ghazani, S.; Marangoni, A. Nutrition and Food Grains in Encyclopedia of Food Grains; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Nosenko, T.; Kot, T.; Kichshenko, V. Rape Seeds as a Source of Feed and Food Proteins. Pol. J. Food Nutr. Sci. 2014, 64, 109. [Google Scholar] [CrossRef]
- Murphy, D.J. The biotechnological utilisation of oilseeds. Acta Bot. Gall. 1993, 140, 767–777. [Google Scholar] [CrossRef]
- Wallace, H.; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B. Erucic acid in feed and food. EFSA J. 2016, 14, e04593. [Google Scholar]
- Kramer, J.K.; Sauer, F.D.; Wolynetz, M.S.; Farnworth, E.R.; Johnston, K.M. Effects of dietary saturated fat on erucic acid induced myocardial lipidosis in rats. Lipids 1992, 27, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.; Corner, A.; Davey, K.; Kramer, J.; Mahadevan, S.; Sauer, F. Cardiac lesions in rats fed rapeseed oils. Can. J. Comp. Med. 1975, 39, 261. [Google Scholar]
- Przybylski, R.; Mag, T.; Eskin, N.; McDonald, B. Canola oil. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Milton, QLD, Australia, 2005; Volume 2, pp. 1–95. [Google Scholar]
- Slominski, B.A.; Kienzle, H.D.; Jiang, P.; Campbell, L.D.; Pickard, M.; Rakow, G. Chemical composition and nutritive value of canola-quality Sinapis alba mustared. In Proceedings of the 10th International Rapeseed Congress, Canberra, ACT, Australia, 26–29 September 1999; pp. 416–421. [Google Scholar]
- Goering, K.; Eslick, R.; Brelsford, D. A search for high erucic acid containing oils in the Cruciferae. Econ. Bot. 1965, 19, 251–256. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Böttcher, C.; Pollmann, S. Plant oxylipins: Plant responses to 12-oxo-phytodienoic acid are governed by its specific structural and functional properties. FEBS J. 2009, 276, 4693–4704. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; To, Q.H. Defense and signalling metabolites of the crucifer Erucastrum canariense: Synchronized abiotic induction of phytoalexins and galacto-oxylipins. Phytochemistry 2017, 139, 18–24. [Google Scholar] [CrossRef]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Meskin, M.S.; Bidlack, W.R.; Davies, A.J.; Omaye, S.T. Phytochemicals in Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Nollet, L.M.L.; Toldra, F. Handbook of Food Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Chahoud, G.; Aude, Y.W.; Mehta, J.L. Dietary recommendations in the prevention and treatment of coronary heart disease: Do we have the ideal diet yet? Am. J. Cardiol. 2004, 94, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Batra, N. 1000 Indian Recipes; Houghton Mifflin Harcourt: Boston, MA, USA, 2011. [Google Scholar]
- Marwede, V.; Schierholt, A.; Möllers, C.; Becker, H.C. Genotype× environment interactions and heritability of tocopherol contents in canola. Crop Sci. 2004, 44, 728–731. [Google Scholar] [CrossRef]
- Carey, F.A.; Giuliano, R.M. Organic Chemistry; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2011. [Google Scholar]
- Steinbrecher, A.; Linseisen, J. Dietary intake of individual glucosinolates in participants of the EPIC-Heidelberg cohort study. Ann. Nutr. Metab. 2009, 54, 87–96. [Google Scholar] [CrossRef] [PubMed]
- CABI Invasive Species Compendium. Available online: www.cabi.org/isc (accessed on 7 November 2019).
- Duke, J.A. Handbook of Energy Crops; Center for New Crops & Plants Products: Sydney, NSW, Australia, 1983. [Google Scholar]
- Guil-Guerrero, J.L.; GIimenea-Martinez, J.J.; Torija-Isasa, M.E. Nutritional composition of wild edible crucifer species. J. Food Biochem. 1999, 23, 283–294. [Google Scholar] [CrossRef]
- Duma, M.; Alsina, I.; Zeipina, S.; Lepse, L.; Dubova, L. Leaf vegetables as source of phytochemicals. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being”, Latvia University of Life Sciences and Technologies, Jelgava, Latvia, 8–9 May 2014; FOODBALT 2014 Conference Proceedings: Jelgava, Latvia, 2014; pp. 262–265. [Google Scholar]
- Mansour, E.H.; Dworschák, E.; Lugasi, A.; Gaál, Ö.; Barna, É.; Gergely, A. Effect of processing on the antinutritive factors and nutritive value of rapeseed products. Food Chem. 1993, 47, 247–252. [Google Scholar] [CrossRef]
- Chopra, R.; Nayar, S.; Chopra, I. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research: New Delhi, India, 1986; pp. 2–79. [Google Scholar]
- Teh, S.-S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Gibney, M.J.; Lanham-New, S.A.; Cassidy, A.; Vorster, H.H. Introduction to Human Nutrition; Wiley-Blackwell: Chichester, UK, 2002. [Google Scholar]
- Claussen, F.A.; Taylor, M.L.; Breeze, M.L.; Liu, K. Measurement of vitamin k1 in commercial canola cultivars from growing locations in north and south America using high-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2015, 63, 1076–1081. [Google Scholar] [CrossRef]
- Bukhari, S.A.B.H. Mustard as Medicinal Plant: Sources, Botanical Features, Genetics and Applications. Sch. Bull. 2021, 7, 123–129. [Google Scholar]
- Bhandari, S.; Jo, J.; Lee, J. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules 2015, 20, 15827. [Google Scholar] [CrossRef]
- Nyerges, C. Foraging Wild Edible Plants of North America: More than 150 Delicious Recipes Using Nature’s Edibles; FalconGuides: Helena, MT, USA, 2016. [Google Scholar]
- Pengelly, A. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine; University of Newcastle: Newcastle, NSW, Australia, 2004; Volume 108, pp. 28–159. [Google Scholar]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Wittstock, U.; Halkier, B.A. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 2002, 7, 263–270. [Google Scholar] [CrossRef]
- Mazumder, A.; Dwivedi, A.; du Plessis, J. Sinigrin and its therapeutic benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Nicolas, M.E.; Bennett, R.N.; Premier, R.R.; Eagling, D.R.; Taylor, P.W.J. Determination of sinigrin and glucoraphanin in Brassica species using a simple extraction method combined with ion-pair HPLC analysis. Sci. Hortic. 2002, 96, 27–41. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Sun, B.; Liu, N.; Zhao, Y.; Yan, H.; Wang, Q. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Hossain, M.R.; Park, J.-I.; Kim, H.R.; Nou, I.-S. Glucosinolate profiles in cabbage genotypes influence the preferential feeding of diamondback moth (Plutella xylostella). Front. Plant Sci. 2017, 8, 1244. [Google Scholar] [CrossRef]
- Sugiyama, R.; Hirai, M.Y. Atypical Myrosinase as a Mediator of Glucosinolate Functions in Plants. Front. Plant Sci. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Pérez, F.R.; Reichelt, M.; Gershenzon, J.; Heckel, D.G. Phylloplane location of glucosinolates in Barbarea spp. (Brassicaceae) and misleading assessment of host suitability by a specialist herbivore. New Phytol. 2011, 189, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Mithen, R. Glucosinolates–biochemistry, genetics and biological activity. Plant Growth Regul. 2001, 34, 91–103. [Google Scholar] [CrossRef]
- Benkeblia, N. Omics Technologies and Crop Improvement; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef] [PubMed]
- Liener, I. Toxic Constituents of Plant Foodstuffs; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Son, H.-R.; Oh, S.-K.; Tsukamoto, C.; Choi, M.-R. ACE and α-glucosidase inhibitory activity of the glucosinolates in olsan Leaf mustard pickle during storage. KSBB J. 2016, 31, 165–170. [Google Scholar] [CrossRef]
- Tu, A. Handbook of Natural Toxins: Food Poisoning; Taylor & Francis: Abingdon, UK, 1992. [Google Scholar]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef]
- Park, M.-H.; Arasu, M.V.; Park, N.-Y.; Choi, Y.-J.; Lee, S.-W.; Al-Dhabi, N.A.; Kim, J.B.; Kim, S.-J. Variation of glucoraphanin and glucobrassicin: Anticancer components in Brassica during processing. Food Sci. Technol. 2013, 33, 624–631. [Google Scholar] [CrossRef]
- Peterson, C.J.; Tsao, R.; Coats, J.R. Glucosinolate aglucones and analogues: Insecticidal properties and a QSAR. Pestic. Sci. 1998, 54, 35–42. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Rask, L.; Andréasson, E.; Ekbom, B.; Eriksson, S.; Pontoppidan, B.; Meijer, J. Myrosinase: Gene family evolution and herbivore defense in Brassicaceae. In Plant Molecular Evolution; Springer: Berlin/Heidelberg, Germany, 2000; pp. 93–113. [Google Scholar]
- Nastruzzi, C.; Cortesi, R.; Esposito, E.; Menegatti, E.; Leoni, O.; Iori, R.; Palmieri, S. In vitro cytotoxic activity of some glucosinolate-derived products generated by myrosinase hydrolysis. J. Agric. Food Chem. 1996, 44, 1014–1021. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Li, Y.; Shi, Y.; Zhang, Y. Enhanced inhibition of urinary bladder cancer growth and muscle invasion by allyl isothiocyanate and celecoxib in combination. Carcinogenesis 2013, 34, 2593–2599. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, S.-Q.; Zhang, J.; Huang, G.-Y.; Chen, L.-Y.; Zhao, F.-Y. Chemical composition, antimicrobial property and microencapsulation of Mustard (Sinapis alba) seed essential oil by complex coacervation. Food Chem. 2014, 165, 560–568. [Google Scholar] [CrossRef]
- Gupta, M. Pharmacological properties and traditional therapeutic uses of important Indian spices: A review. Int. J. Food Prop. 2010, 13, 1092–1116. [Google Scholar] [CrossRef]
- Gupta, M.; Shaw, B. Uses of medicinal plants in Panchakarma Ayurvedic therapy. Indian J. Tradit. Knowl. 2009, 8, 372–378. [Google Scholar]
- Dubey, N.K. Natural Products in Plant Pest Management; Centre for Agriculture and Bioscience International: Wallingford, UK, 2010. [Google Scholar]
- El-Beltagi, H.E.-D.S.; Mohamed, A.A. Variations in fatty acid composition, glucosinolate profile and some phytochemical contents in selected oil seed rape (Brassica napus L.) cultivars. Grasas Aceites 2010, 61, 143–150. [Google Scholar]
- Evans, W.C. Trease and Evans’ Pharmacognosy, 14th ed.; WB Saunders Company Ltd.: London, UK, 1997. [Google Scholar]
- Velasco, P.; Soengas, P.; Vilar, M.; Cartea, M.E.; del Rio, M. Comparison of glucosinolate profiles in leaf and seed tissues of different Brassica napus crops. J. Am. Soc. Hortic. Sci. 2008, 133, 551–558. [Google Scholar] [CrossRef]
- Xiao, D.; Srivastava, S.K.; Lew, K.L.; Zeng, Y.; Hershberger, P.; Johnson, C.S.; Trump, D.L.; Singh, S.V. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 2003, 24, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Fauduet, H.; Coic, J.; Lessire, M.; Quinsac, A.; Ribaillier, D.; Rollin, P. Rapeseed meal upgrading—Pilot scale preparation of rapeseed meal materials with high or low glucosinolate contents. Anim. Feed Sci. Technol. 1995, 56, 99–109. [Google Scholar] [CrossRef]
- Oh, S.; Tsukamoto, C.; Kim, K.; Choi, M. Investigation of glucosinolates, and the antioxidant activity of Dolsan leaf mustard kimchi extract using HPLC and LC-PDA-MS/MS. J. Food Biochem. 2017, 41, e12366. [Google Scholar] [CrossRef]
- Horbowicz, M. The occurrence, role and contents of glucosinolates in Brassica vegetables. Vegetetable Crops Res. Bull. 2003, 58, 23–40. [Google Scholar]
- Stoner, G.D.; Morse, M.A. Isothiocyanates and plant polyphenols as inhibitors of lung and esophageal cancer. Cancer Lett. 1997, 114, 113–119. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kim, H.-W.; Jeon, J.-H.; Lee, H.-S. Acaricidal constituents isolated from Sinapis alba L. seeds and structure—Activity relationships. J. Agric. Food Chem. 2008, 56, 9962–9966. [Google Scholar] [CrossRef] [PubMed]
- Ernst, I.M.; Palani, K.; Esatbeyoglu, T.; Schwarz, K.; Rimbach, G. Synthesis and Nrf2-inducing activity of the isothiocyanates iberverin, iberin and cheirolin. Pharmacol. Res. 2013, 70, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R. Bioactive Foods and Extracts: Cancer Treatment and Prevention; Taylor & Francis: Melbourne, VIC, Australia, 2010. [Google Scholar]
- Shapiro, T.A.; Fahey, J.W.; Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Stephenson, K.K.; Wade, K.L.; Ye, L.; Talalay, P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr. Cancer 2006, 55, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Raymond, S.M. Brassica Extracts or Sulforaphane in Combination with Resveratrol as Antitumor Agents. GB2363571A, 2 January 2000. [Google Scholar]
- Jadhav, U.; Ezhilarasan, R.; Vaughn, S.F.; Berhow, M.A.; Mohanam, S. Dietary isothiocyanate iberin inhibits growth and induces apoptosis in human glioblastoma cells. J. Pharmacol. Sci. 2007, 103, 247–251. [Google Scholar] [CrossRef]
- Al-Gendy, A.; El-Gindi, O.; Hafez, A.S.; Ateya, A. Glucosinolates, volatile constituents and biological activities of Erysimum corinthium Boiss. (Brassicaceae). Food Chem. 2010, 118, 519–524. [Google Scholar] [CrossRef]
- Avato, P.; D’Addabbo, T.; Leonetti, P.; Argentieri, M. Nematicidal potential of Brassicaceae. Phytochem. Rev. 2013, 12, 791–802. [Google Scholar] [CrossRef]
- Sayed, G.; Louveaux, A.; Mavratzotis, M.; Rollin, P.; Quinsac, A. Effects of glucobrassicin, epiprogoitrin and related breakdown products on locusts feeding: Schouwia purpurea and desert locust relationships. Entomol. Exp. Appl. 1996, 78, 231–236. [Google Scholar] [CrossRef]
- Guo, W.; Yuan, L.; Chen, P.; Guo, J. Determination of glucosinolates in rapeseeds by liquid chromatography–electrospray mass spectrometry. Anal. Lett. 2005, 38, 343–351. [Google Scholar] [CrossRef]
- Bunting, E.S. Production and Utilization of Protein in Oilseed Crops. In Proceedings of the Seminar in the EEC Programme of Coordination of Research on the Improvement of the Production of Plant Proteins, Institut für Pflanzenbau und Pflanzenzüchting, Braunschweig, Germany, 8–10 July 1980; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Nilsson, T.; Rosenfeld, H.J. Symposium on Quality of Vegetables: Ås, Norway, 18–22 June 1984; International Society for Horticultural Science: Leuven, Belgium, 1984. [Google Scholar]
- Vageeshbabu, H.; Chopra, V. Genetic and biotechnological approaches for reducing glucosinolates from rapeseed-mustard meal. J. Plant Biochem. Biotechnol. 1997, 6, 53–62. [Google Scholar] [CrossRef]
- Lai, R.-H.; Miller, M.J.; Jeffery, E. Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food Funct. 2010, 1, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef]
- Prashar, A.; Siddiqui, F.; Singh, A.K. Synthetic and green vegetable isothiocyanates target red blood leukemia cancers. Fitoterapia 2012, 83, 255–265. [Google Scholar] [CrossRef]
- Mesimeri, I.-D.; Revelou, P.-K.; Constantinou-Kokotou, V.; Kokotou, M.G. Determination of phenethyl isothiocyanate, erucin, iberverin, and erucin nitrile concentrations in healthy and Pieris rapae-infected broccoli tissues using gas chromatography-mass spectrometry. Chemosensors 2024, 12, 16. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Wallig, M.A.; Juvik, J.A.; Klein, B.P.; Kushad, M.M.; Jeffery, E.H. Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea. J. Agric. Food Chem. 2001, 49, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Jeffery, E.H. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem. 2001, 49, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Mota, V.; Saavedra, M.; Rosa, E.; Bennett, R. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Herz, C.; Schlotz, N.; Kupke, F.; Bartolomé Rodríguez, M.M.; Schreiner, M.; Rohn, S.; Lamy, E. The Brassica epithionitrile 1-cyano-2, 3-epithiopropane triggers cell death in human liver cancer cells in vitro. Mol. Nutr. Food Res. 2015, 59, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Kaufmann, M.; Kupke, F.; Hackl, T.; Kroh, L.W.; Rohn, S.; Schreiner, M. Brassica vegetables as sources of epithionitriles: Novel secondary products formed during cooking. Food Chem. 2018, 245, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, Nitriles, and Epithionitriles from Glucosinolates Are Affected by Genotype and Developmental Stage in Brassica oleracea Varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef]
- Kumar, S.; Andy, A. Health promoting bioactive phytochemicals from Brassica. Int Food Res. J. 2012, 19, 141–152. [Google Scholar]
- Gland, A. Content and Pattern of Glucosinolates in Resynthesised Rapeseed. In Production and Utilization of Protein in Oilseed Crops, Proceedings of a Seminar in the EEC Programme of Coordination of Research on the Improvement of the Production of Plant Proteins, Institut für Pflanzenbau und Pflanzenzüchting, Braunschweig, Germany, 8–10 July 1980; Bunting, E.S., Ed.; Springer: Dordrecht, The Netherlands, 1981; pp. 127–135. [Google Scholar]
- Font, R.; del Río-Celestino, M.; Cartea, E.; de Haro-Bailón, A. Quantification of glucosinolates in leaves of leaf rape (Brassica napus ssp. pabularia) by near-infrared spectroscopy. Phytochemistry 2005, 66, 175–185. [Google Scholar]
- Hrncirik, K.; Valusek, J.; Velisek, J. Investigation of ascorbigen as a breakdown product of glucobrassicin autolysis in Brassica vegetables. Eur. Food Res. Technol. 2001, 212, 576–581. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Rouse, M.; Zhang, J.; Chatterjee, S.; Nagarkatti, P.S.; Nagarkatti, M. Dietary Indoles Suppress Delayed-Type Hypersensitivity by Inducing a Switch from Proinflammatory Th17 Cells to Anti-Inflammatory Regulatory T Cells through Regulation of MicroRNA. J. Immunol. 2016, 196, 1108–1122. [Google Scholar] [CrossRef]
- Patterson, C. Mustard: Protein, Mucilage and Bioactives; The Pathfinders: Sydney, NSW, Australia, 2016. [Google Scholar]
- Natella, F.; Maldini, M.; Leoni, G.; Scaccini, C. Glucosinolates redox activities: Can they act as antioxidants? Food Chem. 2014, 149, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Zaharia, I.L. Sinalbins A and B, phytoalexins from Sinapis alba: Elicitation, isolation, and synthesis. Phytochemistry 2000, 55, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Sano, M.; Ogawa, K.; Hirose, M.; Goshima, H.; Shirai, T. Involvement of toxicity as an early event in urinary bladder carcinogenesis induced by phenethyl isothiocyanate, benzyl isothiocyanate, and analogues in F344 rats. Toxicol. Pathol. 2003, 31, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Thies, W. Isolation of sinigrin and glucotropaeolin from Cruciferous seeds. Lipid Fett 1988, 90, 311–314. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Berhow, M.A. Allelochemicals isolated from tissues of the invasive weed garlic mustard (Alliaria petiolata). J. Chem. Ecol. 1999, 25, 2495–2504. [Google Scholar] [CrossRef]
- Stan, S.D.; Singh, S.V.; Whitcomb, D.C.; Brand, R.E. Phenethyl isothiocyanate inhibits proliferation and induces apoptosis in pancreatic cancer cells in vitro and in a MIAPaca2 xenograft animal model. Nutr. Cancer 2014, 66, 747–755. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Bullen, C.K.; Villabona-Rueda, A.F.; Thompson, E.A.; Turner, M.L.; Merino, V.F.; Yan, Y.; Kim, J.; Davis, S.L.; Komm, O. Sulforaphane exhibits antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses in vitro and in mice. Commun. Biol. 2022, 5, 242. [Google Scholar] [CrossRef]
- Treasure, K.; Harris, J.; Williamson, G. Exploring the anti-inflammatory activity of sulforaphane. Immunol. Cell Biol. 2023, 101, 805–828. [Google Scholar] [CrossRef]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Reichelt, M.; Hidalgo, W.; Agnolet, S.; Schneider, B. Tissue-specific distribution of secondary metabolites in rapeseed (Brassica napus L.). PLoS ONE 2012, 7, e48006. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef]
- Kozlowska, H.; Rotkiewicz, D.A.; Zadernowski, R.; Sosulski, F.W. Phenolic acids in rapeseed and mustard. J. Am. Oil Chem. Soc. 1983, 60, 1119–1123. [Google Scholar] [CrossRef]
- Koubaa, M.; Mhemdi, H.; Sanlaville, Q.; Vorobiev, E. Recovery of Oil, Erucic Acid, and Phenolic Compounds from Rapeseed and Rocket Seeds. Chem. Eng. Technol. 2016, 39, 1431–1437. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Volume 9: Modified Stems, Roots, Bulbs; Springer: Dordrecht, The Netherlands, 2015; Volume 9. [Google Scholar]
- Dubie, J.; Stancik, A.; Morra, M.; Nindo, C. Antioxidant extraction from mustard (Brassica juncea) seed meal using high-intensity ultrasound. J. Food Sci. 2013, 78, E542–E548. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. Natural Antioxidants: Chemistry, Health Effects, and Applications; The American Oil Chemists Society: Urbana, IL, USA, 1997. [Google Scholar]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Wijesundera, C.; Ceccato, C.; Fagan, P.; Shen, Z. Seed roasting improves the oxidative stability of canola (B. napus) and mustard (B. juncea) seed oils. Eur. J. Lipid Sci. Technol. 2008, 110, 360–367. [Google Scholar] [CrossRef]
- Siger, A.; Grygier, A.; Bąkowska, E.; Szczechowiak-Pigłas, J.; Bartkowiak-Broda, I. Phytochemical content of roasted seeds of three white mustard (Sinapis alba L.) varieties differing in their glucosinolate and erucic acid content. Ind. Crops Prod. 2024, 220, 119207. [Google Scholar] [CrossRef]
- Mizani, M.; Yousefi, M.; Rasouli, S.; Sharifan, A.; Bamani, M.M. The effect of different deheating processes on residual myrosinase activity, antimicrobial properties and total phenolic contents of yellow mustard (Sinapis alba). J. Food Biosci. Technol. 2016, 6, 1–12. [Google Scholar]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Hald, C.; Dawid, C.; Tressel, R.; Hofmann, T. Kaempferol 3-O-(2‴-O-sinapoyl-β-sophoroside) causes the undesired bitter taste of canola/rapeseed protein isolates. J. Agric. Food Chem. 2018, 67, 372–378. [Google Scholar] [CrossRef]
- Marnoch, R.; Diosady, L.L. Production of mustard protein isolates from oriental mustard seed (Brassica juncea L.). J. Am. Oil Chem. Soc. 2006, 83, 65–69. [Google Scholar] [CrossRef]
- Anjum, N.A.; Ahmad, I.; Pereira, M.E.; Duarte, A.C.; Umar, S.; Khan, N.A. The Plant Family Brassicaceae: Contribution Towards Phytoremediation; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Dileep, K.; Remya, C.; Cerezo, J.; Fassihi, A.; Pérez-Sánchez, H.; Sadasivan, C. Comparative studies on the inhibitory activities of selected benzoic acid derivatives against secretory phospholipase A 2, a key enzyme involved in the inflammatory pathway. Mol. BioSystems 2015, 11, 1973–1979. [Google Scholar] [CrossRef]
- Aider, M.; Barbana, C. Canola proteins: Composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity—A practical and critical review. Trends Food Sci. Technol. 2011, 22, 21–39. [Google Scholar] [CrossRef]
- Gruis, D.F.; Selinger, D.A.; Curran, J.M.; Jung, R. Redundant proteolytic mechanisms process seed storage proteins in the absence of seed-type members of the vacuolar processing enzyme family of cysteine proteases. Plant Cell 2002, 14, 2863–2882. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Ross, A.R.; Yang, J.; Hegedus, D.D.; Kermode, A.R. Phosphorylation of the 12 S globulin cruciferin in wild-type and abi1-1 mutant Arabidopsis thaliana (thale cress) seeds. Biochem. J. 2007, 404, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Wanasundara, J.P.; McIntosh, T.C.; Perera, S.P.; Withana-Gamage, T.S.; Mitra, P. Canola/rapeseed protein-functionality and nutrition. OCL 2016, 23, D407. [Google Scholar] [CrossRef]
- Schwenke, K. Rapeseed proteins. In New and Developing Sources of Food Proteins; Hudson, J.F., Ed.; Chapman and Hall: London, UK, 1994; pp. 281–306. [Google Scholar]
- Depree, J.; Howard, T.; Savage, G. Flavour and pharmaceutical properties of the volatile sulphur compounds of wasabi (Wasabia japonica). Food Res. Int. 1998, 31, 329–337. [Google Scholar] [CrossRef]
- Gulbransen, G.; Esernio-Jenssen, D. Aspiration of black mustard. J. Toxicol. Clin. Toxicol. 1998, 36, 591–593. [Google Scholar] [CrossRef]
- Jyothi, T.; Sinha, S.; Singh, S.A.; Surolia, A.; Rao, A.A. Napin from Brassica juncea: Thermodynamic and structural analysis of stability. Biochim. Biophys. Acta BBA Proteins Proteom. 2007, 1774, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S. Bioactive Compounds in Canola Meal. Ph.D. Thesis, Charles Sturt University, Bathurst, NSW, Australia, 2015. [Google Scholar]
- Kasprzak, M.; Houdijk, J.; Liddell, S.; Davis, K.; Olukosi, O.; Kightley, S.; White, G.; Wiseman, J. Raspeseed napin and cruciferin are readily digested by poultry. J. Anim. Physiol. Anim. Nutr. 2016, 101, 558–666. [Google Scholar]
- Bérot, S.; Compoint, J.; Larré, C.; Malabat, C.; Guéguen, J. Large scale purification of rapeseed proteins (Brassica napus L.). J. Chromatogr. B 2005, 818, 35–42. [Google Scholar] [CrossRef]
- Bos, C.; Airinei, G.; Mariotti, F.; Benamouzig, R.; Bérot, S.; Evrard, J.; Fénart, E.; Tomé, D.; Gaudichon, C. The poor digestibility of rapeseed protein is balanced by its very high metabolic utilization in humans. J. Nutr. 2007, 137, 594–600. [Google Scholar] [CrossRef]
- Li, J.; Gao, G.; Xu, K.; Chen, B.; Yan, G.; Li, F.; Qiao, J.; Zhang, T.; Wu, X. Genome-Wide Survey and Expression Analysis of the Putative Non-Specific Lipid Transfer Proteins in Brassica rapa L. PLoS ONE 2014, 9, e84556. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Carbohydrates of canola and rapeseed. In Canola and Rapeseed: Production, Chemistry, Nutrition and Processing Technology; Springer: New York, NY, USA, 1990; pp. 211–220. [Google Scholar]
- Biesek, J.; Kuźniacka, J.; Banaszak, M.; Kaczmarek, S.; Adamski, M.; Rutkowski, A.; Zmudzińska, A.; Perz, K.; Hejdysz, M. Growth Performance and Carcass Quality in Broiler Chickens Fed on Legume Seeds and Rapeseed Meal. Animals 2020, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Çakmakli, Ü.; Ünal, M.K. Composition and mineral distribution of rapeseed varieties tested for adaptation in Turkey. Lipid Fett 1988, 90, 386–389. [Google Scholar] [CrossRef]
- Khan, A.; Sankhyan, P.; Kumar, S. Biochemical characterization of Mustard Oil (Brassica campestris L.) with special reference to its fatty acid composition. Asian J. Adv. Basic Sci. 2013, 1, 1–9. [Google Scholar]
- Misra, A.; Kumar, S.; Verma, A.K.; Chanana, N.P.; Das, M.; Dhawan, V.; Dwivedi, P.D. Safety evaluation of genetically modified mustard (V4) seeds in terms of allergenicity. GM Crops Food 2012, 3, 273–282. [Google Scholar] [CrossRef]
- Sharif, R.; Paul, R.; Bhattacharjya, D.; Ahmed, K. Physicochemical characters of oilseeds from selected mustard genotypes. J. Bangladesh Agril. Univ. 2017, 15, 27–40. [Google Scholar] [CrossRef]
- Singh, P. Physico-chemical investigations of Mustard seed (Brassica juncea L). Int. J. Sci. Res. Multidiscip. Stud. 2018, 4, 24–27. [Google Scholar]
- Stojanović, Z.S.; Uletilović, D.D.; Kravić, S.Ž.; Kevrešan, Ž.S.; Grahovac, N.L.; Lončarević, I.S.; Đurović, A.D.; Marjanović Jeromela, A.M. Comparative Study of the Nutritional and Chemical Composition of New Oil Rape, Safflower and Mustard Seed Varieties Developed and Grown in Serbia. Plants 2023, 12, 2160. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.K.E.; El-Newihi, A.M.; Omar, S.M.; Ahmed, Z.S. Assessment of proximate chemical composition, nutritional status, fatty acid composition and antioxidants of curcumin (Zingiberaceae) and mustard seeds powders (Brassicaceae). Food Public Health 2014, 4, 286–292. [Google Scholar]
- British-Pharmacopoeia. British Bharmacopoeia; Spottiswoode and Co.: London, UK, 1867. [Google Scholar]
- Swami, S.S.T. The Ayurveda Encyclopedia: Natural Secrets to Healing, Prevention and Longevity; Sri Satguru Publication: New Delhi, India, 1998. [Google Scholar]
- Hashem, M.; Alamri, S.A.; Alrumman, S.A.; Moustafa, M.F. Suppression of phytopathogenic fungi by plant extract of some weeds and the possible mode of action. Br. Microbiol. Res. J. 2016, 15, 1–13. [Google Scholar] [CrossRef]
- Garland, S. The Complete Book of Herbs & Spices: An Illustrated Guide to Growing and Using Culinary, Aromatic, Cosmetic and Medicinal Plants; Hodder & Stoughton (Aust) Pty Ltd., Sydney & Aukland: Rydalmere, NSW, Australia, 1993. [Google Scholar]
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial activity of mustard essential oil against Escherichia coli O157: H7 and Salmonella typhi. Food Control 2009, 20, 1073–1079. [Google Scholar] [CrossRef]
- Khan, B.; Abraham, A.; Leelamma, S. Hypoglycemic action of Murraya koenigii (curry leaf) and Brassica juncea (mustard): Mechanism of action. Indian J. Biochem. Biophys. 1995, 32, 106. [Google Scholar]
- Srinivasan, K. Plant foods in the management of diabetes mellitus: Spices as beneficial antidiabetic food adjuncts. Int. J. Food Sci. Nutr. 2005, 56, 399–414. [Google Scholar] [CrossRef]
- Zargari, A. Medicinal Plants, 5th ed.; Tehran University Publications: Tehran, Iran, 2001; Volume 1. [Google Scholar]
- Thirumalai, T.; Therasa, S.V.; Elumalai, E.K.; David, E. Hypoglycemic effect of Brassica juncea (seeds) on streptozotocin induced diabetic male albino rat. Asian Pac. J. Trop. Biomed. 2011, 1, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Roia, F.C. The use of plants in hair and scalp preparations. Econ. Bot. 1966, 20, 17–30. [Google Scholar] [CrossRef]
- Chiej, R. Encyclopaedia of Medicinal Plants; Macdonald & Co. (Publishers) Ltd.: London, UK, 1984. [Google Scholar]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Melbourne, VIC, Australia, 2016. [Google Scholar]
- Kayani, S.; Ahmad, M.; Zafar, M.; Sultana, S.; Khan, M.P.Z.; Ashraf, M.A.; Hussain, J.; Yaseen, G. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies–Abbottabad, Northern Pakistan. J. Ethnopharmacol. 2014, 156, 47–60. [Google Scholar] [CrossRef]
- Shah, A.; Marwat, S.K.; Gohar, F.; Khan, A.; Bhatti, K.H.; Amin, M.; Din, N.U.; Ahmad, M.; Zafar, M. Ethnobotanical study of medicinal plants of semi-tribal area of Makerwal & Gulla Khel (lying between Khyber Pakhtunkhwa and Punjab Provinces), Pakistan. Am. J. Plant Sci. 2013, 4, 98–116. [Google Scholar]
- Kloss, J. Back to Eden: The Classic Guide to Medicine, Natural Foods and Home Remedies, 4th ed.; Lifeline Books: Adelaide, SA, Australia, 1974. [Google Scholar]
- Haq, F.; Ahmad, H.; Ullah, R.; Iqbal, Z. Species diversity and ethno botanical classes of the flora of Allai valley district Battagram Pakistan. Int. J. Plant Res. 2012, 2, 111–123. [Google Scholar] [CrossRef]
- Kizilarslan, Ç.; Özhatay, N. Wild plants used as medicinal purpose in the south part of İzmit (northwest Turkey). Turk. J. Pharm. Sci. 2012, 9, 199–218. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Eastwood, M.; Passmore, R. Dietary fibre. Lancet 1983, 322, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Karpozilos, A.; Pavlidis, N. The treatment of cancer in Greek antiquity. Eur. J. Cancer 2004, 40, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Shah, A.H.; Tariq, M.; Ageel, A.M. Studies on herbal aphrodisiacs used in Arab system of medicine. Am. J. Chin. Med. 1989, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Duke, J.A. A Field Guide to Medicinal Plants: Eastern and Central North America; Houghton Mifflin Company: Boston, MA, USA, 1990; 366p, ISBN 395353092. [Google Scholar]
- Hussain, A.; Khan, M.N.; Iqbal, Z.; Sajid, M.S. An account of the botanical anthelmintics used in traditional veterinary practices in Sahiwal district of Punjab, Pakistan. J. Ethnopharmacol. 2008, 119, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.A.; Rodrigues, E.; Mendes, F.R.; Tabach, R.; Gianfratti, B. Treatment of drug dependence with Brazilian herbal medicines. Rev. Bras. Farmacogn. 2006, 16, 690–695. [Google Scholar] [CrossRef]
- Eichel, V.; Schüller, A.; Biehler, K.; Al-Ahmad, A.; Frank, U. Antimicrobial effects of mustard oil-containing plants against oral pathogens: An in vitro study. BMC Complement. Med. Ther. 2020, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.H.; Cho, C.Y.; Ha, K.S.; Lee, J.Y.; Choi, H.Y.; Kwon, Y.I.; Apostolidis, E. In vitro and in vivo anti-hyperglycemic effects of green and red mustard leaves (Brassica juncea var. integrifolia). J. Food Biochem. 2018, 42, e12583. [Google Scholar] [CrossRef]
- Lietzow, J. Biologically active compounds in mustard seeds: A toxicological perspective. Foods 2021, 10, 2089. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Hong, E.; Kim, G.H. Evaluation of Antibacterial Activity of 3-Butenyl, 4-Pentenyl, 2-Phenylethyl, and Benzyl Isothiocyanate in Brassica Vegetables. J. Food Sci. 2010, 75, M412–M416. [Google Scholar] [CrossRef]
- Borek, V.; Morra, M.J.; Brown, P.D.; McCaffrey, J.P. Allelochemicals produced during sinigrin decomposition in soil. J. Agric. Food Chem. 1994, 42, 1030–1034. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J.; McCaffrey, J.P.; Auld, D.L.; Williams, L. Allelochemicals produced during glucosinolate degradation in soil. J. Chem. Ecol. 1991, 17, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Storm, D.R.; Rosenthal, K.S.; Swanson, P.E. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 1977, 46, 723–763. [Google Scholar] [CrossRef]
- Vernon, L.P.; Evett, G.E.; Zeikus, R.D.; Gray, W.R. A toxic thionin from Pyrularia pubera: Purification, properties, and amino acid sequence. Arch. Biochem. Biophys. 1985, 238, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Browne, J.; Crugten, J.V.; Hasan, M.F.; Liu, L.; Barkla, B.J. In Silico, Molecular Docking and In Vitro Antimicrobial Activity of the Major Rapeseed Seed Storage Proteins. Front. Pharmacol. 2020, 11, 1340. [Google Scholar] [CrossRef]
- Ledger, E.V.; Sabnis, A.; Edwards, A.M. Polymyxin and lipopeptide antibiotics: Membrane-targeting drugs of last resort. Microbiology 2022, 168, 001136. [Google Scholar] [CrossRef] [PubMed]
- Drobnica, Ľ.; Zemanova, M.; Nemec, P.; Antoš, K.; Kristian, P.; Štullerová, A.; Knoppova, V. Antifungal activity of isothiocyanates and related compounds I. Naturally occurring isothiocyanates and their analogues. Appl. Microbiol. 1967, 15, 701–709. [Google Scholar] [CrossRef]
- Mari, M.; Leoni, O.; Iori, R.; Cembali, T. Antifungal vapour-phase activity of allyl-isothiocyanate against Penicillium expansum on pears. Plant Pathol. 2002, 51, 231–236. [Google Scholar] [CrossRef]
- Welke, J.E.; Hoeltz, M.; Dottori, H.A.; Noll, I.B. Patulin accumulation in apples during storage by Penicillium expansum and Penicillium griseofulvum Strains. Braz. J. Microbiol. 2011, 42, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Bainard, L.; Brown, P.; Upadhyaya, M. Inhibitory effect of tall hedge mustard (Sisymbrium loeselii) allelochemicals on rangeland plants and arbuscular mycorrhizal fungi. Weed Sci. 2009, 57, 386–393. [Google Scholar] [CrossRef]
- Tesaki, S.; Tanabe, S.; Ono, H.; Fukushi, E.; Kawabata, J.; Watanabe, M. 4-Hydroxy-3-nitrophenylacetic and Sinapic Acids as Antibacterial Compounds from Mustard Seeds. Biosci. Biotechnol. Biochem. 1998, 62, 998–1000. [Google Scholar] [CrossRef]
- Meneguetti, B.T.; Machado, L.d.S.; Oshiro, K.G.N.; Nogueira, M.L.; Carvalho, C.M.E.; Franco, O.L. Antimicrobial Peptides from Fruits and Their Potential Use as Biotechnological Tools—A Review and Outlook. Front. Microbiol. 2017, 7, 2136. [Google Scholar] [CrossRef]
- Munir, A.; Iqbal, S.; Khaliq, B.; Saeed, Q.; Hussain, S.; Shah, K.H.; Ahmad, F.; Mehmood, S.; Ali, Z.; Munawar, A. In Silico Studies and Functional Characterization of a Napin Protein from Seeds of Brassica juncea. Int. J. Agric. Biol. 2019, 22, 1655–1662. [Google Scholar]
- Ngai, P.; Ng, T. A napin-like polypeptide from dwarf Chinese white cabbage seeds with translation-inhibitory, trypsin-inhibitory, and antibacterial activities. Peptides 2004, 25, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.; Ng, T. A napin-like polypeptide with translation-inhibitory, trypsin-inhibitory, antiproliferative and antibacterial activities from kale seeds. Chem. Biol. Drug Des. 2004, 64, 202–208. [Google Scholar] [CrossRef]
- Nioi, C.; Kapel, R.; Rondags, E.; Marc, I. Selective extraction, structural characterisation and antifungal activity assessment of napins from an industrial rapeseed meal. Food Chem. 2012, 134, 2149–2155. [Google Scholar] [CrossRef]
- Rahman, M.; Browne, J.; Crugten, J.V.; Hasan, M.F.; Liu, L.; Barkla, B.J. In silico, molecular docking and in vitro evidences for rapeseed major seed storage proteins as antimicrobial peptides. In Proceedings of the Australasian Grain Science Association 2020 Online Conference, Charles Sturt University, Wagga Wagga, NSW, Australia, 26 August 2020. [Google Scholar]
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B. Identification of Allergenic Epitopes in the Sequences of Rapeseed Seed Proteins. In Proceedings of the First Canadian Peptide and Protein Community Virtual Symposium Session Peptide and Protein Applications in Cosmetic, Agricultural and High-Tech Products, Online, 27–28 May 2021. [Google Scholar]
- Wolfe, B.E.; Rodgers, V.L.; Stinson, K.A.; Pringle, A. The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. J. Ecol. 2008, 96, 777–783. [Google Scholar] [CrossRef]
- Handley, T.N.; Li, W.; Welch, N.G.; O’Brien-Simpson, N.M.; Hossain, M.A.; Wade, J.D. Evaluation of Potential DnaK Modulating Proline-Rich Antimicrobial Peptides Identified by Computational Screening. Front. Chem. 2022, 10, 875233. [Google Scholar] [CrossRef] [PubMed]
- Barciszewski, J.; Maciej, S.; Haertlé, T. Minireview: Analysis of rape seed napin structure and potential roles of the storage protein. J. Protein Chem. 2000, 19, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Rico, M.; Bruix, M.; González, C.; Monsalve, R.I.; Rodríguez, R. 1H NMR assignment and global fold of napin BnIb, a representative 2S albumin seed protein. Biochemistry 1996, 35, 15672–15682. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Mello, É.O.; Carvalho, A.O.; Regente, M.; Pinedo, M.; de La Canal, L.; Rodrigues, R.; Gomes, V.M. Antimicrobial activity and mechanism of action of a thionin-like peptide from Capsicum annuum fruits and combinatorial treatment with fluconazole against Fusarium solani. Pept. Sci. 2017, 108, e23008. [Google Scholar] [CrossRef] [PubMed]
- Ateeq, M.; Adeel, M.M.; Kanwal, A.; Tahir ul Qamar, M.; Saeed, A.; Khaliq, B.; Saeed, Q.; Atiq, M.N.; Bilal, M.; Alharbi, M.; et al. In Silico Analysis and Functional Characterization of Antimicrobial and Insecticidal Vicilin from Moth Bean (Vigna aconitifolia (Jacq.) Marechal) Seeds. Molecules 2022, 27, 3251. [Google Scholar] [CrossRef] [PubMed]
- Barbosa Pelegrini, P.; del Sarto, R.P.; Silva, O.N.; Franco, O.L.; Grossi-de-Sa, M.F. Antibacterial peptides from plants: What they are and how they probably work. Biochem. Res. Int. 2011, 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, M.; Chen, F. Prediction and analysis of antimicrobial peptides from rapeseed protein using in silico approach. J. Food Biochem. 2021, 45, e13598. [Google Scholar] [CrossRef]
- Garcia, T.B.; Soares, A.A.; Costa, J.H.; Costa, H.P.S.; Neto, J.X.S.; Rocha-Bezerra, L.C.B.; Silva, F.D.A.; Arantes, M.R.; Sousa, D.O.B.; Vasconcelos, I.M.; et al. Gene expression and spatiotemporal localization of antifungal chitin-binding proteins during Moringa oleifera seed development and germination. Planta 2019, 249, 1503–1519. [Google Scholar] [CrossRef]
- Rahman, M.; Khatun, A.; Guo, Q.; Baten, A.; Mauleon, R.; King, G.J.; Browne, J.; Crugten, J.V.; Hasan, M.F.; Liu, L.; et al. Indexing and characterisation of a new class of protein from rapeseed Brassica rapa meal. In Research in Science and Engineering (RISE) Conference, 2021; Southern Cross University: Lismore, NSW, Australia, 2021. [Google Scholar]
- Ribeiro, S.F.F.; Taveira, G.B.; Carvalho, A.O.; Dias, G.B.; Da Cunha, M.; Santa-Catarina, C.; Rodrigues, R.; Gomes, V.M. Antifungal and other biological activities of two 2S albumin-homologous proteins against pathogenic fungi. Protein J. 2012, 31, 59–67. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Cammue, B.P.A.; De Bolle, M.F.C.; Thevissen, K.; De Samblanx, G.W.; Osborn, R.W.; Nielson, K. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 1997, 16, 297–323. [Google Scholar] [CrossRef]
- Román, J.; Lagos, A.; Mahn, A.; Quintero, J. The Effect of Broccoli Glucosinolates Hydrolysis Products on Botrytis cinerea: A Potential New Antifungal Agent. Int. J. Mol. Sci. 2024, 25, 7945. [Google Scholar] [CrossRef]
- Jain, S.; Agarwal, S.; Malaiya, S. Antimycotic effect of fixed oils treated with herbal seeds on the growth of fungi causing otomycosis. Anc. Sci. Life 1993, 13, 160–164. [Google Scholar]
- Fotos, P.G.; Hellstein, J.W. Candida and candidosis: Epidemiology, diagnosis, and therapeutic management. Dent. Clin. N. Am. 1992, 36, 857–878. [Google Scholar] [CrossRef] [PubMed]
- Last, W. Heal Yourself the Natural Way Overcome Disease & Create Superior Health; Huckleberry Enterprises: Sydney, NSW, Australia, 2010. [Google Scholar]
- Bajpai, D.; Malaiappan, S.; Rajeshkumar, S. Evaluation of Anti-inflammatory and Antimicrobial Properties of Mustard Seed Extract-Based Hydrogel: An In Vitro Study. Cureus 2023, 15, e45146. [Google Scholar] [CrossRef]
- Rahman, M.; Afroz, M.; Liu, L.; Barkla, B.J. Rapeseed proteins-a treasure trove of bioactive peptides. In Phytochemical Society of Europe-Natural Product Scientists 2020 Summit; Khulna University: Khulna, Bangladesh, 2020. [Google Scholar]
- del Mar Yust, M.; Pedroche, J.; Megías, C.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Rapeseed protein hydrolysates: A source of HIV protease peptide inhibitors. Food Chem. 2004, 87, 387–392. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, S.; Vasudeva, N. In Vivo Assessment of Antihyperglycemic and Antioxidant Activity from Oil of Seeds of Brassica nigra in Streptozotocin Induced Diabetic Rats. Adv. Pharm. Bull. 2013, 3, 359–365. [Google Scholar]
- Anand, P.; Murali, K.; Tandon, V.; Chandra, R.; Murthy, P. Preliminary studies on antihyperglycemic effect of aqueous extract of Brassica nigra (L.) Koch in streptozotocin induced diabetic rats. Indian J. Exp. Biol. 2007, 45, 696. [Google Scholar]
- Grover, J.; Yadav, S.; Vats, V. Effect of feeding Murraya koeingii and Brassica juncea diet kidney functions and glucose levels in streptozotocin diabetic mice. J. Ethnopharmacol. 2003, 85, 1–5. [Google Scholar] [CrossRef]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Kim, K.; Nam, K.; Kurihara, H.; Kim, S. Potent alpha-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Skoneczka, J.; Kingston, D.; Krishnan, A.; Misyak, S.; Guri, A.; Pereira, A.; Carter, A.; Minorsky, P.; Tumarkin, R. Mechanisms of action and medicinal applications of abscisic acid. Curr. Med. Chem. 2010, 17, 467–478. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.; Rahman, M.M.; Hossain, H.; Jahan, I.A.; Nesa, M.L. Antioxidant, antinociceptive and CNS activities of Viscum orientale and high sensitive quantification of bioactive polyphenols by UPLC. Front. Pharmacol. 2016, 7, 176. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Pearson, T.A.; Wan, Y.; Hargrove, R.L.; Moriarty, K.; Fishell, V.; Etherton, T.D. High–monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am. J. Clin. Nutr. 1999, 70, 1009–1015. [Google Scholar] [CrossRef]
- Parillo, M.; Rivellese, A.; Ciardullo, A.; Capaldo, B.; Giacco, A.; Genovese, S.; Riccardi, G. A high-monounsaturated-fat/low-carbohydrate diet improves peripheral insulin sensitivity in non-insulin-dependent diabetic patients. Metabolism 1992, 41, 1373–1378. [Google Scholar] [CrossRef]
- Guévin, N.; Jacques, H.; Nadeau, A.; Galibois, I. Postprandial glucose, insulin, and lipid responses to four meals containing unpurified dietary fiber in non-insulin-dependent diabetes mellitus (NIDDM), hypertriglyceridemic subjects. J. Am. Coll. Nutr. 1996, 15, 389–396. [Google Scholar] [CrossRef]
- Khan, B.; Abraham, A.; Leelamma, S. Anti-oxidant effects of curry leaf, Murraya koenigii and mustard seeds, Brassica juncea in rats fed with high fat diet. Indian J. Exp. Biol. 1997, 35, 148. [Google Scholar]
- Begin, F.; Vachon, C.; Jones, J.; Wood, P.; Savoie, L. Effect of dietary fibers on glycemia and insulinemia and on gastrointestinal function in rats. Can. J. Physiol. Pharmacol. 1989, 67, 1265–1271. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Hypoglycemic and antihyperglycemic effect of Brassica juncea diet and their effect on hepatic glycogen content and the key enzymes of carbohydrate metabolism. Mol. Cell. Biochem. 2002, 241, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, V.; Pandiyan, V.; Vijayarani, K.; Padmanath, K. A study on insulin levels and the expression of glut 4 in streptozotocin (STZ) induced diabetic rats treated with mustard oil diet. Indian J. Clin. Biochem. 2020, 35, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Zeb, A.; Ateeq, M.K. In vivo antidiabetic effects of phenolic compounds of spinach, mustard, and cabbage leaves in mice. Heliyon 2023, 9, e16616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xu, H.; Hong, S.; Song, N.; Xie, J.; Yan, Z.; Wang, R.; Yang, P.; Jiang, X. Rapeseed Protein-Derived Antioxidant Peptide RAP Ameliorates Nonalcoholic Steatohepatitis and Related Metabolic Disorders in Mice. Mol. Pharm. 2019, 16, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Ellegård, L.; Andersson, H.; Bosaeus, I. Rapeseed oil, olive oil, plant sterols, and cholesterol metabolism: An ileostomy study. Eur. J. Clin. Nutr. 2005, 59, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Ghosh, M. Hypolipidemic effect of mustard oil enriched with medium chain fatty acid and polyunsaturated fatty acid. Nutrition 2011, 27, 1183–1193. [Google Scholar] [CrossRef]
- Vahouny, G.V.; Tombes, R.; Cassidy, M.M.; Kritchevsky, D.; Gallo, L.L. Dietary fibers: V. Binding of bile salts, phospholipids and cholesterol from mixed micelles by bile acid sequestrants and dietary fibers. Lipids 1980, 15, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Baumstark, M.W.; Marckmann, P.; Gylling, H.; Sandström, B. An olive oil-rich diet results in higher concentrations of LDL cholesterol and a higher number of LDL subfraction particles than rapeseed oil and sunflower oil diets. J. Lipid Res. 2000, 41, 1901–1911. [Google Scholar] [CrossRef]
- Khan, B.A.; Abraham, A.; Leelamma, S. Murraya koenigii and Brassica juncea—Alterations on lipid profile in 1–2 dimethyl hydrazine induced colon carcinogenesis. Investig. New Drugs 1996, 14, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Abraham, A.; Leelamma, S. Role of Murraya koenigii (curry leaf) and Brassica juncea (Mustard) in lipid peroxidation. Indian J. Physiol. Pharmacol. 1996, 40, 155–158. [Google Scholar]
- Chen, W.-J.L.; Anderson, J.W.; Jennings, D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Exp. Biol. Med. 1984, 175, 215–218. [Google Scholar] [CrossRef]
- Hunninghake, D.B.; Miller, V.T.; LaRosa, J.C.; Kinosian, B.; Brown, V.; Howard, W.; DiSerio, F.; O’Connor, R. Hypocholesterolemic effects of a dietary fiber supplement. Am. J. Clin. Nutr. 1994, 59, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W. Advances in understanding of the role of lipid metabolism in aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef]
- Howard, B.V. Insulin resistance and lipid metabolism. Am. J. Cardiol. 1999, 84, 28–32. [Google Scholar] [CrossRef]
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.-G.; Chen, T.-Y.; Fahey, J.W.; Talalay, P. Keap1-Nrf2 signaling: A target for cancer prevention by sulforaphane. Top. Curr. Chem. 2013, 329, 163–177. [Google Scholar] [PubMed]
- Rahman, M.S. Handbook of Food Preservation; Taylor & Francis: Abingdon, UK, 1999. [Google Scholar]
- Altinoz, M.A.; Bilir, A.; Elmaci, İ. Erucic acid, a component of Lorenzo’s oil and PPAR-δ ligand modifies C6 glioma growth and toxicity of doxorubicin. Experimental data and a comprehensive literature analysis. Chem.-Biol. Interact. 2018, 294, 107–117. [Google Scholar] [CrossRef]
- Ishtiaque, S.; Khan, N.; Siddiqui, M.A.; Siddiqi, R.; Naz, S. Antioxidant potential of the extracts, fractions and oils derived from oilseeds. Antioxidants 2013, 2, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.; Aachary, A.; Thiyam-Holländer, U. Endogenous phenolics in hulls and cotyledons of mustard and canola: A comparative study on its Sinapates and antioxidant capacity. Antioxidants 2014, 3, 544–558. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Alashi, A.; Malomo, S.A.; Girgih, A.T.; Chao, D.; Ju, X.; Aluko, R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef]
- Rahman, M.; Khatun, A.; Nesa, M.L.; Hossain, H.; Jahan, I.A. Bioactive polyphenols from the methanol extract of Cnicus arvensis (L.) Roth demonstrated antinociceptive and central nervous system depressant activities in mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 794729. [Google Scholar] [CrossRef]
- Rahman, M.K.A.; Rahman, S.M.; Rashid, M.A. Antioxidant, antimicrobial and cytotoxic activities of Vitis trifolia Linn. J. Dhaka Int. Univ. 2009, 1, 181–184. [Google Scholar]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. BioMed Res. Int. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Eskin, N.; Raju, J.; Bird, R. Novel mucilage fraction of Sinapis alba L. (mustard) reduces azoxymethane-induced colonic aberrant crypt foci formation in F344 and Zucker obese rats. Phytomedicine 2007, 14, 479–485. [Google Scholar] [CrossRef]
- Wen, C.; Yang, R.; Hu, J.; Jiao, Z.; Yang, Y.; Yang, J.; Li, H.; Zhao, H. Effects of mustard seed on 2, 4-dinitrofluorobenzene-induced allergic contact dermatitis in BALB/c mice. J. South. Med. Univ. 2012, 32, 569–572. [Google Scholar]
- Kesanakurti, D.; Sareddy, G.R.; Babu, P.P.; Kirti, P.B. Mustard NPR1, a mammalian IκB homologue inhibits NF-κB activation in human GBM cell lines. Biochem. Biophys. Res. Commun. 2009, 390, 427–433. [Google Scholar] [CrossRef]
- Yap, J.L.; Worlikar, S.; MacKerell, A.D., Jr.; Shapiro, P.; Fletcher, S. Small molecule inhibitors of the ERK signalling pathway: Towards novel anti-cancer therapeutics. ChemMedChem 2011, 6, 38. [Google Scholar] [CrossRef]
- Kim, J.S.; Han, S.; Kim, H.; Won, S.Y.; Park, H.W.; Choi, H.; Choi, M.; Lee, M.Y.; Ha, I.J.; Lee, S.-G. Anticancer Effects of High Glucosinolate Synthesis Lines of Brassica rapa on Colorectal Cancer Cells. Antioxidants 2022, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Virk, P.; Awad, M.A.; Alanazi, M.M.; Hendi, A.A.; Elobeid, M.; Ortashi, K.M.; Alrowaily, A.W.; Bahlool, T.; Aouaini, F. Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation. Green Process. Synth. 2023, 12, 20228119. [Google Scholar] [CrossRef]
- Savio, A.L.V.; da Silva, G.N.; de Camargo, E.A.; Salvadori, D.M.F. Cell cycle kinetics, apoptosis rates, DNA damage and TP53 gene expression in bladder cancer cells treated with allyl isothiocyanate (mustard essential oil). Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014, 762, 40–46. [Google Scholar] [CrossRef]
- Cui, H.; Bashar, M.A.; Rady, I.; El-Naggar, H.A.; Abd El-Maoula, L.M.; Mehany, A.B. Antiproliferative activity, proapoptotic effect, and cell cycle arrest in human cancer cells of some marine natural product extract. Oxid. Med. Cell. Longev. 2020, 2020, 7948705. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.G.; Hussein, U.K.; Ahmed, A.E.; Kim, K.M.; Mahmoud, H.M.; Hammouda, O.; Jang, K.Y.; Bishayee, A. Mustard seed (Brassica nigra) extract exhibits antiproliferative effect against human lung cancer cells through differential regulation of apoptosis, cell cycle, migration, and invasion. Molecules 2020, 25, 2069. [Google Scholar] [CrossRef]
- Bafna, S.; Pink, J. Mustard Seeds: A Potential Treatment for Colorectal Cancer: 1978. Off. J. Am. Coll. Gastroenterol. ACG 2012, 107, S807. [Google Scholar] [CrossRef]
- Zhang, Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis 2011, 33, 2–9. [Google Scholar] [CrossRef]
- Conaway, C.C.; Wang, C.-X.; Pittman, B.; Yang, Y.-M.; Schwartz, J.E.; Tian, D.; McIntee, E.J.; Hecht, S.S.; Chung, F.-L. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005, 65, 8548–8557. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Vogel, V.; Singh, S.V. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol. Cancer Ther. 2006, 5, 2931–2945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, W.; Dong, X.; Chen, Y.; Lu, Y.; Lin, B.; Guo, J.; Li, M. Benzyl-isothiocyanate Induces Apoptosis and Inhibits migration and invasion of hepatocellular carcinoma cells in vitro. J. Cancer 2017, 8, 240–248. [Google Scholar] [CrossRef]
- Kim, S.-H.; Singh, S.V. p53 Independent Apoptosis by Benzyl Isothiocyanate in Human Breast Cancer Cells Is Mediated by Suppression of XIAP Expression. Cancer Prev. Res. 2010, 3, 718–726. [Google Scholar] [CrossRef]
- Xiao, D.; Powolny, A.A.; Singh, S.V. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J. Biol. Chem. 2008, 283, 30151–30163. [Google Scholar] [CrossRef]
- Wu, C.L.; Huang, A.C.; Yang, J.S.; Liao, C.L.; Lu, H.F.; Chou, S.T.; Ma, C.Y.; Hsia, T.C.; Ko, Y.C.; Chung, J.G. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J. Orthop. Res. 2011, 29, 1199–1209. [Google Scholar] [PubMed]
- Zhang, Y.; Tang, L.; Gonzalez, V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol. Cancer Ther. 2003, 2, 1045–1052. [Google Scholar] [PubMed]
- Malikova, J.; Swaczynova, J.; Kolar, Z.; Strnad, M. Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry 2008, 69, 418–426. [Google Scholar] [CrossRef]
- Steigerova, J.; Oklestkova, J.; Levkova, M.; Rarova, L.; Kolar, Z.; Strnad, M. Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem. Biol. Interact. 2010, 188, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Oklešt′ková, J.; Rárová, L.; Strnad, M. Brassinosteroids and their biological activities. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3851–3871. [Google Scholar]
- Jadhav, U.; Ezhilarasan, R.; Vaughn, S.F.; Berhow, M.A.; Mohanam, S. Iberin induces cell cycle arrest and apoptosis in human neuroblastoma cells. Int. J. Mol. Med. 2007, 19, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Gerhäuser, C.; You, M.; Liu, J.; Moriarty, R.M.; Hawthorne, M.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Cancer chemopreventive potential of sulforamate, a novel analogue of sulforaphane that induces phase 2 drug-metabolizing enzymes. Cancer Res. 1997, 57, 272–278. [Google Scholar] [PubMed]
- Kisbenedek, A.; Szabo, S.; Polyak, E.; Breitenbach, Z.; Bona, A.; Mark, L.; Figler, M. Analysis of trans-resveratrol in oilseeds by high-performance liquid chromatography. Acta Aliment. 2014, 43, 459–464. [Google Scholar] [CrossRef]
- Jin, J.; Sheraliev, G.; Xie, D.; Zhang, W.; Jin, Q.; Wang, X. Characteristics of specialty natural micronutrients in certain oilseeds and oils: Plastochromanol-8, resveratrol, 5-hydroxytryptamine phenylpropanoid amides, lanosterol, ergosterol and cyclolinopeptides. J. Am. Oil Chem. Soc. 2016, 93, 155–170. [Google Scholar] [CrossRef]
- Jiang, H.; Shang, X.; Wu, H.; Huang, G.; Wang, Y.; Al-Holou, S.; Gautam, S.C.; Chopp, M. Combination treatment with resveratrol and sulforaphane induces apoptosis in human U251 glioma cells. Neurochem. Res. 2010, 35, 152–161. [Google Scholar] [CrossRef]
- Tai, A.; Fukunaga, K.; Ohno, A.; Ito, H. Antioxidative properties of ascorbigen in using multiple antioxidant assays. Biosci. Biotechnol. Biochem. 2014, 78, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Huebbe, P.; Konishi, T.; Rahman, M.M.; Nakahara, M.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of ascorbigen versus ascorbic acid: Studies in vitro and in cultured human keratinocytes. J. Agric. Food Chem. 2008, 56, 11694–11699. [Google Scholar] [CrossRef]
- Stec, B. Plant thionins–the structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef]
- Florack, D.; Stiekema, W. Thionins: Properties, possible biological roles and mechanisms of action. Plant Mol. Biol. 1994, 26, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, J.; Wang, Y.; Zhang, J.; Gao, Y.; Yuan, J.; Su, A.; Ju, X. Study on antioxidant activity and amino acid analysis of rapeseed protein hydrolysates. Int. J. Food Prop. 2016, 19, 1899–1911. [Google Scholar] [CrossRef]
- Ferrero, R.L.; Soto-Maldonado, C.; Weinstein-Oppenheimer, C.; Cabrera-Muñoz, Z.; Zúñiga-Hansen, M.E. Antiproliferative Rapeseed Defatted Meal Protein and Their Hydrolysates on MCF-7 Breast Cancer Cells and Human Fibroblasts. Foods 2021, 10, 309. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Yuan, Q.; Xie, H.; Shi, J.; Ju, X. Separation and purification of an anti-tumor peptide from rapeseed (Brassica campestris L.) and the effect on cell apoptosis. Food Funct. 2016, 7, 2239–2248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.X.; Zhang, T.; He, C.; He, R.; Ju, X.; Wu, X.Y. In Situ Proapoptotic Peptide-Generating Rapeseed Protein-Based Nanocomplexes Synergize Chemotherapy for Cathepsin-B Overexpressing Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10, 41056–41069. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, J.; Wang, Z.; Yao, Y.; Atungulu, G.G.; Ju, X.; Wang, L. Absorption and Metabolism of Peptide WDHHAPQLR Derived from Rapeseed Protein and Inhibition of HUVEC Apoptosis under Oxidative Stress. J. Agric. Food Chem. 2018, 66, 5178–5189. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, Z.; Bu, L.; An, C.; Wang, D.; Liu, X.; Zhang, J.; Yang, W.; Deng, B.; Xie, J. Rapeseed protein-derived antioxidant peptide RAP alleviates renal fibrosis through MAPK/NF-κB signaling pathways in diabetic nephropathy. Drug Des. Dev. Ther. 2018, 12, 1255. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Cruciferous Vegetables, Isothiocyanates and Indoles; IARC: Lyon, France, 2004. [Google Scholar]
- Darmstadt, G.L.; Saha, S.K. Traditional practice of oil massage of neonates in Bangladesh. J. Health Popul. Nutr. 2002, 20, 184–188. [Google Scholar]
- Menon, S. 61 Home Remedies for Cold and Cough in Babies. Available online: http://www.bumpsnbaby.com/21-home-remedies-cold-cough-babies/ (accessed on 2 August 2017).
- Hiltz, S. Annas Herbal an Education in the Healing Power of Herbs. Available online: https://www.yumpu.com/en/document/read/12019238/annas-herbal-by-dr-sharon-hiltz-fairmont-state-university (accessed on 12 September 2019).
- Yardley, K. The Good Living Guide to Natural and Herbal Remedies: Simple Salves, Teas, Tinctures, and More; Good Books: New York, NY, USA, 2016. [Google Scholar]
- Mars, B.; Fiedler, C. The Country Almanac of Home Remedies: Time-Tested & Almost Forgotten Wisdom for Treating Hundreds of Common Ailments, Aches & Pains Quickly and Naturally; Fair Winds Press: Beverly, MA, USA, 2014. [Google Scholar]
- Olesen, J. The Headaches; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- WHO. Cardiovascular Diseases (CVDs) Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)/ (accessed on 11 April 2021).
- Mäkinen, S.; Streng, T.; Larsen, L.B.; Laine, A.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antihypertensive properties of potato and rapeseed protein-derived peptides. J. Funct. Foods 2016, 25, 160–173. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Malomo, S.A.; Girgih, A.T.; Aluko, R.E. Blood pressure lowering effects of Australian canola protein hydrolysates in spontaneously hypertensive rats. Food Res. Int. 2014, 55, 281–287. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Aluko, R.E. Kinetics and molecular docking studies of the inhibitions of angiotensin converting enzyme and renin activities by hemp seed (Cannabis sativa L.) peptides. J. Agric. Food Chem. 2014, 62, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Malomo, S.A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Glycinyl-histidinyl-serine (GHS), a novel rapeseed protein-derived peptide has blood pressure-lowering effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2013, 61, 8396–8402. [Google Scholar] [CrossRef]
- Marczak, E.D.; Ohinata, K.; Lipkowski, A.W.; Yoshikawa, M. Arg-Ile-Tyr (RIY) derived from rapeseed protein decreases food intake and gastric emptying after oral administration in mice. Peptides 2006, 27, 2065–2068. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Muir, A. Comparative structural, emulsifying, and biological properties of 2 major canola proteins, cruciferin and napin. J. Food Sci. 2008, 73, C210–C260. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Iwasaki, M.; Usui, H.; Ohinata, K.; Marczak, E.D.; Lipkowski, A.W.; Yoshikawa, M. Rapakinin, an anti-hypertensive peptide derived from rapeseed protein, dilates mesenteric artery of spontaneously hypertensive rats via the prostaglandin IP receptor followed by CCK1 receptor. Peptides 2010, 31, 909–914. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohinata, K.; Lipkowski, A.W.; Yoshikawa, M. Rapakinin, Arg–Ile–Tyr, derived from rapeseed napin, shows anti-opioid activity via the prostaglandin IP receptor followed by the cholecystokinin CCK2 receptor in mice. Peptides 2011, 32, 281–285. [Google Scholar] [CrossRef]
- He, R.; Aluko, R.E.; Ju, X.-R. Evaluating molecular mechanism of hypotensive peptides interactions with renin and angiotensin converting enzyme. PLoS ONE 2014, 9, e91051. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Rozoy, E.; Bazinet, L.; Ju, X.R.; Aluko, R.E. Selective separation and concentration of antihypertensive peptides from rapeseed protein hydrolysate by electrodialysis with ultrafiltration membranes. Food Chem. 2016, 197, 1008–1014. [Google Scholar] [CrossRef]
- He, R.; Malomo, S.A.; Alashi, A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Purification and hypotensive activity of rapeseed protein-derived renin and angiotensin converting enzyme inhibitory peptides. J. Funct. Foods 2013, 5, 781–789. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Yang, Y.; Wang, Z.; Ju, X.; Yuan, J. Rapeseed protein-derived ACE inhibitory peptides LY, RALP and GHS show antioxidant and anti-inflammatory effects on spontaneously hypertensive rats. J. Funct. Foods 2019, 55, 211–219. [Google Scholar] [CrossRef]
- He, R.; Yang, Y.-J.; Wang, Z.; Xing, C.-r.; Yuan, J.; Wang, L.-F.; Udenigwe, C.; Ju, X.-R. Rapeseed protein-derived peptides, LY, RALP, and GHS, modulates key enzymes and intermediate products of renin–angiotensin system pathway in spontaneously hypertensive rat. Npj Sci. Food 2019, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, D.; Zhang, W.; Xie, X.; Dang, Y.; Gao, X. Identification and molecular mechanism of novel ACE inhibitory peptides from broccoli protein. Food Biosci. 2024, 61, 104678. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.; Uddin, K.N.; Ahsan, K.; Shimu, S.N.; Kobra, K.; Shimu, S.A.; Haque, W.; Rahman, T.; Jessy, T.H. Preliminary study on thrombolytic property of thirty six different extracts of eight Bangladeshi medicinal plants with folkloric relevance. Orient. Pharm. Exp. Med. 2016, 16, 311–319. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Cristofaro, M.; Breschi, M.C.; Calderone, V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 2013, 65, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Hollman, P.C. Flavonoids and cardiovascular health: Which compounds, what mechanisms? Am. J. Clin. Nutr. 2008, 88, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Nguemeni, C.; Delplanque, B.; Rovère, C.; Simon-Rousseau, N.; Gandin, C.; Agnani, G.; Nahon, J.L.; Heurteaux, C.; Blondeau, N. Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke. Pharmacol. Res. 2010, 61, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Bourourou, M.; Heurteaux, C.; Blondeau, N. Alpha-linolenic acid given as enteral or parenteral nutritional intervention against sensorimotor and cognitive deficits in a mouse model of ischemic stroke. Neuropharmacology 2016, 108, 60–72. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, H.; Leak, R.K.; Shi, Y.; Hu, X.; Gao, Y.; Chen, J. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Exp. Neurol. 2015, 272, 170–180. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Melbourne, VIC, Australia, 2002. [Google Scholar]
- Ferreira, I.C.F.R.; Morales, P.; Barros, L. Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; John Wiley & Sons Australia Ltd.: Milton, NSW, Australia, 2016. [Google Scholar]
- Harborne, J.B.; Baxter, H. Chemical Dictionary of Economic Plants; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Macarthur, J.G.; Alstead, S. Counter-irritants a method of assessing their effect. Lancet 1953, 262, 1060–1062. [Google Scholar] [CrossRef]
- Agarwal, Y. Reversing Back Pain: Doctors’ Guide to a Healthy Back; Orient Paperbacks: Delhi, India, 2008. [Google Scholar]
- Wynn, S.G.; Fougère, B. Veterinary Herbal Medicine; Mosby Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Morisset, M.; Moneret-Vautrin, D.A.; Maadi, F.; Fremont, S.; Guenard, L.; Croizier, A.; Kanny, G. Prospective study of mustard allergy: First study with double-blind placebo-controlled food challenge trials (24 cases). Allergy 2003, 58, 295–299. [Google Scholar] [CrossRef]
- Nicholson, W. The British Encyclopedia, or Dictionary of Arts and Sciences Comprising an Accurate and Popular View of the Present Improved State of Human Knowledge; Oxford University: Oxford, UK, 1809. [Google Scholar]
- Manchester, A.-F. Allergy information for: Mustard (Brassica nigra, Brassica juncea, Brassica hirta, Sinapis alba). Available online: http://research.bmh.manchester.ac.uk/informall/allergenic-food/?FoodId=56/ (accessed on 2 August 2017).
- Du Val, J. A Family Manual: In Which Are Found Directions for the Use of His Family Antispasmodic, in More Than Twenty Diseases, to Wit Asiatic Cholera; John W. Woods: Baltimore, MD, USA, 1851. [Google Scholar]
- Yabanoglu, H.; Akbulut, S.; Karakayali, F. Phytocontact dermatitis due to mustard seed mimicking burn injury: Report of a case. Case Rep. Med. 2012, 2012, 519215. [Google Scholar] [CrossRef] [PubMed]
- Dineeen, P.; Homan, W.P.; Grafe, W.R. Tuberculous peritonitis: 43 years’ expereince in diagnosis and treatment. Ann. Surg. 1976, 184, 717. [Google Scholar] [CrossRef]
- Brown, P.O. The Complete Herbalist; Editorial MAXTOR: Valladolid, Spain, 1872. [Google Scholar]
- Hoffmann, D. Healthy Bones and Joints: A Natural Approach to Treating Arthritis, Osteoporosis, Tendinitis, Myalgia & Bursitis; Storey Publishing, LLC.: North Adams, MA, USA, 2017. [Google Scholar]
- Erbay, M.Ş.; Anil, S.; Melikoglu, G. Plants used as painkiller in folk medicine in turkey-istomachache. Marmara Pharm. J. 2017, 21, 741–755. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, A.K.; Gupta, R.K.; Neelabh; Dwivedi, P.D. A comprehensive review on mustard-induced allergy and implications for human health. Clin. Rev. Allergy Immunol. 2017, 57, 39–54. [Google Scholar] [CrossRef]
- Guimaraes, M.Z.; Jordt, S.-E. TRPA1: A sensory channel of many talents. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W., Ed.; Talyor and Francis: Abingdon, UK, 2006; pp. 151–161. [Google Scholar]
- Jordt, S.-E.; Bautista, D.M.; Chuang, H.-h.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Smith, C.H.; Barker, J.; Morris, R.W.; MacDonald, D.M.; Lee, T. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J. Immunol. 1993, 151, 3274–3282. [Google Scholar] [CrossRef]
- Rahman, M.S.; Jahan, N.; Rahman, S.M.A.; Rashid, M.A. Analgesic and antidepressant activities of Brassica rapa subspecies chinensis (L.) Hanelt on Swiss-albino mice model. Bangladesh Med. Res. Counc. Bull. 2016, 41, 7. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.R.; Dutton, S.P.; Truswell, A.S. Salicylates in foods. J Am. Diet. Assoc. 1985, 85, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Food choice as a key management strategy for functional gastrointestinal symptoms. Am. J. Gastroenterol. 2012, 107, 657–666. [Google Scholar] [CrossRef]
- Ateya, A.; Al-Gendy, A.; Kotob, S.; Hafez, A. Chemical constituents, antioxidant, antimicrobial and antiinflammatory activities of Erysimum corinthium boiss (Brassicaceae). Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1601–1609. [Google Scholar]
- da Silva, K.A.S.; Paszcuk, A.F.; Passos, G.F.; Silva, E.S.; Bento, A.F.; Meotti, F.C.; Calixto, J.B. Activation of cannabinoid receptors by the pentacyclic triterpene α, β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain 2011, 152, 1872–1887. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer chemoprevention through dietary antioxidants: Progress and promise. Antioxid. Redox Signal. 2008, 10, 475–510. [Google Scholar] [PubMed]
- Davidson, R.; Jupp, O.; Bao, Y.; MacGregor, A.; Donell, S.; Cassidy, A.; Clark, I. Can sulforaphane prevent the onset or slow the progression of osteoarthritis? Nutr. Bull. 2016, 41, 175–179. [Google Scholar] [CrossRef]
- Sanossian, N.; Saver, J.L.; Navab, M.; Ovbiagele, B. High-density lipoprotein cholesterol. Stroke 2007, 38, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Chamorro, P.A.F.; Riego, G.; Leánez, S.; Pol, O. Treatment with Sulforaphane Produces Antinociception and Improves Morphine Effects during Inflammatory Pain in Mice. J. Pharmacol. Exp. Ther. 2017, 363, 293–302. [Google Scholar] [CrossRef]
- Khaket, T.P.; Redhu, D.; Dhanda, S.; Singh, J. In Silico evaluation of potential DPP-III inhibitor precursors from dietary proteins. Int. J. Food Prop. 2015, 18, 499–507. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.; Greenwald, R.; Hochberg, M. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J. Australian Medical Association Guide to Medicine and Drugs; Reader’s Digest (Australia) Pty Ltd.: Sydney, NSW, Australia, 1990. [Google Scholar]
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis; Medpharm: Guildford, UK, 2004. [Google Scholar]
- Ali, F.B.; Heidari, F. Transformation of manitol 1-phosphate dehydrogenase (MTLD) gene into canola (Brassica napus L.) to improve salinity tolerance. Acta Medica Mediterr. 2017, 33, 935–943. [Google Scholar]
- Al-Snafi, A.E. Chemical constituents and pharmacological importance of Agropyron repens—A review. Res. J. Pharmacol. Toxicol. 2015, 1, 37–41. [Google Scholar]
- Tiyagi, S.A.; Alam, M.M. Efficacy of oil-seed cakes against plant-parasitic nematodes and soil-inhabiting fungi on mungbean and chickpea. Bioresour. Technol. 1995, 51, 233–239. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Rahman, M. Chemical and Biological Screening of Ecbolium linneanum (Family-Acanthaceae) Leaves and Stems Extracts. Ph.D. Thesis, Khulna University, Khulna, Bangladesh, 2014. [Google Scholar]
- Basha, S.N.; Rekha, R.; Saleh, S.; Yemane, S. Evaluation of in vitro anthelmintic activities of Brassica nigra, Ocimum basilicum and Rumex abyssinicus. Pharmacogn. J. 2011, 3, 88–92. [Google Scholar] [CrossRef]
- Sayre, R.; Patrick, Z.; Thorpe, H. Identification of a selective nematicidal component in extracts of plant residues decomposing in soil. Nematologica 1965, 11, 263–268. [Google Scholar]
- Singh, R.; Sitaramaiah, K. Control of plant parasitic nematodes with organic soil amendments. PANS Pest Artic. News Summ. 1970, 16, 287–297. [Google Scholar]
- Butool, F.; Haseeb, A.; Shukla, P. Management of root-knot nematode, Meloidogyne incognita, infesting Egyptian henbane, Hyoscyamus muticus L., by the use of nematicides and oilcakes. Int. J. Pest Manag. 1998, 44, 199–202. [Google Scholar] [CrossRef]
- Linford, M. The feeding of the root-knot nematode in root tissue and nutrient solution. Phytopathology 1937, 27, 824–835. [Google Scholar]
- Quiroz-Castañeda, R.E.; Dantán-González, E. Control of avian coccidiosis: Future and present natural alternatives. BioMed Res. Int. 2015, 2015, 430610. [Google Scholar] [CrossRef] [PubMed]
- VanderJagt, D.J.; Freiberger, C.; Vu, H.-T.N.; Mounkaila, G.; Glew, R.S.; Glew, R.H. The trypsin inhibitor content of 61 wild edible plant foods of Niger. Plant Foods Hum. Nutr. 2000, 55, 335–346. [Google Scholar] [CrossRef]
- Woods, D.; Downey, R. Mucilage from yellow mustard. Can. J. Plant Sci. 1980, 60, 1031–1033. [Google Scholar] [CrossRef]
- Bown, D. Encyclopaedia of Herbs and Their Uses; Dorling Kindersley: London, UK, 1995. [Google Scholar]
- Ghanbari, R.; Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I converting enzyme (ACE) inhibitory and anti-oxidant activities of sea cucumber (Actinopyga lecanora) hydrolysates. Int. J. Mol. Sci. 2015, 16, 28870–28885. [Google Scholar] [CrossRef]
- Esposito, D.; Komarnytsky, S.; Shapses, S.; Raskin, I. Anabolic effect of plant brassinosteroid. FASEB J. 2011, 25, 3708–3719. [Google Scholar] [CrossRef]
- Mitrović, P.M.; Stamenković, O.S.; Banković-Ilić, I.; Djalović, I.G.; Nježić, Z.B.; Farooq, M.; Siddique, K.H.M.; Veljković, V.B. White Mustard (Sinapis alba L.) Oil in Biodiesel Production: A Review. Front. Plant Sci. 2020, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, G.; Heaney, R. Glucosinolates and their breakdown products in cruciferous crops, foods and feedingstuffs. Food Chem. 1983, 11, 249–271. [Google Scholar]
- Malik, A.H.; Khuroo, A.A.; Dar, G.; Khan, Z. Ethnomedicinal uses of some plants in the Kashmir Himalaya. Indian J. Tradit. Knowl. IJTK 2011, 10, 28. [Google Scholar]
- Nameth, I. Medicinal Plants and Drugs; Europian Union: Brussels, Belgium, 2012. [Google Scholar]
- Takeguchi, N.; Nishimura, Y.; Watanabe, T.; Mori, Y.; Morii, M. A pungent ingredient of mustard, allylisothiocyanate, inhibits (H+ + K+)-ATPase. Biochem Biophys. Res. Commun. 1983, 112, 464–468. [Google Scholar] [CrossRef]
- Agarwal, K.; Gupta, A.; Pushkarna, R.; Bhargava, S. Effects of massage & use of oil on growth, blood flow & sleep pattern in infants. Indian J. Med. Res. 2000, 112, 212. [Google Scholar]
- Paulún, F. 50 Ways to Boost Your Metabolism: How Mustard, Red Wine, and Days at the Beach Can Help You Lose Weight & Stay Healthy; Skyhorse Publishing: New York, NY, USA, 2013. [Google Scholar]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; Aluko, R.E.; Strappe, P. Effects of canola proteins and hydrolysates on adipogenic differentiation of C3H10T/2 mesenchymal stem cells. Food Chem. 2015, 185, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Aachary, A.A.; Thiyam, U. A pursuit of the functional nutritional and bioactive properties of canola proteins and peptides. Crit. Rev. Food Sci. Nutr. 2012, 52, 965–979. [Google Scholar]
- Lim, J.-S.; Im, J.-H.; Han, X.; Men, X.; Oh, G.; Fu, X.; Hwang, W.; Choi, S.-I.; Lee, O.-H. The Mechanism of the Anti-Obesity Effects of a Standardized Brassica juncea Extract in 3T3-L1 Preadipocytes and High-Fat Diet-Induced Obese C57BL/6J Mice. Nutrients 2024, 16, 846. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Kim, H.-D.; Lee, Y.-H. Anti-obesity Effects of Treatment with Air-classified Brassica juncea L. Extract in High-Fat Diet-Induced Obese C57BL/6J Mice. J. Agric. Life Environ. Sci. 2021, 33, 379–390. [Google Scholar]
- Lee JaeJoon, L.J.; Kim HyunA, K.H.; Lee JooMin, L.J. The effects of Brassica juncea L. leaf extract on obesity and lipid profiles of rats fed a high-fat/high-cholesterol diet. Nutr. Res. Pract. 2018, 12, 298–306. [Google Scholar] [PubMed]
- Jamila, N.; Khan, N.; Hwang, I.M.; Choi, J.Y.; Nho, E.Y.; Khan, S.N.; Atlas, A.; Kim, K.S. Determination of macro, micro, trace essential, and toxic elements in Garcinia cambogia fruit and its anti-obesity commercial products. J. Sci. Food Agric. 2019, 99, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zheng, D.; He, L.; Chen, J. The Lipid-Metabolism-Associated Anti-Obesity Properties of Rapeseed Diacylglycerol Oil. Nutrients 2024, 16, 2003. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.; Keshavarz, S.A.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Amini, H.; Chamari, M.; Djazayery, A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010, 178, 112–115. [Google Scholar] [CrossRef]
- Pace, T.W.; Hu, F.; Miller, A.H. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007, 21, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar]
- Aubourg, P.; Adamsbaum, C.; Lavallard-Rousseau, M.-C.; Rocchiccioli, F.; Cartier, N.; Jambaque, I.; Jakobezak, C.; Lemaitre, A.; Boureau, F.; Wolf, C. A two-year trial of oleic and erucic acids (“Lorenzo’s oil”) as treatment for adrenomyeloneuropathy. N. Engl. J. Med. 1993, 329, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Poulos, A.; Gibson, R.; Sharp, P.; Beckman, K.; Rattan-Smith, P.G. Very long chain fatty acids in X-linked adrenoleukodystrophy brain after treatment with Lorenzo’s oil. Ann. Neurol. 1994, 36, 741–746. [Google Scholar] [CrossRef]
- Aubourg, P. Efficacy of Lorenzo’s Oil in Adrenoleukodystrophy. CNS Drugs 1994, 1, 323–329. [Google Scholar] [CrossRef]
- Jackson, C.; Paliyath, G. Functional Foods and Ingredients. In Handbook of Food Products Manufacturing: Principles, Bakery, Beverages, Cereals, Cheese, Confectionary, Fats, Fruits, and Functional Foods; Hui, Y., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Pałgan, K.; Żbikowska-Gotz, M.; Bartuzi, Z. Dangerous anaphylactic reaction to mustard. Arch Med. Sci. 2018, 14, 477–479. [Google Scholar] [CrossRef]
- Sirvent, S.; Akotenou, M.; Cuesta-Herranz, J.; Vereda, A.; Rodríguez, R.; Villalba, M.; Palomares, O. The 11S globulin Sin a 2 from yellow mustard seeds shows IgE cross-reactivity with homologous counterparts from tree nuts and peanut. Clin. Transl. Allergy 2012, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.-Y.; Wanasundara, J.P. Quantitative detection of allergenic protein Sin a 1 from yellow mustard (Sinapis alba L.) seeds using enzyme-linked immunosorbent assay. J. Agric. Food Chem. 2008, 56, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Oñaderra, M.; Monsalve, R.I.; Mancheño, J.M.; Villalba, M.; Pozo, A.M.; Gavilanes, J.G.; Rodriguez, R. Food mustard allergen interaction with phospholipid vesicles. Eur. J. Biochem. 1994, 225, 609–615. [Google Scholar] [PubMed]
- Puumalainen, T.J.; Puustinen, A.; Poikonen, S.; Turjanmaa, K.; Palosuo, T.; Vaali, K. Proteomic identification of allergenic seed proteins, napin and cruciferin, from cold-pressed rapeseed oils. Food Chem. 2015, 175, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Untersmayr, E.; Jensen-Jarolim, E. Mechanisms of type I food allergy. Pharmacol. Ther. 2006, 112, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.; Singh, S.; Brito, M. Thyroid, diet, and alternative approaches. J. Clin. Endocrinol. Metab. 2022, 107, 2973–2981. [Google Scholar]
- Richards, I.S.; Bourgeois, M. Principles and Practice of Toxicology in Public Health; Jones & Bartlett Learning, LLC: Burlington, MA, USA, 2013. [Google Scholar]
- Felker, P.; Bunch, R.; Leung, A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in Brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016, 74, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, G.; Jansman, A. Use of EC produced oil seeds in animal feeds. Recent Adv. Anim. Nutr. 1994, 128, 31–56. [Google Scholar]
- Khan, R.A.; Asad, T.; Feroz, Z.; Ahmed, M. In vivo examination of the anticoagulant effect of the Brassica oleracea methanol extract. Arch. Biol. Sci. 2015, 67, 631–638. [Google Scholar] [CrossRef]
- Smith, R.; Watt, W.; Lawson, W.; Rice-Evans, C. Red cell membrane changes in a Heinz body anaemia (kale anaemia). In Protides of the Biological Fluids; Elsevier: Amsterdam, The Netherlands, 1982; Volume 29, pp. 125–128. [Google Scholar]
- Braine, K. Grazing Brassicas and Livestock Health. Available online: https://www.lls.nsw.gov.au/regions/murray/articles,-plans-and-publications/production-advice-june-2021/grazing-brassicas-and-livestock-health (accessed on 8 August 2024).
- Benevenga, N.; Case, G.; Steele, R. Occurrence and metabolism of S-methyl-L-cysteine and S-methyl-L-cysteine sulfoxide in plants and their toxicity and metabolism in animals. Toxic. Plant Orig. 1989, 3, 203–228. [Google Scholar]
- Gustine, D.L. Determination of S-methyl cysteine sulfoxide in Brassica extracts by high-performance liquid chromatography. J. Chromatogr. A 1985, 319, 450–453. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The importance of thiamine (vitamin B1) in humans. Biosci. Rep. 2023, 43, BSR20230374. [Google Scholar]
- FitzGerald, G.A.; Healy, C.; Daugherty, J. Thromboxane A2 biosynthesis in human disease. Fed. Proc. 1987, 46, 154–158. [Google Scholar] [PubMed]
- Hossain, M.K.; Khatun, A.; Rahman, M.; Akter, M.N.; Chowdhury, S.A.; Alam, S.M.M. Characterization of the effect of drug-drug interaction on protein binding in concurrent administration of sulfamethoxazol and diclofenac sodium using bovine serum albumin. Adv. Pharm. Bull. 2016, 6, 589–595. [Google Scholar] [CrossRef]
- Ijiri, Y.; Ishii, H.; Yamamoto, J. Diet of fruits and vegetables with experimental antithrombotic effect may be beneficial to humans in the prevention of arterial thrombotic diseases. Int. J. Drug Dev. Res. 2016, 8, 12–16. [Google Scholar]
- Arjariya, A.; Chaurasia, K. Some medicinal plants among the tribes of Chhatarpur district (MP) India. Ecoprint. Int. J. Ecol. 2009, 16, 43–50. [Google Scholar] [CrossRef]
- Akbari, A.; Wu, J. Cruciferin nanoparticles: Preparation, characterization and their potential application in delivery of bioactive compounds. Food Hydrocoll. 2016, 54, 107–118. [Google Scholar] [CrossRef]
- Aluko, R.E.; McIntosh, T. Polypeptide profile and functional properties of defatted meals and protein isolates of canola seeds. J. Sci. Food Agric. 2001, 81, 391–396. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O. Technological and bioactive functionalities of canola meal proteins and hydrolysates. Food Rev. Int. 2013, 29, 231–260. [Google Scholar]
- Wang, F.; Yang, Y.; Ju, X.; Udenigwe, C.C.; He, R. Polyelectrolyte complex nanoparticles from chitosan and acylated rapeseed cruciferin protein for curcumin delivery. J. Agric. Food Chem. 2018, 66, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, R.X.; Zhang, C.; Dai, C.; Ju, X.; He, R. Fabrication of stable and self-assembling rapeseed protein nanogel for hydrophobic curcumin delivery. J. Agric. Food Chem. 2019, 67, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Berhow, M.; Jeffery, E. Comparison of bioactivity between sulforaphane and neoglucobrassicin hydrolysis product in murine and human cell lines (372.5). FASEB J. 2014, 28, 372–375. [Google Scholar] [CrossRef]
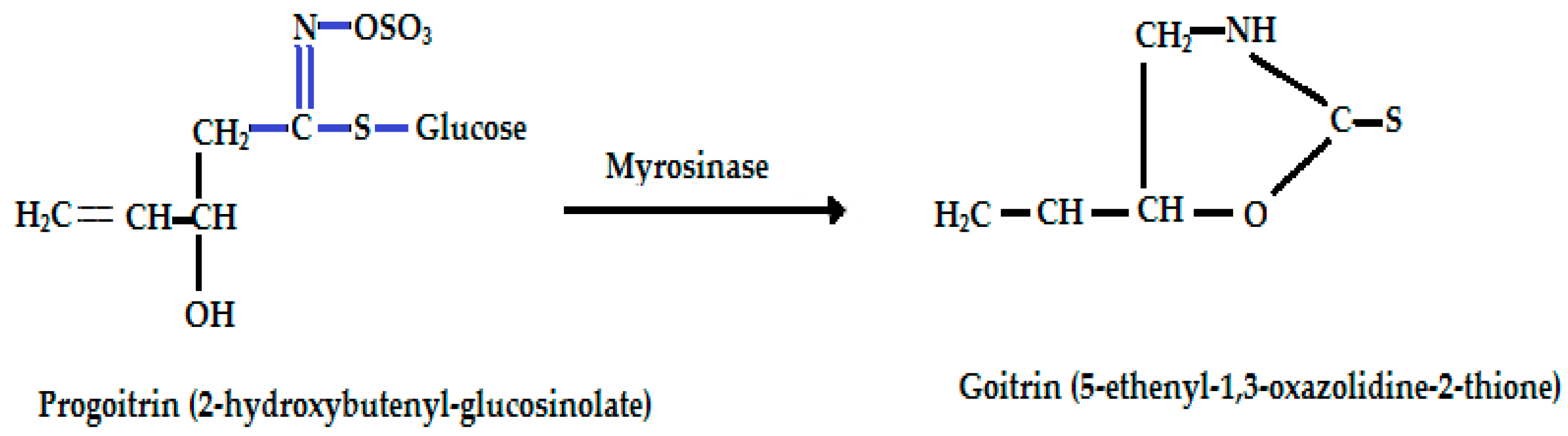
| Scientific Name | Common Name | Habitat | References |
|---|---|---|---|
| Alliaria petiolata (M. Bieb.) Cavara and Grande, Syn: Alliaire officinalis | Garlic mustard, hedge garlic mustard, and jack-by-the-hedge mustard. | A biennial herb, native to various regions of Africa, temperate and tropical Asia, and Europe, and naturalized to the United States and Canada. | [5,6,7] |
| Brassica rapa var. campestris L. | Field mustard, wild turnip mustard, bird rape, turnip rape, and winter mustard. | A winter annual or biennial plant native to central Asia and Europe. | [8,9,10,11] |
| Brassica carinata A. Braun. | Ethiopian mustard and Abyssinian mustard. | Traditional African vegetable cultivated in Ethiopian highlands. | [7,12] |
| Sinapis alba Boiss. (Hook f. & Th.), Syn: B. hirta Moench, B. alba Linn. | White mustard, yellow mustard, and rai mustard. | Best known mustard in Europe, grown in the Mediterranean region, India, and China. | [13,14] |
| Brassica juncea (L.) Czerniak. Coss., Syn: B. integrifolia | Brown mustard, Asian mustard, Oriental Mustard, Chinese mustard, Indian mustard, leaf mustard, giant red, sarepta mustard, Asiatic mustard, mustard green, and wild Brazilian mustard. | An annual herb native to Eastern and Southern Asia. It is widely cultivated throughout India, central Africa, south Russia, and steppes of the northeast Caspian Sea. It is a natural hybrid of B. rapa and B. nigra. | [15,16,17,18,19] |
| Brassica napus (var. napus or var. oleifera) | Rapeseed, oilseed rape, colza, rutabaga, swede, Swedish turnip, leaf rape winter oilseed rape, and summer rape. | Native to South-East Asia and Eurasia and is most commonly grown in northern temperate regions including northern Asia, Japan, Korea, Northern China, Scandinavia, and Russia. Dominant species in Europe. | [8,20,21,22,23] |
| Brassica nigra (L.) W.D.J. Koch. Syn.: Sinapis nigra, B. sinapioides | Black mustard, brown mustard, cadlock, Scurvy mustard, Senvil mustard, short-pod mustard, true mustard, and warlock. | An annual herb native to most parts of Europe, the Mediterranean region, and other parts of north Africa, and has been naturalized in Great Britain and North America. | [15,24,25,26,27] |
| Brassica rapa L. | Oilseed rape, rape oilseed, kale rape, rapa, rappi, keblock, colza, bird rape, rape mustard, field mustard, or rapeseed. | The most cultivated oilseed in many countries like Australia, Canada, and Poland. | [28,29] |
| Calepina irregularis (Asso) Thellung. | White ball mustard, smooth ball mustard. | Native to the countries in central Asia, the Mediterranean basin and naturalized in central Europe, North America, and Australia. | [30,31] |
| Camelina sativa | Camelina, false flax, wild flax, gold-of-pleasure, linseed dodder, German sesame, and Siberian oilseed. | Central Asia and northern Europe | [32,33] |
| Erysimum corinthium | Wormseed mustard and wallflower. | Native to temperate Eurasia (Southeastern Europe), North Africa and Macaronesia, and North America south to Costa Rica. | [34] |
| Erysimum repandum L. | Spreading wallflower, bushy wallflower, and spreading treacle-mustard. | NF | |
| Neslia paniculata (L.) Desv. Syn.: Myagrum paniculatum | Ball mustard, common ball mustard, yellow weed, neslia, neslie, or moutarde. | Native Eurosiberian southern-temperate species commonly naturalized in South Australia, Canada, Europe, and Asia, South America. | [35,36] |
| Sisymbrium officinale L. Scop., Syn.: Erysimum officinale | Erysimum, English watercress, mustard, hedge mustard, St. Barbara’s Hedge Mustard, common hedge, singer’s plant, and thalictroc. | An annual or biennial mustard found on roadsides and wastelands as a weed of arable land in Eurasia, the Mediterranean area, northern Africa, Scandinavia to north Africa, and Asia and naturalized in New Zealand. | [37,38,39,40,41] |
| Sisymbrium orientale Syn.: S. columnae Jacq. | Asian hedge mustard, Indian hedge mustard, eastern rocket, oriental wild rocket, and London rocket. | Native to Europe, Asia, and north Africa, and further introduced in much of the rest of the world, including parts of North America and Australia. | [37,42,43] |
| Sisymbrium erysimoides Desf. | Smooth mustard, and Mediterranean rocket. | A desert plant native to Middle Eastern Arab countries and naturalized in Australia, North America, and New Zealand. | [44,45,46] |
| Fatty Acid | Alliaria petiolata | Sinapis alba | B. carinata | B. juncea | B. napus | B. nigra | B. rapa | Calepina irregularis | Camelina sativa | Erysimum repandum | Neslia paniculata | Sisymbrium erysimoides | S. officinale | S. orientale | Canola (low-erucic acid) B. napus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total oil content (% od seed weight) | 15–30 | 25–36 | 30–47 | 25–45 | 40–50 | 21–40 | 25–45 | 16–36 | 38–43 | 20.1–40 | 15–25 | 30 | 7–12 | 22–30 | 33–50 |
| Saturated fatty acids | |||||||||||||||
| Palmitic acid (C16:0) | 3–4 | 2–7 | 2–12 | 2–4.5 | 2–7 | 2–4 | 2–4.5 | 0–15 | 4–8 | 7.0 | 3–17 | 10–15 | 2–8.0 | 8–10 | 3.3–6 |
| Stearic acid (C18:0) | 0.3–0.5 | 0.1–3 | 2–3.5 | 1–2 | 0.5–2.5 | 1–2 | 1.5–2.5 | 2–3 | 2.1–2.3 | 2.0 | 1–4 | 0.5–1 | 0.3–1.5 | 0.1–1 | 1.1–2.5 |
| Behenic acid (C22:0) | ND | 0.5–1 | ND | 0–1 | 0–1 | ND | ND | ND | 0.3 | 0.7 | ND | ND | ND | ND | ~2.0 |
| Lignoceric acid (C24:0) | ND | ND | ND | 0–1 | 0–1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ~0.2 |
| Arachidic acid (C20:0) | 0.4–1 | 0.5–1 | ND | 0.5–1.5 | 0.1–2.5 | 0.5–1.5 | ND | ND | 1–1.8 | 2.0 | ND | ND | ND | ND | 0.2–0.8 |
| Unsaturated fatty acids | |||||||||||||||
| Monounsaturated fatty acids | |||||||||||||||
| Palmitoleic acid (C16:1) | ND | 0.1–0.2 | 0.1–0.2 | 0.2–0.5 | 0.2–1.5 | 0.1–0.2 | 0.1–2.0 | ND | 0.07–0.1 | ND | ND | ND | ND | ND | 0.1–0.6 |
| Oleic acid (C18:1) | 6–9 | 15–39 | 7–19 | 8–33 | 8–63 | 9–23 | 11–62 | 0.1–2 | 12.7–14.8 | 7.0 | 9–36 | 10–15 | 1–7.5 | 3–7 | 52–66.9 |
| Gadoleic/eicosenoic acid (20:1) | 4–5 | 6–12 | 5–9 | 5–12 | 1–13 | 5–8 | 1–11 | ND | 11.1–12.8 | 9.0 | ND | 3–4 | ND | 1–4 | ND |
| Erucic acid (C22:1) | 41–49 | 23–51 | 35–45 | 22–55 | 14–55 | 27–46 | 9–51 | 3–7 | 2.3–3.5 | 17.0 | 0.1–1 | 15–20 | 8–17 | 11–18 | 0.3–1.6 |
| Nervonic acid (C24:1) | 6–8 | 1.2–3 | ND | 0–2.5 | 0–2 | ND | ND | 0.1–1 | ND | 1.0 | 0.1–0.3 | ND | 0.5–1.5 | 1–0.6 | ND |
| Polyunsaturated fatty acids | |||||||||||||||
| Linoleic acid (C18:2) | 18–22 | 7–33 | 15–22 | 12–21 | 12–21 | 6–17 | 10–13 | ND | 2.4–24.3 | 17.0 | ND | 15–20 | ND | 10–14 | 16.1–28.8 |
| Eicosadienoic acid (C20:2) | 0.5–1 | ND | ND | 0.3–1 | 0.5 | 3–4 | ND | ND | 11.1–12.8 | 2.0 | ND | ND | ND | ND | ~0.1 |
| Linolenic acid (C18:3) | 4–7 | 8–20 | 16–20 | 8–20 | 8–14 | 3–15 | 1–17 | 7–26 | 33.7–36.9 | 33.0 | 4–10 | 20–31 | 18–30 | 35–42 | 6.4–14.1 |
| Roughanic acid (C16:3) | ND | ND | ND | ND | 0.7–1 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Other fatty acids | 1.7 | 0.1–2.2 | ND | 6–8 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Total MUFA | ND | 72.19 | 62.49 | 66.56 | ND | 60.8 | ND | 46.5 | 30–36 | ND | ND | 38.4 | 39.8 | ND | ND |
| Total PUFA | ND | 2.05 | 32.65 | 29.59 | ND | 31.5 | ND | 39.6 | 50–60 | ND | ND | 38.9 | 47.2 | ND | ND |
| Total SFA | ND | 4.48 | 4.57 | 3.82 | ND | 6.7 | ND | 13.5 | 13.3 | ND | ND | 21.4 | 13 | ND | <7 |
| TUFA/TSFA | ND | ND | ND | 10.4 | ND | 13.8 | ND | 6.5 | ND | ND | ND | 3.6 | 6.7 | ND | ND |
| Total polyphenol content | ND | 72.56 | 23.5 | 21.02 | 47.48 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| References | [92] | [93] | [93] | [93,94] | [95] | [94] | [86] | [31,94] | [96,97,98] | [35] | [94] | [35] | [38,39,94] | [99] | [63,100,101,102] |
| Vitamin | Mustard Species (Amount per 100 g) | References |
|---|---|---|
| Vitamin A/carotenoid | Alliaria petiolata (contains more vitamin A than spinach, 13.3 mg), Sianpis alba, B. juncea (27 mg), B. nigra, Sisymbrium erysimoides | [16,19,35,44,122,123,124,125] |
| Vitamin B Complex | [126] | |
| Thiamine | B. napus (0.8 mg) | [126] |
| Riboflavin | B. napus (0.3 mg) | [126] |
| Niacin | B. napus (8.1 mg) | [126] |
| Pyridoxine | B. napus (1.9 mg) | [126] |
| Vitamin C/Ascorbic acid | Alliaria petiolata (contains more vitamin C than orange—261 mg), B. nigra, Erysimum repandum, S. officinale, B. juncea (72–89 mg) | [6,122,123,124,125,127], USDA National Nutrient data base |
| Vitamin E/tocopherols (α-, β-, γ-, and δ-tocopherol, γ tocopherol is predominant) | Sinapis alba, Canola (B. napus) seed oil: (α tocopherols 12 mg, γ tocopherols 21.3 mg) | [35,128] |
| Vitamin K | All rapeseeds including B. juncea (0.3 mg). Canola seed (B. napus) oil (70–150 µg) | [16,129], USDA National Nutrient data base, [130,131] |
| Traditional Use | Mustards | References |
|---|---|---|
| Anti-microbial activity | Alliaria petiolata, Sisymbrium officinale, S. erysimoides, B. hirta, and B. nigra | [6,39,123,158,216,250,251,252] |
| Antidiabetic activity | B. juncea, and B. nigra | [253,254] |
| Treatment for vitamin C deficiency | A. petiolata, B. rapa, B. napus, and Erysimum repandum are antiscorbutic | [6,21,123,255] |
| Diuretic activity | A. petiolata, B. juncea, B. napus, B. nigra, B. rapa, S. officinale, and S. orientale | [6,21,38,40,41,123,255,256,257,258,259] |
| Expectorant activity | A. petiolata, S. orientale, S. officinale, and S. erysimoides | [6,38,40,41,258,259,260,261] |
| Stimulant activity | A. petiolata, S. alba, B. juncea, and B. nigra | [6,256,257,262] |
| Analgesic activity | B. rapa var. campestris, B. juncea, B. napus, S. erysimoides, S. officinale, B. carinata, Neslia paniculata, and Calepina irregularis | [21,44,123,160,242,256,263,264] |
| Activity in cold and flu | Sinapis alba, S. officinale, S. erysimoides, B. napus, and B. nigra | [39,251,258,260,261] |
| Anti-catarrhal activity | Sinapis alba, S. officinale, S. erysimoides, B. napus, and B. nigra | [39,251,258,260,261,265] |
| Bronchitis | S. officinale, S. orientale, and S. erysimoides | [259,260,261] |
| Anti-asthmatic activity | S. officinale | [38,39,40,41,258] |
| Emetic activity | Sinapis alba, B. nigra, B. juncea, S. officinale, and B. nigra | [123,256,266] |
| Anti-cancer activity | B. juncea, B. napus, B. rapa, S. officinale, and B compestris | [39,123,158,256,267] |
| Effect on bowl | Sinapis alba, B. nigra, B. juncea, and S. officinale are used as laxatives. B. nigra is used as a carminative | [123,256,257,262,266] |
| Rubefacient | B. rapa, B. juncea, and S. officinale | [24,30,39,256,268] |
| Galactagogue | B. juncea | [256] |
| Anti-gout potential | B. napus, and B. rapa | [21,123,255] |
| Use in gall stone | B. napus, and B. rapa | [162,255,265] |
| Use against alopecia | B. nigra | [257] |
| Anti-dandruff activity | B. nigra | [257] |
| Use in neuralgia | B. nigra | [257] |
| Anti-spasmodic activity | B. nigra, and S. officinale | [257] |
| Aphrodisiac activity | B. rapa, and B. nigra | [24,268] |
| Use in hepatic and kidney colic | B. rapa | [162,255] |
| Anti-inflammatory activity | B. rapa, S. erysimoides, and S. officinale | [44,255,269] |
| Anthelmintic activity | S. orientale, and B. rapa var. campestris | [259,270] |
| Remedial use in fever | S. orientale, and Erysimum repandum | [127,259] |
| Use in dysentery | S. orientale | [259] |
| Anti-addiction activity | S. officinale | [271] |
| Use in the disorders voice and throat | S. officinale | [40] |
| Appetising and digestive activities | S. officinale, B. nigra, and B. juncea | [40,41,256,257,258] |
| Snake bite antidote | B. rapa var. campestris, and S. officinale | [40,41,258,270] |
| Skin disorders | Neslia paniculata | [35,263] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae Mustards: Phytochemical Constituents, Pharmacological Effects, and Mechanisms of Action against Human Disease. Int. J. Mol. Sci. 2024, 25, 9039. https://doi.org/10.3390/ijms25169039
Rahman M, Khatun A, Liu L, Barkla BJ. Brassicaceae Mustards: Phytochemical Constituents, Pharmacological Effects, and Mechanisms of Action against Human Disease. International Journal of Molecular Sciences. 2024; 25(16):9039. https://doi.org/10.3390/ijms25169039
Chicago/Turabian StyleRahman, Mahmudur, Amina Khatun, Lei Liu, and Bronwyn J. Barkla. 2024. "Brassicaceae Mustards: Phytochemical Constituents, Pharmacological Effects, and Mechanisms of Action against Human Disease" International Journal of Molecular Sciences 25, no. 16: 9039. https://doi.org/10.3390/ijms25169039







