Systematic Investigation of Aluminum Stress-Related Genes and Their Critical Roles in Plants
Abstract
1. Introduction
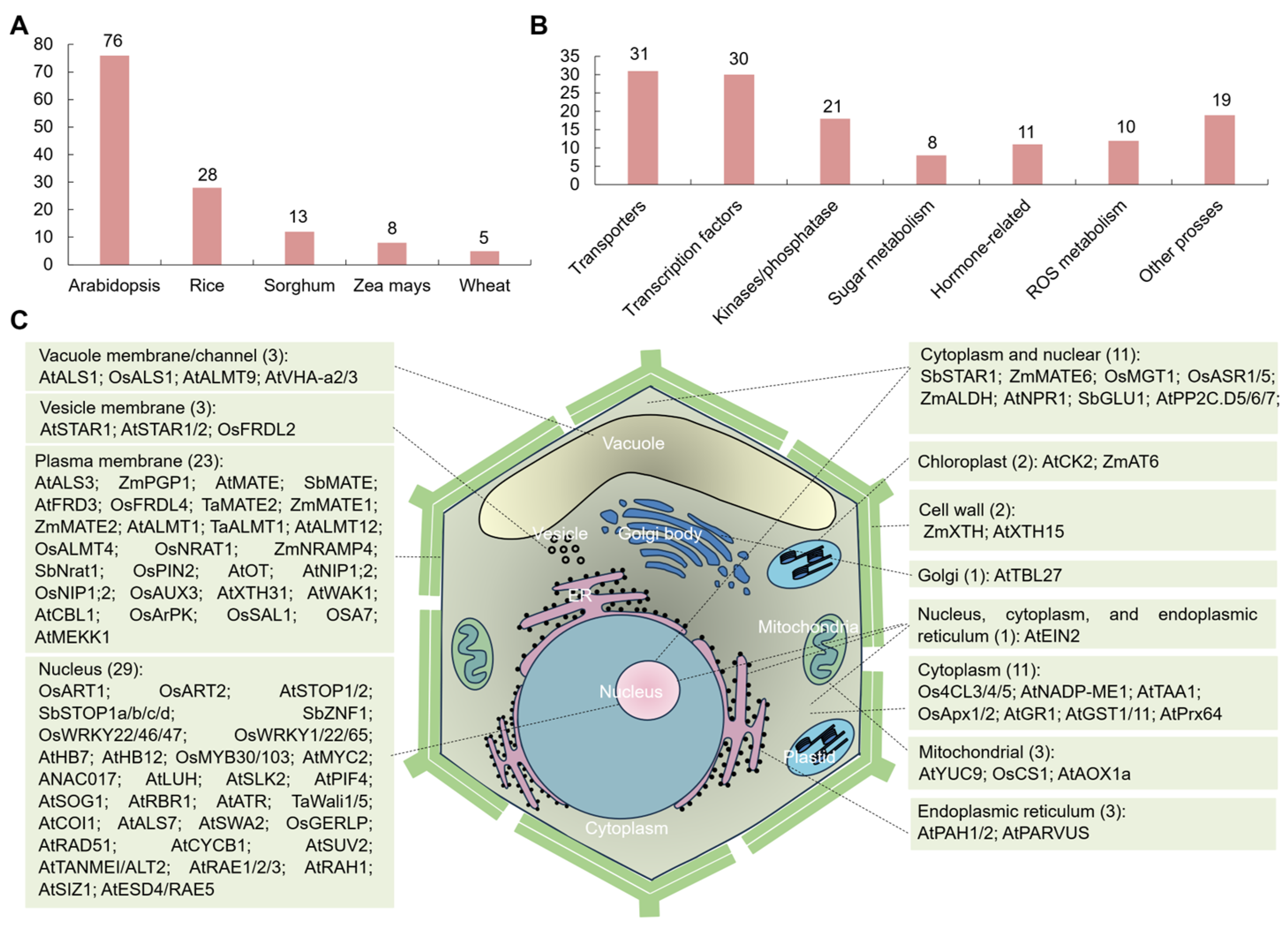
2. Overview of Al-SR Genes in Plants
3. Al-SR Genes and Their Essential Roles in Plants
3.1. Transporters
3.2. Transcription Factor
3.3. Kinases/Phosphatase
3.4. Sugar Metabolism
3.5. Hormone-Related Genes
3.6. ROS Metabolism
3.7. Other Processes
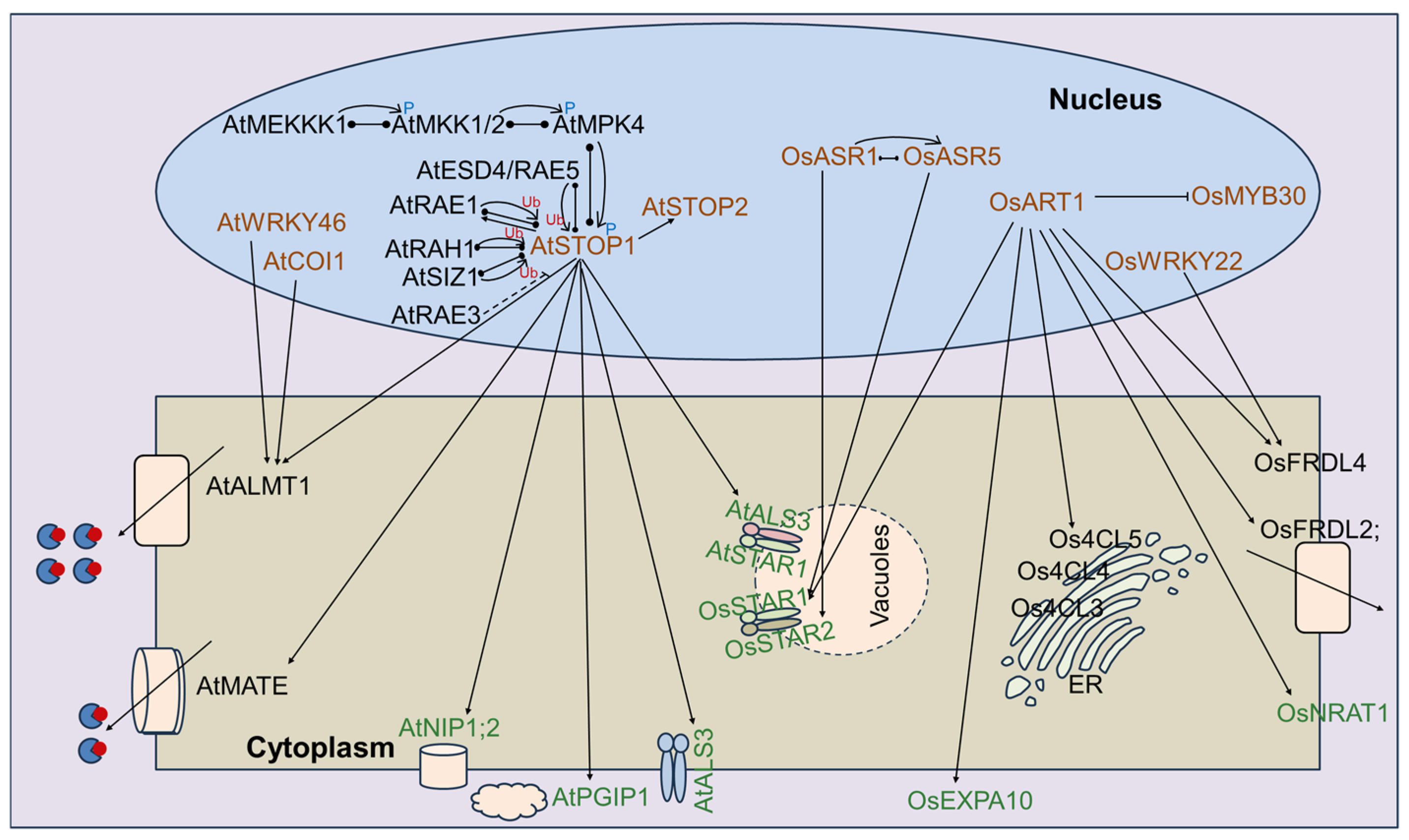
4. The Primary Molecular Regulatory Network for the Cloned Al Stress-Related Genes in Plants
4.1. STOP1-Related Pathway in Arabidopsis
4.2. ART1-Related Pathway in Rice
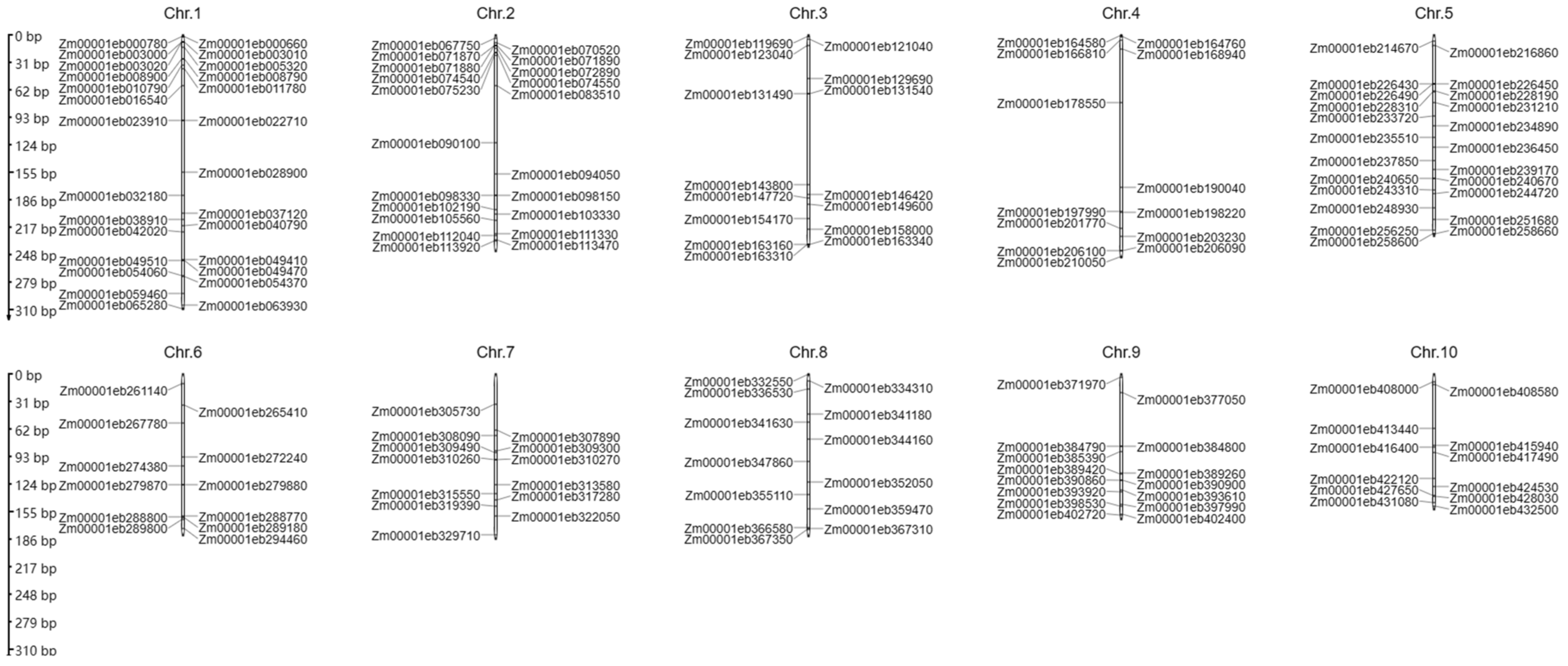
5. Prediction of Putative Al Stress-Related Genes in Maize
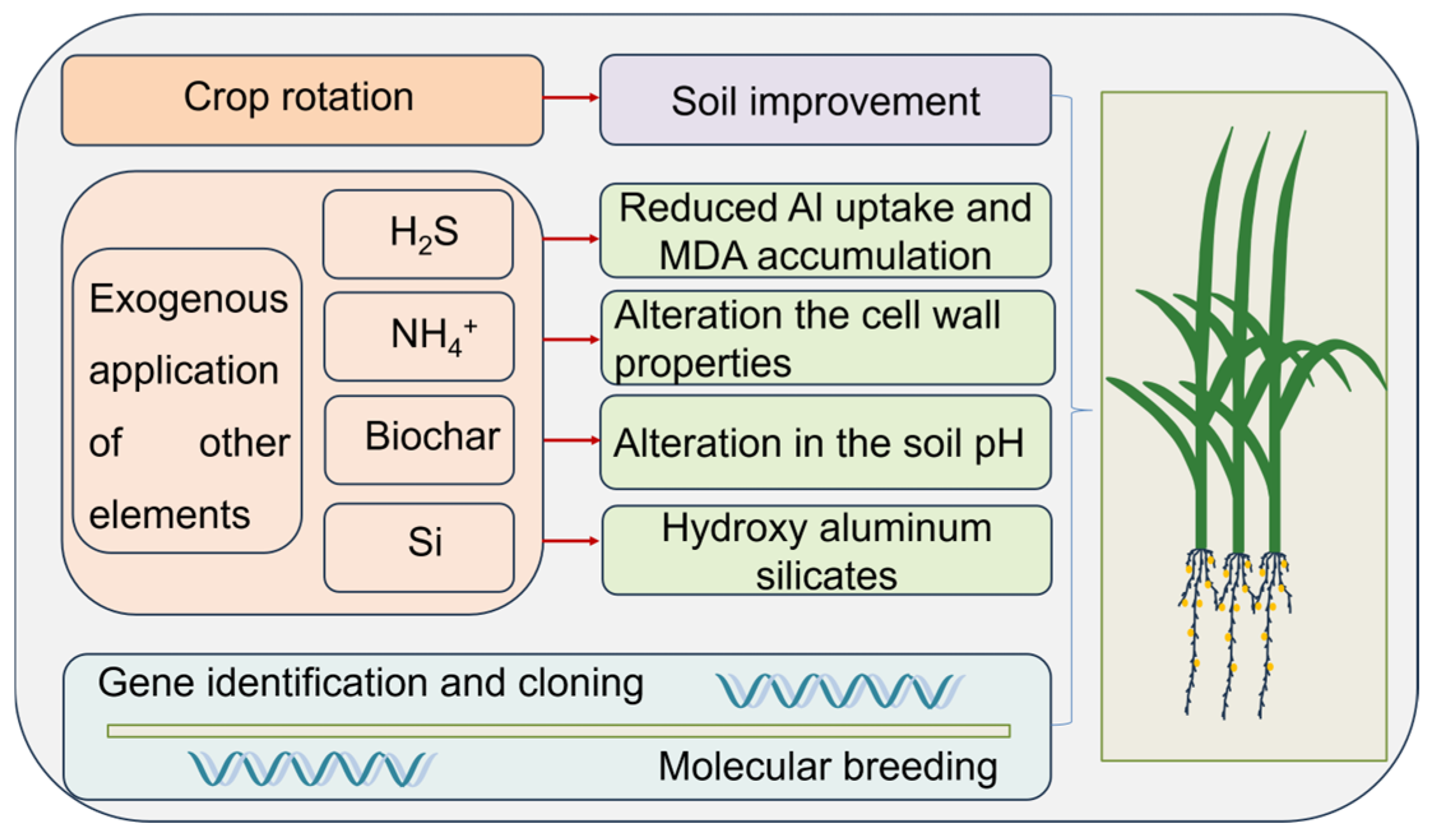
6. Potential Applications to Alleviate Al Stress in Crop Production
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, M.; Yuan, M.M.; Wang, E.; Bai, Y.; Crowther, T.W.; Zhou, J.; Ma, Z.; Zhang, L.; Wang, Y.; et al. Root microbiota confers rice resistance to aluminium toxicity and phosphorus deficiency in acidic soils. Nat. Food 2023, 4, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 2007, 264, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.K.; Yadav, V.; Vaculik, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Horst, W.J.; Wang, Y.; Eticha, D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Guo, J.; Zhou, F.; Singh, S.; Xu, X.; Xie, Q.; Yang, Z.; Huang, C. F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, S.; Tian, H.; Wu, W.; Robert, H.S.; Ding, Z. Local transcriptional control of YUCCA regulates auxin promoted root-growth inhibition in response to aluminium stress in Arabidopsis. PLoS Genet. 2016, 12, e1006360. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, X.; Han, X.; Tang, R.; Chu, M.; Yang, Y.; Yang, Y.; Zhao, F.; Fu, A.; Luan, S.; et al. A defective vacuolar proton pump enhances aluminum tolerance by reducing vacuole sequestration of organic acids. Plant Physiol. 2019, 181, 743–761. [Google Scholar] [CrossRef]
- Che, J.; Tsutsui, T.; Yokosho, K.; Yamaji, N.; Ma, J.F. Functional characterization of an aluminum (Al)-inducible transcription factor, ART2, revealed a different pathway for Al tolerance in rice. New Phytol. 2018, 220, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Arbelaez, J.D.; Maron, L.G.; Jobe, T.O.; Pineros, M.A.; Famoso, A.N.; Rebelo, A.R.; Singh, N.; Ma, Q.; Fei, Z.; Kochian, L.V.; et al. ALUMINUM RESISTANCE TRANSCRIPTION FACTOR 1 (ART1) contributes to natural variation in aluminum resistance in diverse genetic backgrounds of rice (O. sativa). Plant Direct. 2017, 1, e00014. [Google Scholar] [CrossRef]
- Godon, C.; Mercier, C.; Wang, X.; David, P.; Richaud, P.; Nussaume, L.; Liu, D.; Desnos, T. Under phosphate starvation conditions, Fe and Al trigger accumulation of the transcription factor STOP1 in the nucleus of Arabidopsis root cells. Plant J. 2019, 99, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Huang, C. Activation and activity of STOP1 in aluminium resistance. J. Exp. Bot. 2021, 72, 2269–2272. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, Y.; Koyama, H.; Kobayashi, M. STOP1, a Cys2/His2 type zinc-finger protein, plays critical role in acid soil tolerance in Arabidopsis. Plant Signal. Behav. 2008, 3, 128–130. [Google Scholar] [CrossRef]
- Le Poder, L.; Mercier, C.; Fevrier, L.; Duong, N.; David, P.; Pluchon, S.; Nussaume, L.; Desnos, T. Uncoupling aluminum toxicity from aluminum signals in the STOP1 pathway. Front. Plant Sci. 2022, 13, 785791. [Google Scholar] [CrossRef]
- Sun, L.M.; Che, J.; Ma, J.F.; Shen, R.F. Expression level of transcription factor ART1 is responsible for differential aluminum tolerance in indica rice. Plants 2021, 10, 634. [Google Scholar] [CrossRef]
- Yamaji, N.; Huang, C.F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell. 2009, 21, 3339–3349. [Google Scholar] [CrossRef]
- Tsutsui, T.; Yamaji, N.; Ma, J.F. Identification of a cis-acting element of ART1, a C2H2-type zinc-finger transcription factor for aluminum tolerance in rice. Plant Physiol. 2011, 156, 925–931. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Growth and nutrient concentrations of common bean, lowland rice, corn, soybean, and wheat at different soil pH on an Inceptisol. J. Plant Nutr. 1999, 22, 1495–1507. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Zhao, S.; Huang, L.; Hao, L. Arabidopsis ein2-1 and npr1-1 response to Al stress. Bull. Environ. Contam. Toxicol. 2014, 93, 78–83. [Google Scholar] [CrossRef]
- Guan, K.; Yang, Z.; Zhan, M.; Zheng, M.; You, J.; Meng, X.; Li, H.; Gao, J. Two sweet sorghum (Sorghum bicolor L.) WRKY transcription factors promote aluminum tolerance via the reduction in callose deposition. Int. J. Mol. Sci. 2023, 24, 288. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, Y.; Li, J.; Wang, S.; Zhan, M.; Zheng, M.; Li, H.; Yang, Z. Identification of a bacterial-type ATP-binding cassette transporter implicated in aluminum tolerance in sweet sorghum (Sorghum bicolor L.). Plant Signal. Behav. 2021, 16, 1916211. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ryan, P.R.; Liu, C.; Li, H.; Hu, W.; Yan, W.; Huang, Y.; He, W.; Luo, B.; Zhang, X.; et al. ZmMATE6 from maize encodes a citrate transporter that enhances aluminum tolerance in transgenic Arabidopsis thaliana. Plant Sci. 2021, 311, 111016. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012, 159, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Arenhart, R.A.; Schunemann, M.; Bucker, N.L.; Margis, R.; Wang, Z.Y.; Margis-Pinheiro, M. Rice ASR1 and ASR5 are complementary transcription factors regulating aluminium responsive genes. Plant Cell Environ. 2016, 39, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Du Hanmei, L.C.J.X. Overexpression of the aldehyde dehydrogenase gene ZmALDH confers aluminum tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 477. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, W.L.; You, J.F.; Bian, M.D.; Qin, X.M.; Yu, H.; Liu, Q.; Ryan, P.R.; Yang, Z.M. Transgenic Arabidopsis thaliana plants expressing a β-1,3-glucanase from sweet sorghum (Sorghum bicolor L.) show reduced callose deposition and increased tolerance to aluminium toxicity. Plant Cell Environ. 2015, 38, 1178–1188. [Google Scholar] [CrossRef]
- Xie, W.; Liu, S.; Gao, H.; Wu, J.; Liu, D.; Kinoshita, T.; Huang, C.F. PP2C.D phosphatase SAL1 positively regulates aluminum resistance via restriction of aluminum uptake in rice. Plant Physiol. 2023, 192, 1498–1516. [Google Scholar] [CrossRef]
- Ren, H.; Park, M.Y.; Spartz, A.K.; Wong, J.H.; Gray, W.M. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet. 2018, 14, e1007455. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Devanna, B.N.; Jaswal, R.; Singh, P.K.; Kapoor, R.; Jain, P.; Kumar, G.; Sharma, Y.; Samantaray, S.; Sharma, T.R. Role of transporters in plant disease resistance. Physiol. Plant. 2021, 171, 849–867. [Google Scholar] [CrossRef]
- Huang, C.; Yamaji, N.; Ma, J.F. Knockout of a bacterial-type ATP-binding cassette transporter gene, AtSTAR1, results in increased aluminum sensitivity in Arabidopsis. Plant Physiol. 2010, 153, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Mitani, N.; Yano, M.; Nagamura, Y.; Ma, J.F. Bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef]
- Larsen, P.B.; Cancel, J.; Rounds, M.; Ochoa, V. Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 2007, 225, 1447–1458. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Chen, Z.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, X.; Li, C.; Zhang, B.; Zhang, C.; Zhang, X.; Ding, Z. Auxin efflux carrier ZmPGP1 mediates root growth inhibition under aluminum stress. Plant Physiol. 2018, 177, 819–832. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Shaff, J.; Liang, C.; Jia, X.; Li, Z.; Magalhaes, J.; Kochian, L.V. A promoter-swap strategy between the AtALMT and AtMATE genes increased Arabidopsis aluminum resistance and improved carbon-use efficiency for aluminum resistance. Plant J. 2012, 71, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Kusunoki, K.; Maruyama, H.; Enomoto, T.; Tokizawa, M.; Iuchi, S.; Kobayashi, M.; Kochian, L.V.; Koyama, H.; Kobayashi, Y. A single-population GWAS identified AtMATE expression level polymorphism caused by promoter variants is associated with variation in aluminum tolerance in a local Arabidopsis population. Plant Direct 2020, 4, e00250. [Google Scholar] [CrossRef]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef]
- Green, L.S.; Rogers, E.E. FRD3 controls iron localization in Arabidopsis. Plant Physiol. 2004, 136, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. Functional analysis of a MATE gene OsFRDL2 revealed its involvement in Al-induced secretion of citrate, but a lower contribution to Al tolerance in rice. Plant Cell Physiol. 2016, 57, 976–985. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Ma, J.F. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 2011, 68, 1061–1069. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, Z.Q.; Yokosho, K.; Ding, B.; Fan, W.; Gong, Q.Q.; Li, G.X.; Wu, Y.R.; Yang, J.L.; Ma, J.F.; et al. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018, 219, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Caniato, F.F.; Hamblin, M.T.; Guimaraes, C.T.; Zhang, Z.; Schaffert, R.E.; Kochian, L.V.; Magalhaes, J.V. Association mapping provides insights into the origin and the fine structure of the sorghum aluminum tolerance locus, AltSB. PLoS ONE 2014, 9, e87438. [Google Scholar] [CrossRef]
- Carvalho, G.; Schaffert, R.E.; Malosetti, M.; Viana, J.H.M.; Menezes, C.B.; Silva, L.A.; Guimaraes, C.T.; Coelho, A.M.; Kochian, L.V.; van Eeuwijk, F.A.; et al. The citrate transporter SbMATE is a major asset for sustainable grain yield for sorghum cultivated on acid soils. G3 Genes Genom. Genet. 2016, 6, 475–484. [Google Scholar] [CrossRef]
- Hufnagel, B.; Guimaraes, C.T.; Craft, E.J.; Shaff, J.E.; Schaffert, R.E.; Kochian, L.V.; Magalhaes, J.V. Exploiting sorghum genetic diversity for enhanced aluminum tolerance: Allele mining based on the AltSB locus. Sci. Rep. 2018, 8, 10094. [Google Scholar] [CrossRef]
- Magalhaes, J.V.; Liu, J.; Guimaraes, C.T.; Lana, U.G.; Alves, V.M.; Wang, Y.H.; Schaffert, R.E.; Hoekenga, O.A.; Pineros, M.A.; Shaff, J.E.; et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 2007, 39, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.O.; Lana, U.G.; Pineros, M.A.; Alves, V.M.; Guimaraes, C.T.; Liu, J.; Zheng, Y.; Zhong, S.; Fei, Z.; Maron, L.G.; et al. Incomplete transfer of accessory loci influencing SbMATE expression underlies genetic background effects for aluminum tolerance in sorghum. Plant J. 2013, 73, 276–288. [Google Scholar] [CrossRef]
- Melo, J.O.; Martins, L.; Barros, B.A.; Pimenta, M.R.; Lana, U.; Duarte, C.; Pastina, M.M.; Guimaraes, C.T.; Schaffert, R.E.; Kochian, L.V.; et al. Repeat variants for the SbMATE transporter protect sorghum roots from aluminum toxicity by transcriptional interplay in cis and trans. Proc. Natl. Acad. Sci. USA 2019, 116, 313–318. [Google Scholar] [CrossRef]
- Rupak Doshi, A.P.M.M. Functional characterization and discovery of modulators of SbMATE, the agronomically important aluminium tolerance transporter from Sorghum bicolor. Sci. Rep. 2017, 7, 17996. [Google Scholar] [CrossRef]
- Zhou, G.; Pereira, J.F.; Delhaize, E.; Zhou, M.; Magalhaes, J.V.; Ryan, P.R. Enhancing the aluminium tolerance of barley by expressing the citrate transporter genes SbMATE and FRD3. J. Exp. Bot. 2014, 65, 2381–2390. [Google Scholar] [CrossRef]
- Sivaguru, M.; Liu, J.; Kochian, L.V. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013, 76, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Maron, L.G.; Guimaraes, C.T.; Kirst, M.; Albert, P.S.; Birchler, J.A.; Bradbury, P.J.; Buckler, E.S.; Coluccio, A.E.; Danilova, T.V.; Kudrna, D.; et al. Aluminum tolerance in maize is associated with higher MATE1 gene copy number. Proc. Natl. Acad. Sci. USA 2013, 110, 5241–5246. [Google Scholar] [CrossRef] [PubMed]
- Matonyei, T.K.; Barros, B.A.; Guimaraes, R.; Ouma, E.O.; Cheprot, R.K.; Apolinario, L.C.; Ligeyo, D.O.; Costa, M.; Were, B.A.; Kisinyo, P.O.; et al. Aluminum tolerance mechanisms in Kenyan maize germplasm are independent from the citrate transporter ZmMATE1. Sci. Rep. 2020, 10, 7320. [Google Scholar] [CrossRef]
- Garcia-Oliveira, A.L.; Benito, C.; Guedes-Pinto, H.; Martins-Lopes, P. Molecular cloning of TaMATE2 homoeologues potentially related to aluminium tolerance in bread wheat (Triticum aestivum L.). Plant Biol. 2018, 20, 817–824. [Google Scholar] [CrossRef]
- Hoekenga, O.A.; Maron, L.G.; Pineros, M.A.; Cançado, G.M.A.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Hoekenga, O.A.; Itoh, H.; Nakashima, M.; Saito, S.; Shaff, J.E.; Maron, L.G.; Piñeros, M.A.; Kochian, L.V.; Koyama, H. Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiol. 2007, 145, 843–852. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsuchiya, Y.; Ariyoshi, M.; Ryan, P.R.; Yamamoto, Y. A chimeric protein of aluminum-activated malate transporter generated from wheat and Arabidopsis shows enhanced response to trivalent cations. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 1427–1435. [Google Scholar] [CrossRef]
- Tokizawa, M.; Kobayashi, Y.; Saito, T.; Kobayashi, M.; Iuchi, S.; Nomoto, M.; Tada, Y.; Yamamoto, Y.Y.; Koyama, H. SENSITIVE TO PROTON RHIZOTOXICITY1, CALMODULIN BINDING TRANSCRIPTION ACTIVATOR2, and other transcription factors are involved in ALUMINUM-ACTIVATED MALATE TRANSPORTER1 expression. Plant Physiol. 2015, 167, 991–1003. [Google Scholar] [CrossRef]
- Zhang, J.; Martinoia, E.; De Angeli, A. Cytosolic nucleotides block and regulate the Arabidopsis vacuolar anion channel AtALMT9. J. Biol. Chem. 2014, 289, 25581–25589. [Google Scholar] [CrossRef]
- Gilliham, M.; Xu, B. γ-aminobutyric acid may directly or indirectly regulate Arabidopsis ALMT9. Plant Physiol. 2022, 190, 1570–1573. [Google Scholar] [CrossRef]
- Zhang, J.; Baetz, U.; Krügel, U.; Martinoia, E.; De Angeli, A. Identification of a probable pore-forming domain in the multimeric vacuolar anion channel AtALMT91. Plant Physiol. 2013, 163, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, T.; Sasaki, T.; Tsuchiya, Y.; Ryan, P.R.; Delhaize, E.; Yamamoto, Y. An extracellular hydrophilic carboxy-terminal domain regulates the activity of TaALMT1, the aluminum-activated malate transport protein of wheat. Plant J. 2010, 64, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, M.; Delhaize, E.; Ryan, P.R. Altered expression of a malate-permeable anion channel, OsALMT4, disrupts mineral nutrition. Plant Physiol. 2017, 175, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, M.; Estavillo, G.M.; Delhaize, E.; White, R.G.; Zhou, M.; Ryan, P.R. Altered expression of the malate-permeable anion channel OsALMT4 reduces the growth of rice under low radiance. Front. Plant. Sci. 2018, 9, 542. [Google Scholar] [CrossRef]
- Raman, H.; Ryan, P.R.; Raman, R.; Stodart, B.J.; Zhang, K.; Martin, P.; Wood, R.; Sasaki, T.; Yamamoto, Y.; Mackay, M.; et al. Analysis of TaALMT1 traces the transmission of aluminum resistance in cultivated common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2008, 116, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Liu, J.; Dong, D.; Jia, X.; McCouch, S.R.; Kochian, L.V. Natural variation underlies alterations in nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc. Natl. Acad. Sci. USA 2014, 111, 6503–6508. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, G.; Li, P.; Wang, Z.; Fu, S.; Zhang, X.; Chen, X.; Shi, M.; Ming, Z.; Xia, J. Bioinformatic and functional analysis of a key determinant underlying the substrate selectivity of the Al transporter, Nrat1. Front. Plant Sci. 2018, 9, 606. [Google Scholar] [CrossRef]
- Tao, Y.; Niu, Y.; Wang, Y.; Chen, T.; Naveed, S.A.; Zhang, J.; Xu, J.; Li, Z. Genome-wide association mapping of aluminum toxicity tolerance and fine mapping of a candidate gene for Nrat1 in rice. PLoS ONE 2018, 13, e0198589. [Google Scholar] [CrossRef]
- Lu, M.; Wang, Z.; Fu, S.; Yang, G.; Shi, M.; Lu, Y.; Wang, X.; Xia, J. Functional characterization of the SbNrat1 gene in sorghum. Plant Sci. 2017, 262, 18–23. [Google Scholar] [CrossRef]
- Li, H.; Wang, N.; Hu, W.; Yan, W.; Jin, X.; Yu, Y.; Du, C.; Liu, C.; He, W.; Zhang, S. ZmNRAMP4 enhances the tolerance to aluminum stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 8162. [Google Scholar] [CrossRef]
- Wu, D.; Shen, H.; Yokawa, K.; Baluska, F. Alleviation of aluminium-induced cell rigidity by overexpression of OsPIN2 in rice roots. J. Exp. Bot. 2014, 65, 5305–5315. [Google Scholar] [CrossRef]
- Wu, D.; Shen, H.; Yokawa, K.; Baluska, F. Overexpressing OsPIN2 enhances aluminium internalization by elevating vesicular trafficking in rice root apex. J. Exp. Bot. 2015, 66, 6791–6801. [Google Scholar] [CrossRef]
- Wang, M.; Qiao, J.; Yu, C.; Chen, H.; Sun, C.; Huang, L.; Li, C.; Geisler, M.; Qian, Q.; Jiang, A.; et al. The auxin influx carrier, OsAUX3, regulates rice root development and responses to aluminium stress. Plant Cell Environ. 2019, 42, 1125–1138. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, P.; Luo, X.; Peng, W.; Liu, Z.; Xie, G.; Wang, M.; An, F. An oxalate transporter gene, AtOT, enhances aluminum tolerance in Arabidopsis thaliana by regulating oxalate efflux. Int. J. Mol. Sci. 2023, 24, 4516. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, E.; Wu, G.; Bai, Q.; Xu, F.; Ji, X.; Li, C.; Li, L.; Liu, J. The roles of selectivity filters in determining aluminum transport by AtNIP1;2. Plant Signal. Behav. 2021, 16, 1991686. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, R.; Li, D.; Jia, X.; Zhou, D.; Li, J.; Lyi, S.M.; Hou, S.; Huang, Y.; Kochian, L.V.; et al. NIP1;2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. Proc Natl. Acad. Sci. USA 2017, 114, 5047–5052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, S.; Li, C.; Hu, T.; Hou, S.; Bai, Q.; Ji, X.; Xu, F.; Guo, C.; Huang, M.; et al. The plasma membrane-localized OsNIP1;2 mediates internal aluminum detoxification in rice. Front. Plant. Sci. 2022, 13, 970270. [Google Scholar] [CrossRef]
- Gabrielson, K.M.; Cancel, J.D.; Morua, L.F.; Larsen, P.B. Identification of dominant mutations that confer increased aluminium tolerance through mutagenesis of the Al-sensitive Arabidopsis mutant, als3-1. J. Exp. Bot. 2006, 57, 943–951. [Google Scholar] [CrossRef]
- Maron, L.G.; Pineros, M.A.; Guimaraes, C.T.; Magalhaes, J.V.; Pleiman, J.K.; Mao, C.; Shaff, J.; Belicuas, S.N.; Kochian, L.V. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J. 2010, 61, 728–740. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. Retrotransposon-mediated aluminum tolerance through enhanced expression of the citrate transporter OsFRDL4. Plant Physiol. 2016, 172, 2327–2336. [Google Scholar] [CrossRef]
- Zhang, W.H.; Ryan, P.R.; Sasaki, T.; Yamamoto, Y.; Sullivan, W.; Tyerman, S.D. Characterization of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana tabacum L.) cells. Plant Cell Physiol. 2008, 49, 1316–1330. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Sasaki, T.; Sivaguru, M.; Yamamoto, Y.; Osawa, H.; Ahn, S.J.; Matsumoto, H. Evidence for the plasma membrane localization of Al-activated malate transporter (ALMT1). Plant Cell Physiol. 2005, 46, 812–816. [Google Scholar] [CrossRef]
- Raman, H.; Zhang, K.; Cakir, M.; Appels, R.; Garvin, D.F.; Maron, L.G.; Kochian, L.V.; Moroni, J.S.; Raman, R.; Imtiaz, M.; et al. Molecular characterization and mapping of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivum L.). Genome 2005, 48, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, X.Q.; Chen, R.F.; Dong, X.Y.; Lan, P.; Ma, J.F.; Shen, R.F. Altered cell wall properties are responsible for ammonium-reduced aluminium accumulation in rice roots. Plant Cell Environ. 2015, 38, 1382–1390. [Google Scholar] [CrossRef]
- Luchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef]
- Huang, S.; Gao, J.; You, J.; Liang, Y.; Guan, K.; Yan, S.; Zhan, M.; Yang, Z. Identification of STOP1-like proteins associated with aluminum tolerance in sweet sorghum (Sorghum bicolor L.). Front. Plant Sci. 2018, 9, 258. [Google Scholar] [CrossRef]
- Garcia-Oliveira, A.L.; Benito, C.; Prieto, P.; de Andrade, M.R.; Rodrigues-Pousada, C.; Guedes-Pinto, H.; Martins-Lopes, P. Molecular characterization of TaSTOP1 homoeologues and their response to aluminium and proton (H+) toxicity in bread wheat (Triticum aestivum L.). BMC Plant Biol. 2013, 13, 134. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ohyama, Y.; Kobayashi, Y.; Ito, H.; Iuchi, S.; Fujita, M.; Zhao, C.; Tanveer, T.; Ganesan, M.; Kobayashi, M.; et al. STOP2 activates transcription of several genes for Al- and Low pH-tolerance that are regulated by STOP1 in Arabidopsis. Mol. Plant 2014, 7, 311–322. [Google Scholar] [CrossRef]
- Ding, Z.J.; Yan, J.Y.; Xu, X.Y.; Li, G.X.; Zheng, S.J. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013, 76, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Yan, J.Y.; Ren, J.Y.; Sun, L.; Xu, C.; Li, G.X.; Ding, Z.J.; Zheng, S.J. A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. J. Integr. Plant Biol. 2020, 62, 1176–1192. [Google Scholar] [CrossRef]
- Arenhart, R.A.; Bai, Y.; de Oliveira, L.F.; Neto, L.B.; Schunemann, M.; Maraschin, F.S.; Mariath, J.; Silverio, A.; Sachetto-Martins, G.; Margis, R.; et al. New insights into aluminum tolerance in rice: The ASR5 protein binds the STAR1 promoter and other aluminum-responsive genes. Mol. Plant 2014, 7, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Rafael Augusto Arenharta, R.M.M.M. The rice ASR5 protein: A putative role in the response to aluminum photosynthesis disturbance. Plant. Signal. Behav. 2012, 10, 1263–1266. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Guo, S.; Yuan, X.; Zhao, S.; Tian, H.; Dai, S.; Kong, X.; Ding, Z. AtHB7/12 regulate root growth in response to aluminum stress. Int. J. Mol. Sci. 2020, 21, 4080. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Gao, J.; You, J.; Guan, K.; Zheng, M.; Meng, X.; Li, H.; Yang, Z. The transcription factor SbHY5 mediates light to promote aluminum tolerance by activating SbMATE and SbSTOP1s expression. Plant Physiol. Biochem. 2023, 205, 108197. [Google Scholar] [CrossRef]
- Gao, L.J.; Liu, X.P.; Gao, K.K.; Cui, M.Q.; Zhu, H.H.; Li, G.X.; Yan, J.Y.; Wu, Y.R.; Ding, Z.J.; Chen, X.W.; et al. ART1 and putrescine contribute to rice aluminum resistance via OsMYB30 in cell wall modification. J. Integr. Plant Biol. 2023, 65, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tao, Y.; Huang, J.; Liu, Y.S.; Yang, X.Z.; Jing, H.K.; Shen, R.F.; Zhu, X.F. The MYB transcription factor MYB103 acts upstream of RICHOME BIREFRINGENCE-LIKE27 in regulating aluminum sensitivity by modulating theO-acetylation level of cell wall xyloglucan in Arabidopsis thaliana. Plant J. 2022, 111, 529–545. [Google Scholar] [CrossRef]
- Tao, Y.; Wan, J.X.; Liu, Y.S.; Yang, X.Z.; Shen, R.F.; Zhu, X.F. The NAC transcription factor ANAC017 regulates aluminum tolerance by regulating the cell wall-modifying genes. Plant Physiol. 2022, 189, 2517–2534. [Google Scholar] [CrossRef]
- Chen, P.; Sjogren, C.A.; Larsen, P.B.; Schnittger, A. A multi-level response to DNA damage induced by aluminium. Plant J. 2019, 98, 479–491. [Google Scholar] [CrossRef]
- Sjogren, C.A.; Bolaris, S.C.; Larsen, P.B. Aluminum-Dependent Terminal Differentiation of the Arabidopsis Root Tip Is Mediated through an ATR-, ALT2-, and SOG1-Regulated Transcriptional Response. Plant Cell 2015, 27, 2501–2515. [Google Scholar] [CrossRef]
- Yang, Z.; He, C.; Ma, Y.; Herde, M.; Ding, Z. Jasmonic acid enhances Al-induced root growth inhibition. Plant Physiol. 2016, 173, 1420–1433. [Google Scholar] [CrossRef]
- Geng, X.; Horst, W.J.; Golz, J.F.; Lee, J.E.; Ding, Z.; Yang, Z.B. LEUNIG_HOMOLOG transcriptional co-repressor mediates aluminium sensitivity through PECTIN METHYLESTERASE46-modulated root cell wall pectin methylesterification in Arabidopsis. Plant J. 2017, 90, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; DeBowles, D.; Esfandiari, E.; Dean, G.; Carpita, N.C.; Haughn, G.W. The Arabidopsis transcription factor LUH/MUM1 is required for extrusion of seed coat mucilage. Plant Physiol. 2011, 156, 491–502. [Google Scholar] [CrossRef]
- Biedermann, S.; Harashima, H.; Chen, P.; Heese, M.; Bouyer, D.; Sofroni, K.; Schnittger, A. The retinoblastoma homolog RBR1 mediates localization of the repair protein RAD51 to DNA lesions in Arabidopsis. EMBO J. 2017, 36, 1279–1297. [Google Scholar] [CrossRef]
- Sivaguru, M.; Ezaki, B.; Zheng-Hui, H.; Tong, H. Aluminum-induced gene expression and protein localization of a cell wall-associated receptor kinase in Arabidopsis. Plant Physiol. 2003, 132, 2256–2266. [Google Scholar] [CrossRef]
- Wei, P.; Demulder, M.; David, P.; Eekhout, T.; Yoshiyama, K.O.; Nguyen, L.; Vercauteren, I.; Eeckhout, D.; Galle, M.; De Jaeger, G.; et al. Arabidopsis casein kinase 2 triggers stem cell exhaustion under Al toxicity and phosphate deficiency through activating the DNA damage response pathway. Plant Cell 2021, 33, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhou, F.; Zhang, Y.; Singh, S.; Huang, C.F. Degradation of STOP1 mediated by the F-box proteins RAH1 and RAE1 balances aluminum resistance and plant growth in Arabidopsis thaliana. Plant J. 2021, 106, 493–506. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, J.; Zhang, Y.; Huang, C. The THO/TREX complex component RAE2/TEX1 is involved in the regulation of aluminum resistance and low phosphate response in Arabidopsis. Front. Plant Sci. 2021, 12, 698443. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Gao, H.; Li, S.; Wang, Z.; Huang, C. Mutation of HPR1 encoding a component of the THO/TREX complex reduces STOP1 accumulation and aluminium resistance in Arabidopsis thaliana. New Phytol. 2020, 228, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhang, J.; Zhang, Y.; Fan, N.; van den Burg, H.A.; Huang, C.F. Regulation of aluminum resistance in arabidopsis involves the SUMOylation of the zinc finger transcription factor STOP1. Plant Cell 2020, 32, 3921–3938. [Google Scholar] [CrossRef]
- Motoda, H.; Sasaki, T.; Kano, Y.; Ryan, P.R.; Delhaize, E.; Matsumoto, H.; Yamamoto, Y. The membrane topology of ALMT1, an aluminum-activated malate transport protein in wheat (Triticum aestivum). Plant Signal. Behav. 2007, 2, 467–472. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, J.; Liu, J.; Wang, J.; Ding, Z.; Tian, H. SIZ1 negatively regulates aluminum resistance by mediating the STOP1–ALMT1 pathway in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Singh, S.; Zhang, J.; Fang, Q.; Li, C.; Wang, J.; Zhao, C.; Wang, P.; Huang, C.F. The MEKK1-MKK1/2-MPK4 cascade phosphorylates and stabilizes STOP1 to confer aluminum resistance in Arabidopsis. Mol. Plant. 2023, 16, 337–353. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kobayashi, Y.; Watanabe, T.; Shaff, J.E.; Ohta, H.; Kochian, L.V.; Wagatsuma, T.; Kinraide, T.B.; Koyama, H. Molecular and physiological analysis of Al3+ and H+ rhizotoxicities at moderately acidic conditions. Plant Physiol. 2013, 163, 180–192. [Google Scholar] [CrossRef]
- Sjogren, C.A.; Larsen, P.B. SUV2, which encodes an ATR-related cell cycle checkpoint and putative plant ATRIP, is required for aluminium-dependent root growth inhibition in Arabidopsis. Plant Cell Environ. 2017, 40, 1849–1860. [Google Scholar] [CrossRef]
- Liu, X.P.; Gao, L.J.; She, B.T.; Li, G.X.; Wu, Y.R.; Xu, J.M.; Ding, Z.J.; Ma, J.F.; Zheng, S.J. A novel kinase subverts aluminium resistance by boosting ornithine decarboxylase-dependent putrescine biosynthesis. Plant Cell Environ. 2022, 45, 2520–2532. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. An Al-inducible expansin gene, OsEXPA10 is involved in root cell elongation of rice. Plant J. 2016, 88, 132–142. [Google Scholar] [CrossRef]
- Du, H.; Hu, X.; Yang, W.; Hu, W.; Yan, W.; Li, Y.; He, W.; Cao, M.; Zhang, X.; Luo, B.; et al. ZmXTH, a xyloglucan endotransglucosylase/hydrolase gene of maize, conferred aluminum tolerance in Arabidopsis. J. Plant Physiol. 2021, 266, 153520. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lei, G.J.; Wang, Z.W.; Shi, Y.Z.; Braam, J.; Li, G.X.; Zheng, S.J. Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol. 2013, 162, 1947–1955. [Google Scholar] [CrossRef]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z.; et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 2012, 24, 4731–4747. [Google Scholar] [CrossRef]
- Zhu, X.F.; Sun, Y.; Zhang, B.C.; Mansoori, N.; Wan, J.X.; Liu, Y.; Wang, Z.W.; Shi, Y.Z.; Zhou, Y.H.; Zheng, S.J. TRICHOME BIREFRINGENCE-LIKE27 affects aluminum sensitivity by modulating the O-Acetylation of xyloglucan and aluminum-binding capacity in Arabidopsis. Plant Physiol. 2014, 166, 181–189. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wan, J.X.; Wu, Q.; Zhao, X.S.; Zheng, S.J.; Shen, R.F. PARVUS affects aluminium sensitivity by modulating the structure of glucuronoxylan in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 1916–1925. [Google Scholar] [CrossRef]
- Gao, J.; Yan, S.; Yu, H.; Zhan, M.; Guan, K.; Wang, Y.; Yang, Z. Sweet sorghum (Sorghum bicolor L.) SbSTOP1 activates the transcription of a β-1,3-glucanase gene to reduce callose deposition under Al toxicity: A novel pathway for Al tolerance in plants. Biosci. Biotechnol. Biochem. 2019, 83, 446–455. [Google Scholar] [CrossRef]
- Liu, S.; Gao, H.; Wu, X.; Fang, Q.; Chen, L.; Zhao, F.J.; Huang, C.F. Isolation and characterization of an aluminum-resistant mutant in rice. Rice 2016, 9, 60. [Google Scholar] [CrossRef]
- Yang, Z.; Geng, X.; He, C.; Zhang, F.; Wang, R.; Horst, W.J.; Ding, Z. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 2014, 26, 2889–2904. [Google Scholar] [CrossRef]
- Kong, W.; Li, Y.; Zhang, M.; Jin, F.; Li, J. A Novel Arabidopsis microRNA promotes IAA biosynthesis via the indole-3-acetaldoxime pathway by suppressing superroot1. Plant Cell Physiol. 2015, 56, 715–726. [Google Scholar] [CrossRef]
- Delarue, M.; Prinsen, E.; Onckelen, H.V.; Caboche, M.; Bellini, C. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J. 1998, 14, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.B.; Caverzan, A.; Teixeira, F.K.; Lazzarotto, F.; Silveira, J.A.G.; Ferreira-Silva, S.L.; Abreu-Neto, J.; Margis, R.; Margis-Pinheiro, M. Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 2010, 71, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Mano, J.; Tanaka, K.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S. High level of reduced glutathione contributes to detoxification of lipid peroxide-derived reactive carbonyl species in transgenic Arabidopsis overexpressing glutathione reductase under aluminum stress. Physiol. Plant. 2017, 161, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, B.; Suzuki, M.; Motoda, H.; Kawamura, M.; Nakashima, S.; Matsumoto, H. Mechanism of gene expression of Arabidopsis glutathione S-transferase, AtGST1, and AtGST11 in response to aluminum stress1. Plant Physiol. 2004, 134, 1672–1682. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Z.; How, J.; Xu, H.; Chen, L.; Li, K. Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant’s tolerance to aluminum stress. Plant Mol. Biol. 2017, 95, 157–168. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Wang, Y.; Xing, D. Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria-dependent programmed cell death induced by aluminium phytotoxicity in Arabidopsis. J. Exp. Bot. 2014, 65, 4465–4478. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, Y.; Qu, M.; Li, Y.; Hu, X.; Yang, W.; Li, H.; He, W.; Ding, J.; Liu, C.; et al. A maize ZmAT6 gene confers aluminum tolerance via reactive oxygen species scavenging. Front. Plant. Sci. 2020, 11, 1016. [Google Scholar] [CrossRef]
- Badia, M.B.; Maurino, V.G.; Pavlovic, T.; Arias, C.L.; Pagani, M.A.; Andreo, C.S.; Saigo, M.; Drincovich, M.F.; Gerrard, W.M. Loss of function of Arabidopsis NADP-malic enzyme 1 results in enhanced tolerance to aluminum stress. Plant J. 2020, 101, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Mangeon, A.; Pardal, R.; Menezes-Salgueiro, A.D.; Duarte, G.L.; de Seixas, R.; Cruz, F.P.; Cardeal, V.; Magioli, C.; Ricachenevsky, F.K.; Margis, R.; et al. AtGRP3 is implicated in root size and aluminum response pathways in Arabidopsis. PLoS ONE 2016, 11, e0150583. [Google Scholar] [CrossRef]
- Ligaba-Osena, A.; Fei, Z.; Liu, J.; Xu, Y.; Shaff, J.; Lee, S.C.; Luan, S.; Kudla, J.; Kochian, L.; Pineros, M. Loss-of-function mutation of the calcium sensor CBL1 increases aluminum sensitivity in Arabidopsis. New Phytol. 2017, 214, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Nezames, C.D.; Ochoa, V.; Larsen, P.B. Mutational loss of Arabidopsis SLOW WALKER2 results in reduced endogenous spermine concomitant with increased aluminum sensitivity. Funct. Plant Biol. 2012, 40, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Miftahudin, M.; Roslim, D.I.; Fendiyanto, M.H.; Satrio, R.D.; Zulkifli, A.; Umaiyah, E.I.; Chikmawati, T.; Sulistyaningsih, Y.C.; Suharsono, S.; Hartana, A.; et al. OsGERLP: A novel aluminum tolerance rice gene isolated from a local cultivar in Indonesia. Plant Physiol. Biochem. 2021, 162, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Nezames, C.D.; Sjogren, C.A.; Barajas, J.F.; Larsen, P.B. The Arabidopsis cell cycle checkpoint regulators TANMEI/ALT2 and ATR mediate the active process of aluminum-dependent root growth inhibition. Plant Cell 2012, 24, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, R.K.; Enomoto, T.; Ito, H.; Nakano, Y.; Yanase, E.; Watanabe, T.; Sadhukhan, A.; Iuchi, S.; Kobayashi, M.; Panda, S.K.; et al. Expression GWAS of PGIP1 identifies STOP1-dependent and STOP1-independent regulation of PGIP1 in aluminum stress signaling in Arabidopsis. Front. Plant Sci. 2021, 12, 774687. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, L.; Liao, Y.; Luo, Z.; Wang, H.; Wang, P.; Zhao, H.; Xia, J.; Huang, C.F. Dysfunction of the 4-coumarate:coenzyme A ligase 4CL4 impacts aluminum resistance and lignin accumulation in rice. Plant J. 2020, 104, 1233–1250. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, A.Y.; Dong, X.Y.; Shen, R.F.; Zhao, X.Q. Involvement of the 4-coumarate:coenzyme A ligase 4CL4 in rice phosphorus acquisition and rhizosphere microbe recruitment via root growth enlargement. Planta 2023, 258, 7. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Shen, J.; Li, L. Functional characterization of evolutionarily divergent 4-coumarate: Coenzyme A ligases in rice. Plant Physiol. 2011, 157, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, W.; Zhang, B.; Zhang, S.; Wang, W.; Ming, F. One novel mitochondrial citrate synthase from Oryza sativa L. can enhance aluminum tolerance in transgenic tobacco. Mol. Biotechnol. 2009, 42, 299–305. [Google Scholar] [CrossRef]
- Garg, B.; Puranik, S.; Tuteja, N.; Prasad, M. Abiotic stress-responsive expression of wali1 and wali5 genes from wheat. Plant Signal. Behav. 2012, 7, 1393–1396. [Google Scholar] [CrossRef]
- Wan, X.; Wu, S.; Li, Z.; Dong, Z.; An, X.; Ma, B.; Tian, Y.; Li, J. Maize genic male-sterility genes and their applications in hybrid breeding: Progress and perspectives. Mol. Plant 2019, 12, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, H.; Chen, D.; Zhang, H.; Sun, M.; Chen, S.; Qin, Z.; Ding, Z.; Dai, S. Cysteine-rich receptor-like protein kinases: Emerging regulators of plant stress responses. Trends Plant. Sci. 2023, 28, 776–794. [Google Scholar] [CrossRef]
- Vishal Varshney, J.S.V.M. Unlocking the plant ER stress code: IRE1-proteasome signaling cohort takes the lead. Trends Plant Sci. 2024, 6, 610–612. [Google Scholar] [CrossRef]
- Mercier, C.; Roux, B.; Have, M.; Le Poder, L.; Duong, N.; David, P.; Leonhardt, N.; Blanchard, L.; Naumann, C.; Abel, S.; et al. Root responses to aluminium and iron stresses require the SIZ1 SUMO ligase to modulate the STOP1 transcription factor. Plant J. 2021, 108, 1507–1521. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, J.; Yang, D.L.; Huang, C.F. The SUMO E3 ligase SIZ1 partially regulates STOP1 SUMOylation and stability in Arabidopsis thaliana. Plant Signal. Behav. 2021, 16, 1899487. [Google Scholar] [CrossRef]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef]
- Breia, R.; Conde, A.; Badim, H.; Fortes, A.M.; Geros, H.; Granell, A. Plant SWEETs: From sugar transport to plant-pathogen interaction and more unexpected physiological roles. Plant Physiol. 2021, 186, 836–852. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Scandalios, J.G. The rise of ROS. Trends Biochem. Sci. 2022, 9, 483–486. [Google Scholar] [CrossRef]
- Henrichs, S.; Wang, B.; Fukao, Y.; Zhu, J.; Charrier, L.; Bailly, A.; Oehring, S.C.; Linnert, M.; Weiwad, M.; Endler, A.; et al. Regulation of ABCB1/PGP1-catalysed auxin transport by linker phosphorylation. EMBO J. 2012, 31, 2965–2980. [Google Scholar] [CrossRef] [PubMed]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Dawood, M.; Cao, F.; Jahangir, M.M.; Zhang, G.; Wu, F. Alleviation of aluminum toxicity by hydrogen sulfide is related to elevated ATPase, and suppressed aluminum uptake and oxidative stress in barley. J. Hazard. Mater. 2012, 209–210, 121–128. [Google Scholar] [CrossRef]
- Wang, Y.; Stass, A.; Horst, W.J. Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol. 2004, 136, 3762–3770. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
| No. | Gene Names | Gene ID | Encoded Proteins | Biological Function | References |
|---|---|---|---|---|---|
| Transporters | |||||
| 1 | AtSTAR1 | At1g67940 | ABC transporter | AtSTAR1 is involved in the basic detoxification of Al in Arabidopsis. | [31] |
| 2 | OsSTAR1 | Os06g0695800 | ABC transporter | OsSTAR1 interacts with OsSTAR2 and is involved in the detoxification of Al. | [32] |
| 3 | SbSTAR1 | SORBI_3010G246200 | ABC transporter | SbSTAR1 enhances Al tolerance by regulating hemicellulose content in the root cell wall. | [20,21] |
| 4 | OsSTAR2 | Os05g0119000 | ABC transporter | OsSTAR1 interacts with OsSTAR2 and is involved in the detoxification of Al. | [32] |
| 6 | AtALS1 | At5g39040 | ABC transporter | Contributes to Al redistribution between the cytoplasm and vacuoles and to symplastic Al detoxification. | [33] |
| 7 | OsALS1 | Os03g0755100 | ABC transporter | Responsible for sequestrating Al into vacuoles. | [34] |
| 5 | AtALS3 | At2g37330 | ABC transporter | Required for Al resistance/tolerance and distribution to gather Al away from sensitive tissues to protect the growing root from the toxic effects of Al. | [78] |
| 8 | ZmPGP1 | Zm00001eb038710 | ABC transporter | ZmPGP1 regulates Al stress and is associated with reduced auxin accumulation in root tips. | [35] |
| 9 | AtMATE | At5g52450 | Multidrug and toxic compound extrusion (MATE) family protein | Increased Al resistance of the transgenic plants and enhanced carbon-use efficiency for Al resistance. | [36,37] |
| 10 | SbMATE | SORBI_3009G106960 | Multidrug and toxic compound extrusion (MATE) family protein | SbMATE is associated with the induction of Al tolerance via enhanced root citrate exudation. | [20,43,44,45,46,47,49,50,51] |
| 11 | ZmMATE1 | Zm00001eb261140 | Multidrug and toxic compound extrusion (MATE) family protein | Maize lines with a higher ZmMATE1 copy number are Al-tolerant. | [52,53,79] |
| 12 | AtFRDL3 | At3g08040 | Multidrug and toxic compound extrusion (MATE) family protein | AtFRD3 confers tolerance to aluminum. | [38,39] |
| 13 | OsFRDL2 | Os10g0206800 | Multidrug and toxic compound extrusion (MATE) family protein | OsFRDL2 is involved in the Al-induced secretion of citrate. | [40] |
| 14 | TaMATE2 | TraesCS1A02G305200, TraesCS1B02G315900, TraesCS1D02G304800 | Multidrug and toxic compound extrusion (MATE) family protein | TaMATE2 is involved in Al tolerance in bread wheat. | [54] |
| 15 | ZmMATE2 | Zm00001eb219790 | Multidrug and toxic compound extrusion (MATE) family protein | ZmMATE2 is involved in a novel Al tolerance mechanism. | [79] |
| 16 | ZmMATE6 | Zm00001eb230490 | Multidrug and toxic compound extrusion (MATE) family protein | ZmMATE6 displays a greater Al-activated release of citrate from the roots and is significantly resistant to Al toxicity. | [22] |
| 17 | OsFRDL4 | Os01g0919100 | Multidrug and toxic compound extrusion (MATE) family protein | OsFRDL4 protein was able to transport citrate and was activated by Al. | [41,42,80] |
| 18 | AtALMT1 | At1g08430 | Malate transporter | AtALMT1 confers acid–soil tolerance by releasing malate from roots and enhances the response to trivalent cations. | [36,37,55,56,57,58] |
| 19 | TaALMT1 | TraesCS2A02G297900 | Malate transporter | TaALMT1 confers acid–soil tolerance by releasing malate from roots, enhances response to trivalent cations, and is permeable not only to malate but also to other physiologically relevant anions. | [57,62,65,81,82,83] |
| 20 | OsALMT4 | Os01g0221600 | Malate transporter | OsALMT4 facilitates malate efflux from cells and protects plants from Al stress, and its expression is altered in low-light environments. | [63,64] |
| 21 | AtALMT9 | At3g18440 | Malate transporter | AtALMT9 is a tetramer, and the TMa5 domains of its subunits contribute to form the pores of anion channels. | [60,61] |
| 22 | AtALMT12 | At4g17970 | Malate transporter | An anion transporter involved in stomatal closure. | [62,63] |
| 23 | OsNrat1 | Os02g0131800 | Metal transporter | The preliminary step to sequester Al3+ into vacuoles and thus relieve Al toxicity. | [66,67,68] |
| 24 | SbNrat1 | SORBI_3004G029900 | Metal transporter | Selectively transports Al3+ and is involved in basic Al tolerance in sorghum. | [69] |
| 25 | ZmNRAMP4 | Zm00001d015133 | Metal transporter | ZmNRAMP4 enhances Al tolerance via cytoplasmic sequestration of Al in maize. | [70] |
| 26 | OsMGT1 | Os01g0869200 | Magnesium transporter | Al induces the upregulation of OsMGT1 to increase the Mg content in cells, thereby preventing the binding of Al to enzymes and other cellular components and enhancing the aluminum tolerance of rice. | [23] |
| 27 | OsPIN2 | Os06g0660200 | Auxin transporter | Overexpression of OsPIN2 altered the distribution of Al3+ in apical cells, as indicated by a significant increase in the content of Al3+ in the cytosol and a decrease in the cell wall. | [71,72] |
| 31 | OsAUX3 | Os05g0447200 | Auxin transporter | Involved in Al-induced inhibition of root growth. | [73] |
| 28 | AtOT | At4g09580 | Oxalate transporter | Oxalate involves AtOT to enhance oxalic acid resistance and aluminum tolerance. | [74] |
| 29 | AtNIP1;2 | At4g18910 | Aquaporins | AtNIP1;2 mediates Al uptake and demonstrates critical roles of the constriction regions for transport activities. | [75,84] |
| 30 | OsNIP1;2 | Os01g0202800 | Aquaporins | OsNIP1;2 confers internal Al detoxification via taking out the root cell wall’s Al, sequestering it to the root cell’s vacuole, and re-distributing it to the above-ground tissues. | [77] |
| Transcription factors | |||||
| 1 | OsART1 | Os12g0170400 | Zinc finger transcription factor | Regulates the expression of genes related to Al tolerance in rice. | [9,10,15,16,17] |
| 2 | OsART2 | Os04g0165200 | Zinc finger transcription factor | The expression of OsART2 is rapidly induced by Al in the roots of wild-type rice, and the knockout of OsART2 increases sensitivity to Al toxicity. | [9] |
| 3 | AtSTOP1 | At1g34370 | Zinc finger transcription factor | AtSTOP1 binding to the consensus motif in the promoters of AtSTOP2, AtALMT1, AtGDH1, and AtGDH2 with high affinity to drive their expression. Fe2/3+ and Al3+ act similarly to increase the stability of STOP1 and its accumulation in the nucleus, where it activates the expression of AtALMT1. | [11,12,14,85] |
| 4 | SbSTOP1a | SORBI_3001G020200 | Zinc finger transcription factor | SbSTOP1 plays an important role in Al tolerance in sweet sorghum and extends our understanding of the complex regulatory mechanisms of STOP1-like proteins in response to Al toxicity. | [86] |
| 5 | SbSTOP1b | SORBI_3004G188300 | Zinc finger transcription factor | ||
| 6 | SbSTOP1c | SORBI_3007G166000 | Zinc finger transcription factor | ||
| 7 | SbSTOP1d | SORBI_3003G370700 | Zinc finger transcription factor | ||
| 8 | TaSTOP1 | TraesCS3A02G381900, TraesCS3B02G414500, TraesCS3D02G375000 | Zinc finger transcription factor | TaSTOP1 could be a potential candidate gene for genomic-assisted breeding for Al tolerance in bread wheat. | [87] |
| 9 | AtSTOP2 | At5g22890 | Zinc finger transcription factor | STOP2 is a physiologically minor isoform of STOP1 and activates the expression of genes regulated by STOP1. | [88] |
| 10 | SbZNF1 | SORBI_3009G151400 | Zinc finger transcription factor | SbWRKY1 and SbZNF1 transcriptional activation of SbMATE. | [48] |
| 11 | SbWRKY1 | SORBI_3009G174300 | WRKY transcription factor | ||
| 12 | OsWRKY22 | Os01g0820400 | WRKY transcription factor | OsWRKY22 promotes Al-induced increases in OsFRDL4 expression, thus enhancing Al-induced citrate secretion and Al tolerance in rice. | [42] |
| 13 | AtWRKY46 | At2g46400 | WRKY transcription factors | Regulating aluminum-induced malate secretion. | [89] |
| 14 | AtWRKY47 | At4g01720 | WRKY transcription factor | WRKY47 is required for root growth under both normal and Al stress conditions via direct regulation of cell wall modification genes. | [90] |
| 15 | SbWRKY22 | SORBI_3002G418500 | WRKY transcription factor | OE-SbWRKY22/65 plants enhance Al tolerance by reducing callose deposition in roots. | [20] |
| 16 | SbWRKY65 | SORBI_3003G285500 | WRKY transcription factor | ||
| 17 | OsASR1 | Os02g0543000 | ASR (abscisic acid, stress, ripening-induced) family transcription factors | ASR1 and ASR5 act in concert and complementarily regulate gene expression in Al response. | [24] |
| 18 | OsASR5 | Os11g0167800 | ASR (abscisic acid, stress, ripening-induced) family transcription factors | OsASR5 is sequestered in the chloroplasts as an inactive transcription factor that could be released to the nucleus in response to Al to regulate genes related to photosynthesis. | [24,91,92] |
| 19 | AtHB7 | At2g46680 | HD-Zip I transcription factors | AtHB7 and AtHB12 oppositely regulate Al resistance by enacting Al accumulation in root cell walls, enabling homodimers or heterodimers in response to Al stress. | [93] |
| 20 | AtHB12 | At3g61890 | HD-Zip I transcription factors | ||
| 21 | SbHY5 | SORBI_3004G085600 | Basic-leucine zipper (bZIP) transcription factor family protein | SbHY5 confers Al tolerance in plants by modulating Al-SR gene expression. | [94] |
| 22 | OsMYB30 | Os09g0431300 | MYB transcription factor | OsART1 confers Put-promoted Al resistance via the repression of OsMYB30-regulated modification of cell wall properties in rice. | [95] |
| 23 | AtMYB103 | At1g63910 | MYB transcription factor | AtMYB103 acts upstream of AtTBL27 to positively regulate Al resistance by modulating the O-acetylation of the cell wall XyG. | [96] |
| 24 | AtNAC017 | At1g34190 | NAC transcription factors | Regulates Al tolerance in Arabidopsis by positively regulating the expression of AtXTH31. | [97] |
| 25 | AtSOG1 | At1g25580 | NAC transcription factors | Suppressed growth reduction in plants on Al-containing media. sog1 mutants are sensitive to Al. | [98,99] |
| 26 | AtMYC2 | At1g32640 | bHLH transcription factor | Upregulated in response to Al stress in root tips. | [100] |
| 27 | AtLUH | At2g32700 | Groucho-like family of transcriptional corepressor | Promotes Al accumulation in the root cell wall. | [101,102] |
| 28 | AtSLK2 | At5g62090 | SEUSS-like | The atslk2 mutants responded to Al in a similar way as LUH mutants, suggesting that a LUH–SLK2 complex represses the expression of AtPME46. | [101] |
| 29 | AtPIF4 | At2g43010 | Phytochrome interacting factor | AtPIF4 promotes Al-inhibited primary root growth by regulating the local expression of YUCs and auxin signal in the root apex TZ. | [7] |
| 30 | AtRBR1 | At3g12280 | Retinoblastoma protein | RBR1 is targeted to DNA break sites in a CDKB1 activity-dependent manner and partially co-localizes with RAD51 at damage sites. | [103] |
| Kinases/phosphatase | |||||
| 1 | AtWAK1 | At1g21250 | Cell wall-associated receptor kinase | OE-AtWAK1 shows an enhanced Al tolerance in terms of root growth. | [104] |
| 2 | AtCK2 | At4g17640 | Casein kinase | AtCK2 controls the DDR pathway through phosphorylation of SOG1. | [105] |
| 3 | AtRAE1 | At5g01720 | F-box protein | RAH1 and/or RAE1 participate in the regulation of Al resistance and plant growth, and also function as an E3 ligase in the regulation of STOP1 stability. | [6,106] |
| 6 | AtRAH1 | At5g27920 | F-box protein | [106] | |
| 4 | AtRAE2 | At5g56130 | Core component of the THO complex | The atrae2 mutant is less sensitive to Al; RAE2 regulates AtALMT1 and modulates low Pi response. | [107] |
| 5 | AtRAE3/AtHPR1 | At5g09860 | THO/TREX complex | Mutation of RAE3 reduces Al resistance and low phosphate response. | [107,108] |
| 7 | AtESD4/RAE5 | At4g15880 | SMALL UBIQUITIN-LIKE MODIFIER | Mutation of ESD4 increases the level of STOP1 SUMOylation. | [109] |
| 8 | AtSIZ1 | At5g60410 | SUMO E3 ligase | AtSIZ1 regulates Al resistance and low Pi response through the modulation of AtALMT1 expression. “SIZ1–STOP1–ALMT1” is involved in root growth response to Al stress. | [106,109,110,111] |
| 9 | AtMEKK1 | At4g08500 | Mitogen-activated protein kinase (MAPK) kinase kinase kinases | MEKK1-MKK1/2-MPK4 cascade is important for Al signaling and confers Al resistance through phosphorylation-mediated enhancement of STOP1 accumulation in Arabidopsis. | [112] |
| 10 | AtMKK1 | At4g26070 | MAP kinase kinases | ||
| 11 | AtMKK2 | At4g29810 | MAP kinase kinases | ||
| 12 | AtMPK4 | At4g01370 | MAP kinases | ||
| 13 | OsSAL1 | Os06g0717800 | PP2C.D phosphatase | osals1 increased PM H+-ATPase activity and Al uptake, causing hypersensitivity to internal Al toxicity. | [27] |
| 14 | AtPP2C.D5 | At4g38520 | PP2C.D phosphatase | The atpp2c.d5d6d7 triple mutant was more resistant to Al than WT. | [27,28] |
| 15 | AtPP2C.D6 | At3g51370 | PP2C.D phosphatase | ||
| 16 | AtPP2C.D7 | At5g66080 | PP2C.D phosphatase | ||
| 17 | OsA7 | Os04g0656100 | H+-ATPase | OsSAL1 interacts with OsA7 to negatively regulate the PM H+-ATPase function. | [27] |
| 18 | AtPAH1 | At3g09560 | Phosphatidate phosphatase | The pah1/pah2 double mutant shows enhanced Al susceptibility under low-P conditions. | [113] |
| 19 | AtPAH2 | At5g42870 | Phosphatidate phosphatase | ||
| 20 | AtATR | At5g40820 | Plant ATRIP | SUV2 may be a phosphorylation target of ATR. | [114] |
| 21 | OsArPK | Os06g0693000 | Al-related protein kinase | OsArPK expression is induced by longer exposure to a high Al concentration in the roots. | [115] |
| Sugar metabolism | |||||
| 1 | OsEXPA10 | Os04g0583500 | Al-inducible expansin gene | The root cell wall of the knockout lines accumulated less Al than that in the wild type. | [116] |
| 2 | ZmXTH | Zm00001eb414340 | Xyloglucan endotransglucosylase/hydrolase | Overexpression of ZmXTH in Arabidopsis enhanced its tolerance to Al toxicity by reducing Al accumulation in its roots and cell wall. | [117] |
| 3 | AtXTH15 | At4g14130 | Xyloglucan endotransglucosylase/hydrolases | The atxth15 showed enhanced Al resistance. | [118] |
| 4 | AtXTH31 | At3g44990 | Endotransglucosylase-hydrolase | AtXTH31 affects Al sensitivity by modulating cell wall xyloglucan content and Al binding capacity. | [119] |
| 5 | AtTBL27 | At1g70230 | XyG O-acetyltransferase | Modulation of the O-acetylation level of XyG influences the Al sensitivity in Arabidopsis by affecting the Al-binding capacity in hemicellulose. | [96,120] |
| 6 | AtPME46 | At5g04960 | Pectin methylesterase | AtPME46 was found to reduce Al binding to cell walls and alleviate Al-induced root growth inhibition by decreasing PME enzyme activity. | [101] |
| 7 | AtPARVUS | At1g19300 | Glucuronoxylan | The altered properties of hemicellulose contribute to decrease Al accumulation in parvus mutant. | [121] |
| 8 | SbGLU1 | SORBI_3002G402700 | β-1,3-glucanase enzyme | β-1,3-glucanase reduced callose deposition and increased tolerance to aluminum toxicity. | [20,26,122] |
| Hormone-related | |||||
| 1 | AtEIN2 | At5g03280 | Ethylene signaling | Double mutant ein2-1/npr1-1 displayed more sensitivity to Al stress than wild-type plants. | [19] |
| 2 | AtYUC9 | At1g04180 | Flavin monooxygenase-like protein | YUCs regulated local auxin biosynthesis in the root apex TZ, mediating root growth inhibition in response to Al stress. | [123] |
| 3 | AtYUC8 | At4g28720 | Flavin monooxygenase-like protein | ||
| 4 | AtYUC7 | At2g33230 | Flavin monooxygenase-like protein | ||
| 5 | AtYUC3 | At1g04610 | Flavin monooxygenase-like protein | ||
| 6 | AtYUC5 | At5g43890 | Flavin monooxygenase-like protein | ||
| 7 | AtTAA1 | At1g70560 | Trp aminotransferase | TAA1 is specifically upregulated in the root apex TZ in response to Al treatment. | [7,124] |
| 8 | AtCOI1 | At2g39940 | Coronatine-insensitive | AtCOI1-mediated Al-induced root growth inhibition under Al stress controlled by ethylene. | [100] |
| 9 | AtSUR1 | At2g20610 | Tyrosine transaminase family protein | SUR1 promotes IAA biosynthesis via the indole-3-acetaldoxime pathway, superroot2, and superroot1 mutant increased Al sensitivity. | [118,125] |
| 10 | AtSUR2 | At4g31500 | Cytochrome P450 CYP83B1 | SUR2 may be involved in the control of auxin conjugation, and the superroot2 and superroot1 mutant had increased aluminum sensitivity. | [118,126] |
| 11 | AtNPR1 | At1g64280 | Regulatory protein | Double mutant ein2-1/npr1-1 displayed more sensitivity to Al stress than wild-type plants. | [19] |
| ROS metabolism | |||||
| 1 | OsApx1 | Os03g0285700 | Ascorbate peroxidases | Apx1/2-silenced plants also showed increased H2O2 accumulation under control and stress situations and presented higher tolerance to a toxic concentration of Al when compared to WT. | [127] |
| 2 | OsApx2 | Os07g0694700 | Ascorbate peroxidases | ||
| 3 | AtGR1 | At3g24170 | Glutathione reductase | GR, an efficient approach to enhance Al tolerance, maintained GSH and reinforced dual detoxification functions in plants. | [128] |
| 4 | AtGST1 | At1g02930 | Glutathione S-transferase | Gene expression in response to Al stresses. | [129] |
| 5 | AtGST11 | At1g02920 | Glutathione S-transferase | ||
| 6 | AtPrx64 | At5g42180 | Peroxidases | The AtPrx64 gene increases the root growth and reduces Al accumulation and ROS in roots. | [130] |
| 7 | AtAOX1a | At3g22370 | Alternative oxidase | AtAOX1a alleviates Al-induced PCD by maintaining mitochondrial function and promoting the expression of protective functional genes. | [131] |
| 8 | ZmAT6 | Zm00001eb154120 | - | ZmAT6 confers aluminum tolerance via reactive oxygen species scavenging. | [132] |
| 9 | ZmALDH | Zm00001d017418 | Aldehyde dehydrogenase | ZmALDH participates in Al-induced oxidative stress and Al accumulation in roots. | [25] |
| 10 | AtNADP-ME1 | At2g19900 | NADP-dependent malic enzyme | NADP-ME1 is involved in adjusting the malate levels in the root apex, and its loss results in an increased content of this organic acid. | [133] |
| Other processes | |||||
| 1 | AtGRP3 | At2g05520 | Glycine-rich protein | AtGRP3 functions in root size determination during development and in Al stress. | [134] |
| 2 | AtCBL1 | At4g17615 | Calcineurin B-like calcium sensors | Mutation of CBL1 suppresses root malate efflux. | [135] |
| 3 | AtALS7 | At1g72480 | Ribosomal biogenesis factor | The atals7–1 is related to the expression of the S-adenosylmethionine recycling factor and reduced levels of endogenous polyamines. | [136] |
| 4 | AtSWA2 | At1g72440 | CCAAT-box binding factor | AtSWA2 is required for normal gametogenesis and mitotic progression. | [136] |
| 5 | OsGERLP | Os03g0168900 | Ribosomal L32-like protein | Low expression of OsGERLP caused the gene-silenced rice to be sensitive to Al, while high expression induced the Al tolerance in transgenic tobacco. | [137] |
| 6 | AtVHA-a2 | At2g21410 | Subunit of the vacuolar H+-ATPase (V-ATPase) | The vha-a2 vha-a3 mutants displayed less sensitivity with lower Al accumulation in the roots compared to the wild-type plants when grown under excessive Al3+. | [8] |
| 7 | AtVHA-a3 | At4g39080 | Subunit of the vacuolar H+-ATPase (V-ATPase) | ||
| 8 | AtRAD51 | At3g22880 | DNA repair (Rad51) family protein | RBR1 targets DNA break sites in CDKB1-CYCB1 complexes in an activity-dependent manner and partially co-localizes with RAD51 at damage sites. | [103] |
| 9 | AtCYCB1 | At4g37490 | Cyclin | ||
| 10 | AtSUV2 | At5g45610 | Putative plant ATRIP homolog | Loss of SUV2 reverses hypersensitivity of als3-1 to Al. SUV2 detects Al damage in an ATR-dependent manner and is required for Al-dependent cell cycle arrest and terminal differentiation. | [114] |
| 11 | AtTANMEI/ALT2 | At4g29860 | WD40 protein | ALT2 is required for active stoppage of root growth after Al exposure. | [138] |
| 12 | AtPGIP1 | At5g06860 | P450-dependent monooxygenases | Involved in STOP1-dependent regulation in phosphoinositide signaling pathway, and regulates PGIP1 expression under Al stress | [139] |
| 13 | AtALT1 | At1g35290 | Thioesterase | The alt1 mutant positively impacts Al resistance in a manner dependent on pH adjustment. | [78] |
| 14 | OsRAL1/4CL4 | Os06g0656500 | 4-Coumarate: coenzyme A ligase | 4-coumaric acid and ferulic acid reduce Al binding to hemicellulose and consequently enhances Al resistance in ral1/4cl4 mutants. | [140,141] |
| 15 | Os4CL3 | Os02g0177600 | 4-Coumarate: coenzyme A ligase | 4CL3 is involved in the regulation of lignin accumulation and Al resistance. | [140,142] |
| 16 | Os4CL5 | Os08g0448000 | 4-Coumarate: coenzyme A ligase | Enhances resistance of os4cl5 mutant to Al. | [142] |
| 17 | OsCS1 | Os02g0194100 | Citrate synthase | OsCS1 is induced by Al toxicity | [143] |
| 18 | TaWali1 | TraesCS1A02G115900 | - | The Tawali1 and Tawali5 mutants have a generalized response for Al stress. | [144] |
| 19 | TaWali5 | TraesCS1D02G265800 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; Wu, J.; Liang, W. Systematic Investigation of Aluminum Stress-Related Genes and Their Critical Roles in Plants. Int. J. Mol. Sci. 2024, 25, 9045. https://doi.org/10.3390/ijms25169045
Fang C, Wu J, Liang W. Systematic Investigation of Aluminum Stress-Related Genes and Their Critical Roles in Plants. International Journal of Molecular Sciences. 2024; 25(16):9045. https://doi.org/10.3390/ijms25169045
Chicago/Turabian StyleFang, Chaowei, Jiajing Wu, and Weihong Liang. 2024. "Systematic Investigation of Aluminum Stress-Related Genes and Their Critical Roles in Plants" International Journal of Molecular Sciences 25, no. 16: 9045. https://doi.org/10.3390/ijms25169045
APA StyleFang, C., Wu, J., & Liang, W. (2024). Systematic Investigation of Aluminum Stress-Related Genes and Their Critical Roles in Plants. International Journal of Molecular Sciences, 25(16), 9045. https://doi.org/10.3390/ijms25169045






