The Beneficial Effects of Prenatal Biotin Supplementation in a Rat Model of Intrauterine Caloric Restriction to Prevent Cardiometabolic Risk in Adult Female Offspring
Abstract
1. Introduction
2. Results
2.1. Food Intake and Body Weight in Dams and Body Weight Gain, Caloric Intake, Feed Conversion Ratio, and Lee’s Index in Female Offspring
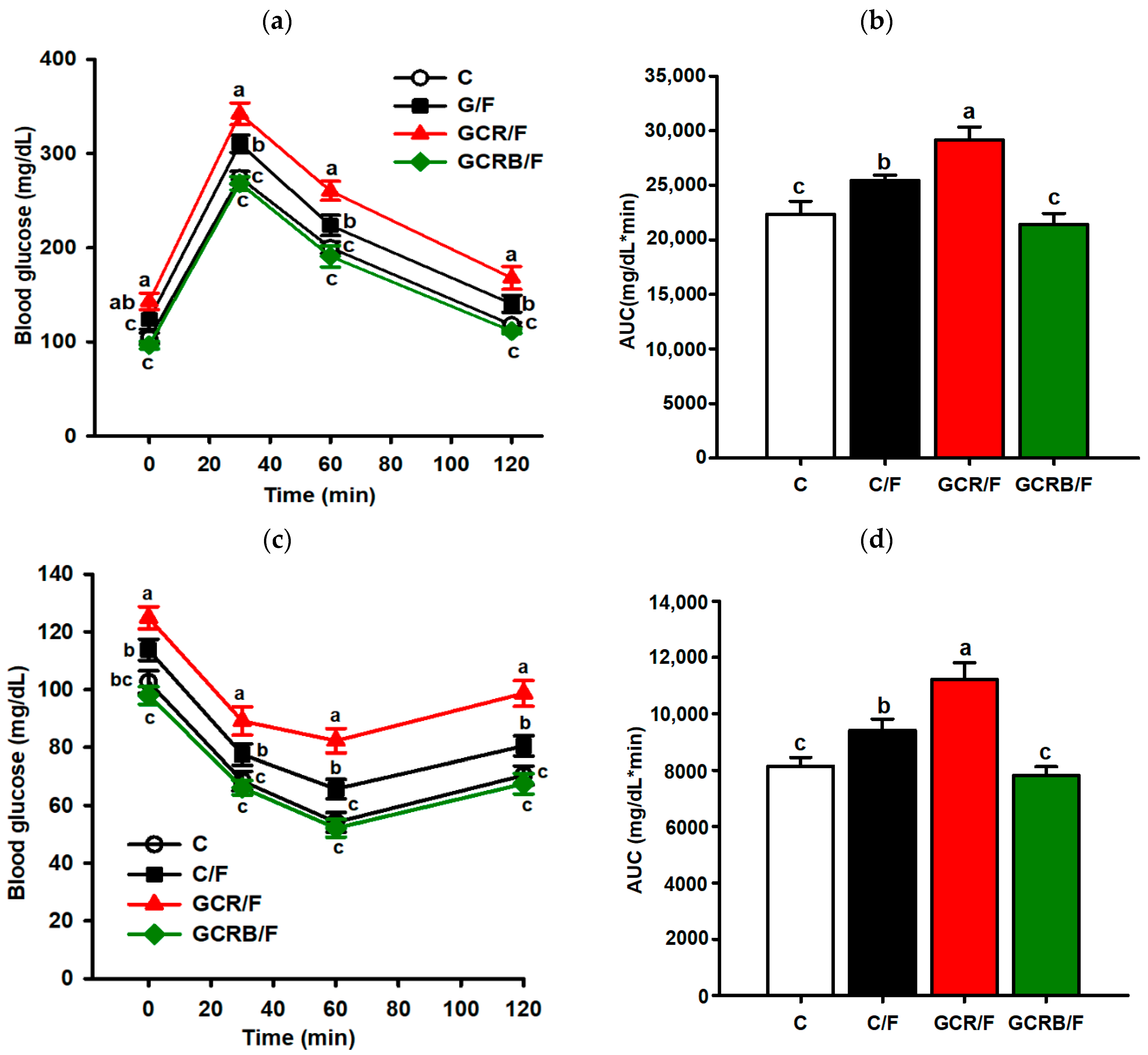
2.2. Intraperitoneal Glucose Tolerance and Insulin Resistance Testing
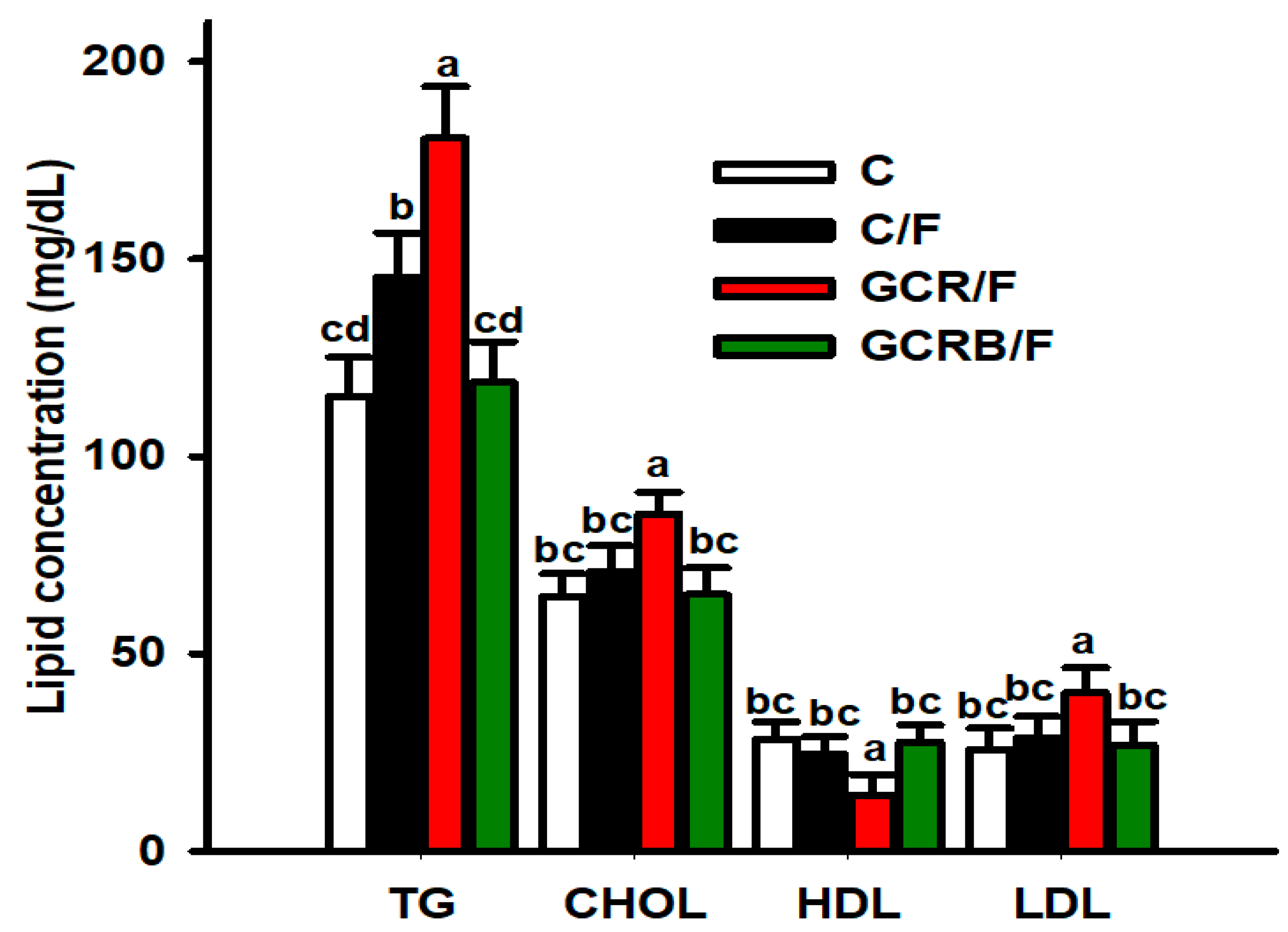
2.3. Serum Lipid Profile Parameters
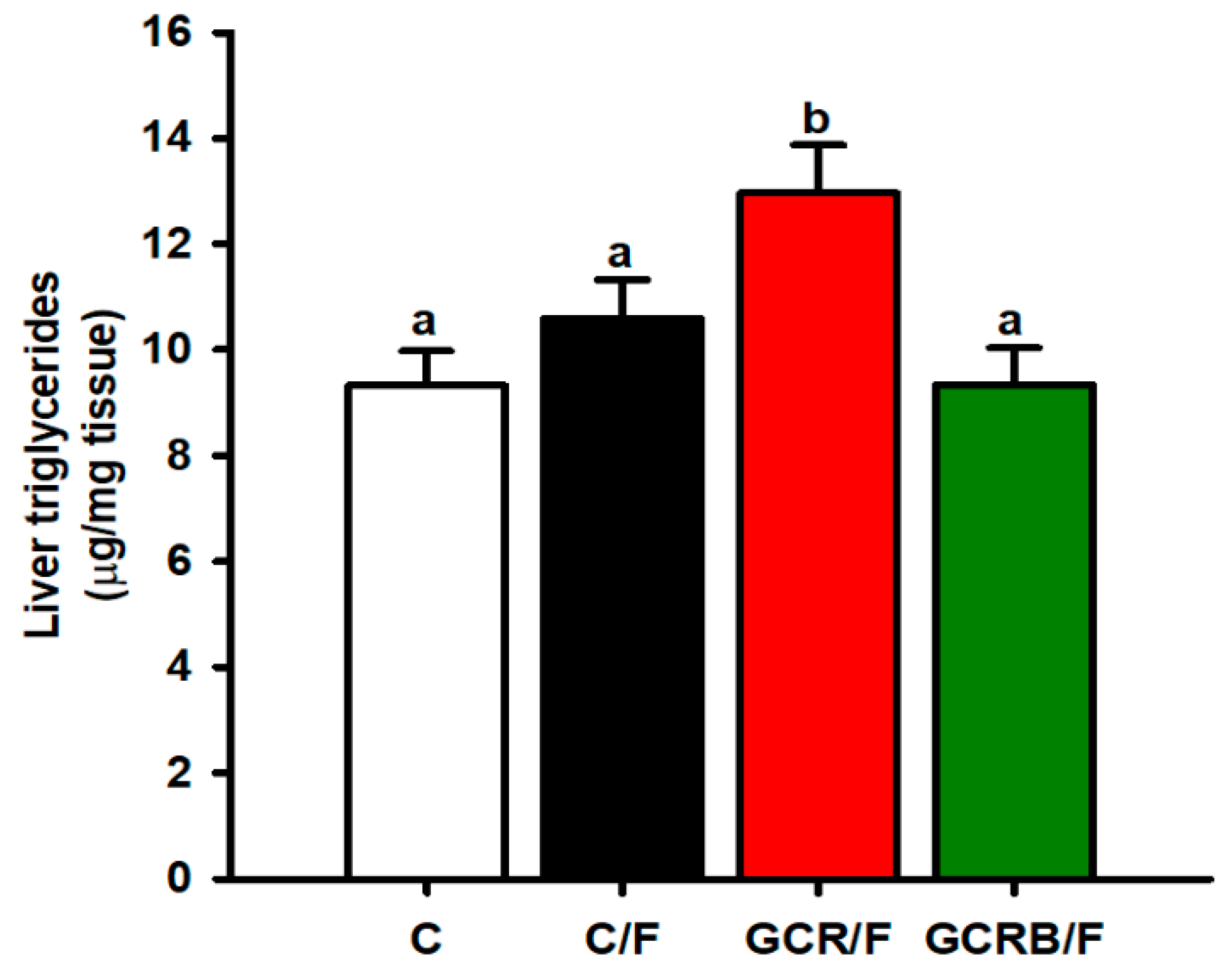
2.4. Hepatic Triglyceride Quantification
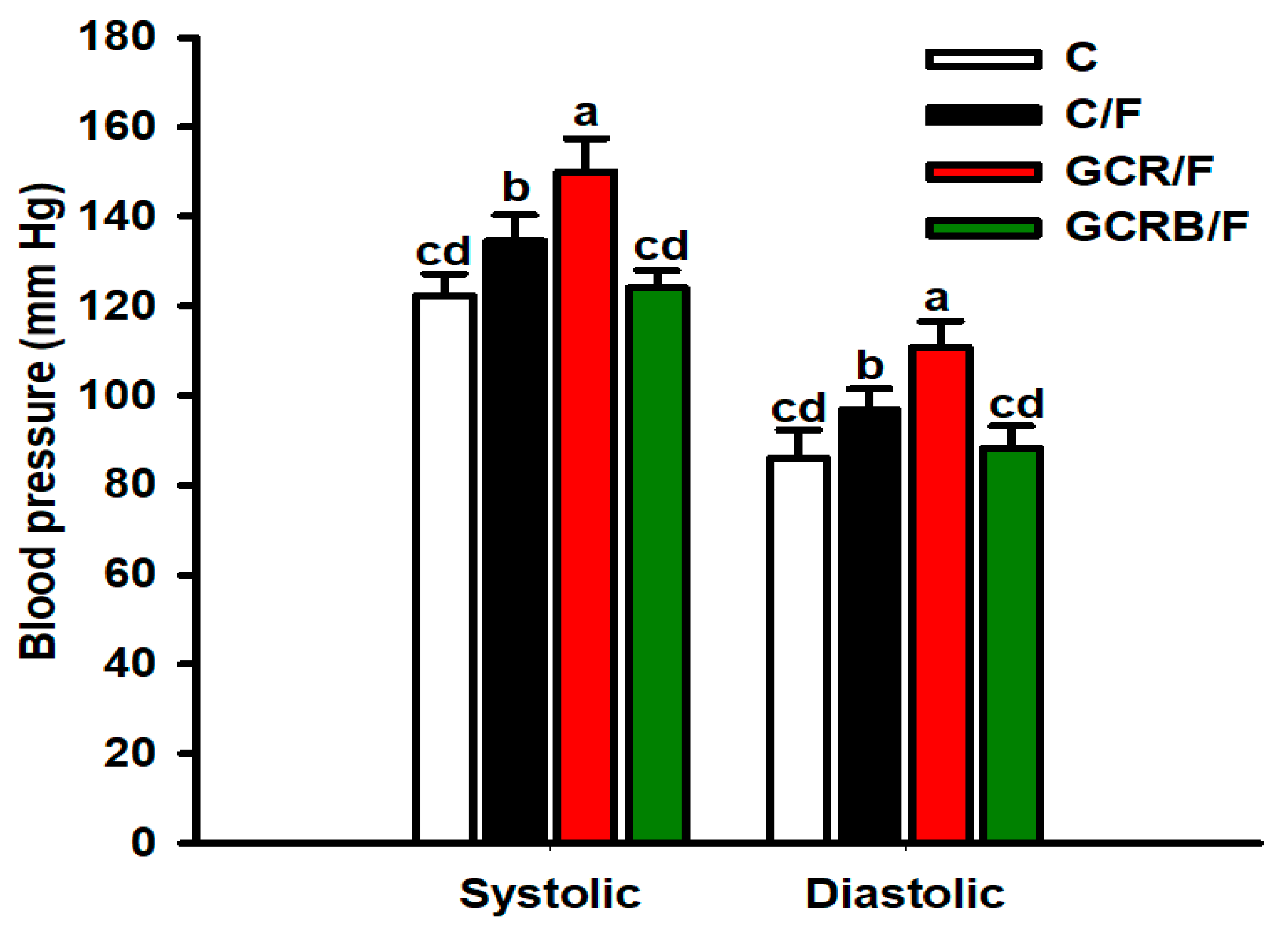
2.5. Arterial Blood Pressure Measurement
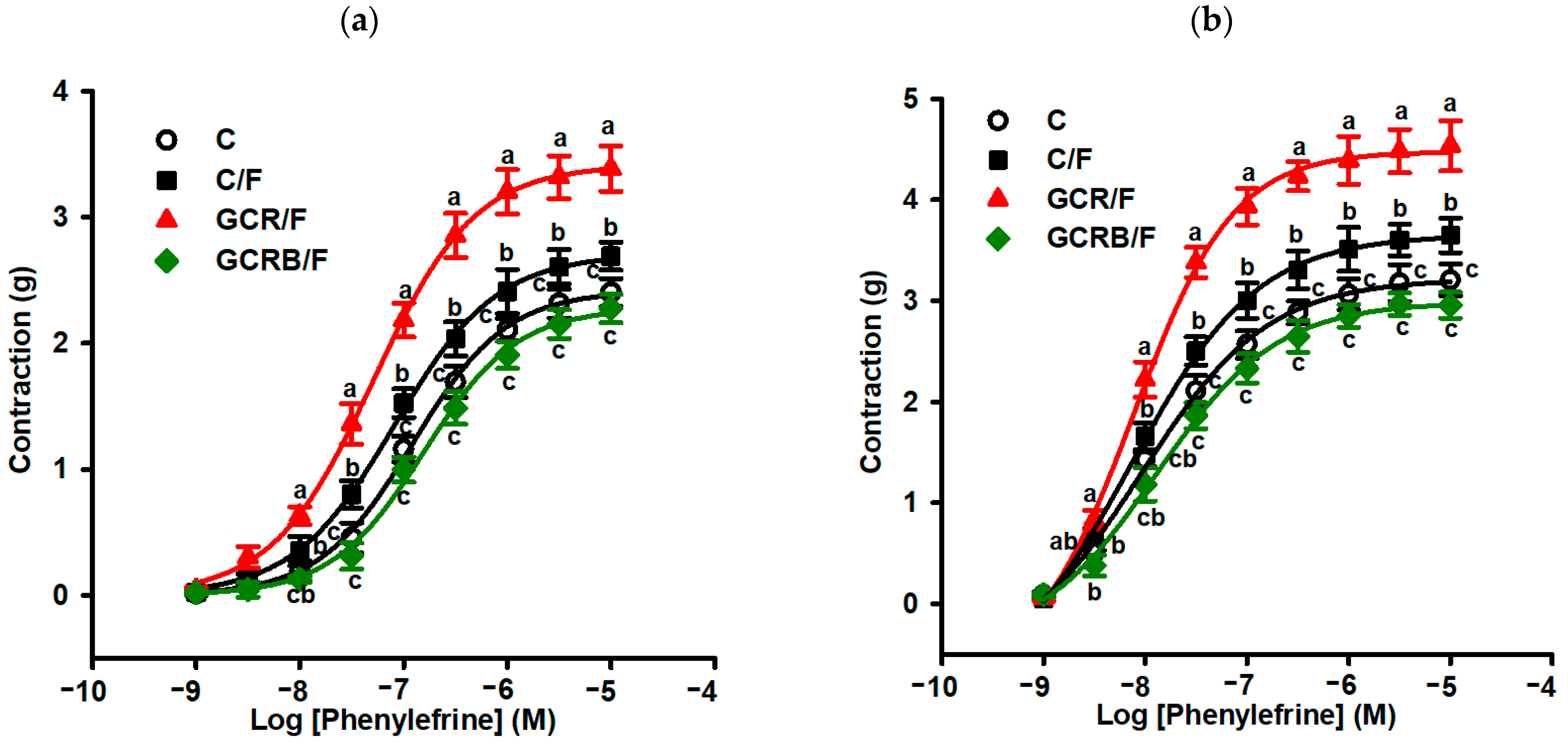
2.6. Measurement of Vascular Reactivity
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
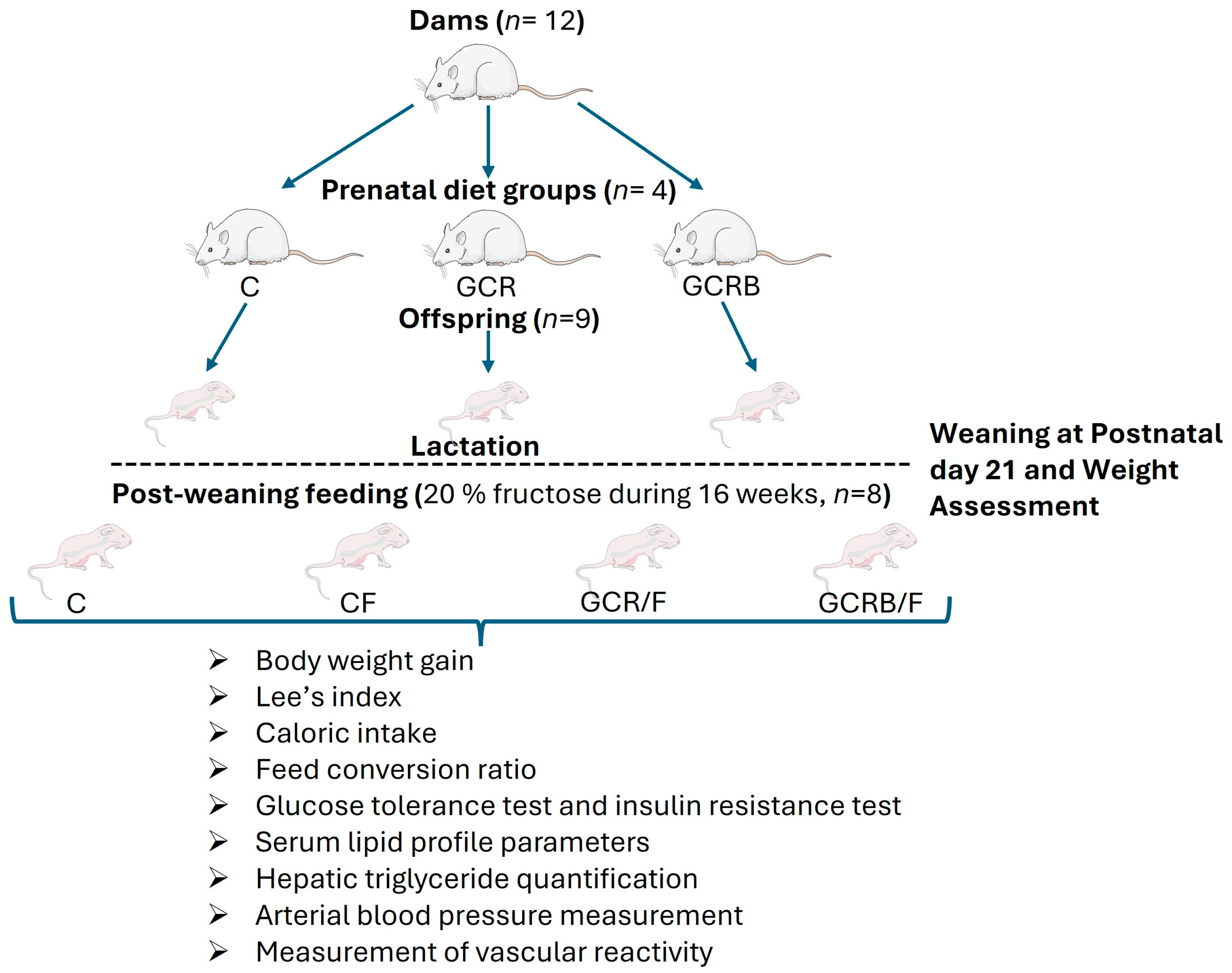
4.2. Experimental Design
4.3. Assessment of Food Intake, Body Weight, Body Weight Gain, Caloric Intake, Feed Conversion Ratio, and Lee’s Index
4.4. Assessment of Intraperitoneal Glucose Tolerance and Insulin Resistance Testing
4.5. Serum Triglyceride, Total Cholesterol, LDL, and HDL Concentration Analysis
4.6. Measurement of Hepatic Triglyceride
4.7. Blood Pressure Measurement
4.8. Measurement of Vascular Reactivity in Aortic Rings
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Beckett, A.; Scott, J.R.; Chater, A.M.; Ferrandino, L.; Aldous, J.W.F. The Prevalence of Metabolic Syndrome and Its Components in Firefighters: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6814. [Google Scholar] [CrossRef]
- Hildreth, J.; Vickers, M.; Buklijas, T.; Bay, J. Understanding the importance of the early-life period for adult health: A systematic review. J. Dev. Orig. Health Dis. 2023, 14, 166–174. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Irakoze, L.; Manirakiza, A.; Zhang, Y.; Liu, J.; Li, J.; Nkengurutse, L.; Deng, S.; Xiao, X. Metabolic Syndrome in Offspring of Parents with Metabolic Syndrome: A Meta-Analysis. Obes. Facts 2021, 14, 148–162. [Google Scholar] [CrossRef]
- Mutamba, A.K.; He, X.; Wang, T. Therapeutic advances in overcoming intrauterine growth restriction induced metabolic syndrome. Front. Pediatr. 2023, 10, 1040742. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Z.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan. J. Obs. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Aiken, C.E.; Ozanne, S.E. Sex differences in developmental programming models. Reproduction 2013, 145, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Cauzzo, C.; Chiavaroli, V.; Di Valerio, S.; Chiarelli, F. Birth size, growth trajectory and later cardio-metabolic risk. Front. Endocrinol. 2023, 14, 1187261. [Google Scholar] [CrossRef]
- Vickers, M.H. Utility of preclinical models of altered maternal nutrition to support the developmental origins of health and disease hypothesis. Clin. Sci. 2022, 136, 711–714. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Aguilera-Méndez, A.; Boone-Villa, D.; Nieto-Aguilar, R.; Villafaña-Rauda, S.; Molina, A.S.; Sobrevilla, J.V. Role of vitamins in the metabolic syndrome and cardiovascular disease. Pflug. Arch. 2022, 474, 117–140. [Google Scholar] [CrossRef]
- Aguilera-Méndez, A.; Espino-García, R.; Toledo-López, Z.; Hernández-Gallegosa, Z.; Villafaña-Raudab, S.; Nieto-Aguilar, R.; Serrato-Ochoa, D.; Manuel-Jacobo, G.C. Biotin improves relaxation of rat aortic rings in combination with antihypertensive drugs. PharmaNutrition 2019, 8, 100147. [Google Scholar] [CrossRef]
- Aguilera-Mendez, A.; Hernández-Equihua, M.G.; Rueda-Rocha, A.C.; Guajardo-López, C.; Nieto-Aguilar, R.; Serrato-Ochoa, D.; Ruíz Herrera, L.F.; Guzmán-Nateras, J.A. Protective effect of supplementation with biotin against high-fructose-induced metabolic syndrome in rats. Nutr. Res. 2018, 57, 86–96. [Google Scholar] [CrossRef]
- Riverón, N.L.; Fernandez, M.C. Pharmacological Effects of Biotin in Animals. Mini Rev. Med. Chem. 2017, 17, 529–540. [Google Scholar] [CrossRef]
- do Carmo Franco, M.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sertie, R.; Kang, M.; Antipenko, J.P.; Liu, X.; Maianu, L.; Habegger, K.; Garvey, W.T. In utero nutritional stress as a cause of obesity: Altered relationship between body fat, leptin levels and caloric intake in offspring into adulthood. Life Sci. 2020, 254, 117764. [Google Scholar] [CrossRef]
- Michońska, I.; Łuszczki, E.; Zielińska, M.; Oleksy, Ł.; Stolarczyk, A.; Dereń, K. Nutritional Programming: History, Hypotheses, and the Role of Prenatal Factors in the Prevention of Metabolic Diseases-A Narrative Review. Nutrients 2022, 14, 4422. [Google Scholar] [CrossRef]
- Durda-Masny, M.; Stróżewska, W.; Szwed, A. Catch-Up Growth as a Risk Factor for Rapid Weight Gain, Earlier Menarche and Earlier Pubertal Growth Spurt in Girls Born Small for Gestational Age (SGA)-A Longitudinal Study. Int. J. Environ. Res. Public Health 2022, 19, 16808. [Google Scholar] [CrossRef] [PubMed]
- Oke, S.L.; Sohi, G.; Hardy, D.B. Perinatal protein restriction with postnatal catch-up growth leads to elevated p66Shc and mitochondrial dysfunction in the adult rat liver. Reproduction 2020, 159, 27–39. [Google Scholar] [CrossRef]
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Mohd Yusof, M.R.; Haji Suhaimi, F. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. BioMed Res. Int. 2014, 2014, 263897. [Google Scholar] [CrossRef]
- Wickramasinghe, V.P.; Arambepola, C. The effect of foetal and early childhood growth on metabolic derangements of Sri Lankan children. BMC Pediatr. 2023, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.N.; Rajindrajith, S. Fetal programming of obesity and type 2 diabetes. World J. Diabetes 2022, 13, 482–497. [Google Scholar] [CrossRef]
- Aguilera-Méndez, A. Esteatosis hepática no alcohólica: Una enfermedad silente [Nonalcoholic hepatic steatosis: A silent disease]. Rev. Med. Inst. Mex. Seguro Soc. 2019, 56, 544–549. [Google Scholar]
- Deegan, D.F.; Nigam, P.; Engel, N. Sexual Dimorphism of the Heart: Genetics, Epigenetics, and Development. Front. Cardiovasc. Med. 2021, 8, 668252. [Google Scholar] [CrossRef] [PubMed]
- Talbot, C.P.J.; Dolinsky, V.W. Sex differences in the developmental origins of cardiometabolic disease following exposure to maternal obesity and gestational diabetes 1. Appl. Physiol. Nutr. Metab. 2019, 44, 687–695. [Google Scholar] [CrossRef]
- Boone-Villa, D.; Aguilera-Méndez, A.; Miranda-Cervantes, A.; Fernandez-Mejia, C. Effects of Biotin Supplementation in the Diet on Adipose Tissue cGMP Concentrations, AMPK Activation, Lipolysis, and Serum-Free Fatty Acid Levels. J. Med. Food 2015, 18, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Méndez, A.; Fernández-Mejía, C. The hypotriglyceridemic effect of biotin supplementation involves increased levels of cGMP and AMPK activation. Biofactors 2012, 38, 387–394. [Google Scholar] [CrossRef]
- Lazo-de-la-Vega-Monroy, M.L.; Larrieta, E.; Tixi-Verdugo, W.; Ramírez-Mondragón, R.; Hernández-Araiza, I.; German, M.S. Effects of dietary biotin supplementation on glucagon production, secretion, and action. Nutrition 2017, 43–44, 47–53. [Google Scholar] [CrossRef]
- More, V.R.; Lao, J.; McLaren, D.G.; Cumiskey, A.M.; Murphy, B.A.; Chen, Y. Glucagon like receptor 1/ glucagon dual agonist acutely enhanced hepatic lipid clearance and suppressed de novo lipogenesis in mice. PLoS ONE 2017, 12, e0186586. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Kamiyama, M.; Kamiyama, S.; Horiuchi, K.; Ohinata, K.; Shirakawa, H.; Furukawa, Y.; Komai, M. Antihypertensive effect of biotin in stroke-prone spontaneously hypertensive rats. Br. J. Nutr. 2008, 99, 756–763. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Early-Life Programming and Reprogramming of Adult Kidney Disease and Hypertension: The Interplay between Maternal Nutrition and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3572. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Elkins, R.; Yallampalli, U.; Yallampalli, C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J. Vasc. Res. 2009, 46, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Safi-Stibler, S.; Gabory, A. Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin. Cell Dev. Biol. 2020, 97, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Khajebishak, Y.; Alivand, M.; Faghfouri, A.H.; Moludi, J.; Payahoo, L. The effects of vitamins and dietary pattern on epigenetic modification of non-communicable diseases. Int. J. Vitam. Nutr. Res. 2023, 93, 362–377. [Google Scholar] [CrossRef]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
| Variables | Dams Group | |||||
|---|---|---|---|---|---|---|
| C | GCR | GCRB | ||||
| Food intake (g/24 h) | 21.5 ± 1.4 a | 17.3 ± 0.87 b | 17.6 ± 0.92 b | |||
| Litter size | 11 ± 0.85 a | 10 ± 1.08 a | 11 ± 0.93 a | |||
| Males | 5 ± 0.21 a | 4 ± 0.38 a | 5 ± 0.27 a | |||
| Females | 6 ± 0.3 a | 6 ± 0.56 a | 6 ± 0.33 a | |||
| Initial body weight (g) | 188.8 ± 2.7 a | 190.5 ± 3.1 a | 187.1 ± 3.5 a | |||
| Final body weight (g) | 257.6 ± 5.8 a | 224.3 ± 7.5 b | 254.5 ± 6.1 a | |||
| Offspring Group | ||||||
| C | C/F | GCR/F | GCRB/F | |||
| Food intake (g/24 h) | 21.8 ± 1.1 a | 15.1 ± 0.95 b | 16.5 ± 0.81 b | 15.8 ± 0.85 b | ||
| Caloric intake (kcal/g/day) | 69.76 ± 2.5 a | 84.24 ± 3.7 b | 83.55 ± 3.3 b | 81.94 ± 3.1 b | ||
| Feed conversion ratio (g/g) | 5.70 ± 0.8 a | 3.75 ± 0.6 b | 4.18 ± 0.7 bc | 4.04 ± 0.9 bc | ||
| BW at weaning (g) | 53.5 ± 2.8 a | 52.0 ± 3.1 a | 41.8 ± 3.2 b | 45.2 ± 2.9 b | ||
| Final body weight (g) | 283.2 ± 7.5 a | 293.5 ± 9.9 b | 278.6 ± 9.4 a | 279.8 ± 8.2 a | ||
| Body weight gain (g/day) | 3.82 ± 0.1 a | 4.02 ± 0.1 b | 3.94 ± 0.3 b | 3.91 ± 0.2 b | ||
| Lee’s index | 0.30 ± 0.011 a | 0.31 ± 0.08 b | 0.30 ± 0.09 a | 0.30 ± 0.07 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera-Méndez, A.; Figueroa-Fierros, I.; Ruiz-Pérez, X.; Godínez-Hernández, D.; Saavedra-Molina, A.; Rios-Chavez, P.; Villafaña, S.; Boone-Villa, D.; Ortega-Cuellar, D.; Gauthereau-Torres, M.Y.; et al. The Beneficial Effects of Prenatal Biotin Supplementation in a Rat Model of Intrauterine Caloric Restriction to Prevent Cardiometabolic Risk in Adult Female Offspring. Int. J. Mol. Sci. 2024, 25, 9052. https://doi.org/10.3390/ijms25169052
Aguilera-Méndez A, Figueroa-Fierros I, Ruiz-Pérez X, Godínez-Hernández D, Saavedra-Molina A, Rios-Chavez P, Villafaña S, Boone-Villa D, Ortega-Cuellar D, Gauthereau-Torres MY, et al. The Beneficial Effects of Prenatal Biotin Supplementation in a Rat Model of Intrauterine Caloric Restriction to Prevent Cardiometabolic Risk in Adult Female Offspring. International Journal of Molecular Sciences. 2024; 25(16):9052. https://doi.org/10.3390/ijms25169052
Chicago/Turabian StyleAguilera-Méndez, Asdrubal, Ian Figueroa-Fierros, Xóchilt Ruiz-Pérez, Daniel Godínez-Hernández, Alfredo Saavedra-Molina, Patricia Rios-Chavez, Santiago Villafaña, Daniel Boone-Villa, Daniel Ortega-Cuellar, Marcia Yvette Gauthereau-Torres, and et al. 2024. "The Beneficial Effects of Prenatal Biotin Supplementation in a Rat Model of Intrauterine Caloric Restriction to Prevent Cardiometabolic Risk in Adult Female Offspring" International Journal of Molecular Sciences 25, no. 16: 9052. https://doi.org/10.3390/ijms25169052
APA StyleAguilera-Méndez, A., Figueroa-Fierros, I., Ruiz-Pérez, X., Godínez-Hernández, D., Saavedra-Molina, A., Rios-Chavez, P., Villafaña, S., Boone-Villa, D., Ortega-Cuellar, D., Gauthereau-Torres, M. Y., Nieto-Aguilar, R., & Palomera-Sanchez, Z. (2024). The Beneficial Effects of Prenatal Biotin Supplementation in a Rat Model of Intrauterine Caloric Restriction to Prevent Cardiometabolic Risk in Adult Female Offspring. International Journal of Molecular Sciences, 25(16), 9052. https://doi.org/10.3390/ijms25169052






