tRF-Gly-GCC in Atretic Follicles Promotes Ferroptosis in Granulosa Cells by Down-Regulating MAPK1
Abstract
1. Introduction
2. Results
2.1. The Occurrence of Ferroptosis in Sows during Follicular Atresia
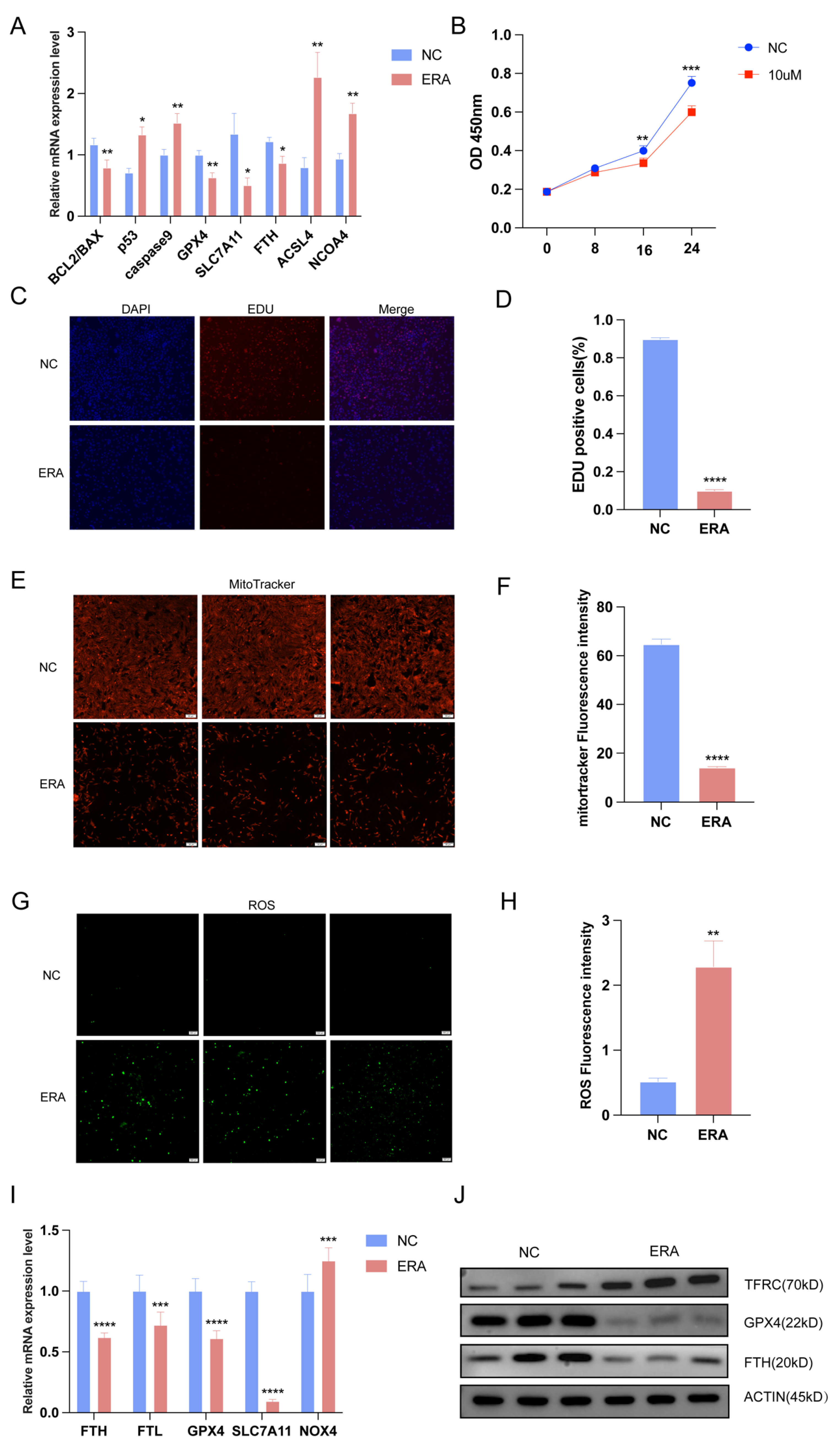
2.2. Ferroptosis Inducers Inhibit Granulosa Cell Proliferation and Promote Ferroptosis of Granulosa Cell
2.3. Differential Expression of tRFs in Healthy Follicles after Treatment with Ferroptosis Inducer Erastin
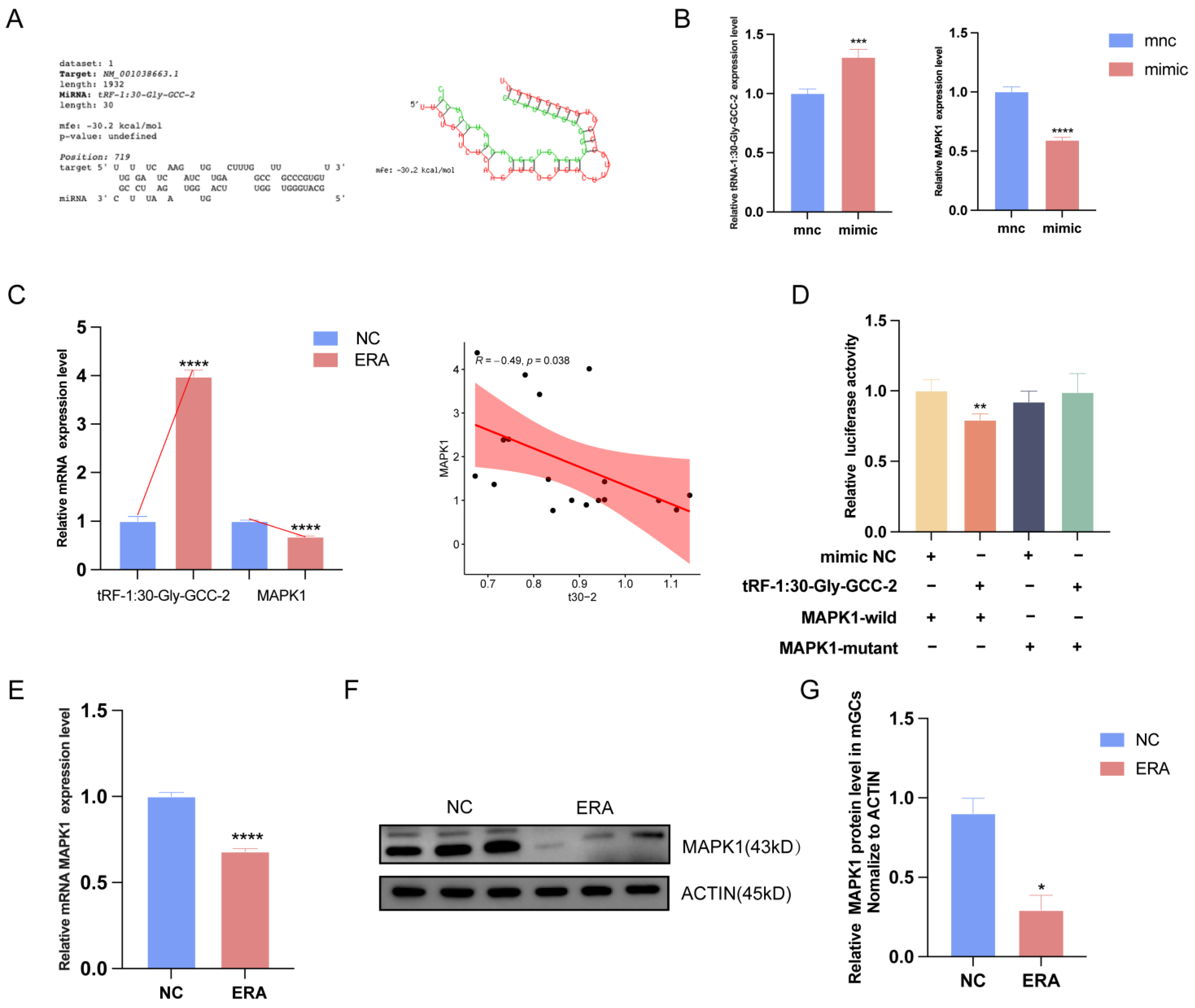
2.4. MAPK1 Is a Positional Target of tRF-1:30-Gly-GCC-2
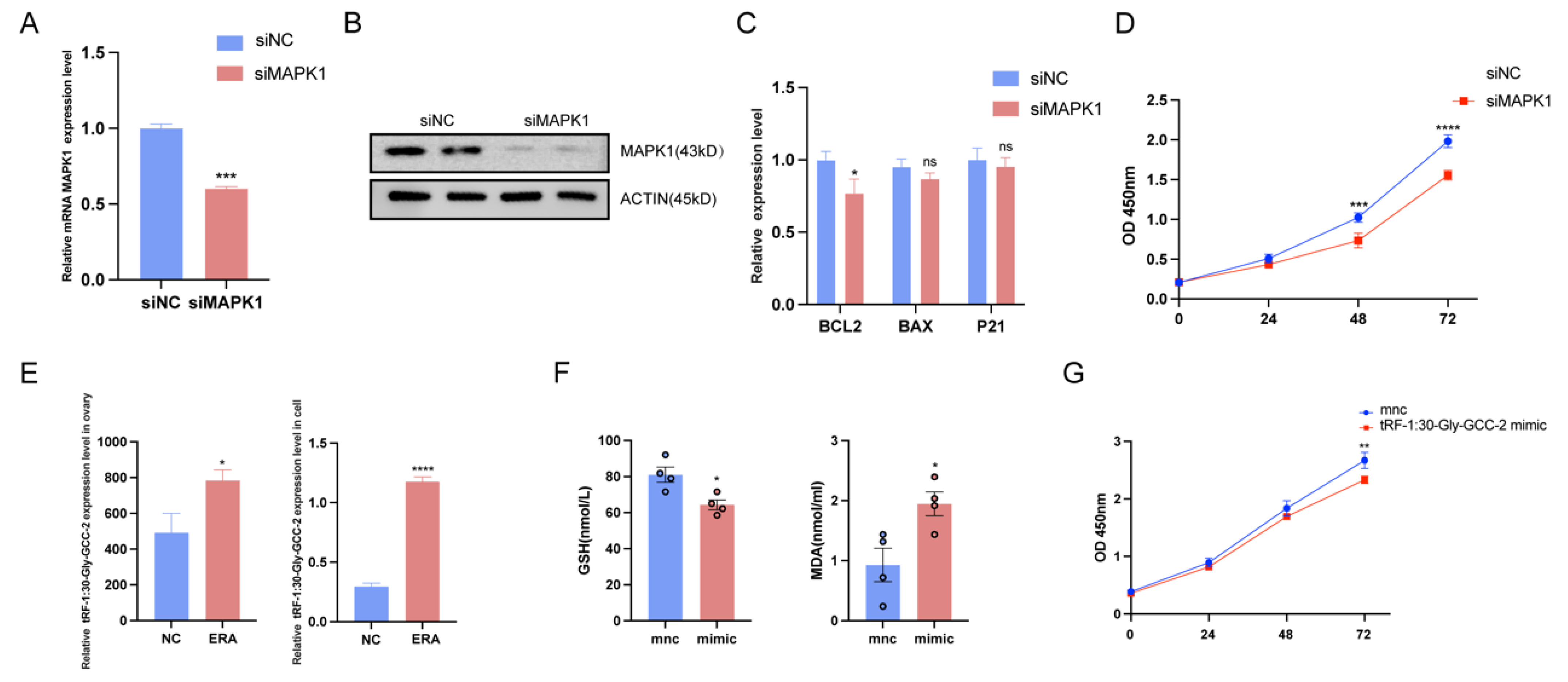
2.5. Overexpression of tRF-1:30-Gly-GCC-2 or MAPK1 Knockdown Inhibited Granulosa Cell Proliferation
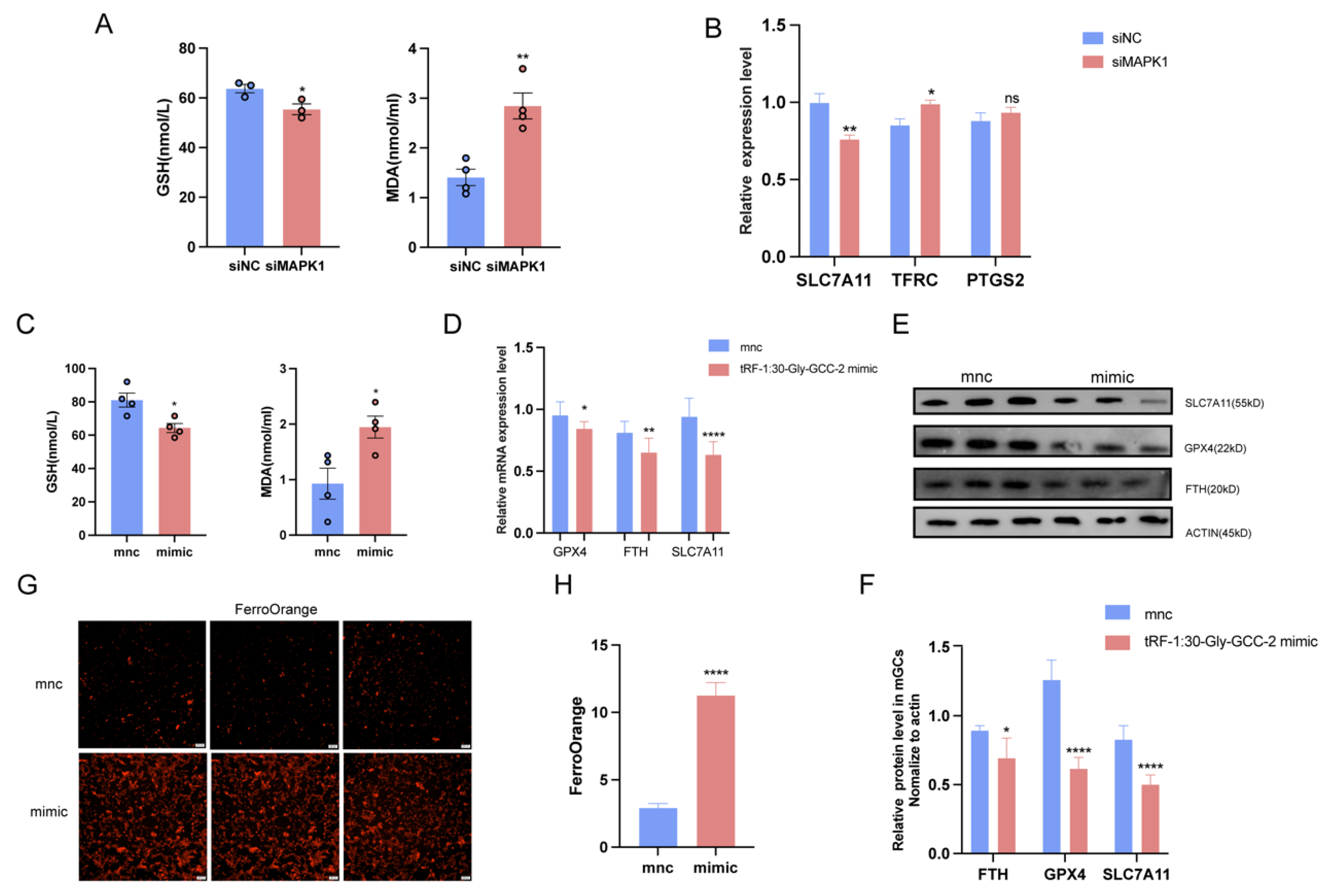
2.6. tRF-1:30-Gly-GCC-2 Inhibits Granular Cell Proliferation and Promotes Ferroptosis by Targeting MAPK1
3. Discussion
4. Materials and Methods
4.1. Dissection, Culture, and Treatment of Porcine Follicles
4.1.1. Preparation of Follicle Samples
4.1.2. Handling of Follicle Samples
4.2. Cell Culture
4.3. RNA Extraction and Reverse Transcription
4.4. Real-Time Fluorescent Quantitative PCR
4.5. Assessment of Reduced Glutathione (GSH), Malondialdehyde (MDA), and Iron Levels
4.6. Immunofluorescence
4.6.1. EDU
4.6.2. Mitotracker
4.6.3. ROS
4.7. Cell Viability Assay
4.8. Western Blot
4.9. Criteria for Determining the Degree of Follicular Atresia
4.10. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beltran-Alcrudo, D.; Falco, J.R.; Raizman, E. Transboundary spread of pig diseases: The role of international trade and travel. BMC Vet. Res. 2019, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Stringer, J.M.; Alesi, L.R.; Winship, A.L. Beyond apoptosis: Evidence of other regulated cell death pathways in the ovary throughout development and life. Hum. Reprod. Update 2023, 29, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Sung, Y.-W.; Hsueh, Y.-W. Growth hormone in fertility and infertility: Mechanisms of action and clinical applications. Front. Endocrinol. 2022, 13, 1040503. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bialecka, M.; Moustakas, I. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nat. Commun. 2019, 10, 3164. [Google Scholar] [CrossRef]
- Gougeon, A. Some aspects of the dynamics of ovarian follicular growth in the human. Acta Eur. Fertil. 1989, 20, 185–192. [Google Scholar] [PubMed]
- Duffy, D.M.; Ko, C.; Jo, M. Ovulation: Parallels With Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zhang, S. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 2021, 11, 3052–3059. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Wu, Y. The emerging role of ferroptosis in female reproductive disorders. Biomed. Pharmacother. 2023, 166, 115415. [Google Scholar] [CrossRef]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 99, 979–997. [Google Scholar] [CrossRef]
- Wen, J.-T.; Huang, Z.-H.; Li, Q.-H. Research progress on the tsRNA classification, function, and application in gynecological malignant tumors. Cell Death Discov. 2021, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.; Shi, J. Origins and evolving functionalities of tRNA-derived small RNAs. Trends Biochem. Sci. 2021, 46, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ji, X. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Roskoski, R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-B.; Baek, K.-H. E3 ligases and deubiquitinating enzymes regulating the MAPK signaling pathway in cancers. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188736. [Google Scholar] [CrossRef]
- Spears, N.; Lopes, F.; Stefansdottir, A. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef]
- Prerna, K.; Dubey, V.K. Beclin1-mediated interplay between autophagy and apoptosis: New understanding. Int. J. Biol. Macromol. 2022, 204, 258–273. [Google Scholar] [CrossRef]
- Gielecińska, A.; Kciuk, M.; Yahya, E.B. Apoptosis, necroptosis, and pyroptosis as alternative cell death pathways induced by chemotherapeutic agents? Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 189024. [Google Scholar] [CrossRef]
- Kaur, S.; Kurokawa, M. Regulation of Oocyte Apoptosis: A View from Gene Knockout Mice. Int. J. Mol. Sci. 2023, 24, 1345. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef]
- Zheng, D.; Liu, J.; Piao, H. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, D.; Gu, W. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef] [PubMed]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Tang, R.; Xiao, M. Targeting cell death pathways for cancer therapy: Recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy. 2021, 17, 4266–4285. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Li, Q. Cloning and Characterization of Yak DHODH Gene and Its Functional Studies in a Bisphenol S-Induced Ferroptosis Model of Fetal Fibroblasts. Animals 2023, 13, 3832. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tong, X.; Zhang, Y. Decreased Expression of EZH2 in Granulosa Cells Contributes to Endometriosis-Associated Infertility by Targeting IL-1R2. Endocrinology 2022, 164, bqac210. [Google Scholar] [CrossRef] [PubMed]
- Canipari, R. Oocyte--granulosa cell interactions. Hum. Reprod. Update 2000, 6, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Feng, Y.; Li, H. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Bammidi, S.; Hose, S.; Handa, J.T. Thermal Shift Assay in Ferroptosis. Methods Mol. Biol. 2023, 2712, 179–186. [Google Scholar]
- Wei, R.; Zhao, Y.; Wang, J. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 2021, 17, 2703–2717. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Siegal, E.; Gregory, R.I. tRNA dysregulation and disease. Nat. Rev. Genet. 2022, 23, 651–664. [Google Scholar] [CrossRef]
- Fu, M.; Gu, J.; Wang, M. Emerging roles of tRNA-derived fragments in cancer. Mol. Cancer 2023, 22, 30. [Google Scholar] [CrossRef]
- Xiang, J.; Jiang, M.; Du, X. The role of Hippo pathway in ferroptosis. Front. Oncol. 2022, 12, 1107505. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yu, Y.; Xie, E. Hepatic HDAC3 Regulates Systemic Iron Homeostasis and Ferroptosis via the Hippo Signaling Pathway. Research 2023, 6, 0281. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Cui, Y. Bioinformatics Analysis Identifies Potential Ferroptosis Key Genes in the Pathogenesis of Intracerebral Hemorrhage. Front. Neurosci. 2021, 15, 661663. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Gan, M.; Wu, S.; He, Y.; Feng, J.; Jing, Y.; Li, J.; Chen, Q.; Tong, J.; Kang, L.; et al. tRF-Gly-GCC in Atretic Follicles Promotes Ferroptosis in Granulosa Cells by Down-Regulating MAPK1. Int. J. Mol. Sci. 2024, 25, 9061. https://doi.org/10.3390/ijms25169061
Pan Y, Gan M, Wu S, He Y, Feng J, Jing Y, Li J, Chen Q, Tong J, Kang L, et al. tRF-Gly-GCC in Atretic Follicles Promotes Ferroptosis in Granulosa Cells by Down-Regulating MAPK1. International Journal of Molecular Sciences. 2024; 25(16):9061. https://doi.org/10.3390/ijms25169061
Chicago/Turabian StylePan, Yuheng, Mailin Gan, Shuang Wu, Yuxu He, Jinkang Feng, Yunhong Jing, Jiaxin Li, Qian Chen, Jiang Tong, Lingfan Kang, and et al. 2024. "tRF-Gly-GCC in Atretic Follicles Promotes Ferroptosis in Granulosa Cells by Down-Regulating MAPK1" International Journal of Molecular Sciences 25, no. 16: 9061. https://doi.org/10.3390/ijms25169061
APA StylePan, Y., Gan, M., Wu, S., He, Y., Feng, J., Jing, Y., Li, J., Chen, Q., Tong, J., Kang, L., Chen, L., Zhao, Y., Niu, L., Zhang, S., Wang, Y., Zhu, L., & Shen, L. (2024). tRF-Gly-GCC in Atretic Follicles Promotes Ferroptosis in Granulosa Cells by Down-Regulating MAPK1. International Journal of Molecular Sciences, 25(16), 9061. https://doi.org/10.3390/ijms25169061








