Estrogen Receptors: A New Frontier in Alzheimer’s Disease Therapy
Abstract
:1. Introduction
2. The Role of Estrogen Receptors in Alzheimer’s Disease: Molecular Mechanisms, Therapeutic Potential, and Biomarkers
3. Therapeutic Compounds Targeting Estrogen Signaling in Alzheimer’s Disease
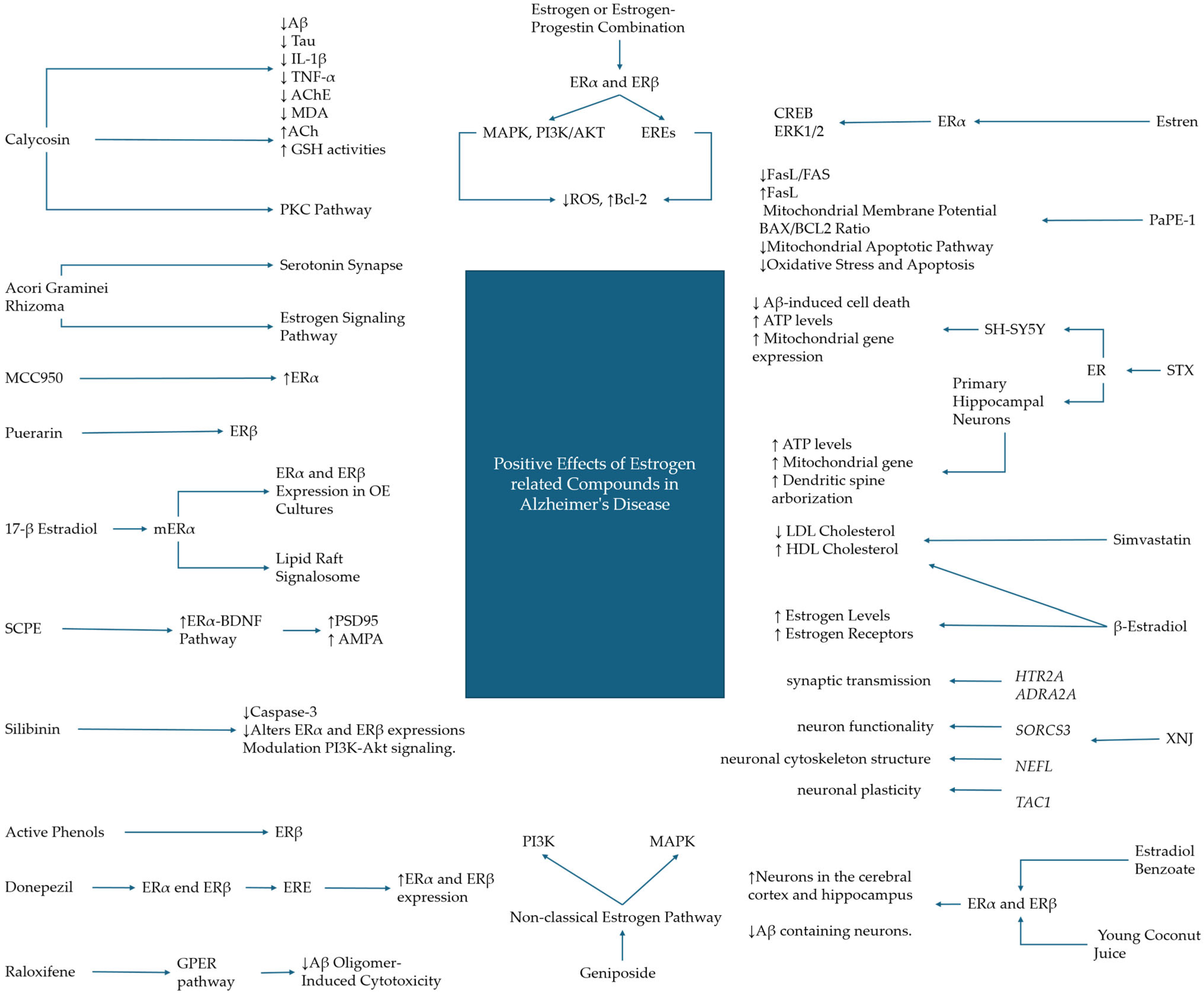
3.1. Hormonal and Hormonal Replacement Treatments
3.2. Phytocompounds
3.3. Synthetic and Non-Hormonal Estrogenic Modulators in Alzheimer’s Disease Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade-Moraes, C.H.; Oliveira-Pinto, A.V.; Castro-Fonseca, E.; da Silva, C.G.; Guimarães, D.M.; Szczupak, D.; Parente-Bruno, D.R.; Carvalho, L.R.; Polichiso, L.; Gomes, B.V.; et al. Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain 2013, 136, 3738–3752. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lendel, C. Extracellular protein components of amyloid plaques and their roles in Alzheimer’s disease pathology. Mol. Neurodegener. 2021, 16, 59. [Google Scholar] [CrossRef]
- Murray, C.E.; Gami-Patel, P.; Gkanatsiou, E.; Brinkmalm, G.; Portelius, E.; Wirths, O.; Heywood, W.; Blennow, K.; Ghiso, J.; Holton, J.L.; et al. The presubiculum is preserved from neurodegenerative changes in Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 62. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Montero-Crespo, M.; Domínguez-Álvaro, M.; Alonso-Nanclares, L.; DeFelipe, J.; Blazquez-Llorca, L. Three-dimensional analysis of synaptic organization in the hippocampal CA1 field in Alzheimer’s disease. Brain 2021, 144, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Rahman, M.M.; Jakaria, M.; Rahman, M.S.; Hossain, M.S.; Islam, A.; Ahmed, M.; Mathew, B.; Omar, U.M.; Barreto, G.E.; et al. Estrogen Signaling in Alzheimer’s Disease: Molecular Insights and Therapeutic Targets for Alzheimer’s Dementia. Mol. Neurobiol. 2020, 57, 2654–2670. [Google Scholar] [CrossRef]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Liss, J.L.; Seleri Assunção, S.; Cummings, J.; Atri, A.; Geldmacher, D.S.; Candela, S.F.; Devanand, D.P.; Fillit, H.M.; Susman, J.; Mintzer, J.; et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: A review and synthesis. J. Internal Med. 2021, 290, 310–334. [Google Scholar] [CrossRef]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Rusanen, M.; Rovio, S.; Ngandu, T.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Midlife smoking, apolipoprotein E and risk of dementia and Alzheimer’s disease: A population-based cardiovascular risk factors, aging and dementia study. Dement. Geriatr. Cognit. Disord. 2010, 30, 277–284. [Google Scholar] [CrossRef]
- Nianogo, R.A.; Rosenwohl-Mack, A.; Yaffe, K.; Carrasco, A.; Hoffmann, C.M.; Barnes, D.E. Risk Factors Associated With Alzheimer Disease and Related Dementias by Sex and Race and Ethnicity in the US. JAMA Neurol. 2022, 79, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Sando, S.B.; Melquist, S.; Cannon, A.; Hutton, M.L.; Sletvold, O.; Saltvedt, I.; White, L.R.; Lydersen, S.; Aasly, J.O. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central Norway. BMC Neurol. 2008, 8, 9. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Iso-Markku, P.; Kujala, U.M.; Knittle, K.; Polet, J.; Vuoksimaa, E.; Waller, K. Physical activity as a protective factor for dementia and Alzheimer’s disease: Systematic review, meta-analysis and quality assessment of cohort and case–control studies. Br. J. Sports Med. 2022, 56, 701–709. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Kivimaki, M. Midlife obesity, related behavioral factors, and the risk of dementia in later life. Neurology 2020, 94, 53–54. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Perez, E.; Wang, X.; Yang, S.; Wen, Y.; Singh, M. The potential for estrogens in preventing Alzheimer’s disease and vascular dementia. Therap. Adv. Neurol. Disord. 2009, 2, 31–49. [Google Scholar] [CrossRef]
- Scudiero, R.; Verderame, M. Gene expression profile of estrogen receptors alpha and beta in rat brain during aging and following high fat diet. C. R. Biol. 2017, 340, 372–378. [Google Scholar] [CrossRef]
- Sharma, P.K.; Thakur, M.K. Expression of estrogen receptor (ER) alpha and beta in mouse cerebral cortex: Effect of age, sex and gonadal steroids. Neurobiol. Aging 2006, 27, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Maioli, S.; Leander, K.; Nilsson, P.; Nalvarte, I. Estrogen receptors and the aging brain. Essays Biochem. 2021, 65, 913–925. [Google Scholar] [CrossRef]
- Waters, E.M.; Yildirim, M.; Janssen, W.G.; Lou, W.Y.; McEwen, B.S.; Morrison, J.H.; Milner, T.A. Estrogen and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011, 1379, 86–97. [Google Scholar] [CrossRef]
- Mehra, R.D.; Sharma, K.; Nyakas, C.; Vij, U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: A qualitative and quantitative study. Brain Res. 2005, 1056, 22–35. [Google Scholar] [CrossRef]
- Lan, Y.L.; Zhao, J.; Li, S. Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2015, 43, 1137–1148. [Google Scholar] [CrossRef]
- Oveisgharan, S.; Yang, J.; Yu, L.; Burba, D.; Bang, W.; Tasaki, S.; Grodstein, F.; Wang, Y.; Zhao, J.; De Jager, P.L.; et al. Estrogen Receptor Genes, Cognitive Decline, and Alzheimer Disease. Neurology 2023, 100, e1474–e1487. [Google Scholar] [CrossRef]
- Ostlund, H.; Keller, E.; Hurd, Y.L. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann. N. Y. Acad. Sci. 2003, 1007, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, M.K.; Gustafsson, J.A.; Keller, E.; Hurd, Y.L. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: Distinct distribution pattern to ERalpha mRNA. J. Clin. Endocrinol. Metabol. 2000, 85, 3840–3846. [Google Scholar] [CrossRef]
- Urdinguio, R.G.; Sanchez-Mut, J.V.; Esteller, M. Epigenetic mechanisms in neurological diseases: Genes, syndromes, and therapies. Lancet Neurol. 2009, 8, 1056–1072. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Fouse, S.; Fan, G. Epigenetic regulation of neural gene expression and neuronal function. Pediatr. Res. 2007, 61, 58R–63R. [Google Scholar] [CrossRef]
- Westberry, J.M.; Trout, A.L.; Wilson, M.E. Epigenetic Regulation of Estrogen Receptor α Gene Expression in the Mouse Cortex during Early Postnatal Development. Endocrinology 2010, 151, 731–740. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, S.; Wei, M.; Vlantis, A.C.; Chan, J.Y.K.; van Hasselt, C.A.; Li, D.; Zeng, X.; Xue, L.; Tong, M.C.F.; et al. The Isoforms of Estrogen Receptor Alpha and Beta in Thyroid Cancer. Front. Oncol. 2022, 12, 916804. [Google Scholar] [CrossRef]
- Nagler, J.J.; Cavileer, T.; Sullivan, J.; Cyr, D.G.; Rexroad, C., 3rd. The complete nuclear estrogen receptor family in the rainbow trout: Discovery of the novel ERalpha2 and both ERbeta isoforms. Gene 2007, 392, 164–173. [Google Scholar] [CrossRef]
- Pace, P.; Taylor, J.; Suntharalingam, S.; Coombes, R.C.; Ali, S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J. Biol. Chem. 1997, 272, 25832–25838. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.; Matthews, J. A new class of estrogen receptor beta-selective activators. Mol. Interv. 2010, 10, 133–136. [Google Scholar] [CrossRef]
- Nilsson, S.; Gustafsson, J.A. Biological role of estrogen and estrogen receptors. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- Dama, A.; Baggio, C.; Boscaro, C.; Albiero, M.; Cignarella, A. Estrogen Receptor Functions and Pathways at the Vascular Immune Interface. Int. J. Mol. Sci. 2021, 22, 4254. [Google Scholar] [CrossRef] [PubMed]
- Younesi, E.; Hofmann-Apitius, M. A network model of genomic hormone interactions underlying dementia and its translational validation through serendipitous off-target effect. J. Transl. Med. 2013, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.Z.; Zhao, Y.G.; Zhang, Y.Y.; He, L.; Zhao, J.K.; Liu, M.Y.; Liu, Y.; Zhang, J.Q. Nuclear and membrane estrogen receptor antagonists induce similar mTORC2 activation-reversible changes in synaptic protein expression and actin polymerization in the mouse hippocampus. CNS Neurosci. Ther. 2018, 24, 495–507. [Google Scholar] [CrossRef]
- Xiong, Y.S.; Liu, F.F.; Liu, D.; Huang, H.Z.; Wei, N.; Tan, L.; Chen, J.G.; Man, H.Y.; Gong, C.X.; Lu, Y.; et al. Opposite effects of two estrogen receptors on tau phosphorylation through disparate effects on the miR-218/PTPA pathway. Aging Cell 2015, 14, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, F.; Jiang, S.; Siedlak, S.L.; Shen, L.; Perry, G.; Wang, X.; Tang, B.; Zhu, X. Estrogen receptor-α is localized to neurofibrillary tangles in Alzheimer’s disease. Sci. Rep. 2016, 6, 20352. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Marín, R.; Torrealba, E.; Santos, G.; Díaz, M. Neuronal ER-Signalosome Proteins as Early Biomarkers in Prodromal Alzheimer’s Disease Independent of Amyloid-β Production and Tau Phosphorylation. Front. Mol. Neurosci. 2022, 15, 879146. [Google Scholar] [CrossRef]
- Buchhave, P.; Minthon, L.; Zetterberg, H.; Wallin, A.K.; Blennow, K.; Hansson, O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch. Gen. Psychiatry 2012, 69, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.J.; Chen, T.F.; Hu, C.J.; Yan, S.H.; Sun, Y.; Liu, B.H.; Chang, Y.T.; Yang, C.C.; Yang, S.Y. Nanoparticle-based immunomagnetic assay of plasma biomarkers for differentiating dementia and prodromal states of Alzheimer’s disease—A cross-validation study. Nanomed. Nanotechnol. Biol. Med 2020, 28, 102182. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, L. Cell type-specific potential pathogenic genes and functional pathways in Alzheimer’s Disease. BMC Neurol. 2021, 21, 381. [Google Scholar] [CrossRef]
- Ishunina, T.A.; Swaab, D.F. Estrogen receptor α splice variant TADDI in the human supraoptic nucleus: An effect on neuronal size and changes in pneumonia. Neuro Endocrinol. Lett. 2021, 42, 128–132. [Google Scholar]
- Iacobas, D.A.; Iacobas, S.; Nebieridze, N.; Velíšek, L.; Velíšková, J. Estrogen Protects Neurotransmission Transcriptome During Status Epilepticus. Front. Neurosci. 2018, 12, 332. [Google Scholar] [CrossRef]
- Morelli, A.; Sarchielli, E.; Guarnieri, G.; Coppi, E.; Pantano, D.; Comeglio, P.; Nardiello, P.; Pugliese, A.M.; Ballerini, L.; Matucci, R.; et al. Young Human Cholinergic Neurons Respond to Physiological Regulators and Improve Cognitive Symptoms in an Animal Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2017, 11, 339. [Google Scholar] [CrossRef]
- Pooley, A.E.; Luong, M.; Hussain, A.; Nathan, B.P. Neurite outgrowth promoting effect of 17-β estradiol is mediated through estrogen receptor alpha in an olfactory epithelium culture. Brain Res. 2015, 1624, 19–27. [Google Scholar] [CrossRef]
- Duong, P.; Tenkorang, M.A.A.; Trieu, J.; McCuiston, C.; Rybalchenko, N.; Cunningham, R.L. Neuroprotective and neurotoxic outcomes of androgens and estrogens in an oxidative stress environment. Biol. Sex Differ. 2020, 11, 12. [Google Scholar] [CrossRef]
- Canerina-Amaro, A.; Hernandez-Abad, L.G.; Ferrer, I.; Quinto-Alemany, D.; Mesa-Herrera, F.; Ferri, C.; Puertas-Avendano, R.A.; Diaz, M.; Marin, R. Lipid raft ER signalosome malfunctions in menopause and Alzheimer’s disease. Front. Biosci. 2017, 9, 111–126. [Google Scholar] [CrossRef]
- Boyle, C.P.; Raji, C.A.; Erickson, K.I.; Lopez, O.L.; Becker, J.T.; Gach, H.M.; Kuller, L.H.; Longstreth, W., Jr.; Carmichael, O.T.; Riedel, B.C.; et al. Estrogen, brain structure, and cognition in postmenopausal women. Hum. Brain Mapp. 2021, 42, 24–35. [Google Scholar] [CrossRef]
- Meng, Q.; Chao, Y.; Zhang, S.; Ding, X.; Feng, H.; Zhang, C.; Liu, B.; Zhu, W.; Li, Y.; Zhang, Q.; et al. Attenuation of estrogen and its receptors in the post-menopausal stage exacerbates dyslipidemia and leads to cognitive impairment. Mol. Brain 2023, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.K.; Xiao, Y.; Zhong, S.M.; Huang, Y.X.; Chen, Q.W.; Zhou, Y.Q.; Guo, J.Y.; Yang, C. Study on the Mechanism of Acori Graminei Rhizoma in the Treatment of Alzheimer’s Disease Based on Network Pharmacology and Molecular Docking. BioMed Res. Int. 2021, 2021, 5418142. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Li, Y.; Cai, G.; Cao, M.; Li, L. Integrated analysis and network pharmacology approaches to explore key genes of Xingnaojing for treatment of Alzheimer’s disease. Brain Behav. 2020, 10, e01610. [Google Scholar] [CrossRef]
- Zhao, D.P.; Lei, X.; Wang, Y.Y.; Xue, A.; Zhao, C.Y.; Xu, Y.M.; Zhang, Y.; Liu, G.L.; Geng, F.; Xu, H.D.; et al. Sagacious confucius’ pillow elixir ameliorates Dgalactose induced cognitive injury in mice via estrogenic effects and synaptic plasticity. Front. Pharmacol. 2022, 13, 971385. [Google Scholar] [CrossRef]
- Song, X.; Liu, B.; Cui, L.; Zhou, B.; Liu, L.; Liu, W.; Yao, G.; Xia, M.; Hayashi, T.; Hattori, S.; et al. Estrogen Receptors Are Involved in the Neuroprotective Effect of Silibinin in Aβ(1-42)-Treated Rats. Neurochem. Res. 2018, 43, 796–805. [Google Scholar] [CrossRef]
- Li, L.; Xue, Z.; Chen, L.; Chen, X.; Wang, H.; Wang, X. Puerarin suppression of Aβ(1-42)-induced primary cortical neuron death is largely dependent on ERβ. Brain Res. 2017, 1657, 87–94. [Google Scholar] [CrossRef]
- Song, L.; Li, X.; Bai, X.X.; Gao, J.; Wang, C.Y. Calycosin improves cognitive function in a transgenic mouse model of Alzheimer’s disease by activating the protein kinase C pathway. Neural Regener. Res. 2017, 12, 1870–1876. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Ding, H.; Jin, C.; Chen, J.; Zhao, Y.; Li, X.; Chen, W.; Sun, P.; Tan, Y.; et al. Geniposide, the component of the Chinese herbal formula Tongluojiunao, protects amyloid-β peptide (1-42-mediated death of hippocampal neurons via the non-classical estrogen signaling pathway. Neural Regener. Res. 2014, 9, 474–480. [Google Scholar] [CrossRef]
- Balit, T.; Abdel-Wahhab, M.A.; Radenahmad, N. Young Coconut Juice Reduces Some Histopathological Changes Associated with Alzheimer’s Disease through the Modulation of Estrogen Receptors in Orchidectomized Rat Brains. J. Aging Res. 2019, 2019, 7416419. [Google Scholar] [CrossRef]
- Park, H.; McEachon, J.D., 2nd; Pollock, J.A. Synthesis and characterization of hydrogen peroxide activated estrogen receptor beta ligands. Bioorg. Med. Chem. 2019, 27, 2075–2082. [Google Scholar] [CrossRef]
- Fekete, C.; Vastagh, C.; Dénes, Á.; Hrabovszky, E.; Nyiri, G.; Kalló, I.; Liposits, Z.; Sárvári, M. Chronic Amyloid β Oligomer Infusion Evokes Sustained Inflammation and Microglial Changes in the Rat Hippocampus via NLRP3. Neuroscience 2019, 405, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Imamura, O.; Arai, M.; Dateki, M.; Oishi, K.; Takishima, K. Donepezil-induced oligodendrocyte differentiation is mediated through estrogen receptors. J. Neurochem. 2020, 155, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; O’Neill, K.; Brinton, R.D. Estrogenic agonist activity of ICI 182780 (Faslodex) in hippocampal neurons: Implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. J. Pharmacol. Exp. Therap. 2006, 319, 1124–1132. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Potapov, K.; Kim, S.; Peppercorn, K.; Tate, W.P.; Ábrahám, I.M. Treatment of beta amyloid 1-42 (Aβ(1-42))-induced basal forebrain cholinergic damage by a non-classical estrogen signaling activator in vivo. Sci. Rep. 2016, 6, 21101. [Google Scholar] [CrossRef]
- Wnuk, A.; Przepiórska, K.; Rzemieniec, J.; Pietrzak, B.; Kajta, M. Selective Targeting of Non-nuclear Estrogen Receptors with PaPE-1 as a New Treatment Strategy for Alzheimer’s Disease. Neurotox. Res. 2020, 38, 957–966. [Google Scholar] [CrossRef]
- Gray, N.E.; Zweig, J.A.; Kawamoto, C.; Quinn, J.F.; Copenhaver, P.F. STX, a Novel Membrane Estrogen Receptor Ligand, Protects Against Amyloid-β Toxicity. J. Alzheimer’s Dis. JAD 2016, 51, 391–403. [Google Scholar] [CrossRef]
- Ma, F.; Liu, D. 17β-trenbolone, an anabolic-androgenic steroid as well as an environmental hormone, contributes to neurodegeneration. Toxicol. Appl. Pharmacol. 2015, 282, 68–76. [Google Scholar] [CrossRef]
- Bang, Y.; Lim, J.; Kim, S.S.; Jeong, H.M.; Jung, K.K.; Kang, I.H.; Lee, K.Y.; Choi, H.J. Aroclor1254 interferes with estrogen receptor-mediated neuroprotection against beta-amyloid toxicity in cholinergic SN56 cells. Neurochem. Int. 2011, 59, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Nohara, T.; Tsuji, M.; Oguchi, T.; Momma, Y.; Ohashi, H.; Nagata, M.; Ito, N.; Yamamoto, K.; Murakami, H.; Kiuchi, Y. Neuroprotective Potential of Raloxifene via G-Protein-Coupled Estrogen Receptors in Aβ-Oligomer-Induced Neuronal Injury. Biomedicines 2023, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
| Experimental Model | Biological Specimen | Pathway | Compound/Therapy | Ref. |
|---|---|---|---|---|
| In silico | - | Estrogen signaling pathway and insulin signaling pathway | - | [39] |
| Adult female C57/BL6 mice (in vivo and in vitro) | hippocampus | ERs in hippocampal synaptic plasticity, particularly examining the mTORC2 pathway’s involvement in actin polymerization and synaptic protein expression | ER antagonists (MPP, PHTPP for nERs; G15 for mER) and the mTORC2 activator A-443654 | [40] |
| 18-month-old male Tg2576 mice (in vivo and in vitro) | HEK293/tau cells and mouse N2a cells | miR-218/PTPa pathway | ICI 182780 | [41] |
| Human postmortem brain tissues and M17 human neuroblastoma cells (in vitro) | Hippocampal and cortical tissues | estrogen signaling pathway | - | [42] |
| Cohort of human subjects categorized into three groups: cognitively normal healthy controls (HC), mild cognitive impairment (MCI), and subjective memory complaints (SMC) (Clinical samples) | CSF samples collected from the participants | The pathways related to cellular signaling and neuronal survival, including interactions involving proteins like ERα, IGF-1Rβ, and VDAC | - | [43] |
| snRNA-seq (clinical samples (in vitro)) | Cortex samples from 42 AD pathology subjects and 39 normal controls | Immune response and neuroinflammation Mitochondrial dysfunction across various cell types Estrogen signaling pathway disruption Oxidative stress response | - | [46] |
| 58 control patients aged 20–94 years without neurological or psychiatric disorders, and 26 patients with AD aged 54–94 years. (clinical samples (in vitro)) | Hypothalamic sections containing the SON were sourced from the Netherlands Brain Bank | Estrogen receptor signaling, particularly involving a splice variant of ERα, known as TADDI, and its effect on neuronal morphology and metabolic activity in the SON | - | [47] |
| Experimental Model | Biological Specimen | Pathway | Compound/Therapy | Type of Compound | Ref. |
|---|---|---|---|---|---|
| Female Sprague Dawley rats, 8–9 weeks old, OVX (in vivo and in vitro) | Hippocampal DG tissue | Interaction and protection of synaptic transmission pathways by E2, including glutamatergic, GABAergic, dopaminergic, cholinergic, and serotonergic pathways. ESG significantly engaged. | 17β-estradiol benzoate | Hormonal and Hormonal replacing treatments | [48] |
| in vivo and in vitro | Human cholinergic neurons isolated from the NBM of 12-week-old human fetuses. Male Wistar rats subjected to a NBM lesion induced | Nicotinic and muscarinic receptors; NGF/TrkA and estrogens | NGF and 17-β-estradiol | Hormonal and hormonal replacement treatments | [49] |
| Olfactory epithelium culture (in vivo and in vitro) | Rat olfactory epithelial cells | Estrogen receptor alpha pathway | 17-β estradiol | Hormonal and Hormonal replacement treatments | [50] |
| N27 and PC12 neuronal cell lines C6 glial cell line (in vivo and in vitro) | Female rat-derived N27 cells Male rat-derived PC12 and C6 cells Human hippocampal tissue | Estrogen receptor pathway Membrane-associated AR45 pathway Oxidative stress pathway mediated by H2O2 | Testosterone 17β-estradiol | Hormonal and hormonal replacement treatments | [51] |
| Human brain samples from three groups: premenopausal women (<50 years old), postmenopausal women (>65 years old), and AD patients (ADV/VI stages) (in vitro) | Human frontal cortex tissue. | ERα signalosome pathway, involving interactions with IGF-1Rb and VDAC1 | 17β-estradiol | Hormonal and hormonal replacement treatments | [52] |
| 562 females: Patient with cognitive impairment (MCI and AD: 137 cognitively normal controls: 425 (clinical model) | Brain structure and volume, as assessed using high-resolution structural MRI | Estrogen receptor pathways. Interaction effects between HT and BMI on brain volume. Possible involvement of genetic variations in estrogen receptor expression. | Estrogen (specifically, the effects of HT involving CEE and other forms of exogenous estrogen). | Hormonal and hormonal replacement treatments | [53] |
| Ovx LDLR−/− mice SH-SY5Y cells treated with PA (in vivo and in vitro) | Mouse hippocampal tissue. SH-SY5Y cells | ERα, ERβ, and GPER signaling pathways. Lipid metabolism pathways involving dyslipidemia and its effects on cognitive functions | β-Estradiol simvastatin AB23A | Hormonal and hormonal replacement treatments | [54] |
| Network pharmacology molecular docking (bioinformatic modeling) | Acori Graminei Rhizoma | Alzheimer’s disease pathway Serotonin synapses Estrogen signaling pathway Dopaminergic synapses PI3K-Akt signaling pathway | Acori Graminei Rhizoma | Phytocompounds | [55] |
| Integrated microarray analysis using gene expression datasets from the GEO database (bioinformatic modeling) | Brain tissue samples from AD patients and normal controls | Neuroactive ligand–receptor interaction Regulation of the actin cytoskeleton Estrogen signaling pathway Notch signaling pathway | XNJ | Phytocompounds | [56] |
| KM mice and SD rats (in vivo and in vitro) | Brain and hippocampal tissue | The estrogen signaling pathway, synaptic signaling pathway, ERα-BDNF pathway, actin cytoskeleton regulation, and MAPK signaling pathway | SCPE | Phytocompounds | [57] |
| Scopolamine-induced memory impairment model in mice (in vivo and in vitro) | brain tissue of mice. | The antioxidant pathway, anti-inflammatory pathway, immunomodulatory pathway, and apoptotic pathway | Silybum marianum (milk thistle) | Phytocompounds | [58] |
| Primary cortical neurons from rat embryos (in vivo and in vitro) | Primary cortical neurons exposed to Aβ1–42 | Estrogen receptor (ER) pathway | Puerarin | Phytocompounds | [59] |
| Transgenic mouse model (APP/PS1 transgenic mice) (in vivo and in vitro) | Male APP/PS1 transgenic mice aged 7–8 months old. Male C57BL/6 mice of the same age as controls. | Protein kinase C pathway | Calycosin | Phytocompounds | [60] |
| Primary cultured hippocampal neurons from rats. (in vivo and in vitro) | Primary hippocampal neurons extracted from Sprague Dawley rats. | The non-classical estrogen signaling pathway, PI3K pathway, And MAPK pathway | Geniposide | Phytocompounds | [61] |
| Adult male Wistar rats ORX and treated with various doses of YCJ or EB (in vivo and in vitro) | Brains of adult male Wistar rats | NF200 PV ERα and ERβ) Aβ accumulation | YCJ, rich in β-sitosterol with estrogenic properties | Phytocompounds | [62] |
| Masking of endogenous estrogens and the ERβ-selective agonist DPN as boronic acid pinacol esters (chemical synthesis experiments) | Derivatives of estrone and estradiol. Non-steroidal ERβ-selective agonist DPN. Cellular environment for ERβ transcriptional activity assays. | Activation of ERα and ERβ Modulation of ERβ transcriptional activity in response to pathological H2O2 levels. | DPN, an ERβ-selective agonist. | Synthetic and non-hormonal estrogenic modulators | [63] |
| Middle-aged Long–Evans rats (in vivo and in vitro) | Rat hippocampus | NF-kB activation NLRP3 Inflammasome pathway Esr1 Scn1a | AbO MCC950 | Synthetic and Non-Hormonal Estrogenic Modulators | [64] |
| miPSC-NSCs (in vitro) | Oligodendrocytes, neurons, and astrocytes | ER signaling pathway | Donepezil ICI 182780, MPP and PHTPP | Synthetic and non-hormonal estrogenic modulators | [65] |
| Hippocampal neurons (in vitro) | Rat hippocampal neurons | Estrogen receptor pathway | ICI 182780 (Faslodex) | Synthetic and non-hormonal estrogenic nodulators | [66] |
| OVX female C57BL/6J mice and nERα KO mice (in vivo and in vitro) | Brain tissue, specifically BFC neurons, NBM, and somatosensory cortex | MAPK/CREB Signaling Pathway; Components: ERK1/2, CREB; Activation: Phosphorylation of ERK1/2 and CREB in BFC neurons | 4-estren-3α, 17β-diol (estren) | Synthetic and non-hormonal estrogenic modulators | [67] |
| Primary neocortical cultures from E15 embryos of CD-1® IGS Swiss mice (in vivo and in vitro) | Mouse neocortical neurons | Apoptotic pathways (mitochondrial and external); oxidative stress pathway; MAPK and mTOR signaling pathways | PaPE-1 ((S)-5-(4-hydroxy-3,5-dimethyl-phenyl)-indan-1-ol) | Synthetic and non-hormonal estrogenic modulators | [68] |
| MC65 and SH-SY5Y neuroblastoma cell lines, as well as primary hippocampal neurons from WT and Tg2576 mice (in vivo and in vitro) | MC65 and SH-SY5Y and primary hippocampal neurons isolated from embryonic WT and Tg2576 mice | The protective effects of STX are mediated via GqMER, involving pathways such as ERK/MAPK, PKCδ/PKA, and PI3K signaling | STX | Synthetic and non-hormonal estrogenic modulators | [69] |
| Adult and pregnant Wistar rats (in vivo and in vitro) | Adult rat brains (hippocampus), fetal brains, and primary hippocampal neurons | Aβ42 accumulation, caspase-3 activity, presenilin-1 protein expression, and androgen- and estrogen receptor-mediated pathways | 17β-trenbolone | Synthetic and non-hormonal estrogenic modulators | [70] |
| C57BL/6 (in vivo and in vitro) | The SN56 cells used are derived from murine C57BL/6 neurons, representing a model for cholinergic neuronal studies | ER signaling, Tau phosphorylation, and JNK activation | A1254 and E2 | Synthetic and non-hormonal estrogenic modulators | [71] |
| SH-SY5Y (in vitro) | Aβo and SH-SY5Y cells | GPER, ER, oxidative stress pathways, NMDA receptor, L-type voltage-gated calcium channels, and phospholipid peroxidation pathways | Raloxifene, estradiol, fulvestrant, and G-15 | Synthetic and non-hormonal estrogenic modulators | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipriano, G.L.; Mazzon, E.; Anchesi, I. Estrogen Receptors: A New Frontier in Alzheimer’s Disease Therapy. Int. J. Mol. Sci. 2024, 25, 9077. https://doi.org/10.3390/ijms25169077
Cipriano GL, Mazzon E, Anchesi I. Estrogen Receptors: A New Frontier in Alzheimer’s Disease Therapy. International Journal of Molecular Sciences. 2024; 25(16):9077. https://doi.org/10.3390/ijms25169077
Chicago/Turabian StyleCipriano, Giovanni Luca, Emanuela Mazzon, and Ivan Anchesi. 2024. "Estrogen Receptors: A New Frontier in Alzheimer’s Disease Therapy" International Journal of Molecular Sciences 25, no. 16: 9077. https://doi.org/10.3390/ijms25169077






