Monitoring Fruit Growth and Development in Apricot (Prunus armeniaca L.) through Gene Expression Analysis
Abstract
1. Introduction
2. Results
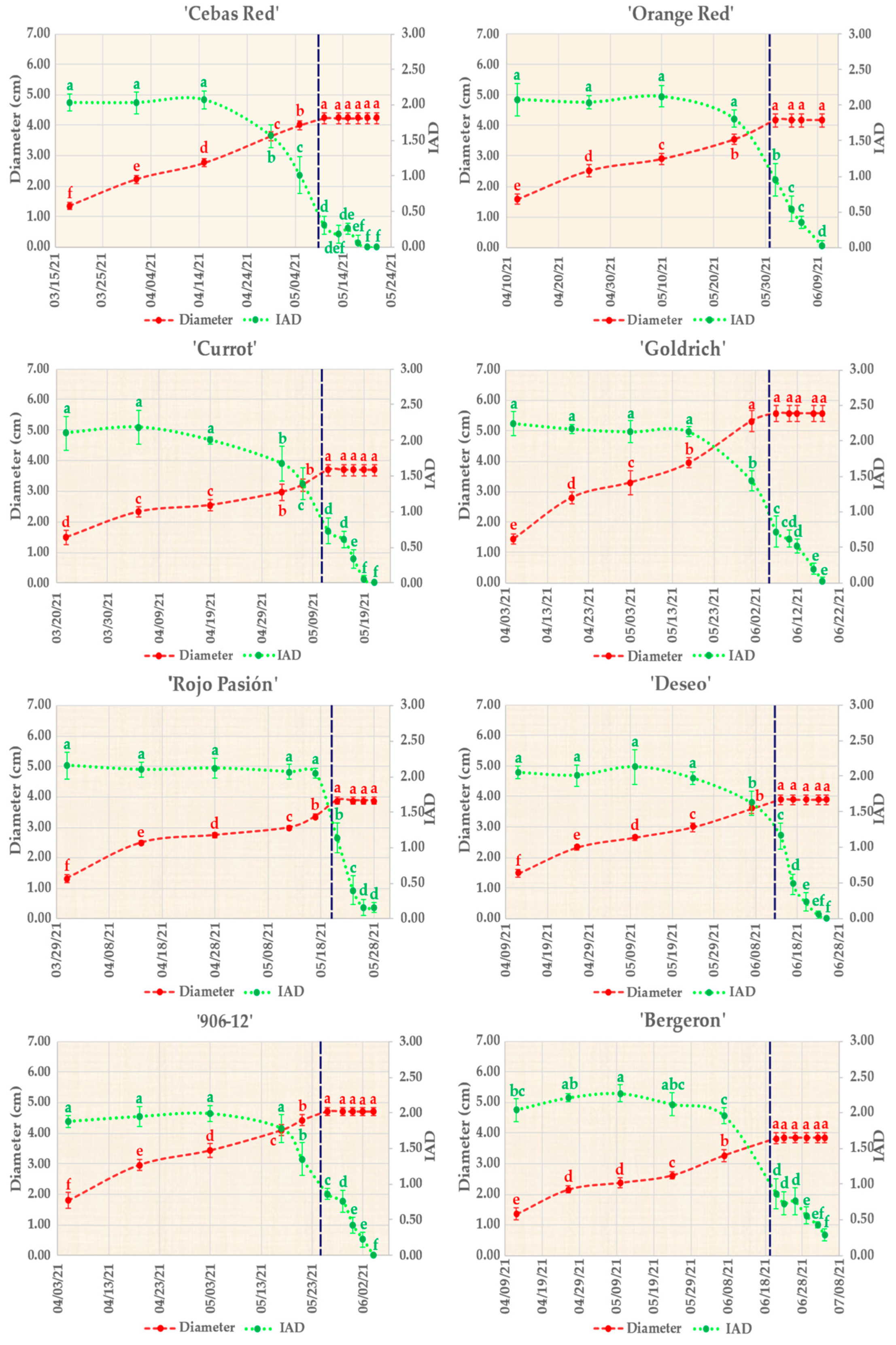
2.1. Pomological Monitoring of Fruit Development and Ripening
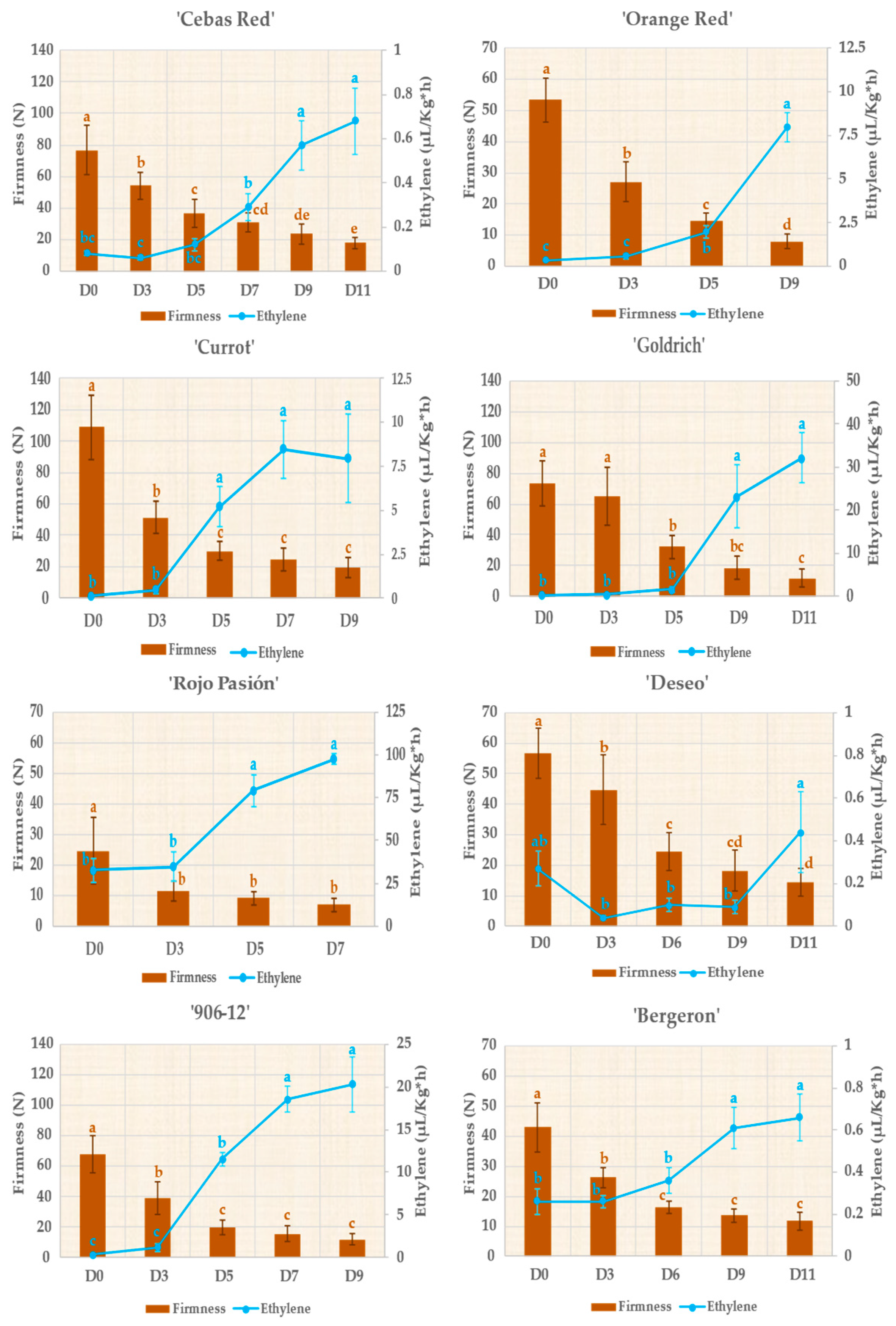
2.2. Monitoring Fruit Quality Traits during Postharvest
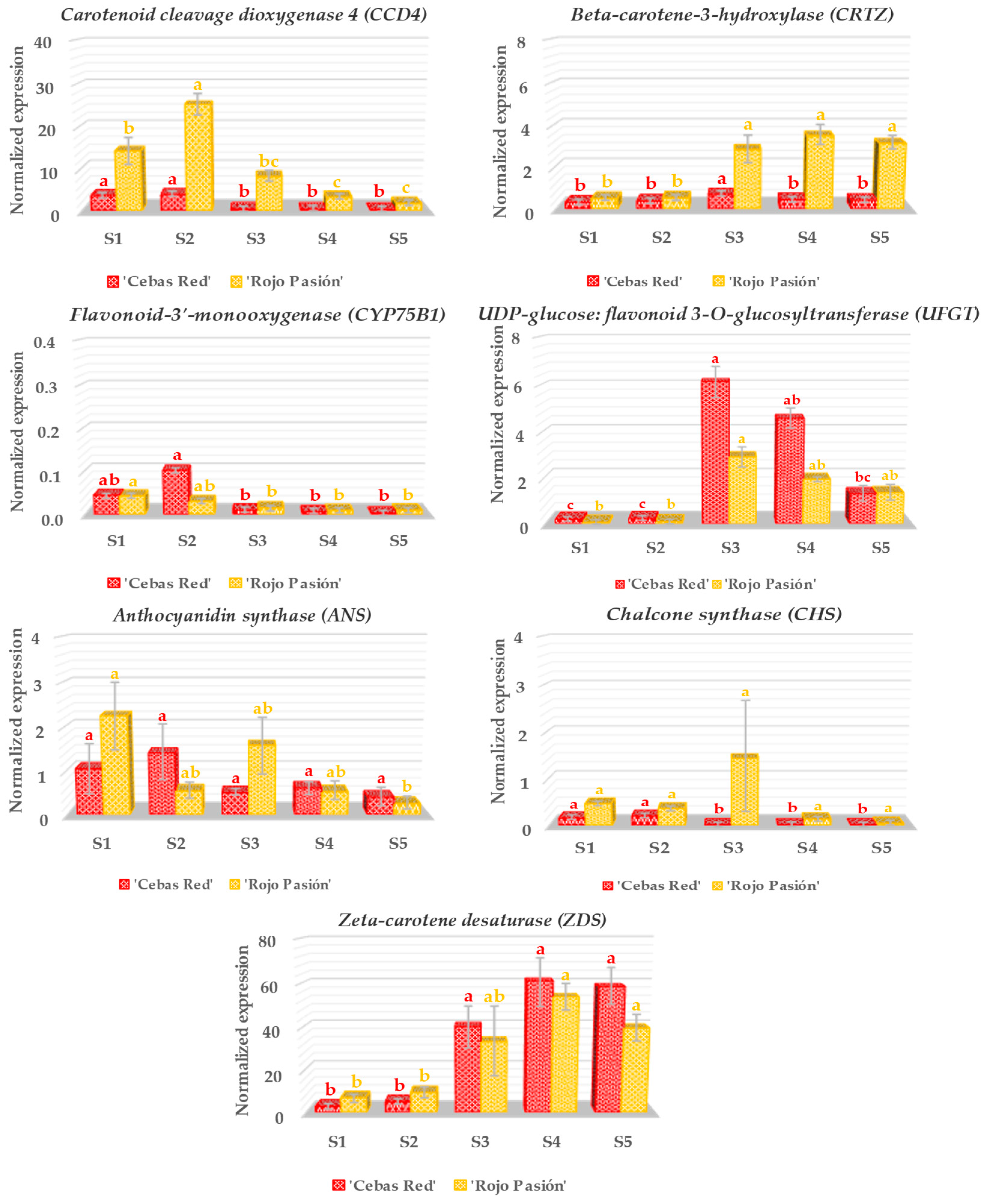
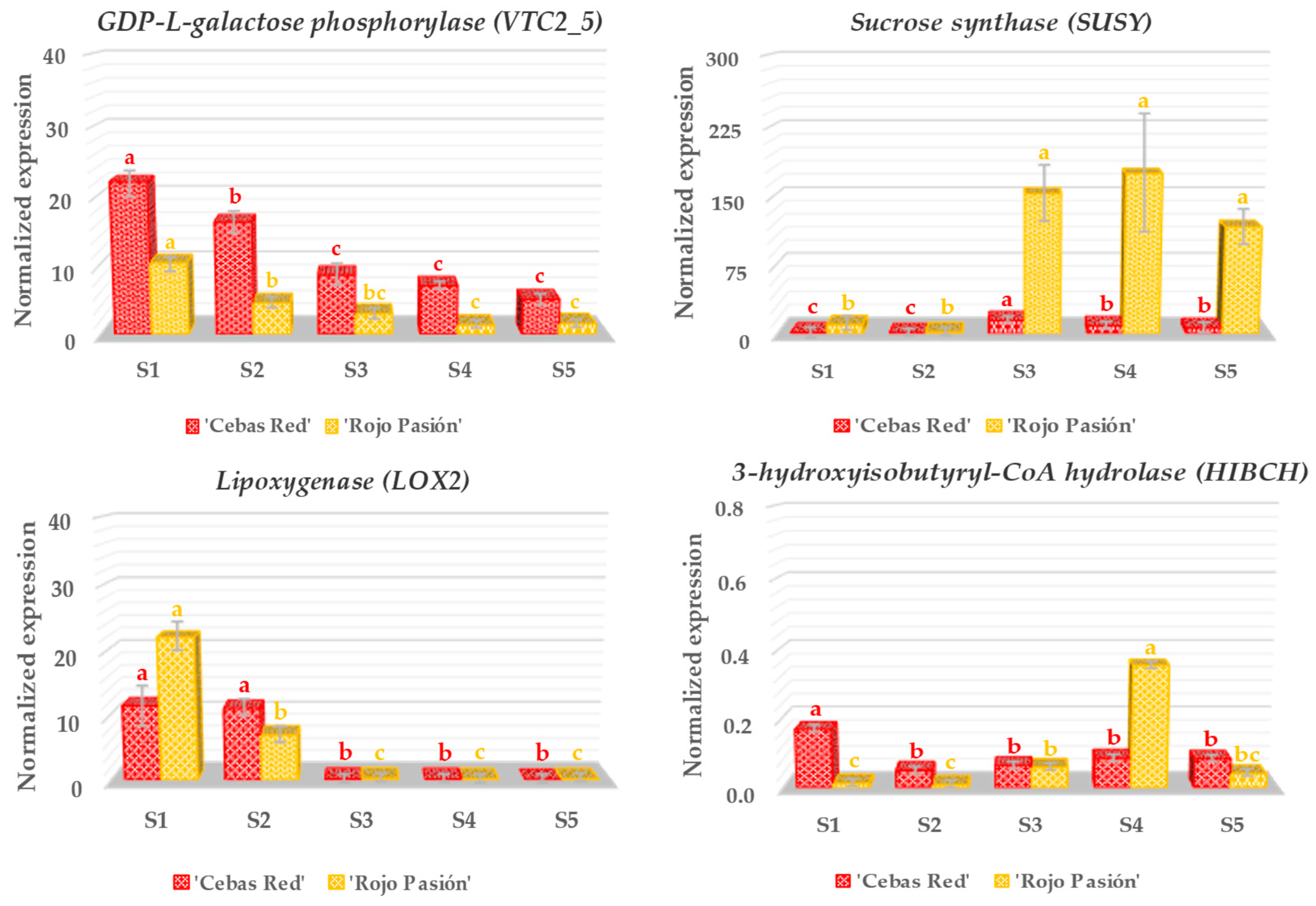
2.3. Gene Expression Analysis in Relation to Fruit Development and Ripening
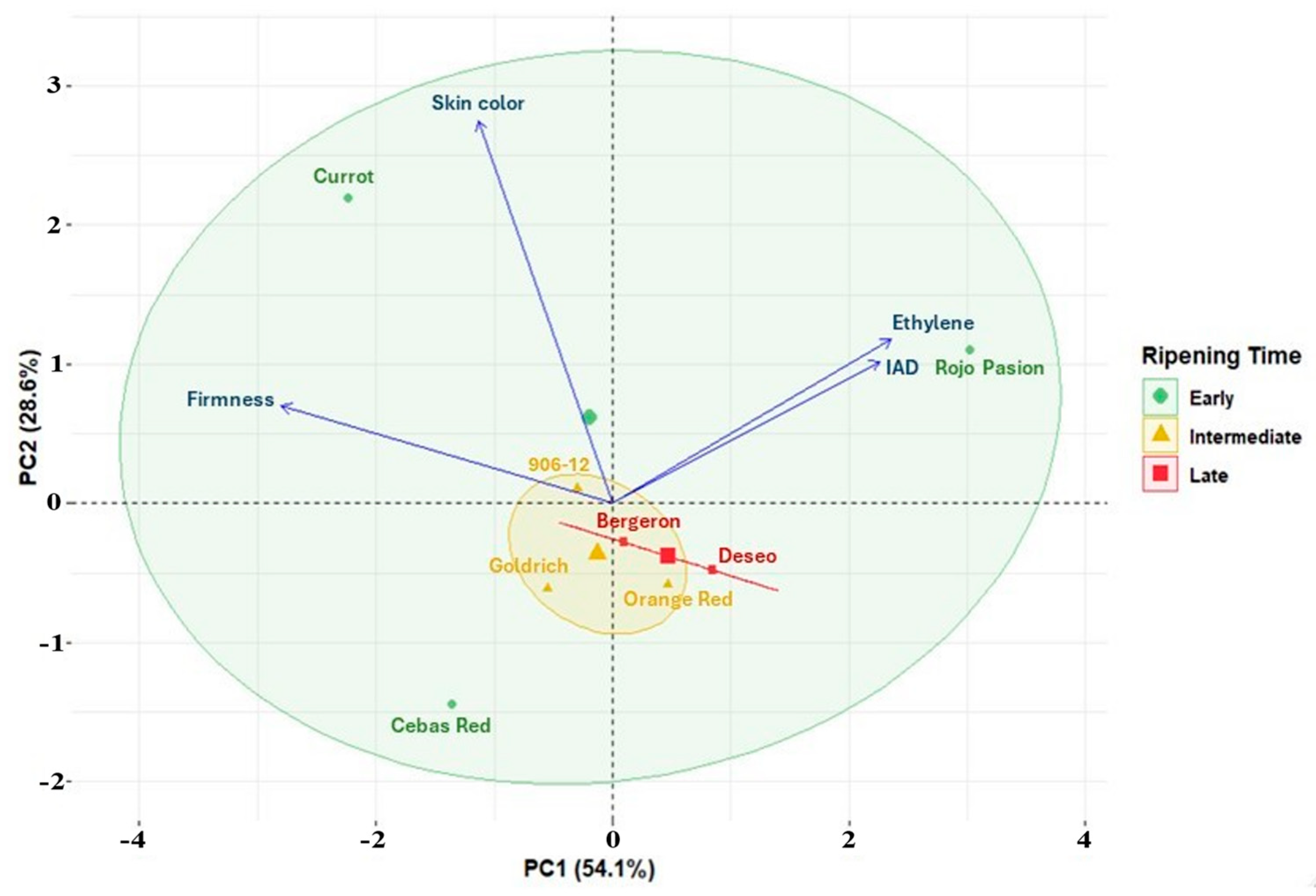
3. Discussion
3.1. Monitoring Fruit Development, Ripening, and Shelf Life
3.2. Analysis of Genes Linked to Fruit Growth and Ripening
3.3. Analysis of Genes Linked to Fruit Color
3.4. Analysis of Genes Linked to the Nutraceutical Properties
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design and Testing
4.3. Preharvest Analysis
4.4. Postharvest Analysis
4.5. RNA Isolation and Purification
4.6. Gene Expression Analysis Using RT-qPCR
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarıdaş, M.A.; Ağçam, E.; Ünal, N.; Akyıldız, A.; Kargı, S.P. Comprehensive Quality Analyses of Important Apricot Varieties Produced in Türkiye. J. Food Compos. Anal. 2024, 125, 105791. [Google Scholar] [CrossRef]
- Juhnevica-Radenkova, K.; Krasnova, I.; Seglina, D.; Kaufmane, E.; Gravite, I.; Valdovska, A.; Radenkovs, V. Biochemical Profile and Antioxidant Activity of Dried Fruit Produced from Apricot Cultivars Grown in Latvia. Horticulturae 2024, 10, 205. [Google Scholar] [CrossRef]
- Zaurov, D.E.; Molnar, T.J.; Eisenman, S.W.; Ford, T.M.; Mavlyanova, R.F.; Capik, J.M.; Funk, C.R.; Goffreda, J.C. Genetic Resources of Apricots (Prunus armeniaca L.) in Central Asia. HortScience 2013, 48, 681–691. [Google Scholar] [CrossRef]
- Ru, S.; Main, D.; Evans, K.; Peace, C. Current Applications, Challenges, and Perspectives of Marker-Assisted Seedling Selection in Rosaceae Tree Fruit Breeding. Tree Genet. Genomes 2015, 11, 8. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 1 April 2024).
- Salazar, J.A.; Ruiz, D.; Campoy, J.A.; Sánchez-Pérez, R.; Crisosto, C.H.; Martínez-García, P.J.; Blenda, A.; Jung, S.; Main, D.; Martínez-Gómez, P.; et al. Quantitative Trait Loci (QTL) and Mendelian Trait Loci (MTL) Analysis in Prunus: A Breeding Perspective and Beyond. Plant Mol. Biol. Rep. 2014, 32, 1–18. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Dondini, L.; Martínez-Gómez, P.; Ruiz, D. Identification of QTLs Linked to Fruit Quality Traits in Apricot (Prunus armeniaca L.) and Biological Validation through Gene Expression Analysis Using QPCR. Mol. Breed. 2019, 39, 28. [Google Scholar] [CrossRef]
- Moustafa, K.; Cross, J. Production, Pomological and Nutraceutical Properties of Apricot. J. Food Sci. Technol. 2019, 56, 12–23. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Rezaei, M.; Rohani, A.; Lawson, S.; Fatahi, R. Towards Modeling Growth of Apricot Fruit: Finding a Proper Growth Model. Hortic. Environ. Biotechnol. 2023, 64, 209–222. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Sun, L.; Du, D.; Cheng, T.; Pan, H.; Yang, W.; Wang, J. Genome-Wide Identification, Characterisation and Expression Analysis of the MADS-Box Gene Family in Prunus mume. Mol. Genet. Genom. 2014, 289, 903–920. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit Ripening Phenomena—An Overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Fatima, T.; Bashir, O.; Gani, G.; Bhat, T.; Jan, N. Nutritional and Health Benefits of Apricots. Int. J. Unani Integr. Med. 2018, 2, 5–9. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Cipriano, L.; Nazzaro, F. Apricots: Biochemistry and Functional Properties. Curr. Opin. Food Sci. 2018, 19, 23–29. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, F.; Zhao, Y.; Shi, L.; Zhu, X. Cloning and Expression Analysis of Polygalacturonase and Pectin Methylesterase Genes during Softening in Apricot (Prunus armeniaca L.) Fruit. Sci. Hortic. 2019, 256, 108607. [Google Scholar] [CrossRef]
- González-Agüero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-León, M.A.; Defilippi, B.G. Differential Expression Levels of Aroma-Related Genes during Ripening of Apricot (Prunus armeniaca L.). Plant Physiol. Biochem. 2009, 47, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, C.; Li, W.; Qu, Z.; Zeng, M.; Xi, W. Transcriptional Regulatory Networks Controlling Taste and Aroma Quality of Apricot (Prunus armeniaca L.) Fruit during Ripening. BMC Genom. 2019, 20, 45. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Rubio, P.; Infante, R.; Campos-Vargas, R.; Manríquez, D.; González-Agüero, M.; Defilippi, B.G. Ethylene Biosynthesis in Apricot: Identification of a Ripening-Related 1-Aminocyclopropane-1-Carboxylic Acid Synthase (ACS) Gene. Postharvest Biol. Technol. 2012, 63, 85–90. [Google Scholar] [CrossRef]
- Marty, I.; Bureau, S.; Sarkissian, G.; Gouble, B.; Audergon, J.M.; Albagnac, G. Ethylene Regulation of Carotenoid Accumulation and Carotenogenic Gene Expression in Colour-Contrasted Apricot Cultivars (Prunus armeniaca). J. Exp. Bot. 2005, 56, 1877–1886. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Hegedűs, A. Review of the Molecular Genetics of Flavonoid Biosynthesis in Fruits. Acta Aliment. 2011, 40, 150–163. [Google Scholar] [CrossRef]
- Xi, W.; Feng, J.; Liu, Y.; Zhang, S.; Zhao, G. The R2R3-MYB Transcription Factor PaMYB10 Is Involved in Anthocyanin Biosynthesis in Apricots and Determines Red Blushed Skin. BMC Plant Biol. 2019, 19, 287. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Nicolás-Almansa, M.; Razi, M.; Rubio, M.; Ruiz, D.; Martínez-Gómez, P. Molecular Bases of Fruit Quality in Prunus Species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective. Int. J. Mol. Sci. 2021, 22, 333. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Rasori, A.; Bassi, D.; Geuna, F.; Ramina, A.; Tonutti, P.; Bonghi, C. Comparative Transcript Profiling of Apricot (Prunus armeniaca L.) Fruit Development and on-Tree Ripening. Tree Genet Genomes 2011, 7, 609–616. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Analysis of Metabolites and Gene Expression Changes Relative to Apricot (Prunus armeniaca L.) Fruit Quality During Development and Ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, B.E.; Salazar, J.A.; Egea, J.A.; Rubio, M.; Martínez-Gómez, P.; Ruiz, D. Monitoring Apricot (Prunus armeniaca L.) Ripening Progression through Candidate Gene Expression Analysis. Int. J. Mol. Sci. 2022, 23, 4575. [Google Scholar] [CrossRef]
- Kan, J.; Yuan, N.; Lin, J.; Li, H.; Yang, Q.; Wang, Z.; Shen, Z.; Ying, Y.; Li, X.; Cao, F. Seed Germination and Growth Improvement for Early Maturing Pear Breeding. Plants 2023, 12, 4120. [Google Scholar] [CrossRef]
- Infante, R.; Rubio, P.; Contador, L.; Noferini, M.; Costa, G. Determination of Harvest Maturity of D’Agen Plums Using the Chlorophyll Absorbance Index. Cienc. Investig. Agrar. 2011, 38, 199–203. [Google Scholar] [CrossRef]
- Ortuño-Hernández, G.; Ruiz, D.; Martínez-Gómez, P.; Salazar, J.A. Differentially Methylated DNA Regions in Apricot (Prunus armeniaca L.) and Japanese Plum (Prunus salicina L.) during Fruit Ripening after Ethylene-Related Treatments. Sci. Hortic. 2024, 330, 113052. [Google Scholar] [CrossRef]
- Ortuño-Hernández, G.; Fernández, M.; Martínez-Gómez, P.; Ruiz, D.; Salazar, J.A. Ripening-Related Gene Expression Analysis Revealed the Molecular Impact of 1-MCP Application on Apricot Fruit Softening, Color, Aroma, and Antioxidant Capacity. Postharvest Biol. Technol. 2024, 216, 113037. [Google Scholar] [CrossRef]
- Leida, C.; Ríos, G.; Soriano, J.M.; Pérez, B.; Llácer, G.; Crisosto, C.H.; Badenes, M.L. Identification and Genetic Characterization of an Ethylene-Dependent Polygalacturonase from Apricot Fruit. Postharvest Biol. Technol. 2011, 62, 26–34. [Google Scholar] [CrossRef]
- Grimplet, J.; Romieu, C.; Audergon, J.M.; Marty, I.; Albagnac, G.; Lambert, P.; Bouchet, J.P.; Terrier, N. Transcriptomic Study of Apricot Fruit (Prunus armeniaca) Ripening among 13 006 Expressed Sequence Tags. Physiol. Plant 2005, 125, 281–292. [Google Scholar] [CrossRef]
- Salazar, J.A.; Ruiz, D.; Zapata, P.; Martínez-García, P.J.; Martínez-Gómez, P. Whole Transcriptome Analyses of Apricots and Japanese Plum Fruits after 1-MCP (Ethylene-Inhibitor) and Ethrel (Ethylene-Precursor) Treatments Reveal New Insights into the Physiology of the Ripening Process. Int. J. Mol. Sci. 2022, 23, 11045. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, W.; Geng, W.; Zhao, S.; Pan, Y.; Fan, G.; Zhang, S.; Wang, Y.; Liao, K. Transcriptome Analysis Insight into Ethylene Metabolism and Pectinase Activity of Apricot (Prunus armeniaca L.) Development and Ripening. Sci. Rep. 2021, 11, 13569. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Moshe, F.; Ruth, B.-A. The Effect of Synthetic Auxins on Fruit Development, Quality and Final Fruit Size in ‘Canino’ Apricot (Prunus armeniaca L.). J. Hortic. Sci. Biotechnol. 2007, 82, 335–340. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Wang, N.; He, H.; Wen, B.; Zhang, R.; Fu, X.; Xiao, W.; Li, D.; Li, L.; et al. Genome-Wide Identification and Characterization of the Prunus Persica Ferredoxin Gene Family and Its Role in Improving Heat Tolerance. Plant Physiol. Biochem. 2022, 179, 108–119. [Google Scholar] [CrossRef]
- Taji, T.; Takahashi, S.; Shinozaki, K. Inositols and Their Metabolites in Abiotic and Biotic Stress Responses. In Biology of Inositols and Phosphoinositides: Subcellular Biochemistry; Majumder, A.L., Biswas, B.B., Eds.; Springer: Boston, MA, USA, 2006; pp. 239–264. ISBN 978-0-387-27600-7. [Google Scholar]
- Loewus, F.A. Inositol and Plant Cell Wall Polysaccharide Biogenesis. In Biology of Inositols and Phosphoinositides: Subcellular Biochemistry; Majumder, A.L., Biswas, B.B., Eds.; Springer: Boston, MA, USA, 2006; pp. 21–45. ISBN 978-0-387-27600-7. [Google Scholar]
- Torabinejad, J.; Gillaspy, G.E. Functional Genomics of Inositol Metabolism. In Biology of Inositols and Phosphoinositides: Subcellular Biochemistry; Majumder, A.L., Biswas, B.B., Eds.; Springer: Boston, MA, USA, 2006; pp. 47–70. ISBN 978-0-387-27600-7. [Google Scholar]
- Bianchi, V.J.; Rubio, M.; Trainotti, L.; Verde, I.; Bonghi, C.; Martínez-Gómez, P. Prunus Transcription Factors: Breeding Perspectives. Front. Plant Sci. 2015, 6, 443. [Google Scholar] [CrossRef]
- Liu, G.-S.; Li, H.-L.; Grierson, D.; Fu, D.-Q. NAC Transcription Factor Family Regulation of Fruit Ripening and Quality: A Review. Cells 2022, 11, 525. [Google Scholar] [CrossRef]
- Zhuo, X.; Zheng, T.; Zhang, Z.; Zhang, Y.; Jiang, L.; Ahmad, S.; Sun, L.; Wang, J.; Cheng, T.; Zhang, Q. Genome-Wide Analysis of the NAC Transcription Factor Gene Family Reveals Differential Expression Patterns and Cold-Stress Responses in the Woody Plant Prunus mume. Genes 2018, 9, 494. [Google Scholar] [CrossRef]
- Dhar, M.K.; Mishra, S.; Bhat, A.; Chib, S.; Kaul, S. Plant Carotenoid Cleavage Oxygenases: Structure–Function Relationships and Role in Development and Metabolism. Brief. Funct. Genom. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Varghese, R.; Kumar, S.U.; Doss, C.G.P.; Siva, R. Unraveling the Versatility of CCD4: Metabolic Engineering, Transcriptomic and Computational Approaches. Plant Sci. 2021, 310, 110991. [Google Scholar] [CrossRef]
- Egea, J.; Dicenta, F.; Burgos, L. ‘Rojo Pasión’ Apricot. HortScience 2004, 39, 1490–1491. [Google Scholar] [CrossRef]
- Pott, D.M.; Durán-Soria, S.; Osorio, S.; Vallarino, J.G. Combining Metabolomic and Transcriptomic Approaches to Assess and Improve Crop Quality Traits. CABI Agric. Biosci. 2021, 2, 1. [Google Scholar] [CrossRef]
- Lado, J.; Zacarías, L.; Rodrigo, M.J. Regulation of Carotenoid Biosynthesis During Fruit Development. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 161–198. ISBN 978-3-319-39126-7. [Google Scholar]
- Ohmiya, A.; Kato, M.; Shimada, T.; Nashima, K.; Kishimoto, S.; Nagata, M. Molecular Basis of Carotenoid Accumulation in Horticultural Crops. Hortic. J. 2019, 88, 135–149. [Google Scholar] [CrossRef]
- Muhammad, N.; Luo, Z.; Yang, M.; Li, X.; Liu, Z.; Liu, M. The Joint Role of the Late Anthocyanin Biosynthetic UFGT-Encoding Genes in the Flowers and Fruits Coloration of Horticultural Plants. Sci. Hortic. 2022, 301, 111110. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; dos Santos, A.L.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the Genetic Regulation of Anthocyanin Biosynthesis in Plants—Tools for Breeding Purple Cultivars of Fruits and Vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Celi, G.E.A.; Gratão, P.L.; Lanza, M.G.D.B.; Reis, A.R. dos Physiological and Biochemical Roles of Ascorbic Acid on Mitigation of Abiotic Stresses in Plants. Plant Physiol. Biochem. 2023, 202, 107970. [Google Scholar] [CrossRef]
- Castro, J.C.; Castro, C.G.; Cobos, M. Genetic and Biochemical Strategies for Regulation of L-Ascorbic Acid Biosynthesis in Plants through the L-Galactose Pathway. Front. Plant Sci. 2023, 14, 1099829. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Strobbe, S.; Van Der Straeten, D.; Zhang, C. Regulation of Plant Vitamin Metabolism: Backbone of Biofortification for the Alleviation of Hidden Hunger. Mol. Plant 2021, 14, 40–60. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Brown, G.S.; Walker, T.D. Indicators of Maturity in Apricots Using Biplot Multivariate Analysis. J. Sci. Food Agric. 1990, 53, 321–331. [Google Scholar] [CrossRef]
- Le Provost, G.; Herrera, R.; Paiva, J.A.P.; Chaumeil, P.; Salin, F.; Plomion, C. A Micromethod for High Throughput RNA Extraction in Forest Trees. Biol. Res. 2007, 40, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]


| Apricot Cultivar | Trait | Mean ± SD | Apricot Cultivar | Trait | Mean ± SD |
|---|---|---|---|---|---|
| ‘Cebas Red’ | Ripening date | 130 | ‘Orange Red’ | Ripening date | 153 |
 | Fruit weight | 63.03 ± 6.77 |  | Fruit weight | 61.04 ± 6.39 |
| IAD | 0.31 ± 0.13 | IAD | 0.95 ± 0.23 | ||
| Skin color | 74.02 ± 3.39 | Skin color | 77.05 ± 2.97 | ||
| Blush color | 52.80 ± 8.94 | Blush color | 49.87 ± 15.97 | ||
| % Blush color | 24.50 ± 13.22 | % Blush color | 25.50 ± 14.42 | ||
| Flesh color | 73.93 ± 1.03 | Flesh color | 73.93 ± 2.47 | ||
| Firmness | 76.83 ± 15.59 | Firmness | 53.41 ± 7.06 | ||
| Ethylene | 0.08 ± 0.01 | Ethylene | 0.33 ± 0.11 | ||
| SSC | 7.97 ± 0.06 | SSC | 10.40 ± 0.46 | ||
| Acidity | 1.10 ± 0.20 | Acidity | 1.07 ± 0.06 | ||
| ‘Currot’ | Ripening date | 132 | ‘Goldrich’ | Ripening date | 158 |
 | Fruit weight | 36.83 ± 5.52 |  | Fruit weight | 126.63 ± 14.37 |
| IAD | 0.73 ± 0.18 | IAD | 0.72 ± 0.22 | ||
| Skin color | 105.40 ± 2.07 | Skin color | 77.87 ± 1.72 | ||
| Blush color | 82.93 ± 8.91 | Blush color | 69.42 ± 4.53 | ||
| % Blush color | 10.00 ± 5.27 | % Blush color | 13.00 ± 10.85 | ||
| Flesh color | 98.00 ± 3.37 | Flesh color | 72.55 ± 1.19 | ||
| Firmness | 108.90 ± 20.57 | Firmness | 73.42 ± 14.41 | ||
| Ethylene | 0.11 ± 0.08 | Ethylene | 0.35 ± 0.33 | ||
| SSC | 11.07 ± 0.73 | SSC | 10.33 ± 0.06 | ||
| Acidity | 1.76 ± 0.15 | Acidity | 2.65 ± 0.34 | ||
| ‘Rojo Pasión’ | Ripening date | 141 | ‘Deseo’ | Ripening date | 167 |
 | Fruit weight | 71.39 ± 9.59 |  | Fruit weight | 65.29 ± 6.58 |
| IAD | 1.14 ± 0.21 | IAD | 1.16 ± 0.18 | ||
| Skin color | 84.01 ± 3.31 | Skin color | 75.05 ± 2.01 | ||
| Blush color | 77.12 ± 7.66 | Blush color | 52.80 ± 7.32 | ||
| % Blush color | 7.50 ± 4.25 | % Blush color | 17.50 ± 10.34 | ||
| Flesh color | 75.94 ± 1.54 | Flesh color | 73.57 ± 1.44 | ||
| Firmness | 24.65 ± 10.79 | Firmness | 56.61 ± 8.24 | ||
| Ethylene | 32.57 ± 15.69 | Ethylene | 0.27 ± 0.19 | ||
| SSC | 9.60 ± 0.75 | SSC | 12.90 ± 0.44 | ||
| Acidity | 1.13 ± 0.08 | Acidity | 1.40 ± 0.02 | ||
| ‘906-12’ | Ripening date | 146 | ‘Bergeron’ | Ripening date | 174 |
 | Fruit weight | 84.38 ± 5.54 |  | Fruit weight | 55.66 ± 4.66 |
| IAD | 0.86 ± 0.08 | IAD | 0.73 ± 0.16 | ||
| Skin color | 84.45 ± 2.33 | Skin color | 84.59 ± 3.23 | ||
| Blush color | 49.22 ± 10.32 | Blush color | 71.05 ± 8.68 | ||
| % Blush color | 27.50 ± 8.90 | % Blush color | 14.50 ± 7.25 | ||
| Flesh color | 80.58 ± 1.87 | Flesh color | 75.89 ± 2.37 | ||
| Firmness | 67.94 ± 12.19 | Firmness | 42.99 ± 8.26 | ||
| Ethylene | 0.36 ± 0.30 | Ethylene | 0.26 ± 0.13 | ||
| SSC | 11.03 ± 0.21 | SSC | 10.13 ± 0.47 | ||
| Acidity | 1.45 ± 0.08 | Acidity | 1.87 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortuño-Hernández, G.; Sánchez, M.; Ruiz, D.; Martínez-Gómez, P.; Salazar, J.A. Monitoring Fruit Growth and Development in Apricot (Prunus armeniaca L.) through Gene Expression Analysis. Int. J. Mol. Sci. 2024, 25, 9081. https://doi.org/10.3390/ijms25169081
Ortuño-Hernández G, Sánchez M, Ruiz D, Martínez-Gómez P, Salazar JA. Monitoring Fruit Growth and Development in Apricot (Prunus armeniaca L.) through Gene Expression Analysis. International Journal of Molecular Sciences. 2024; 25(16):9081. https://doi.org/10.3390/ijms25169081
Chicago/Turabian StyleOrtuño-Hernández, Germán, María Sánchez, David Ruiz, Pedro Martínez-Gómez, and Juan Alfonso Salazar. 2024. "Monitoring Fruit Growth and Development in Apricot (Prunus armeniaca L.) through Gene Expression Analysis" International Journal of Molecular Sciences 25, no. 16: 9081. https://doi.org/10.3390/ijms25169081
APA StyleOrtuño-Hernández, G., Sánchez, M., Ruiz, D., Martínez-Gómez, P., & Salazar, J. A. (2024). Monitoring Fruit Growth and Development in Apricot (Prunus armeniaca L.) through Gene Expression Analysis. International Journal of Molecular Sciences, 25(16), 9081. https://doi.org/10.3390/ijms25169081








