The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects
Abstract
1. Introduction
2. Discovery, Structure and Some Biophysical Properties
3. Biosynthesis and Phylogenetic Distribution
4. The Metabolic Pathway from Tryptophan to KYNA
4.1. Tryptophan Dioxygenase/Indole Dioxygenase (EC 1.13.11.11 and 1.13.11.52)
4.2. Kynurenine Formamidase (E.C. 3.5.1.9) [83]
4.3. Kynurenine Oxoglutarate Transaminase (E. C. 2.6.1.7)/Kynurenine Aminotransferase (KATs)
4.4. KAT1
4.5. KAT II
4.6. KAT III
4.7. KAT IV
5. Transport of Kynurenic Acid and Related Metabolites
Mammalian kynurenic Acid Transporters SLC22A6 and SLC22A8
6. Bioavailability
7. Concentrations of KYNA in ‘Normal’ Serum and Plasma, and Other Body Fluids/Tissues
7.1. Breast Milk
7.2. Bile
7.3. Intestine
7.4. Gut Microbiota and KYNA
| Tissues—Rodents | ||
| Concentration Range | Comments | Reference |
| Review | [347] | |
| 32 nM increases to → 135 mM after dosing | Gerbil brain | [360] |
| 1–16 mM | Rat ileum | [361] |
| ~40 nM in plasma | Trebled after dosing at 5 mg/kg | [210] |
| Tissues—human | ||
| Concentration range | Comments | Reference |
| Review | [305] | |
| 0.2–0.7 pmol/mg | Brain; 3× increase in Down syndrome | [362] |
| 2–3 pmol/mg | Brain | [353] |
| Up to 1.58 pmol/mg | Brain | [363] and review [347] |
| 1.6 mM | Colon | [359] |
| 10.2 ng/mL | Fetal membrane | [364] |
| 7.6 g/mL | Umbilical Cord | [364] |
| 1 ng/mL | Placenta | [364] |
7.5. Urine
7.6. Feces
8. Nutritional Sources
9. Pharmacokinetics
10. Further Metabolism and Excretion
11. Oxidative Stress
12. Diseases in Which KYNA Levels Are Significantly Altered
13. KYNA and COVID-19
14. Protection against Various Diseases
14.1. Neuroprotection
14.2. Peripheral Protection
| Organ/Tissue/Disease | Comments | Selected References |
|---|---|---|
| Alimentary canal | Protects vs. stress ulcers in rats | [553,554] |
| Cardiovascular disorders | Review | [555] |
| Diabetes, type 2 | Protective of glomerular filtration rate and against end-stage kidney disease in type 2 diabetes | [556] |
| Fibrosis | Protective vs. fibrotic injury after surgery | [550,552] |
| Heart | Protection against ischemia-reperfusion injury | [557,558] |
| Kidney | Protective of glomerular filtration rate and against end-stage kidney disease in type 2 diabetes | [556] |
| Improved kidney function in spontaneously hypertensive and normotensive rats | [559] | |
| Liver | Levels raised in and protective against hexafluoropropylene oxide dimer acid (HFPO-DA) challenge in mice | [560] |
| Protection vs. nonalcoholic fatty liver disease at very high concentrations | [561] | |
| Lung | Protective in an acute lung injury model | [562] |
| Multi-organ | Protection against heatstroke by multiple mechanisms, including an anti-apoptotic effect | [563] |
| Pancreatitis (acute) | Rat study. Significantly protective at 300 mg/kg | [564] |
| Retinal ganglia | Protective against ischemia-reperfusion injury in mice | [565] |
| Sepsis | Protection vs. neutrophil activation and mitochondrial dysfunction in rats | [566] |
| Active at high doses against LPS-induced inflammation/death in mice | [567] | |
| Stroke | Associated with a lower level of risk (but probably also confounded with kynurenine); also protective | [568,569,570] |
| Vascular inflammation | Protective | [571] |
| Wound healing and scarring | Protective, by largely unknown mechanisms. | [550,552,572,573,574] |
14.3. Reported Receptors
15. Role of KYNA in Protecting against Ischemia-Reperfusion Injury
16. Other Factors Known to Affect KYNA Levels
17. Other Effects of KYNA
18. Safety
19. Possible Risks
20. Regulations for Food Supplements and Nutraceuticals
21. Analytics
22. KYNA as a Therapeutic for Chronic Inflammatory Diseases
23. Use of KYNA as an Antioxidant in Processed Foodstuffs
24. KYNA in the Feed of Racing Animals
25. Use of KYNA in Cosmetics
26. Role of KYNA as a Cofactor
27. Cheminformatics of KYNA
28. Predicting the KYNA Interactome
29. Biotechnological Production
30. Conclusions and Forward Look
- Many examples in which its exogenous addition seems to offer benefits of health or of protection against disease
- Evidence that its concentration is relatively low in normal populations
- Safety evidence to the effect that there do not seem to be examples in which hyperactive alleles of KAT enzymes lead to overt disease, and that exogenous KYNA cannot realistically ‘go back’ to L-kynurenine
- Evidence that it is more or less readily bioavailable for entering plasma from the diet rather than simply being produced by compounds such as tryptophan and L-kynurenine that are more easily transported but that can lead to other, potentially toxic molecules.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Ogle, W.O.; Speisman, R.B.; Ormerod, B.K. Potential of treating age-related depression and cognitive decline with nutraceutical approaches: A mini-review. Gerontology 2013, 59, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ragle, R.L.; Sawitzke, A.D. Nutraceuticals in the management of osteoarthritis: A critical review. Drugs Aging 2012, 29, 717–731. [Google Scholar] [CrossRef]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4–8. [Google Scholar] [CrossRef]
- Borghi, C.; Cicero, A.F. Nutraceuticals with a clinically detectable blood pressure-lowering effect: A review of available randomized clinical trials and their meta-analyses. Br. J. Clin. Pharmacol. 2017, 83, 163–171. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Coles, L.S.; Landes, B.; Repine, J.E. Functional benefits of ergothioneine and fruit- and vegetable-derived nutraceuticals: Overview of the supplemental issue contents. Prev. Med. 2012, 54, S4–S8. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Sharif, M.K.; Khalid, R. Nutraceuticals: Myths Versus Realities. In Therapeut Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–21. [Google Scholar]
- Singh, S.; Razak, M.A.; Sangam, S.R.; Viswanath, B.; Begum, P.S.; Rajagopal, S. The Impact of Functional Food and Nutraceuticals in Health. In Therapeut Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 23–47. [Google Scholar]
- Spindler, S.R.; Mote, P.L.; Flegal, J.M. Lifespan effects of simple and complex nutraceutical combinations fed isocalorically to mice. Age 2014, 36, 705–718. [Google Scholar] [CrossRef]
- D’Cunha, N.M.; Georgousopoulou, E.N.; Dadigamuwage, L.; Kellett, J.; Panagiotakos, D.B.; Thomas, J.; McKune, A.J.; Mellor, D.D.; Naumovski, N. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: A 10-year systematic review of randomised controlled trials. Br. J. Nutr. 2018, 119, 280–298. [Google Scholar] [CrossRef]
- Hopper, I.; Connell, C.; Briffa, T.; De Pasquale, C.G.; Driscoll, A.; Kistler, P.M.; Macdonald, P.S.; Sindone, A.; Thomas, L.; Atherton, J.J. Nutraceuticals in Patients With Heart Failure: A Systematic Review. J. Card. Fail. 2020, 26, 166–179. [Google Scholar] [CrossRef]
- Ilari, S.; Proietti, S.; Russo, P.; Malafoglia, V.; Gliozzi, M.; Maiuolo, J.; Oppedisano, F.; Palma, E.; Tomino, C.; Fini, M.; et al. A Systematic Review and Meta-Analysis on the Role of Nutraceuticals in the Management of Neuropathic Pain in In Vivo Studies. Antioxidants 2022, 11, 2361. [Google Scholar] [CrossRef]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical Properties of Syringic Acid in Civilization Diseases-Review. Nutrients 2023, 16, 10. [Google Scholar] [CrossRef]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, R.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; Uberti, F.; Folli, A.; Turco, A.; Curci, C.; d’Abrosca, F.; de Sire, A.; Invernizzi, M. Impact of nutraceuticals and dietary supplements on mitochondria modifications in healthy aging: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 2659–2674. [Google Scholar] [CrossRef] [PubMed]
- Allaqaband, S.; Dar, A.H.; Patel, U.; Kumar, N.; Nayik, G.A.; Khan, S.A.; Ansari, M.J.; Alabdallah, N.M.; Kumar, P.; Pandey, V.K.; et al. Utilization of Fruit Seed-Based Bioactive Compounds for Formulating the Nutraceuticals and Functional Food: A Review. Front. Nutr. 2022, 9, 902554. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: A review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2022, 62, 1242–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, S.; Tang, L.; Gong, J.; Su, D.; Yang, H. Piperine as a Potential Nutraceutical Agent for Managing Diabetes and Its Complications: A Literature Review. J. Med. Food 2023, 26, 693–704. [Google Scholar] [CrossRef]
- Yılmaz, C.; Gökmen, V. Perspective on the Formation, Analysis, and Health Effects of Neuroactive Compounds in Foods. J. Agric. Food Chem. 2021, 69, 13364–13372. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Ruscica, M.; Penson, P.E.; Ferri, N.; Sirtori, C.R.; Pirro, M.; Mancini, G.B.J.; Sattar, N.; Toth, P.P.; Sahebkar, A.; Lavie, C.J.; et al. Impact of nutraceuticals on markers of systemic inflammation: Potential relevance to cardiovascular diseases—A position paper from the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2021, 67, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Colletti, A.; Penson, P.E.; Katsiki, N.; Mikhailidis, D.P.; Toth, P.P.; Gouni-Berthold, I.; Mancini, J.; Marais, D.; Moriarty, P.; et al. Nutraceutical approaches to non-alcoholic fatty liver disease (NAFLD): A position paper from the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2023, 189, 106679. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Thorne, J.L.; Moore, J.B. Ergothioneine: An underrecognised dietary micronutrient required for healthy ageing? Br. J. Nutr. 2023, 129, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Liebig, J. Über Kynurensäure. Ann. Chem. 1853, 86, 125–126. [Google Scholar]
- Pileni, M.P.; Giraud, M.; Santus, R. Kynurenic acid: I. Spectroscopic properties. Photochem. Photobiol. 1979, 30, 251–256. [Google Scholar]
- Zelentsova, E.A.; Sherin, P.S.; Snytnikova, O.A.; Kaptein, R.; Vauthey, E.; Tsentalovich, Y.P. Photochemistry of aqueous solutions of kynurenic acid and kynurenine yellow. Photochem. Photobiol. Sci. 2013, 12, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, D.; Janczak, J.; Pawlak, S.; Zaworotko, M.; Videnova-Adrabinska, V. Tautomeric polymorphism of the neuroactive inhibitor kynurenic acid. Acta Crystallogr. C Struct. Chem. 2019, 75, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Staniszewska, M. Electrochemical Determination of Kynurenine Pathway Metabolites-Challenges and Perspectives. Sensors 2021, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Kuc, D.; Turski, W.A. Presence of kynurenic acid in food and honeybee products. Amino Acids 2009, 36, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kita, A.; Kołodziejczyk, M.; Michalska-Ciechanowska, A.; Brzezowska, J.; Wicha-Komsta, K.; Turski, W. The Effect of Thermal Treatment on Selected Properties and Content of Biologically Active Compounds in Potato Crisps. Appl. Sci. 2022, 12, 555. [Google Scholar] [CrossRef]
- Pileni, M.P.; Giraud, M.; Santus, R. Kynurenic acid: II. Photosensitizing properties. Photochem. Photobiol. 1979, 30, 257–261. [Google Scholar]
- Samanta, A.; Guchhait, N.; Bhattacharya, S.C. Photophysical aspects of biological photosensitizer Kynurenic acid from the perspective of experimental and quantum chemical study. Spectrochim. Acta 2014, 129, 457–465. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B. The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review. Molecules 2020, 25, 4984. [Google Scholar] [CrossRef]
- Kubicova, L.; Hadacek, F.; Bachmann, G.; Weckwerth, W.; Chobot, V. Coordination Complex Formation and Redox Properties of Kynurenic and Xanthurenic Acid Can Affect Brain Tissue Homeodynamics. Antioxidants 2019, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Mitsuhashi, S.; Tomiya, M.; Kawai, J.; Hashimoto, K.; Toyo’oka, T. Determination of rat brain kynurenic acid by column-switching HPLC with fluorescence detection. Biomed. Chromatogr. 2007, 21, 514–519. [Google Scholar] [CrossRef]
- Qi, X.; Fu, K.; Yue, M.; Shou, N.; Yuan, X.; Chen, X.; He, C.; Yang, Y.; Shi, Z. Kynurenic acid mediates bacteria-algae consortium in resisting environmental cadmium toxicity. J. Hazard. Mater. 2023, 444, 130397. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Muhamadali, H.; Winder, C.L.; Dunn, W.B.; Goodacre, R. Unlocking the secrets of the microbiome: Exploring the dynamic microbial interplay with humans through metabolomics and their manipulation for synthetic biology applications. Biochem. J. 2023, 480, 891–908. [Google Scholar] [CrossRef]
- McCann, J.R.; Rawls, J.F. Essential Amino Acid Metabolites as Chemical Mediators of Host-Microbe Interaction in the Gut. Annu. Rev. Microbiol. 2023, 77, 479–497. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sujino, T.; Kanai, T. The tryptophan metabolic pathway of the microbiome and host cells in health and disease. Int. Immunol. 2024. [Google Scholar] [CrossRef]
- Schwarcz, R.; Foo, A.; Sathyasaikumar, K.V.; Notarangelo, F.M. The Probiotic Lactobacillus reuteri Preferentially Synthesizes Kynurenic Acid from Kynurenine. Int. J. Mol. Sci. 2024, 25, 3679. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019, 12, 1178646919852996. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Chen, L.M.; Bao, C.H.; Wu, Y.; Liang, S.H.; Wang, D.; Wu, L.Y.; Huang, Y.; Liu, H.R.; Wu, H.G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflammation 2021, 18, 135. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Spekker, E.; Vécsei, L. Kynurenines, Neuronal Excitotoxicity, and Mitochondrial Oxidative Stress: Role of the Intestinal Flora. Int. J. Mol. Sci. 2024, 25, 1698. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Neuroactive compounds in foods: Occurrence, mechanism and potential health effects. Food Res. Int. 2020, 128, 108744. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Fila, M.; Chojnacki, J.; Pawlowska, E.; Szczepanska, J.; Chojnacki, C.; Blasiak, J. Kynurenine Pathway of Tryptophan Metabolism in Migraine and Functional Gastrointestinal Disorders. Int. J. Mol. Sci. 2021, 22, 10134. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Raes, J.; Lowry, C.A.; Seedat, S.; Hemmings, S.M.J. The Gut Microbiome and Mental Health: Implications for Anxiety- and Trauma-Related Disorders. OMICS 2018, 22, 90–107. [Google Scholar] [CrossRef]
- Rust, C.; Malan-Muller, S.; van den Heuvel, L.L.; Tonge, D.; Seedat, S.; Pretorius, E.; Hemmings, S.M.J. Platelets bridging the gap between gut dysbiosis and neuroinflammation in stress-linked disorders: A narrative review. J. Neuroimmunol. 2023, 382, 578155. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wang, Y.F.; Lei, L.; Zhang, Y. Impacts of microbiota and its metabolites through gut-brain axis on pathophysiology of major depressive disorder. Life Sci. 2024, 351, 122815. [Google Scholar] [CrossRef]
- Ramos-Chávez, L.A.; Lugo Huitrón, R.; González Esquivel, D.; Pineda, B.; Ríos, C.; Silva-Adaya, D.; Sánchez-Chapul, L.; Roldán-Roldán, G.; Pérez de la Cruz, V. Relevance of Alternative Routes of Kynurenic Acid Production in the Brain. Oxid. Med. Cell Longev. 2018, 2018, 5272741. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef]
- Badawy, A.A. Hypothesis kynurenic and quinolinic acids: The main players of the kynurenine pathway and opponents in inflammatory disease. Med. Hypotheses 2018, 118, 129–138. [Google Scholar] [CrossRef]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Stavrum, A.K.; Heiland, I.; Schuster, S.; Puntervoll, P.; Ziegler, M. Model of tryptophan metabolism, readily scalable using tissue-specific gene expression data. J. Biol. Chem. 2013, 288, 34555–34566. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Miggiano, R.; Ferraris, D.M.; Rizzi, M. The Synthesis of Kynurenic Acid in Mammals: An Updated Kynurenine Aminotransferase Structural KATalogue. Front. Mol. Biosci. 2019, 6, 7. [Google Scholar] [CrossRef]
- Kolodziej, L.R.; Paleolog, E.M.; Williams, R.O. Kynurenine metabolism in health and disease. Amino Acids 2011, 41, 1173–1183. [Google Scholar] [CrossRef]
- Proietti, E.; Rossini, S.; Grohmann, U.; Mondanelli, G. Polyamines and Kynurenines at the Intersection of Immune Modulation. Trends Immunol. 2020, 41, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vécsei, L. Kynurenines in the Pathogenesis of Multiple Sclerosis: Therapeutic Perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49, 15–23. [Google Scholar] [CrossRef]
- Parrott, J.M.; O’Connor, J.C. Kynurenine 3-Monooxygenase: An Influential Mediator of Neuropathology. Front. Psychiatry 2015, 6, 116. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Davey, H.M.; Kell, D.B. Flow cytometry and cell sorting of heterogeneous microbial populations: The importance of single-cell analysis. Microbiol. Rev. 1996, 60, 641–696. [Google Scholar] [PubMed]
- Lundberg, E.; Uhlén, M. Creation of an antibody-based subcellular protein atlas. Proteomics 2010, 10, 3984–3996. [Google Scholar] [PubMed]
- Uhlén, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [PubMed]
- Regev, A.; Teichmann, S.A.; Lander, E.S.; Amit, I.; Benoist, C.; Birney, E.; Bodenmiller, B.; Campbell, P.; Carninci, P.; Clatworthy, M.; et al. The Human Cell Atlas. eLife 2017, 6, e27041. [Google Scholar] [CrossRef]
- George, N.; Fexova, S.; Fuentes, A.M.; Madrigal, P.; Bi, Y.; Iqbal, H.; Kumbham, U.; Nolte, N.F.; Zhao, L.; Thanki, A.S.; et al. Expression Atlas update: Insights from sequencing data at both bulk and single cell level. Nucleic Acids Res. 2024, 52, D107–D114. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, R.G.H.; Regev, A.; Teichmann, S.A. Towards a Human Cell Atlas: Taking Notes from the Past. Trends Genet. 2021, 37, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Rood, J.E.; Maartens, A.; Hupalowska, A.; Teichmann, S.A.; Regev, A. Impact of the Human Cell Atlas on medicine. Nat. Med. 2022, 28, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: A compensatory mechanism or a driving force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Zsizsik, B.K.; Hardeland, R. Formation of kynurenic and xanthurenic acids from kynurenine and 3-hydroxykynurenine in the dinoflagellate Lingulodinium polyedrum: Role of a novel, oxidative pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 133, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Pabarcus, M.K.; Casida, J.E. Kynurenine formamidase: Determination of primary structure and modeling-based prediction of tertiary structure and catalytic triad. Biochim. Biophys. Acta 2002, 1596, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Tan, V.; Lovejoy, D.; Braidy, N.; Rowe, D.B.; Brew, B.J.; Guillemin, G.J. Involvement of quinolinic acid in the neuropathogenesis of amyotrophic lateral sclerosis. Neuropharmacology 2017, 112, 346–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sakuma, M.; Deora, G.S.; Levy, C.W.; Klausing, A.; Breda, C.; Read, K.D.; Edlin, C.D.; Ross, B.P.; Wright Muelas, M.; et al. A brain-permeable inhibitor of the neurodegenerative disease target kynurenine 3-monooxygenase prevents accumulation of neurotoxic metabolites. Commun. Biol. 2019, 2, 271. [Google Scholar] [CrossRef]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and alpha-Lipoic Acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.C.; Vezzani, A.; French, E.D.; Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 1984, 48, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.F.; Boegman, R.J.; Beninger, R.J.; Jhamandas, K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience 1997, 78, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Miranda, A.F.; Tanguay, J.J.; Boegman, R.J.; Beninger, R.J.; Jhamandas, K. Modulation of striatal quinolinate neurotoxicity by elevation of endogenous brain kynurenic acid. Br. J. Pharmacol. 1998, 124, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, L.; Pérez-Severiano, F.; González-Esquivel, D.; Elizondo, G.; Segovia, J. Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J. Neurosci. Res. 2015, 93, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Biasibetti-Brendler, H.; Pierozan, P.; Schmitz, F.; Bertó, C.G.; Prezzi, C.A.; Manfredini, V.; Wyse, A.T.S. Kynurenic Acid Restores Nrf2 Levels and Prevents Quinolinic Acid-Induced Toxicity in Rat Striatal Slices. Mol. Neurobiol. 2018, 55, 8538–8549. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Schmitz, F.; Marques, E.P.; Siebert, C.; Wyse, A.T.S. Intrastriatal Quinolinic Acid Administration Impairs Redox Homeostasis and Induces Inflammatory Changes: Prevention by Kynurenic Acid. Neurotox. Res. 2020, 38, 50–58. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Lima, S.; Kumar, S.; Gawandi, V.; Momany, C.; Phillips, R.S. Crystal structure of the Homo sapiens kynureninase-3-hydroxyhippuric acid inhibitor complex: Insights into the molecular basis of kynureninase substrate specificity. J. Med. Chem. 2009, 52, 389–396. [Google Scholar] [CrossRef]
- Wilson, K.; Mole, D.J.; Binnie, M.; Homer, N.Z.; Zheng, X.; Yard, B.A.; Iredale, J.P.; Auer, M.; Webster, S.P. Bacterial expression of human kynurenine 3-monooxygenase: Solubility, activity, purification. Protein Expr. Purif. 2014, 95, 96–103. [Google Scholar] [CrossRef]
- Urbańska, E.M.; Chmiel-Perzyńska, I.; Perzyński, A.; Derkacz, M.; Owe-Larsson, B. Endogenous Kynurenic Acid and Neurotoxicity. In Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–31. [Google Scholar]
- Zinger, A.; Barcia, C.; Herrero, M.T.; Guillemin, G.J. The involvement of neuroinflammation and kynurenine pathway in Parkinson’s disease. Parkinsons Dis. 2011, 2011, 716859. [Google Scholar] [CrossRef]
- Boros, F.A.; Bohár, Z.; Vécsei, L. Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases. Mutat. Res. Rev. Mutat. Res. 2018, 776, 32–45. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef]
- Kacser, H.; Burns, J.A. The control of flux. In Rate Control of Biological Processes. Symposium of the Society for Experimental Biology; Davies, D.D., Ed.; Cambridge University Press: Cambridge, UK, 1973; Volume 27, pp. 65–104. [Google Scholar]
- Heinrich, R.; Rapoport, T.A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur. J. Biochem. 1974, 42, 89–95. [Google Scholar] [PubMed]
- Kell, D.B.; Westerhoff, H.V. Metabolic control theory: Its role in microbiology and biotechnology. FEMS Microbiol. Rev. 1986, 39, 305–320. [Google Scholar]
- Fell, D.A. Understanding the Control of Metabolism; Portland Press: London, UK, 1996. [Google Scholar]
- Fell, D.A.; Saavedra, E.; Rohwer, J. 50 years of Metabolic Control Analysis: Its past and current influence in the biological sciences. Biosystems 2024, 235, 105086. [Google Scholar] [CrossRef]
- Rios-Avila, L.; Nijhout, H.F.; Reed, M.C.; Sitren, H.S.; Gregory, J.F., 3rd. A mathematical model of tryptophan metabolism via the kynurenine pathway provides insights into the effects of vitamin B-6 deficiency, tryptophan loading, and induction of tryptophan 2,3-dioxygenase on tryptophan metabolites. J. Nutr. 2013, 143, 1509–1519. [Google Scholar] [CrossRef]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef]
- Bergmann, F.T.; Hoops, S.; Klahn, B.; Kummer, U.; Mendes, P.; Pahle, J.; Sahle, S. COPASI and its applications in biotechnology. J. Biotechnol. 2017, 261, 215–220. [Google Scholar] [CrossRef]
- Hoops, S.; Sahle, S.; Gauges, R.; Lee, C.; Pahle, J.; Simus, N.; Singhal, M.; Xu, L.; Mendes, P.; Kummer, U. COPASI: A COmplex PAthway SImulator. Bioinformatics 2006, 22, 3067–3074. [Google Scholar] [PubMed]
- Mendes, P.; Hoops, S.; Sahle, S.; Gauges, R.; Dada, J.; Kummer, U. Computational modeling of biochemical networks using COPASI. Methods Mol. Biol. 2009, 500, 17–59. [Google Scholar]
- Keating, S.M.; Waltemath, D.; Konig, M.; Zhang, F.; Drager, A.; Chaouiya, C.; Bergmann, F.T.; Finney, A.; Gillespie, C.S.; Helikar, T.; et al. SBML Level 3: An extensible format for the exchange and reuse of biological models. Mol. Syst. Biol. 2020, 16, e9110. [Google Scholar] [CrossRef]
- Thiele, I.; Swainston, N.; Fleming, R.M.T.; Hoppe, A.; Sahoo, S.; Aurich, M.K.; Haraldsdottír, H.; Mo, M.L.; Rolfsson, O.; Stobbe, M.D.; et al. A community-driven global reconstruction of human metabolism. Nat. Biotechnol. 2013, 31, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Smallbone, K. Striking a balance with Recon 2.1. arXiv 2013, arXiv:1311.5696. [Google Scholar]
- Herrgård, M.J.; Swainston, N.; Dobson, P.; Dunn, W.B.; Arga, K.Y.; Arvas, M.; Blüthgen, N.; Borger, S.; Costenoble, R.; Heinemann, M.; et al. A consensus yeast metabolic network obtained from a community approach to systems biology. Nat. Biotechnol. 2008, 26, 1155–1160. [Google Scholar] [PubMed]
- Ball, H.J.; Jusof, F.F.; Bakmiwewa, S.M.; Hunt, N.H.; Yuasa, H.J. Tryptophan-catabolizing enzymes-party of three. Front. Immunol. 2014, 5, 485. [Google Scholar] [CrossRef]
- Yuasa, H.J.; Ushigoe, A.; Ball, H.J. Molecular evolution of bacterial indoleamine 2,3-dioxygenase. Gene 2011, 485, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.J.; Ball, H.J. Molecular evolution and characterization of fungal indoleamine 2,3-dioxygenases. J. Mol. Evol. 2011, 72, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.J.; Ball, H.J. The evolution of three types of indoleamine 2,3 dioxygenases in fungi with distinct molecular and biochemical characteristics. Gene 2012, 504, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.J.; Ball, H.J. Indoleamine 2,3-dioxygenases with very low catalytic activity are well conserved across kingdoms: IDOs of Basidiomycota. Fungal Genet. Biol. 2013, 56, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.J.; Ball, H.J. Efficient tryptophan-catabolizing activity is consistently conserved through evolution of TDO enzymes, but not IDO enzymes. J. Exp. Zool. B Mol. Dev. Evol. 2015, 324, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.J.; Sugiura, M.; Harumoto, T. A single amino acid residue regulates the substrate affinity and specificity of indoleamine 2,3-dioxygenase. Arch. Biochem. Biophys. 2018, 640, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.C.; Varani, A.M.; Menck, C.F. NAD biosynthesis evolution in bacteria: Lateral gene transfer of kynurenine pathway in Xanthomonadales and Flavobacteriales. Mol. Biol. Evol. 2009, 26, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.J.; Sanchez-Perez, A.; Weiser, S.; Austin, C.J.; Astelbauer, F.; Miu, J.; McQuillan, J.A.; Stocker, R.; Jermiin, L.S.; Hunt, N.H. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene 2007, 396, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Metz, R.; Duhadaway, J.B.; Kamasani, U.; Laury-Kleintop, L.; Muller, A.J.; Prendergast, G.C. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007, 67, 7082–7087. [Google Scholar] [CrossRef] [PubMed]
- Theate, I.; van Baren, N.; Pilotte, L.; Moulin, P.; Larrieu, P.; Renauld, J.C.; Herve, C.; Gutierrez-Roelens, I.; Marbaix, E.; Sempoux, C.; et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol. Res. 2015, 3, 161–172. [Google Scholar] [CrossRef]
- Yamazaki, F.; Kuroiwa, T.; Takikawa, O.; Kido, R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem. J. 1985, 230, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Löb, S.; Konigsrainer, A.; Zieker, D.; Brücher, B.L.; Rammensee, H.G.; Opelz, G.; Terness, P. IDO1 and IDO2 are expressed in human tumors: Levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol. Immunother. 2009, 58, 153–157. [Google Scholar] [CrossRef]
- Girithar, H.N.; Staats Pires, A.; Ahn, S.B.; Guillemin, G.J.; Gluch, L.; Heng, B. Involvement of the kynurenine pathway in breast cancer: Updates on clinical research and trials. Br. J. Cancer 2023, 129, 185–203. [Google Scholar] [CrossRef]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Croitoru-Lamoury, J.; Lamoury, F.M.; Caristo, M.; Suzuki, K.; Walker, D.; Takikawa, O.; Taylor, R.; Brew, B.J. Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS ONE 2011, 6, e14698. [Google Scholar] [CrossRef]
- Sarkar, S.A.; Wong, R.; Hackl, S.I.; Moua, O.; Gill, R.G.; Wiseman, A.; Davidson, H.W.; Hutton, J.C. Induction of indoleamine 2,3-dioxygenase by interferon-gamma in human islets. Diabetes 2007, 56, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Roy, C.R. Host cell depletion of tryptophan by IFNgamma-induced Indoleamine 2,3-dioxygenase 1 (IDO1) inhibits lysosomal replication of Coxiella burnetii. PLoS Pathog. 2019, 15, e1007955. [Google Scholar] [CrossRef]
- Capece, L.; Arrar, M.; Roitberg, A.E.; Yeh, S.R.; Marti, M.A.; Estrin, D.A. Substrate stereo-specificity in tryptophan dioxygenase and indoleamine 2,3-dioxygenase. Proteins 2010, 78, 2961–2972. [Google Scholar] [CrossRef]
- Shimizu, T.; Nomiyama, S.; Hirata, F.; Hayaishi, O. Indoleamine 2,3-dioxygenase. Purification and some properties. J. Biol. Chem. 1978, 253, 4700–4706. [Google Scholar]
- Geng, J.; Liu, A. Heme-dependent dioxygenases in tryptophan oxidation. Arch. Biochem. Biophys. 2014, 544, 18–26. [Google Scholar] [CrossRef]
- Lewis-Ballester, A.; Forouhar, F.; Kim, S.M.; Lew, S.; Wang, Y.; Karkashon, S.; Seetharaman, J.; Batabyal, D.; Chiang, B.Y.; Hussain, M.; et al. Molecular basis for catalysis and substrate-mediated cellular stabilization of human tryptophan 2,3-dioxygenase. Sci. Rep. 2016, 6, 35169. [Google Scholar] [CrossRef]
- Pantouris, G.; Serys, M.; Yuasa, H.J.; Ball, H.J.; Mowat, C.G. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids 2014, 46, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Mayersbach, P.; Becker, K.; Schennach, H.; Fuchs, D.; Gostner, J.M. Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors. Pteridines 2015, 26, 31–36. [Google Scholar] [CrossRef]
- Perez-Castro, L.; Garcia, R.; Venkateswaran, N.; Barnes, S.; Conacci-Sorrell, M. Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J. 2023, 290, 7–27. [Google Scholar] [CrossRef]
- Kudo, Y.; Koh, I.; Sugimoto, J. Localization of Indoleamine 2,3-Dioxygenase-1 and Indoleamine 2,3-Dioxygenase-2 at the Human Maternal-Fetal Interface. Int. J. Tryptophan Res. 2020, 13, 1178646920984163. [Google Scholar] [CrossRef] [PubMed]
- Mandarano, M.; Bellezza, G.; Belladonna, M.L.; Vannucci, J.; Gili, A.; Ferri, I.; Lupi, C.; Ludovini, V.; Falabella, G.; Metro, G.; et al. Indoleamine 2,3-Dioxygenase 2 Immunohistochemical Expression in Resected Human Non-small Cell Lung Cancer: A Potential New Prognostic Tool. Front. Immunol. 2020, 11, 839. [Google Scholar] [CrossRef]
- Trabanelli, S.; Ocadlikova, D.; Ciciarello, M.; Salvestrini, V.; Lecciso, M.; Jandus, C.; Metz, R.; Evangelisti, C.; Laury-Kleintop, L.; Romero, P.; et al. The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells. J. Immunol. 2014, 192, 1231–1240. [Google Scholar] [CrossRef]
- Merlo, L.M.F.; DuHadaway, J.B.; Montgomery, J.D.; Peng, W.D.; Murray, P.J.; Prendergast, G.C.; Caton, A.J.; Muller, A.J.; Mandik-Nayak, L. Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses. Front. Immunol. 2020, 11, 1861. [Google Scholar] [CrossRef]
- Meng, B.; Wu, D.; Gu, J.; Ouyang, S.; Ding, W.; Liu, Z.J. Structural and functional analyses of human tryptophan 2,3-dioxygenase. Proteins 2014, 82, 3210–3216. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, S.; Wright Muelas, M.; Day, P.J.; Lundberg, E.; Kell, D.B. GeneGini: Assessment via the Gini coefficient of reference ‘‘housekeeping’’ genes and diverse human transporter expression profiles. Cell Syst. 2018, 6, 230–244. [Google Scholar]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic tryptophan homeostasis. Front. Mol. Biosci. 2022, 9, 897929. [Google Scholar] [CrossRef]
- Van Baren, N.; Van den Eynde, B.J. Tryptophan-degrading enzymes in tumoral immune resistance. Front. Immunol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Hoffmann, D.; Dvorakova, T.; Stroobant, V.; Bouzin, C.; Daumerie, A.; Solvay, M.; Klaessens, S.; Letellier, M.C.; Renauld, J.C.; van Baren, N.; et al. Tryptophan 2,3-Dioxygenase Expression Identified in Human Hepatocellular Carcinoma Cells and in Intratumoral Pericytes of Most Cancers. Cancer Immunol. Res. 2020, 8, 19–31. [Google Scholar] [CrossRef]
- Boros, F.A.; Vécsei, L. Tryptophan 2,3-dioxygenase, a novel therapeutic target for Parkinson’s disease. Expert. Opin. Ther. Targets 2021, 25, 877–888. [Google Scholar] [CrossRef]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Tavasol, A.; Jazi, K.; Hajibeygi, R.; Shool, S.; Sodeifian, F.; Klegeris, A.; McElhinney, A.; et al. Dynamic changes in metabolites of the kynurenine pathway in Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A systematic Review and meta-analysis. Front. Immunol. 2022, 13, 997240. [Google Scholar] [CrossRef]
- Basran, J.; Rafice, S.A.; Chauhan, N.; Efimov, I.; Cheesman, M.R.; Ghamsari, L.; Raven, E.L. A kinetic, spectroscopic, and redox study of human tryptophan 2,3-dioxygenase. Biochemistry 2008, 47, 4752–4760. [Google Scholar] [CrossRef] [PubMed]
- Pabarcus, M.K.; Casida, J.E. Cloning, expression, and catalytic triad of recombinant arylformamidase. Protein Expr. Purif. 2005, 44, 39–44. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Schuettengruber, B.; Doetzlhofer, A.; Kroboth, K.; Wintersberger, E.; Seiser, C. Alternate activation of two divergently transcribed mouse genes from a bidirectional promoter is linked to changes in histone modification. J. Biol. Chem. 2003, 278, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolsky, V.N.; Bowyer, J.F.; Pabarcus, M.K.; Heflich, R.H.; Williams, L.D.; Doerge, D.R.; Arvidsson, B.; Bergquist, J.; Casida, J.E. Effect of arylformamidase (kynurenine formamidase) gene inactivation in mice on enzymatic activity, kynurenine pathway metabolites and phenotype. Biochim. Biophys. Acta 2005, 1724, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wogulis, M.; Chew, E.R.; Donohoue, P.D.; Wilson, D.K. Identification of formyl kynurenine formamidase and kynurenine aminotransferase from Saccharomyces cerevisiae using crystallographic, bioinformatic and biochemical evidence. Biochemistry 2008, 47, 1608–1621. [Google Scholar] [CrossRef]
- Moscioni, A.D.; Engel, J.L.; Casida, J.E. Kynurenine formamidase inhibition as a possible mechanism for certain teratogenic effects of organophosphorus and methylcarbamate insecticides in chicken embryos. Biochem. Pharmacol. 1977, 26, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Robinson, H.; Li, J. Biochemical identification and crystal structure of kynurenine formamidase from Drosophila melanogaster. Biochem. J. 2012, 446, 253–260. [Google Scholar] [CrossRef]
- Seifert, J.; Pewnim, T. Alteration of mice L-tryptophan metabolism by the organophosphorous acid triester diazinon. Biochem. Pharmacol. 1992, 44, 2243–2250. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Serine hydrolase targets of organophosphorus toxicants. Chem. Biol. Interact. 2005, 157–158, 277–283. [Google Scholar] [CrossRef]
- Schwarcz, R.; Pellicciari, R. Manipulation of Brain Kynurenines: Glial Targets, Neuronal Effects, and Clinical Opportunities. J. Pharm. Exp. Therapeut 2002, 303, 1010. [Google Scholar]
- Bellocchi, D.; Macchiarulo, A.; Carotti, A.; Pellicciari, R. Quantum mechanics/molecular mechanics (QM/MM) modeling of the irreversible transamination of L-kynurenine to kynurenic acid: The round dance of kynurenine aminotransferase II. Biochim. Biophys. Acta 2009, 1794, 1802–1812. [Google Scholar] [CrossRef]
- Koper, K.; Han, S.W.; Pastor, D.C.; Yoshikuni, Y.; Maeda, H.A. Evolutionary origin and functional diversification of aminotransferases. J. Biol. Chem. 2022, 298, 102122. [Google Scholar] [CrossRef]
- Bulfer, S.L.; Brunzelle, J.S.; Trievel, R.C. Crystal structure of Saccharomyces cerevisiae Aro8, a putative alpha-aminoadipate aminotransferase. Protein Sci. 2013, 22, 1417–1424. [Google Scholar] [CrossRef]
- Han, Q.; Fang, J.; Li, J. Kynurenine aminotransferase and glutamine transaminase K of Escherichia coli: Identity with aspartate aminotransferase. Biochem. J. 2001, 360, 617–623. [Google Scholar] [CrossRef]
- Ohashi, K.; Chaleckis, R.; Takaine, M.; Wheelock, C.E.; Yoshida, S. Kynurenine aminotransferase activity of Aro8/Aro9 engage tryptophan degradation by producing kynurenic acid in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 12180. [Google Scholar] [CrossRef]
- Radi, M.S.; Sora, J.E.S.; Kim, S.H.; Sudarsan, S.; Sastry, A.V.; Kell, D.B.; Herrgård, M.J.; Feist, A.M. Membrane transporter identification and modulation via adaptive laboratory evolution. Metab. Eng. 2022, 72, 376–390. [Google Scholar]
- Genestet, C.; Le Gouellec, A.; Chaker, H.; Polack, B.; Guery, B.; Toussaint, B.; Stasia, M.J. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic. Biol. Med. 2014, 73, 400–410. [Google Scholar] [CrossRef]
- Bortolotti, P.; Hennart, B.; Thieffry, C.; Jausions, G.; Faure, E.; Grandjean, T.; Thepaut, M.; Dessein, R.; Allorge, D.; Guery, B.P.; et al. Tryptophan catabolism in Pseudomonas aeruginosa and potential for inter-kingdom relationship. BMC Microbiol. 2016, 16, 137. [Google Scholar] [CrossRef]
- Jansen, R.S.; Mandyoli, L.; Hughes, R.; Wakabayashi, S.; Pinkham, J.T.; Selbach, B.; Guinn, K.M.; Rubin, E.J.; Sacchettini, J.C.; Rhee, K.Y. Aspartate aminotransferase Rv3722c governs aspartate-dependent nitrogen metabolism in Mycobacterium tuberculosis. Nat. Commun. 2020, 11, 1960. [Google Scholar] [CrossRef]
- Han, Q.; Robinson, H.; Cai, T.; Tagle, D.A.; Li, J. Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K. J. Med. Chem. 2009, 52, 2786–2793. [Google Scholar] [CrossRef]
- Nadvi, N.A.; Salam, N.K.; Park, J.; Akladios, F.N.; Kapoor, V.; Collyer, C.A.; Gorrell, M.D.; Church, W.B. High resolution crystal structures of human kynurenine aminotransferase-I bound to PLP cofactor, and in complex with aminooxyacetate. Protein Sci. 2017, 26, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Garavaglia, S.; Montalbano, V.; Walsh, M.A.; Rizzi, M. Crystal structure of human kynurenine aminotransferase II, a drug target for the treatment of schizophrenia. J. Biol. Chem. 2008, 283, 3559–3566. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Cai, T.; Tagle, D.A.; Robinson, H.; Li, J. Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II. Biosci. Rep. 2008, 28, 205–215. [Google Scholar] [CrossRef]
- Tuttle, J.B.; Anderson, M.; Bechle, B.M.; Campbell, B.M.; Chang, C.; Dounay, A.B.; Evrard, E.; Fonseca, K.R.; Gan, X.; Ghosh, S.; et al. Structure-Based Design of Irreversible Human KAT II Inhibitors: Discovery of New Potency-Enhancing Interactions. ACS Med. Chem. Lett. 2013, 4, 37–40. [Google Scholar] [CrossRef]
- Nematollahi, A.; Sun, G.; Harrop, S.J.; Hanrahan, J.R.; Church, W.B. Structure of the PLP-Form of the Human Kynurenine Aminotransferase II in a Novel Spacegroup at 1.83 Å Resolution. Int. J. Mol. Sci. 2016, 17, 446. [Google Scholar] [CrossRef]
- Nematollahi, A.; Church, W.B.; Nadvi, N.A.; Gorrell, M.D.; Sun, G. Homology modeling of human kynurenine aminotransferase III and observations on inhibitor binding using molecular docking. Cent. Nerv. Syst. Agents Med. Chem. 2014, 14, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Chang, H.; Zhou, Y. Recombinant expression, purification and crystallographic studies of the mature form of human mitochondrial aspartate aminotransferase. Biosci. Trends 2016, 10, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Pinto, J.T.; Krasnikov, B.F.; Niatsetskaya, Z.V.; Han, Q.; Li, J.; Vauzour, D.; Spencer, J.P. Substrate specificity of human glutamine transaminase K as an aminotransferase and as a cysteine S-conjugate beta-lyase. Arch. Biochem. Biophys. 2008, 474, 72–81. [Google Scholar] [CrossRef]
- Badillo-Ramírez, I.; Saniger, J.M.; Rivas-Arancibia, S. 5-S-cysteinyl-dopamine, a neurotoxic endogenous metabolite of dopamine: Implications for Parkinson’s disease. Neurochem. Int. 2019, 129, 104514. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Ǻ.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Han, Q.; Li, J.; Li, J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur. J. Biochem. 2004, 271, 4804–4814. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Shurubor, Y.I.; Dorai, T.; Pinto, J.T.; Isakova, E.P.; Deryabina, Y.I.; Denton, T.T.; Krasnikov, B.F. omega-Amidase: An underappreciated, but important enzyme in L-glutamine and L-asparagine metabolism; relevance to sulfur and nitrogen metabolism, tumor biology and hyperammonemic diseases. Amino Acids 2016, 48, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C.; Elmer, G.I.; Bergeron, R.; Albuquerque, E.X.; Guidetti, P.; Wu, H.Q.; Schwarcz, R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 2010, 35, 1734–1742. [Google Scholar] [CrossRef]
- Kapoor, R.; Lim, K.S.; Cheng, A.; Garrick, T.; Kapoor, V. Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1). Brain Res. 2006, 1106, 205–210. [Google Scholar] [CrossRef]
- Barazorda-Ccahuana, H.L.; Zevallos-Delgado, C.; Valencia, D.E.; Gómez, B. Molecular Dynamics Simulation of Kynurenine AminotransferaseType II with Nicotine as a Ligand: A Possible Biochemical Role ofNicotine in Schizophrenia. ACS Omega 2019, 4, 710–717. [Google Scholar] [CrossRef]
- Chang, C.; Fonseca, K.R.; Li, C.; Horner, W.; Zawadzke, L.E.; Salafia, M.A.; Welch, K.A.; Strick, C.A.; Campbell, B.M.; Gernhardt, S.S.; et al. Quantitative Translational Analysis of Brain Kynurenic Acid Modulation via Irreversible Kynurenine Aminotransferase II Inhibition. Mol. Pharmacol. 2018, 94, 823–833. [Google Scholar] [CrossRef]
- Dounay, A.B.; Anderson, M.; Bechle, B.M.; Campbell, B.M.; Claffey, M.M.; Evdokimov, A.; Evrard, E.; Fonseca, K.R.; Gan, X.; Ghosh, S.; et al. Discovery of Brain-Penetrant, Irreversible Kynurenine Aminotransferase II Inhibitors for Schizophrenia. ACS Med. Chem. Lett. 2012, 3, 187–192. [Google Scholar] [CrossRef]
- Dounay, A.B.; Anderson, M.; Bechle, B.M.; Evrard, E.; Gan, X.; Kim, J.Y.; McAllister, L.A.; Pandit, J.; Rong, S.; Salafia, M.A.; et al. PF-04859989 as a template for structure-based drug design: Identification of new pyrazole series of irreversible KAT II inhibitors with improved lipophilic efficiency. Bioorg Med. Chem. Lett. 2013, 23, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, A.; Sun, G.; Jayawickrama, G.S.; Church, W.B. Kynurenine Aminotransferase Isozyme Inhibitors: A Review. Int. J. Mol. Sci. 2016, 17, 946. [Google Scholar] [CrossRef]
- Yoshida, Y.; Fujigaki, H.; Kato, K.; Yamazaki, K.; Fujigaki, S.; Kunisawa, K.; Yamamoto, Y.; Mouri, A.; Oda, A.; Nabeshima, T.; et al. Selective and competitive inhibition of kynurenine aminotransferase 2 by glycyrrhizic acid and its analogues. Sci. Rep. 2019, 9, 10243. [Google Scholar] [CrossRef]
- Erhardt, S.; Schwieler, L.; Nilsson, L.; Linderholm, K.; Engberg, G. The kynurenic acid hypothesis of schizophrenia. Physiol. Behav. 2007, 92, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Birner, A.; Platzer, M.; Bengesser, S.A.; Dalkner, N.; Fellendorf, F.T.; Queissner, R.; Pilz, R.; Rauch, P.; Maget, A.; Hamm, C.; et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS ONE 2017, 12, e0172699. [Google Scholar] [CrossRef]
- Bai, M.Y.; Lovejoy, D.B.; Guillemin, G.J.; Kozak, R.; Stone, T.W.; Koola, M.M. Galantamine-Memantine Combination and Kynurenine Pathway Enzyme Inhibitors in the Treatment of Neuropsychiatric Disorders. Complex. Psychiatry 2021, 7, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Fukuwatari, T. Possibility of Amino Acid Treatment to Prevent the Psychiatric Disorders via Modulation of the Production of Tryptophan Metabolite Kynurenic Acid. Nutrients 2020, 12, 1403. [Google Scholar] [CrossRef]
- Yu, P.; Di Prospero, N.A.; Sapko, M.T.; Cai, T.; Chen, A.; Melendez-Ferro, M.; Du, F.; Whetsell, W.O., Jr.; Guidetti, P.; Schwarcz, R.; et al. Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol. Cell Biol. 2004, 24, 6919–6930. [Google Scholar] [CrossRef]
- Hallen, A.; Jamie, J.F.; Cooper, A.J. Lysine metabolism in mammalian brain: An update on the importance of recent discoveries. Amino Acids 2013, 45, 1249–1272. [Google Scholar] [CrossRef]
- Madadi, S.; Pasbakhsh, P.; Tahmasebi, F.; Mortezaee, K.; Khanehzad, M.; Boroujeni, F.B.; Noorzehi, G.; Kashani, I.R. Astrocyte ablation induced by La-aminoadipate (L-AAA) potentiates remyelination in a cuprizone demyelinating mouse model. Metab. Brain Dis. 2019, 34, 593–603. [Google Scholar] [CrossRef]
- Okuno, E.; Tsujimoto, M.; Nakamura, M.; Kido, R. 2-Aminoadipate-2-oxoglutarate aminotransferase isoenzymes in human liver: A plausible physiological role in lysine and tryptophan metabolism. Enzyme Protein 1993, 47, 136–148. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan-Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Han, Q.; Liao, C.; Lan, J.; Ding, H.; Zhou, H.; Diao, X.; Li, J. Kynurenine aminotransferase 3/glutamine transaminase L/cysteine conjugate beta-lyase 2 is a major glutamine transaminase in the mouse kidney. Biochem. Biophys. Rep. 2016, 8, 234–241. [Google Scholar] [CrossRef][Green Version]
- Wu, Q.; Huang, J.; Wu, R. Drugs Based on NMDAR Hypofunction Hypothesis in Schizophrenia. Front. Neurosci. 2021, 15, 641047. [Google Scholar] [CrossRef]
- Jayawickrama, G.S.; Sadig, R.R.; Sun, G.; Nematollahi, A.; Nadvi, N.A.; Hanrahan, J.R.; Gorrell, M.D.; Church, W.B. Kynurenine Aminotransferases and the Prospects of Inhibitors for the Treatment of Schizophrenia. Curr. Med. Chem. 2015, 22, 2902–2918. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Li, Z.; Zhang, L.; Tagle, D.A.; Cai, T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene 2006, 365, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Robinson, H.; Cai, T.; Tagle, D.A.; Li, J. Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol. Cell Biol. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Monné, M.; Miniero, D.V.; Iacobazzi, V.; Bisaccia, F.; Fiermonte, G. The mitochondrial oxoglutarate carrier: From identification to mechanism. J. Bioenerg. Biomembr. 2013, 45, 1–13. [Google Scholar] [CrossRef]
- Guidetti, P.; Amori, L.; Sapko, M.T.; Okuno, E.; Schwarcz, R. Mitochondrial aspartate aminotransferase: A third kynurenate-producing enzyme in the mammalian brain. J. Neurochem. 2007, 102, 103–111. [Google Scholar] [CrossRef]
- Han, Q.; Robinson, H.; Cai, T.; Tagle, D.A.; Li, J. Biochemical and structural characterization of mouse mitochondrial aspartate aminotransferase, a newly identified kynurenine aminotransferase-IV. Biosci. Rep. 2011, 31, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392.e375. [Google Scholar] [CrossRef] [PubMed]
- Palzkill, V.R.; Thome, T.; Murillo, A.L.; Khattri, R.B.; Ryan, T.E. Increasing plasma L-kynurenine impairs mitochondrial oxidative phosphorylation prior to the development of atrophy in murine skeletal muscle: A pilot study. Front. Physiol. 2022, 13, 992413. [Google Scholar] [CrossRef]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef]
- Alam, S.; Doherty, E.; Ortega-Prieto, P.; Arizanova, J.; Fets, L. Membrane transporters in cell physiology, cancer metabolism and drug response. Dis. Model. Mech. 2023, 16, dmm050404. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Swainston, N.; Pir, P.; Oliver, S.G. Membrane transporter engineering in industrial biotechnology and whole-cell biocatalysis. Trends Biotechnol. 2015, 33, 237–246. [Google Scholar] [PubMed]
- Dobson, P.D.; Kell, D.B. Carrier-mediated cellular uptake of pharmaceutical drugs: An exception or the rule? Nat. Rev. Drug Disc. 2008, 7, 205–220. [Google Scholar]
- Kell, D.B.; Dobson, P.D. The cellular uptake of pharmaceutical drugs is mainly carrier-mediated and is thus an issue not so much of biophysics but of systems biology. In Proceedings of the Institut Beilstein Symposium on Systems Chemistry, Bozen, Italy, 26–30 May 2008; Hicks, M.G., Kettner, C., Eds.; Logos Verlag: Berlin, Germany, 2009; pp. 149–168. [Google Scholar]
- Kell, D.B.; Dobson, P.D.; Oliver, S.G. Pharmaceutical drug transport: The issues and the implications that it is essentially carrier-mediated only. Drug Disc. Today 2011, 16, 704–714. [Google Scholar]
- Kell, D.B.; Dobson, P.D.; Bilsland, E.; Oliver, S.G. The promiscuous binding of pharmaceutical drugs and their transporter-mediated uptake into cells: What we (need to) know and how we can do so. Drug Disc. Today 2013, 18, 218–239. [Google Scholar]
- Kell, D.B. Finding novel pharmaceuticals in the systems biology era using multiple effective drug targets, phenotypic screening, and knowledge of transporters: Where drug discovery went wrong and how to fix it. FEBS J. 2013, 280, 5957–5980. [Google Scholar] [PubMed]
- Kell, D.B.; Oliver, S.G. How drugs get into cells: Tested and testable predictions to help discriminate between transporter-mediated uptake and lipoidal bilayer diffusion. Front. Pharmacol. 2014, 5, 231. [Google Scholar]
- Kell, D.B. The transporter-mediated cellular uptake of pharmaceutical drugs is based on their metabolite-likeness and not on their bulk biophysical properties: Towards a systems pharmacology. Perspect. Sci. 2015, 6, 66–83. [Google Scholar] [CrossRef]
- Kell, D.B. How drugs pass through biological cell membranes—A paradigm shift in our understanding? Beilstein Mag. 2016, 2. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [PubMed]
- Dickens, D.; Rädisch, S.; Chiduza, G.N.; Giannoudis, A.; Cross, M.J.; Malik, H.; Schaeffeler, E.; Sison-Young, R.L.; Wilkinson, E.L.; Goldring, C.E.; et al. Cellular uptake of the atypical antipsychotic clozapine is a carrier-mediated process. Mol. Pharm. 2018, 15, 3557–3572. [Google Scholar] [CrossRef]
- Kell, D.B. The transporter-mediated cellular uptake and efflux of pharmaceutical drugs and biotechnology products: How and why phospholipid bilayer transport is negligible in real biomembranes. Molecules 2021, 26, 5629. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.A.; Clemencon, B.; Burrier, R.E.; Bruford, E.A. The ABCs of membrane transporters in health and disease (SLC series): Introduction. Mol. Aspects Med. 2013, 34, 95–107. [Google Scholar] [CrossRef]
- Anonymous. SLC Tables. Available online: http://www.bioparadigms.org/slc/intro.htm (accessed on 27 August 2019).
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Jindal, S.; Yang, L.; Day, P.J.; Kell, D.B. Involvement of multiple influx and efflux transporters in the accumulation of cationic fluorescent dyes by Escherichia coli. BMC Microbiol. 2019, 19, 195, also bioRxiv 603688v603681. [Google Scholar] [CrossRef]
- Ter Beek, J.; Guskov, A.; Slotboom, D.J. Structural diversity of ABC transporters. J. Gen. Physiol. 2014, 143, 419–435. [Google Scholar] [CrossRef]
- Lewinson, O.; Livnat-Levanon, N. Mechanism of Action of ABC Importers: Conservation, Divergence, and Physiological Adaptations. J. Mol. Biol. 2017, 429, 606–619. [Google Scholar] [CrossRef]
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687. [Google Scholar] [CrossRef]
- Bush, K.T.; Wu, W.; Lun, C.; Nigam, S.K. The drug transporter OAT3 (SLC22A8) regulates endogenous metabolite flow through the gut-liver-kidney axis. J. Biol. Chem. 2017, 292, 15789–15803. [Google Scholar] [CrossRef]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Wright Muelas, M.; Mughal, F.; O’Hagan, S.; Day, P.J.; Kell, D.B. The role and robustness of the Gini coefficient as an unbiased tool for the selection of Gini genes for normalising expression profiling data. Sci. Rep. 2019, 9, 17960. [Google Scholar] [CrossRef]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A.; Kuroki, Y.; Urata, T.; Mori, N.; Fukuwatari, T. Inhibition of Large Neutral Amino Acid Transporters Suppresses Kynurenic Acid Production Via Inhibition of Kynurenine Uptake in Rodent Brain. Neurochem. Res. 2016, 41, 2256–2266. [Google Scholar] [CrossRef]
- Patel, W.; Rimmer, L.; Smith, M.; Moss, L.; Smith, M.A.; Snodgrass, H.R.; Pirmohamed, M.; Alfirevic, A.; Dickens, D. Probenecid Increases the Concentration of 7-Chlorokynurenic Acid Derived from the Prodrug 4-Chlorokynurenine within the Prefrontal Cortex. Mol. Pharm. 2021, 18, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Patel, W.; Shankar, R.G.; Smith, M.A.; Snodgrass, H.R.; Pirmohamed, M.; Jorgensen, A.L.; Alfirevic, A.; Dickens, D. Role of Transporters and Enzymes in Metabolism and Distribution of 4-Chlorokynurenine (AV-101). Mol. Pharm. 2024, 21, 550–563. [Google Scholar] [CrossRef]
- Miller, J.M.; MacGarvey, U.; Beal, M.F. The effect of peripheral loading with kynurenine and probenecid on extracellular striatal kynurenic acid concentrations. Neurosci. Lett. 1992, 146, 115–118. [Google Scholar] [CrossRef]
- Vécsei, L.; Miller, J.; MacGarvey, U.; Beal, M.F. Kynurenine and probenecid inhibit pentylenetetrazol- and NMDLA-induced seizures and increase kynurenic acid concentrations in the brain. Brain Res. Bull. 1992, 28, 233–238. [Google Scholar] [CrossRef]
- Moroni, F.; Russi, P.; Lombardi, G.; Beni, M.; Carla, V. Presence of kynurenic acid in the mammalian brain. J. Neurochem. 1988, 51, 177–180. [Google Scholar] [CrossRef]
- Shepard, P.D.; Joy, B.; Clerkin, L.; Schwarcz, R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology 2003, 28, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Robotka, H.; Rózsa, É.; Ágoston, M.; Szénási, G.; Gigler, G.; Marosi, M.; Kis, Z.; Farkas, T.; Vécsei, L.; et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 2008, 32, 302–308. [Google Scholar] [CrossRef]
- Silva-Adaya, D.; Pérez-De La Cruz, V.; Villeda-Hernández, J.; Carrillo-Mora, P.; González-Herrera, I.G.; García, E.; Colín-Barenque, L.; Pedraza-Chaverrí, J.; Santamaría, A. Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: Implications of modulating kynurenate as a protective strategy. Neurotoxicol Teratol. 2011, 33, 303–312. [Google Scholar] [CrossRef]
- Russel, F.G.M.; Koenderink, J.B.; Masereeuw, R. Multidrug resistance protein 4 (MRP4/ABCC4): A versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol. Sci. 2008, 29, 200–207. [Google Scholar] [CrossRef]
- Mendes, P.; Girardi, E.; Superti-Furga, G.; Kell, D.B. Why most transporter mutations that cause antibiotic resistance are to efflux pumps rather than to import transporters. bioRxiv 2020, 2020.2001.2016.909507v909501. [Google Scholar] [CrossRef]
- Li, X.-Z.; Elkins, C.A.; Zgurskaya, H.I. (Eds.) Efflux-Mediated Antimicrobial Resistance in Bacteria: Mechanisms, Regulation and Clinical Implications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Piddock, L.J.V. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Piddock, L.J.V. The 2019 Garrod Lecture: MDR efflux in Gram-negative bacteria-how understanding resistance led to a new tool for drug discovery. J. Antimicrob. Chemother. 2019, 74, 3128–3134. [Google Scholar] [CrossRef] [PubMed]
- Poku, V.O.; Iram, S.H. A critical review on modulators of Multidrug Resistance Protein 1 in cancer cells. PeerJ 2022, 10, e12594. [Google Scholar] [CrossRef]
- Bharathiraja, P.; Yadav, P.; Sajid, A.; Ambudkar, S.V.; Prasad, N.R. Natural medicinal compounds target signal transduction pathways to overcome ABC drug efflux transporter-mediated multidrug resistance in cancer. Drug Resist. Updat. 2023, 71, 101004. [Google Scholar] [CrossRef]
- Grixti, J.; O’Hagan, S.; Day, P.J.; Kell, D.B. Enhancing drug efficacy and therapeutic index through cheminformatics-based selection of small molecule binary weapons that improve transporter-mediated targeting: A cytotoxicity system based on gemcitabine. Front. Pharmacol. 2017, 8, 155. [Google Scholar] [CrossRef]
- Sajid, A.; Rahman, H.; Ambudkar, S.V. Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters. Nat. Rev. Cancer 2023, 23, 762–779. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wu, Z.X.; Yang, Y.; Teng, Q.X.; Li, Y.D.; Lei, Z.N.; Jani, K.A.; Kaushal, N.; Chen, Z.S. ATP-binding cassette (ABC) transporters in cancer: A review of recent updates. J. Evid. Based Med. 2021, 14, 232–256. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, H.A.; van den Heuvel, L.P.; Ringens, L.H.; Dankers, A.C.; Russel, F.G.; Wetzels, J.F.; Hoenderop, J.G.; Masereeuw, R. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS ONE 2011, 6, e18438. [Google Scholar] [CrossRef]
- Dankers, A.C.A.; Mutsaers, H.A.M.; Dijkman, H.B.P.M.; van den Heuvel, L.P.; Hoenderop, J.G.; Sweep, F.C.G.J.; Russel, F.G.M.; Masereeuw, R. Hyperuricemia influences tryptophan metabolism via inhibition of multidrug resistance protein 4 (MRP4) and breast cancer resistance protein (BCRP). Biochim. Biophys. Acta 2013, 1832, 1715–1722. [Google Scholar] [CrossRef]
- Stafim da Cunha, R.; Azevedo, C.A.B.; Falconi, C.A.; Ruiz, F.F.; Liabeuf, S.; Carneiro-Ramos, M.S.; Stinghen, A.E.M. The Interplay between Uremic Toxins and Albumin, Membrane Transporters and Drug Interaction. Toxins 2022, 14, 177. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, F.; Xin, M.; Gou, X.; Wang, X.; Wu, X. Albumin-bound kynurenic acid is an appropriate endogenous biomarker for assessment of the renal tubular OATs-MRP4 channel. J. Pharm. Anal. 2023, 13, 1205–1220. [Google Scholar] [CrossRef]
- Frechen, S.; Rostami-Hodjegan, A. Quality Assurance of PBPK Modeling Platforms and Guidance on Building, Evaluating, Verifying and Applying PBPK Models Prudently under the Umbrella of Qualification: Why, When, What, How and By Whom? Pharm. Res. 2022, 39, 1733–1748. [Google Scholar] [CrossRef]
- Murata, Y.; Neuhoff, S.; Rostami-Hodjegan, A.; Takita, H.; Al-Majdoub, Z.M.; Ogungbenro, K. In Vitro to In Vivo Extrapolation Linked to Physiologically Based Pharmacokinetic Models for Assessing the Brain Drug Disposition. AAPS J. 2022, 24, 28. [Google Scholar] [CrossRef]
- Superti-Furga, G.; Lackner, D.; Wiedmer, T.; Ingles-Prieto, A.; Barbosa, B.; Girardi, E.; Goldman, U.; Gürtl, B.; Klavins, K.; Klimek, C.; et al. The RESOLUTE consortium: Unlocking SLC transporters for drug discovery. Nat. Rev. Drug Discov. 2020, 19, 429–430. [Google Scholar] [CrossRef]
- Wright Muelas, M.; Roberts, I.; Mughal, F.; O’Hagan, S.; Day, P.J.; Kell, D.B. An untargeted metabolomics strategy to measure differences in metabolite uptake and excretion by mammalian cell lines. Metabolomics 2020, 16, 107. [Google Scholar] [CrossRef]
- Cheung, L.; Flemming, C.L.; Watt, F.; Masada, N.; Yu, D.M.; Huynh, T.; Conseil, G.; Tivnan, A.; Polinsky, A.; Gudkov, A.V.; et al. High-throughput screening identifies Ceefourin 1 and Ceefourin 2 as highly selective inhibitors of multidrug resistance protein 4 (MRP4). Biochem. Pharmacol. 2014, 91, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Sahores, A.; Rodríguez González, A.; Yaneff, A.; May, M.; Gómez, N.; Monczor, F.; Fernández, N.; Davio, C.; Shayo, C. Ceefourin-1, a MRP4/ABCC4 inhibitor, induces apoptosis in AML cells enhanced by histamine. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130322. [Google Scholar] [CrossRef]
- Marcantoni, E.; Allen, N.; Cambria, M.R.; Dann, R.; Cammer, M.; Lhakhang, T.; O’Brien, M.P.; Kim, B.; Worgall, T.; Heguy, A.; et al. Platelet Transcriptome Profiling in HIV and ATP-Binding Cassette Subfamily C Member 4 (ABCC4) as a Mediator of Platelet Activity. JACC Basic. Transl. Sci. 2018, 3, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Rius, M.; Hummel-Eisenbeiss, J.; Keppler, D. ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4). J. Pharmacol. Exp. Ther. 2008, 324, 86–94. [Google Scholar] [CrossRef]
- Van de Ven, R.; Scheffer, G.L.; Reurs, A.W.; Lindenberg, J.J.; Oerlemans, R.; Jansen, G.; Gillet, J.P.; Glasgow, J.N.; Pereboev, A.; Curiel, D.T.; et al. A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood 2008, 112, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249. [Google Scholar] [CrossRef]
- Gekeler, V.; Ise, W.; Sanders, K.H.; Ulrich, W.R.; Beck, J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem. Biophys. Res. Commun. 1995, 208, 345–352. [Google Scholar] [CrossRef]
- Takeuchi, K.; Shibata, M.; Kashiyama, E.; Umehara, K. Expression levels of multidrug resistance-associated protein 4 (MRP4) in human leukemia and lymphoma cell lines, and the inhibitory effects of the MRP-specific inhibitor MK-571 on methotrexate distribution in rats. Exp. Ther. Med. 2012, 4, 524–532. [Google Scholar] [CrossRef]
- Low, F.G.; Shabir, K.; Brown, J.E.; Bill, R.M.; Rothnie, A.J. Roles of ABCC1 and ABCC4 in Proliferation and Migration of Breast Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 7664. [Google Scholar] [CrossRef]
- Copsel, S.; Garcia, C.; Diez, F.; Vermeulem, M.; Baldi, A.; Bianciotti, L.G.; Russel, F.G.M.; Shayo, C.; Davio, C. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J. Biol. Chem. 2011, 286, 6979–6988. [Google Scholar] [CrossRef]
- Britz, H.; Hanke, N.; Taub, M.E.; Wang, T.; Prasad, B.; Fernandez, E.; Stopfer, P.; Nock, V.; Lehr, T. Physiologically Based Pharmacokinetic Models of Probenecid and Furosemide to Predict Transporter Mediated Drug-Drug Interactions. Pharm. Res. 2020, 37, 250. [Google Scholar] [CrossRef]
- Maeda, K.; Tian, Y.; Fujita, T.; Ikeda, Y.; Kumagai, Y.; Kondo, T.; Tanabe, K.; Nakayama, H.; Horita, S.; Kusuhara, H.; et al. Inhibitory effects of p-aminohippurate and probenecid on the renal clearance of adefovir and benzylpenicillin as probe drugs for organic anion transporter (OAT) 1 and OAT3 in humans. Eur. J. Pharm. Sci. 2014, 59, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Calcagno, A.M.; Hladky, S.B.; Ambudkar, S.V.; Barrand, M.A. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J. 2005, 272, 4725–4740. [Google Scholar] [CrossRef] [PubMed]
- Ritter, C.A.; Jedlitschky, G.; Meyer zu Schwabedissen, H.; Grube, M.; Kock, K.; Kroemer, H.K. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab. Rev. 2005, 37, 253–278. [Google Scholar] [CrossRef]
- Wen, J.; Luo, J.; Huang, W.; Tang, J.; Zhou, H.; Zhang, W. The Pharmacological and Physiological Role of Multidrug-Resistant Protein 4. J. Pharmacol. Exp. Ther. 2015, 354, 358–375. [Google Scholar] [CrossRef]
- Nigam, S.K.; Granados, J.C. OAT, OATP, and MRP Drug Transporters and the Remote Sensing and Signaling Theory. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 637–660. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.K.; van den Heuvel, J.J.M.W.; Koenderink, J.B.; Russel, F.G.M. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J. Pharmacol. Exp. Ther. 2007, 320, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.D.; Esslinger, C.S.; Thompson, C.M.; Bridges, R.J. Substituted quinolines as inhibitors of L-glutamate transport into synaptic vesicles. Neuropharmacology 1998, 37, 839–846. [Google Scholar] [CrossRef]
- Kanai, Y.; Clemencon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Aspects Med. 2013, 34, 108–120. [Google Scholar] [CrossRef]
- Magi, S.; Piccirillo, S.; Amoroso, S.; Lariccia, V. Excitatory Amino Acid Transporters (EAATs): Glutamate Transport and Beyond. Int. J. Mol. Sci. 2019, 20, 5674. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Aspects Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466–485. [Google Scholar] [CrossRef]
- Bosshart, P.D.; Charles, R.P.; Garibsingh, R.A.; Schlessinger, A.; Fotiadis, D. SLC16 Family: From Atomic Structure to Human Disease. Trends Biochem. Sci. 2021, 46, 28–40. [Google Scholar] [CrossRef]
- Tan, K.M.; Tint, M.T.; Kothandaraman, N.; Michael, N.; Sadananthan, S.A.; Velan, S.S.; Fortier, M.V.; Yap, F.; Tan, K.H.; Gluckman, P.D.; et al. The Kynurenine Pathway Metabolites in Cord Blood Positively Correlate With Early Childhood Adiposity. J. Clin. Endocrinol. Metab. 2022, 107, e2464–e2473. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Schwarcz, R. Restraint Stress during Pregnancy Rapidly Raises Kynurenic Acid Levels in Mouse Placenta and Fetal Brain. Dev. Neurosci. 2016, 38, 458–468. [Google Scholar] [CrossRef]
- Goeden, N.; Notarangelo, F.M.; Pocivavsek, A.; Beggiato, S.; Bonnin, A.; Schwarcz, R. Prenatal Dynamics of Kynurenine Pathway Metabolism in Mice: Focus on Kynurenic Acid. Dev. Neurosci. 2017, 39, 519–528. [Google Scholar] [CrossRef]
- Lin, L.; Lemieux, G.A.; Enogieru, O.J.; Giacomini, K.M.; Ashrafi, K. Neural production of kynurenic acid in Caenorhabditis elegans requires the AAT-1 transporter. Genes Dev. 2020, 34, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Galluccio, M.; Console, L.; Pochini, L.; Indiveri, C. The Human SLC7A5 (LAT1): The Intriguing Histidine/Large Neutral Amino Acid Transporter and Its Relevance to Human Health. Front. Chem. 2018, 6, 243. [Google Scholar] [CrossRef]
- Cappoli, N.; Jenkinson, M.D.; Dello Russo, C.; Dickens, D. LAT1, a novel pharmacological target for the treatment of glioblastoma. Biochem. Pharmacol. 2022, 201, 115103. [Google Scholar] [CrossRef]
- Kanai, Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol. Ther. 2022, 230, 107964. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, K.; Ohgaki, R.; Okanishi, H.; Okuda, S.; Xu, M.; Endou, H.; Kanai, Y. Pharmacologic inhibition of LAT1 predominantly suppresses transport of large neutral amino acids and downregulates global translation in cancer cells. J. Cell Mol. Med. 2022, 26, 5246–5256. [Google Scholar] [CrossRef] [PubMed]
- Sekine, A.; Okamoto, M.; Kanatani, Y.; Sano, M.; Shibata, K.; Fukuwatari, T. Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. Springerplus 2015, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Sone, Y.; Mitsuhashi, S.; Tomiya, M.; Toyo’oka, T. Alteration of kynurenic acid concentration in rat plasma following optically pure kynurenine administration: A comparative study between enantiomers. Chirality 2009, 21, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Cruz, V.; Amori, L.; Sathyasaikumar, K.V.; Wang, X.D.; Notarangelo, F.M.; Wu, H.Q.; Schwarcz, R. Enzymatic transamination of D-kynurenine generates kynurenic acid in rat and human brain. J. Neurochem. 2012, 120, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kaihara, M.; Price, J.M. The conversion of kynurenic acid to quinaldic acid by humans and rats. J. Biol. Chem. 1956, 223, 705–708. [Google Scholar]
- Turska, M.; Rutyna, R.; Paluszkiewicz, M.; Terlecka, P.; Dobrowolski, A.; Pelak, J.; Turski, M.P.; Muszynska, B.; Dabrowski, W.; Kocki, T.; et al. Presence of kynurenic acid in alcoholic beverages—Is this good news, or bad news? Med. Hypotheses 2019, 122, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Jędruchniewicz, K. Dietary Kynurenine Pathway Metabolites-Source, Fate, and Chromatographic Determinations. Int. J. Mol. Sci. 2023, 24, 16304. [Google Scholar] [CrossRef] [PubMed]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Turska, J. A Review of the Health Benefits of Food Enriched with Kynurenic Acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.; Csapó, E.; Majláth, Z.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I.; Knapp, L.; Toldi, J.; Vécsei, L.; Dékány, I. Targeting of the kynurenic acid across the blood-brain barrier by core-shell nanoparticles. Eur. J. Pharm. Sci. 2016, 86, 67–74. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, S.; Kell, D.B. The apparent permeabilities of Caco-2 cells to marketed drugs: Magnitude, and independence from both biophysical properties and endogenite similarities. PeerJ 2015, 3, e1405. [Google Scholar] [PubMed]
- Füvesi, J.; Somlai, C.; Németh, H.; Varga, H.; Kis, Z.; Farkas, T.; Károly, N.; Dobszay, M.; Penke, Z.; Penke, B.; et al. Comparative study on the effects of kynurenic acid and glucosamine-kynurenic acid. Pharmacol. Biochem. Behav. 2004, 77, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Robotka, H.; Németh, H.; Somlai, C.; Vécsei, L.; Toldi, J. Systemically administered glucosamine-kynurenic acid, but not pure kynurenic acid, is effective in decreasing the evoked activity in area CA1 of the rat hippocampus. Eur. J. Pharmacol. 2005, 513, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Hornok, V.; Bujdosó, T.; Toldi, J.; Nagy, K.; Demeter, I.; Fazakas, C.; Krizbai, I.; Vécsei, L.; Dékány, I. Preparation and properties of nanoscale containers for biomedical application in drug delivery: Preliminary studies with kynurenic acid. J. Neural Transm. 2012, 119, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hornok, V.; Amin, K.W.K.; Kovács, A.N.; Juhász, Á.; Katona, G.; Balogh, G.T.; Csapó, E. Increased blood-brain barrier permeability of neuroprotective drug by colloidal serum albumin carriers. Colloids Surf. B Biointerfaces 2022, 220, 112935. [Google Scholar] [CrossRef]
- Juhász, A.; Ungor, D.; Varga, N.; Katona, G.; Balogh, G.T.; Csapó, E. Lipid-Based Nanocarriers for Delivery of Neuroprotective Kynurenic Acid: Preparation, Characterization, and BBB Transport. Int. J. Mol. Sci. 2023, 24, 14251. [Google Scholar] [CrossRef] [PubMed]
- Monfared, Y.K.; Pedrazzo, A.R.; Mahmoudian, M.; Caldera, F.; Zakeri-Milani, P.; Valizadeh, H.; Cavalli, R.; Matencio, A.; Trotta, F. Oral supplementation of solvent-free kynurenic acid/cyclodextrin nanosponges complexes increased its bioavailability. Colloids Surf. B Biointerfaces 2023, 222, 113101. [Google Scholar] [CrossRef]
- Deák, Á.; Csapó, E.; Juhász, Á.; Dékány, I.; Janovák, L. Anti-ulcerant kynurenic acid molecules intercalated Mg/Al-layered double hydroxide and its release study. Appl. Clay Sci. 2018, 156, 28–35. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavalli, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115168. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Tang, R.M.Y.; Yew, T.S.Z.; Lim, K.H.C.; Halliwell, B. Administration of Pure Ergothioneine to Healthy Human Subjects: Uptake, Metabolism, and Effects on Biomarkers of Oxidative Damage and Inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.C.; The SCOPE Consortium; Brown, L.W.; Ortea, P.; Tuytten, R.; Kell, D.B. Relationship between the concentration of ergothioneine in plasma and the likelihood of developing pre-eclampsia. Biosci. Rep. 2023, 43, BSR20230160. [Google Scholar] [CrossRef]
- Uwai, Y.; Honjo, H.; Iwamoto, K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol. Res. 2012, 65, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Uwai, Y.; Hara, H.; Iwamoto, K. Transport of Kynurenic Acid by Rat Organic Anion Transporters rOAT1 and rOAT3: Species Difference between Human and Rat in OAT1. Int. J. Tryptophan Res. 2013, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [PubMed]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important disease marker, and is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xi, K.; Zhang, L.; Han, M.; Wang, Q.; Liu, X. Tryptophan metabolites and gut microbiota play an important role in pediatric migraine diagnosis. J. Headache Pain 2024, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.J.; Jonkers, D.M.; Kruimel, J.W.; Leue, C.; Masclee, A.A. Decreased levels of kynurenic acid in the intestinal mucosa of IBS patients: Relation to serotonin and psychological state. J. Psychosom. Res. 2013, 74, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Bakker, L.; Choe, K.; Eussen, S.; Ramakers, I.; van den Hove, D.L.A.; Kenis, G.; Rutten, B.P.F.; Verhey, F.R.J.; Kohler, S. Relation of the kynurenine pathway with normal age: A systematic review. Mech. Ageing Dev. 2024, 217, 111890. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Vermeiren, Y.; Van Faassen, M.; van der Ley, C.; Nollen, E.A.A.; Kema, I.P.; De Deyn, P.P. Age- and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J. Neurochem. 2019, 151, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.; Chung, T.; Lovett, J.; Ward, C.; Joca, H.; Yang, H.; Khadeer, M.; Tian, J.; Xue, Q.L.; Le, A.; et al. Kynurenines link chronic inflammation to functional decline and physical frailty. JCI Insight 2020, 5, e136091. [Google Scholar] [CrossRef]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Grant, R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011, 278, 4425–4434. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Wright Muelas, M.; Taylor, J.M.; Davison, A.S.; Winder, C.L.; Goodacre, R.; Kell, D.B. Quantitative LC-MS study of compounds found predictive of COVID-19 severity and outcome. Metabolomics 2023, 19, 87. [Google Scholar] [CrossRef]
- Theiler-Schwetz, V.; Trummer, C.; Grubler, M.R.; Keppel, M.H.; Zittermann, A.; Tomaschitz, A.; Marz, W.; Meinitzer, A.; Pilz, S. Associations of Parameters of the Tryptophan-Kynurenine Pathway with Cardiovascular Risk Factors in Hypertensive Patients. Nutrients 2023, 15, 256. [Google Scholar] [CrossRef]
- Aarsland, T.I.; Landaas, E.T.; Hegvik, T.A.; Ulvik, A.; Halmoy, A.; Ueland, P.M.; Haavik, J. Serum concentrations of kynurenines in adult patients with attention-deficit hyperactivity disorder (ADHD): A case-control study. Behav. Brain Funct. 2015, 11, 36. [Google Scholar] [CrossRef]
- Dudzińska, E.; Szymona, K.; Kloc, R.; Gil-Kulik, P.; Kocki, T.; Świstowska, M.; Bogucki, J.; Kocki, J.; Urbanska, E.M. Increased expression of kynurenine aminotransferases mRNA in lymphocytes of patients with inflammatory bowel disease. Therap Adv. Gastroenterol. 2019, 12, 1756284819881304. [Google Scholar] [CrossRef]
- Fukushima, T.; Mitsuhashi, S.; Tomiya, M.; Iyo, M.; Hashimoto, K.; Toyo’oka, T. Determination of kynurenic acid in human serum and its correlation with the concentration of certain amino acids. Clin. Chim. Acta 2007, 377, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Iłźecka, J.; Kocki, T.; Stelmasiak, Z.; Turski, W.A. Endogenous protectant kynurenic acid in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2003, 107, 412–418. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhou, C.; Wei, Y.Y.; Li, H.H.; Lei, W.; Boeldt, D.S.; Wang, K.; Zheng, J. Differential Distribution of Tryptophan-Metabolites in Fetal and Maternal Circulations During Normotensive and Preeclamptic Pregnancies. Reprod. Sci. 2022, 29, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine metabolism in plasma and in red blood cells in Parkinson’s disease. J. Neurol. Sci. 2005, 239, 31–35. [Google Scholar] [CrossRef]
- Hu, L.J.; Li, X.F.; Hu, J.Q.; Ni, X.J.; Lu, H.Y.; Wang, J.J.; Huang, X.N.; Lin, C.X.; Shang, D.W.; Wen, Y.G. A Simple HPLC-MS/MS Method for Determination of Tryptophan, Kynurenine and Kynurenic Acid in Human Serum and its Potential for Monitoring Antidepressant Therapy. J. Anal. Toxicol. 2017, 41, 37–44. [Google Scholar] [CrossRef]
- Özdemir, M.; Abusoglu, S.; Baldane, S.; Kıraç, C.O.; Unlu, A.; Onmaz, D.E.; Çelik, M.; Abusoglu, G. Analyzing serum tryptophan metabolites in patients with gestational diabetes mellitus. Rev. Rom. Med. Lab. 2023, 31, 251–262. [Google Scholar] [CrossRef]
- Murthi, P.; Wallace, E.M.; Walker, D.W. Altered placental tryptophan metabolic pathway in human fetal growth restriction. Placenta 2017, 52, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Tuka, B.; Nyári, A.; Cseh, E.K.; Körtési, T.; Veréb, D.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; Tajti, J.; Vécsei, L. Clinical relevance of depressed kynurenine pathway in episodic migraine patients: Potential prognostic markers in the peripheral plasma during the interictal period. J. Headache Pain 2021, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Gulaj, E.; Pawlak, K.; Bien, B.; Pawlak, D. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv. Med. Sci. 2010, 55, 204–211. [Google Scholar] [CrossRef]
- Savitz, J. Blood versus cerebrospinal fluid: Kynurenine pathway metabolites in depression. Brain Behav. Immun. 2022, 101, 333–334. [Google Scholar] [CrossRef]
- Savitz, J.; Ford, B.N.; Kuplicki, R.; Khalsa, S.; Teague, T.K.; Paulus, M.P. Acute administration of ibuprofen increases serum concentration of the neuroprotective kynurenine pathway metabolite, kynurenic acid: A pilot randomized, placebo-controlled, crossover study. Psychopharmacology 2022, 239, 3919–3927. [Google Scholar] [CrossRef]
- Tuka, B.; Körtési, T.; Nánási, N.; Tömösi, F.; Janáky, T.; Veréb, D.; Szok, D.; Tajti, J.; Vécsei, L. Cluster headache and kynurenines. J. Headache Pain 2023, 24, 35. [Google Scholar] [CrossRef]
- Sellgren, C.M.; Gracias, J.; Jungholm, O.; Perlis, R.H.; Engberg, G.; Schwieler, L.; Landen, M.; Erhardt, S. Peripheral and central levels of kynurenic acid in bipolar disorder subjects and healthy controls. Transl. Psychiatry 2019, 9, 37. [Google Scholar] [CrossRef]
- Apalset, E.M.; Gjesdal, C.G.; Ueland, P.M.; Midttun, O.; Ulvik, A.; Eide, G.E.; Meyer, K.; Tell, G.S. Interferon (IFN)-gamma-mediated inflammation and the kynurenine pathway in relation to bone mineral density: The Hordaland Health Study. Clin. Exp. Immunol. 2014, 176, 452–460. [Google Scholar] [CrossRef]
- Tang, J.; Shen, H.; Zhao, X.; Holenarsipur, V.K.; Mariappan, T.T.; Zhang, Y.; Panfen, E.; Zheng, J.; Humphreys, W.G.; Lai, Y. Endogenous Plasma Kynurenic Acid in Human: A Newly Discovered Biomarker for Drug-Drug Interactions Involving Organic Anion Transporter 1 and 3 Inhibition. Drug Metab. Dispos. 2021, 49, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic Acid in the digestive system-new facts, new challenges. Int. J. Tryptophan Res. 2013, 6, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Amirkhani, A.; Heldin, E.; Markides, K.E.; Bergquist, J. Quantitation of tryptophan, kynurenine and kynurenic acid in human plasma by capillary liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2002, 780, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, P.; Zhu, D. Optimization of Zn2+-containing mobile phase for simultaneous determination of kynurenine, kynurenic acid and tryptophan in human plasma by high performance liquid chromatography. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2010, 878, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Long-Smith, C.; Moloney, G.M.; Morkl, S.; O’Mahony, S.M.; Cryan, J.F.; Clarke, G.; Dinan, T.G. The immune-kynurenine pathway in social anxiety disorder. Brain Behav. Immun. 2022, 99, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, R.M.; Bjørke-Monsen, A.L.; Midttun, Ø.; Nygård, O.; Pedersen, E.R.; Ulvik, A.; Magnus, P.; Gjessing, H.K.; Vollset, S.E.; Ueland, P.M. Maternal tryptophan and kynurenine pathway metabolites and risk of preeclampsia. Obstet. Gynecol. 2012, 119, 1243–1250. [Google Scholar] [CrossRef]
- González-Sánchez, M.; Jiménez, J.; Narváez, A.; Antequera, D.; Llamas-Velasco, S.; Martín, A.H.; Arjona, J.A.M.; López de Munain, A.; Bisa, A.L.; Marco, M.P.; et al. Kynurenic Acid Levels are Increased in the CSF of Alzheimer’s Disease Patients. Biomolecules 2020, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Swartz, K.J.; Matson, W.R.; MacGarvey, U.; Ryan, E.A.; Beal, M.F. Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high-performance liquid chromatography with fluorometric and coulometric electrode array detection. Anal. Biochem. 1990, 185, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Kepplinger, B.; Baran, H.; Kainz, A.; Ferraz-Leite, H.; Newcombe, J.; Kalina, P. Age-related increase of kynurenic acid in human cerebrospinal fluid-IgG and beta2-microglobulin changes. Neurosignals 2005, 14, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.G.; Christen, S.; Bellac, C.L.; Fontes, F.L.; Souza, F.R.; Grandgirard, D.; Leib, S.L.; Agnez-Lima, L.F. The kynurenine pathway is involved in bacterial meningitis. J. Neuroinflammation 2014, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Milart, P.; Paluszkiewicz, P.; Dobrowolski, P.; Tomaszewska, E.; Smolinska, K.; Debinska, I.; Gawel, K.; Walczak, K.; Bednarski, J.; Turska, M.; et al. Kynurenic acid as the neglected ingredient of commercial baby formulas. Sci. Rep. 2019, 9, 6108. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yang, S.; Lu, Z.; Li, G.; Wu, S.; Wu, D.R.; Liu, J.; Zhou, B.; Wang, H.D.; et al. The Beneficial Effects of Edible Kynurenic Acid from Marine Horseshoe Crab (Tachypleus tridentatus) on Obesity, Hyperlipidemia, and Gut Microbiota in High-Fat Diet-Fed Mice. Oxid. Med. Cell Longev. 2021, 2021, 8874503. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszynski, S.; Kuc, D.; Dobrowolski, P.; Lamorski, K.; Smolinska, K.; Donaldson, J.; Swietlicka, I.; Mielnik-Blaszczak, M.; Paluszkiewicz, P.; et al. Chronic dietary supplementation with kynurenic acid, a neuroactive metabolite of tryptophan, decreased body weight without negative influence on densitometry and mandibular bone biomechanical endurance in young rats. PLoS ONE 2019, 14, e0226205. [Google Scholar] [CrossRef]
- Paluszkiewicz, P.; Zgrajka, W.; Saran, T.; Schabowski, J.; Piedra, J.L.; Fedkiv, O.; Rengman, S.; Pierzynowski, S.G.; Turski, W.A. High concentration of kynurenic acid in bile and pancreatic juice. Amino Acids 2009, 37, 637–641. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Heng, B.; Guillemin, G.J. The Gut Microbiota, Kynurenine Pathway, and Immune System Interaction in the Development of Brain Cancer. Front. Cell Dev. Biol. 2020, 8, 562812. [Google Scholar] [CrossRef]
- Arsenescu, R.; Arsenescu, V.; Zhong, J.; Nasser, M.; Melinte, R.; Dingle, R.W.; Swanson, H.; de Villiers, W.J. Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.M.; Shepherd, D.M. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol. Sci. 2011, 120, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Korecka, A.; Dona, A.; Lahiri, S.; Tett, A.J.; Al-Asmakh, M.; Braniste, V.; D’Arienzo, R.; Abbaspour, A.; Reichardt, N.; Fujii-Kuriyama, Y.; et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes 2016, 2, 16014. [Google Scholar] [CrossRef]
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.M.; Fish, K.; Fu, Y.X.; Zhou, L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 2012, 36, 92–104. [Google Scholar] [CrossRef]
- Shi, L.Z.; Faith, N.G.; Nakayama, Y.; Suresh, M.; Steinberg, H.; Czuprynski, C.J. The aryl hydrocarbon receptor is required for optimal resistance to Listeria monocytogenes infection in mice. J. Immunol. 2007, 179, 6952–6962. [Google Scholar] [CrossRef]
- Sutter, C.H.; Bodreddigari, S.; Campion, C.; Wible, R.S.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol. Sci. 2011, 124, 128–137. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy-Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Dong, F.; Hao, F.; Murray, I.A.; Smith, P.B.; Koo, I.; Tindall, A.M.; Kris-Etherton, P.M.; Gowda, K.; Amin, S.G.; Patterson, A.D.; et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes 2020, 12, 1–24. [Google Scholar] [CrossRef]
- Anzai, H.; Yoshimoto, S.; Okamura, K.; Hiraki, A.; Hashimoto, S. IDO1-mediated Trp-kynurenine-AhR signal activation induces stemness and tumor dormancy in oral squamous cell carcinomas. Oral. Sci. Internat 2022, 19, 31–43. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Aleti, G.; Troyer, E.A.; Hong, S. G protein-coupled receptors: A target for microbial metabolites and a mechanistic link to microbiome-immune-brain interactions. Brain Behav. Immun. Health 2023, 32, 100671. [Google Scholar] [CrossRef]
- Sanders, K.M. G protein-coupled receptors in gastrointestinal physiology. IV. Neural regulation of gastrointestinal smooth muscle. Am. J. Physiol. 1998, 275, G1-7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Ma, C.; Chen, K.; Jiang, M.; Vasu, R.; Liu, R.; Zhao, Y.; Zhang, H. Roles of G Protein-Coupled Receptors (GPCRs) in Gastrointestinal Cancers: Focus on Sphingosine 1-Shosphate Receptors, Angiotensin II Receptors, and Estrogen-Related GPCRs. Cells 2021, 10, 2988. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Bing, X.; Xu, C.; Qiu, J.; Shen, J.; Huang, J.; Li, J.; Liu, P.; Xie, B. GPR35-mediated kynurenic acid sensing contributes to maintenance of gut microbiota homeostasis in ulcerative colitis. FEBS Open Bio 2023, 13, 1415–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, D.; Zhang, Y.; Chen, J.; Zhang, Y.; Liao, C.; Qin, S.; Tian, Y.; Zhang, Z.; Xu, F. Functional metabolomics reveal the role of AHR/GPR35 mediated kynurenic acid gradient sensing in chemotherapy-induced intestinal damage. Acta Pharm. Sin. B 2021, 11, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Sujino, T.; Harada, Y.; Ashida, H.; Yoshimatsu, Y.; Yonemoto, Y.; Nemoto, Y.; Tomura, M.; Melhem, H.; Niess, J.H.; et al. The gut microbiota-induced kynurenic acid recruits GPR35-positive macrophages to promote experimental encephalitis. Cell Rep. 2023, 42, 113005. [Google Scholar] [CrossRef]
- Pedraz-Petrozzi, B.; Marszalek-Grabska, M.; Kozub, A.; Szalaj, K.; Trzpil, A.; Stachniuk, A.; Lamade, E.K.; Gilles, M.; Deuschle, M.; Turski, W.A.; et al. LC-MS/MS-based quantification of tryptophan, kynurenine, and kynurenic acid in human placental, fetal membranes, and umbilical cord samples. Sci. Rep. 2023, 13, 12554. [Google Scholar] [CrossRef]
- Yan, J.; Kuzhiumparambil, U.; Bandodkar, S.; Solowij, N.; Fu, S. Development and validation of a simple, rapid and sensitive LC-MS/MS method for the measurement of urinary neurotransmitters and their metabolites. Anal. Bioanal. Chem. 2017, 409, 7191–7199. [Google Scholar] [CrossRef] [PubMed]
- Oluwagbemigun, K.; Anesi, A.; Clarke, G.; Schmid, M.; Mattivi, F.; Nothlings, U. An Investigation into the Temporal Reproducibility of Tryptophan Metabolite Networks Among Healthy Adolescents. Int. J. Tryptophan Res. 2021, 14, 11786469211041376. [Google Scholar] [CrossRef]
- Pritchard, J.B.; Miller, D.S. Mechanisms mediating renal secretion of organic anions and cations. Physiol. Rev. 1993, 73, 765–796. [Google Scholar] [CrossRef]
- Sekine, T.; Cha, S.H.; Endou, H. The multispecific organic anion transporter (OAT) family. Pflugers Arch. 2000, 440, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Luo, S.; Zhai, J.; Chen, Y. A novel causative role of imbalanced kynurenine pathway in ulcerative colitis: Upregulation of KMO and KYNU promotes intestinal inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166929. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Bartnik, M.; Kocki, T.; Turski, W.A. Distribution, synthesis, and absorption of kynurenic acid in plants. Planta Med. 2011, 77, 858–864. [Google Scholar] [CrossRef]
- Starratt, A.N.; Caveney, S. Quinoline-2-carboxylic acids from Ephedra species. Phytochemistry 1996, 42, 1477–1478. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 1998, 15, 595–606. [Google Scholar]
- Zgrajka, W.; Turska, M.; Rajtar, G.; Majdan, M.; Parada-Turska, J. Kynurenic acid content in anti-rheumatic herbs. Ann. Agric. Environ. Med. 2013, 20, 800–802. [Google Scholar] [PubMed]
- Catchpole, G.S.; Beckmann, M.; Enot, D.P.; Mondhe, M.; Zywicki, B.; Taylor, J.; Hardy, N.; Smith, A.; King, R.D.; Kell, D.B.; et al. Hierarchical metabolomics demonstrates substantial compositional similarity between genetically modified and conventional potato crops. Proc. Natl. Acad. Sci. USA 2005, 102, 14458–14462. [Google Scholar] [PubMed]
- Turski, M.P.; Kaminski, P.; Zgrajka, W.; Turska, M.; Turski, W.A. Potato- an important source of nutritional kynurenic acid. Plant Foods Hum. Nutr. 2012, 67, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Turski, M.P.; Chwil, S.; Turska, M.; Chwil, M.; Kocki, T.; Rajtar, G.; Parada-Turska, J. An exceptionally high content of kynurenic acid in chestnut honey and flowers of chestnut tree. J. Food Composition Anal. 2016, 48, 67–72. [Google Scholar] [CrossRef]
- Truchado, P.; Martos, I.; Bortolotti, L.; Sabatini, A.G.; Ferreres, F.; Tomas-Barberan, F.A. Use of quinoline alkaloids as markers of the floral origin of chestnut honey. J. Agric. Food Chem. 2009, 57, 5680–5686. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, W.; May, J.; Scaysbrook, T.; Whatley, F.R. Identification by gas chromatography-mass spectrometry of 150 compounds in propolis. Z. Naturforsch. C 1991, 46, 111–121. [Google Scholar]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Alternat Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cheah, I.K.; Ng, M.M.; Li, J.; Chan, S.M.; Lim, S.L.; Mahendran, R.; Kua, E.H.; Halliwell, B. The association between mushroom consumption and Mild Cognitive Impairment: A community-based cross-sectional study in Singapore. J. Alzheimers Dis. 2019, 68, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Saral, Ö. An Investigation into Chestnut Honeys from Artvin Province in Turkiye: Their Physicochemical Properties, Phenolic Profiles and Antioxidant Activities. Chem. Biodivers. 2023, 20, e202201162. [Google Scholar] [CrossRef]
- Terzo, S.; Calvi, P.; Nuzzo, D.; Picone, P.; Galizzi, G.; Caruana, L.; Di Carlo, M.; Lentini, L.; Puleio, R.; Mule, F.; et al. Preventive Impact of Long-Term Ingestion of Chestnut Honey on Glucose Disorders and Neurodegeneration in Obese Mice. Nutrients 2022, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Calvi, P.; Nuzzo, D.; Picone, P.; Allegra, M.; Mule, F.; Amato, A. Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice. Int. J. Mol. Sci. 2023, 24, 3467. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Kaltalioglu, K.; Erisgin, Z.; Coskun-Cevher, S.; Kolayli, S. Protective effects of aqueous extracts of some honeys against HCl/ethanol-induced gastric ulceration in rats. J. Food Biochem. 2019, 43, e13054. [Google Scholar] [CrossRef]
- Saral, Ö.; Yıldız, O.; Aliyazıcıoğlu, R.; Yuluğ, E.; Canpolat, S.; Öztürk, F.; Kolaylı, S. Apitherapy products enhance the recovery of CCL4-induced hepatic damages in rats. Turk. J. Med. Sci. 2016, 46, 194–202. [Google Scholar] [CrossRef]
- Seyhan, M.F.; Yılmaz, E.; Timirci-Kahraman, Ö.; Saygılı, N.; Kısakesen, H.I.; Eronat, A.P.; Ceviz, A.B.; Gazioğglu, S.B.; Yılmaz-Aydoğan, H.; Öztürk, O. Anatolian honey is not only sweet but can also protect from breast cancer: Elixir for women from Artemis to present. IUBMB Life 2017, 69, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.B.; Kim, S.G.; Kim, Y.S.; Kim, B.; Han, S.M.; Lee, H.J.; Choi, H.M.; Choi, J.G. Castanea crenata honey reduces influenza infection by activating the innate immune response. Front. Immunol. 2023, 14, 1157506. [Google Scholar] [CrossRef]
- Oskay, G.S.; Oskay, D.; Ardac, N. Investigation of the Effect of Chestnut Honey and Curcumin Combination on Lifespan in the Experimental Heat Stress Model of Honey Bee. Biol. Bull. 2023, 50, 1393–1400. [Google Scholar] [CrossRef]
- Turska, M.; Pelak, J.; Turski, M.P.; Kocki, T.; Dukowski, P.; Plech, T.; Turski, W. Fate and distribution of kynurenic acid administered as beverage. Pharmacol. Rep. 2018, 70, 1089–1096. [Google Scholar] [CrossRef]
- Kaihara, M.; Price, J.M. The metabolism of quinaldic acid, kynurenic acid, and xanthurenic acid in the rabbit. J. Biol. Chem. 1962, 237, 1727–1729. [Google Scholar]
- Horibata, K.; Taniuchi, H.; Tashiro, M.; Kuno, S.; Hayaishi, O. The metabolism of kynurenic acid. II. Tracer experiments on the mechanism of kynurenic acid degradation and glutamic acid synthesis by Pseudomonas extracts. J. Biol. Chem. 1961, 236, 2991–2995. [Google Scholar]
- Kell, D.B.; Pretorius, E. No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. 2018, 93, 1518–1557. [Google Scholar] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Frontiers Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: Evidence, mechanisms, and therapeutic implications. Biochem. J. 2022, 479, 1653–1708. [Google Scholar] [PubMed]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar]
- Kell, D.B. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch. Toxicol. 2010, 577, 825–889. [Google Scholar]
- Ramírez Ortega, D.; Ugalde Muñiz, P.E.; Blanco Ayala, T.; Vázquez Cervantes, G.I.; Lugo Huitrón, R.; Pineda, B.; Gonzalez Esquivel, D.F.; Pérez de la Cruz, G.; Pedraza Chaverri, J.; Sánchez Chapul, L.; et al. On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Worton, S.A.; Greenwood, S.L.; Wareing, M.; Heazell, A.E.; Myers, J. The kynurenine pathway; A new target for treating maternal features of preeclampsia? Placenta 2019, 84, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Reyes Ocampo, J.; Lugo Huitrón, R.; González-Esquivel, D.; Ugalde-Muñiz, P.; Jiménez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Ríos, C.; Pérez de la Cruz, V. Kynurenines with neuroactive and redox properties: Relevance to aging and brain diseases. Oxid. Med. Cell Longev. 2014, 2014, 646909. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, M.; Shayan, M.; Khalilzadeh, M.; Soltani, Z.E.; Jafari-Sabet, M.; Ghasemi, M.; Dehpour, A.R. Kynurenine pathway and its role in neurologic, psychiatric, and inflammatory bowel diseases. Mol. Biol. Rep. 2023, 50, 10409–10425. [Google Scholar] [CrossRef] [PubMed]
- Darlington, L.G.; Mackay, G.M.; Forrest, C.M.; Stoy, N.; George, C.; Stone, T.W. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur. J. Neurosci. 2007, 26, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Kozlowska, A.; Thoene, M.; Lepiarczyk, E.; Grzegorzewski, W.J. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharmacol. 2016, 67, 3–19. [Google Scholar]
- Sekine, A.; Fukuwatari, T. Acute liver failure increases kynurenic acid production in rat brain via changes in tryptophan metabolism in the periphery. Neurosci. Lett. 2019, 701, 14–19. [Google Scholar] [CrossRef]
- Hartai, Z.; Juhász, A.; Rimanóczy, Á.; Janáky, T.; Donkó, T.; Dux, L.; Penke, B.; Tóth, G.K.; Janka, Z.; Kálmán, J. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochem. Int. 2007, 50, 308–313. [Google Scholar] [CrossRef]
- Heyes, M.P.; Saito, K.; Crowley, J.S.; Davis, L.E.; Demitrack, M.A.; Der, M.; Dilling, L.A.; Elia, J.; Kruesi, M.J.; Lackner, A.; et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992, 115, 1249–1273. [Google Scholar] [CrossRef]
- Knapskog, A.B.; Aksnes, M.; Edwin, T.H.; Ueland, P.M.; Ulvik, A.; Fang, E.F.; Eldholm, R.S.; Halaas, N.B.; Saltvedt, I.; Giil, L.M.; et al. Higher concentrations of kynurenic acid in CSF are associated with the slower clinical progression of Alzheimer’s disease. Alzheimers Dement. 2023, 19, 5573–5582. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, S.; He, Y.; Xu, M.; Qiao, X.; Zhu, Y.; Wu, W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Markers 2022, 2022, 9484217. [Google Scholar] [CrossRef]
- Zapolski, T.; Kaminska, A.; Kocki, T.; Wysokinski, A.; Urbanska, E.M. Aortic stiffness-Is kynurenic acid a novel marker? Cross-sectional study in patients with persistent atrial fibrillation. PLoS ONE 2020, 15, e0236413. [Google Scholar] [CrossRef]
- Cavaleri, D.; Crocamo, C.; Morello, P.; Bartoli, F.; Carra, G. The Kynurenine Pathway in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Blood Concentrations of Tryptophan and Its Catabolites. J. Clin. Med. 2024, 13, 583. [Google Scholar] [CrossRef]
- Liu, H.; Ding, L.; Zhang, H.; Mellor, D.; Wu, H.; Zhao, D.; Wu, C.; Lin, Z.; Yuan, J.; Peng, D. The Metabolic Factor Kynurenic Acid of Kynurenine Pathway Predicts Major Depressive Disorder. Front. Psychiatry 2018, 9, 552. [Google Scholar] [CrossRef]
- Colpo, G.D.; Venna, V.R.; McCullough, L.D.; Teixeira, A.L. Systematic Review on the Involvement of the Kynurenine Pathway in Stroke: Pre-clinical and Clinical Evidence. Front. Neurol. 2019, 10, 778. [Google Scholar] [CrossRef]
- Bartoli, F.; Cioni, R.M.; Cavaleri, D.; Callovini, T.; Crocamo, C.; Misiak, B.; Savitz, J.B.; Carra, G. The association of kynurenine pathway metabolites with symptom severity and clinical features of bipolar disorder: An overview. Eur. Psychiatry 2022, 65, e82. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Dabrowski, W.; Langner, E.; Zgrajka, W.; Piłat, J.; Kocki, T.; Rzeski, W.; Turski, W.A. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand. J. Gastroenterol. 2011, 46, 903–912. [Google Scholar] [CrossRef]
- Walczak, K.; Turski, W.A.; Rajtar, G. Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids 2014, 46, 2393–2401. [Google Scholar] [CrossRef]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic acid and cancer: Facts and controversies. Cell Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Perugino, F.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain 2015, 17, 27. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2015, 17, 47. [Google Scholar] [CrossRef]
- Badawy, A.A. The kynurenine pathway of tryptophan metabolism: A neglected therapeutic target of COVID-19 pathophysiology and immunotherapy. Biosci. Rep. 2023, 43, BSR20230595. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Fedele, R.; Cevenini, A.; Pontillo, M.; Barra, L.; Ruoppolo, M. COVIDomics: The Proteomic and Metabolomic Signatures of COVID-19. Int. J. Mol. Sci. 2022, 23, 2414. [Google Scholar] [CrossRef]
- Tezcan, D.; Onmaz, D.E.; Sivrikaya, A.; Körez, M.K.; Hakbilen, S.; Gülcemal, S.; Yılmaz, S. Kynurenine pathway of tryptophan metabolism in patients with familial Mediterranean fever. Mod. Rheumatol. 2023, 33, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Al Saedi, A.; Chow, S.; Vogrin, S.; Guillemin, G.J.; Duque, G. Association Between Tryptophan Metabolites, Physical Performance, and Frailty in Older Persons. Int. J. Tryptophan Res. 2022, 15, 11786469211069951. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F.; Matson, W.R.; Storey, E.; Milbury, P.; Ryan, E.A.; Ogawa, T.; Bird, E.D. Kynurenic acid concentrations are reduced in Huntington’s disease cerebral cortex. J. Neurol. Sci. 1992, 108, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.; Piryatinsky, V.; Birnberg, T.; Hingaly, T.; Raymond, E.; Kashi, R.; Amit-Romach, E.; Caballero, I.S.; Towfic, F.; Ator, M.A.; et al. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2016, 113, E6145–E6152. [Google Scholar] [CrossRef]
- Shen, H.; Xu, X.; Bai, Y.; Wang, X.; Wu, Y.; Zhong, J.; Wu, Q.; Luo, Y.; Shang, T.; Shen, R.; et al. Therapeutic potential of targeting kynurenine pathway in neurodegenerative diseases. Eur. J. Med. Chem. 2023, 251, 115258. [Google Scholar] [CrossRef]
- Tsuji, A.; Ikeda, Y.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. Int. J. Mol. Sci. 2023, 24, 5742. [Google Scholar] [CrossRef]
- Li, P.; Zheng, J.; Bai, Y.; Wang, D.; Cui, Z.; Li, Y.; Zhang, J.; Wang, Y. Characterization of kynurenine pathway in patients with diarrhea-predominant irritable bowel syndrome. Eur. J. Histochem. 2020, 64, 3132. [Google Scholar] [CrossRef]
- Chojnacki, C.; Błońska, A.; Konrad, P.; Chojnacki, M.; Podogrocki, M.; Poplawski, T. Changes in Tryptophan Metabolism on Serotonin and Kynurenine Pathways in Patients with Irritable Bowel Syndrome. Nutrients 2023, 15, 1262. [Google Scholar] [CrossRef]
- Pawlak, D.; Pawlak, K.; Malyszko, J.; Mysliwiec, M.; Buczko, W. Accumulation of toxic products degradation of kynurenine in hemodialyzed patients. Int. Urol. Nephrol. 2001, 33, 399–404. [Google Scholar] [CrossRef]
- Krupa, A.; Krupa, M.M.; Pawlak, K. Kynurenine Pathway-An Underestimated Factor Modulating Innate Immunity in Sepsis-Induced Acute Kidney Injury? Cells 2022, 11, 2604. [Google Scholar] [CrossRef]
- Mor, A.; Kalaska, B.; Pawlak, D. Kynurenine Pathway in Chronic Kidney Disease: What’s Old, What’s New, and What’s Next? Int. J. Tryptophan Res. 2020, 13, 1178646920954882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Plasma kynurenic acid/tryptophan ratio: A sensitive and reliable biomarker for the assessment of renal function. Ren. Fail. 2013, 35, 648–653. [Google Scholar] [CrossRef]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef]
- Francis, H.M.; Stevenson, R.J.; Tan, L.S.Y.; Ehrenfeld, L.; Byeon, S.; Attuquayefio, T.; Gupta, D.; Lim, C.K. Kynurenic acid as a biochemical factor underlying the association between Western-style diet and depression: A cross-sectional study. Front. Nutr. 2022, 9, 945538. [Google Scholar] [CrossRef]
- Li, C.C.; Ye, F.; Xu, C.X.; Jiang, N.; Chang, Q.; Liu, X.M.; Pan, R.L. Tryptophan-kynurenine metabolic characterization in the gut and brain of depressive-like rats induced by chronic restraint stress. J. Affect. Disord. 2023, 328, 273–286. [Google Scholar] [CrossRef]
- Fila, M.; Chojnacki, J.; Derwich, M.; Chojnacki, C.; Pawlowska, E.; Blasiak, J. Urine 5-Hydroxyindoleacetic Acid Negatively Correlates with Migraine Occurrence and Characteristics in the Interictal Phase of Episodic Migraine. Int. J. Mol. Sci. 2024, 25, 5471. [Google Scholar] [CrossRef]
- Biringer, R.G. Migraine signaling pathways: Amino acid metabolites that regulate migraine and predispose migraineurs to headache. Mol. Cell Biochem. 2022, 477, 2269–2296. [Google Scholar] [CrossRef] [PubMed]
- Hartai, Z.; Klivényi, P.; Janáky, T.; Penke, B.; Dux, L.; Vécsei, L. Kynurenine metabolism in multiple sclerosis. Acta Neurol. Scand. 2005, 112, 93–96. [Google Scholar] [CrossRef]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef]
- Sandi, D.; Fricska-Nagy, Z.; Bencsik, K.; Vécsei, L. Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy-Kynurenines Are Important Players. Molecules 2021, 26, 3423. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, K.; Bartosik-Psujek, H.; Dobosz, B.; Kocki, T.; Grieb, P.; Giovannoni, G.; Turski, W.A.; Stelmasiak, Z. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci. Lett. 2002, 331, 63–65. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Panahi, H.K.S.; Kavyani, B.; Heng, B.; Tan, V.; Braidy, N.; Guillemin, G.J. The Role of Kynurenine Pathway and NAD(+) Metabolism in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Aging Dis. 2022, 13, 698–711. [Google Scholar] [CrossRef]
- Kavyani, B.; Lidbury, B.A.; Schloeffel, R.; Fisher, P.R.; Missailidis, D.; Annesley, S.J.; Dehhaghi, M.; Heng, B.; Guillemin, G.J. Could the kynurenine pathway be the key missing piece of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) complex puzzle? Cell Mol. Life Sci. 2022, 79, 412. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, B.; Ahn, S.B.; Missailidis, D.; Annesley, S.J.; Fisher, P.R.; Schloeffel, R.; Guillemin, G.J.; Lovejoy, D.B.; Heng, B. Dysregulation of the Kynurenine Pathway, Cytokine Expression Pattern, and Proteomics Profile Link to Symptomology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Mol. Neurobiol. 2024, 61, 3771–3787. [Google Scholar] [CrossRef]
- Shi, T.; Shi, Y.; Gao, H.; Ma, Y.; Wang, Q.; Shen, S.; Shao, X.; Gong, W.; Chen, X.; Qin, J.; et al. Exercised accelerated the production of muscle-derived kynurenic acid in skeletal muscle and alleviated the postmenopausal osteoporosis through the Gpr35/NFκB p65 pathway. J. Orthop. Transl. 2022, 35, 1–12. [Google Scholar] [CrossRef]
- Lim, C.K.; Fernández-Gomez, F.J.; Braidy, N.; Estrada, C.; Costa, C.; Costa, S.; Bessede, A.; Fernandez-Villalba, E.; Zinger, A.; Herrero, M.T.; et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2017, 155, 76–95. [Google Scholar] [CrossRef]
- Ogawa, T.; Matson, W.R.; Beal, M.F.; Myers, R.H.; Bird, E.D.; Milbury, P.; Saso, S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 1992, 42, 1702–1706. [Google Scholar] [CrossRef]
- Pires, A.S.; Gupta, S.; Barton, S.A.; Vander Wall, R.; Tan, V.; Heng, B.; Phillips, J.K.; Guillemin, G.J. Temporal Profile of Kynurenine Pathway Metabolites in a Rodent Model of Autosomal Recessive Polycystic Kidney Disease. Int. J. Tryptophan Res. 2022, 15, 11786469221126063. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mu, L.; Zhang, C.; Long, X.; Zhang, Y.; Li, R.; Zhao, Y.; Qiao, J. Abnormal Activation of Tryptophan-Kynurenine Pathway in Women With Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 877807. [Google Scholar] [CrossRef]
- Van Zundert, S.K.; Broekhuizen, M.; Smit, A.J.; van Rossem, L.; Mirzaian, M.; Willemsen, S.P.; Danser, A.J.; De Rijke, Y.B.; Reiss, I.K.; Merkus, D.; et al. The Role of the Kynurenine Pathway in the (Patho) physiology of Maternal Pregnancy and Fetal Outcomes: A Systematic Review. Int. J. Tryptophan Res. 2022, 15, 11786469221135545. [Google Scholar] [CrossRef]
- Cai, Z.; Tian, S.; Klein, T.; Tu, L.; Geenen, L.W.; Koudstaal, T.; van den Bosch, A.E.; de Rijke, Y.B.; Reiss, I.K.M.; Boersma, E.; et al. Kynurenine metabolites predict survival in pulmonary arterial hypertension: A role for IL-6/IL-6Ralpha. Sci. Rep. 2022, 12, 12326. [Google Scholar] [CrossRef]
- Linderholm, K.R.; Skogh, E.; Olsson, S.K.; Dahl, M.L.; Holtze, M.; Engberg, G.; Samuelsson, M.; Erhardt, S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2012, 38, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef]
- Kozak, R.; Campbell, B.M.; Strick, C.A.; Horner, W.; Hoffmann, W.E.; Kiss, T.; Chapin, D.S.; McGinnis, D.; Abbott, A.L.; Roberts, B.M.; et al. Reduction of brain kynurenic acid improves cognitive function. J. Neurosci. 2014, 34, 10592–10602. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Kegel, M.E.; Johansson, V.; Wetterberg, L.; Bhat, M.; Schwieler, L.; Cannon, T.D.; Schuppe-Koistinen, I.; Engberg, G.; Landén, M.; Hultman, C.M.; et al. Kynurenic acid and psychotic symptoms and personality traits in twins with psychiatric morbidity. Psychiatry Res. 2017, 247, 105–112. [Google Scholar] [CrossRef]
- Chen, W.; Tian, Y.; Gou, M.; Wang, L.; Tong, J.; Zhou, Y.; Feng, W.; Li, Y.; Chen, S.; Liu, Y.; et al. Role of the immune-kynurenine pathway in treatment-resistant schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 130, 110926. [Google Scholar] [CrossRef]
- Skorobogatov, K.; Autier, V.; Foiselle, M.; Richard, J.R.; Boukouaci, W.; Wu, C.L.; Raynal, S.; Carbonne, C.; Laukens, K.; Meysman, P.; et al. Kynurenine pathway abnormalities are state-specific but not diagnosis-specific in schizophrenia and bipolar disorder. Brain Behav. Immun. Health 2023, 27, 100584. [Google Scholar] [CrossRef]
- Eryavuz Onmaz, D.; Tezcan, D.; Abusoglu, S.; Sak, F.; Humeyra Yerlikaya, F.; Yilmaz, S.; Abusoglu, G.; Kazim Korez, M.; Unlu, A. Impaired kynurenine metabolism in patients with primary Sjögren’s syndrome. Clin. Biochem. 2023, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eryavuz Onmaz, D.; Tezcan, D.; Yilmaz, S.; Onmaz, M.; Unlu, A. Altered kynurenine pathway metabolism and association with disease activity in patients with systemic lupus. Amino Acids 2023, 55, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Chmiel-Perzyńska, I.; Perzyński, A.; Urbańska, E.M. Experimental diabetes mellitus type 1 increases hippocampal content of kynurenic acid in rats. Pharmacol. Rep. 2014, 66, 1134–1139. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol. Neurobiol. 2015, 52, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Kiluk, M.; Lewkowicz, J.; Pawlak, D.; Tankiewicz-Kwedlo, A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk-Review. J. Clin. Med. 2021, 10, 2484. [Google Scholar] [CrossRef]
- Sofia, M.A.; Ciorba, M.A.; Meckel, K.; Lim, C.K.; Guillemin, G.J.; Weber, C.R.; Bissonnette, M.; Pekow, J.R. Tryptophan Metabolism through the Kynurenine Pathway is Associated with Endoscopic Inflammation in Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1471–1480. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef]
- Roberts, I.; Wright Muelas, M.; Taylor, J.M.; Davison, A.S.; Xu, Y.; Grixti, J.M.; Gotts, N.; Sorokin, A.; Goodacre, R.; Kell, D.B. Untargeted metabolomics of COVID-19 patient serum reveals potential prognostic markers of both severity and outcome. Metabolomics 2022, 18, 6. [Google Scholar] [CrossRef]
- Cihan, M.; Doğan, Ö.; Ceran Serdar, C.; Altunçekiç Yıldırım, A.; Kurt, C.; Serdar, M.A. Kynurenine pathway in Coronavirus disease (COVID-19): Potential role in prognosis. J. Clin. Lab. Anal. 2022, 36, e24257. [Google Scholar] [CrossRef]
- Kucukkarapinar, M.; Yay-Pence, A.; Yildiz, Y.; Buyukkoruk, M.; Yaz-Aydin, G.; Deveci-Bulut, T.S.; Gulbahar, O.; Senol, E.; Candansayar, S. Psychological outcomes of COVID-19 survivors at sixth months after diagnose: The role of kynurenine pathway metabolites in depression, anxiety, and stress. J. Neural Transm. 2022, 129, 1077–1089. [Google Scholar] [CrossRef]
- Sindelar, M.; Stancliffe, E.; Schwaiger-Haber, M.; Anbukumar, D.S.; Adkins-Travis, K.; Goss, C.W.; O’Halloran, J.A.; Mudd, P.A.; Liu, W.C.; Albrecht, R.A.; et al. Longitudinal metabolomics of human plasma reveals prognostic markers of COVID-19 disease severity. Cell Rep. Med. 2021, 2, 100369. [Google Scholar] [CrossRef]
- Cai, Y.; Kim, D.J.; Takahashi, T.; Broadhurst, D.I.; Yan, H.; Ma, S.; Rattray, N.J.W.; Casanovas-Massana, A.; Israelow, B.; Klein, J.; et al. Kynurenic acid may underlie sex-specific immune responses to COVID-19. Sci. Signal 2021, 14, eabf8483. [Google Scholar] [CrossRef] [PubMed]
- Almulla, A.F.; Supasitthumrong, T.; Tunvirachaisakul, C.; Algon, A.A.A.; Al-Hakeim, H.K.; Maes, M. The tryptophan catabolite or kynurenine pathway in COVID-19 and critical COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 615. [Google Scholar] [CrossRef]
- Holmes, E.; Wist, J.; Masuda, R.; Lodge, S.; Nitschke, P.; Kimhofer, T.; Loo, R.L.; Begum, S.; Boughton, B.; Yang, R.; et al. Incomplete Systemic Recovery and Metabolic Phenoreversion in Post-Acute-Phase Nonhospitalized COVID-19 Patients: Implications for Assessment of Post-Acute COVID-19 Syndrome. J. Proteome Res. 2021, 20, 3315–3329. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Shishkova, E.; Miller, I.J.; Balnis, J.; Bernstein, M.N.; Peters-Clarke, T.M.; Meyer, J.G.; Quan, Q.; Muehlbauer, L.K.; Trujillo, E.A.; et al. Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Syst. 2020, 12, 23–40e27. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Stangl, M.; Börner, N.; Bösch, F.; Durner, J.; Drunin, G.; Buhl, J.L.; Abendroth, D. Kynurenine serves as useful biomarker in acute, Long- and Post-COVID-19 diagnostics. Front. Immunol. 2022, 13, 1004545. [Google Scholar] [CrossRef]
- Mangge, H.; Herrmann, M.; Meinitzer, A.; Pailer, S.; Curcic, P.; Sloup, Z.; Holter, M.; Prüller, F. Increased Kynurenine Indicates a Fatal Course of COVID-19. Antioxidants 2021, 10, 1960. [Google Scholar] [CrossRef]
- Michaelis, S.; Zelzer, S.; Schnedl, W.J.; Baranyi, A.; Meinitzer, A.; Enko, D. Assessment of tryptophan and kynurenine as prognostic markers in patients with SARS-CoV-2. Clin. Chim. Acta 2022, 525, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, S.; Zelzer, S.; Schneider, C.; Schnedl, W.J.; Baranyi, A.; Meinitzer, A.; Herrmann, M.; Enko, D. Alteration of the kynurenine pathway is inversely associated with the humoral immune response in patients with SARS-CoV-2. Clin. Chim. Acta 2022, 537, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, F.C.; Virseda-Berdices, A.; Resino, S.; Ryan, P.; Martinez-Gonzalez, O.; Perez-Garcia, F.; Martin-Vicente, M.; Brochado-Kith, O.; Blancas, R.; Bartolome-Sanchez, S.; et al. Metabolic Profiling at COVID-19 Onset Shows Disease Severity and Sex-Specific Dysregulation. Front. Immunol. 2022, 13, 925558. [Google Scholar] [CrossRef]
- Dewulf, J.P.; Martin, M.; Marie, S.; Oguz, F.; Belkhir, L.; De Greef, J.; Yombi, J.C.; Wittebole, X.; Laterre, P.F.; Jadoul, M.; et al. Urine metabolomics links dysregulation of the tryptophan-kynurenine pathway to inflammation and severity of COVID-19. Sci. Rep. 2022, 12, 9959. [Google Scholar] [CrossRef]
- Brown, M.; Dunn, W.B.; Ellis, D.I.; Goodacre, R.; Handl, J.; Knowles, J.D.; O’Hagan, S.; Spasic, I.; Kell, D.B. A metabolome pipeline: From concept to data to knowledge. Metabolomics 2005, 1, 39–51. [Google Scholar]
- Kell, D.B.; Oliver, S.G. Here is the evidence, now what is the hypothesis? The complementary roles of inductive and hypothesis-driven science in the post-genomic era. Bioessays 2004, 26, 99–105. [Google Scholar] [PubMed]
- Li, R.W.S.; Yang, C.; Sit, A.S.M.; Kwan, Y.W.; Lee, S.M.Y.; Hoi, M.P.M.; Chan, S.W.; Hausman, M.; Vanhoutte, P.M.; Leung, G.P.H. Uptake and Protective Effects of Ergothioneine in Human Endothelial Cells. J. Pharmacol. Exp. Ther. 2014, 350, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. The unusual amino acid L-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010, 17, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta 2012, 1822, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Connick, J.H.; Heywood, G.C.; Sills, G.J.; Thompson, G.G.; Brodie, M.J.; Stone, T.W. Nicotinylalanine increases cerebral kynurenic acid content and has anticonvulsant activity. Gen. Pharmacol. 1992, 23, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol. Sci. 2000, 21, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kocki, T.; Wielosz, M.; Turski, W.A.; Urbanska, E.M. Enhancement of brain kynurenic acid production by anticonvulsants--novel mechanism of antiepileptic activity? Eur. J. Pharmacol. 2006, 541, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Huang, X.F.; Newell, K.A. The kynurenine pathway in major depression: What we know and where to next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Yan, J.; Kothur, K.; Innes, E.A.; Han, V.X.; Jones, H.F.; Patel, S.; Tsang, E.; Webster, R.; Gupta, S.; Troedson, C.; et al. Decreased cerebrospinal fluid kynurenic acid in epileptic spasms: A biomarker of response to corticosteroids. EBioMedicine 2022, 84, 104280. [Google Scholar] [CrossRef]
- Thaler, S.; Choragiewicz, T.J.; Rejdak, R.; Fiedorowicz, M.; Turski, W.A.; Tulidowicz-Bielak, M.; Zrenner, E.; Schuettauf, F.; Zarnowski, T. Neuroprotection by acetoacetate and beta-hydroxybutyrate against NMDA-induced RGC damage in rat--possible involvement of kynurenic acid. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1729–1735. [Google Scholar] [CrossRef]
- Chmiel-Perzyńska, I.; Kloc, R.; Perzyński, A.; Rudzki, S.; Urbańska, E.M. Novel aspect of ketone action: Beta-hydroxybutyrate increases brain synthesis of kynurenic acid in vitro. Neurotox. Res. 2011, 20, 40–50. [Google Scholar] [CrossRef]
- Salvati, P.; Ukmar, G.; Dho, L.; Rosa, B.; Cini, M.; Marconi, M.; Molinari, A.; Post, C. Brain concentrations of kynurenic acid after a systemic neuroprotective dose in the gerbil model of global ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 741–752. [Google Scholar] [CrossRef]
- Andiné, P.; Lehmann, A.; Ellren, K.; Wennberg, E.; Kjellmer, I.; Nielsen, T.; Hagberg, H. The excitatory amino acid antagonist kynurenic acid administered after hypoxic-ischemia in neonatal rats offers neuroprotection. Neurosci. Lett. 1988, 90, 208–212. [Google Scholar] [CrossRef]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The Mechanism of the Neuroprotective Effect of Kynurenic Acid in the Experimental Model of Neonatal Hypoxia-Ischemia: The Link to Oxidative Stress. Antioxidants 2021, 10, 1775. [Google Scholar] [CrossRef]
- Gigler, G.; Szénási, G.; Simó, A.; Lévay, G.; Hársing, L.G., Jr.; Sas, K.; Vécsei, L.; Toldi, J. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur. J. Pharmacol. 2007, 564, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Robotka, H.; Sas, K.; Ágoston, M.; Rózsa, É.; Szénási, G.; Gigler, G.; Vécsei, L.; Toldi, J. Neuroprotection achieved in the ischaemic rat cortex with L-kynurenine sulphate. Life Sci. 2008, 82, 915–919. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Vohra, M.; Lemieux, G.A.; Lin, L.; Ashrafi, K. Kynurenic acid accumulation underlies learning and memory impairment associated with aging. Genes Dev. 2018, 32, 14–19. [Google Scholar] [CrossRef]
- Fejes, A.; Párdutz, Á.; Toldi, J.; Vécsei, L. Kynurenine metabolites and migraine: Experimental studies and therapeutic perspectives. Curr. Neuropharmacol. 2011, 9, 376–387. [Google Scholar] [CrossRef]
- Párdutz, Á.; Fejes, A.; Bohár, Z.; Tar, L.; Toldi, J.; Vécsei, L. Kynurenines and headache. J. Neural Transm. 2012, 119, 285–296. [Google Scholar] [CrossRef]
- Oláh, G.; Heredi, J.; Menyhart, A.; Czinege, Z.; Nagy, D.; Fuzik, J.; Kocsis, K.; Knapp, L.; Krucsó, E.; Géllert, L.; et al. Unexpected effects of peripherally administered kynurenic acid on cortical spreading depression and related blood-brain barrier permeability. Drug Des. Devel Ther. 2013, 7, 981–987. [Google Scholar] [CrossRef][Green Version]
- Körtési, T.; Spekker, E.; Vécsei, L. Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms. Cells 2022, 11, 3795. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Franco, N.F.; Ng, M.L.; Pai, S.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Current Evidence for a Role of the Kynurenine Pathway of Tryptophan Metabolism in Multiple Sclerosis. Front. Immunol. 2016, 7, 246. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Rojewska, E.; Ciapała, K.; Mika, J. Kynurenic acid and zaprinast diminished CXCL17-evoked pain-related behaviour and enhanced morphine analgesia in a mouse neuropathic pain model. Pharmacol. Rep. 2019, 71, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Samavati, R.; Zádor, F.; Szűcs, E.; Tuka, B.; Martos, D.; Veres, G.; Gáspár, R.; Mándity, I.M.; Fülöp, F.; Vécsei, L.; et al. Kynurenic acid and its analogue can alter the opioid receptor G-protein signaling after acute treatment via NMDA receptor in rat cortex and striatum. J. Neurol. Sci. 2017, 376, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ciapała, K.; Mika, J.; Rojewska, E. The Kynurenine Pathway as a Potential Target for Neuropathic Pain Therapy Design: From Basic Research to Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11055. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Korimová, A.; Cížková, D.; Toldi, J.; Vécsei, L.; Vanický, I. Protective effects of glucosamine-kynurenic acid after compression-induced spinal cord injury in the rat. Centr Eur. J. Biol. 2012, 7, 996–1004. [Google Scholar] [CrossRef]
- Alme, K.N.; Ulvik, A.; Askim, T.; Assmus, J.; Mollnes, T.E.; Naik, M.; Naess, H.; Saltvedt, I.; Ueland, P.M.; Knapskog, A.B. Neopterin and kynurenic acid as predictors of stroke recurrence and mortality: A multicentre prospective cohort study on biomarkers of inflammation measured three months after ischemic stroke. BMC Neurol. 2021, 21, 476. [Google Scholar] [CrossRef]
- Vieira, J.P.P.; Karampatsi, D.; Vercalsteren, E.; Darsalia, V.; Patrone, C.; Duarte, J.M.N. Nuclear magnetic resonance spectroscopy reveals biomarkers of stroke recovery in a mouse model of obesity-associated type 2 diabetes. Biosci. Rep. 2024, 44, BSR20240249. [Google Scholar] [CrossRef]
- Mangas, A.; Heredia, M.; Riolobos, A.; De la Fuente, A.; Criado, J.M.; Yajeya, J.; Geffard, M.; Coveñas, R. Overexpression of kynurenic acid and 3-hydroxyanthranilic acid after rat traumatic brain injury. Eur. J. Histochem. 2018, 62, 278–284. [Google Scholar] [CrossRef]
- Suma, T.; Koshinaga, M.; Fukushima, M.; Kano, T.; Katayama, Y. Effects of in situ administration of excitatory amino acid antagonists on rapid microglial and astroglial reactions in rat hippocampus following traumatic brain injury. Neurol. Res. 2008, 30, 420–429. [Google Scholar] [CrossRef]
- Kell, D.B.; Laubscher, G.J.; Pretorius, E. A central role for amyloid fibrin microclots in long COVID/PASC: Origins and therapeutic implications. Biochem. J. 2022, 479, 537–559. [Google Scholar] [CrossRef]
- Pretorius, E.; Kell, D.B. A perspective on how microscopy imaging of fibrinaloid microclots and platelet pathology may be applied in clinical investigations. Semin. Thromb. Haemost. 2024, 50, 537–551. [Google Scholar] [CrossRef]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormal coagulation. Trends Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef]
- Kell, D.B.; Khan, M.A.; Kane, B.; Lip, G.Y.H.; Pretorius, E. Possible role of fibrinaloid microclots in Postural Orthostatic Tachycardia Syndrome (POTS): Focus on Long COVID. J. Pers. Med. 2024, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Ćorović, A.; Scully, P.; Moon, J.C. Myocardial Amyloidosis: The Exemplar Interstitial Disease. JACC Cardiovasc. Imaging 2019, 12, 2345–2356. [Google Scholar] [CrossRef]
- Pucci, A.; Aimo, A.; Musetti, V.; Barison, A.; Vergaro, G.; Genovesi, D.; Giorgetti, A.; Masotti, S.; Arzilli, C.; Prontera, C.; et al. Amyloid Deposits and Fibrosis on Left Ventricular Endomyocardial Biopsy Correlate With Extracellular Volume in Cardiac Amyloidosis. J. Am. Heart Assoc. 2021, 10, e020358. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: Lessons from and for blood clotting. Progr Biophys. Mol. Biol. 2017, 123, 16–41. [Google Scholar] [CrossRef]
- Pretorius, E.; Mbotwe, S.; Bester, J.; Robinson, C.J.; Kell, D.B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J. R. Soc. Interface 2016, 123, 20160539. [Google Scholar] [CrossRef]
- Pretorius, E.; Page, M.J.; Hendricks, L.; Nkosi, N.B.; Benson, S.R.; Kell, D.B. Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: Assessment with novel Amytracker™ stains. J. R. Soc. Interface 2018, 15, 20170941. [Google Scholar]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef]
- Nabai, L.; Ghahary, A.; Jackson, J. Localized Controlled Release of Kynurenic Acid Encapsulated in Synthetic Polymer Reduces Implant-Induced Dermal Fibrosis. Pharmaceutics 2022, 14, 1546. [Google Scholar] [CrossRef]
- Papp, A.; Hartwell, R.; Evans, M.; Ghahary, A. The Safety and Tolerability of Topically Delivered Kynurenic Acid in Humans. A Phase 1 Randomized Double-Blind Clinical Trial. J. Pharm. Sci. 2018, 107, 1572–1576. [Google Scholar] [CrossRef]
- Poormasjedi-Meibod, M.S.; Pakyari, M.; Jackson, J.K.; Salimi Elizei, S.; Ghahary, A. Development of a nanofibrous wound dressing with an antifibrogenic properties in vitro and in vivo model. J. Biomed. Mater. Res. A 2016, 104, 2334–2344. [Google Scholar] [CrossRef]
- Glavin, G.B.; Pinsky, C. Kynurenic acid attenuates experimental ulcer formation and basal gastric acid secretion in rats. Res. Commun. Chem. Pathol. Pharmacol. 1989, 64, 111–119. [Google Scholar]
- Glavin, G.B.; Bose, R.; Pinsky, C. Kynurenic acid protects against gastroduodenal ulceration in mice injected with extracts from poisonous Atlantic shellfish. Prog. Neuropsychopharmacol. Biol. Psychiatry 1989, 13, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Liu, J.; Zhang, X.D.; Song, Z. Kynurenic Acid Acts as a Signaling Molecule Regulating Energy Expenditure and Is Closely Associated With Metabolic Diseases. Front. Endocrinol. 2022, 13, 847611. [Google Scholar] [CrossRef]
- Liu, J.J.; Ching, J.; Wee, H.N.; Liu, S.; Gurung, R.L.; Lee, J.; M, Y.; Zheng, H.; Lee, L.S.; Ang, K.; et al. Plasma Tryptophan-Kynurenine Pathway Metabolites and Risk for Progression to End-Stage Kidney Disease in Patients with Type 2 Diabetes. Diabetes Care 2023, 46, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Bigelman, E.; Pasmanik-Chor, M.; Dassa, B.; Itkin, M.; Malitsky, S.; Dorot, O.; Pichinuk, E.; Kleinberg, Y.; Keren, G.; Entin-Meer, M. Kynurenic acid, a key L-tryptophan-derived metabolite, protects the heart from an ischemic damage. PLoS ONE 2023, 18, e0275550. [Google Scholar] [CrossRef]
- Kamel, R.; Baetz, D.; Gueguen, N.; Lebeau, L.; Barbelivien, A.; Guihot, A.L.; Allawa, L.; Gallet, J.; Beaumont, J.; Ovize, M.; et al. Kynurenic Acid: A Novel Player in Cardioprotection against Myocardial Ischemia/Reperfusion Injuries. Pharmaceuticals 2023, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Bądzyńska, B.; Zakrocka, I.; Sadowski, J.; Turski, W.A.; Kompanowska-Jezierska, E. Effects of systemic administration of kynurenic acid and glycine on renal haemodynamics and excretion in normotensive and spontaneously hypertensive rats. Eur. J. Pharmacol. 2014, 743, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Dai, J.; Sheng, N. Kynurenic Acid Plays a Protective Role in Hepatotoxicity Induced by HFPO-DA in Male Mice. Environ. Sci. Technol. 2024, 58, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Pyun, D.H.; Kim, T.J.; Kim, M.J.; Hong, S.A.; Abd El-Aty, A.M.; Jeong, J.H.; Jung, T.W. Endogenous metabolite, kynurenic acid, attenuates nonalcoholic fatty liver disease via AMPK/autophagy- and AMPK/ORP150-mediated signaling. J. Cell Physiol. 2021, 236, 4902–4912. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.C.; Chen, R.F.; Yeh, Y.S.; Lin, M.T.; Hsieh, J.H.; Chen, S.H. Kynurenic acid attenuates multiorgan dysfunction in rats after heatstroke. Acta Pharmacol. Sin. 2011, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Balla, Z.; Kormányos, E.S.; Kui, B.; Bálint, E.R.; Für, G.; Orján, E.M.; Iványi, B.; Vécsei, L.; Fülöp, F.; Varga, G.; et al. Kynurenic Acid and Its Analogue SZR-72 Ameliorate the Severity of Experimental Acute Necrotizing Pancreatitis. Front. Immunol. 2021, 12, 702764. [Google Scholar] [CrossRef]
- Nahomi, R.B.; Nam, M.H.; Rankenberg, J.; Rakete, S.; Houck, J.A.; Johnson, G.C.; Stankowska, D.L.; Pantcheva, M.B.; MacLean, P.S.; Nagaraj, R.H. Kynurenic Acid Protects Against Ischemia/Reperfusion-Induced Retinal Ganglion Cell Death in Mice. Int. J. Mol. Sci. 2020, 21, 1795. [Google Scholar] [CrossRef] [PubMed]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabo, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect Against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front. Immunol. 2021, 12, 717157. [Google Scholar] [CrossRef]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Teunis, C.J.; Stroes, E.S.G.; Boekholdt, S.M.; Wareham, N.J.; Murphy, A.J.; Nieuwdorp, M.; Hazen, S.L.; Hanssen, N.M.J. Tryptophan metabolites and incident cardiovascular disease: The EPIC-Norfolk prospective population study. Atherosclerosis 2023, 387, 117344. [Google Scholar] [CrossRef] [PubMed]
- Mangas, A.; Yajeya, J.; González, N.; Ruiz, I.; Pernia, M.; Geffard, M.; Coveñas, R. Gemst: A taylor-made combination that reverts neuroanatomical changes in stroke. Eur. J. Histochem. 2017, 61, 2790. [Google Scholar] [CrossRef]
- Mangas, A.; Yajeya, J.; González, N.; Ruiz, I.; Duleu, S.; Geffard, M.; Coveñas, R. Overexpression of kynurenic acid in stroke: An endogenous neuroprotector? Ann. Anat. 2017, 211, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.; Berg, M.; Matic, L.; Polyzos, K.P.; Forteza, M.J.; Hjorth, S.A.; Schwartz, T.W.; Paulsson-Berne, G.; Hansson, G.K.; Hedin, U.; et al. Evidence that a deviation in the kynurenine pathway aggravates atherosclerotic disease in humans. J. Intern. Med. 2021, 289, 53–68. [Google Scholar] [CrossRef]
- Matysik-Woźniak, A.; Turski, W.A.; Turska, M.; Paduch, R.; Łańcut, M.; Piwowarczyk, P.; Czuczwar, M.; Rejdak, R. Kynurenic Acid Accelerates Healing of Corneal Epithelium In Vitro and In Vivo. Pharmaceuticals 2021, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Berman, B.; Fischer, D.L.; Han, H.; Gade, A.; Arnold, D.; Lawson, A. A Randomized, Double-Blind, Active- and Placebo-Controlled Trial Evaluating a Novel Topical Treatment for Keloid Scars. J. Drugs Dermatol. 2021, 20, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Poormasjedi-Meibod, M.S.; Hartwell, R.; Kilani, R.T.; Ghahary, A. Anti-scarring properties of different tryptophan derivatives. PLoS ONE 2014, 9, e91955. [Google Scholar] [CrossRef]
- Sorkin, E.M.; Heel, R.C. Guanfacine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs 1986, 31, 301–336. [Google Scholar] [CrossRef]
- van Stralen, J.; Gill, S.K.; Reaume, C.J.; Handelman, K. A retrospective medical chart review of clinical outcomes in children and adolescents with attention-deficit/hyperactivity disorder treated with guanfacine extended-release in routine Canadian clinical practice. Child. Adolesc. Psychiatry Ment. Health 2021, 15, 55. [Google Scholar] [CrossRef]
- Yu, S.; Shen, S.; Tao, M. Guanfacine for the Treatment of Attention-Deficit Hyperactivity Disorder: An Updated Systematic Review and Meta-Analysis. J. Child. Adolesc. Psychopharmacol. 2023, 33, 40–50. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Ishizawa, Y.; Xie, Z. Scientific rationale for the use of alpha2A-adrenoceptor agonists in treating neuroinflammatory cognitive disorders. Mol. Psychiatry 2023, 28, 4540–4552. [Google Scholar] [CrossRef]
- Kapolka, N.J.; Taghon, G.J.; Rowe, J.B.; Morgan, W.M.; Enten, J.F.; Lambert, N.A.; Isom, D.G. DCyFIR: A high-throughput CRISPR platform for multiplexed G protein-coupled receptor profiling and ligand discovery. Proc. Natl. Acad. Sci. USA 2020, 117, 13117–13126. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Cuartero, M.I.; de la Parra, J.; Garcia-Culebras, Á.; Ballesteros, I.; Lizasoain, I.; Moro, M.A. The Kynurenine Pathway in the Acute and Chronic Phases of Cerebral Ischemia. Curr. Pharm. Des. 2016, 22, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, L.; Qi, Y.; Zhang, J.; Zhu, H.; Tang, J. Intestinal Flora-Derived Kynurenic Acid Protects Against Intestinal Damage Caused by Candida albicans Infection via Activation of Aryl Hydrocarbon Receptor. Front. Microbiol. 2022, 13, 934786. [Google Scholar] [CrossRef]
- Badawy, A.A. Immunotherapy of COVID-19 with poly (ADP-ribose) polymerase inhibitors: Starting with nicotinamide. Biosci. Rep. 2020, 40, BSR20202856. [Google Scholar] [CrossRef]
- Siddiqui, T.; Bhattarai, P.; Popova, S.; Cosacak, M.I.; Sariya, S.; Zhang, Y.; Mayeux, R.; Tosto, G.; Kizil, C. KYNA/Ahr Signaling Suppresses Neural Stem Cell Plasticity and Neurogenesis in Adult Zebrafish Model of Alzheimer’s Disease. Cells 2021, 10, 2748. [Google Scholar] [CrossRef]
- Shi, Y.; Zeng, Z.; Yu, J.; Tang, B.; Tang, R.; Xiao, R. The aryl hydrocarbon receptor: An environmental effector in the pathogenesis of fibrosis. Pharmacol. Res. 2020, 160, 105180. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gallego, N.; Sánchez-Madrid, F.; Cibrian, D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells 2021, 10, 3176. [Google Scholar] [CrossRef]
- Dadvar, S.; Ferreira, D.M.S.; Cervenka, I.; Ruas, J.L. The weight of nutrients: Kynurenine metabolites in obesity and exercise. J. Intern. Med. 2018, 284, 519–533. [Google Scholar] [CrossRef]
- Wyant, G.A.; Yu, W.; Doulamis, I.P.; Nomoto, R.S.; Saeed, M.Y.; Duignan, T.; McCully, J.D.; Kaelin, W.G., Jr. Mitochondrial remodeling and ischemic protection by G protein-coupled receptor 35 agonists. Science 2022, 377, 621–629. [Google Scholar] [CrossRef]
- Milligan, G. Orthologue selectivity and ligand bias: Translating the pharmacology of GPR35. Trends Pharmacol. Sci. 2011, 32, 317–325. [Google Scholar] [CrossRef]
- Mackenzie, A.E.; Lappin, J.E.; Taylor, D.L.; Nicklin, S.A.; Milligan, G. GPR35 as a Novel Therapeutic Target. Front. Endocrinol. 2011, 2, 68. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, P.; Fan, H.; Zhang, C.; Yu, P.; Liang, X.; Chen, Y. GPR35 acts a dual role and therapeutic target in inflammation. Front. Immunol. 2023, 14, 1254446. [Google Scholar] [CrossRef]
- Ahmadi, S.; Muth-Selbach, U.; Lauterbach, A.; Lipfert, P.; Neuhuber, W.L.; Zeilhofer, H.U. Facilitation of spinal NMDA receptor currents by spillover of synaptically released glycine. Science 2003, 300, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Danysz, W.; Quack, G.; Hartmann, S.; Lorenz, B.; Wollenburg, C.; Baran, L.; Przegalinski, E.; Kostowski, W.; Krzascik, P.; et al. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: Electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 1997, 283, 1264–1275. [Google Scholar] [PubMed]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, G.A.; Cunningham, K.A.; Lin, L.; Mayer, F.; Werb, Z.; Ashrafi, K. Kynurenic acid is a nutritional cue that enables behavioral plasticity. Cell 2015, 160, 119–131. [Google Scholar] [CrossRef]
- Bagasrawala, I.; Zecevic, N.; Radonjić, N.V. N-Methyl D-Aspartate Receptor Antagonist Kynurenic Acid Affects Human Cortical Development. Front. Neurosci. 2016, 10, 435. [Google Scholar] [CrossRef]
- Mok, M.H.S.; Fricker, A.C.; Weil, A.; Kew, J.N.C. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology 2009, 57, 242–249. [Google Scholar] [CrossRef]
- Erhardt, S.; Olsson, S.K.; Engberg, G. Pharmacological manipulation of kynurenic acid: Potential in the treatment of psychiatric disorders. CNS Drugs 2009, 23, 91–101. [Google Scholar] [CrossRef]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy States. Int. J. Tryptophan Res. 2009, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef] [PubMed]
- Dobelis, P.; Staley, K.J.; Cooper, D.C. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE 2012, 7, e41108. [Google Scholar] [CrossRef]
- Steiner, L.; Gold, M.; Mengel, D.; Dodel, R.; Bach, J.P. The endogenous alpha7 nicotinic acetylcholine receptor antagonist kynurenic acid modulates amyloid-beta-induced inflammation in BV-2 microglial cells. J. Neurol. Sci. 2014, 344, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Torosyan, R.; Huang, S.; Bommi, P.V.; Tiwari, R.; An, S.Y.; Schonfeld, M.; Rajendran, G.; Kavanaugh, M.A.; Gibbs, B.; Truax, A.D.; et al. Hypoxic preconditioning protects against ischemic kidney injury through the IDO1/kynurenine pathway. Cell Rep. 2021, 36, 109547. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, G.; Kargaran, S.; Engelhardt, B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418794184. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gu, X.; Ma, Z. Mitochondrial Quality Control in Cerebral Ischemia-Reperfusion Injury. Mol. Neurobiol. 2021, 58, 5253–5271. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Invest. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Andreadou, I.; Oelze, M.; Davidson, S.M.; Hausenloy, D.J. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic. Biol. Med. 2021, 163, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef]
- Aigner, F.; Maier, H.T.; Schwelberger, H.G.; Wallnofer, E.A.; Amberger, A.; Obrist, P.; Berger, T.; Mak, T.W.; Maglione, M.; Margreiter, R.; et al. Lipocalin-2 regulates the inflammatory response during ischemia and reperfusion of the transplanted heart. Amer J. Transplant. 2007, 7, 779–788. [Google Scholar]
- Jaeschke, H.; Woolbright, B.L. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant. Rev. 2012, 26, 103–114. [Google Scholar] [CrossRef]
- Situmorang, G.R.; Sheerin, N.S. Ischaemia reperfusion injury: Mechanisms of progression to chronic graft dysfunction. Pediatr. Nephrol. 2019, 34, 951–963. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Williamson, R.D.; McCarthy, F.P.; Manna, S.; Groarke, E.; Kell, D.B.; Kenny, L.C.; McCarthy, C.M. L-(+)-Ergothioneine Significantly Improves the Clinical Characteristics of Preeclampsia in the Reduced Uterine Perfusion Pressure Rat Model. Hypertension 2020, 75, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Taracha, E.; Czarna, M.; Turzynska, D.; Sobolewska, A.; Maciejak, P. Long-term disruption of tissue levels of glutamate and glutamatergic neurotransmission neuromodulators, taurine and kynurenic acid induced by amphetamine. Psychopharmacology 2024, 241, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef]
- Bednarz, K.; Kozieł, K.; Urbańska, E.M. Novel Activity of Oral Hypoglycemic Agents Linked with Decreased Formation of Tryptophan Metabolite, Kynurenic Acid. Life 2024, 14, 127. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Zhang, L.D.; Luo, X.; Wu, L.L.; Chen, Z.W.; Wei, G.H.; Zhang, K.Q.; Du, Z.A.; Li, R.Z.; So, K.F.; et al. All roads lead to Rome—A review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1210–1227. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wang, Q.; Wang, Y.; Lou, S. The function of previously unappreciated exerkines secreted by muscle in regulation of neurodegenerative diseases. Front. Mol. Neurosci. 2023, 16, 1305208. [Google Scholar] [CrossRef]
- Lim, A.; Harijanto, C.; Vogrin, S.; Guillemin, G.; Duque, G. Does Exercise Influence Kynurenine/Tryptophan Metabolism and Psychological Outcomes in Persons With Age-Related Diseases? A Systematic Review. Int. J. Tryptophan Res. 2021, 14, 1178646921991119. [Google Scholar] [CrossRef]
- Joisten, N.; Walzik, D.; Schenk, A.; Metcalfe, A.J.; Belen, S.; Schaaf, K.; Jacko, D.; Gehlert, S.; Spiliopoulou, P.; Garzinsky, A.M.; et al. Acute exercise activates the AHR in peripheral blood mononuclear cells in an intensity-dependent manner. Am. J. Physiol. Cell Physiol. 2024, 327, C438–C445. [Google Scholar] [CrossRef] [PubMed]
- Juhas, U.; Reczkowicz, J.; Kortas, J.A.; Zychowska, M.; Pilis, K.; Ziemann, E.; Cytrych, I.; Antosiewicz, J.; Borkowska, A. Eight-day fasting modulates serum kynurenines in healthy men at rest and after exercise. Front. Endocrinol. 2024, 15, 1403491. [Google Scholar] [CrossRef]
- Louvrou, V.; Solianik, R.; Brazaitis, M.; Erhardt, S. Exploring the effect of prolonged fasting on kynurenine pathway metabolites and stress markers in healthy male individuals. Eur. J. Clin. Nutr. 2024. [Google Scholar] [CrossRef]
- Tomczyk, T.; Urbańska, E.M. Experimental hypothyroidism raises brain kynurenic acid—Novel aspect of thyroid dysfunction. Eur. J. Pharmacol. 2020, 883, 173363. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Smith, D.G.; Kerr, S.J.; Smythe, G.A.; Kapoor, V.; Armati, P.J.; Brew, B.J. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 2000, 5, 108–111. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Cullen, K.M.; Lim, C.K.; Smythe, G.A.; Garner, B.; Kapoor, V.; Takikawa, O.; Brew, B.J. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007, 27, 12884–12892. [Google Scholar] [CrossRef]
- Żarnowski, T.; Chorągiewicz, T.; Tulidowicz-Bielak, M.; Thaler, S.; Rejdak, R.; Żarnowski, I.; Turski, W.A.; Gasior, M. Ketogenic diet increases concentrations of kynurenic acid in discrete brain structures of young and adult rats. J. Neural Transm. 2012, 119, 679–684. [Google Scholar] [CrossRef]
- Żarnowski, T.; Tulidowicz-Bielak, M.; Żarnowska, I.; Mitosek-Szewczyk, K.; Wnorowski, A.; Jozwiak, K.; Gasior, M.; Turski, W.A. Kynurenic Acid and Neuroprotective Activity of the Ketogenic Diet in the Eye. Curr. Med. Chem. 2017, 24, 3547–3558. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Łanocha, M.; Bednarski, M.; Marcinkowska, M. MM165—A Small Hybrid Molecule Modulates the Kynurenine Pathway and Attenuates Lipopolysaccharide-Induced Memory Deficits and Inflammation. Neurochem. Res. 2024, 49, 1200–1211. [Google Scholar] [CrossRef]
- Kupjetz, M.; Patt, N.; Joisten, N.; Ueland, P.M.; McCann, A.; Gonzenbach, R.; Bansi, J.; Zimmer, P. The serum kynurenine pathway metabolic profile is associated with overweight and obesity in multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 72, 104592. [Google Scholar] [CrossRef]
- Bjørke-Monsen, A.L.; Varsi, K.; Sakkestad, S.T.; Ulvik, A.; Ebbing, C.; Ueland, P.M. Lower levels of the neuroprotective tryptophan metabolite, kynurenic acid, in users of estrogen contraceptives. Sci. Rep. 2023, 13, 16370. [Google Scholar] [CrossRef]
- de Bie, J.; Lim, C.K.; Guillemin, G.J. Progesterone Alters Kynurenine Pathway Activation in IFN-gamma-Activated Macrophages—Relevance for Neuroinflammatory Diseases. Int. J. Tryptophan Res. 2016, 9, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, Z.; Lv, Y.; Ge, C.; Luo, X.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Hericium erinaceus polysaccharides ameliorate nonalcoholic fatty liver disease via gut microbiota and tryptophan metabolism regulation in an aged laying hen model. Int. J. Biol. Macromol. 2024, 273, 132735. [Google Scholar] [CrossRef]
- Klausing, A.D.; Fukuwatari, T.; Bucci, D.J.; Schwarcz, R. Stress-induced impairment in fear discrimination is causally related to increased kynurenic acid formation in the prefrontal cortex. Psychopharmacology 2020, 237, 1931–1941. [Google Scholar] [CrossRef]
- Yan, L.; Wang, W.J.; Cheng, T.; Yang, D.R.; Wang, Y.J.; Wang, Y.Z.; Yang, F.Z.; So, K.F.; Zhang, L. Hepatic kynurenic acid mediates phosphorylation of Nogo-A in the medial prefrontal cortex to regulate chronic stress-induced anxiety-like behaviors in mice. Acta Pharmacol. Sin. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Deora, G.S.; Kantham, S.; Chan, S.; Dighe, S.N.; Veliyath, S.K.; McColl, G.; Parat, M.O.; McGeary, R.P.; Ross, B.P. Multifunctional Analogs of Kynurenic Acid for the Treatment of Alzheimer’s Disease: Synthesis, Pharmacology, and Molecular Modeling Studies. ACS Chem. Neurosci. 2017, 8, 2667–2675. [Google Scholar] [CrossRef]
- Szabo, M.; Lajkó, N.; Dulka, K.; Szatmári, I.; Fülöp, F.; Mihály, A.; Vécsei, L.; Gulya, K. Kynurenic Acid and Its Analog SZR104 Exhibit Strong Antiinflammatory Effects and Alter the Intracellular Distribution and Methylation Patterns of H3 Histones in Immunochallenged Microglia-Enriched Cultures of Newborn Rat Brains. Int. J. Mol. Sci. 2022, 23, 1079. [Google Scholar] [CrossRef]
- Baris, E.; Simsek, O.; Yoca, O.U.; Demir, A.B.; Tosun, M. Effects of kynurenic acid and choline on lipopolysaccharide-induced cyclooxygenase pathway. Turk. J. Biochem. 2023, 48, 311–318. [Google Scholar] [CrossRef]
- Małaczewska, J.; Siwicki, A.K.; Wócik, R.M.; Kaczorek, E.; Turski, W.A. Effect of oral administration of kynurenic acid on the activity of the peripheral blood leukocytes in mice. Cent. Eur. J. Immunol. 2014, 39, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Małaczewska, J.; Siwicki, A.K.; Wójcik, R.M.; Turski, W.A.; Kaczorek, E. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes—in vitro and ex vivo studies. Cent. Eur. J. Immunol. 2016, 41, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lajkó, N.; Kata, D.; Szabó, M.; Mátyás, A.; Dulka, K.; Földesi, I.; Fülöp, F.; Gulya, K.; Vécsei, L.; Mihály, A. Sensitivity of Rodent Microglia to Kynurenines in Models of Epilepsy and Inflammation In Vivo and In Vitro: Microglia Activation is Inhibited by Kynurenic Acid and the Synthetic Analogue SZR104. Int. J. Mol. Sci. 2020, 21, 9333. [Google Scholar] [CrossRef] [PubMed]
- Csáti, A.; Edvinsson, L.; Vécsei, L.; Toldi, J.; Fülöp, F.; Tajti, J.; Warfvinge, K. Kynurenic acid modulates experimentally induced inflammation in the trigeminal ganglion. J. Headache Pain 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Pang, X.; Yang, Y. Hydrogels containing KYNA promote angiogenesis and inhibit inflammation to improve the survival rate of multi-territory perforator flaps. Biomed. Pharmacother. 2024, 174, 116454. [Google Scholar] [CrossRef]
- Robinson, K.S.L.; Stewart, A.M.; Cachat, J.; Landsman, S.; Gebhardt, M.; Kalueff, A.V. Psychopharmacological effects of acute exposure to kynurenic acid (KYNA) in zebrafish. Pharmacol. Biochem. Behav. 2013, 108, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Jeong, S.H.; Ha, S.E.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Won, C.K.; Heo, J.D.; Kim, H.W.; Kim, G.S. Cellular Regulation of Kynurenic Acid-Induced Cell Apoptosis Pathways in AGS Cells. Int. J. Mol. Sci. 2022, 23, 8894. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Ha, S.E.; Park, M.Y.; Jeong, S.H.; Bhosale, P.B.; Abusaliya, A.; Won, C.K.; Heo, J.D.; Ahn, M.; Seong, J.K.; et al. Identification of Kynurenic Acid-Induced Apoptotic Biomarkers in Gastric Cancer-Derived AGS Cells through Next-Generation Transcriptome Sequencing Analysis. Nutrients 2023, 15, 193. [Google Scholar] [CrossRef]
- Lun, J.; Li, Y.; Gao, X.; Gong, Z.; Chen, X.; Zou, J.; Zhou, C.; Huang, Y.; Zhou, B.; Huang, P.; et al. Kynurenic acid blunts A1 astrocyte activation against neurodegeneration in HIV-associated neurocognitive disorders. J. Neuroinflamm. 2023, 20, 87. [Google Scholar] [CrossRef]
- Młotkowska, P.; Misztal, T.; Kowalczyk, P.; Marciniak, E. Effect of kynurenic acid on enzymatic activity of the DNA base excision repair pathway in specific areas of the sheep brain. Sci. Rep. 2024, 14, 15506. [Google Scholar] [CrossRef]
- Wejksza, K.; Rzeski, W.; Turski, W.A. Kynurenic acid protects against the homocysteine-induced impairment of endothelial cells. Pharmacol. Rep. 2009, 61, 751–756. [Google Scholar] [CrossRef]
- Lajkó, E.; Tuka, B.; Fülöp, F.; Krizbai, I.; Toldi, J.; Magyar, K.; Vécsei, L.; Köhidai, L. Kynurenic acid and its derivatives are able to modulate the adhesion and locomotion of brain endothelial cells. J. Neural Transm. 2018, 125, 899–912. [Google Scholar] [CrossRef]
- Di Serio, C.; Cozzi, A.; Angeli, I.; Doria, L.; Micucci, I.; Pellerito, S.; Mirone, P.; Masotti, G.; Moroni, F.; Tarantini, F. Kynurenic acid inhibits the release of the neurotrophic fibroblast growth factor (FGF)-1 and enhances proliferation of glia cells, in vitro. Cell Mol. Neurobiol. 2005, 25, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Pereira, E.F.; Bruno, J.P.; Pellicciari, R.; Albuquerque, E.X.; Schwarcz, R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 2010, 40, 204–210. [Google Scholar] [CrossRef]
- Bądzyńska, B.; Zakrocka, I.; Turski, W.A.; Olszyński, K.H.; Sadowski, J.; Kompanowska-Jezierska, E. Kynurenic acid selectively reduces heart rate in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, X.; Su, D.; Ren, Y.; Cheng, F.; Yao, Y.; Shi, G.; Ji, Y.; Chen, S.; Shi, P.; et al. Therapeutic efcacy of human adipose mesenchymal stem cells in Crohn’s colon fbrosis is improved by IFN-γ and kynurenic acid priming through indoleamine 2,3-dioxygenase-1 signaling. Cell Res. Ther. 2022, 13, 465. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, J.; Sun, J.L.; Ahn, S.H.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Kim, H.C.; Shim, J.H.; Shin, S.; Jeong, J.H. Administration of kynurenic acid reduces hyperlipidemia-induced inflammation and insulin resistance in skeletal muscle and adipocytes. Mol. Cell Endocrinol. 2020, 518, 110928. [Google Scholar] [CrossRef] [PubMed]
- Salimi Elizei, S.; Poormasjedi-Meibod, M.S.; Wang, X.; Kheirandish, M.; Ghahary, A. Kynurenic acid downregulates IL-17/1L-23 axis in vitro. Mol. Cell Biochem. 2017, 431, 55–65. [Google Scholar] [CrossRef]
- Klein, C.; Patte-Mensah, C.; Taleb, O.; Bourguignon, J.J.; Schmitt, M.; Bihel, F.; Maitre, M.; Mensah-Nyagan, A.G. The neuroprotector kynurenic acid increases neuronal cell survival through neprilysin induction. Neuropharmacology 2013, 70, 254–260. [Google Scholar] [CrossRef]
- Tiszlavicz, Z.; Németh, B.; Fülöp, F.; Vécsei, L.; Tápai, K.; Ocsovszky, I.; Mándi, Y. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-alpha (TNF-alpha) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 447–455. [Google Scholar] [CrossRef]
- Mándi, Y.; Endrész, V.; Mosolygo, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lörinczi, B.; Balog, A.; Vécsei, L. The Opposite Effects of Kynurenic Acid and Different Kynurenic Acid Analogs on Tumor Necrosis Factor-alpha (TNF-alpha) Production and Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Expression. Front. Immunol. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Balog, A.; Varga, B.; Fülöp, F.; Lantos, I.; Toldi, G.; Vécsei, L.; Mándi, Y. Kynurenic Acid Analog Attenuates the Production of Tumor Necrosis Factor-alpha, Calgranulins (S100A 8/9 and S100A 12), and the Secretion of HNP1-3 and Stimulates the Production of Tumor Necrosis Factor-Stimulated Gene-6 in Whole Blood Cultures of Patients With Rheumatoid Arthritis. Front. Immunol. 2021, 12, 632513. [Google Scholar] [CrossRef]
- Joshi, P.; Perni, M.; Limbocker, R.; Mannini, B.; Casford, S.; Chia, S.; Habchi, J.; Labbadia, J.; Dobson, C.M.; Vendruscolo, M. Two human metabolites rescue a C. elegans model of Alzheimer’s disease via a cytosolic unfolded protein response. Commun. Biol. 2021, 4, 843. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wang, Z.; Tian, Y.; Li, C.; Jin, F.; Li, J.; Wang, L. Perivascular brown adipocytes-derived kynurenic acid relaxes blood vessel via endothelium PI3K-Akt-eNOS pathway. Biomed. Pharmacother. 2022, 150, 113040. [Google Scholar] [CrossRef]
- Hare, S.M.; Adhikari, B.M.; Mo, C.; Chen, S.; Wijtenburg, S.A.; Seneviratne, C.; Kane-Gerard, S.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Schwarcz, R.; et al. Tryptophan challenge in individuals with schizophrenia and healthy controls: Acute effects on circulating kynurenine and kynurenic acid, cognition and cerebral blood flow. Neuropsychopharmacology 2023, 48, 1594–1601. [Google Scholar] [CrossRef]
- Turski, W.A.; Malaczewska, J.; Marciniak, S.; Bednarski, J.; Turski, M.P.; Jablonski, M.; Siwicki, A.K. On the toxicity of kynurenic acid in vivo and in vitro. Pharmacol. Rep. 2014, 66, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.; Wnorowski, A.; Smolinska, K.; Walczyna, B.; Turski, W.; Kocki, T.; Paluszkiewicz, P.; Parada-Turska, J. Kynurenic Acid Protects against Thioacetamide-Induced Liver Injury in Rats. Anal. Cell Pathol. 2018, 2018, 1270483. [Google Scholar] [CrossRef]
- Loikas, P.; Hilakivi, I. Effects of kynurenic acid and ketamine on neonatal sleep in rats. Pharmacol. Toxicol. 1989, 64, 185–189. [Google Scholar] [CrossRef]
- Bespalov, A.; Dumpis, M.; Piotrovsky, L.; Zvartau, E. Excitatory amino acid receptor antagonist kynurenic acid attenuates rewarding potential of morphine. Eur. J. Pharmacol. 1994, 264, 233–239. [Google Scholar] [CrossRef]
- Majerova, P.; Olesova, D.; Golisova, G.; Buralova, M.; Michalicova, A.; Vegh, J.; Piestansky, J.; Bhide, M.; Hanes, J.; Kovac, A. Analog of kynurenic acid decreases tau pathology by modulating astrogliosis in rat model for tauopathy. Biomed. Pharmacother. 2022, 152, 113257. [Google Scholar] [CrossRef]
- Okuno, A.; Fukuwatari, T.; Shibata, K. Urinary excretory ratio of anthranilic acid/kynurenic acid as an index of the tolerable amount of tryptophan. Biosci. Biotechnol. Biochem. 2008, 72, 1667–1672. [Google Scholar] [CrossRef]
- Okuno, A.; Fukuwatari, T.; Shibata, K. High tryptophan diet reduces extracellular dopamine release via kynurenic acid production in rat striatum. J. Neurochem. 2011, 118, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, C.; Fukuwatari, T.; Sano, M.; Saito, K.; Sasaki, S.; Shibata, K. Supplementing healthy women with up to 5.0 g/d of L-tryptophan has no adverse effects. J. Nutr. 2013, 143, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, C.; Sano, M.; Fukuwatari, T.; Shibata, K. Time-dependent effects of L-tryptophan administration on urinary excretion of L-tryptophan metabolites. J. Nutr. Sci. Vitaminol. 2014, 60, 255–260. [Google Scholar] [CrossRef][Green Version]
- Al-Karagholi, M.A.; Hansen, J.M.; Abou-Kassem, D.; Hansted, A.K.; Ubhayasekera, K.; Bergquist, J.; Vecsei, L.; Jansen-Olesen, I.; Ashina, M. Phase 1 study to access safety, tolerability, pharmacokinetics, and pharmacodynamics of kynurenine in healthy volunteers. Pharmacol. Res. Perspect. 2021, 9, e00741. [Google Scholar] [CrossRef]
- Jauch, D.A.; Sethy, V.H.; Weick, B.G.; Chase, T.N.; Schwarcz, R. Intravenous administration of L-kynurenine to rhesus monkeys: Effect on quinolinate and kynurenate levels in serum and cerebrospinal fluid. Neuropharmacology 1993, 32, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Mestres, J.; Gregori-Puigjané, E.; Valverde, S.; Solé, R.V. The topology of drug-target interaction networks: Implicit dependence on drug properties and target families. Mol. Biosyst. 2009, 5, 1051–1057. [Google Scholar]
- Hokari, M.; Wu, H.Q.; Schwarcz, R.; Smith, Q.R. Facilitated brain uptake of 4-chlorokynurenine and conversion to 7-chlorokynurenic acid. Neuroreport 1996, 8, 15–18. [Google Scholar] [CrossRef]
- Wallace, M.; White, A.; Grako, K.A.; Lane, R.; Cato, A.J.; Snodgrass, H.R. Randomized, double-blind, placebo-controlled, dose-escalation study: Investigation of the safety, pharmacokinetics, and antihyperalgesic activity of l-4-chlorokynurenine in healthy volunteers. Scand. J. Pain. 2017, 17, 243–251. [Google Scholar] [CrossRef]
- Park, L.T.; Kadriu, B.; Gould, T.D.; Zanos, P.; Greenstein, D.; Evans, J.W.; Yuan, P.; Farmer, C.A.; Oppenheimer, M.; George, J.M.; et al. A Randomized Trial of the N-Methyl-d-Aspartate Receptor Glycine Site Antagonist Prodrug 4-Chlorokynurenine in Treatment-Resistant Depression. Int. J. Neuropsychopharmacol. 2020, 23, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J. Biochemical Individuality; John Wiley: New York, NY, USA, 1956. [Google Scholar]
- Ioannidis, J.P.A.; Trikalinos, T.A. Early extreme contradictory estimates may appear in published research: The Proteus phenomenon in molecular genetics research and randomized trials. J. Clin. Epidemiol. 2005, 58, 543–549. [Google Scholar]
- Ioannidis, J.P.A. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar]
- Ioannidis, J.P.A. Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005, 294, 218–228. [Google Scholar]
- Büki, A.; Kekesi, G.; Horvath, G.; Vécsei, L. A Potential Interface between the Kynurenine Pathway and Autonomic Imbalance in Schizophrenia. Int. J. Mol. Sci. 2021, 22, 10016. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Wiggins, A.K.A.; Grantham, A.; Anderson, G.H. Optimizing foods for special dietary use in Canada: Key outcomes and recommendations from a tripartite workshop. Appl. Physiol. Nutr. Metab. 2019, 44, 1258–1265. [Google Scholar] [CrossRef]
- Bansal, R.; Dhiman, A. Nutraceuticals: A Comparative Analysis of Regulatory Framework in Different Countries of the World. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1654–1663. [Google Scholar] [CrossRef]
- Blaze, J. A Comparison of Current Regulatory Frameworks for Nutraceuticals in Australia, Canada, Japan, and the United States. Innov. Pharm. 2021, 12. [Google Scholar] [CrossRef]
- Thakur, S.; Gupta, M.M.; Sharma, D. Nutraceuticals regulation: An overview of the regulatory frameworks in USA, EU, and Japan. In Nutraceutical Fruits and Foods for Neurodegenerative Disorders; Elsevier: Amsterdam, The Netherlands, 2023; pp. 421–440. [Google Scholar]
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Oliver, S.G. The metabolome 18 years on: A concept comes of age. Metabolomics 2016, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, N.; Knowles, J.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Dunn, W.B.; Erban, A.; Weber, R.J.M.; Creek, D.J.; Brown, M.; Breitling, R.; Hankemeier, T.; Goodacre, R.; Neumann, S.; Kopka, J.; et al. Mass Appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 2013, 9, S44–S66. [Google Scholar]
- Möller, M.; Du Preez, J.L.; Harvey, B.H. Development and validation of a single analytical method for the determination of tryptophan, and its kynurenine metabolites in rat plasma. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2012, 898, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Patel, V.D.; Shamsi, S.A.; Miller, A.; Liu, A.; Powell, M. Simultaneous separation and detection of nine kynurenine pathway metabolites by reversed-phase liquid chromatography-mass spectrometry: Quantitation of inflammation in human cerebrospinal fluid and plasma. Anal. Chim. Acta 2023, 1278, 341659. [Google Scholar] [CrossRef] [PubMed]
- Abusoglu, S.; Eryavuz Onmaz, D.; Abusoglu, G.; Humeyra Yerlikaya, F.; Unlu, A. Measurement of kynurenine pathway metabolites by tandem mass spectrometry. J. Mass. Spectrom. Adv. Clin. Lab. 2023, 28, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Fuertig, R.; Ceci, A.; Camus, S.M.; Bezard, E.; Luippold, A.H.; Hengerer, B. LC-MS/MS-based quantification of kynurenine metabolites, tryptophan, monoamines and neopterin in plasma, cerebrospinal fluid and brain. Bioanalysis 2016, 8, 1903–1917. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.; Pires, A.S.; Chow, S.; Krishnamurthy, S.; Bonnell, B.; Bustamante, S.; Guillemin, G.J. Stability Studies of Kynurenine Pathway Metabolites in Blood Components Define Optimal Blood Processing Conditions. Int. J. Tryptophan Res. 2023, 16, 11786469231213521. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Umino, M.; Sakamoto, T.; Onozato, M. A review of chromatographic methods for bioactive tryptophan metabolites, kynurenine, kynurenic acid, quinolinic acid, and others, in biological fluids. Biomed. Chromatogr. 2022, 36, e5308. [Google Scholar] [CrossRef]
- Mitsuhashi, S.; Fukushima, T.; Arai, K.; Tomiya, M.; Santa, T.; Imai, K.; Toyo’oka, T. Development of a column-switching high-performance liquid chromatography for kynurenine enantiomers and its application to a pharmacokinetic study in rat plasma. Anal. Chim. Acta 2007, 587, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Pi, L.G.; Tang, A.G.; Mo, X.M.; Luo, X.B.; Ao, X. More rapid and sensitive method for simultaneous determination of tryptophan and kynurenic acid by HPLC. Clin. Biochem. 2009, 42, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Luchetti, D.; Schaeffer, E.; Cutrone, J. Determination of kynurenic acid in rat cerebrospinal fluid by HPLC with fluorescence detection. Biomed. Chromatogr. 2016, 30, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; Ares, A.M.; Bernal, J.; Nozal, M.J.; Bernal, J.L. Simultaneous determination of tryptophan, kynurenine, kynurenic and xanthurenic acids in honey by liquid chromatography with diode array, fluorescence and tandem mass spectrometry detection. J. Chromatogr. A 2011, 1218, 7592–7600. [Google Scholar] [CrossRef] [PubMed]
- Sadok, I.; Gamian, A.; Staniszewska, M.M. Chromatographic analysis of tryptophan metabolites. J. Sep. Sci. 2017, 40, 3020–3045. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.; Petronijević, N.; Stašević, M.; Stašević Karličić, I.; Velimirović, M.; Stojković, T.; Ristić, S.; Stojković, M.; Milić, N.; Nikolić, T. Decreased Plasma Levels of Kynurenine and Kynurenic Acid in Previously Treated and First-Episode Antipsychotic-Naive Schizophrenia Patients. Cells 2023, 12, 2814. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, K.; Iinuma, F.; Watanabe, M. Fluorometric determination of urinary kynurenic acid by flow injection analysis equipped with a “bypass line”. Anal. Biochem. 1990, 190, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, M.; Mawatari, K.I.; Morooka, A.; Yasuda, M.; Fukuuchi, T.; Yamaoka, N.; Kaneko, K.; Nakagomi, K.; Oku, N. Simultaneous Determination of Kynurenine and Kynurenic Acid by High-Performance Liquid Chromatography Photoirradiation System Using a Mobile Phase Containing 18-crown-6. Int. J. Tryptophan Res. 2019, 12, 1178646919834551. [Google Scholar] [CrossRef] [PubMed]
- Odo, J.; Funai, T.; Hirai, A. Spectrofluorometric determination of kynurenic acid with horseradish peroxidase in the presence of hydrogen peroxide. Anal. Sci. 2007, 23, 317–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Odo, J.; Inoguchi, M.; Aoki, H.; Sogawa, Y.; Nishimura, M. Fluorescent derivatization of aromatic carboxylic acids with horseradish peroxidase in the presence of excess hydrogen peroxide. Anal. Sci. 2015, 31, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lin, L.; Ye, J. Human metabolite detection by surface-enhanced Raman spectroscopy. Mater. Today Bio 2022, 13, 100205. [Google Scholar] [CrossRef]
- Murphy, M.P.; O’Neill, L.A.J. A break in mitochondrial endosymbiosis as a basis for inflammatory diseases. Nature 2024, 626, 271–279. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Zinellu, A. A systematic review and meta-analysis of the kynurenine pathway of tryptophan metabolism in rheumatic diseases. Front. Immunol. 2023, 14, 1257159. [Google Scholar] [CrossRef]
- Encarnacion, A.B.; Fagutao, F.; Hirono, I.; Ushio, H.; Ohshima, T. Effects of ergothioneine from mushrooms (Flammulina velutipes) on melanosis and lipid oxidation of kuruma shrimp (Marsupenaeus japonicus). J. Agric. Food Chem. 2010, 58, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, A.B.; Fagutao, F.; Hirayama, J.; Terayama, M.; Hirono, I.; Ohshima, T. Edible mushroom (Flammulina velutipes) extract inhibits melanosis in Kuruma shrimp (Marsupenaeus japonicus). J. Food Sci. 2011, 76, C52-58. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, A.B.; Fagutao, F.; Shozen, K.; Hirono, I.; Ohshima, T. Biochemical intervention of ergothioneine-rich edible mushroom (Flammulina velutipes) extract inhibits melanosis in crab (Chionoecetes japonicus). Food Chem. 2011, 127, 1594–1599. [Google Scholar] [CrossRef]
- Staniszewska, M.; Kowalik, S.; Sadok, I.; Kędzierski, W. The Influence of Exercise Intensity on Tryptophan Metabolites in Thoroughbred Horses. Pharmaceuticals 2023, 16, 107. [Google Scholar] [CrossRef]
- Mago, Y.; Sharma, Y.; Thakran, Y.; Mishra, A.; Tewari, S.; Kataria, N. Next-Generation Organic Beauty Products Obtained from Algal Secondary Metabolites: A Sustainable Development in Cosmeceutical Industries. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, A.; Kaur, J.; Kumari, S.; Garg, M.; Sindhu, R.K.; Rahman, M.H.; Akhtar, M.F.; Tagde, P.; Najda, A.; et al. Bioactive-Based Cosmeceuticals: An Update on Emerging Trends. Molecules 2022, 27, 828. [Google Scholar] [CrossRef]
- Vaishampayan, P.; Rane, M.M. Herbal nanocosmecuticals: A review on cosmeceutical innovation. J. Cosmet. Dermatol. 2022, 21, 5464–5483. [Google Scholar] [CrossRef]
- Lee, C.M. Fifty years of research and development of cosmeceuticals: A contemporary review. J. Cosmet. Dermatol. 2016, 15, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Epstein, H. Cosmeceuticals and polyphenols. Clin. Dermatol. 2009, 27, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Norins, A.L. Free radical formation in the skin following exposure to ultraviolet light. J. Investig. Dermatol. 1962, 39, 445–448. [Google Scholar]
- Chen, X.; Yang, C.; Jiang, G. Research progress on skin photoaging and oxidative stress. Postepy Dermatol. Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Bissonnette, R.; Stein Gold, L.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad. Dermatol. 2021, 84, 1059–1067. [Google Scholar] [CrossRef]
- Bissonnette, R.; Saint-Cyr Proulx, E.; Jack, C.; Maari, C. Tapinarof for psoriasis and atopic dermatitis: 15 years of clinical research. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1168–1174. [Google Scholar] [CrossRef]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2019, 20, 5424. [Google Scholar] [CrossRef]
- Tsuji, G.; Hashimoto-Hachiya, A.; Matsuda-Taniguchi, T.; Takai-Yumine, A.; Takemura, M.; Yan, X.; Furue, M.; Nakahara, T. Natural Compounds Tapinarof and Galactomyces Ferment Filtrate Downregulate IL-33 Expression via the AHR/IL-37 Axis in Human Keratinocytes. Front. Immunol. 2022, 13, 745997. [Google Scholar] [CrossRef]
- Hwang, J.; Newton, E.M.; Hsiao, J.; Shi, V.Y. Aryl hydrocarbon receptor/nuclear factor E2-related factor 2 (AHR/NRF2) signalling: A novel therapeutic target for atopic dermatitis. Exp. Dermatol. 2022, 31, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, E.H.; Bergboer, J.G.M.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.J.J.; Hato, S.V.; van der Valk, P.G.M.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.J.M.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, K.M.; Jung, C.H. Diosmin restores the skin barrier by targeting the aryl hydrocarbon receptor in atopic dermatitis. Phytomedicine 2021, 81, 153418. [Google Scholar] [CrossRef]
- Li, X.; Gianoulis, T.A.; Yip, K.Y.; Gerstein, M.; Snyder, M. Extensive in vivo metabolite-protein interactions revealed by large-scale systematic analyses. Cell 2010, 143, 639–650. [Google Scholar]
- Li, X.; Snyder, M. Metabolites as global regulators: A new view of protein regulation: Systematic investigation of metabolite-protein interactions may help bridge the gap between genome-wide association studies and small molecule screening studies. Bioessays 2011, 33, 485–489. [Google Scholar] [PubMed]
- Kell, D.B. Metabolites do social networking. Nat. Chem. Biol. 2011, 7, 7–8. [Google Scholar]
- Piazza, I.; Kochanowski, K.; Cappelletti, V.; Fuhrer, T.; Noor, E.; Sauer, U.; Picotti, P. A map of protein-metabolite interactions reveals principles of chemical communication. Cell 2018, 172, 358–372.e323. [Google Scholar] [CrossRef]
- Malinovska, L.; Cappelletti, V.; Kohler, D.; Piazza, I.; Tsai, T.H.; Pepelnjak, M.; Stalder, P.; Dorig, C.; Sesterhenn, F.; Elsasser, F.; et al. Proteome-wide structural changes measured with limited proteolysis-mass spectrometry: An advanced protocol for high-throughput applications. Nat. Protoc. 2023, 18, 659–682. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Lo, H.W.; Korivi, M.; Tsai, Y.C.; Tang, M.J.; Yang, H.L. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes. Free Radic. Biol. Med. 2015, 86, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Zalachoras, I.; Hollis, F.; Ramos-Fernández, E.; Trovo, L.; Sonnay, S.; Geiser, E.; Preitner, N.; Steiner, P.; Sandi, C.; Morató, L. Therapeutic potential of glutathione-enhancers in stress-related psychopathologies. Neurosci. Biobehav. Rev. 2020, 114, 134–155. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Vudhya Gowrisankar, Y.; Chen, X.Z.; Yang, Y.C.; Yang, H.L. The Antiaging Activity of Ergothioneine in UVA-Irradiated Human Dermal Fibroblasts via the Inhibition of the AP-1 Pathway and the Activation of Nrf2-Mediated Antioxidant Genes. Oxid. Med. Cell Longev. 2020, 2020, 2576823. [Google Scholar] [CrossRef]
- Bernardo, V.S.; Torres, F.F.; de Paula, C.P.; de Oliveira da Silva, J.P.M.; de Almeida, E.A.; da Cunha, A.F.; da Silva, D.G.H. Potential Cytoprotective and Regulatory Effects of Ergothioneine on Gene Expression of Proteins Involved in Erythroid Adaptation Mechanisms and Redox Pathways in K562 Cells. Genes 2022, 13, 2368. [Google Scholar] [CrossRef] [PubMed]
- Dare, A.; Elrashedy, A.A.; Channa, M.L.; Nadar, A. Cardioprotective Effects and in-silico Antioxidant Mechanism of L-Ergothioneine in Experimental Type-2 Diabetic Rats. Cardiovasc. Hematol. Agents Med. Chem. 2022, 20, 133–147. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jin, B.-R.; Lee, C.-D.; Kim, D.; Lee, A.Y.; Lee, S.; An, H.-J. Anti-Inflammatory Effect of Chestnut Honey and Cabbage Mixtures Alleviates Gastric Mucosal Damage. Nutrients 2024, 16, 389. [Google Scholar] [CrossRef]
- Pierozan, P.; Biasibetti-Brendler, H.; Schmitz, F.; Ferreira, F.; Pessoa-Pureur, R.; Wyse, A.T.S. Kynurenic Acid Prevents Cytoskeletal Disorganization Induced by Quinolinic Acid in Mixed Cultures of Rat Striatum. Mol. Neurobiol. 2018, 55, 5111–5124. [Google Scholar] [CrossRef]
- Yeager, R.L.; Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicol. Sci. 2009, 111, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef]
- Furue, M.; Ishii, Y.; Tsukimori, K.; Tsuji, G. Aryl Hydrocarbon Receptor and Dioxin-Related Health Hazards-Lessons from Yusho. Int. J. Mol. Sci. 2021, 22, 708. [Google Scholar] [CrossRef]
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814. [Google Scholar] [CrossRef]
- Vogel, C.F.; Khan, E.M.; Leung, P.S.; Gershwin, M.E.; Chang, W.L.; Wu, D.; Haarmann-Stemmann, T.; Hoffmann, A.; Denison, M.S. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: A role for nuclear factor-κB. J. Biol. Chem. 2014, 289, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.E.; Ihekwaba, A.E.C.; Elliott, M.; Gibney, C.A.; Foreman, B.E.; Nelson, G.; See, V.; Horton, C.A.; Spiller, D.G.; Edwards, S.W.; et al. Oscillations in NF-κB signalling control the dynamics of gene expression. Science 2004, 306, 704–708. [Google Scholar]
- Kell, D.B. Metabolomics, modelling and machine learning in systems biology: Towards an understanding of the languages of cells. The 2005 Theodor Bücher lecture. FEBS J 2006, 273, 873–894. [Google Scholar]
- Ashall, L.; Horton, C.A.; Nelson, D.E.; Paszek, P.; Ryan, S.; Sillitoe, K.; Harper, C.V.; Spiller, D.G.; Unitt, J.F.; Broomhead, D.S.; et al. Pulsatile stimulation determines timing and specificity of NFkappa-B-dependent transcription. Science 2009, 324, 242–246. [Google Scholar] [PubMed]
- Bento, A.P.; Hersey, A.; Félix, E.; Landrum, G.; Gaulton, A.; Atkinson, F.; Bellis, L.J.; De Veij, M.; Leach, A.R. An open source chemical structure curation pipeline using RDKit. J. Cheminform 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, S.; Swainston, N.; Handl, J.; Kell, D.B. A ‘rule of 0.5’ for the metabolite-likeness of approved pharmaceutical drugs. Metabolomics 2015, 11, 323–339. [Google Scholar] [CrossRef]
- O’Hagan, S.; Kell, D.B. Understanding the foundations of the structural similarities between marketed drugs and endogenous human metabolites. Front. Pharmacol. 2015, 6, 105. [Google Scholar] [CrossRef][Green Version]
- O’Hagan, S.; Kell, D.B. The KNIME workflow environment and its applications in Genetic Programming and machine learning. Genetic Progr Evol. Mach. 2015, 16, 387–391. [Google Scholar] [CrossRef][Green Version]
- O’Hagan, S.; Kell, D.B. Consensus rank orderings of molecular fingerprints illustrate the ‘most genuine’ similarities between marketed drugs and small endogenous human metabolites, but highlight exogenous natural products as the most important ‘natural’ drug transporter substrates. ADMET & DMPK 2017, 5, 85–125. [Google Scholar] [CrossRef]
- Lin, X.M.; Li, H.; Wang, C.; Peng, X.X. Proteomic analysis of nalidixic acid resistance in Escherichia coli: Identification and functional characterization of OM proteins. J. Proteome Res. 2008, 7, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Munro, L.J.; Kell, D.B. Analysis of a library of E. coli transporter knockout strains to identify transport pathways of antibiotics. Antibiotics 2022, 11, 1129. [Google Scholar] [CrossRef]
- Ren, X.; Yan, C.X.; Zhai, R.X.; Xu, K.; Li, H.; Fu, X.J. Comprehensive survey of target prediction web servers for Traditional Chinese Medicine. Heliyon 2023, 9, e19151. [Google Scholar] [CrossRef] [PubMed]
- Hagg, A.; Kirschner, K.N. Open-Source Machine Learning in Computational Chemistry. J. Chem. Inf. Model. 2023, 63, 4505–4532. [Google Scholar] [CrossRef]
- Kell, D.B.; Samanta, S.; Swainston, N. Deep learning and generative methods in cheminformatics and chemical biology: Navigating small molecule space intelligently. Biochem. J. 2020, 477, 4559–4580. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Gallo, K.; Goede, A.; Preissner, R.; Gohlke, B.O. SuperPred 3.0: Drug classification and target prediction—A machine learning approach. Nucleic Acids Res. 2022, 50, W726–W731. [Google Scholar] [CrossRef]
- Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 1988, 28, 31–36. [Google Scholar]
- Temperini, C.; Innocenti, A.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase activators: Kinetic and X-ray crystallographic study for the interaction of D- and L-tryptophan with the mammalian isoforms I-XIV. Bioorg Med. Chem. 2008, 16, 8373–8378. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; Blanchard, R.K.; McCormack, W.T.; Cousins, R.J. cDNA array analysis identifies thymic LCK as upregulated in moderate murine zinc deficiency before T-lymphocyte population changes. J. Nutr. 2001, 131, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Lörinczi, B.; Csámpai, A.; Fülöp, F.; Szatmári, I. Synthesis of New C-3 Substituted Kynurenic Acid Derivatives. Molecules 2020, 25, 937. [Google Scholar] [CrossRef]
- Simon, P.; Lörinczi, B.; Hetényi, A.; Szatmári, I. Novel Eco-friendly, One-Pot Method for the Synthesis of Kynurenic Acid Ethyl Esters. ACS Omega 2023, 8, 17966–17975. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Ambatwar, R.; Datusalia, A.K.; Khatik, G.L. Convenient One-Pot Synthesis of Kynurenic Acid Ethyl Ester and Exploration to Direct Synthesis of Neuroprotective Kynurenic Acid and Amide Derivatives. J. Org. Chem. 2023, 88, 10494–10500. [Google Scholar] [CrossRef] [PubMed]
- van der Hoek, S.A.; Darbani, B.; Zugaj, K.; Prabhala, B.K.; Biron, M.B.; Randelovic, M.; Medina, J.B.; Kell, D.B.; Borodina, I. Engineering the yeast Saccharomyces cerevisiae for the production of L-(+)-ergothioneine. Front. Bioeng. Biotechnol. 2019, 7, 262. [Google Scholar] [CrossRef]
- Van der Hoek, S.A.; Rusnák, M.; Wang, G.; Stanchev, L.D.; de Fátima Alvez, L.; Jessop-Fabre, M.M.; Paramasivan, K.; Jacobsen, I.H.; Sonnenschein, N.; Martínez, J.L.; et al. Engineering precursor supply for the high-level production of ergothioneine in Saccharomyces cerevisiae. Metab. Eng. 2022, 70, 129–142. [Google Scholar]
- van der Hoek, S.A.; Rusnák, M.; Jacobsen, I.H.; Martínez, J.L.; Kell, D.B.; Borodina, I. Engineering ergothioneine production in Yarrowia lipolytica. FEBS Lett. 2022, 596, 1356–1364. [Google Scholar] [CrossRef]
- Sharma, K.; Ghiffary, M.R.; Lee, G.; Kim, H.U. Efficient production of an antitumor precursor actinocin and other medicinal molecules from kynurenine pathway in Escherichia coli. Metab. Eng. 2023, 81, 144–156. [Google Scholar] [CrossRef]
- Dei Cas, M.; Vigentini, I.; Vitalini, S.; Laganaro, A.; Iriti, M.; Paroni, R.; Foschino, R. Tryptophan Derivatives by Saccharomyces cerevisiae EC1118: Evaluation, Optimization, and Production in a Soybean-Based Medium. Int. J. Mol. Sci. 2021, 22, 472. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Kinetic evaluation of the formation of tryptophan derivatives in the kynurenine pathway during wort fermentation using Saccharomyces pastorianus and Saccharomyces cerevisiae. Food Chem. 2019, 297, 124975. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Turski, W.; Kocki, T.; Rakicka-Pustułka, M.; Rymowicz, W. An efficient method for production of kynurenic acid by Yarrowia lipolytica. Yeast 2020, 37, 541–547. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Ziuzia, P.; Pierwola, J.; Szymanski, K.; Wróbel-Kwiatkowska, M.; Lazar, Z. The microbial production of kynurenic acid using Yarrowia lipolytica yeast growing on crude glycerol and soybean molasses. Front. Bioeng. Biotechnol. 2022, 10, 936137. [Google Scholar] [CrossRef]
- Matusiewicz, M.; Wróbel-Kwiatkowska, M.; Niemiec, T.; Świderek, W.; Kosieradzka, I.; Rosińska, A.; Niwińska, A.; Rakicka-Pustułka, M.; Kocki, T.; Rymowicz, W.; et al. Effect of Yarrowia lipolytica yeast biomass with increased kynurenic acid content on selected metabolic indicators in mice. PeerJ 2023, 11, e15833. [Google Scholar] [CrossRef]
- Aburto, J.M.; Villavicencio, F.; Basellini, U.; Kjaergaard, S.; Vaupel, J.W. Dynamics of life expectancy and life span equality. Proc. Natl. Acad. Sci. USA 2020, 117, 5250–5259. [Google Scholar] [CrossRef]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Singh-Manoux, A.; Tabak, A.G.; Jokela, M.; Virtanen, M.; Ferrie, J.E.; Marmot, M.G.; Shipley, M.J.; Kivimaki, M. Overall diet history and reversibility of the metabolic syndrome over 5 years: The Whitehall II prospective cohort study. Diabetes Care 2010, 33, 2339–2341. [Google Scholar] [CrossRef]
- Lagström, H.; Stenholm, S.; Akbaraly, T.; Pentti, J.; Vahtera, J.; Kivimäki, M.; Head, J. Diet quality as a predictor of cardiometabolic disease-free life expectancy: The Whitehall II cohort study. Am. J. Clin. Nutr. 2020, 111, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. Spermine and gene methylation: A mechanism of lifespan extension induced by polyamine-rich diet. Amino Acids 2020, 52, 213–224. [Google Scholar] [CrossRef]
- Smith, E.; Ottosson, F.; Hellstrand, S.; Ericson, U.; Orho-Melander, M.; Fernandez, C.; Melander, O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart 2020, 106, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Tang, R.M.Y.; Cheah, I.K. Diet-Derived Antioxidants: The Special Case of Ergothioneine. Annu. Rev. Food Sci. Technol. 2023, 14, 323–345. [Google Scholar] [CrossRef]
- Tian, X.; Cioccoloni, G.; Sier, J.H.; Naseem, K.M.; Thorne, J.L.; Moore, J.B. Ergothioneine supplementation in people with metabolic syndrome (ErgMS): Protocol for a randomised, double-blind, placebocontrolled pilot study. Pilot. Feas Stud. 2021, 7, 193. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, recent developments. Redox Biol. 2021, 42, 101868. [Google Scholar] [CrossRef]
- Beelman, R.B.; Phillips, A.T.; Richie, J.P., Jr.; Ba, D.M.; Duiker, S.W.; Kalaras, M.D. Health Consequences of Improving the Content of Ergothioneine in the Food Supply. FEBS Lett. 2022, 596, 1231–1240. [Google Scholar] [CrossRef]
- Ey, J.; Schömig, E.; Taubert, D. Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Agrawal, D.C.; Dhanasekaran, M. (Eds.) Medicinal Mushrooms: Recent Progress in Research and Development; Springer: Singapore, 2019. [Google Scholar]
- Uffelman, C.N.; Doenges, K.A.; Armstrong, M.L.; Quinn, K.; Reisdorph, R.M.; Tang, M.; Krebs, N.F.; Reisdorph, N.A.; Campbell, W.W. Metabolomics Profiling of White Button, Crimini, Portabella, Lion’s Mane, Maitake, Oyster, and Shiitake Mushrooms Using Untargeted Metabolomics and Targeted Amino Acid Analysis. Foods 2023, 12, 2985. [Google Scholar] [CrossRef]
- Tsiantas, K.; Tsiaka, T.; Koutrotsios, G.; Siapi, E.; Zervakis, G.I.; Kalogeropoulos, N.; Zoumpoulakis, P. On the Identification and Quantification of Ergothioneine and Lovastatin in Various Mushroom Species: Assets and Challenges of Different Analytical Approaches. Molecules 2021, 26, 1832. [Google Scholar] [CrossRef]
- Mitsuyama, H.; May, J.M. Uptake and antioxidant effects of ergothioneine in human erythrocytes. Clin. Sci. 1999, 97, 407–411. [Google Scholar]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, D.; Hartmann, L.; Flögel, S. The Ergothioneine Transporter (ETT): Substrates and Locations, an Inventory. FEBS Lett. 2022, 596, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.W.; Buitrago, D.; Stecula, A.; Ngo, H.X.; Chien, H.C.; Zou, L.; Koleske, M.L.; Giacomini, K.M. Deorphaning a solute carrier 22 family member, SLC22A15, through functional genomic studies. FASEB J. 2020, 34, 15734–15752. [Google Scholar] [CrossRef]
- Wang, L.Z.; Thuya, W.L.; Toh, D.S.; Lie, M.G.; Lau, J.Y.; Kong, L.R.; Wan, S.C.; Chua, K.N.; Lee, E.J.; Goh, B.C. Quantification of L-ergothioneine in human plasma and erythrocytes by liquid chromatography-tandem mass spectrometry. J. Mass. Spectrom. 2013, 48, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Lazar, A.; Grimberg, G.; Jung, N.; Rubbert, A.; Delank, K.S.; Perniok, A.; Erdmann, E.; Schömig, E. Association of rheumatoid arthritis with ergothioneine levels in red blood cells: A case control study. J. Rheumatol. 2006, 33, 2139–2145. [Google Scholar]
- Flegel, W.A.; Srivastava, K.; Sissung, T.M.; Goldspiel, B.R.; Figg, W.D. Pharmacogenomics with red cells: A model to study protein variants of drug transporter genes. Vox Sang. 2021, 116, 141–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Gonzalez-Gutierrez, G.; Legg, K.A.; Walsh, B.J.C.; Pis Diez, C.M.; Edmonds, K.A.; Giedroc, D.P. Discovery and structure of a widespread bacterial ABC transporter specific for ergothioneine. Nat. Commun. 2022, 13, 7586. [Google Scholar] [CrossRef]
- Dumitrescu, D.G.; Gordon, E.M.; Kovalyova, Y.; Seminara, A.B.; Duncan-Lowey, B.; Forster, E.R.; Zhou, W.; Booth, C.J.; Shen, A.; Kranzusch, P.J.; et al. A microbial transporter of the dietary antioxidant ergothioneine. Cell 2022, 185, 4526–4540.e18. [Google Scholar] [CrossRef]
- Alamgir, K.M.; Masuda, S.; Fujitani, Y.; Fukuda, F.; Tani, A. Production of ergothioneine by Methylobacterium species. Front. Microbiol. 2015, 6, 1185. [Google Scholar] [CrossRef]
- Pepelnjak, M.; de Souza, N.; Picotti, P. Detecting Protein-Small Molecule Interactions Using Limited Proteolysis-Mass Spectrometry (LiP-MS). Trends Biochem. Sci. 2020, 45, 919–920. [Google Scholar] [CrossRef]
- Piazza, I.; Beaton, N.; Bruderer, R.; Knobloch, T.; Barbisan, C.; Chandat, L.; Sudau, A.; Siepe, I.; Rinner, O.; de Souza, N.; et al. A machine learning-based chemoproteomic approach to identify drug targets and binding sites in complex proteomes. Nat. Commun. 2020, 11, 4200. [Google Scholar] [CrossRef] [PubMed]
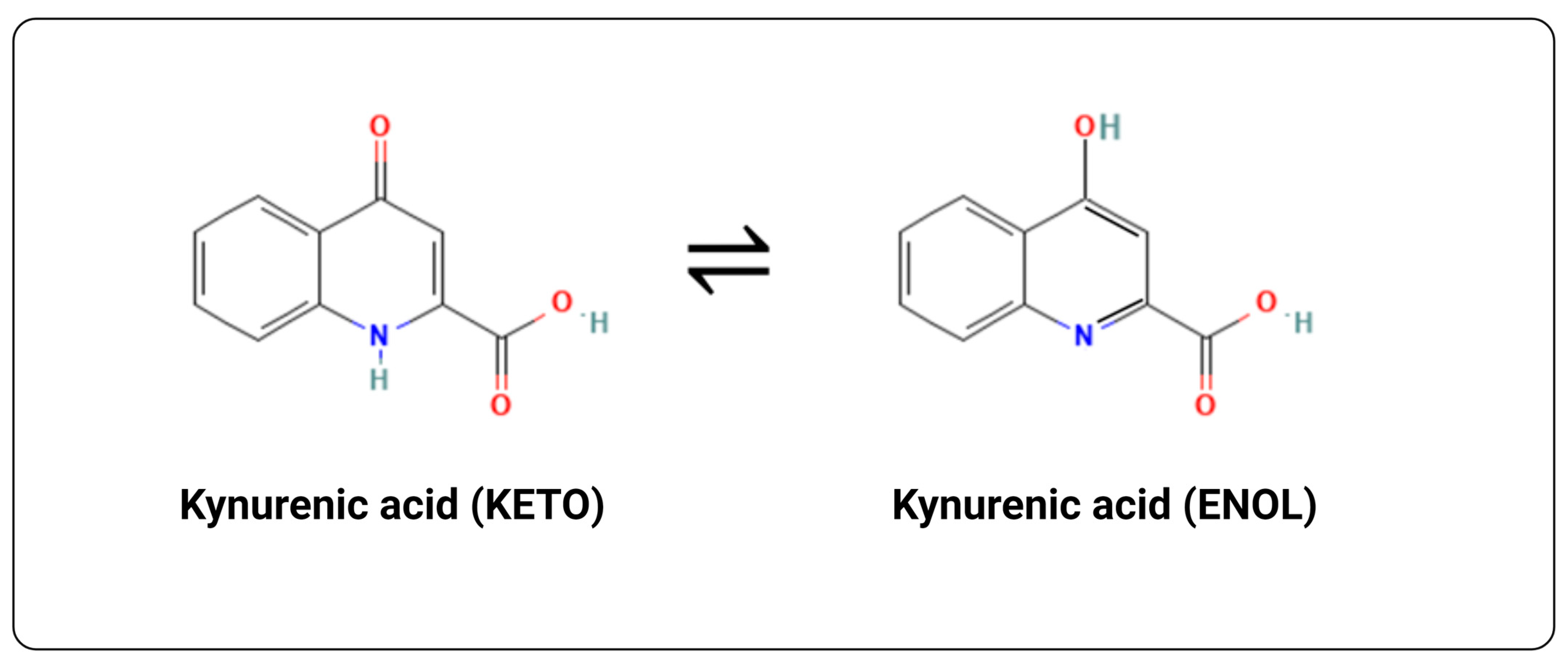
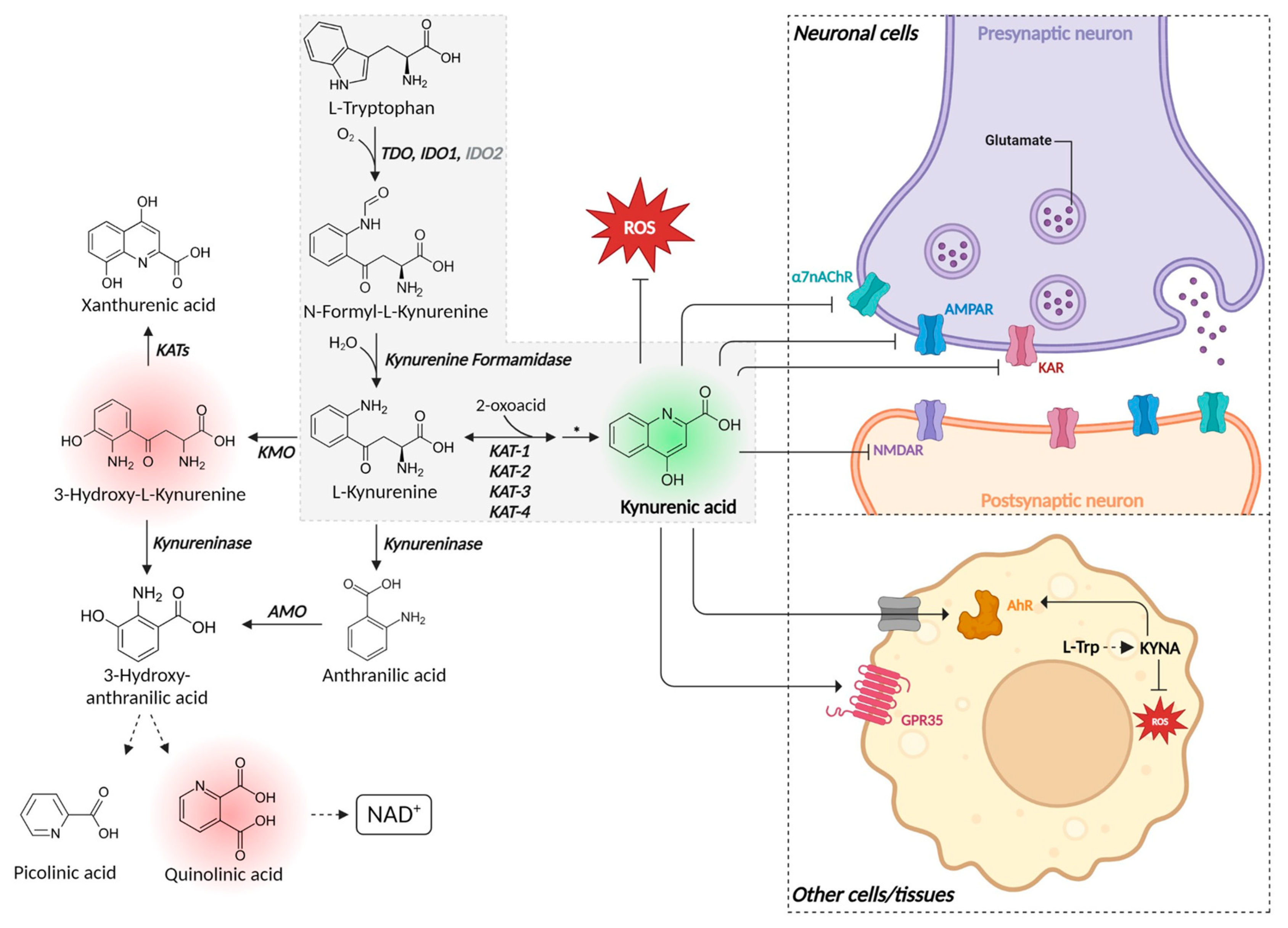
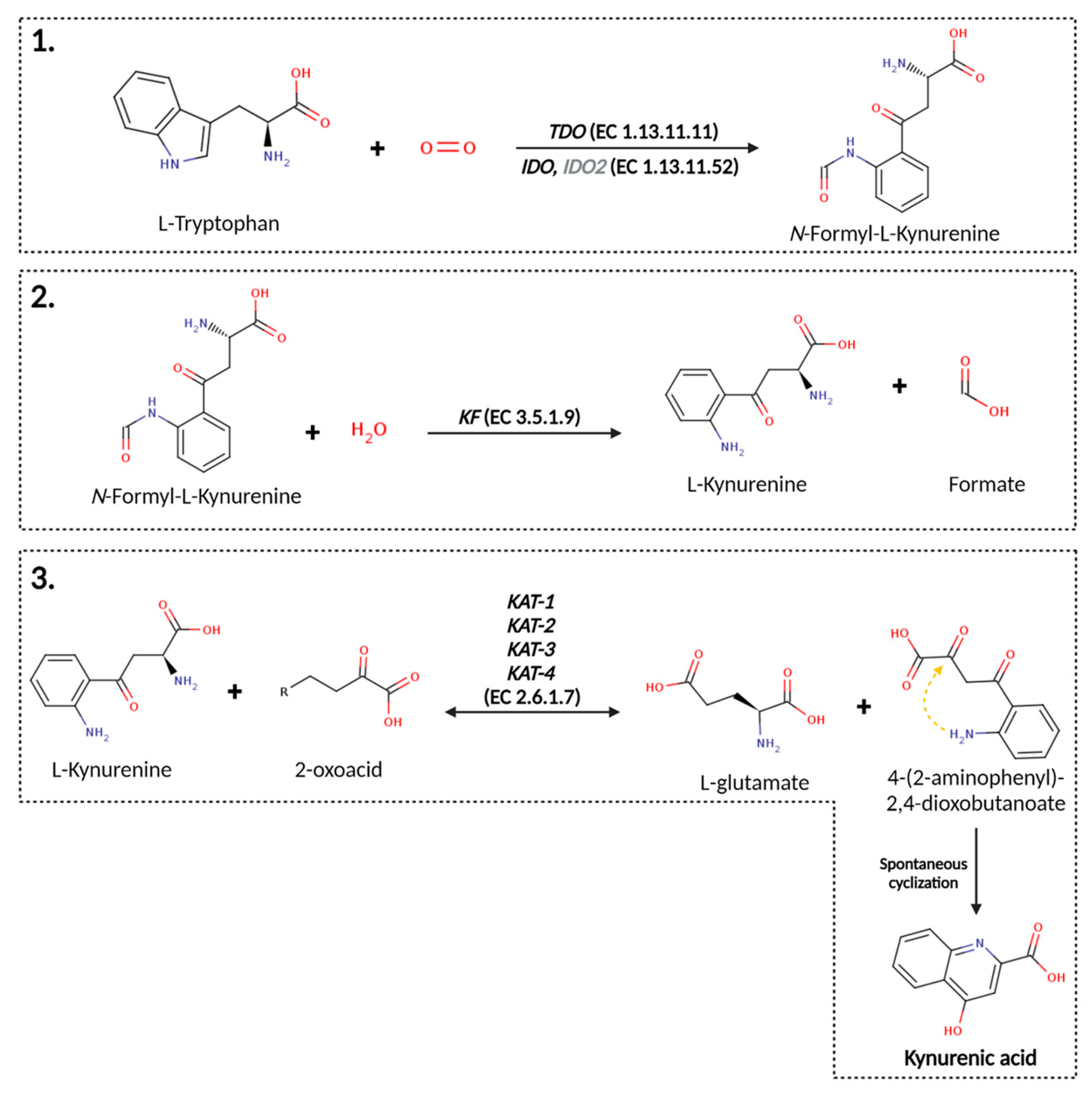
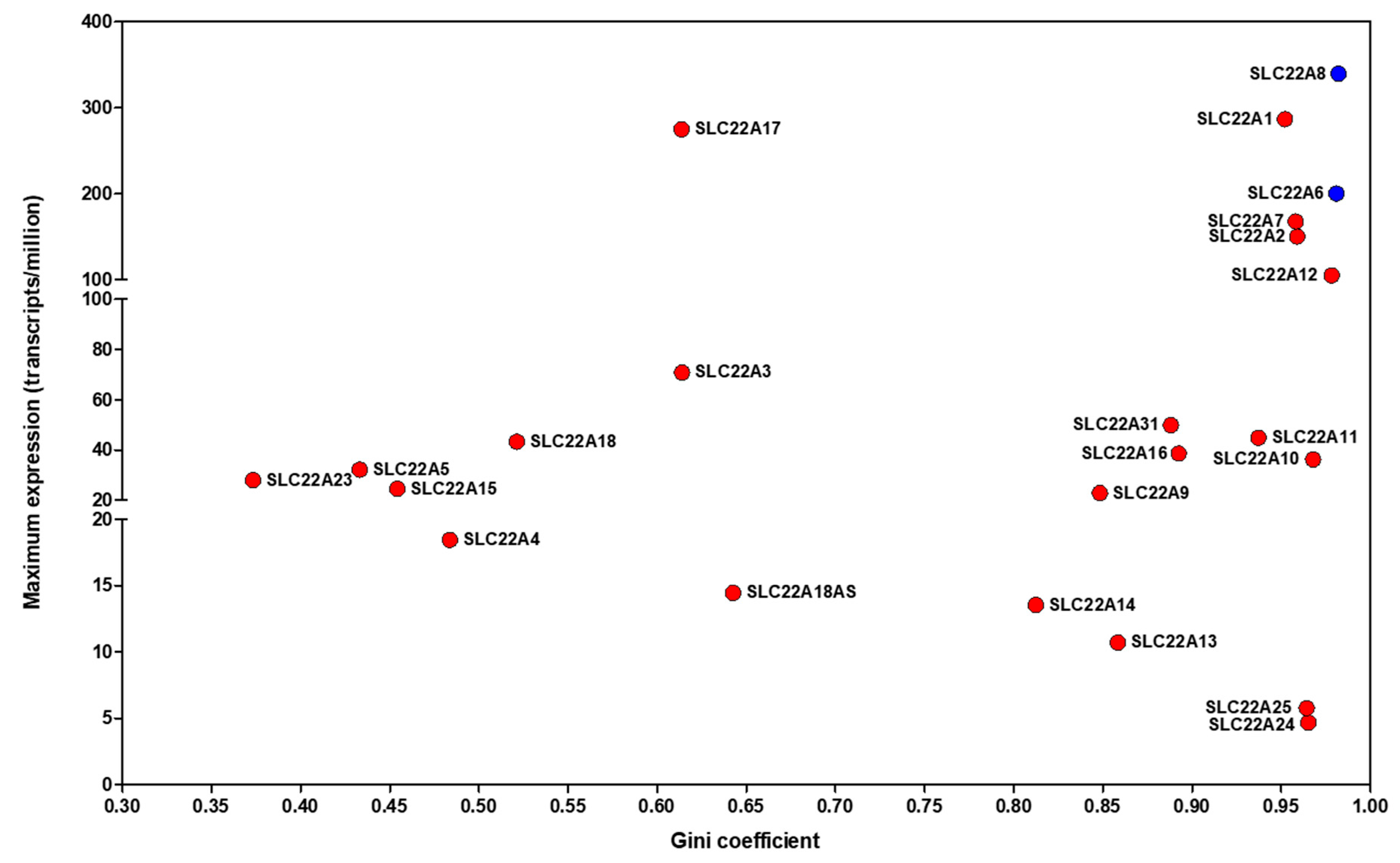

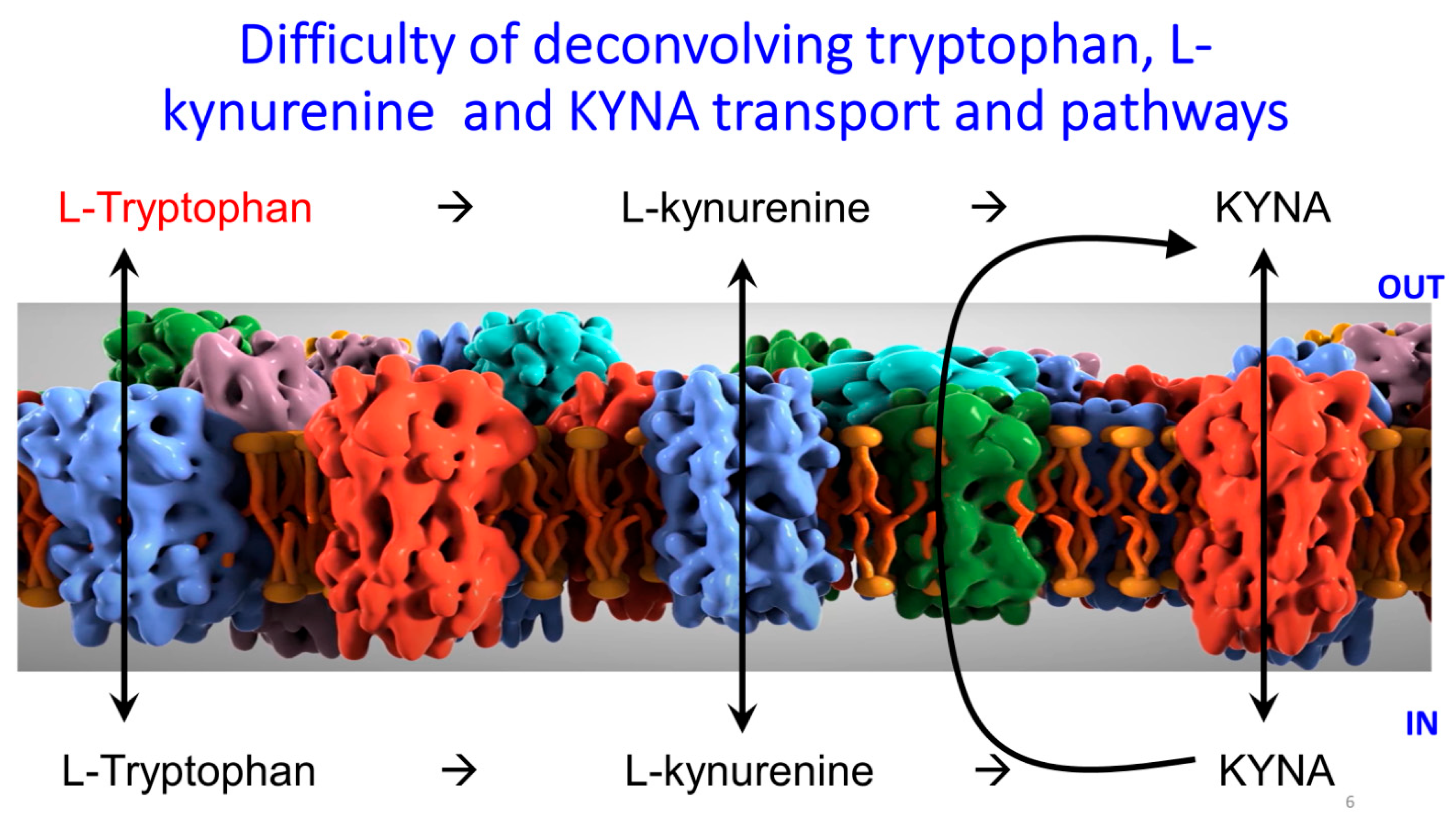
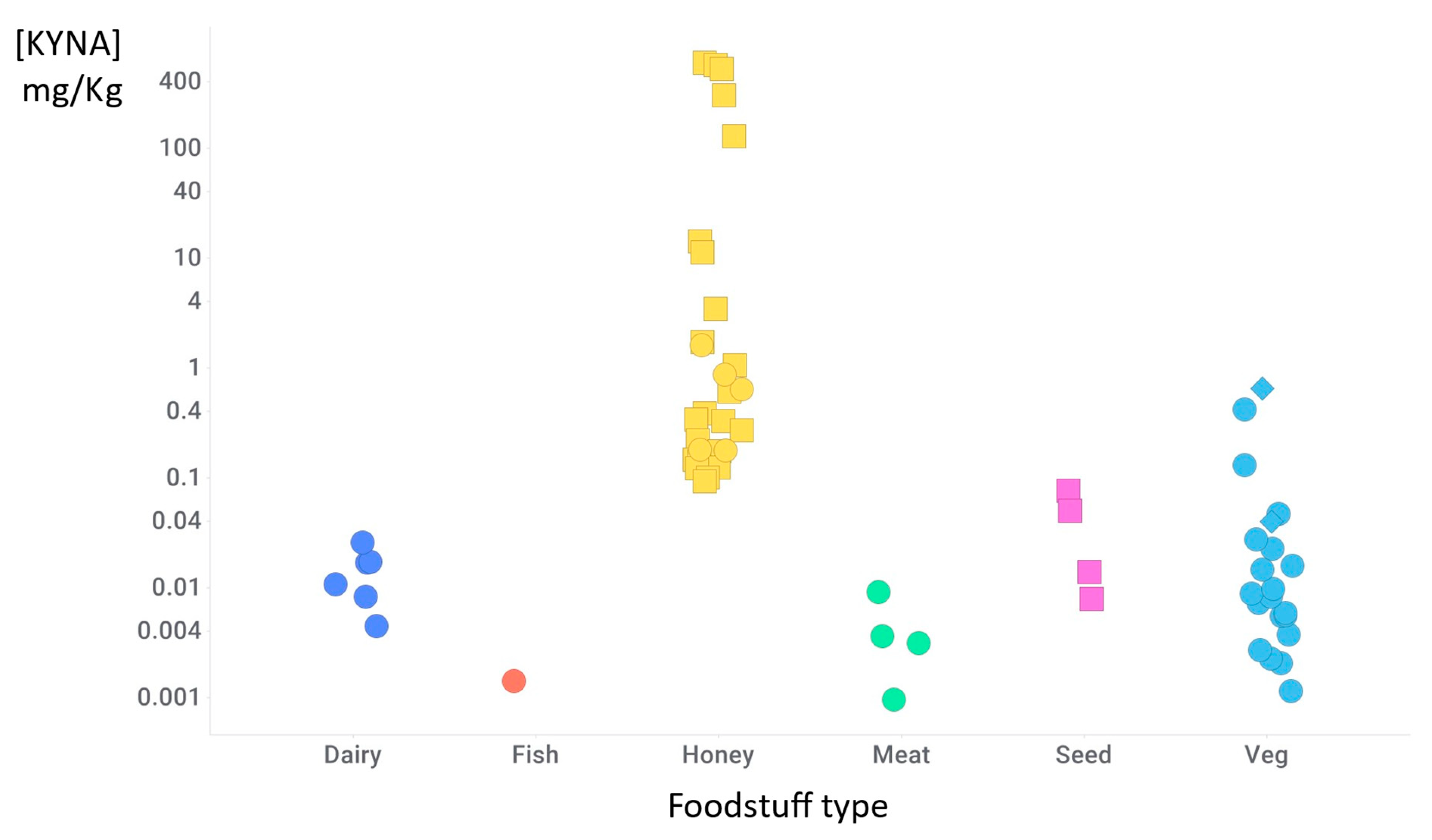

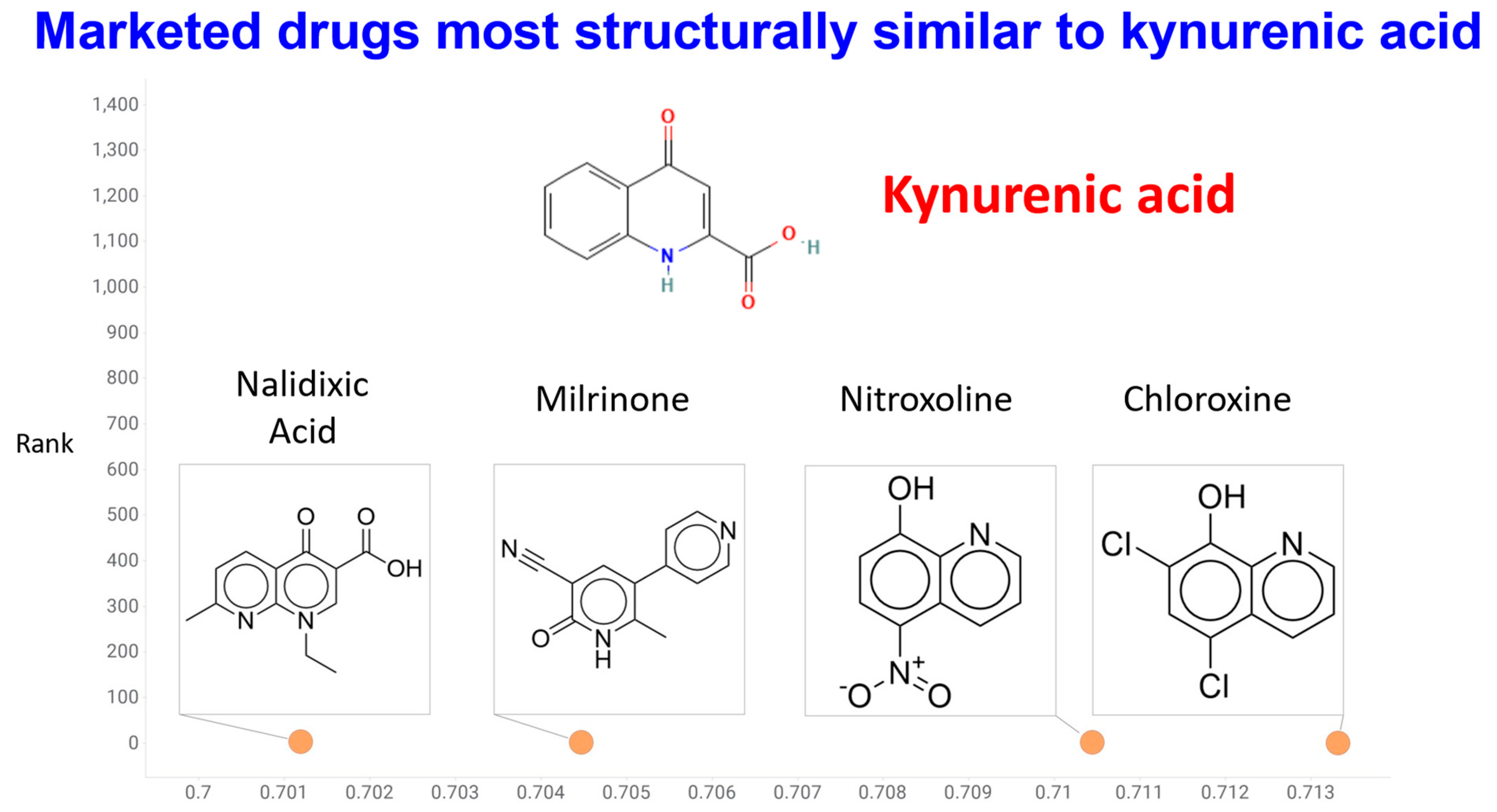
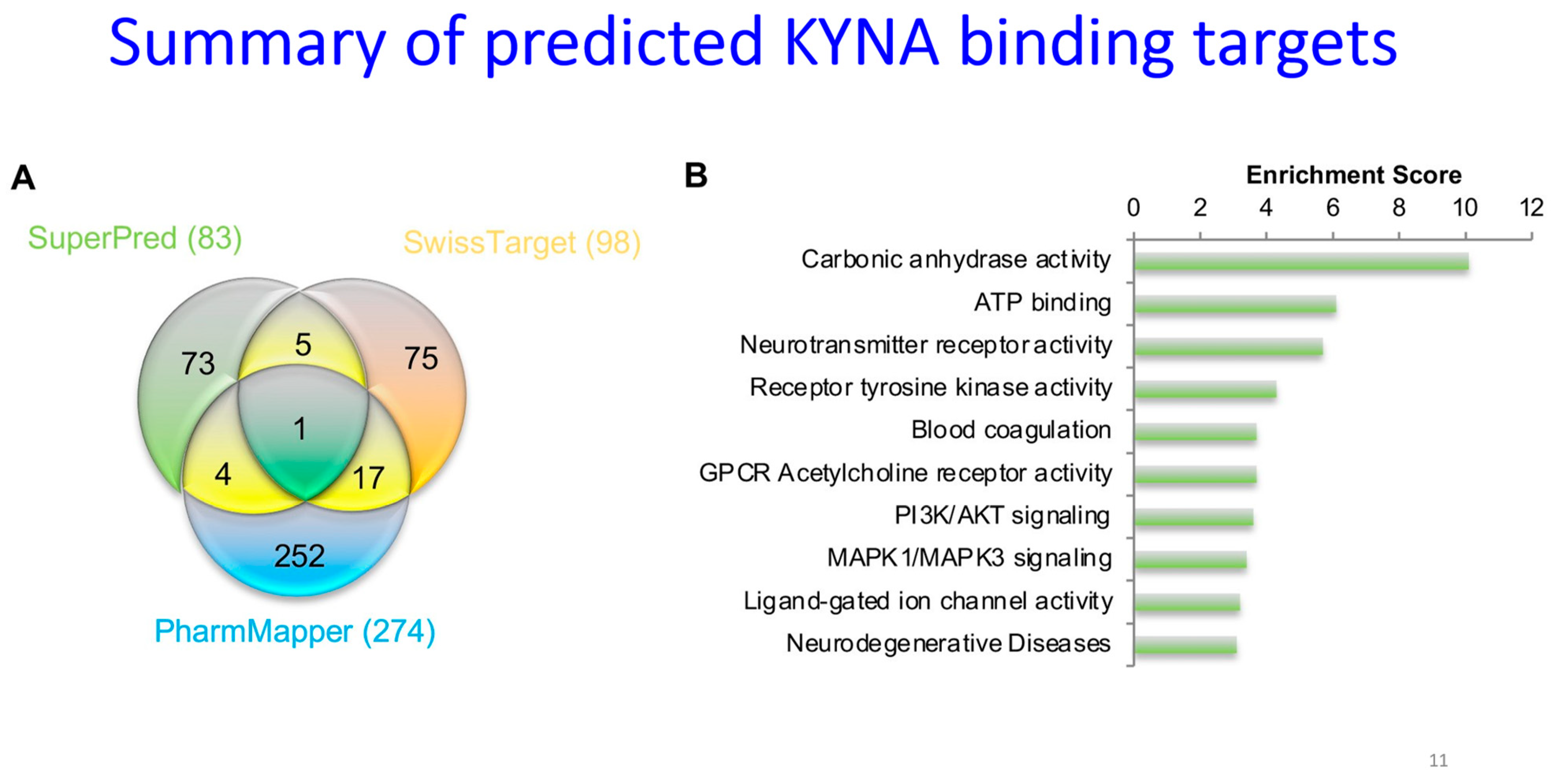
| Molecule | Comments | Selected References |
|---|---|---|
| Ceefourin-1 | Highly selective inhibitor of ABCC4 | [271,272,273] |
| Leukotrienes B4/C4 | Km 0.1–5.6 μM | [274,275,276] |
| MK-571 (Verlukast) | Inhibitor. Also a quinoline with a carboxylate group. Commonly more potent than probenecid. Also inhibits MRP1. | [266,277,278,279] |
| Probenecid | Inhibits multiple transporters. Ki for SLC22A6/8~15 μM | [280,281,282] |
| Quercetin | Ki 1 μM (and other related polyphenols) | [283] |
| Reviews | [251,284,285,286] | |
| Sulindac | Ki 2 μM | [287] |
| Urate | Pertinent in gout and kidney disease | [263] |
| Serum | ||
|---|---|---|
| Concentration Range | Comments | References |
| 42 nM median | Approximately doubled in severe, acute COVID-19 | [331] |
| 42 nM (35–54 nM IQR) | Increase with age noted | [332] |
| 47 nM median | Marginally lower in ADHD | [333] |
| 38 nM mean | Unchanged by inflammatory bowel diseases | [334] |
| 10-60 nM | [335] | |
| 60 nM median | Lower in ALS (ca 40 nM) | [336] |
| 23 nM | No different in pre-eclampsia | [337] |
| 38 nM | Higher in erythrocytes of Parkinson’s disease, implying synthesis or concentration | [338] |
| 28 nM average | Insignificantly lower in depression | [339] |
| Mean 25 nM, 16 nM in gestational diabetes (GD) | L-kynureine was higher in the GD individuals | [340] |
| 100 nM | Maternal serum; 3–4-fold higher in cord blood | [341] |
| Plasma | ||
| Concentration range | Comments | References |
| 40 nM | 15% decrease in migraine | [342] |
| ~30 nM | [343] | |
| ~40 nM | No effect in depression | [344] |
| 30–40 nM | Small increase with ibuprofen | [345] |
| ~40 nM | Increased 63% after endurance exercise (150 km road race) | [215] |
| 44 nM median | No change in migraines | [346] |
| 10–80 nM | Median 38 nM; no difference in bipolar individuals | [347] |
| 39–54 nM median | Slightly greater with age and male gender | [348] |
| ~50 nM | Increased 3-fold when SLC22A6/8 inhibited by addition of probenecid | [349] |
| 18–350 nM | Pregnant women, 18–20 weeks, NB concentrated 20-fold in cord blood | [294] |
| 4–60 nM | A summary of multiple measurements | [350] |
| 23 nM mean | [351] | |
| ~20 nM | Chinese population | [352] |
| 70 nM in controls, 104 nM in those with social anxiety disorder | Increased with age in controls, but no relation with age in social anxiety disorder | [353] |
| Mean 21 nM, 23 nM in women with pre-eclampsia (PE) | Strongly influenced by BMI, that may have been a confounder; raised level suggested as a response to the PE rather than a cause | [354] |
| CSF | ||
| Concentration range | Comments | References |
| ~20 nM in control | Increased 3-fold in Alzheimer’s | [355] |
| 1–4 nM | Medan 38 nM; no difference in bipolar individuals | [336] |
| 5 nM | Stable post mortem | [356] |
| 2–5 nM | Strong positive correlation with age | [357] |
| 1–50 nM | Can be raised strongly by certain alleles of KAT II | [358] |
| Vegetable | Comments | Selected References |
|---|---|---|
| Broccoli | 0.41 mg/kg | [307] |
| Chestnut honey | 129–601 mg/kg | [392] |
| Chestnut honey | ~400 mg/kg | [393] |
| Chestnut honey | Up to 2000 mg/kg | [308] |
| Flower honeys (various) | 0.1–2 mg/kg | [392] |
| Herbs of various kinds | Dandelion leaves 0.5 mg/kgww St John’s wort 32 μg/dose | [386] |
| Horseshoe crab extract | 1200 mg/kg (0.12%) | [360] |
| Potato tubers | 0.1–3.2 mg/kg | [307] |
| 0.3–3 mg/kg across 16 cultivars | [391] | |
| 3× greater in purple potatoes | [37] | |
| Review | [308] |
| Disease or Syndrome | Source, and Raised or Lowered | Selected References |
|---|---|---|
| Acute liver failure | Raised in rat brain as a consequence, via increase in kynurenine in the periphery | [424] |
| Alzheimer’s dementia | Plasma one third lower Plasma marginal effect | [343,355] |
| Serum lower | [425] | |
| CSF lower | [328,426] | |
| CSF higher, but seemingly protective vs. disease progression | [427] | |
| Review | [428] | |
| Aortic stiffness | Correlation of KYNA levels in patients with atrial fibrillation | [429] |
| Attention deficit hyperactivity disorder (ADHD) | Significantly lowered (meta-analysis of 650 individuals) | [430] |
| Bipolar depression | ~40% decreased vs. controls | [431] |
| Review; very variable, mostly lower | [432,433] | |
| Cancers | Very heterogeneous. Inhibition of proliferation observed at very high doses. | [434,435,436] |
| Cluster headaches and migraines | Serum ~one third lower | [437,438] |
| COVID-19 | Raised in serum, especially in more severe acute cases | Reviewed by [439,440], and see next section |
| Familial Mediterranean fever | KYNA decreased | [441] |
| Frailty | Lowered in frailty or little change | [329,442] |
| Huntington’s disease | Cerebral cortex—four-fold reduction. One molecule (laquinimod) targeting the aryl hydrocarbon receptor in clinical trials | [443,444,445] |
| Inflammatory bowel disease | Seen as a protective mechanism via raised levels of KATs | [334,446] |
| Irritable bowel syndrome | Lowered in serum | [447] |
| Lowered in urine | [448] | |
| Kidney disease | Normal serum level of 28 nM increased to 336 nM in renal insufficiency | [449] |
| Significantly raised in non-survivors of septic shock with acute kidney injury | [450] | |
| Chronic kidney disease, plasma levels can exceed 500 nM, along with high levels of other tryptophan metabolites | [263,451] | |
| End-stage kidney disease, plasma levels can exceed 1 μM | [263] | |
| Significantly raised in renal failure | [452] | |
| Major depressive disorder | 30% reduced over healthy controls; seen as the only metabolic biomarker that is both diagnostic and predictive. ~40% decreased over controls Antidepressant activity in mice (at high concentrations) Considered to be related to poor Western diet | [431,453,454,455] |
| Lowered in cortex, raised in serum of rats undergoing chronic restraint stress | [456] | |
| Migraine | 15% lower KYNA/KYN halved | [342,457] |
| Significantly lower, acting via multiple receptors | [458] | |
| Multiple sclerosis | Plasma 45 → 77 nM | [459] |
| Erythrocytes 38 → 63 nM | [459] | |
| Raised in relapsing-remitting MS, lowered in primary and secondary progressive. Quinolinic acid seems to be the real culprit here. | [460,461] | |
| Lowered in CSF vs. other neurological diseases | [462] | |
| Myalgic encephalopathy/chronic fatigue syndrome (ME/CFS) | Significantly lowered in some tissues, raised in others | [463,464,465] |
| Osteoporosis | Potential for treatment | [466] |
| Parkinson’s disease (Review [467]) | Frontal cortex—~one third of controls | [468] |
| Plasma | [338] | |
| Serum | ||
| Substantia nigra—less than half that of controls | [468] | |
| CSF lower | [328] | |
| Polycystic kidney disease | Significantly raised | [469] |
| Polycystic ovary syndrome | Roughly doubled | [470] |
| Pre-eclampsia | Very nonlinear, but significantly raised (especially in those with high BMI) in one Norwegian birth cohort study | [354] |
| No effect in a variety of other studies reviewed in: | [337,471] | |
| Pulmonary arterial hypertension | Raised, as were a great many other L-kynurenine pathway metabolites | [472] |
| Schizophrenia and bipolar disorder | Raised significantly (though usually so are other molecules such as L-kynurenine (which may be the real effector and/or changed by inhibitors of KATII) Lowered in some studies Meta-analysis implies no real or obvious difference | [196,198,199,433,473,474,475,476,477,478,479] |
| Sjögren’s syndrome | KYNA somewhat raised | [480] |
| Systemic lupus erythematosus (Lupus) | Many kynurenine pathway metabolites raised | [481] |
| Type 1 diabetes | Steptozotocin-induced diabetes in rats led to a modest increase in KYNA | [482] |
| Type 2 diabetes | Raised from 36 to 46 nM | [483] |
| Said to be raised during progression | [484] | |
| Ulcerative colitis | Raised, seen as likely coming from changes in microbial gut metabolism. Protection considered to be via GPR35 | [377,485] |
| System | Comments | Selected References |
|---|---|---|
| Anticonvulsants | Possible model of mediation via KYNA | [509,510,511] |
| Depression | Considered neuroprotective in depression | [512] |
| Epileptic spasms | Lower KYNA in epileptic spams that in other non-inflammatory neurological diseases | [513] |
| Excitotoxic challenges | KYNA protective | [514,515] |
| Experimental autoimmune encephalomyelitis | Protective against a Th17 response | [379] |
| Ischemia-reperfusion | Gerbil brain. Massive doses led to very high intracerebral concentrations of KYNA and neuroprotection | [516] |
| Highly protective in a model of hypoxic ischemia in neonatal rats | [517,518] | |
| Kynurenine sulphate produces KYNA that is neuroprotective in gerbils and rats | [519,520] | |
| Memory enhancement | Effective at lower doses in mice Opposite effect in C. elegans | [521,522] |
| Migraine | Seems to be protective via inhibition of glutaminergic neurons | [523,524,525,526] |
| Multiple sclerosis | Considered protective | [527,528] |
| Plasma 45 → 77 nM | [459] | |
| Erythrocytes 38 → 63 nM | [459] | |
| Pain | Protective against neuropathic pain, and enhances effectiveness of morphine | [529] |
| Antinociceptive in inflammatory pain | [530] | |
| Reviews | [531,532,533] | |
| Spinal cord injury (SCI) | Protective (with glucosamine) against SCI in rats | [534] |
| Stroke | Mostly protective if given ahead of experimental stroke. Naturally protective against death after adjusting for inflammation Higher levels associated with better recovery | [432,535,536] |
| Traumatic brain injury | KYNA is overexpressed, attenuates this in rats | [537,538] |
| Putative Receptor | Comments | Selected References |
|---|---|---|
| Adrenoceptor alpha 2B (ADRA2B)—Putative ligand | Note that guanfacine is an FDA-approved agonist, used successfully vs. attention deficit hyperactivity disorder (and interestingly also vs. hypertension [575]) | [576,577] |
| Review | [578] | |
| Identified in a high-throughput CRISPR screen | [579] | |
| Aryl hydrocarbon receptor (AhR)—Agonist | Induces various pathways, including IL-6 production, at 100 nM | [580,581] |
| Protects against intestinal C. albicans infection via AhR | [582] | |
| Possible role in COVID-19 | [439,583] | |
| Protective against acute lung injury | [562] | |
| Removing AhR raises KYNA levels, and these are neuroprotective against excitotoxic insults | [93] | |
| KYNA affect neural plasticity via AhR in zebrafish | [584] | |
| Involved in fibrosis and skin disease | [585] [586] | |
| G-protein-coupled receptor 35 (GPR35)—Agonist | Regulates energy metabolism and ablates weight gain on high-fat diet in mice | [92,213,360,587] |
| Protection against ischemic injury (at 5 mg/kg, ≡26 μM if homogeneous) | [588] | |
| Reviews | [589,590,591] | |
| Glutamate receptor (GAR)—antagonist | Many papers relating to migraines, e.g., | [523,524,525,526] |
| Hydroxycarboxylic acid receptor 3 (HCAR3)—putative ligand | Identified in a high-throughput CRISPR screen | [579] |
| N-methyl D-aspartate (NMDA) receptor (especially the strychnine-sensitive glycine-binding site) (involved in pain [592])—antagonist | EC50 7 μM though binding curves and very complex effects | [69,593,594] |
| Potential role in nutritional signalling | [595] | |
| 10 nM can affect differentiation of cortical cells | [596] | |
| Electrophysiological effects observable at high concentrations | [597] | |
| Reviews | [598,599,600] | |
| alpha-7 nicotinic acetylcholine receptor (α7nAChR)—purported antagonist | Electrophysiological effects not observed even at high concentrations | [597] |
| Active at 7 μM in hippocampal neurons | [601] | |
| No physiological effects observed with KYNA | [602] | |
| Lowers inflammatory cytokine production and Abeta phagocytosis | [603] |
| Substance | Comments | Selected References |
|---|---|---|
| Amphetamines | Significant decrease after dosing with amphetamine | [616] |
| Anthocyanins (in blackberry extract) | Microbiome said to be responsible for increasing KYNA levels (though in the LC-MS data neither the apparent retention time nor the reported mass of the positive molecular ion (m/z = 208; true is 190) underlying this is that of KYNA) | [617] |
| Antidiabetic agents | Glibenclamide and metformin both decrease KYNA levels, likely by different mechanisms | [618] |
| Exercise | Exercise can stimulate the production of katG enzymes and thereby raise KYNA (and lower central L-kynureine), making KYNA an ‘exerkine’. Data are somewhat mixed | [619,620,621] |
| KAT4 was especially strongly stimulated in endurance exercise, leading in some cases to more than a 60% increase in plasma KYNA levels | [215] | |
| Exercise increases KYNA and its activation of the AhR receptor | [622] | |
| Fasting | 8 day fasting increased KYNA levels. Effect observable even at 2 days | [623,624] |
| Hypothyroidism | Experimentally induced hypothyroidism leads to brain KYNA levels being raised | [625] |
| Insulin signalling | Tested in C. elegans, caused increases in KYNA | [522] |
| Interferon-γ | Massive increase in KYNA in neurons and astrocytes | [626,627] |
| Ketone bodies | β-hydroxybutyrate stimulates KYNA synthesis and provides neuroprotection | [514,515,628,629] |
| LPS treatment | Lowers brain KYNA | [630] |
| Obesity | Higher serum levels of several KP metabolites, including KYNA; in some cases, may simply reflect higher fluxes from raised dietary intake | [631] |
| Estrogens (as oral contraceptive agents) | Decreased from a median of 58 → 33 nM in plasma | [632] |
| Progesterone partial reverses interferon-γ-induced decrease in KYNA | [633] | |
| Hericium erinaceus polysaccharides | Hepatoprotective vs. non-alcoholic fatty liver disease, and increase KYNA levels | [634] |
| Stress | Stress increases KYNA levels in rats, who show lower cognitive skills; unclear whether KYNA response is causally involved or an attempt to counteract. KYNA was not added independently Hepatic KYNA lowers anxiety-induced stress | [635,636] |
| Pathway | Comments | Selected References |
|---|---|---|
| Amyloid (Aβ) fibrillation and toxicity in C. elegans | Inhibited by KYNA, as it was by some simple analogues | [637] |
| Anti-inflammatory | Includes effects on histone methylation | [638] |
| Reverses effects of LPS in macrophage cultures | [639] | |
| Lowers inflammatory phagocytic response in mouse macrophages | [640,641] | |
| Lowers LPS-induced inflammation | [642] | |
| Lowers experimentally induced inflammation in the trigeminal ganglion | [643] | |
| Hydrogels containing KYNA lowers experimentally induced inflammation | [644] | |
| Anxiolytic (reduces anxiety) | Notable effects in zebrafish at 105 μM (20 mg/L) | [645] |
| Apoptosis | Induced by KYNA | [646] |
| 270 genes differentially expressed after exposure to 0.25 mM KYNA | [647] | |
| Astrocyte activation | Inhibitory and protects against HIV-induced cognitive loss | [648] |
| DNA excision repair | KYNA increases pathway transcription | [649] |
| Endothelial damage (induced by homocysteine) | Protective | [650] |
| Enhances endothelial adhesion and spreading | [651] | |
| Fibroblast growth factor release from HUVEC cells | Inhibited at “low” concentrations (1 μM) of KYNA, while proliferation rate increased. | [652] |
| Glutamate release | Lowered by KYNA | [653] |
| Hypertension | Heart rate lowered by KYNA in spontaneously hypertensive rats | [654] |
| Indoleamine 2,3-dioxygenase induction | Signalling role | [562,655] |
| Insulin resistance | Protective in high-fat-diet-induction model | [656] |
| Interleukins | Lowered IL17/IL23 at high concentrations | [657] |
| Mitochondrial induction | Acts as a cardioprotective | [557] |
| Neprilysin | Neprilysin degrades amyloids, and KYNA induces its synthesis and is neuroprotective | [658] |
| TNF-α production | Decreased at very high concentrations | [659,660,661] |
| Unfolded protein response | KYNA inhibits, and is protective in a C. elegans Alzheimer’s model | [662] |
| Vasculature | Induces vascular relaxation in endothelial cells | [663] |
| Organism | Dose and Comment | Selected References |
|---|---|---|
| Gerbils | 400–1600 mg/kg; protected against ischemia-reperfusion injury | [516] |
| Mice | 250 mg/L in drinking water, ≡25 mg/kg/d, has no toxic effects | [640,641] |
| 25–250 mg/L drinking water 3–21 d; well tolerated. | [665] | |
| Rat | 500 mg/kg i.p.—protected against thioacetamide-induced liver injury | [666] |
| 300 mg/kg i.p. in young rats prolonged wakefulness | [323,667] | |
| 150 mg/kg lowers morphine-conditioned reward behavior | [668] | |
| 25 mg/kg/d in drinking water ≡ ~250 mg/L, assists healthy growth in rat babies | [359] | |
| 300 mg/kg i.p. protect against mussel toxin | [554] | |
| 200 mg/kg kynuramine | [669] | |
| 300 mg/kg vs. acute pancreatitis | [564] | |
| 300 mg/kg in young rats was neuroprotective | [517,520] | |
| As much as 5% of diets, with large KYNA excretion, small decrease in weight gain, but seemingly without major ill effects. | [670,671] | |
| Mammalian cell lines | ||
| Murine RAW 264.7 macrophages | 100 μM KYNA is protective against LPS challenges | [639] |
| Murine BV-2 microglial cells | No effect on viability of 100 μM KYNA | [603] |
| Rat splenocytes | No effects on viability of proliferation at 500 μM | [641] |
| Humans | Topical application; no safety issues observed. Most secreted in urine | [551] |
| General Biological Area | Comments | Selected References |
|---|---|---|
| Cardiovascular disease | Marginally associated with (a 10% increase) in all-cause, but likely confounded with L-kynurenine that is more significant | [568] |
| Schizophrenia | KYNA levels often raised, though unclear if this is a cause or an attempted detoxification of raised L-kynurenine. Some studies showed them lowered; overall unclear. See also the text. | [196,473,474,476,478,684] |
| Protein Names | UniProt ID | Entry Name | Predicted to Bind KYNA by |
|---|---|---|---|
| Thyroid hormone receptor alpha | P10827 | THA_HUMAN | SuperPred, SwissTarget, PharmMapper |
| Glutathione S-transferase P | P09211 | GSTP1_HUMAN | SuperPred, PharmMapper |
| Inosine-5′-monophosphate dehydrogenase 2 | P12268 | IMDH2_HUMAN | SuperPred, PharmMapper |
| Galectin-3 | P17931 | LEG3_HUMAN | SuperPred, PharmMapper |
| Histone deacetylase 8 | Q9BY41 | HDAC8_HUMAN | SuperPred, PharmMapper |
| Gamma-aminobutyric acid receptor subunit alpha-1 | P14867 | GBRA1_HUMAN | SuperPred, SwissTarget |
| D-amino-acid oxidase | P14920 | OXDA_HUMAN | SuperPred, SwissTarget |
| Amine oxidase (flavin-containing) A | P21397 | AOFA_HUMAN | SuperPred, SwissTarget |
| Aryl hydrocarbon receptor | P35869 | AHR_HUMAN | SuperPred, SwissTarget |
| Dual specificity protein kinase CLK4 | Q9HAZ1 | CLK4_HUMAN | SuperPred, SwissTarget |
| Prothrombin Thrombin heavy chain | P00734 | THRB_HUMAN | SwissTarget, PharmMapper |
| Renin | P00797 | RENI_HUMAN | SwissTarget, PharmMapper |
| Carbonic anhydrase 1 | P00915 | CAH1_HUMAN | SwissTarget, PharmMapper |
| Carbonic anhydrase 2 | P00918 | CAH2_HUMAN | SwissTarget, PharmMapper |
| Thymidylate synthase | P04818 | TYSY_HUMAN | SwissTarget, PharmMapper |
| Lymphocyte specific tyrosine kinase | P06239 | LCK_HUMAN | SwissTarget, PharmMapper |
| Neprilysin | P08473 | NEP_HUMAN | SwissTarget, PharmMapper |
| Leukotriene A-4 hydrolase | P09960 | LKHA4_HUMAN | SwissTarget, PharmMapper |
| Thyroid hormone receptor beta | P10828 | THB_HUMAN | SwissTarget, PharmMapper |
| Angiotensin-converting enzyme | P12821 | ACE_HUMAN | SwissTarget, PharmMapper |
| Farnesyl pyrophosphate synthase | P14324 | FPPS_HUMAN | SwissTarget, PharmMapper |
| Neutrophil collagenase | P22894 | MMP8_HUMAN | SwissTarget, PharmMapper |
| Macrophage metalloelastase | P39900 | MMP12_HUMAN | SwissTarget, PharmMapper |
| Aldo-keto reductase family 1 member C3 | P42330 | AK1C3_HUMAN | SwissTarget, PharmMapper |
| Mitogen-activated protein kinase 10 | P53779 | MK10_HUMAN | SwissTarget, PharmMapper |
| Peroxisome proliferator-activated receptor alpha | Q07869 | PPARA_HUMAN | SwissTarget, PharmMapper |
| Estrogen receptor beta (ER-beta) | Q92731 | ESR2_HUMAN | SwissTarget, PharmMapper |
| Organism | Genetic Modification(s) | Titer | Conditions and Comments | References |
|---|---|---|---|---|
| Escherichia coli | Remove competing pathway, enhance SAM pathway | 350 mg/mL | Main target was actinocin | [780] |
| Saccharomyces cerevisiae | None, but a chemical defined medium including 400 mg/L tryptophan was used | 9 mg/L | [781] | |
| ~1.5 μM (~280 ng/mL) | [782] | |||
| Yarrowia lipolytica | None | 21 μg/mL in culture broth or 494 μg/g cell dry weight | trp-supplemented media | [783,784,785] |
| Property | Ergothioneine | Kynurenic Acid |
|---|---|---|
| Overall status as a nutraceutical | Fairly well established [1,18,29,508,791,792,794,795] | Emerging [86,99,308,533] |
| Biosynthesized endogenously | No | Yes |
| Largest dietary source | Mushrooms (fruiting bodies of basidiomycetes) [29,796,797,798,799] | Chestnut honey [308,392] |
| Highest natural product level | ~9 g/kg [29,800] | ~0.6 g/kg [308,392] |
| Approximate serum/plasma level in ‘healthy’ populations | ~260 ng/mL, ~1.1 μM | ~40 nM |
| Degree of concentration in erythrocytes | Maybe as much as 10 times [801] | Possibly 3× but unclear how calculated [425]; no increase in a related study [459] |
| Known ‘uptake’ transporters in humans | Relatively specific and concentrative e.g., SLC22A4 [802,803], SLC22A15 [804] | Those known are non-specific and non-concentrative [235,236] |
| Concentrated in erythrocytes as a kind of ‘buffer’ for plasma | Significantly (up to ten-fold [801,805]), probably accumulated via SLC22A4 [806] | Much less so (note that RBC express ABCC4 [807]) |
| Known ‘efflux’ (pump) transporters in humans | Not known | ABCC4 [242,696] and possibly ABCG2 [263,264] |
| Known transporters in microorganisms | Several, e.g., [778,808,809] | None seemingly published |
| Thermostability | High (can be extracted at 95 °C [810]) | High (stable to boiling and frying [37]), and in blood samples [699] |
| Pharmacokinetics studies | Somewhat, with human feeding trials for 35 d [319] | Not really initiated orally |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, L.d.F.; Moore, J.B.; Kell, D.B. The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects. Int. J. Mol. Sci. 2024, 25, 9082. https://doi.org/10.3390/ijms25169082
Alves LdF, Moore JB, Kell DB. The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects. International Journal of Molecular Sciences. 2024; 25(16):9082. https://doi.org/10.3390/ijms25169082
Chicago/Turabian StyleAlves, Luana de Fátima, J. Bernadette Moore, and Douglas B. Kell. 2024. "The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects" International Journal of Molecular Sciences 25, no. 16: 9082. https://doi.org/10.3390/ijms25169082
APA StyleAlves, L. d. F., Moore, J. B., & Kell, D. B. (2024). The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects. International Journal of Molecular Sciences, 25(16), 9082. https://doi.org/10.3390/ijms25169082






