Assessment of DNA/RNA Defend Pro: An Inactivating Sample Collection Buffer for Enhanced Stability, Extraction-Free PCR, and Rapid Antigen Testing of Nasopharyngeal Swab Samples
Abstract
1. Introduction
2. Results
2.1. Rapid Antigen Testing (RAT)
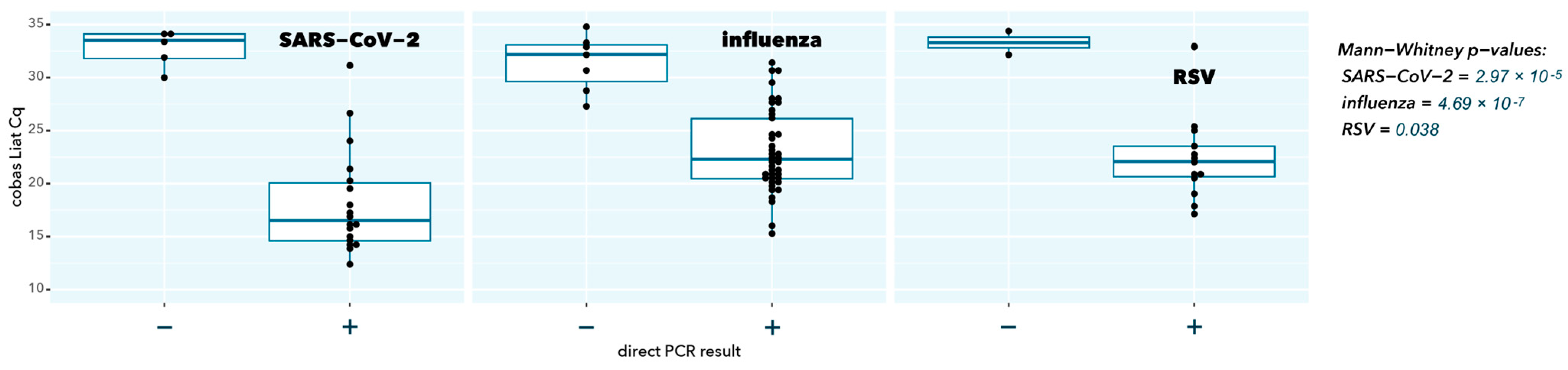
2.2. Direct RT-qPCR
2.3. RNA Stability
3. Discussion and Conclusions
4. Materials and Methods
4.1. Sample Collection
4.2. Nucleic Acid Amplification Testing
4.3. Rapid Antigen Testing
4.4. Extraction-Free Direct RT-qPCR
4.5. Stability Testing
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Diagnostic Testing for SARS-CoV-2, Interim Guidance. Available online: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2 (accessed on 10 March 2024).
- Verwilt, J.; Vandesompele, J. Evaluation of efficiency and sensitivity of 1D and 2D sample pooling strategies for SARS-CoV-2 RT-qPCR screening purposes. Sci. Rep. 2022, 12, 6603. [Google Scholar] [CrossRef] [PubMed]
- Bouttell, J.; Hawkins, N. Evaluation of Triage Tests When Existing Test Capacity Is Constrained: Application to Rapid Diagnostic Testing in COVID-19. Med. Decis. Making 2021, 41, 978–987. [Google Scholar] [CrossRef] [PubMed]
- van Bockel, D.; Munier, C.M.L.; Turville, S.; Badman, S.G.; Walker, G.; Stella, A.O.; Aggarwal, A.; Yeang, M.; Condylios, A.; Kelleher, A.D.; et al. Evaluation of Commercially Available Viral Transport Medium (VTM) for SARS-CoV-2 Inactivation and Use in Point-of-Care (POC) Testing. Viruses 2020, 12, 1208. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Bashir, R. Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Ultrasensitive Detection of SARS-CoV-2 in Saliva and Viral Transport Medium Clinical Samples. Anal. Chem. 2021, 93, 7797–7807. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.L.; Seedhouse, C.H. Comparative effects of viral-transport-medium heat inactivation upon downstream SARS-CoV-2 detection in patient samples. J. Med. Microbiol. 2021, 70, 001301. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.J.; Keynan, Y. Extraction-free RT-LAMP to detect SARS-CoV-2 is less sensitive but highly specific compared to standard RT-PCR in 101 samples. J. Clin. Virol. 2021, 136, 104764. [Google Scholar] [CrossRef] [PubMed]
- Toptan, H.; Karabay, O. Evaluation of Using Direct Viral Transport Medium Samples without Nucleic Acid Isolation for SARS-CoV-2 Diagnosis by RT-PCR. Clin. Lab. 2022, 68, 2213. [Google Scholar] [CrossRef] [PubMed]
- Krijger, P.H.L.; Tanenbaum, M.E. A public-private partnership model for COVID-19 diagnostics. Nat. Biotechnol. 2021, 39, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Avalos, C.; Sandino, A.M. Analysis by real-time PCR of five transport and conservation mediums of nasopharyngeal swab samples to COVID-19 diagnosis in Santiago of Chile. J. Med. Virol. 2022, 94, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- InActiv Blue. InActiv Blue Buffer. Available online: https://www.inactivblue.com/inactiv-blue-buffer/ (accessed on 19 August 2024).
- InActiv Blue. DNA/RNA Defend Pro Buffer. Available online: https://www.inactivblue.com/product-dna-rna-defend-pro/ (accessed on 10 March 2024).
- Deprez, S.; Steyaert, S. Evaluation of a novel respiratory virus inactivating buffer for parallel RT-qPCR and quick antigen testing. medRxiv 2024. [Google Scholar] [CrossRef]
- Cobas SARS-CoV-2 & Influenza A/B [Package Insert V01]; Roche Molecular Systems, Inc.: Pleasanton, CA, USA, 2020.
- Fomsgaard, A.S.; Rosenstierne, M.W. An alternative workflow for molecular detection of SARS-CoV-2—Escape from the NA extraction kit-shortage, Copenhagen, Denmark, March 2020. Eurosurveillance 2020, 25, 2000398. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.W.; Chan, W.M. Evaluation of simple nucleic acid extraction methods for the detection of SARS-CoV-2 in nasopharyngeal and saliva specimens during global shortage of extraction kits. J. Clin. Virol. 2020, 129, 104519. [Google Scholar] [CrossRef] [PubMed]
- Samantha, A. Byrnes, Multiplexed and Extraction-Free Amplification for Simplified SARS-CoV-2 RT-PCR Tests. Anal. Chem. 2021, 93, 4160–4165. [Google Scholar] [CrossRef]
- InActiv Blue. DNA/RNA Defend Pro Instructions for Use (IFU). Available online: https://www.inactivblue.com/files/Instructions%20for%20use%20-%20RUO%20-%20DRDP%20-%20final%20version.pdf (accessed on 10 March 2024).
- Bustin, S.A.; Shipley, G.L. RT-qPCR Detection of SARS-CoV-2: No Need for a Dedicated Reverse Transcription Step. Int. J. Mol. Sci. 2022, 23, 1303. [Google Scholar] [CrossRef] [PubMed]
- Ngetsa, C.; Osoti, V. Validation of saline, PBS and a locally produced VTM at varying storage conditions to detect the SARS-CoV-2 virus by qRT-PCR. PLoS ONE 2023, 18, e0280685. [Google Scholar] [CrossRef]
- FDA List of Transport Media for COVID-19 Diagnostic Testing. Available online: https://www.dhs.gov/sites/default/files/publications/2021-hqfo-00070_-_records.pdf (accessed on 16 August 2024).
- Cobas® Influenza A/B & RSV, Nucleic Acid Test for Use on the cobas®Liat® System, Packages Insert. Available online: https://elabdoc-prod.roche.com/eLD/api/downloads/157bf550-b366-ed11-1a91-005056a71a5d?countryIsoCode=be (accessed on 27 May 2024).
- Cobas® SARS-CoV-2, Nucleic Acid Test for Use on the cobas®Liat® System, Packages Insert. Available online: https://elabdoc-prod.roche.com/eLD/api/downloads/480d0c41-74cd-ed11-1d91-005056a71a5d?countryIsoCode=be (accessed on 27 May 2024).
- Takara, One Step PrimeScript™ III RT-qPCR Mix, Product Malual. Available online: https://www.takarabio.com/documents/User%20Manual/RR601A/RR601A_UM.pdf (accessed on 27 May 2024).
- Bustin, S.A.; Benes, V. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; Li, Y. Switching gears for an influenza pandemic: Validation of a duplex reverse transcriptase PCR assay for simultaneous detection and confirmatory identification of pandemic (H1N1) 2009 influenza virus. J. Clin. Microbiol. 2009, 47, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- van Elden, L.J.; Nijhuis, M. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J. Clin. Microbiol. 2001, 39, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Colella, M. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. J. Clin. Microbiol. 2003, 41, 149–154. [Google Scholar] [CrossRef] [PubMed]


| Cobas Liat Positive | Cobas Liat Negative | Total | |
|---|---|---|---|
| rapid antigen test positive | 40 | 0 | 40 |
| rapid antigen test negative | 48 | 7 | 55 |
| total | 88 | 7 | 95 |
| Cobas Liat Positive | Cobas Liat Negative | Total | |
|---|---|---|---|
| direct RT-qPCR positive | 72 | 0 | 72 |
| direct RT-qPCR negative | 16 | 7 | 23 |
| total | 88 | 7 | 95 |
| SARS-CoV-2 | Influenza A | RSV A | |
|---|---|---|---|
| number of samples | 19 | 40 | 13 |
| mean delta-Cq (SEM) | −7.7 (0.45) | −9.61 (0.28) | −8.92 (0.61) |
| r (Spearman) | 0.940 | 0.916 | 0.791 |
| p (Spearman) | 1.10 × 10−6 | 2.20 × 10−16 | 2.08 × 10−3 |
| Samples Positive | |||||
|---|---|---|---|---|---|
| Day | |||||
| Target | Temperature | 0 | 1 | 3 | 7 |
| SARS-CoV-2 | 4 °C | 9 | 9 | 9 | 9 |
| 20 °C | 9 | 9 | 9 | 9 | |
| 37 °C | 9 | 9 | 9 | 9 | |
| influenza A | 4 °C | 13 | 13 | 13 | 13 |
| 20 °C | 13 | 13 | 13 | 13 | |
| 37 °C | 13 | 13 | 13 | 13 | |
| RSV A | 4 °C | 9 | 9 | 9 | 9 |
| 20 °C | 9 | 9 | 9 | 9 | |
| 37 °C | 9 | 9 | 8 | 9 | |
| Delta-Cq (SEM) | |||||
|---|---|---|---|---|---|
| Day | |||||
| Target | Temperature | 0 | 1 | 3 | 7 |
| SARS-CoV-2 | 4 °C | 0.00 (0.00) | 0.29 (0.59) | −0.10 (0.53) | 1.84 (0.35) |
| 20 °C | 0.00 (0.00) | 1.51 (0.62) | 1.51 (0.43) | 2.73 (0.58) | |
| 37 °C | 0.00 (0.00) | 1.66 (0.71) | 1.22 (0.63) | 2.01 (0.62) | |
| influenza A | 4 °C | 0.00 (0.00) | 0.04 (0.51) | −0.61 (0.49) | 0.92 (1.00) |
| 20 °C | 0.00 (0.00) | −1.36 (0.78) | 2.35 (0.43) | 1.09 (0.88) | |
| 37 °C | 0.00 (0.00) | 0.03 (0.77) | 1.19 (0.82) | −0.54 (1.02) | |
| RSV A | 4 °C | 0.00 (0.00) | 0.08 (0.70) | 1.00 (0.45) | 0.85 (0.69) |
| 20 °C | 0.00 (0.00) | 0.88 (0.70) | 0.28 (0.90) | 2.51 (0.61) | |
| 37 °C | 0.00 (0.00) | −0.67 (0.95) | 0.76 (0.90) | 6.59 (1.96) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claeys, M.; Al Obaidi, S.; Bruyland, K.; Vandecandelaere, I.; Vandesompele, J. Assessment of DNA/RNA Defend Pro: An Inactivating Sample Collection Buffer for Enhanced Stability, Extraction-Free PCR, and Rapid Antigen Testing of Nasopharyngeal Swab Samples. Int. J. Mol. Sci. 2024, 25, 9097. https://doi.org/10.3390/ijms25169097
Claeys M, Al Obaidi S, Bruyland K, Vandecandelaere I, Vandesompele J. Assessment of DNA/RNA Defend Pro: An Inactivating Sample Collection Buffer for Enhanced Stability, Extraction-Free PCR, and Rapid Antigen Testing of Nasopharyngeal Swab Samples. International Journal of Molecular Sciences. 2024; 25(16):9097. https://doi.org/10.3390/ijms25169097
Chicago/Turabian StyleClaeys, Mikhail, Saif Al Obaidi, Karen Bruyland, Ilse Vandecandelaere, and Jo Vandesompele. 2024. "Assessment of DNA/RNA Defend Pro: An Inactivating Sample Collection Buffer for Enhanced Stability, Extraction-Free PCR, and Rapid Antigen Testing of Nasopharyngeal Swab Samples" International Journal of Molecular Sciences 25, no. 16: 9097. https://doi.org/10.3390/ijms25169097
APA StyleClaeys, M., Al Obaidi, S., Bruyland, K., Vandecandelaere, I., & Vandesompele, J. (2024). Assessment of DNA/RNA Defend Pro: An Inactivating Sample Collection Buffer for Enhanced Stability, Extraction-Free PCR, and Rapid Antigen Testing of Nasopharyngeal Swab Samples. International Journal of Molecular Sciences, 25(16), 9097. https://doi.org/10.3390/ijms25169097






