GSDMD-Dependent Neutrophil Extracellular Traps Mediate Portal Vein Thrombosis and Associated Fibrosis in Cirrhosis
Abstract
1. Introduction
2. Results
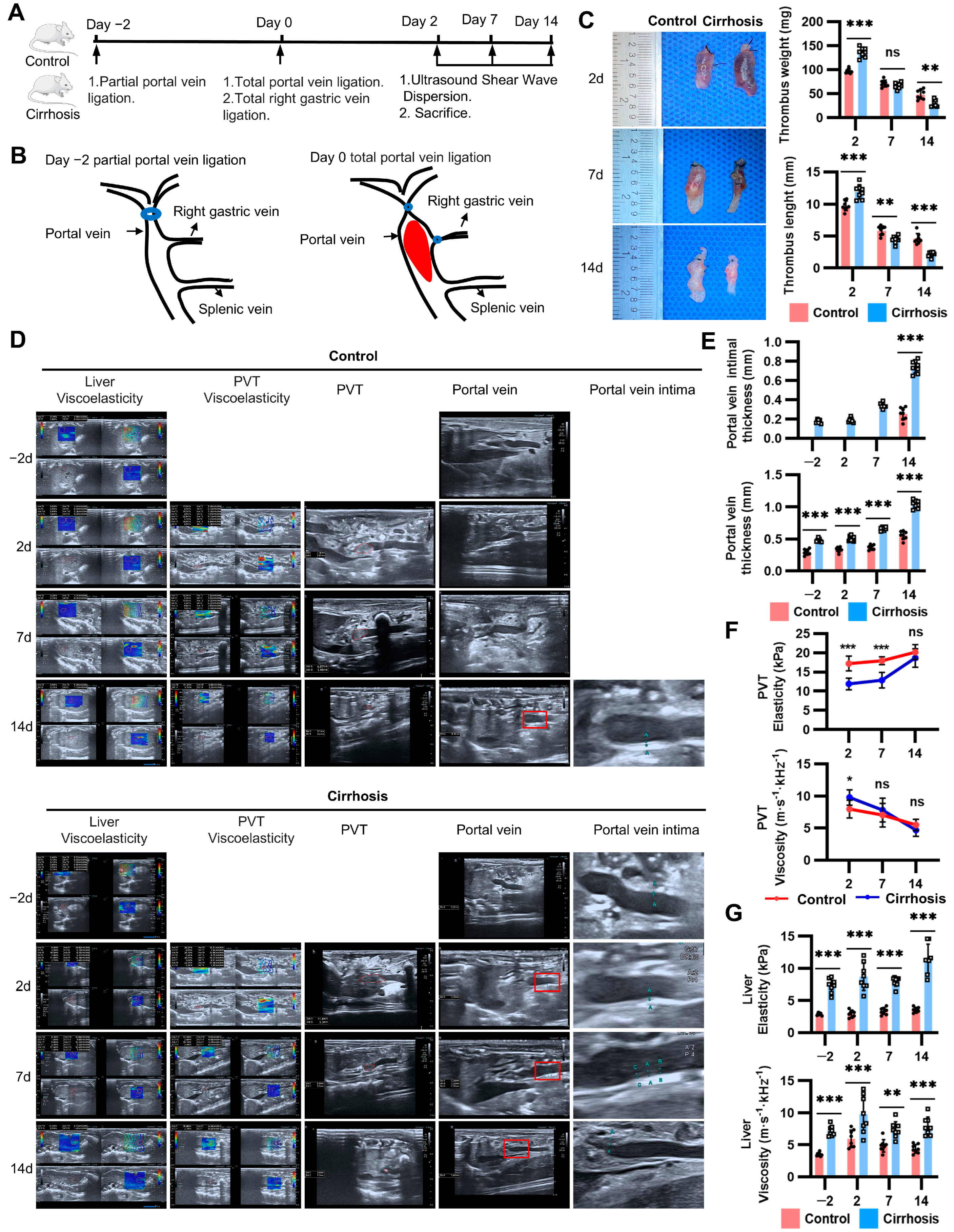
2.1. Natural Course of PVT in the New Model
2.2. Thrombus Instability Explained the Spontaneous Resolution of PVT in Cirrhosis
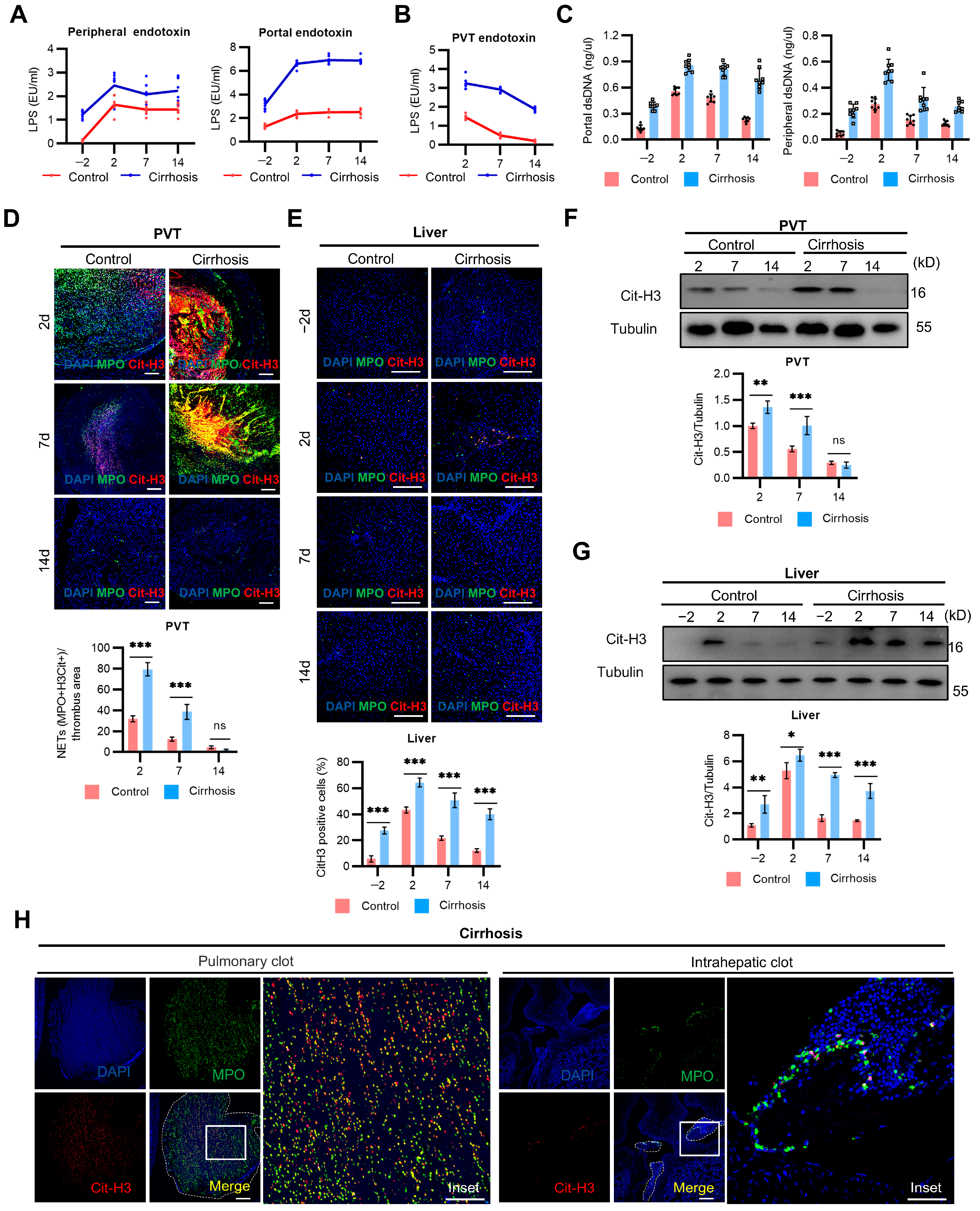
2.3. NETs Mediate PVT and Associated Inflammation in Cirrhosis
2.4. Caspase-4-Dependent Activation of Neutrophil GSDMD Promotes NETs Formation in Cirrhosis
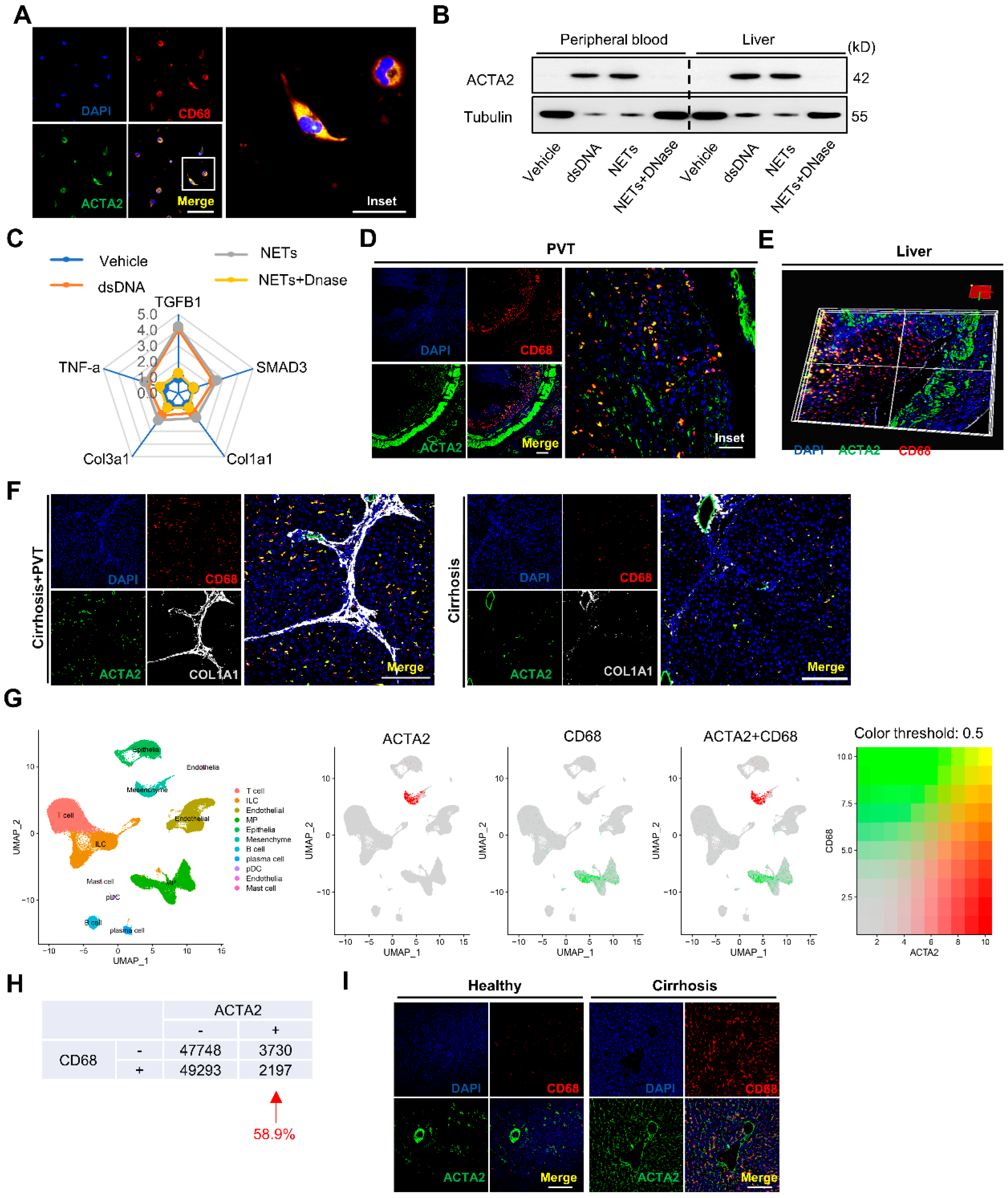
2.5. NETs Directly Promote Macrophage to Myofibroblast Transdifferentiation in Cirrhosis
2.6. GSDMD Inhibition with Disulfiram Inhibited Cirrhotic PVT and Associated Fibrosis
3. Discussion
4. Materials and Methods
4.1. Animal Models of Cirrhosis
4.2. PVT Model
4.3. DVT Model
4.4. Human Sample Collection
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costache, R.S.; Dragomirică, A.S.; Dumitraș, E.A.; Mariana, J.; Căruntu, A.; Popescu, A.; Costache, D.O. Portal vein thrombosis: A concise review. Exp. Ther. Med. 2021, 22, 759. [Google Scholar] [CrossRef]
- Carneiro, C.; Brito, J.; Bilreiro, C.; Barros, M.; Bahia, C.; Santiago, I.; Caseiro-Alves, F. All about portal vein: A pictorial display to anatomy, variants and physiopathology. Insights Imaging 2019, 10, 38. [Google Scholar] [CrossRef]
- Pandian, N.K.R.; Walther, B.K.; Suresh, R.; Cooke, J.P.; Jain, A. Microengineered human vein-chip recreates venous valve Architecture and its contribution to thrombosis. Small 2020, 16, 2003401. [Google Scholar] [CrossRef]
- Senzolo, M.; Garcia-Tsao, G.; García-Pagán, J.C. Current knowledge and management of portal vein thrombosis in cirrhosis. J. Hepatol. 2021, 75, 442–453. [Google Scholar] [CrossRef]
- Northup, P.G.; Garcia-Pagan, J.C.; Garcia-Tsao, G.; Intagliata, N.M.; Superina, R.A.; Roberts, L.N.; Lisman, T.; Valla, D.C. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the american association for the study of liver diseases. Hepatology 2021, 73, 366–413. [Google Scholar] [CrossRef]
- Driever, E.G.; von Meijenfeldt, F.A.; Adelmeijer, J.; de Haas, R.J.; Heuvel, M.C.v.D.; Nagasami, C.; Weisel, J.W.; Fondevila, C.; Porte, R.J.; Blasi, A.; et al. Nonmalignant portal vein thrombi in patients with cirrhosis consist of intimal fibrosis with or without a fibrin-rich thrombus. Hepatology 2021, 75, 898–911. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.-Y.; Wang, X.-B.; Zheng, X.; Huang, Y.; Chen, J.; Meng, Z.-J.; Gao, Y.-H.; Qian, Z.-P.; Liu, F.; et al. Prevalence and clinical significance of portal vein thrombosis in patients with cirrhosis and acute decompensation. Clin. Gastroenterol. Hepatol. 2020, 18, 2564–2572.e1. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Okugawa, H.; Takahashi, M.; Yokosuka, O. De novoportal vein thrombosis in virus-related cirrhosis: Predictive factors and long-term outcomes. Off. J. Am. Coll. Gastroenterol.|ACG 2013, 108, 568–574. [Google Scholar] [CrossRef]
- Nery, F.; Chevret, S.; Condat, B.; de Raucourt, E.; Boudaoud, L.; Rautou, P.; Plessier, A.; Roulot, D.; Chaffaut, C.; Bourcier, V.; et al. Causes and consequences of portal vein thrombosis in 1243 patients with cirrhosis: Results of a longitudinal study. Hepatology 2015, 61, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Zanetto, A.; Rodriguez-Kastro, K.-I.; Germani, G.; Ferrarese, A.; Cillo, U.; Burra, P.; Senzolo, M. Mortality in liver transplant recipients with portal vein thrombosis—An updated meta-analysis. Transpl. Int. 2018, 31, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, L.; Payancé, A.; Plessier, A.; D’alteroche, L.; Ronot, M.; Paradis, V.; Valla, D.; Rautou, P.-E. Management of splanchnic vein thrombosis. JHEP Rep. 2023, 5, 100667. [Google Scholar] [CrossRef]
- Qi, X.; Guo, X.; Yoshida, E.M.; Méndez-Sánchez, N.; De Stefano, V.; Tacke, F.; Mancuso, A.; Sugawara, Y.; Yang, S.-S.; Teschke, R.; et al. Transient portal vein thrombosis in liver cirrhosis. BMC Med. 2018, 16, 83. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, S.; Jiang, Y.-Y.; Ma, J.-Q.; Zhang, W.; Gu, J.-Y.; Wang, J.; Chen, S.-Y. A Preclinical porcine model of portal vein thrombosis in liver cirrhosis. BioMed Res. Int. 2020, 2020, 3086906. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Deng, T.; Luo, D.; Deng, Z.; Peng, C.; Chen, G.; Xiong, G. A new modified canine model of portal vein thrombosis. Acta Biochim. Biophys. Sin. 2021, 53, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, S.; Zhang, Y.; Wang, L.; Yan, C.; Xu, Z.; Zhao, Q.; Qi, X. Neutrophil extracellular traps formation may be involved in the association of propranolol with the development of portal vein thrombosis. Thromb. Res. 2024, 238, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dong, S.; Li, M.; Tao, Y.; Lv, J.; Liu, C. A new model of portal vein thrombosis in rats with cirrhosis induced by partial portal vein ligation plus carbon tetrachloride and intervened with rivaroxaban. BMC Gastroenterol. 2024, 24, 161. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Campreciós, G.; Pérez-Campuzano, V.; Orts, L.; García-Pagán, J.C.; Hernández-Gea, V. The pathophysiology of portal vein thrombosis in cirrhosis: Getting deeper into virchow’s triad. J. Clin. Med. 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Zocco, M.A.; Di Stasio, E.; De Cristofaro, R.; Novi, M.; Ainora, M.E.; Ponziani, F.; Riccardi, L.; Lancellotti, S.; Santoliquido, A.; Flore, R.; et al. Thrombotic risk factors in patients with liver cirrhosis: Correlation with MELD scoring system and portal vein thrombosis development. J. Hepatol. 2009, 51, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Turon, F.; Driever, E.G.; Baiges, A.; Cerda, E.; García-Criado, Á.; Gilabert, R.; Bru, C.; Berzigotti, A.; Nuñez, I.; Orts, L.; et al. Predicting portal thrombosis in cirrhosis: A prospective study of clinical, ultrasonographic and hemostatic factors. J. Hepatol. 2021, 75, 1367–1376. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y.; Bae, J.S.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S.; Kim, H.; Lee, K.B.; Han, J.K. Shear-wave dispersion slope from US shear-wave elastography: Detection of allograft damage after liver transplantation. Radiology 2019, 293, 327–333. [Google Scholar] [CrossRef]
- Huang, C.; Chen, P.; Shih, C. Estimating the viscoelastic modulus of a thrombus using an ultrasonic shear-wave approach. Med Phys. 2013, 40, 042901. [Google Scholar] [CrossRef]
- Jang, J.K.; Lee, E.S.; Seo, J.W.; Kim, Y.R.; Kim, S.Y.; Cho, Y.Y.; Lee, D.H. Two-Dimensional Shear-Wave Elastography and US Attenuation Imaging for Nonalcoholic Steatohepatitis Diagnosis: A Cross-Sectional, Multicenter Study. Radiology 2022, 305, 118–126. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Leppkes, M.; Lindemann, A.; Gößwein, S.; Paulus, S.; Roth, D.; Hartung, A.; Liebing, E.; Zundler, S.; Gonzalez-Acera, M.; Patankar, J.V.; et al. Neutrophils prevent rectal bleeding in ulcerative colitis by peptidyl-arginine deiminase-4-dependent immunothrombosis. Gut 2022, 71, 2414–2429. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Yao, M.; Ma, J.; Wu, D.; Fang, C.; Wang, Z.; Guo, T.; Mo, J. Neutrophil extracellular traps mediate deep vein thrombosis: From mechanism to therapy. Front. Immunol. 2023, 14, 1198952. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Di Campli, M.-P.; Azouz, A.; Assabban, A.; Scaillet, J.; Splittgerber, M.; Van Keymeulen, A.; Libert, F.; Remmelink, M.; Le Moine, A.; Lemaitre, P.; et al. The mononuclear phagocyte system contributes to fibrosis in post-transplant obliterans bronchiolitis. Eur. Respir. J. 2021, 57, 2000344. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.-K.; Nikolic-Paterson, D.J.; Lan, H.-Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Zhuang, T.; Chen, M.-H.; Wu, R.-X.; Wang, J.; Hu, X.-D.; Meng, T.; Wu, A.-H.; Yang, Y.-F.; Lei, Y.; Hu, D.-H.; et al. ALKBH5-mediated m6A modification of IL-11 drives macrophage-to-myofibroblast transition and pathological cardiac fibrosis in mice. Nat. Commun. 2024, 15, 1995. [Google Scholar] [CrossRef]
- Newman, A.A.C.; Serbulea, V.; Baylis, R.A.; Shankman, L.S.; Bradley, X.; Alencar, G.F.; Owsiany, K.; Deaton, R.A.; Karnewar, S.; Shamsuzzaman, S.; et al. Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRβ and bioenergetic mechanisms. Nat. Metab. 2021, 3, 166–181. [Google Scholar] [CrossRef]
- Carnevale, R.; Raparelli, V.; Nocella, C.; Bartimoccia, S.; Novo, M.; Severino, A.; De Falco, E.; Cammisotto, V.; Pasquale, C.; Crescioli, C.; et al. Gut-derived endotoxin stimulates factor VIII secretion from endothelial cells. Implications for hypercoagulability in cirrhosis. J. Hepatol. 2017, 67, 950–956. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell 2020, 180, 941–955. [Google Scholar] [CrossRef]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef]
- Bosch, J.; Iwakiri, Y. The portal hypertension syndrome: Etiology, classification, relevance, and animal models. Hepatol. Int. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Khismatullin, R.R.; Abdullayeva, S.; Peshkova, A.D.; Sounbuli, K.; Evtugina, N.G.; Litvinov, R.I.; Weisel, J.W. Extent of intravital contraction of arterial and venous thrombi and pulmonary emboli. Blood Adv. 2022, 6, 1708–1718. [Google Scholar] [CrossRef]
- Guerrero, A.; del Campo, L.; Piscaglia, F.; Scheiner, B.; Han, G.; Violi, F.; Ferreira, C.-N.; Téllez, L.; Reiberger, T.; Basili, S.; et al. Anticoagulation improves survival in patients with cirrhosis and portal vein thrombosis: The IMPORTAL competing-risk meta-analysis. J. Hepatol. 2023, 79, 69–78. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 151–166. [Google Scholar] [CrossRef]
- Hodson, M.E. Aerosolized Dornase Alfa (rhDNase) for Therapy of Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, S70–S74. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Jenne, C.N.; Surewaard, B.G.J.; Thanabalasuriar, A.; Lee, W.-Y.; Sanz, M.-J.; Mowen, K.; Opdenakker, G.; Kubes, P. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 2015, 6, 6673. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.M.S.; Wanderley, C.W.S.; Veras, F.P.; Sonego, F.; Nascimento, D.C.; Gonçalves, A.V.; Martins, T.V.; Cólon, D.F.; Borges, V.F.; Brauer, V.S.; et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood 2021, 138, 2702–2713. [Google Scholar] [CrossRef]
- Cvek, B. Nonprofit drugs as the salvation of the world’s healthcare systems: The case of Antabuse (disulfiram). Drug Discov. Today 2012, 17, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Cerri, G.G.; A Alves, V.; Magalhães, A. Hepatosplenic schistosomiasis mansoni: Ultrasound manifestations. Radiology 1984, 153, 777–780. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, Y.; Chien, Y.; Zhu, Y.; Huang, X.; Wu, L.; Ai, Y.; Jiang, S.; Li, F.; Chen, S. GSDMD-Dependent Neutrophil Extracellular Traps Mediate Portal Vein Thrombosis and Associated Fibrosis in Cirrhosis. Int. J. Mol. Sci. 2024, 25, 9099. https://doi.org/10.3390/ijms25169099
Che Y, Chien Y, Zhu Y, Huang X, Wu L, Ai Y, Jiang S, Li F, Chen S. GSDMD-Dependent Neutrophil Extracellular Traps Mediate Portal Vein Thrombosis and Associated Fibrosis in Cirrhosis. International Journal of Molecular Sciences. 2024; 25(16):9099. https://doi.org/10.3390/ijms25169099
Chicago/Turabian StyleChe, Ying, Youjung Chien, Yuli Zhu, Xiaoquan Huang, Ling Wu, Yingjie Ai, Siyu Jiang, Feng Li, and Shiyao Chen. 2024. "GSDMD-Dependent Neutrophil Extracellular Traps Mediate Portal Vein Thrombosis and Associated Fibrosis in Cirrhosis" International Journal of Molecular Sciences 25, no. 16: 9099. https://doi.org/10.3390/ijms25169099
APA StyleChe, Y., Chien, Y., Zhu, Y., Huang, X., Wu, L., Ai, Y., Jiang, S., Li, F., & Chen, S. (2024). GSDMD-Dependent Neutrophil Extracellular Traps Mediate Portal Vein Thrombosis and Associated Fibrosis in Cirrhosis. International Journal of Molecular Sciences, 25(16), 9099. https://doi.org/10.3390/ijms25169099





