Integrated Morphological, Comparative Transcriptomic, and Metabolomic Analyses Reveal Mechanisms Underlying Seasonal Patterns of Variation in Spines of the Giant Spiny Frog (Quasipaa spinosa)
Abstract
1. Introduction
2. Results
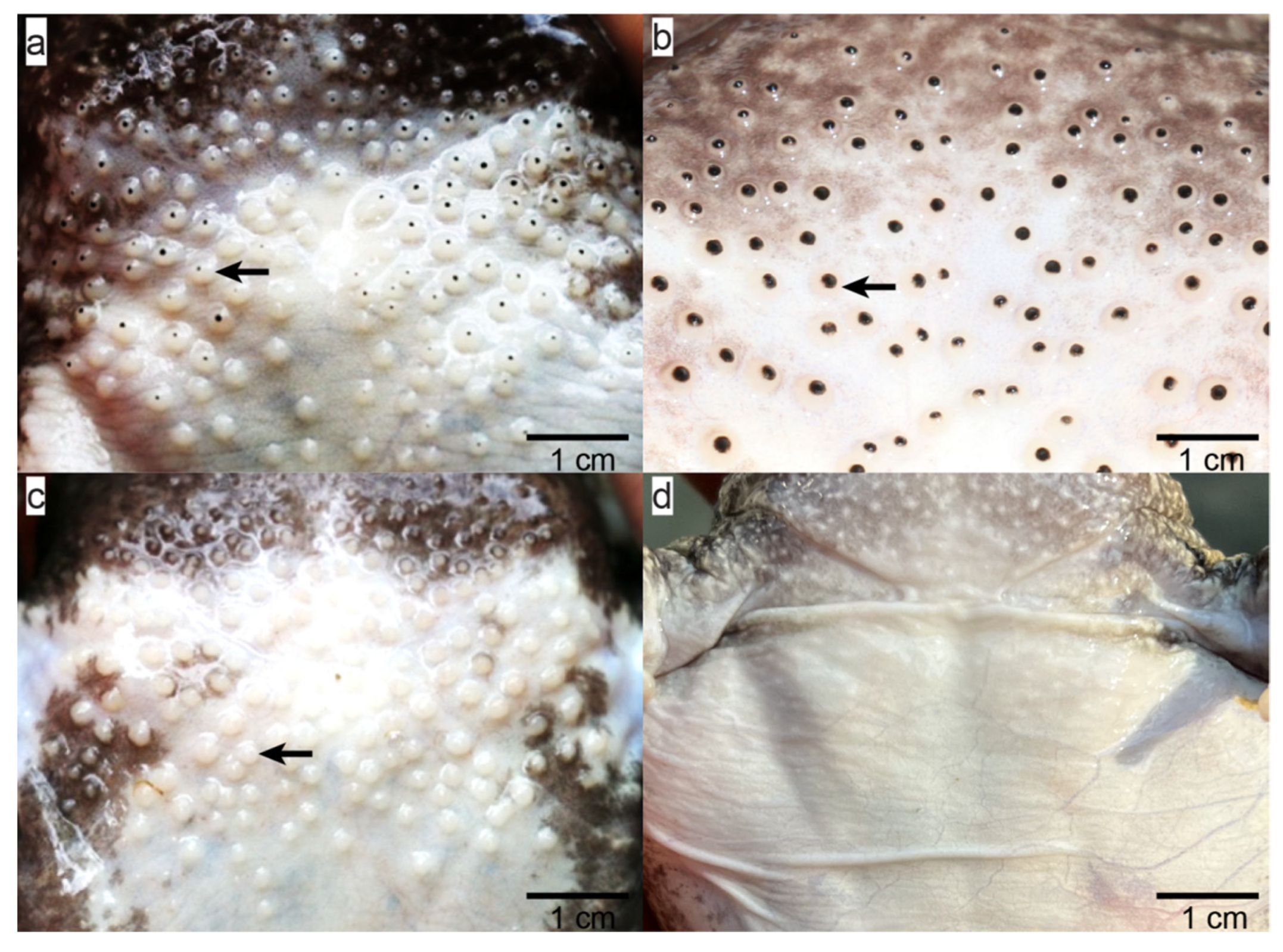
2.1. Morphological Differences in Quasipaa spinosa Skin across Different Periods
2.2. Correlation Analysis between Spine Polymorphism and Body Morphometric Indices
2.3. Analysis of the Skin Transcriptome in Quasipaa spinosa
2.3.1. Transcriptome Data Quality Control
2.3.2. Mining DEGs in Skin Transcriptomes
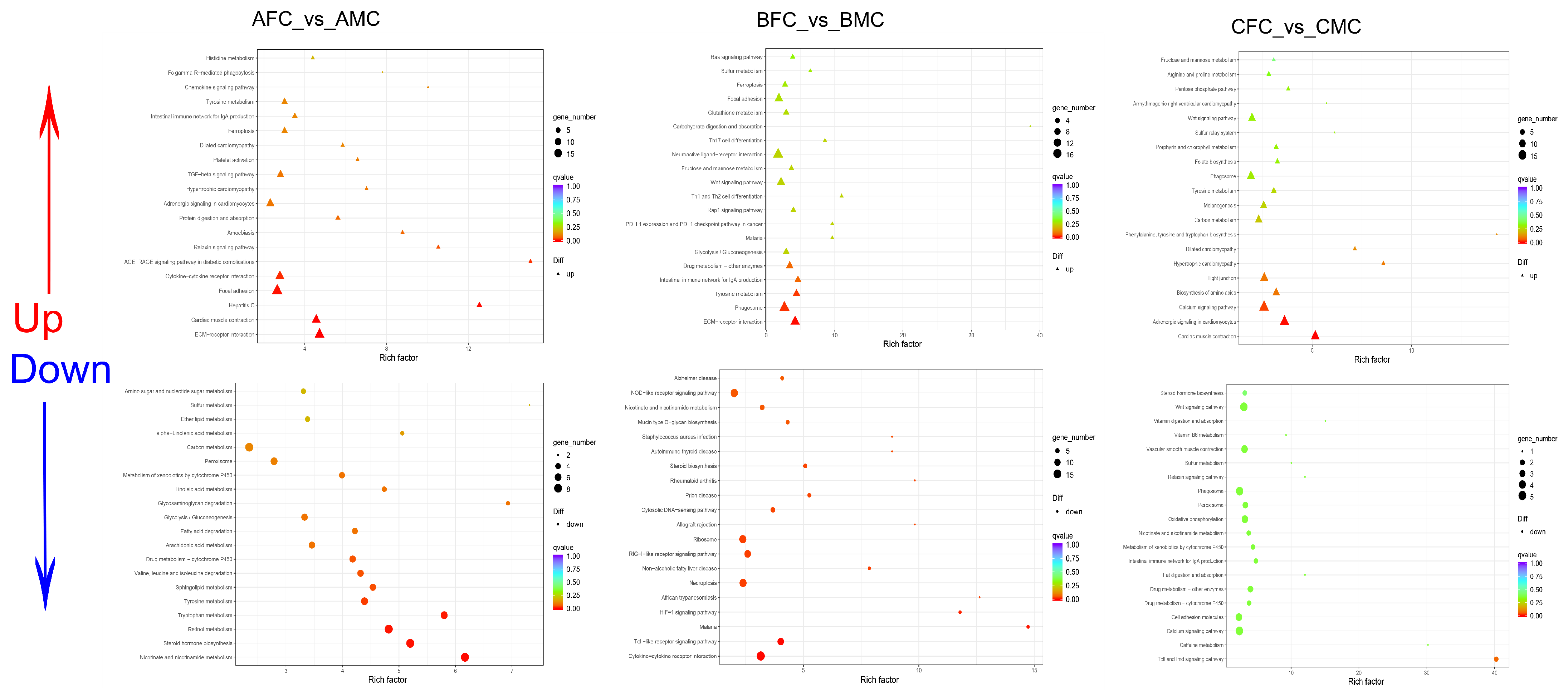
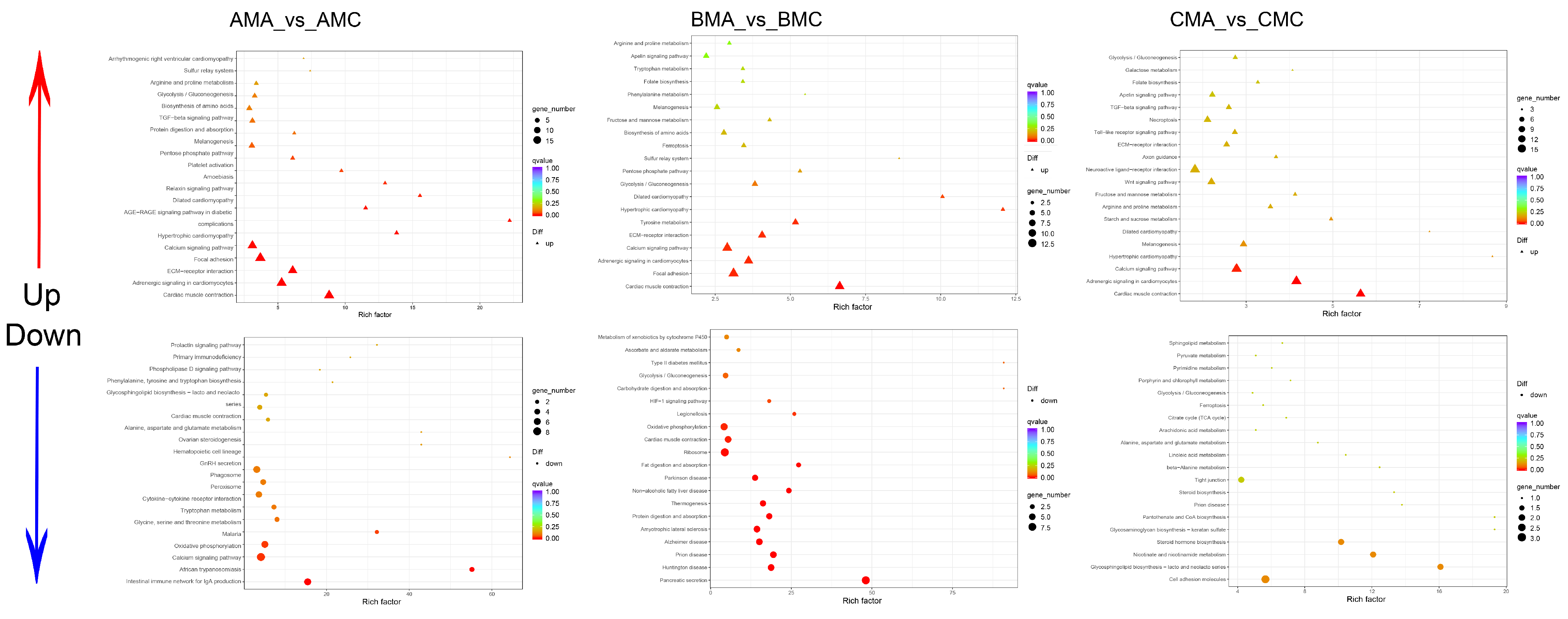
2.3.3. Functional Categorization of DEGs in the Skin Transcriptome
2.3.4. Analysis of Gene Expression Differences Related to Cell Proliferation and Keratin Synthesis among Comparison Groups
2.4. Validation of DEGs through qPCR
2.5. Comparative Metabolomic Analysis of Thoracic and Abdominal Skin in Quasipaa spinosa
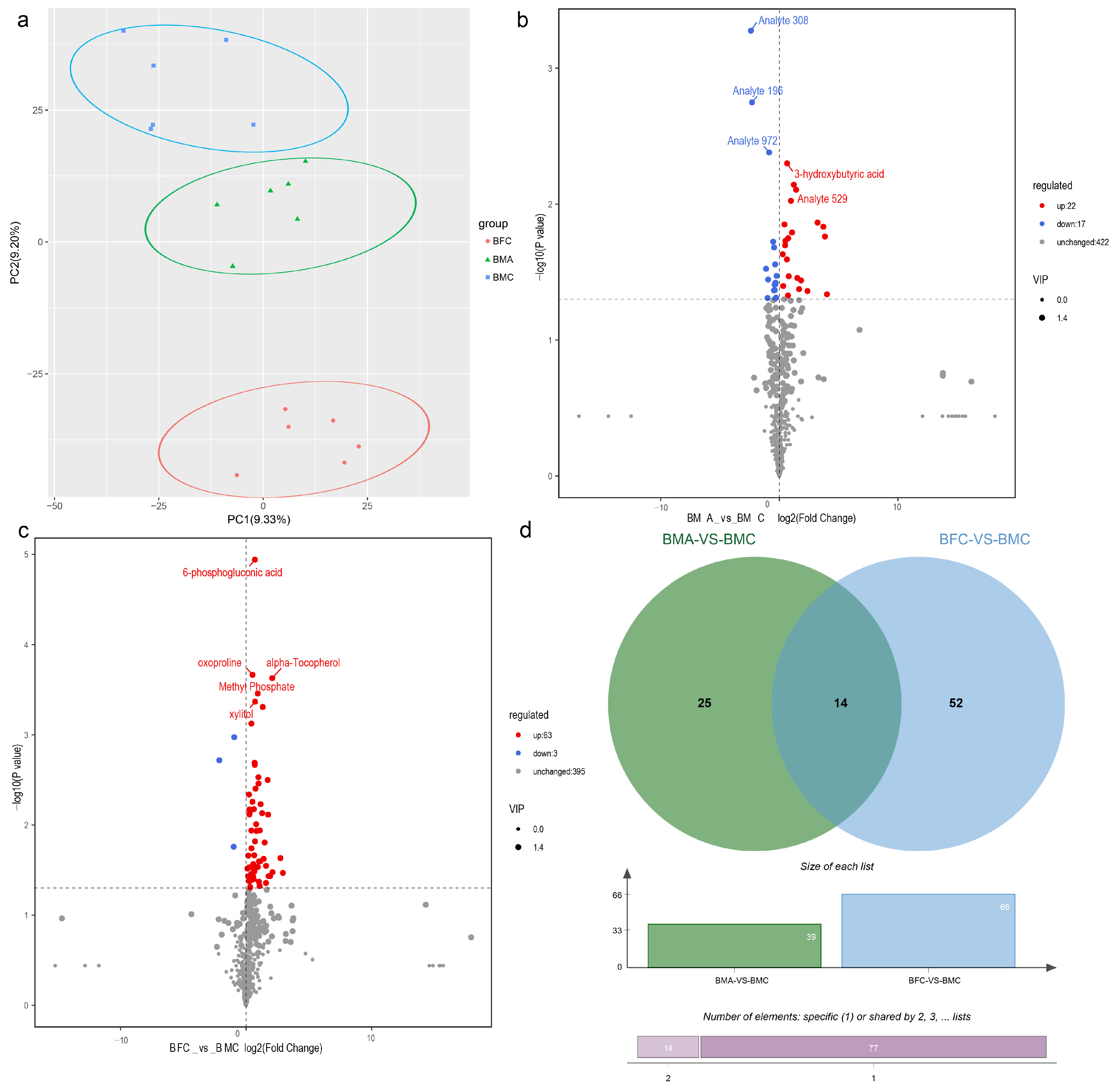
2.5.1. Identification of Differentially Expressed Metabolites (DEMs)
2.5.2. DEM Functional Categorization through KEGG Enrichment Analysis
2.6. Integrated Analysis of DEGs and DEMs
3. Discussion
3.1. Biological Functions and Morphological Characteristics of Spines
3.2. Seasonal Variation in Spines Is Driven by a Complex Regulatory Network
3.3. Joint Analysis of DEGs and DEMs Underscores the Role of Citric Acid Metabolism in Maintaining Spine Morphology
4. Materials and Methods
4.1. Sample Collection and Experimental Design
4.2. Morphometric and Spine Parameters Measurement
4.3. HE Staining and Histology
4.4. RNA-Seq Library Construction and Sequencing
4.5. Validation of RNA-Seq Data by qRT-PCR
4.6. Metabolite Extraction and Analysis
4.7. Comprehensive Metabolome and Transcriptome Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMC | Pre-breeding period male thoracic skin |
| AFC | Pre-breeding period female thoracic skin |
| BMC | Breeding period male thoracic skin |
| BFC | Breeding period female thoracic skin |
| CMC | Late reproductive period male thoracic skin |
| CFC | Late reproductive period female thoracic skin |
| AMA | Pre-breeding period male abdominal skin |
| BMA | Breeding period male abdominal skin |
| CMA | Late reproductive period male abdominal skin |
| BM | Body mass |
| SVL | Snout–vent length |
| FLAH | Forearm plus hand length |
| CLA | Forearm circumference |
| HL | Hand length |
| LL | Hind limb length |
| TL | Tibia length |
| TW | Tibia width |
| FL | Foot length |
| SD | Spine density |
| ASEH | Average spine elevation height |
References
- Mei, Z. Discussing the Classification of the Giant Spiny Frog (Quasipaa spinosa) Based on Morphological and Molecular Biological Characteristics. Master’s Thesis, Zhejiang Normal University, Jinhua, China, 2019. [Google Scholar]
- Carvajal-Castro, J.D.; López-Aguirre, Y.; Ospina-L, A.M.; Santos, J.C.; Rojas, B.; Vargas-Salinas, F. Much more than a clasp: Evolutionary patterns of amplexus diversity in anurans. Biol. J. Linn. Soc. 2020, 129, 652–663. [Google Scholar] [CrossRef]
- Luna, M.C.; Mcdiarmid, R.W.; Faivovich, J. From erotic excrescences to pheromone shots: Structure and diversity of nuptial pads in anurans. Biol. J. Linn. Soc. 2018, 124, 403–446. [Google Scholar] [CrossRef]
- Zheng, P.; Gong, Y.; Wang, B.; Yu, H.; Huang, S.; Liao, X.; Jiang, J.; Ran, J.; Xie, F. Love Hug—Functional Validation of Nuptial Pad-Secreted Pheromone in Anurans. Animals 2024, 14, 1550. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Y.; Hou, J.L.; Li, J.; Feng, X.L.; Liu, X.H.; Li, D.L.; Xiang, J.G.; Li, J.H. Comparative profiling of the skin and gut microbiota during metamorphosis in Chinese spiny frogs (Quasipaa spinosa) highlights microbial communities involved in disease pathogenesis. Aquaculture 2024, 585, 740726. [Google Scholar] [CrossRef]
- Iwai, N. Morphology, function and evolution of the pseudothumb in the Otton frog. J. Zool. 2013, 289, 127–133. [Google Scholar] [CrossRef]
- Lawson, L.P.; Loader, S.P.; Lyakurwa, J.V.; Liedtke, H.C. Diversification of spiny-throated reed frogs (Anura: Hyperoliidae) with the description of a new, range-restricted species from the Ukaguru mountains, Tanzania. PLoS ONE 2023, 18, e0277535. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.Y.; Yang, J.H. Limb malformation in a lesser spiny frog, Quasipaa exilispinosa (Liu & Hu 1975) (Anura: Dicroglossidae). Reptiles Amphib. 2023, 30, e18418. [Google Scholar]
- Nascimento, F.A.C.D.; Vilela, B.; Matias Dubeux, M.J.; Galdino, J.Y.A.; de Araújo-Neto, J.V.; Leal, F.; de Sá, R. Reproductive biology and sexual dimorphism of the poorly known frog Chiasmocleis alagoana (Microhylidae, Gastrophryninae), with an updated diagnosis for the species. Stud. Neotrop. Fauna Environ. 2022, 57, 96–110. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Li, J.; Huang, L.; Kazitsa, E.G.; Wu, H. Transcriptome analysis reveals the genetic basis underlying the seasonal development of keratinized nuptial spines in Leptobrachium boringii. BMC Genom. 2016, 17, 978. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, Z.; Ming, Y.; Jian, J.; Jiang, S.; Zhang, D.; Zhang, J.; Zheng, S.; Fang, X.; Yang, Y. A chromosomal level genome sequence for Quasipaa spinosa (Dicroglossidae) reveals chromosomal evolution and population diversity. Mol. Ecol. Resour. 2022, 22, 1545–1558. [Google Scholar] [CrossRef]
- Che, J.; Hu, J.S.; Zhou, W.W.; Murphy, R.W.; Papenfuss, T.J.; Chen, M.Y.; Rao, D.Q.; Li, P.P.; Zhang, Y.P. Phylogeny of the Asian spiny frog tribe Paini (Family Dicroglossidae) sensu Dubois. Mol. Phylogenet. Evol. 2009, 50, 59–73. [Google Scholar] [CrossRef]
- Che, J.; Zhou, W.W.; Hu, J.S.; Yan, F.; Papenfuss, T.J.; Wake, D.B.; Zhang, Y.P. Spiny frogs (Paini) illuminate the history of the Himalayan region and Southeast Asia. Proc. Natl. Acad. Sci. USA 2010, 107, 13765–13770. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, G. Multi-view representation learning with Kolmogorov-Smirnov to predict default based on imbalanced and complex dataset. Inf. Sci. 2022, 596, 380–394. [Google Scholar] [CrossRef]
- Liu, J.; Xue, R.; Wang, Y.; Li, D.; Yan, Q.; Yang, J. The near-complete mitogenome sequence of the Omei horned toad Megophrys omeimontis Liu, 1950 (Anura, Megophryidae). Mitochondrial DNA Part A 2016, 27, 2389–2390. [Google Scholar] [CrossRef]
- Kröner, L.; Lötters, S.; Hopp, M.T. Insights into caudate amphibian skin secretions with a focus on the chemistry and bioactivity of derived peptides. Biol. Chem. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Su, S.; Wang, Y.; Wang, H.; Huang, W.; Chen, J.; Xing, J.; Xu, P.; Yuan, X.; Huang, C.; Zhou, Y. Comparative expression analysis identifies the respiratory transition-related miRNAs and their target genes in tissues of metamorphosing Chinese giant salamander (Andrias davidianus). BMC Genom. 2018, 19, 406. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Fang, J.; Zheng, S.; Wang, Z.; Jiao, Y.; Xia, P.; Wu, H.; Ma, Z.; Hao, L. Peptides isolated from amphibian skin secretions with emphasis on antimicrobial peptides. Toxins 2022, 14, 722. [Google Scholar] [CrossRef]
- Larsen, E.H. Dual skin functions in amphibian osmoregulation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 253, 110869. [Google Scholar] [CrossRef]
- Woodley, S.K.; Staub, N.L. Pheromonal communication in urodelan amphibians. Cell Tissue Res. 2021, 383, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.F.; Zheng, R.Q.; Liu, C.T.; Cheng, B. Sexual dimorphism and female reproductive characteristics in the Paa spinosa of Pingjiang country Hu’nan Province. J. Zhejiang Norm. Univ. Nat. Sci. Ed. 2008, 31, 220–224. [Google Scholar]
- Yang, C.; Fu, Y.; Zhu, W.D.; Shen, T.J.K.; Si, L.G. Sex-related differences reflected in the effect of morphological traits on body weight and net body weight of an economic animal, Paa spinosa in streams of siming mountains. Oceanol. Limnol. Sin. 2015, 46, 679–686. [Google Scholar]
- Yu, B.G.; Zheng, R.Q.; Zhang, Y.; Liu, C.T. Geographic variation in body size and sexual size dimorphism in the giant spiny frog Paa spinosa (David, 1875) (Anura: Ranoidae). J. Nat. Hist. 2010, 44, 1729–1741. [Google Scholar] [CrossRef]
- Long, J.H. Sexual dimorphism and fecundity of Quasipaa spinosa. Chin. J. Wildl. 2021, 42, 817–824. [Google Scholar]
- Si, L.G.; Fu, Y.; Li, H.P.; Zhu, W.D.; Shen, T.J.K. Morphological differences between bisexual parents of economic frog Paa spinosa in streams of siming mountains. Oceanol. Limnol. Sin. 2015, 46, 853–861. [Google Scholar]
- Yu, T.L.; Li, Y.; Zhang, J.D. Evolution of sexual dimorphism in the plateau brown frog fails to obey Rensch’s rule. Herpetol. J. 2022, 32, 27–33. [Google Scholar] [CrossRef]
- Bernardy, J.V.; Melo, I.; Llusia, D.; Diniz-Filho, J.A.F.; Bastos, R.P. Female preferences for dominant frequency in frogs: Constraints and impact on sexual size dimorphism. Behav. Ecol. Sociobiol. 2024, 78, 4. [Google Scholar] [CrossRef]
- Solano, F. Metabolism and functions of amino acids in the skin. Amino Acids Nutr. Health Amino Acids Syst. Funct. Health 2020, 7, 187–199. [Google Scholar]
- Shenoy, M.; Abdul, N.S.; Qamar, Z.; Al Bahri, B.M.; Al Ghalayini, K.Z.; Kakti, A. Collagen structure, synthesis, and its applications: A systematic review. Cureus 2022, 14, e24856. [Google Scholar] [CrossRef]
- Chun, Y.S.; Kim, S.A.; Kim, Y.H.; Lee, J.H.; Shetty, A.A.; Kim, S.J. Autologous collagen-induced chondrogenesis: From bench to clinical development. Medicina 2023, 59, 530. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L.J. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Di Russo, J.; Magin, T.M.; Leube, R.E. A keratin code defines the textile nature of epithelial tissue architecture. Curr. Opin. Cell Biol. 2023, 85, 102236. [Google Scholar] [CrossRef]
- Ortega-Recalde, O.; Silgado, D.; Fetiva, C.; Fonseca, D.J.; Laissue, P. Transcriptomic analysis of skin in a case of ichthyosis curth-macklin caused by a KRT1 mutation. Br. J. Dermatol. 2016, 175, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Lin, M.; Lee, M.; Yan, W.; Wang, H.; Lin, Z. Nonsense mutations in KRT1 caused recessive epidermolytic palmoplantar keratoderma with knuckle pads. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Steen, K.; Chen, D.; Wang, F.; Majumdar, R.; Chen, S.; Kumar, S.; Lombard, D.B.; Weigert, R.; Zieman, A.G.; Parent, C.A. A role for keratins in supporting mitochondrial organization and function in skin keratinocytes. Mol. Biol. Cell 2020, 31, 1103–1111. [Google Scholar] [CrossRef]
- Depianto, D.; Kerns, M.L.; Dlugosz, A.A.; Coulombe, P.A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet. 2010, 42, 910–914. [Google Scholar] [CrossRef]
- Pang, B.; Zhu, Z.; Xiao, C.; Luo, Y.; Fang, H.; Bai, Y.; Sun, Z.; Ma, J.; Dang, E.; Wang, G. Keratin 17 is required for lipid metabolism in keratinocytes and benefits epidermal permeability barrier homeostasis. Front. Cell Dev. Biol. 2022, 9, 779257. [Google Scholar] [CrossRef]
- Hara, Y.; Kume, S.; Kataoka, Y.; Watanabe, N. Changes in TCA cycle and TCA cycle-related metabolites in plasma upon citric acid administration in rats. Heliyon 2021, 7, e08431. [Google Scholar] [CrossRef] [PubMed]
- Cerri, G.C.; Santos, S.H.; Bader, M.; Santos, R.A.S. Brown adipose tissue transcriptome unveils an important role of the beta-alanine/alamandine receptor, MrgD, in metabolism. J. Nutr. Biochem. 2023, 114, 109268. [Google Scholar] [CrossRef]
- Painelli, V.D.S.; Nemezio, K.M.; Jéssica, A.; Franchi, M.; Andrade, I.; Riani, L.A.; Saunders, B.; Sale, C.; Harris, R.C.; Gualano, B. High-intensity interval training augments muscle carnosine in the absence of dietary beta-alanine intake. Med. Sci. Sports Exercise 2018, 50, 2242–2252. [Google Scholar] [CrossRef]
- Lee, Y.S.; Shon, H.; Rockenhaus, A.E. Glucose-1-phosphate regulates orphan nuclear receptor NR4A1 and apoptosis. FASEB J. 2022, 36, e22435. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Brinza, I.; Honceriu, I.; Mihasan, M.; Hritcu, L. Insights into pharmacological activities of nicotine and 6-hydroxy-l-nicotine, a bacterial nicotine derivative: A systematic review. Biomolecules 2023, 14, 23. [Google Scholar] [CrossRef]
- Wei, L.; Wang, R.; Lin, K.; Jin, X.; Li, L.; Wazir, J.; Pu, W.; Lian, P.; Lu, R.; Song, S. Creatine modulates cellular energy metabolism and protects against cancer cachexia-associated muscle wasting. Front. Pharmacol. 2022, 13, 1086662. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, X.; Su, X.; Ji, B.; Chang, Y.; Huang, Q.; Zhang, Y.; Wang, X.; Wang, P. Extracellular vehicles from commensal skin malassezia restricta inhibit staphylococcus aureus proliferation and biofilm formation. ACS Infect. Dis. 2024, 10, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Feraudi, M.; Gärtner, C.; Kolb, J.; Weicker, H. Bioluminescent and fluorometric techniques for determinations of 19 metabolites of ADP/ATP-dependent transformations in energy metabolism in 200 (or 400) mg muscle. Clin. Chem. Lab. Med. 1983, 45, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shi, M.; Xing, J.; Liu, S.; Liu, Z.; Song, F. Treatment effects of radix ginseng-Schisandra chinensis herb pair on Alzheimer’s disease: An investigation of MS-based metabolomics investigation. J. Pharm. Biomed. Anal. 2022, 220, 115007. [Google Scholar] [CrossRef]
- Bezerra, A.M.; Citeli, N.; Galvão, A.; Carvalho-e-Silva, S.P. Minimizing the damage: A methodological proposal to remove the brains of anurans and squamates. Iheringia Série Zool. 2022, 112, e2022013. [Google Scholar] [CrossRef]
- Fei, L. Chinese Amphibian Search and Illustrations; Sichuan Science and Technology Press: Chengdu, China, 2020. [Google Scholar]
- Watters, J.L.; Cummings, S.T.; Flanagan, R.L.; Siler, C.D. Review of morphometric measurements used in anuran species descriptions and recommendations for a standardized approach. Zootaxa 2016, 4072, 477–495. [Google Scholar] [CrossRef]
- Garda, A.A.; Santana, D.J.; Sao-Pedro, V.A. Taxonomic characterization of paradoxical frogs (Anura, Hylidae, Pseudae): Geographic distribution, external morphology, and morphometry. Zootaxa 2010, 2666, 1–28. [Google Scholar] [CrossRef]
- Chmykhova, N.; Vasilev, D.; Veselkin, N. Serotonergic innervation of the frog spinal cord and involvement of 5-HT5A receptors in the modulation of miniature glycinergic postsynaptic potentials of lumbar motoneurons. J. Evol. Biochem. Physiol. 2024, 60, 22–44. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Avashthi, H.; Bhati, J.; Mittal, S.; Srivastava, A.; Budhlakoti, N.; Kumar, A.; Ramteke, P.W.; Mishra, D.C.; Kumar, A. Transcriptome data analysis using a de novo assembly approach. Genom. Cereal Crops 2022, 14, 195–209. [Google Scholar]
- Shahjaman, M.; Mollah, M.M.H.; Rahman, M.R.; Islam, S.M.S.; Mollah, M.N.H. Robust identification of differentially expressed genes from RNA-seq data. Genomics 2020, 112, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Y.; Zhang, W.; Zeng, Y.; Zhao, J.; Zhang, J.; Yao, T.; Li, H.; Shen, X.; Wu, G. Different software processing affects the peak picking and metabolic pathway recognition of metabolomics data. J. Chromatogr. A 2023, 1687, 463700. [Google Scholar] [CrossRef] [PubMed]






| Primer Name | Primer Sequence (5′–3′) |
|---|---|
| QsCOL1A2-F | CTGGTTCATCTGGTGGTGGA |
| QsCOL1A2-R | TCGGGGTGGCTGAGTCTTA |
| QsKRT12-F | CAAGAGAAGCAATGGGGAAG |
| QsKRT12-R | AAACCAGAACAAGGAGGGATG |
| QsTNC-F | GTTTCGCCGTTGTCTCTAAGG |
| QsTNC-R | AACTGTCAAGGAGGTTGCTCTG |
| QsENO1-F | ACTCGGTCACGGAGCCAATCT |
| QsENO1-R | AGCCAGTGCAGGAATCCAGGTA |
| QsMYL10-F | AAGCGGACAGATTCAGCCAAGA |
| QsMYL10-R | CGCTACTCCTGGTCCTTCTCCT |
| QsTNN-F | TTGGTCCAGTTCCTCCTCCTCT |
| QsTNN-R | TTCTCCGTCTTCCGTGGGTTTG |
| QsKRT-F | TTGCCCACTGTGCTTATTCC |
| QsKRT-R | TTCTCTTCCATTCGCTTCTGAT |
| QsTHBS4-F | GCCCAAATCTACAACCACCC |
| QsTHBS4-R | CAATGAACCTCCCATGACCAC |
| QsTYRP1-F | GGCAGAAGTACTAACCACAACG |
| QsTYRP1-R | GAGAAGAGCCACATCTAAACGC |
| Qsβ-Actin-F | CTGCTGAGCGTGAGATT |
| Qsβ-Actin-R | GGCTGGAAGAGGGTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, G.; Jiang, Z.-Y.; Shi, N.; Xiong, Y.-G.; Zheng, R.-Q. Integrated Morphological, Comparative Transcriptomic, and Metabolomic Analyses Reveal Mechanisms Underlying Seasonal Patterns of Variation in Spines of the Giant Spiny Frog (Quasipaa spinosa). Int. J. Mol. Sci. 2024, 25, 9128. https://doi.org/10.3390/ijms25169128
Wan G, Jiang Z-Y, Shi N, Xiong Y-G, Zheng R-Q. Integrated Morphological, Comparative Transcriptomic, and Metabolomic Analyses Reveal Mechanisms Underlying Seasonal Patterns of Variation in Spines of the Giant Spiny Frog (Quasipaa spinosa). International Journal of Molecular Sciences. 2024; 25(16):9128. https://doi.org/10.3390/ijms25169128
Chicago/Turabian StyleWan, Gang, Ze-Yuan Jiang, Nuo Shi, Yi-Ge Xiong, and Rong-Quan Zheng. 2024. "Integrated Morphological, Comparative Transcriptomic, and Metabolomic Analyses Reveal Mechanisms Underlying Seasonal Patterns of Variation in Spines of the Giant Spiny Frog (Quasipaa spinosa)" International Journal of Molecular Sciences 25, no. 16: 9128. https://doi.org/10.3390/ijms25169128
APA StyleWan, G., Jiang, Z.-Y., Shi, N., Xiong, Y.-G., & Zheng, R.-Q. (2024). Integrated Morphological, Comparative Transcriptomic, and Metabolomic Analyses Reveal Mechanisms Underlying Seasonal Patterns of Variation in Spines of the Giant Spiny Frog (Quasipaa spinosa). International Journal of Molecular Sciences, 25(16), 9128. https://doi.org/10.3390/ijms25169128






