Genome-Wide Association Studies Reveal That the Abietane Diterpene Isopimaric Acid Promotes Rice Growth through Inhibition of Defense Pathways
Abstract
1. Introduction
2. Results
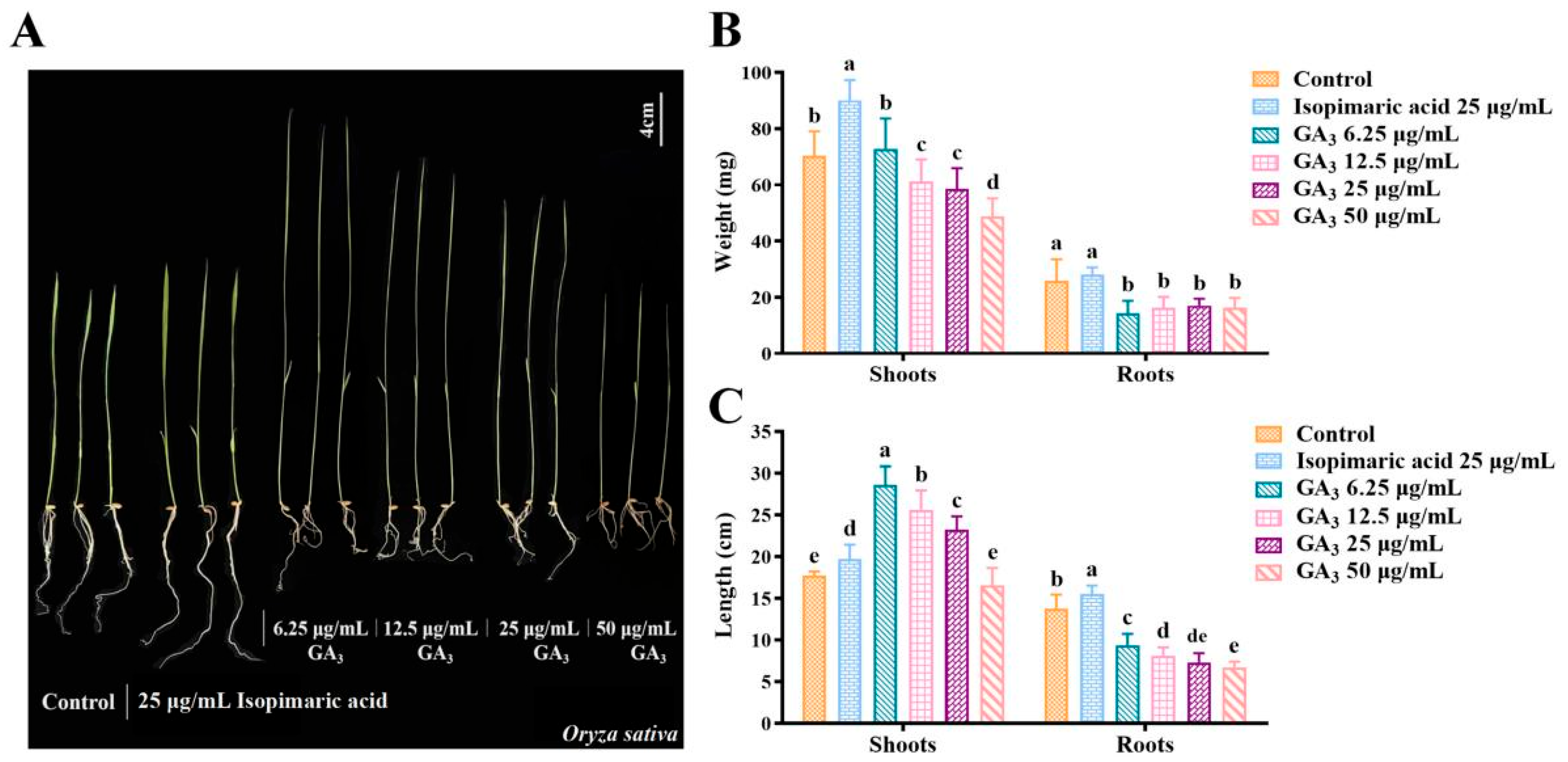
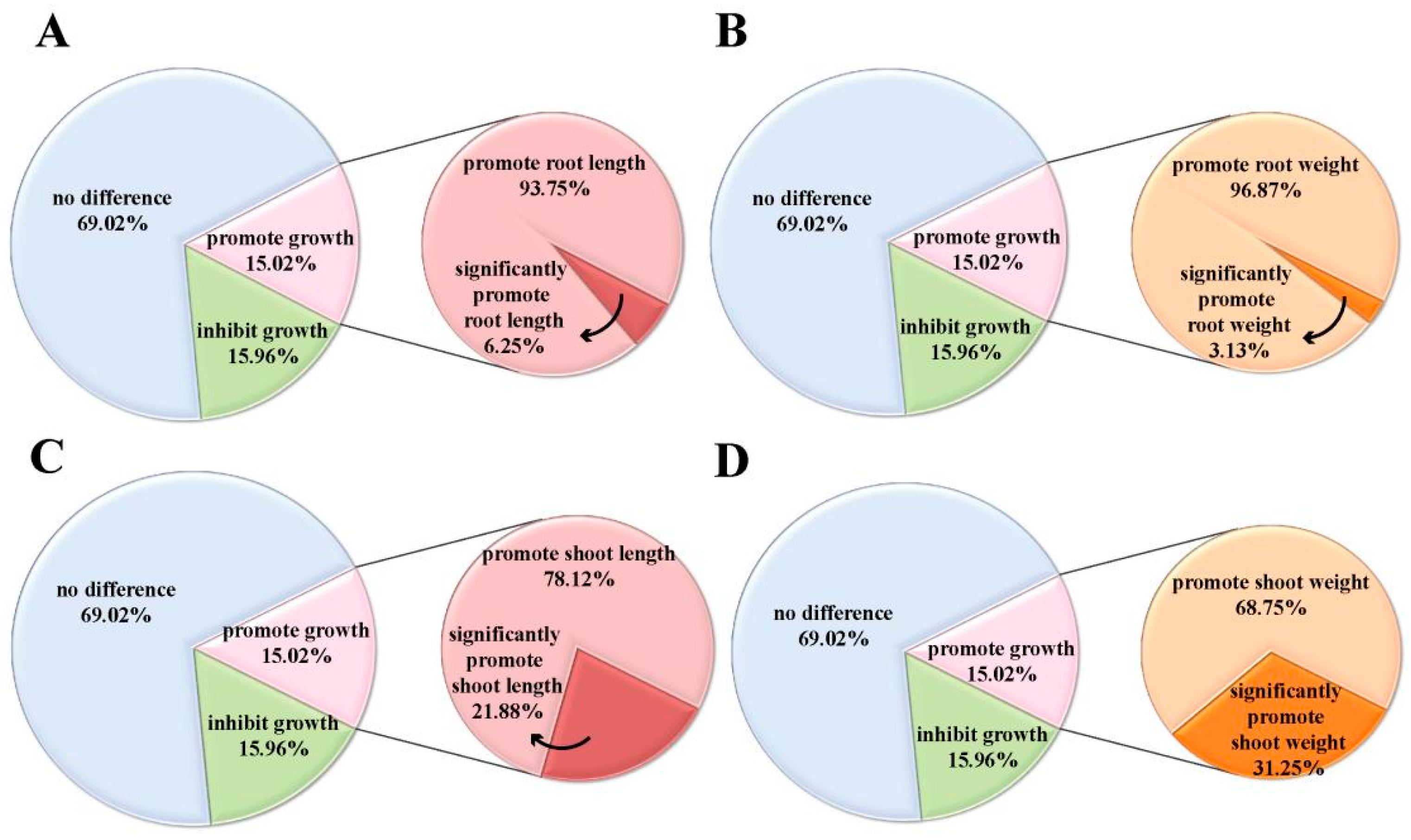
2.1. Isopimaric Acid Promotes the Growth of Nipponbare Rice
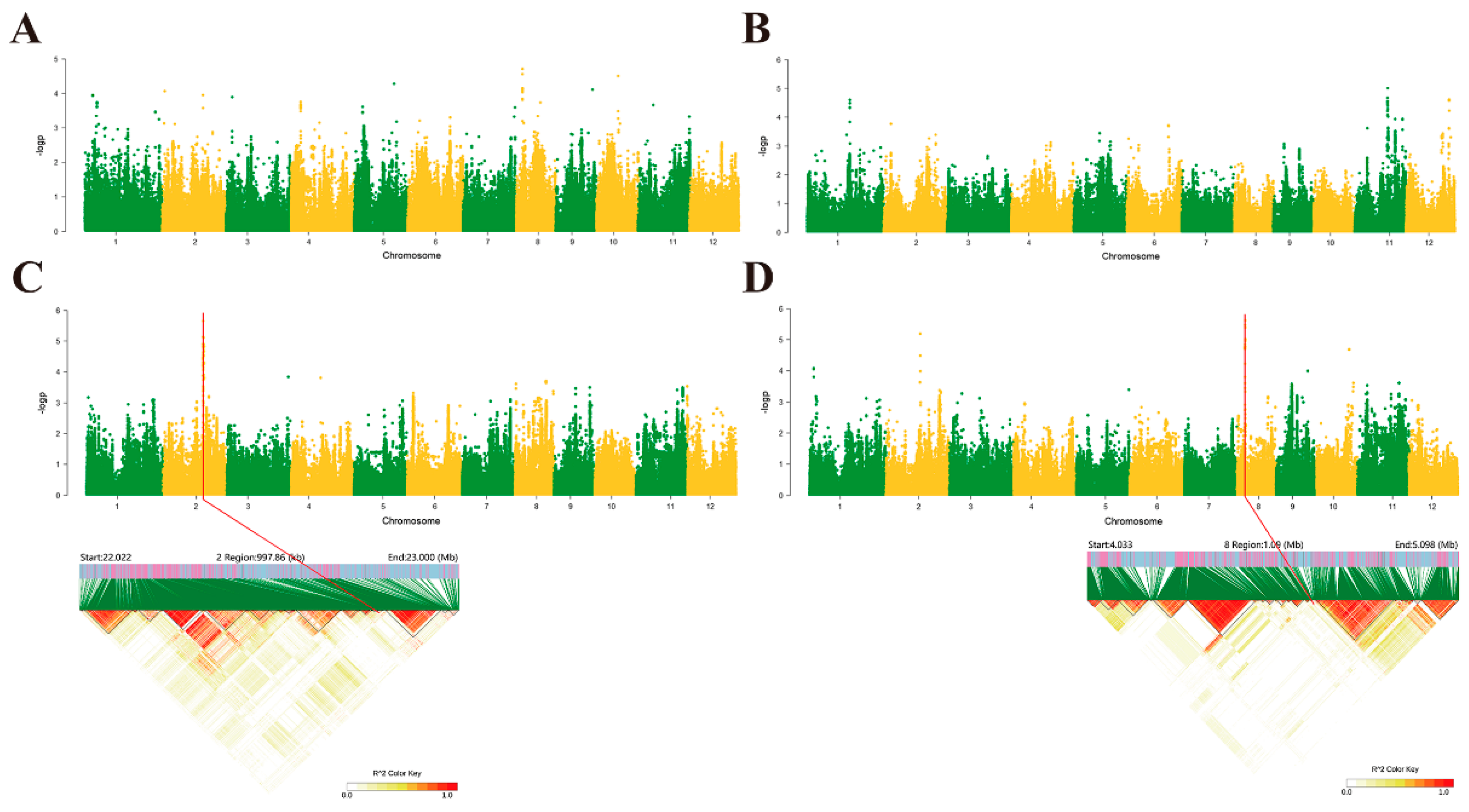
2.2. Genome-Wide Association Studies in Rice Following Induction Treatment with Isopimaric Acid
2.3. Haplotype Analysis and Tissue-Specific Expression of Candidate Genes
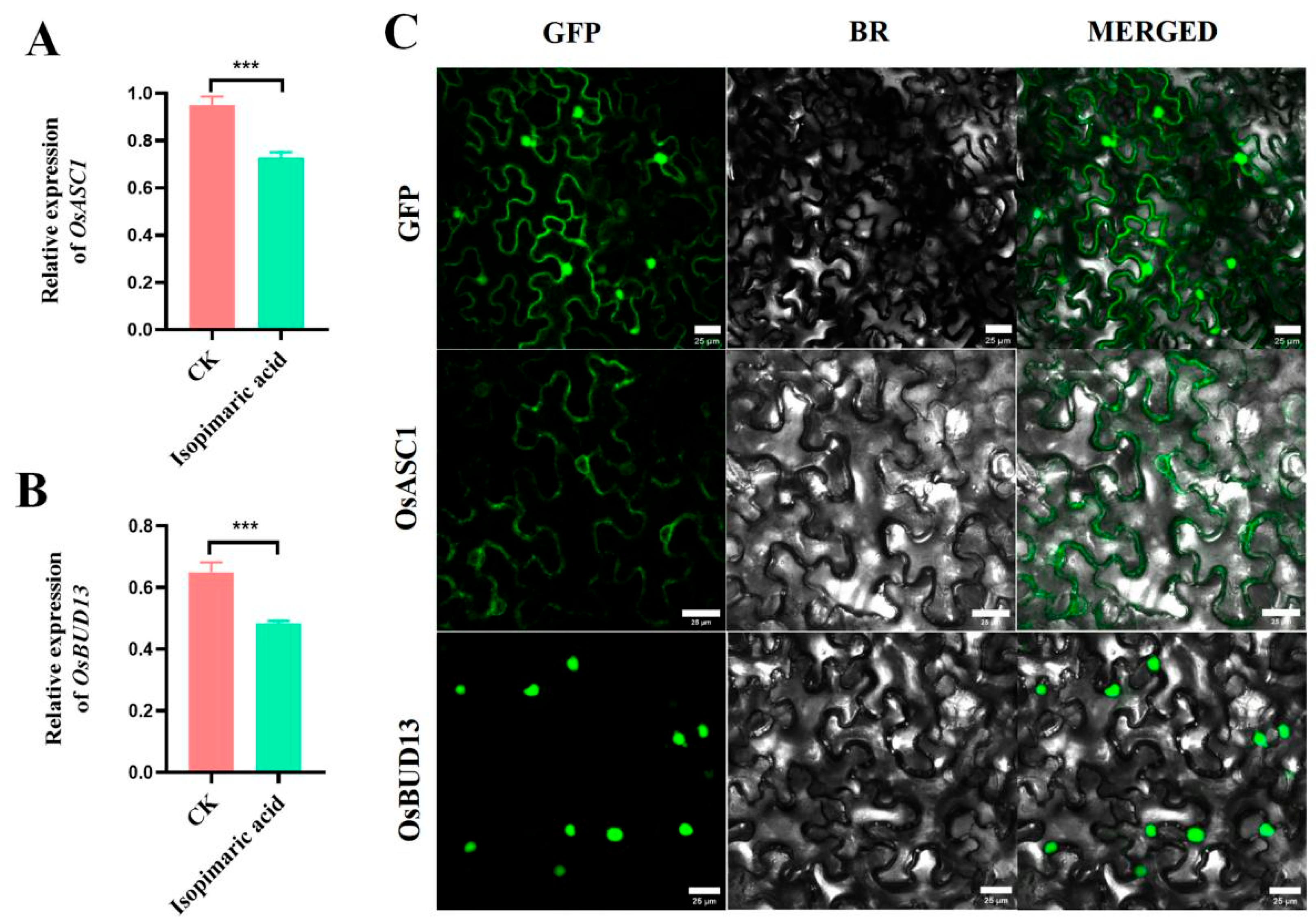
2.4. Expression Analysis and Subcellular Localization of OSASC1 and OSBUD13
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Genome-Wide Association Studies
4.3. Candidate Gene Retrieval and Haplotype Analysis
4.4. Rna Extraction and Rt-Qpcr Analysis
4.5. Gene Cloning and Vector Construction
4.6. Subcellular Localization
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Small, C.C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Flasiński, M.; Hąc, W.K. Natural vs synthetic auxin: Studies on the interactions between plant hormones and biological membrane lipids. Environ. Res. 2014, 133, 123–134. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Farjaminezhad, R.; Garoosi, G. New biological trends on cell and callus growth and azadirachtin production in Azadirachta indica. 3 Biotech 2019, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Didi, D.A.; Su, S.; Sam, F.E.; Tiika, R.J.; Zhang, X. Effect of plant growth regulators on osmotic regulatory substances and antioxidant enzyme activity of Nitraria tangutorum. Plants 2022, 11, 2559. [Google Scholar] [CrossRef]
- An, L.; Ma, J.; Qin, D.; Wang, H.; Yuan, Y.; Li, H.; Na, R.; Wu, X. Novel strategy to decipher the regulatory mechanism of 1-Naphthaleneacetic acid in strawberry maturation. J. Agric. Food Chem. 2019, 67, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Kaewchangwat, N.; Thanayupong, E.; Jarussophon, S.; Niamnont, N.; Yata, T.; Prateepchinda, S.; Unger, O.; Han, B.H.; Suttisintong, K. Coumarin-caged compounds of 1-Naphthaleneacetic acid as light-responsive controlled-release plant root stimulators. J. Agric. Food Chem. 2020, 68, 6268–6279. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qiu, J.; Zhao, X.; Feng, X. 6-Benzylaminopurine exposure induced development toxicity and behaviour alteration in zebrafish (Danio rerio). Environ. Pollut. 2021, 278, 116887. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Zhang, Z.; Jiang, G.; Feng, L.; Duan, X.; Jiang, Y. 6-Benzylaminopurine improves the quality of harvested litchi fruit. Postharvest Biol. Technol. 2018, 143, 137–142. [Google Scholar] [CrossRef]
- Vinale, F.; Strakowska, J.; Mazzei, P.; Piccolo, A.; Marra, R.; Lombardi, N.; Manganiello, G.; Pascale, A.; Woo, S.L.; Lorito, M. Cremenolide, a new antifungal, 10-member lactone from Trichoderma cremeum with plant growth promotion activity. Nat. Prod. Res. 2016, 30, 2575–2581. [Google Scholar] [CrossRef]
- Wang, A.Q.; Hua, J.; Wang, Y.Y.; Zhang, G.S.; Luo, S.H. Stereoisomers of non-volatile acetylbutanediol metabolites produced by Bacillus velezensis WRN031 improved root elongation of maize and rice. J. Agric. Food Chem. 2020, 68, 6308–6315. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Chang, S.T. Bioactivity and characterization of exudates from Cryptomeria japonica bark. Wood Sci. Technol. 2014, 48, 831–840. [Google Scholar] [CrossRef]
- Massaro, F.C.; Brooks, P.R.; Wallace, H.M.; Russell, F.D. Cerumen of australian stingless bees (Tetragonula carbonaria): Gas chromatography-mass spectrometry fingerprints and potential anti-inflammatory properties. Sci. Nat. 2011, 98, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Berman, A.; Shani, E. Plant hormone transport and localization: Signaling Molecules on the Move. Annu. Rev. Plant Biol. 2023, 74, 453–479. [Google Scholar] [CrossRef]
- Mielecki, J.; Gawroński, P.; Karpiński, S. Aux/IAA11 is required for UV-AB tolerance and auxin sensing in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 5708. [Google Scholar] [CrossRef]
- Li, T.; Xu, Y.; Zhang, L.; Ji, Y.; Tan, D.; Yuan, H.; Wang, A. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 2017, 29, 1316–1334. [Google Scholar] [CrossRef]
- Song, C.; Guan, Y.; Zhang, D.; Tang, X.; Chang, Y. Integrated mRNA and miRNA transcriptome analysis suggests a regulatory network for UV–B-controlled terpenoid synthesis in fragrant woodfern (Dryopteris fragrans). Int. J. Mol. Sci. 2022, 23, 5708. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y. Roles of brassinosteroids in plant reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Hartwig, T.; Banf, M.; Prietsch, G.P.; Zhu, J.Y.; Mora-Ramírez, I.; Schippers, J.H.M.; Snodgrass, S.J.; Seetharam, A.S.; Huettel, B.; Kolkman, J.M.; et al. Hybrid allele-specific ChIP-seq analysis identifies variation in brassinosteroid-responsive transcription factor binding linked to traits in maize. Genome Biol. 2023, 24, 108. [Google Scholar] [CrossRef] [PubMed]
- Chaiwanon, J.; Garcia, V.J.; Cartwright, H.; Sun, Y.; Wang, Z.Y. Immunophilin-like FKBP42/TWISTED DWARF1 interacts with the receptor kinase BRI1 to regulate brassinosteroid signaling in Arabidopsis. Mol. Plant 2016, 9, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Aftab, T. Phytohormones, plant growth regulators and signaling molecules: Cross-talk and stress responses. Plant Cell Rep. 2021, 40, 1301–1303. [Google Scholar] [CrossRef]
- Hauser, F.; Waadt, R.; Schroeder, J.I. Evolution of abscisic acid synthesis and signaling mechanisms. Curr. Biol. 2011, 21, R346–R355. [Google Scholar] [CrossRef]
- Wilkinson, S.; Kudoyarova, G.R.; Veselov, D.S.; Arkhipova, T.N.; Davies, W.J. Plant hormone interactions: Innovative targets for crop breeding and management. J. Exp. Bot. 2012, 63, 3499–3509. [Google Scholar] [CrossRef]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Fang, J.; Xu, F.; Wang, W.; Sun, X.; Chu, J.; Cai, B.; Feng, Y.; Chu, C. CYTOKININ OXIDASE/DEHYDROGENASE4 Integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol. 2014, 165, 1035–1046. [Google Scholar] [CrossRef]
- Yu, X.; Cui, X.; Wu, C.; Shi, S.; Yan, S. Salicylic acid inhibits gibberellin signaling through receptor interactions. Mol. Plant 2022, 15, 1759–1771. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, F.; Wang, Z.; Zhuo, C.; Hu, K.; Li, X.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; et al. Transcription factor WRKY28 curbs WRKY33-mediated resistance to Sclerotinia sclerotiorum in Brassica napus. Plant Physiol. 2022, 190, 2757–2774. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z.; Li, H.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y. Phytochrome A and B function antagonistically to regulate cold tolerance via abscisic acid-dependent jasmonate signaling. Plant Physiol. 2016, 170, 459–471. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, Y.; Xiong, B.; Dai, L.; Huang, S.; Dong, T.; Sun, G.; Liao, L.; Deng, Q.; Wang, X.; et al. Effects of exogenous methyl jasmonate on the synthesis of endogenous jasmonates and the regulation of photosynthesis in citrus. Physiol. Plant. 2020, 170, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Zhang, X.; Wu, F.; Feng, H.; Deng, L.; Xu, L.; Zhang, M.; Wang, Q.; Li, C. Transcriptional mechanism of jasmonate receptor COI1-mediated delay of flowering yime in Arabidopsis. Plant Cell 2015, 27, 2814–2828. [Google Scholar] [PubMed]
- Wang, H.; Li, S.; Li, Y.A.; Xu, Y.; Wang, Y.; Zhang, R.; Sun, W.; Chen, Q.; Wang, X.J.; Li, C.; et al. MED25 connects enhancer–promoter looping and MYC2-dependent activation of jasmonate signalling. Nat. Plants 2019, 5, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.P.; Li, J.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.E.; Tao, Y.; Chory, J.; Ballaré, C.L. Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc. Natl. Acad. Sci. USA 2009, 106, 4935–4940. [Google Scholar] [CrossRef]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]

| Chr | Genes | Gene Name | Gene Position | Description |
|---|---|---|---|---|
| 2 | LOC_Os02g37080 | Os02g0581300 | 22407730..22412305 | ASC1, putative, expressed |
| 2 | LOC_Os02g37130 | Os02g0582200 | 22445010..22448965 | expressed protein |
| 2 | LOC_Os02g37250 | Os02g0583700 | 22509550..22510620 | hypothetical protein |
| 2 | LOC_Os02g37260 | Os02g0584200 | 22529056..22531379 | hypothetical protein |
| 2 | LOC_Os02g37309 | Os02g0584900 | 22556916..22558121 | expressed protein |
| 2 | LOC_Os02g37320 | Os02g0585100 | 22558881..22559609 | heavy metal-associated domain containing protein, expressed |
| 2 | LOC_Os02g37410 | / | 22595368..22596778 | expressed protein |
| 2 | LOC_Os02g37430 | Os02g0586500 | 22602418..22605754 | LSM domain-containing protein, expressed |
| 2 | LOC_Os02g37440 | Os02g0586600 | 22607190..22609208 | expressed protein |
| 2 | LOC_Os02g37460 | / | 22614291..22615805 | retrotransposon protein, putative, Ty3-gypsy subclass |
| 2 | LOC_Os02g37500 | Os02g0587300 | 22636061..22636963 | hypothetical protein |
| 2 | LOC_Os02g37540 | Os02g0587800 | 22650959..22651932 | virulence factor, pectin lyase fold family protein |
| 2 | LOC_Os02g37620 | / | 22702356..22705354 | transposon protein, putative, CACTA, En/Spm sub-class |
| 2 | LOC_Os02g37654 | Os02g0589000 | 22722799..22733189 | lecithine cholesterol acyltransferase, putative, expressed |
| 2 | LOC_Os02g37750 | Os02g0589900 | 22761941..22778592 | lecithine cholesterol acyltransferase, putative, expressed |
| 2 | LOC_Os02g37760 | Os02g0590000 | 22782750..22783121 | diacylglycerol acyltransferase domain containing protein |
| 2 | LOC_Os02g37790 | / | 22797600..22798855 | retrotransposon protein, putative, unclassified, expressed |
| Chr | Genes | Gene Name | Gene Position | Description |
|---|---|---|---|---|
| 8 | LOC_Os08g07870 | / | 4424985..4429988 | retrotransposon protein, putative, unclassified, expressed |
| 8 | LOC_Os08g07880 | Os08g0176100 | 4430918..4434203 | phosphopantothenate–cysteine ligase, putative, expressed |
| 8 | LOC_Os08g07930 | Os08g0176633 | 4468741..4472698 | resistance protein, putative, expressed |
| 8 | LOC_Os08g08080 | Os08g0178300 | 4586478..4591217 | BUD13, putative, expressed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Bai, L.; Huang, J.; Peng, L.; Hua, J.; Luo, S. Genome-Wide Association Studies Reveal That the Abietane Diterpene Isopimaric Acid Promotes Rice Growth through Inhibition of Defense Pathways. Int. J. Mol. Sci. 2024, 25, 9161. https://doi.org/10.3390/ijms25179161
Luo X, Bai L, Huang J, Peng L, Hua J, Luo S. Genome-Wide Association Studies Reveal That the Abietane Diterpene Isopimaric Acid Promotes Rice Growth through Inhibition of Defense Pathways. International Journal of Molecular Sciences. 2024; 25(17):9161. https://doi.org/10.3390/ijms25179161
Chicago/Turabian StyleLuo, Xiaomeng, Liping Bai, Jiaqi Huang, Luying Peng, Juan Hua, and Shihong Luo. 2024. "Genome-Wide Association Studies Reveal That the Abietane Diterpene Isopimaric Acid Promotes Rice Growth through Inhibition of Defense Pathways" International Journal of Molecular Sciences 25, no. 17: 9161. https://doi.org/10.3390/ijms25179161
APA StyleLuo, X., Bai, L., Huang, J., Peng, L., Hua, J., & Luo, S. (2024). Genome-Wide Association Studies Reveal That the Abietane Diterpene Isopimaric Acid Promotes Rice Growth through Inhibition of Defense Pathways. International Journal of Molecular Sciences, 25(17), 9161. https://doi.org/10.3390/ijms25179161





