Identification of Novel Independent Correlations between Cellular Components of the Immune System and Strain-Related Indices of Myocardial Dysfunction in CKD Patients and Kidney Transplant Recipients without Established Cardiovascular Disease
Abstract
:1. Introduction
2. Results
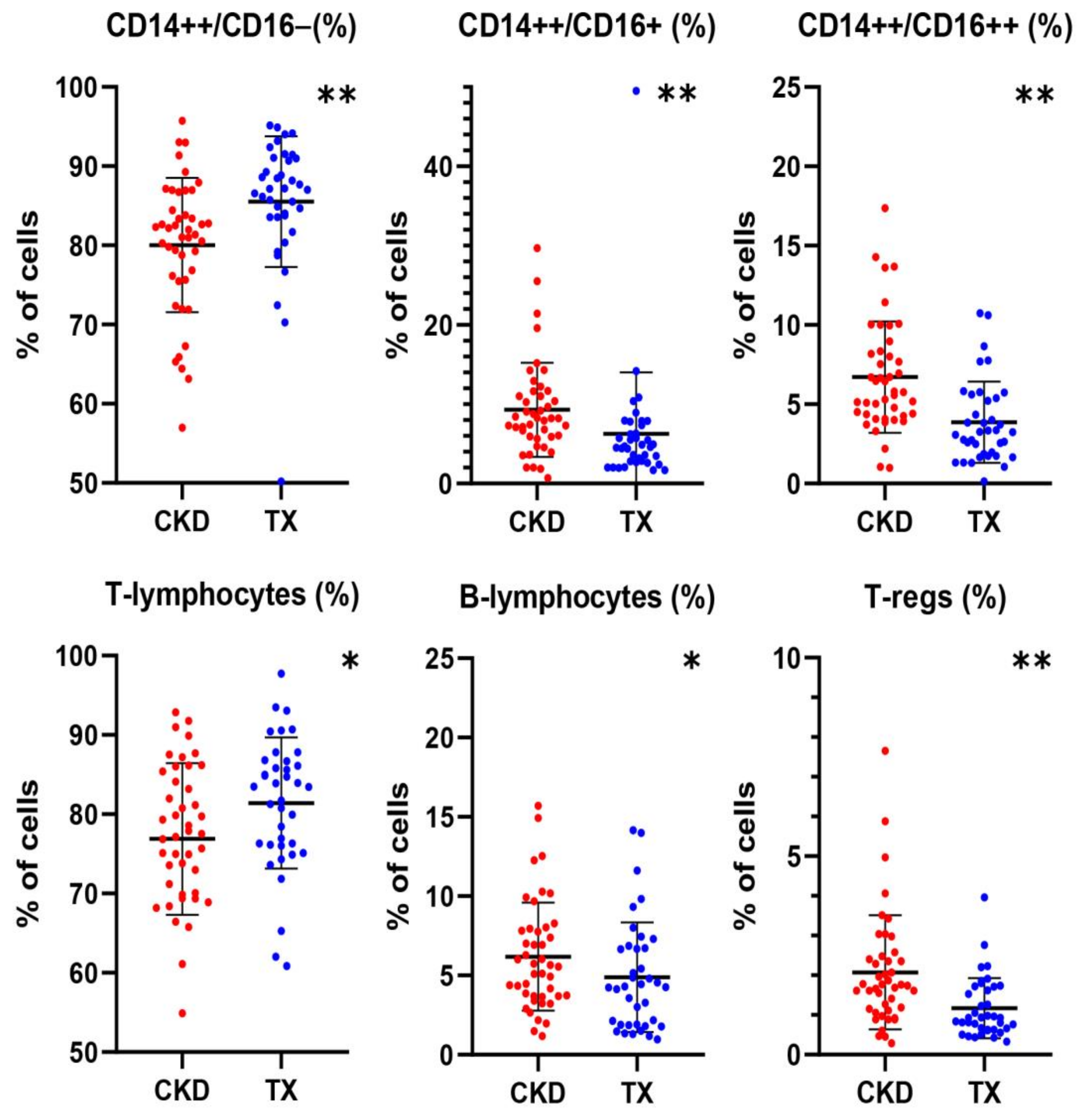
2.1. Differences in the Profile of Immune Cell Subset Expression between CKD Patients and KTRs
2.2. Correlations of Immune Cell Subsets with Clinical and Laboratory Parameters in CKD Patients and KTRs
2.3. Correlations of Immune Cells with Classical and Novel Indices of Left Ventricular Function in CKD Patients
2.4. Correlations of Immune Cells with Classical and Novel Indices of Left Ventricular Function in KTRs
3. Discussion
4. Materials and Methods
4.1. Study Cohort
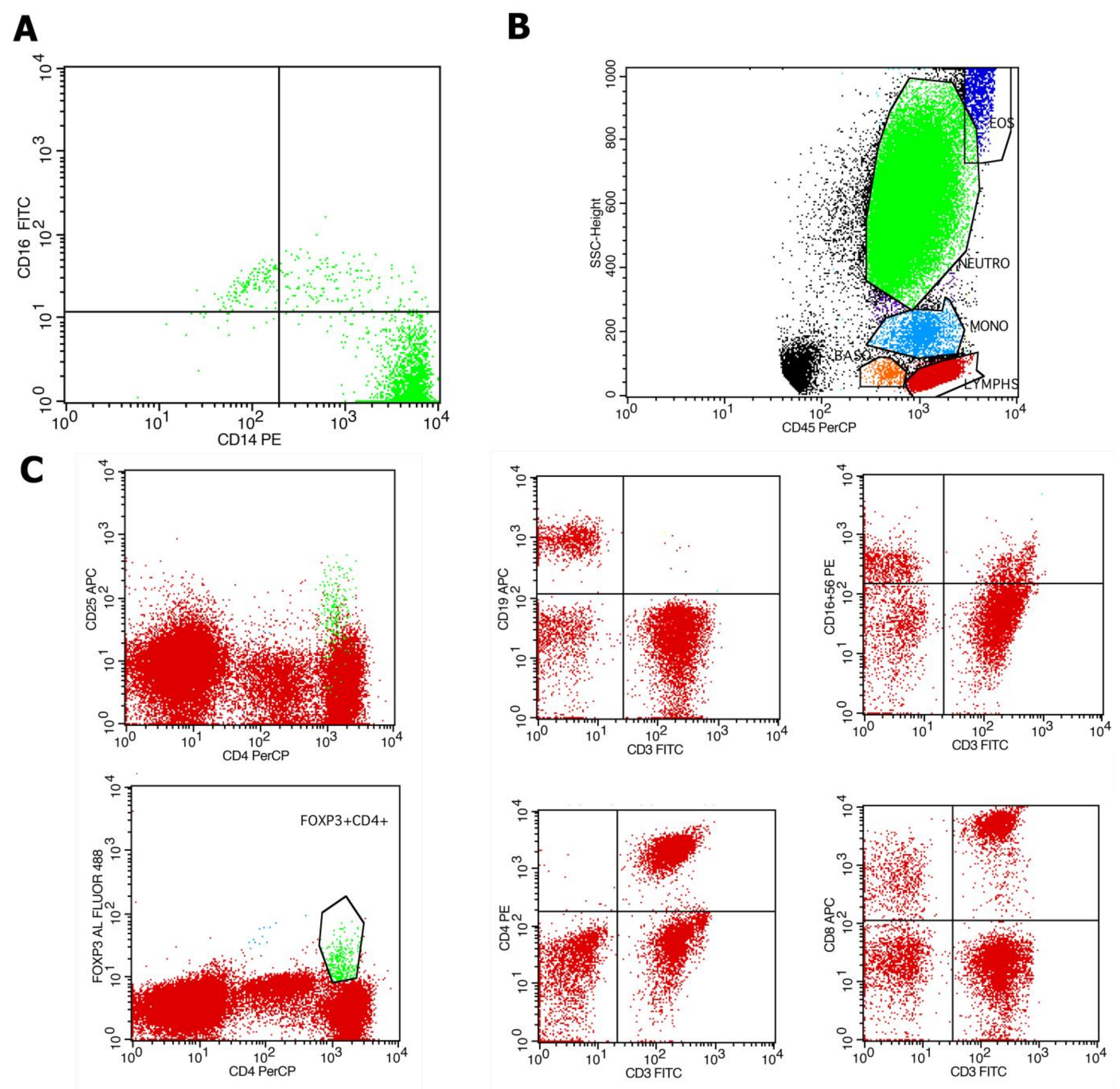
4.2. Evaluation of Immune Cell Subpopulations by Flow Cytometry
4.3. Echocardiographic Evaluation
4.4. Clinical and Laboratory Assessment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| CD14++CD16+ (N) | NK Cells (N) | T-Lymphocytes (N) |
|---|---|---|
| Phosphorus rho = 0.436, p = 0.003 | UPCR rho = −0.302, p = 0.04 Hemoglobin rho = 0.319, p = 0.03 Ferritin rho = 0.307, p = 0.04 Calcium rho = 0.329, p = 0.03 iPTH rho = −0.327, p = 0.03 | eGFR rho = 0.328, p = 0.03 Creatinine rho = −0.361, p = 0.02 Hemoglobin rho = 0.437, p = 0.003 CRP rho = −0.344, p = 0.02 |
| CD14++CD16+ (%) | Lymphocytes (N) | CD4+ T-Cells (N) |
| Phosphorus rho = 0.410, p = 0.006 | eGFR rho = 0.388, p = 0.009 Urea rho = −0.374, p = 0.01 Creatinine rho = −0.408, p = 0.006 Hemoglobin rho = 0.469, p = 0.001 Calcium rho = 0.378, p = 0.01 | eGFR rho = 0.495, p = 0.001 Urea rho = −0.381, p = 0.01, Creatinine rho = −0.505, p <0.001 Hemoglobin rho = 0.522, p < 0.001 Calcium rho = 0.371, p = 0.01 PTH rho = −0.324, p = 0.03 |
| CD14+CD16++ (N) | Lymphocytes (%) | CD8+ T Cells (N) |
| Urea rho = 0.298, p = 0.005 | UPCR rho = −0.439, p = 0.003 eGFR rho = 0.395, p = 0.008 Urea rho = −0.408, p = 0.006 Creatinine rho = −0.475, p = 0.001 Hemoglobin rho = 0.370, p = 0.013 Calcium rho = 0.478, p = 0.008 | Urea rho = −0.345, p = 0.02 Ferritin rho = −0.318, p = 0.04 |
| CD14++% | NK Cells (%) | CD4+ T-Cells (%) |
|---|---|---|
| Hemoglobin rho = −0.37, p = 0.05 Glucose rho = −0.33, p = 0.04 Calcium rho = −0.39, p = 0.014 | ESR rho = 0.393, p = 0.01 | Tacrolimus C0 rho = 0.708, p < 0.001 |
| CD14++CD16− (N) | Lymphocytes (N) | CD8+ T Cells (N) |
| iPTH rho = −0.328, p = 0.04 | eGFR rho = 0.359 p = 0.02 Cyclosporine C0 rho = −0.594, p = 0.02 | Hemoglobin rho = 0.358, p = 0.02 eGFR rho = 0.362, p = 0.02 |
| CD14++CD16+ (%) | Lymphocytes (%) | B-Lymphocytes (N) |
| Phosphorus rho = 0.33, p = 0.04 | eGFR rho = 0.359 p = 0.02 Cyclosporine C0 rho = −0.594, p = 0.02 | Phosphorus rho = −0.430, p = 0.007 Urea rho = −0.426, p = 0.008 Creatinine rho = −0.369, p = 0.02 UPCR rho = −0.335, p = 0.039 Cyclosporine C0 rho = 0.686, p = 0.007 |
| CD14+CD16++ (N) | T-Lymphocytes (N) | B-Lymphocytes (%) |
| Calcium rho = 0.388, p =0.01 | eGFR rho = 0.376 p = 0.02 Cyclosporine C0 Rho = −0.550, p = 0.04 Tacrolimus C0 rho = 0.439, p = 0.03 | Phosphorus rho = −0.411, p = 0.01 Urea rho = −0.349, p = 0.03 UPCR rho = −0.405, p = 0.01 |
| CD14+CD16++ (%) | T-Lymphocytes (%) | Tregs (%) |
| Calcium rho = 0.388, p = 0.01 | Cyclosporine C2 rho = 0.609, p = 0.02 Tacrolimus C0 rho = 0.419, p = 0.047 | Tacrolimus C0 rho = 0.504, p = 0.01 |
| NK Cells (N) | CD4+ T Cells (N) | |
| ESR rho = 0.395, p = 0.01 Calcium rho = 0.356, p = 0.028 | iPTH (N) rho = −0.340, p = 0.03 |
| CKD Patients | KTRs | |||
|---|---|---|---|---|
| Baseline | Δ | Baseline | Δ | |
| LAVI, mL/m2 | 32.6 ± 10.2 | NA | 32.2 ± 10.6 | NA |
| LVMI, g/m2 | 112.3 ± 36.5 | NA | 99.09 (84.3–134.6) | NA |
| RWT | 0.46 (0.42–0.54) | NA | 0.46 (0.38–0.51) | NA |
| EF, % | 68 ± 7 | 3 ± 9 | 65 ± 7 | 10 ± 7 |
| TAPSE, cm | 2.5 ± 0.43 | 0.05 ± 0.44 | 2.2 (1.9–2.4) | 0.14 ±0.46 |
| MAPSE septal, cm | 1.3 (1.2–1.6) | −0.26 ± 0.35 | 1.32 ± 0.29 | 0.14 ± 0.24 |
| MAPSE lateral, cm | 1.7 ± 0.3 | 0.43 ± 0.32 | 1.6 ± 0.3 | 0.029 ± 0.27 |
| E/A | 0.80 (0.65–97) | −0.05 (−0.13–0.09) | 0.91 ±0.24 | −0.05 (−0.19–0.16) |
| E’ average, cm | 0.09 ± 0.01 | 0.012 ± 0.020 | 0.088 ± 0.018 | 0.023 (0.010–0.040) |
| E/E’ | 8.4 ± 2.8 | 0.1 (−1.3–1.5) | 8.8 (7.6–9.6) | −1.2 (−2.7–0.7) |
| Sm, cm/s | 0.08 (0.06–0.09) | 0.01 ± 0.02 | 0.08 (0.07–0.09) | 0.02 (0–0.04) |
| Sl, cm/s | 0.09 (0.08–0.11) | 0.02 (0.00–0.02) | 0.09 (0.08–0.1) | 0.017 (±0.024) |
| GLS, % | −20.3 ± 3.1 | −1.9 ± 4.1 | −21.1 (−21.9–−18.1) | −2.5 ± 3.3 |
| GRS, % | 27.9 ±15.3 | 0.0 (−11.0–15.7) | 21.9 (13.2–37.4) | −1.84 ±21.4 |
| GCS, % | −25.9 (−30.6–−21.0) | −3.0 ± 7.8 | −28.7 ± 7.0 | −1.73 ± 9.2 |
| TWIST, degrees | 9.1 ± 4.3 | −1.1 (−2.7–3.2) | 6.2 (3.4–9.5) | 3.2 ± 6.7 |
| UNTWIST, degrees/s | −77.6 ± 34.1 | −10.5 ± 40.2 | −55.0 (−88.9–−34.4) | −51.4 ± 48 |
References
- Patel, N.; Yaqoob, M.M.; Aksentijevic, D. Cardiac metabolic remodelling in chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Coppolino, G.; De Nicola, L.; Serra, R.; Garofalo, C.; Andreucci, M.; Bolignano, D. Unraveling Cardiovascular Risk in Renal Patients: A New Take on Old Tale. Front. Cell Dev. Biol. 2019, 7, 314. [Google Scholar] [CrossRef]
- Gross, M.L.; Ritz, E. Hypertrophy and fibrosis in the cardiomyopathy of uremia– beyond coronary heart disease. Semin. Dial. 2008, 21, 308–318. [Google Scholar] [CrossRef]
- Dobre, M.A.; Ahlawat, S.; Schelling, J.R. Chronic kidney disease associated cardiomyopathy: Recent advances and future perspectives. Curr. Opin. Nephrol. Hypertens. 2024, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Hsu, C.; Li, Y.; Mishra, R.K.; Keane, M.; Rosas, S.E.; Dries, D.; Xie, D.; Chen, J.; He, J.; et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J. Am. Soc. Nephrol. 2012, 23, 1725–1734. [Google Scholar] [CrossRef]
- Pluta, A.; Stróżecki, P.; Krintus, M.; Odrowąż-Sypniewska, G.; Manitius, J. Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1–3. Ren. Fail. 2015, 37, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Lakkas, L.; Naka, K.K.; Bechlioulis, A.; Duni, A.; Moustakli, M.; Balafa, O.; Theodorou, I.; Katsouras, C.S.; Dounousi, E.; Michalis, L.K. Coronary microcirculation and left ventricular diastolic function but not myocardial deformation indices are impaired early in patients with chronic kidney disease. Echocardiography 2023, 40, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Panoulas, V.F.; Sulemane, S.; Konstantinou, K.; Bratsas, A.; Elliott, S.J.; Dawson, D.; Frankel, A.H.; Nihoyannopoulos, P. Early detection of subclinical left ventricular myocardial dysfunction in patients with chronic kidney disease. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 539–548. [Google Scholar] [CrossRef]
- Krishnasamy, R.; Isbel, N.M.; Hawley, C.M.; Pascoe, E.M.; Burrage, M.; Leano, R.; Haluska, B.A.; Marwick, T.H.; Stanton, T. Left ventricular global longitudinal strain (GLS) is a superior predictor of all-cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS ONE 2015, 10, e0127044. [Google Scholar] [CrossRef]
- Hawwa, N.; Shrestha, K.; Hammadah, M.; Yeo, P.S.D.; Fatica, R.; Tang, W.H.W. Reverse Remodeling and Prognosis Following Kidney Transplantation in Contemporary Patients With Cardiac Dysfunction. J. Am. Coll. Cardiol. 2015, 66, 1779–1787. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.; Park, J.B.; Lee, J.; Huh, K.H.; Hong, G.R.; Ha, J.W.; Choi, J.O.; Shim, C.Y. Changes in Cardiac Structure and Function after Kidney Transplantation: A New Perspective Based on Strain Imaging. J. Cardiovasc. Imaging 2023, 31, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Harb, S.; Hawwa, N.; Tang, W.; Nakhoul, G.; Fatica, R.; Popovic, Z.; Jaber, W. Impact of end-stage renal disease on left and right ventricular mechanics: Does kidney transplantation reverse the abnormalities? JACC Cardiovasc. Imaging 2017, 10, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Lakkas, L.; Naka, K.K.; Bechlioulis, A.; Girdis, I.; Duni, A.; Koutlas, V.; Moustakli, M.; Katsouras, C.S.; Dounousi, E.; Michalis, L.K. The prognostic role of myocardial strain indices and dipyridamole stress test in renal transplantation patients. Echocardiography 2020, 37, 62–70. [Google Scholar] [CrossRef]
- Rangaswami, J.; Mathew, R.O.; Parasuraman, R.; Tantisattamo, E.; Lubetzky, M.; Rao, S.; Yaqub, M.S.; Birdwell, K.A.; Bennett, W.; Dalal, P.; et al. Cardiovascular disease in the kidney transplant recipient: Epidemiology, diagnosis and management strategies. Nephrol. Dial. Transplant. 2019, 34, 760–773. [Google Scholar] [CrossRef]
- Goyal, A.; Chatterjee, K.; Mathew, R.O.; Sidhu, M.S.; Bangalore, S.; McCullough, P.A.; Rangaswami, J. In-hospital mortality and major adverse cardiovascular events after kidney transplantation in the United States. Cardiorenal Med. 2019, 9, 51–60. [Google Scholar] [CrossRef]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
- Baci, D.; Bosi, A.; Parisi, L.; Buono, G.; Mortara, L.; Ambrosio, G.; Bruno, A. Innate Immunity Effector Cells as Inflammatory Drivers of Cardiac Fibrosis. Int. J. Mol. Sci. 2020, 21, 7165. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, B.J.; Lip, G.Y.; Shantsila, E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur. J. Heart Fail. 2011, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Duni, A.; Naka, K.K.; Vartholomatos, G.; Zoccali, C. The Innate Immune System and Cardiovascular Disease in ESKD: Monocytes and Natural Killer Cells. Curr. Vasc. Pharmacol. 2021, 19, 63–76. [Google Scholar] [CrossRef]
- Putko, B.N.; Wang, Z.; Lo, J.; Anderson, T.; Becher, H.; Dyck, J.R.B.; Kassiri, Z.; Oudit, G. Alberta HEART Investigators. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: Evidence for a divergence in pathophysiology. PLoS ONE 2014, 9, e99495. [Google Scholar] [CrossRef]
- Mewhort, H.E.; Lipon, B.D.; Svystonyuk, D.A.; Teng, G.; Guzzardi, D.G.; Silva, C.; Yong, V.W.; Fedak, P.W. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-beta1. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H716–H724. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.; Starlinger, P.; Zajc, P.; Alidzanovic, L.; Maier, T.; Buchberger, E.; Pop, L.; Gruenberger, B.; Gruenberger, T.; Brostjan, C. Monocytes with angiogenic potential are selectively induced by liver resection and accumulate near the site of liver regeneration. BMC Immunol. 2014, 15, 50. [Google Scholar] [CrossRef]
- Nevers, T.; Salvador, A.M.; Grodecki-Pena, A.; Knapp, A.; Velázquez, F.; Aronovitz, M.; Kapur, N.K.; Karas, R.H.; Blanton, R.M.; Alcaide, P. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ. Heart Fail. 2015, 8, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Blanton, R.M.; Carrillo-Salinas, F.J.; Alcaide, P. T-cell recruitment to the heart: Friendly guests or unwelcome visitors? Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H124–H140. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef]
- Heine, G.H.; Ulrich, C.; Seibert, E.; Seiler, S.; Marell, J.; Reichart, B.; Krause, M.; Schlitt, A.; Köhler, H.; Girndt, M. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008, 73, 622–629. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Große-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef]
- Winterberg, P.D.; Robertson, J.M.; Kelleman, M.S.; George, R.P.; Ford, M.L. T Cells Play a Casual Role in Diastolic Dysfunction during Uremic Cardiomyopathy. J. Am. Soc. Nephrol. 2019, 30, 407–420. [Google Scholar] [CrossRef]
- Crépin, T.; Legendre, M.; Carron, C.; Vachey, C.; Courivaud, C.; Rebibou, J.M.; Ferrand, C.; Laheurte, C.; Vauchy, C.; Gaiffe, E.; et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol. Dial. Transplant. 2020, 35, 624–632. [Google Scholar] [CrossRef]
- Ducloux, D.; Courivaud, C.; Bamoulid, J.; Bisaccia, V.; Roubiou, C.; Crepin, T.; Gaugler, B.; Laheurte, C.; Rebibou, J.M.; Chalopin, J.M.; et al. Alloimmune responses and atherosclerotic disease after kidney transplantation. Transplantation 2015, 99, 220–225. [Google Scholar] [CrossRef]
- Duni, A.; Kitsos, A.; Bechlioulis, A.; Markopoulos, G.S.; Lakkas, L.; Baxevanos, G.; Mitsis, M.; Vartholomatos, G.; Naka, K.K.; Dounousi, E. Differences in the Profile of Circulating Immune Cell Subsets in Males with Type 2 Cardiorenal Syndrome versus CKD Patients without Established Cardiovascular Disease. Biomedicines 2023, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Koenig, W.; Libby, P.; Everett, B.M.; Lefkowitz, M.; Thuren, T.; Cornel, J.H. Inhibition of Interleukin-1β by Canakinumab and Cardiovascular Outcomes in Patients With Chronic Kidney Disease. J. Am. Coll. Cardiol. 2018, 71, 2405–2414. [Google Scholar] [PubMed]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef]
- Righetto, M.; Mancini, M.; Modonutti, D.; Calpista, A.; Beltrami, P.; Dal Moro, F. Patients with renal transplant and moderate-to-severe LUTS benefit from urodynamic evaluation and early transurethral resection of the prostate. World J. Urol. 2021, 39, 4397–4404. [Google Scholar] [CrossRef] [PubMed]
- Mongirdienė, A.; Liobikas, J. Phenotypic and Functional Heterogeneity of Monocyte Subsets in Chronic Heart Failure Patients. Biology 2022, 11, 195. [Google Scholar] [CrossRef]
- Loperena, R.; Van Beusecum, J.P.; Itani, H.A.; Engel, N.; Laroumanie, F.; Xiao, L.; Elijovich, F.; Laffer, C.L.; Gnecco, J.S.; Noonan, J.; et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: Roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc. Res. 2018, 114, 1547–1563. [Google Scholar] [CrossRef]
- Delaney, J.A.C.; Olson, N.C.; Sitlani, C.M.; Fohner, A.E.; Huber, S.A.; Landay, A.L.; Heckbert, S.R.; Tracy, R.P.; Psaty, B.M.; Feinstein, M.; et al. Natural killer cells, gamma delta T cells and classical monocytes are associated with systolic blood pressure in the multi-ethnic study of atherosclerosis (MESA). BMC Cardiovasc. Disord. 2021, 21, 45. [Google Scholar] [CrossRef]
- Shimoni, S.; Meledin, V.; Bar, I.; Fabricant, J.; Gandelman, G.; George, J. Circulating CD14(+) monocytes in patients with aortic stenosis. J. Geriatr. Cardiol. 2016, 13, 81–87. [Google Scholar]
- Tsujioka, H.; Imanishi, T.; Ikejima, H.; Kuroi, A.; Takarada, S.; Tanimoto, T.; Kitabata, H.; Okochi, K.; Arita, Y.; Ishibashi, K. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 130–138. [Google Scholar] [CrossRef]
- Martins, S.; António, N.; Rodrigues, R.; Carvalheiro, T.; Tomaz, C.; Gonçalves, L.; Paiva, A. Role of monocytes and dendritic cells in cardiac reverse remodelling after cardiac resynchronization therapy. BMC Cardiovasc. Disord. 2023, 23, 558. [Google Scholar] [CrossRef] [PubMed]
- Barisione, C.; Garibaldi, S.; Ghigliotti, G.; Fabbi, P.; Altieri, P.; Casale, M.C.; Spallarossa, P.; Bertero, G.; Balbi, M.; Corsiglia, L.; et al. CD14CD16 monocyte subset levels in heart failure patients. Dis. Markers. 2010, 28, 115–124. [Google Scholar] [CrossRef]
- Suzuki, A.; Fukuzawa, K.; Yamashita, T.; Yoshida, A.; Sasaki, N.; Emoto, T.; Takei, A.; Fujiwara, R.; Nakanishi, T.; Yamashita, S.; et al. Circulating intermediate CD14++CD16+monocytes are increased in patients with atrial fibrillation and reflect the functional remodelling of the left atrium. Europace 2017, 19, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Heinzmann, D.; Fuß, S.; Ungern-Sternberg, S.V.; Schreieck, J.; Gawaz, M.; Gramlich, M.; Seizer, P. TGFβ Is Specifically Upregulated on Circulating CD14++ CD16+ and CD14+ CD16++ Monocytes in Patients with Atrial Fibrillation and Severe Atrial Fibrosis. Cell Physiol. Biochem. 2018, 49, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Boidin, M.; Lip, G.Y.H.; Shantsila, A.; Thijssen, D.; Shantsila, E. Dynamic changes of monocytes subsets predict major adverse cardiovascular events and left ventricular function after STEMI. Sci. Rep. 2023, 13, 48. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.L.; Sun, T.; Liu, J.; Li, P.; Yang, J.C.; Gao, F. Role of circulating CD14++CD16+ monocytes and VEGF-B186 in formation of collateral circulation in patients with hyperacute AMI. Heliyon 2023, 9, e17692. [Google Scholar] [CrossRef]
- Švachová, V.; Krupičková, L.; Novotný, M.; Fialová, M.; Mezerová, K.; Čečrdlová, E.; Lánská, V.; Slavčev, A.; Viklický, O.; Viklický, O.; et al. Changes in phenotypic patterns of blood monocytes after kidney transplantation and during acute rejection. Physiol. Res. 2021, 70, 709–721. [Google Scholar] [CrossRef]
- Ulrich, C.; Heine, G.H.; Gerhart, M.K.; Köhler, H.; Girndt, M. Proinflammatory CD14+CD16+ monocytes are associated with subclinical atherosclerosis in renal transplant patients. Am. J. Transplant. 2008, 8, 103–110. [Google Scholar] [CrossRef]
- Laroumanie, F.; Douin-Echinard, V.; Pozzo, J.; Lairez, O.; Tortosa, F.; Vinel, C.; Delage, C.; Calise, D.; Dutaur, M.; Parini, A.; et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 2014, 129, 2111–2124. [Google Scholar] [CrossRef]
- Sereti, E.; Stamatelopoulos, K.S.; Zakopoulos, N.A.; Evangelopoulou, A.; Mavragani, C.P.; Evangelopoulos, M.E. Hypertension: An immune related disorder? Clin. Immunol. 2019, 212, 108247. [Google Scholar] [CrossRef] [PubMed]
- Itani, H.A.; McMaster, W.G., Jr.; Saleh, M.A.; Nazarewicz, R.R.; Mikolajczyk, T.P.; Kaszuba, A.M.; Konior, A.; Prejbisz, A.; Januszewicz, A.; Norlander, A.E.; et al. Activation of human t cells in hypertension: Studies of humanized mice and hypertensive humans. Hypertension 2016, 68, 123–132. [Google Scholar] [CrossRef]
- Benson, L.N.; Liu, Y.; Deck, K.S.; Mora, C.; Mu, S. Interferon Gamma contributes to the immune mechanisms of hypertension. Kidney360 2022, 3, 164–2173. [Google Scholar] [CrossRef]
- Benson, L.N.; Liu, Y.; Wang, X.; Xiong, Y.; Rhee, S.W.; Guo, Y.; Deck, K.S.; Mora, C.J.; Li, L.X.; Huang, L.; et al. The IFNγ-PDL1 pathway enhances CD8T-DCT interaction to promote hypertension. Circ. Res. 2022, 130, 1550–1564. [Google Scholar] [CrossRef]
- Youn, J.C.; Yu, H.T.; Lim, B.J.; Koh, M.J.; Lee, J.; Chang, D.Y.; Choi, Y.S.; Lee, S.H.; Kang, S.M.; Jang, Y.; et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013, 62, 126–133. [Google Scholar] [CrossRef]
- Ilatovskaya, D.V.; Pitts, C.; Clayton, J.; Domondon, M.; Troncoso, M.; Pippin, S.; DeLeon-Pennell, K.Y. CD8+ T-cells negatively regulate inflammation post-myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H581–H596. [Google Scholar] [CrossRef] [PubMed]
- Gnakamene, J.B.; Safar, M.E.; Levy, B.I.; Escoubet, B. Left ventricular torsion associated with aortic stiffness in hypertension. J. Am. Heart Assoc. 2018, 7, e007427. [Google Scholar] [CrossRef]
- Duni, A.; Vartholomatos, G.; Balafa, O.; Ikonomou, M.; Tseke, P.; Lakkas, L.; Rapsomanikis, K.P.; Kitsos, A.; Theodorou, I.; Pappas, C.; et al. The Association of Circulating CD14++CD16+ Monocytes, Natural Killer Cells and Regulatory T Cells Subpopulations With Phenotypes of Cardiovascular Disease in a Cohort of Peritoneal Dialysis Patients. Front. Med. 2021, 8, 724316. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.; Ligons, D.L.; Barin, J.G.; Wu, L.; Talor, M.V.; Diny, N.; Fontes, J.A.; Gebremariam, E.; Kass, D.A.; Rose, N.R.; et al. Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am. J. Pathol. 2015, 185, 847–861. [Google Scholar] [CrossRef]
- Boukouaci, W.; Lauden, L.; Siewiera, J.; Dam, N.; Hocine, H.R.; Khaznadar, Z.; Tamouza, R.; Borlado, L.R.; Charron, D.; Jabrane-Ferrat, N.; et al. Natural killer cell crosstalk with allogeneic human cardiac-derived stem/progenitor cells controls persistence. Cardiovasc. Res. 2014, 104, 290–302. [Google Scholar] [CrossRef]
- Vacher-Coponat, H.; Brunet, C.; Lyonnet, L.; Bonnet, E.; Loundou, A.; Sampol, J.; Moal, V.; Dussol, B.; Brunet, P.; Berland, Y.; et al. Natural killer cell alterations correlate with loss of renal function and dialysis duration in uraemic patients. Nephrol. Dial. Transplant. 2008, 23, 1406–1414. [Google Scholar] [CrossRef]
- Griveas, I.; Visvardis, G.; Fleva, A.; Papadopoulou, D.; Mitsopoulos, E.; Kyriklidou, P.; Manou, E.; Ginikopoulou, E.; Meimaridou, D.; Paulitou, A.; et al. Comparative analysis of immunophenotypic abnormalities in cellular immunity of uremic patients undergoing either hemodialysis or continuous ambulatory peritoneal dialysis. Ren. Fail. 2005, 27, 279–282. [Google Scholar] [CrossRef]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.U.; Zamorano, J.L. European Association of Echocardiography et al. Stress echocardiography expert consensus statement: European association of echocardiography (eae) (a registered branch of the esc). Eur. J. Echocardiogr. 2008, 9, 415–437. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]


| All Patients (N = 82) | CKD Patients (N = 44) | KTRs (N = 38) | p-Value * | |
|---|---|---|---|---|
| Age (years) | 58 ± 11 | 63 ± 11 | 53 ± 9 | <0.001 |
| Males, N (%) | 28 (64) | 27 (71) | ||
| DM, N (%) | 26 (32) | 17 (39) | 9 (24) | 0.147 |
| Arterial Hypertension, N (%) | 71 (86) | 39 (88) | 32 (84) | 0.558 |
| Transplantation vintage | - | - | 77.5 (58–111) | - |
| eGFR (mL/min/1.73 m2) | 42 (20–57) | 24 (15–41) | 55 (48–72) | <0.001 |
| UPCR (g protein/g creatinine) | 0.32 (0.13–1.92) | 1.31 (0.23–2.61) | 0.16 (0.09–0.56) | <0.001 |
| Hemoglobin (g/dL) | 12.9 ± 2.0 | 12.4 ± 1.9 | 13.4 ± 2.0 | 0.028 |
| Uric acid (mg/dL) | 7.1 ± 1.7 | 7.4 ± 1.8 | 6.8 ± 1.5 | 0.123 |
| ESR (mm/hour) | 24 (13–34) | 30 (21–42) | 15 (12–26) | 0.001 |
| CRP (mg/L) | 3 (2–6) | 3 (2–6) | 3.5 (3–7) | 0.442 |
| Glucose (mg/dL) | 101 (91–115) | 100 (89–114) | 101 (93–118) | 0.429 |
| Albumin (g/dL) | 4.2 (4–4.5) | 4.2 (3.8–4.4) | 4.2 (4–4.5) | 0.253 |
| Total proteins (g/dL) | 7.0 ± 0.6 | 7.1 ± 0.6 | 7.0 ± 0.5 | 0.614 |
| Total cholesterol (mg/dL) | 187 ± 37 | 183 ± 43 | 190 ± 28 | 0.339 |
| Triglycerides (mg/dL) | 144 (113–192) | 150 (113–200) | 142 (113–166) | 0.468 |
| LDL cholesterol (mg/dL) | 109 ± 35 | 108 ± 41 | 109 ± 26 | 0.835 |
| HDL cholesterol (mg/dL) | 46 (40–55) | 43 (36–50) | 50 (44–62) | 0.001 |
| Ferritin (ng/mL) | 65 (40–108) | 79 (48–112) | 57 (31–104) | 0.200 |
| Calcium (mg/dL) | 9.5 (9.1–9.7) | 9.3 (8.8–9.6) | 9.7 (9.4–10.1) | <0.001 |
| Phosphorus (mg/dL) | 3.4 (2.8–4.1) | 3.9 (3.3–4.6) | 2.9 (2.7–3.5) | <0.001 |
| iPTH (pg/mL) | 121 (85–226) | 156 (88–294) | 109 (74–169) | 0.048 |
| Cyclosporine N (%) | - | - | 15 (40) | - |
| Tacrolimus N (%) | - | - | 23 (60) | - |
| Statins N (%) | 57 (70) | 29 (66) | 28 (74) | 0.464 |
| ACEI/ARB N (%) | 48 (58) | 25 (57) | 23 (60) | 0.656 |
| B-blockers N (%) | 49 (60) | 23 (52) | 26 (68) | 0.115 |
| All Patients (N = 82) | CKD Patients (N = 44) | KTRs (N = 38) | p-Value * | |
|---|---|---|---|---|
| WBC (N) | 7485 (6080–9260) | 7045 (5745–8925) | 7710 (6920–10790) | 0.023 |
| Monocytes (N) | 500 (400–600) | 400 (300–600) | 600 (400–800) | 0.001 |
| Monocytes (%) | 6.7 (5.4–7.9) | 6.4 (5.3–7.4) | 7.1 (5.9–8.7) | 0.033 |
| CD14++CD16− (N) | 415 (318–522) | 366 (258–438) | 479 (354–599) | 0.001 |
| CD14++CD16− (%) | 83.7 (79.2–88.2) | 81.7 (75.9–85.6) | 87.1 (83.6–90.1) | <0.001 |
| CD14++CD16+ (N) | 31 (18–48) | 35 (24–53) | 25 (16–45) | 0.095 |
| CD14++CD16+ (%) | 6.5 (3.6–9.2) | 8.2 (5.9–11.3) | 4.6 (2.8–7.3) | <0.001 |
| CD14+CD16++ (N) | 22 (15–32) | 25 (19–36) | 18 (13–28) | 0.012 |
| CD14+CD16++ (%) | 4.6 (3.1–6.9) | 5.8 (4.3–8.2) | 3.2 (1.9–5.4) | <0.001 |
| Lymphocytes (N) | 1845 (1520–2560) | 1790 (1585–2405) | 2000 (1450–2710) | 0.451 |
| Lymphocytes (%) | 25.6 ± 8.0 | 26.4 ± 7.5 | 24.7 ± 8.5 | 0.335 |
| T-lymphocytes (N) | 1474 (1125–2053) | 1376 (1114–1796) | 1732 (1156–2228) | 0.173 |
| T-lymphocytes (%) | 79.0 ± 9.2 | 76.7 ± 9.6 | 81.4 ± 8.3 | 0.026 |
| B-lymphocytes (N) | 91 (48–157) | 94 (61–161) | 88 (33–140) | 0.196 |
| B-lymphocytes (%) | 4.8 (3.2–7.4) | 5.6 (3.7–7.9) | 4.3 1.9–6.7) | 0.042 |
| NK cells (N) | 276 (170–358) | 304 (178–370) | 257 (150–324) | 0.201 |
| NK cells (%) | 14.4 (9.7–18.1) | 16.5 (11.3–19.3) | 13.2 (7.9–18.8) | 0.056 |
| CD4+ T-cells (N) | 830 (608–1187) | 830 (595–1101) | 835 (610–1299) | 0.395 |
| CD4+ T-cells (%) | 46.2 ± 9.9 | 45.2 ± 10.2 | 47.4 ± 9.6 | 0.321 |
| CD8+ T-cells (N) | 582 (447–838) | 567 (411–781) | 612 (448–896) | 0.370 |
| CD8+ T-cells (%) | 32.7 (26.5–37.1) | 32.1 (25.0–37.3) | 33.1 (28.4–37.1) | 0.491 |
| Tregs (N) | 23 (15–39) | 33 (19–48) | 19 (13–28) | 0.002 |
| T Regs (%) | 1.47 (0.81–2.02) | 1.75 (1.13–2.44) | 0.93 (0.63–1.71) | <0.001 |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| R/Rho | p Value | β | p Value | ANOVA R2 p Value | |
| RWT | 0.338, p = 0.001 | ||||
| CD14++CD16− monocytes (N) | 0.638 | 0.000 | 0.447 | 0.004 | |
| B lymphocytes (%) | −0.363 | 0.030 | −0.328 | 0.03 | |
| WBC | 0.41 | 0.013 | −0.92 | 0.67 | |
| Monocytes (N) | 0.559 | 0.000 | −0.02 | 0.997 | |
| CD14+CD16++ monocytes (%) | −0.53 | 0.001 | −0.240 | 0.181 | |
| Lymphocytes (%) | −0.317 | 0.060 | −0.151 | 0.309 | |
| Arterial hypertension | 0.484 | 0.003 | 0.241 | 0.109 | |
| Albumin | −0.441 | 0.007 | −0.176 | 0.298 | |
| Proteins | −0.383 | 0.023 | −0.174 | 0.259 | |
| HDL | −0.356 | 0.033 | −0.176 | 0.258 | |
| Calcium | −0.338 | 0.044 | −0.150 | 0.320 | |
| LVEF | 0.186, p = 0.009 | ||||
| CD4+ T-cells (N) | −0.451 | 0.006 | −0.431 | 0.009 | |
| T-cells No | −0.364 | 0.029 | 0.012 | 0.969 | |
| Lymphocytes (%) | −0.353 | 0.035 | −0.171 | 0.377 | |
| Lymphocytes (N) | −0.345 | 0.04 | |||
| Hemoglobin | −0.317 | 0.059 | −0.191 | 0.260 | |
| ΔMAPSE septal | 0.360, p = 0.001 | ||||
| CD14++CD16+ monocytes (N) | −0.468 | 0.004 | −0.405 | 0.007 | |
| Urea | −0.333 | 0.048 | −0.263 | 0.074 | |
| UPCR | −0.399 | 0.016 | −0.155 | 0.333 | |
| Arterial hypertension | −0.363 | 0.030 | −0.276 | 0.070 | |
| HDL | 0.340 | 0.043 | 0.413 | 0.006 | |
| GRS | |||||
| CD14++CD16+ monocytes (%) | 0.351 | 0.041 | |||
| CD14++CD16+ monocytes (N) | 0.042 | 0.015 | |||
| TWIST | 0.164, p = 0.02 | ||||
| CD8+ T-cells (%) | 0.309 | 0.080 | 0.405 | 0.021 | |
| Glucose | 0.398 | 0.028 | −0.191 | 0.261 | |
| Triglycerides | −0.348 | 0.051 | −0.278 | 0.098 | |
| UNTWIST | 0.135, p = 0.03 | ||||
| CD8+ T-cells (%) | −0.371 | 0.033 | −0.367 | 0.036 | |
| Age | −0.389 | 0.025 | −0.287 | 0.086 | |
| Univariate Correlations | Multivariate Correlations | ||||
|---|---|---|---|---|---|
| R/Rho | p | Multivariate β | p | ANOVA R2 p Value | |
| LVEF | 0.357, p = 0.002 | ||||
| Tregs (%) | −0.384 | 0.033 | −0.341 | 0.033 | |
| Female gender | 0.456 | 0.01 | 0.454 | 0.006 | |
| CD14++CD16+ monocytes (N) | −0.351 | 0.05 | −0.150 | 0.350 | |
| ΔLVEF | 0.319, p = 0.005 | ||||
| Albumin | 0.447 | 0.012 | 0.353 | 0.034 | |
| LDL | 0.381 | 0.034 | 0.354 | 0.06 | |
| CD14++CD16− (%) | −0.447 | 0.036 | −0.174 | 0.179 | |
| T-cells (%) | −0.475 | 0.007 | 0.95 | 0.557 | |
| CD4+ T-cells (N) | −0.483 | 0.006 | −0.378 | 0.024 | |
| CD8+ T-cells (N) | −0.371 | 0.004 | −0.262 | 0.140 | |
| TAPSE | 0.288, p = 0.001 | ||||
| CD8+ T-cells (%) | 0.494 | 0.005 | 0.559 | 0.001 | |
| CD4+ T-cells (N) | −0.456 | 0.001 | −0.043 | 0.805 | |
| Glucose | 0.404 | 0.024 | 0.221 | 0.124 | |
| ESR | 0.349 | 0.050 | 0.239 | 0.161 | |
| MAPSE septal | 0.483, p = 0.000 | ||||
| Arterial Hypertension | −0.364 | 0.044 | −0.13 | 0.414 | |
| HDL | 0.417 | 0.017 | 0.474 | 0.002 | |
| Phosphorus | 0.369 | 0.041 | 0.376 | 0.013 | |
| Albumin | 0.331 | 0.069 | 0.87 | 0.582 | |
| CD4+ T-cells (%) | −0.303 | 0.098 | −0.359 | 0.016 | |
| ΔSm | 0.266, p = 0.01 | ||||
| CD14+CD16++ monocytes (N) | 0.643 | 0.010 | 0.151 | 0.576 | |
| CD14++ monocytes (%) | −0.489 | 0.010 | −0.516 | 0.010 | |
| ΔSl | 0.500, p = 0.000 | ||||
| Age | 0.439 | 0.032 | 0.137 | 0.405 | |
| CD14++CD16− monocytes (%) | −0.747 | 0.000 | −0.707 | 0.000 | |
| C14++CD16+ monocytes (%) | 0.484 | 0.017 | 0.104 | 0.519 | |
| C14+CD16++ monocytes % | 0.466 | 0.029 | −0.068 | 0.762 | |
| Calcium | 0.451 | 0.027 | 0.200 | 0.217 | |
| E/A | 0.350, p = 0.02 | ||||
| Age | −0.466 | 0.008 | −0.481 | 0.004 | |
| Transplant vintage | 0.596 | 0.000 | 0.211 | 0.179 | |
| NK cells (N) | −0.359 | 0.047 | −0.387 | 0.017 | |
| Hemoglobin | −0.429 | 0.016 | −0.140 | 0.435 | |
| GLS | 0.271, p = 0.01 | ||||
| Gender male | 0.443 | 0.017 | 0.434 | 0.015 | |
| NK cells (N) | −0.447 | 0.013 | −0.362 | 0.038 | |
| LDL | 0.384 | 0.036 | 0.180 | 0.311 | |
| ΔGLS | 0.435, p = 0.002 | ||||
| Hypertension | 0.315 | 0.09 | 0.364 | 0.022 | |
| CD14++CD16− monocytes (%) | 0.455 | 0.012 | 0.248 | 0.156 | |
| CD14++CD16+ monocytes (%) | - 0.374 | 0.042 | −0.423 | 0.009 | |
| CD4+ T-cells (%) | 0.386 | 0.035 | 0.403 | 0.012 | |
| CD4+ T-cells (N) | 0.386 | 0.04 | |||
| Tregs (%) | 0.324 | 0.081 | 0.274 | 0.107 | |
| Albumin | −0.320 | 0.085 | −0.260 | 0.097 | |
| TWIST | 0.345, p = 0.003 | ||||
| Monocytes (N) | −0.412 | 0.024 | −0.335 | 0.045 | |
| CD14++CD16+ monocytes (%) | 0.442 | 0.015 | 0.416 | 0.015 | |
| Lymphocytes No | −0.343 | 0.064 | −0.03 | 0.874 | |
| UNTWIST | 0.550, p = 0.009 | ||||
| DM | 0.326 | 0.079 | 0.113 | 0.647 | |
| CD14++CD16+ monocytes (%) | −0.400 | 0.029 | −0.742 | 0.009 | |
| Ferritin | −0.344 | 0.063 | −0.360 | 0.116 | |
| Cyclosporine C0 | −0.545 | 0.083 | −0.198 | 0.436 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duni, A.; Kitsos, A.; Bechlioulis, A.; Lakkas, L.; Markopoulos, G.; Tatsis, V.; Koutlas, V.; Tzalavra, E.; Baxevanos, G.; Vartholomatos, G.; et al. Identification of Novel Independent Correlations between Cellular Components of the Immune System and Strain-Related Indices of Myocardial Dysfunction in CKD Patients and Kidney Transplant Recipients without Established Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 9162. https://doi.org/10.3390/ijms25179162
Duni A, Kitsos A, Bechlioulis A, Lakkas L, Markopoulos G, Tatsis V, Koutlas V, Tzalavra E, Baxevanos G, Vartholomatos G, et al. Identification of Novel Independent Correlations between Cellular Components of the Immune System and Strain-Related Indices of Myocardial Dysfunction in CKD Patients and Kidney Transplant Recipients without Established Cardiovascular Disease. International Journal of Molecular Sciences. 2024; 25(17):9162. https://doi.org/10.3390/ijms25179162
Chicago/Turabian StyleDuni, Anila, Athanasios Kitsos, Aris Bechlioulis, Lampros Lakkas, Georgios Markopoulos, Vasileios Tatsis, Vasileios Koutlas, Eirini Tzalavra, Gerasimos Baxevanos, Georgios Vartholomatos, and et al. 2024. "Identification of Novel Independent Correlations between Cellular Components of the Immune System and Strain-Related Indices of Myocardial Dysfunction in CKD Patients and Kidney Transplant Recipients without Established Cardiovascular Disease" International Journal of Molecular Sciences 25, no. 17: 9162. https://doi.org/10.3390/ijms25179162






