Synergistic Photothermal Therapy and Chemotherapy Enabled by Tumor Microenvironment-Responsive Targeted SWCNT Delivery
Abstract
1. Introduction
2. Results and Discussion
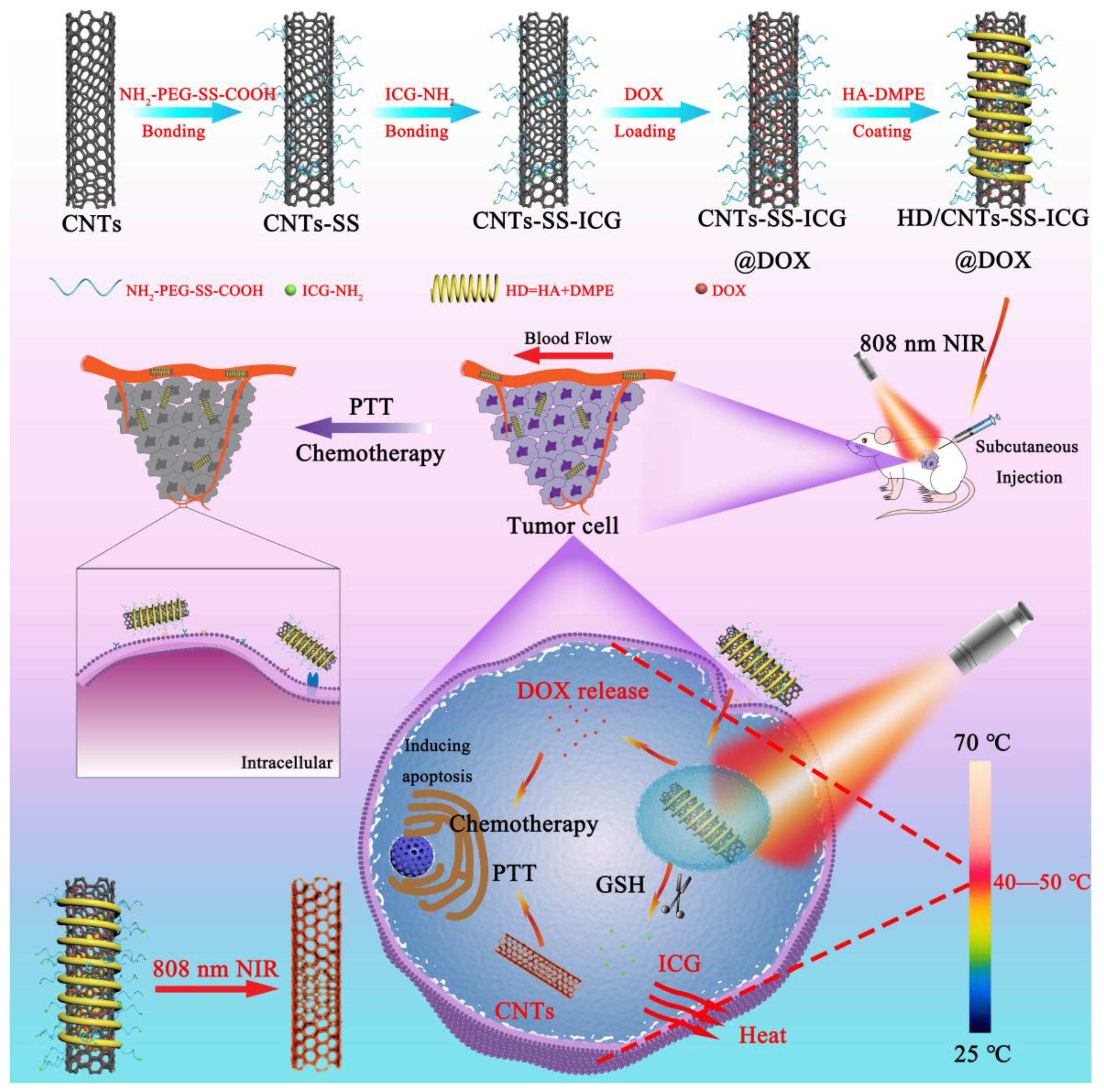
2.1. Characterization of Various SWCNT Nanocarriers
2.2. Loading Efficiency and In Vitro Release of ICG and DOX
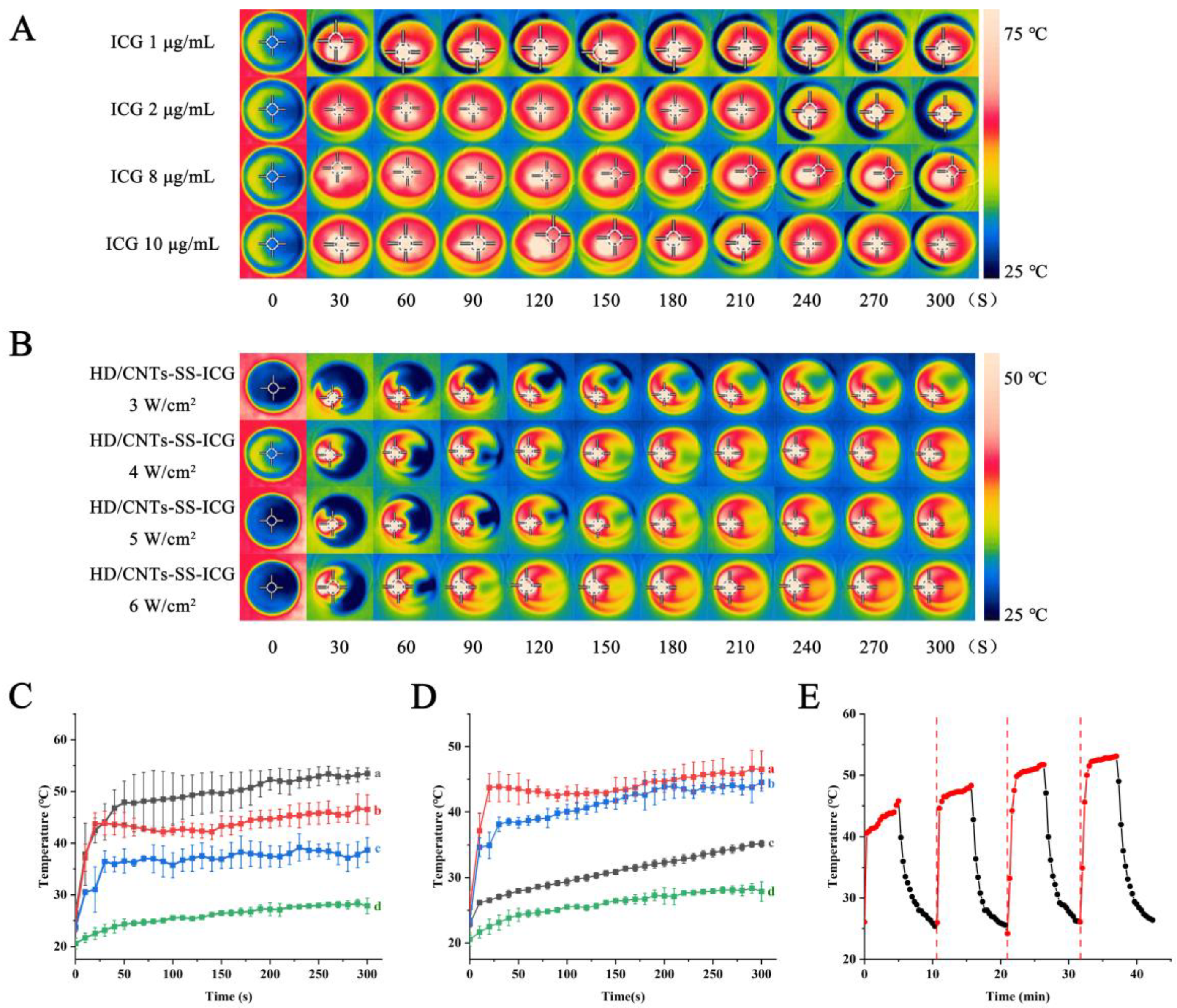
2.3. Photothermal Performance Verification
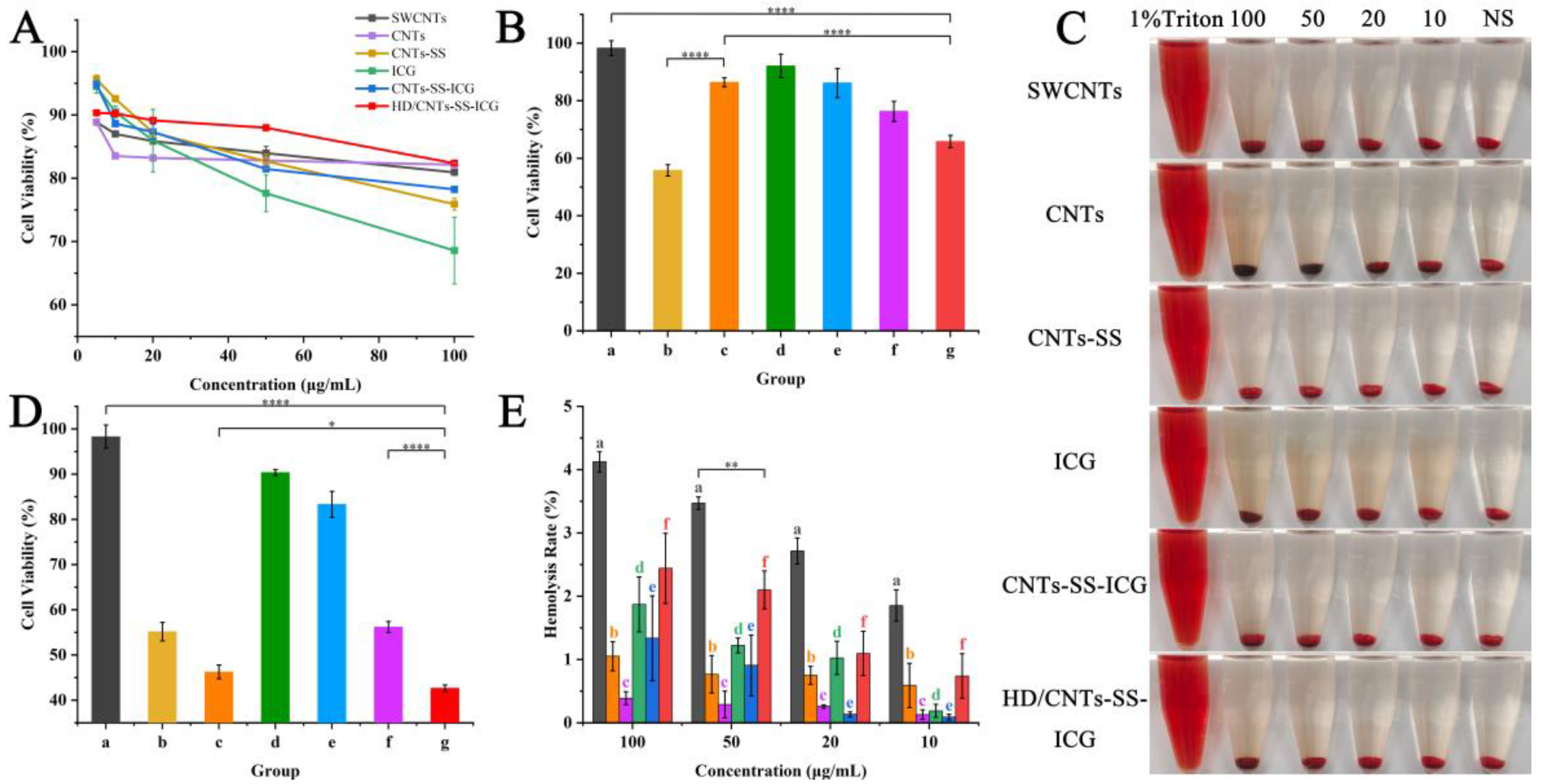
2.4. Cell Viability Assessment
2.5. Cellular Uptake and Intracellular Distribution
2.6. ROS Measurement
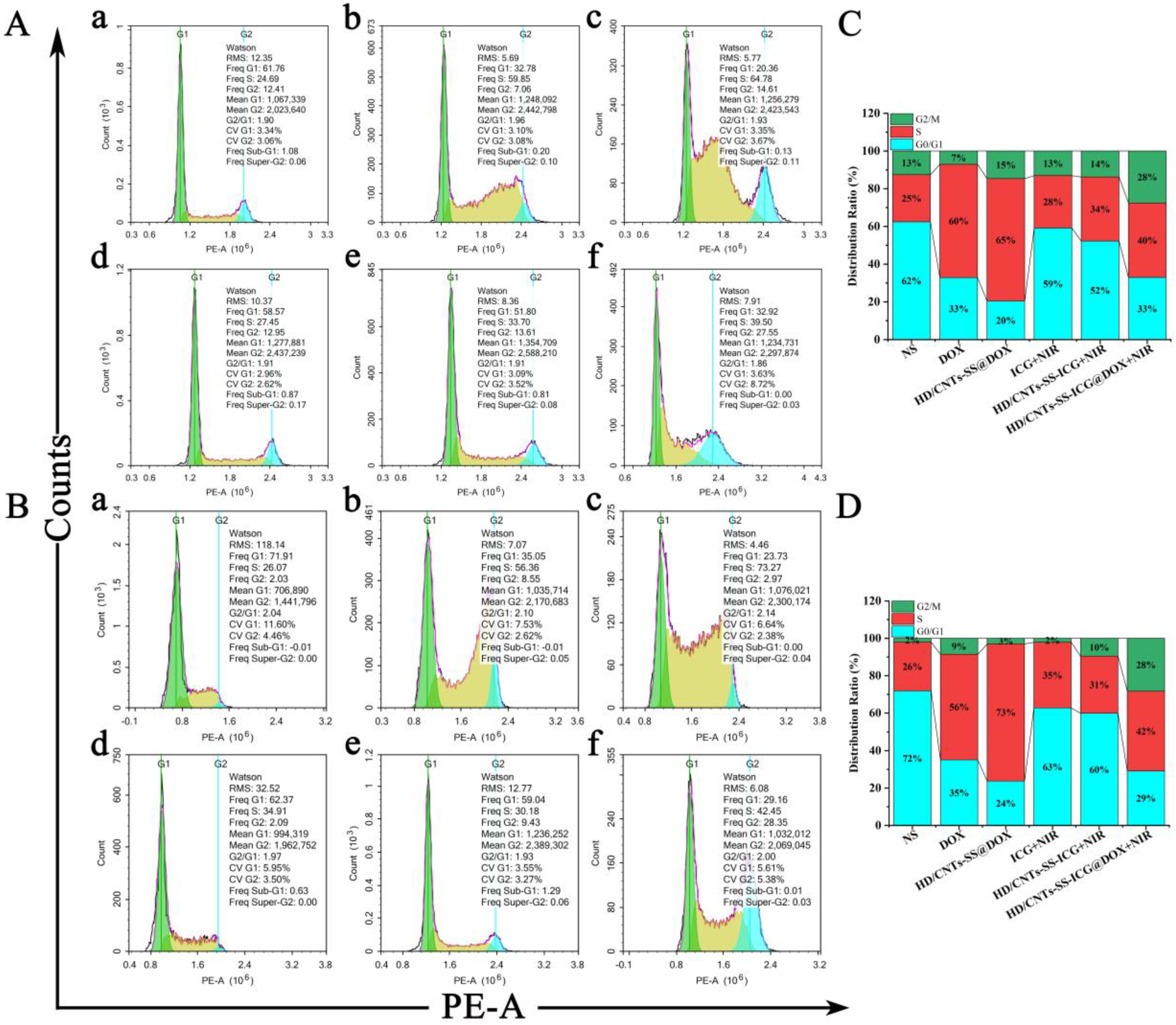
2.7. Cell Apoptosis and Cell Cycle Variation Analysis
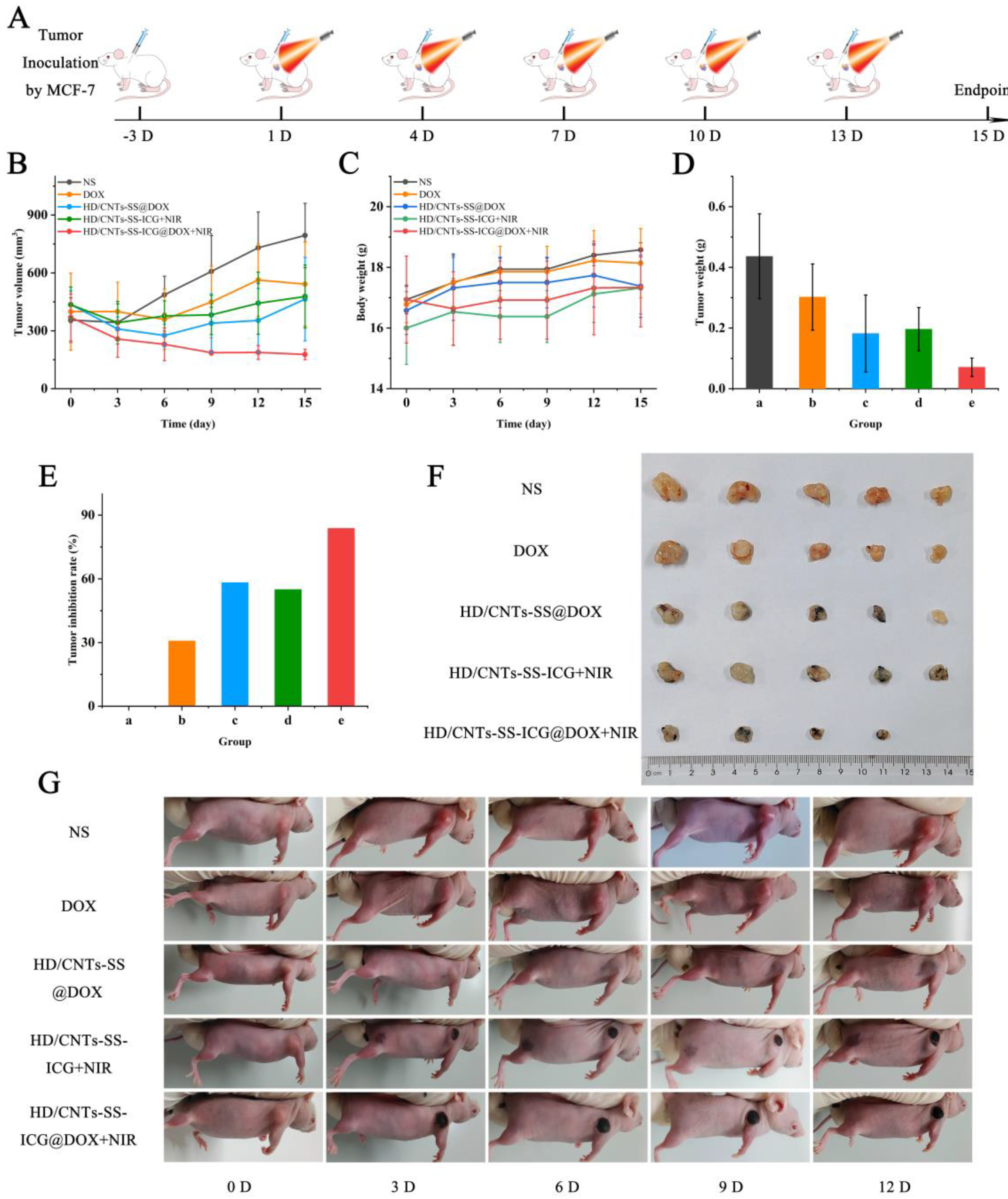
2.8. Evaluation of the Antitumor Efficacy In Vivo
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Experimental Animals
3.4. Development of the Functionalized SWCNT Delivery System
3.4.1. Synthesis of Nano-Sized CNTs
3.4.2. Preparation of CNTs-SS-ICG
3.4.3. Preparation of HD/CNTs-SS-ICG@DOX
3.5. Characterization
3.6. Photothermal Effect Analysis
3.7. Drug Loading and Encapsulation Efficiency
3.8. In Vitro Drug Release
3.9. Cytotoxicity and Hemolysis of Nanoparticles
3.10. In Vitro Antitumor Activity
3.11. Cellular Uptake
3.12. Intracellular Reactive Oxygen Species (ROS) Assessment
3.13. Cell Apoptosis and Cell Cycle Distribution Detection
3.14. In Vivo Antitumor Effect Evaluation
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Yue, Z.; Wang, R.; Yang, K.; Li, S. Nanomaterials: A powerful tool for tumor immunotherapy. Front. Immunol. 2022, 13, 979469. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, W.; Wang, H.; Mao, Y.; Di, D.; Zhao, Q.; Wang, S. Gold nanoparticles gated mesoporous carbon with optimal particle size for photothermal-enhanced thermochemotherapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125212. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Wang, Y.; Zhu, H.; Li, F.; Shen, Y.; Guo, S. A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy. Acta Biomater. 2017, 59, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-J. Redox-Sensitive Mixed Micelles Based on mPEG-SS-PTX as Drug Co-Delivery System. Master’s Thesis, Shandong University, Jinan, China, 2017. Volume 5. [Google Scholar]
- Song, H.; Peng, T.W.; Wang, X.; Li, B.B.; Wang, Y.F.; Song, D.H.; Xu, T.Z.; Liu, X.H. Glutathione-Sensitive Mesoporous Organosilica-Coated Gold Nanorods as Drug Delivery System for Photothermal Therapy-Enhanced Precise Chemotherapy. Front. Chem. 2022, 10, 842682. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold Nanocages as Photothermal Transducers for Cancer Treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Huang, R.; Jiang, J.; Wang, Y.; Zhang, J.; Jiang, H.; Xiang, X.; Chen, W.; Nie, X.; et al. Erythrocyte membrane camouflaged graphene oxide for tumor-targeted photothermal-chemotherapy. Carbon 2019, 146, 660–670. [Google Scholar] [CrossRef]
- Nghia, N.V.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar]
- Wang, X.; Zou, H.; Liu, Q. Effects of Phosphate and Silicate Combined Application on Cadmium Form Changes in Heavy Metal Contaminated Soil. Sustainability 2023, 15, 4503. [Google Scholar] [CrossRef]
- Moon, H.K.; Lee, S.H.; Choi, H.C. In Vivo Near-Infrared Mediated Tumor Destruction by Photothermal Effect of Carbon Nanotubes. Acs Nano 2009, 3, 3707–3713. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Wang, Z.W.; Ping, Y.H.; Miaol, Y.Y.; Xiao, Y.M.; Qu, L.B.; Zhang, L.; Hu, Y.S.; Wang, J.S. PEG/PEI-functionalized single-walled carbon nanotubes as delivery carriers for doxorubicin: Synthesis, characterization, and in vitro evaluation. Beilstein J. Nanotechnol. 2020, 11, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Nariya, P.; Joshi, A.; Vohra, A.; Devkar, R.; Seshadri, S.; Thakore, S. Carbon nanotube embedded cyclodextrin polymer derived injectable nanocarrier: A multiple faceted platform for stimulation of multi-drug resistance reversal. Carbohydr. Polym. 2020, 247, 116751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Cui, Y.; Chu, X.; Sun, B.; Zhou, N.; Shen, J. Magnetofluorescent Fe3O4/carbon quantum dots coated single-walled carbon nanotubes as dual-modal targeted imaging and chemo/photodynamic/photothermal triple-modal therapeutic agents. Chem. Eng. J. 2018, 338, 526–538. [Google Scholar] [CrossRef]
- Barranco, S.C.; Townsend, C.M., Jr.; Weintraub, B.; Beasley, E.G.; MacLean, K.K.; Shaeffer, J.; Liu, N.H.; Schellenberg, K. Changes in glutathione content and resistance to anticancer agents in human stomach cancer cells induced by treatments with melphalan in vitro. Cancer Res. 1990, 50, 3614–3618. [Google Scholar] [PubMed]
- Qu, Y.; Chu, B.; Wei, X.; Lei, M.; Hu, D.; Zha, R.; Zhong, L.; Wang, M.; Wang, F.; Qian, Z. Redox/pH dual-stimuli responsive camptothecin prodrug nanogels for “on-demand” drug delivery. J. Control. Release 2019, 296, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Wen, H.-Y.; Dong, H.-Q.; Xue, W.-M.; Pauletti, G.M.; Cai, X.-J.; Xia, W.-J.; Shi, D.; Li, Y.-Y. Self-assembling nanomicelles of a novel camptothecin prodrug engineered with a redox-responsive release mechanism. Chem. Commun. 2011, 47, 8647–8649. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, J.; Peng, P.; Nassab, N.H.; Smith, B.R. Quantitative Drug Release Monitoring in Tumors of Living Subjects by Magnetic Particle Imaging Nanocomposite. Nano Lett. 2019, 19, 6725–6733. [Google Scholar] [CrossRef]
- Liu, A.; Liu, Y.; Liu, G.; Zhang, A.; Cheng, Y.; Li, Y.; Zhang, L.; Wang, L.; Zhou, H.; Liu, J.; et al. Engineering of surface modified Ti3C2Tx MXene based dually controlled drug release system for synergistic multitherapies of cancer. Chem. Eng. J. 2022, 448, 137691. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Ji, X.; He, X.; Yin, Q.; Zhang, Z.; Shi, J.; Li, Y. Controlled Intracellular Release of Doxorubicin in Multidrug-Resistant Cancer Cells by Tuning the Shell-Pore Sizes of Mesoporous Silica Nanoparticles. Acs Nano 2011, 5, 9788–9798. [Google Scholar] [CrossRef]
- Yang, X.; Fan, B.; Gao, W.; Li, L.; Li, T.; Sun, J.; Peng, X.; Li, X.; Wang, Z.; Wang, B.; et al. Enhanced endosomal escape by photothermal activation for improved small interfering RNA delivery and antitumor effect. Int. J. Nanomed. 2018, 13, 4333–4344. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, T.; Cao, Y.; Sun, J.; Zhou, Q.; Chen, H.; Guo, S.; Wang, Y.; Zhen, Y.; Liang, X.-J.; et al. Temperature-Sensitive Lipid-Coated Carbon Nanotubes for Synergistic Photothermal Therapy and Gene Therapy. Acs Nano 2021, 15, 6517–6529. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Yang, S.; Yang, Y.; Zhang, L.; Cui, L. Synergistic chemotherapy and phototherapy based on red blood cell biomimetic nanomaterials. J. Control. Release 2022, 352, 146–162. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.M.; Deng, K.; Zhou, W.; Li, K.H.; Wang, C.X.; Wang, Q.; Wu, M.; Huang, S.W. GPX4 inhibition synergistically boosts mitochondria targeting nanoartemisinin-induced apoptosis/ferroptosis combination cancer therapy. Biomater. Sci. 2023, 11, 5831–5845. [Google Scholar] [CrossRef]
- Xia, L.; Chu, H.; Cui, N.; Wang, T.; Dong, S.; Cui, S.; Dai, Y.; Wang, D. In vitro and in vivo evaluation of biotin-mediated PEGylated nanostructured lipid as carrier of disulfiram coupled with copper ion. J. Drug Deliv. Sci. Technol. 2019, 51, 651–661. [Google Scholar]
- Shi, S.; Li, H.; Zhang, Y.; Shi, Y.; Zhang, N.; Li, T.; Zhang, Y.; Li, Q.; Duan, P.; Li, Y. Photocatalytic hydrogen evolution and simultaneously converting high-concentration of thiols into disulfides with excellent yield under visible-light. J. Mater. Chem. A 2023, 11, 2726–2736. [Google Scholar] [CrossRef]
- Jamuna, S.; Sadullah, S.M.S.; Ashokkumar, R.; Shanmuganathan, G.; Mozhi, S.S.; Devaraj, N.S. Potential antioxidant and cytoprotective effects of essential oil extracted from Cymbopogon citratus on OxLDL and H2O2 LDL induced Human Peripheral Blood Mononuclear Cells (PBMC). Food Sci. Hum. Wellness 2017, 6, 60–69. [Google Scholar]
- Ge, J.; Jia, Q.; Liu, W.; Lan, M.; Zhou, B.; Guo, L.; Zhou, H.; Zhang, H.; Wang, Y.; Gu, Y.; et al. Carbon Dots with Intrinsic Theranostic Properties for Bioimaging, Red-Light-Triggered Photodynamic/Photothermal Simultaneous Therapy In Vitro and In Vivo. Adv. Healthc. Mater. 2016, 5, 665–675. [Google Scholar] [CrossRef]
- Jeong, S.; Park, J.Y.; Kim, J.M.; Kim, Y. Photothermally enhanced hollow gold nanopigment for water evaporation and sterilization achieved via a photothermal effect. Build. Environ. 2023, 229, 109970. [Google Scholar] [CrossRef]
- Yang, H.; Le, Q.-V.; Shim, G.; Oh, Y.-K.; Shin, Y.K. Molecular engineering of antibodies for site-specific conjugation to lipid polydopamine hybrid nanoparticles. Acta Pharm. Sin. B 2020, 10, 2212–2226. [Google Scholar] [CrossRef]
- Xie, M.; Deng, T.; Li, J.; Shen, H. The camouflage of graphene oxide by red blood cell membrane with high dispersibility for cancer chemotherapy. J. Colloid Interface Sci. 2021, 591, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Tabasi, H.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Nekooei, S.; Nekooei, N.; Ramezani, M.; Alibolandi, M. Surface engineering of hollow gold nanoparticle with mesenchymal stem cell membrane and MUC-1 aptamer for targeted theranostic application against metastatic breast cancer. Eur. J. Pharm. Biopharm. 2023, 187, 76–86. [Google Scholar] [CrossRef]
- Alpdemir, S.; Vural, T.; Kara, G.; Bayram, C.; Haberal, E.; Denkbas, E.B. Magnetically responsive, sorafenib loaded alginate microspheres for hepatocellular carcinoma treatment. IET Nanobiotechnol. 2020, 14, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Larypoor, M. Investigation of HER-3 gene expression under the influence of carbohydrate biopolymers extract of shiitake and reishi in MCF-7 cell line. Mol. Biol. Rep. 2022, 49, 6563–6572. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhang, X.; Wang, J.; Zhao, L.; Zhao, D.; Cheng, M. Design, synthesis and biological evaluation of 2-aminopyrimidine-based LSD1 inhibitors. Bioorg. Chem. 2022, 121, 105699. [Google Scholar] [CrossRef]
- Zuo, C.; Zou, Y.; Gao, G.; Sun, L.; Yu, B.; Guo, Y.; Wang, X.; Han, M. Photothermal combined with intratumoral injection of annonaceous acetogenin nanoparticles for breast cancer therapy. Colloids Surf. B Biointerfaces 2022, 213, 112426. [Google Scholar] [CrossRef]
- Cheng, D.; Ji, Y.; Wang, B.; Wang, Y.; Tang, Y.; Fu, Y.; Xu, Y.; Qian, X.; Zhu, W. Dual-responsive nanohybrid based on degradable silica-coated gold nanorods for triple-combination therapy for breast cancer. Acta Biomater. 2021, 128, 435–446. [Google Scholar] [CrossRef]
- Xu, Z.; Dong, Y.; Peng, F.; Yu, Z.; Zu, Y.; Dai, Z.; Chen, Y.; Wang, J.; Hu, X.; Zhou, Q.; et al. Pigment epithelium-derived factor enhances tumor response to radiation through vasculature normalization in allografted lung cancer in mice. Cancer Gene Ther. 2015, 22, 181–187. [Google Scholar] [CrossRef] [PubMed]
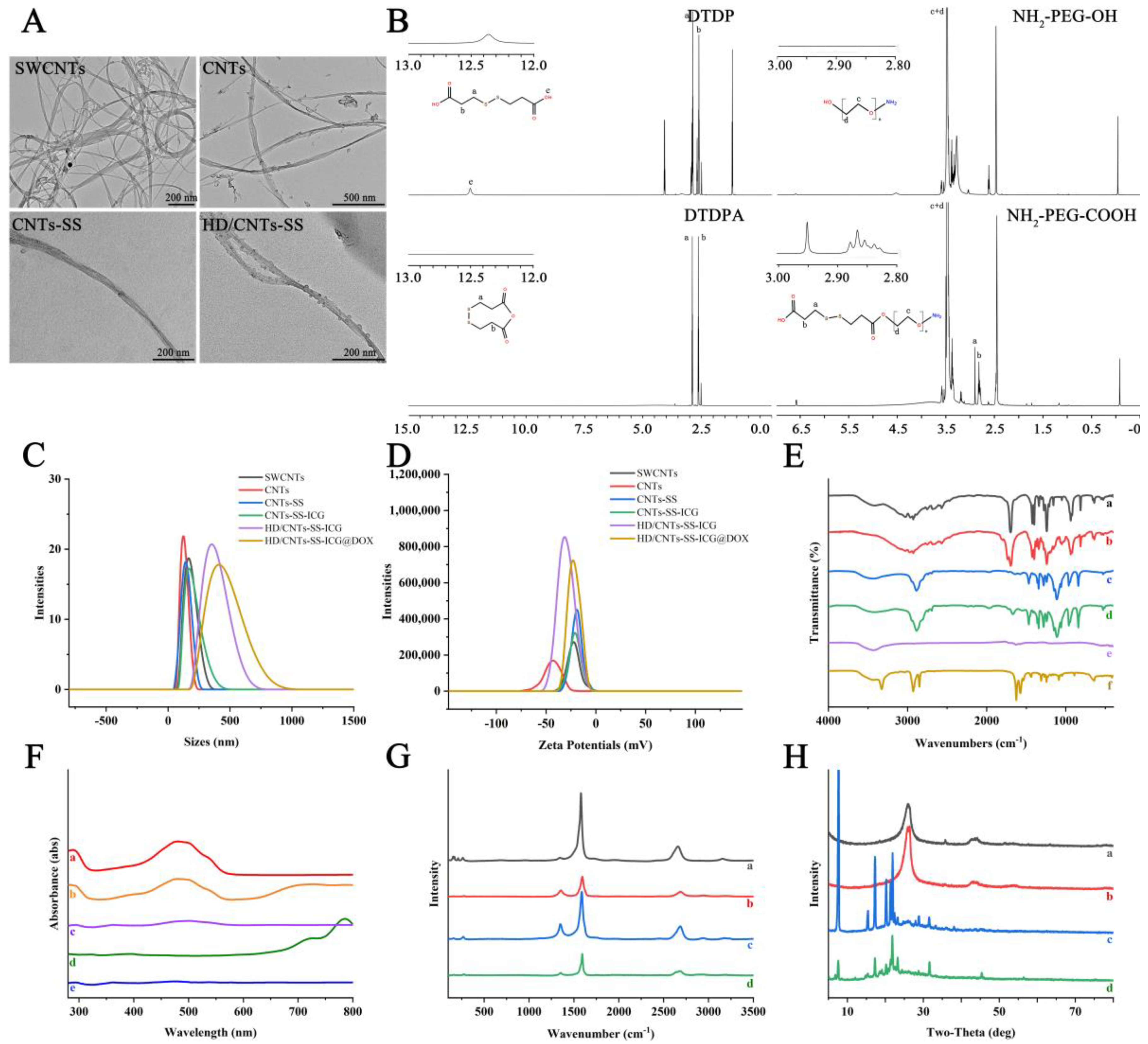




| Samples | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| SWCNTs | 164.2 ± 3.04 | 0.444 | −21.8 ± 1.91 |
| CNTs | 122.4 ± 5.23 | 0.333 | −41.7 ± 2.65 |
| CNTs-SS | 141.8 ± 1.62 | 0.238 | −18.5 ± 0.91 |
| CNTs-SS-ICG | 164.2 ± 2.31 | 0.325 | −12.2 ± 0.83 |
| HD/CNTs-SS-ICG | 342.0 ± 5.11 | 0.349 | −35.1 ± 1.89 |
| HD/CNTs-SS-ICG@DOX | 343.6 ± 3.34 | 0.350 | −18.5 ± 2.44 |
| Samples | Initial Melting Point (°C) | Average (°C) | RSD (%) (a) | Total Melting Point (°C) | Average (°C) | RSD (%) (a) |
|---|---|---|---|---|---|---|
| DTDP (b) | 148.7 | 149.2 | 0.34 | 157.4 | 156.1 | 0.95 |
| 149.7 | 154.5 | |||||
| 149.1 | 156.5 | |||||
| DTDPA (c) | 64.7 | 65.3 | 0.85 | 69.8 | 70.3 | 1.3 |
| 65.8 | 69.7 | |||||
| 65.4 | 71.3 |
| Samples | Encapsulation Efficiency (%) | Drug Loading (μg/mg) |
|---|---|---|
| SWCNTs@DOX | 98.95 | 165.05 |
| CNTs@DOX | 82.33 | 140.97 |
| CNTs-SS@DOX | 60.27 | 101.63 |
| CNTs-SS-ICG@DOX | 53.92 | 90.24 |
| HD/CNTs-SS-ICG@DOX | 88.40 | 55.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Liu, J.; Yuan, H.; Cheng, Q.; Shen, W.; Lv, Y.; Xiao, Y.; Zhang, L.; Li, P. Synergistic Photothermal Therapy and Chemotherapy Enabled by Tumor Microenvironment-Responsive Targeted SWCNT Delivery. Int. J. Mol. Sci. 2024, 25, 9177. https://doi.org/10.3390/ijms25179177
Yang S, Liu J, Yuan H, Cheng Q, Shen W, Lv Y, Xiao Y, Zhang L, Li P. Synergistic Photothermal Therapy and Chemotherapy Enabled by Tumor Microenvironment-Responsive Targeted SWCNT Delivery. International Journal of Molecular Sciences. 2024; 25(17):9177. https://doi.org/10.3390/ijms25179177
Chicago/Turabian StyleYang, Shuoye, Jiaxin Liu, Huajian Yuan, Qianqian Cheng, Weiwei Shen, Yanteng Lv, Yongmei Xiao, Lu Zhang, and Peng Li. 2024. "Synergistic Photothermal Therapy and Chemotherapy Enabled by Tumor Microenvironment-Responsive Targeted SWCNT Delivery" International Journal of Molecular Sciences 25, no. 17: 9177. https://doi.org/10.3390/ijms25179177
APA StyleYang, S., Liu, J., Yuan, H., Cheng, Q., Shen, W., Lv, Y., Xiao, Y., Zhang, L., & Li, P. (2024). Synergistic Photothermal Therapy and Chemotherapy Enabled by Tumor Microenvironment-Responsive Targeted SWCNT Delivery. International Journal of Molecular Sciences, 25(17), 9177. https://doi.org/10.3390/ijms25179177






