Comparative Long Non-Coding Transcriptome Analysis of Three Contrasting Barley Varieties in Response to Aluminum Stress
Abstract
:1. Introduction
2. Results
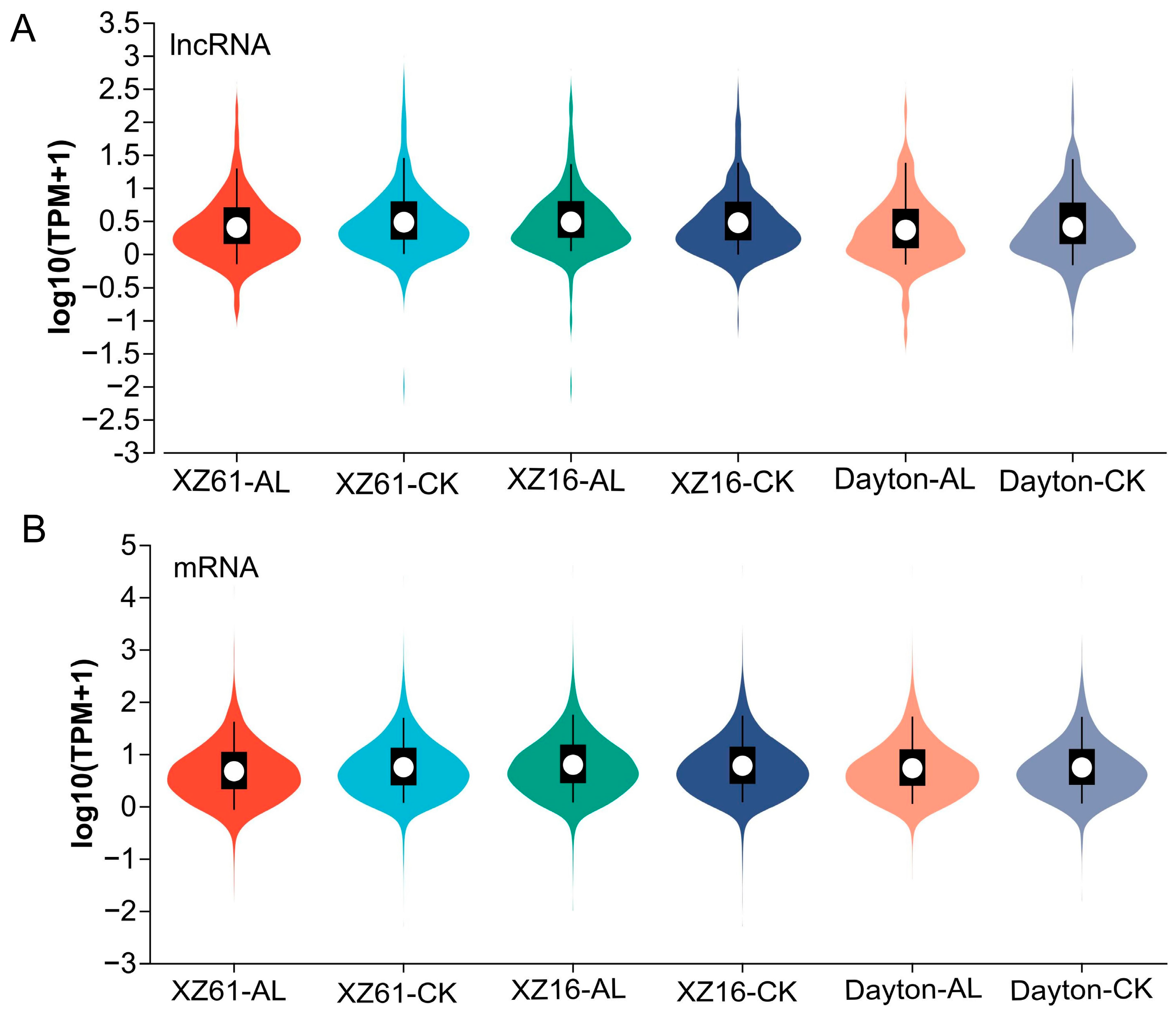
2.1. LncRNAs in Response to Aluminum Stress
2.2. Target Prediction and Family Classification of LncRNAs
2.3. Identification of sRNA Precursor in LncRNAs
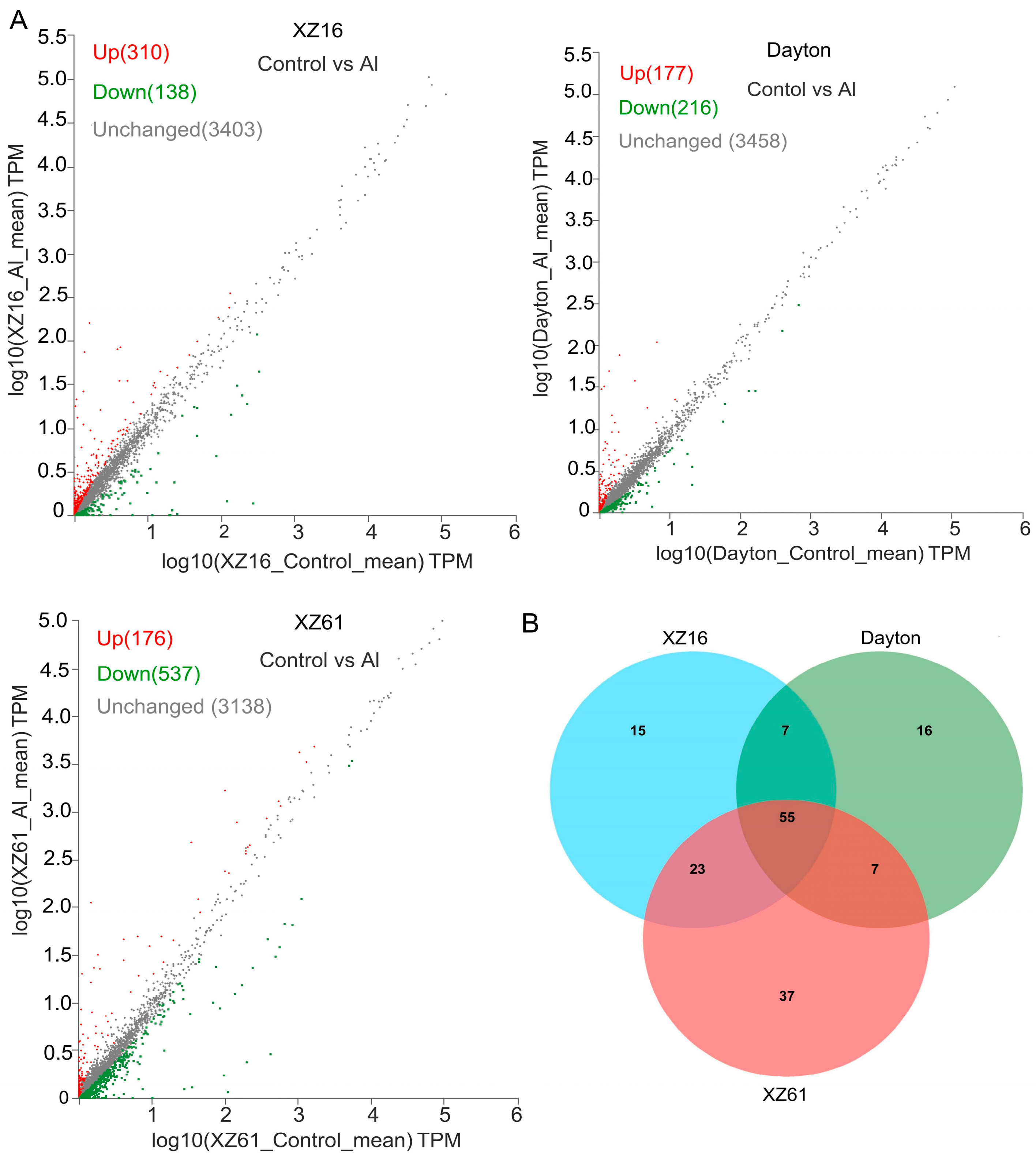
2.4. Expression Profile Analysis of LncRNA in Three Barley Genotypes
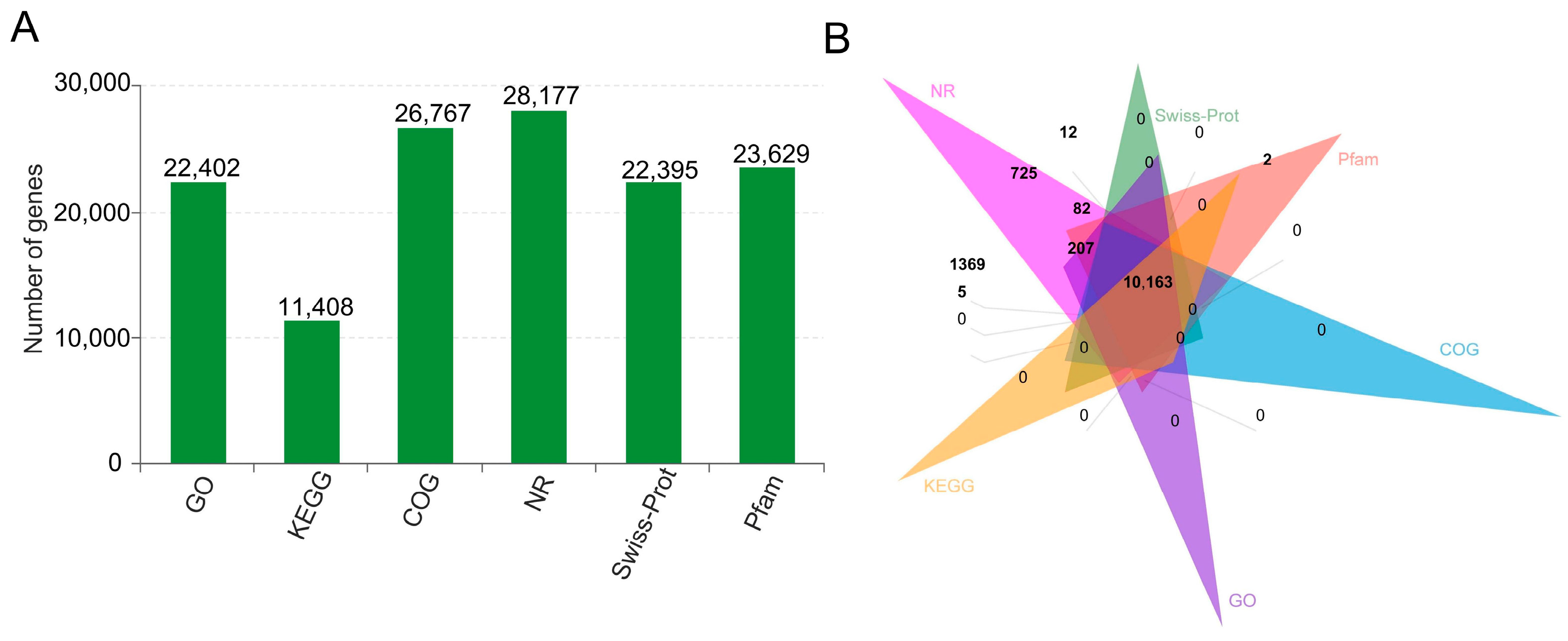
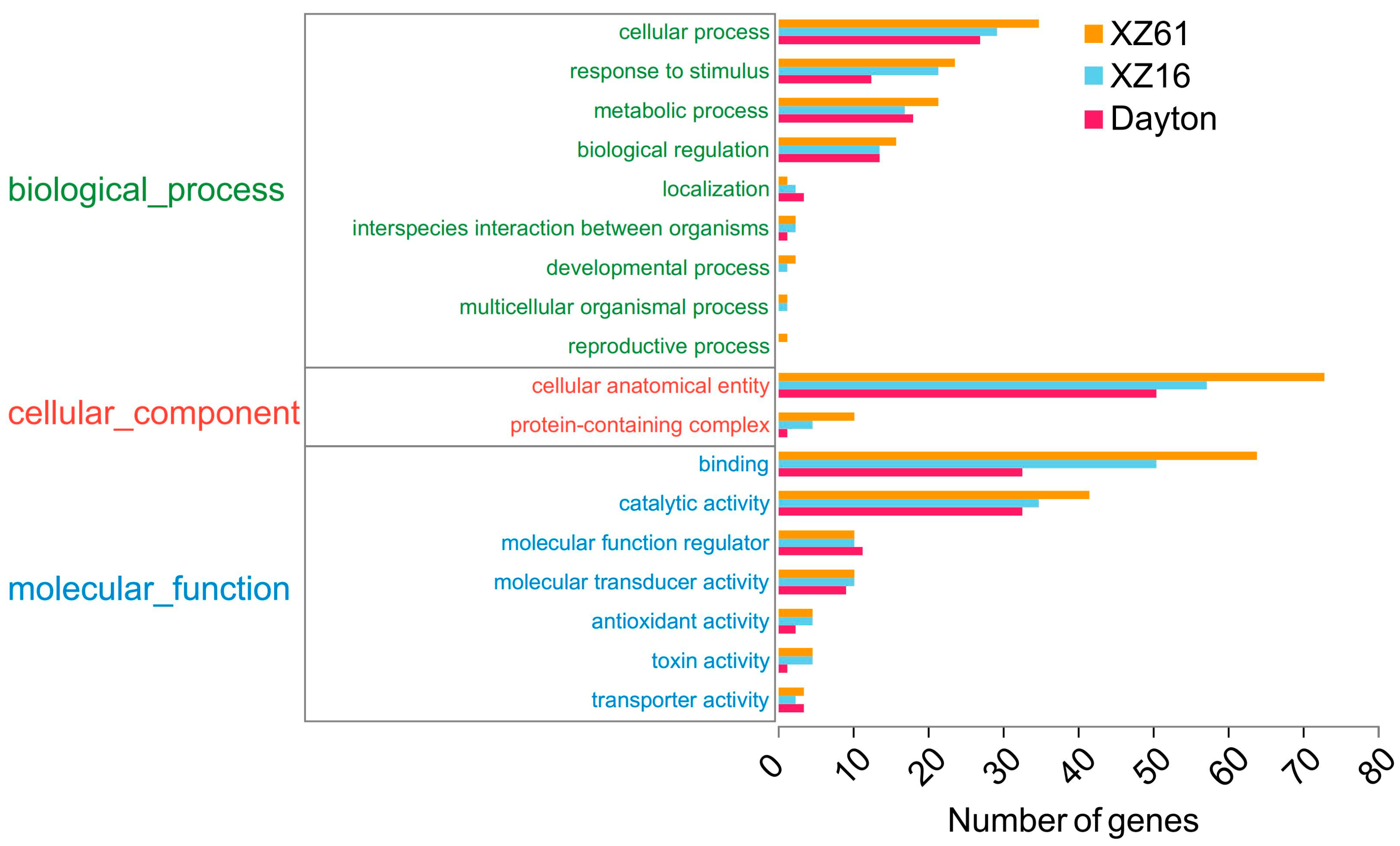
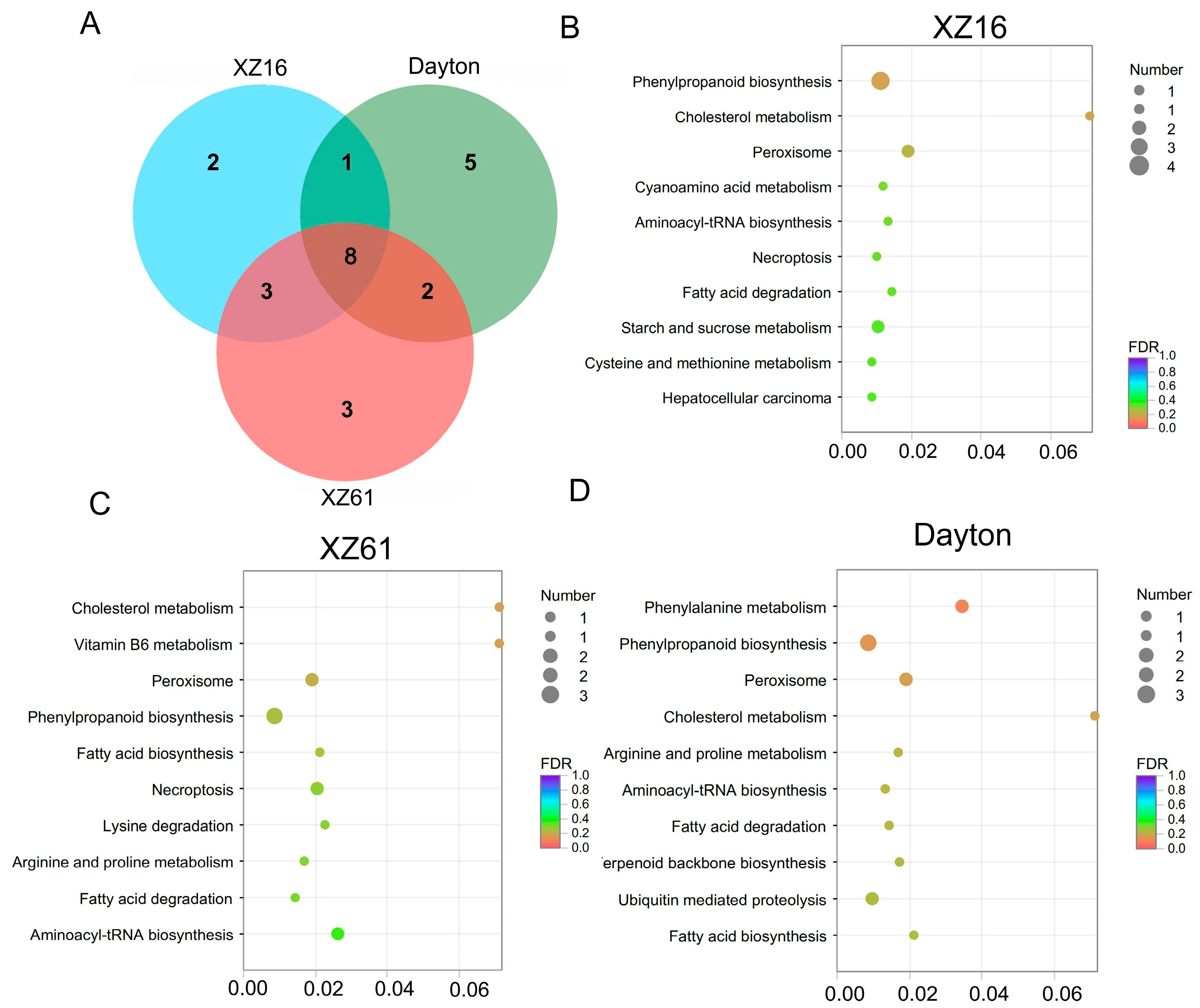
2.5. Annotation and Enrichment Analysis of LncRNA Target Genes
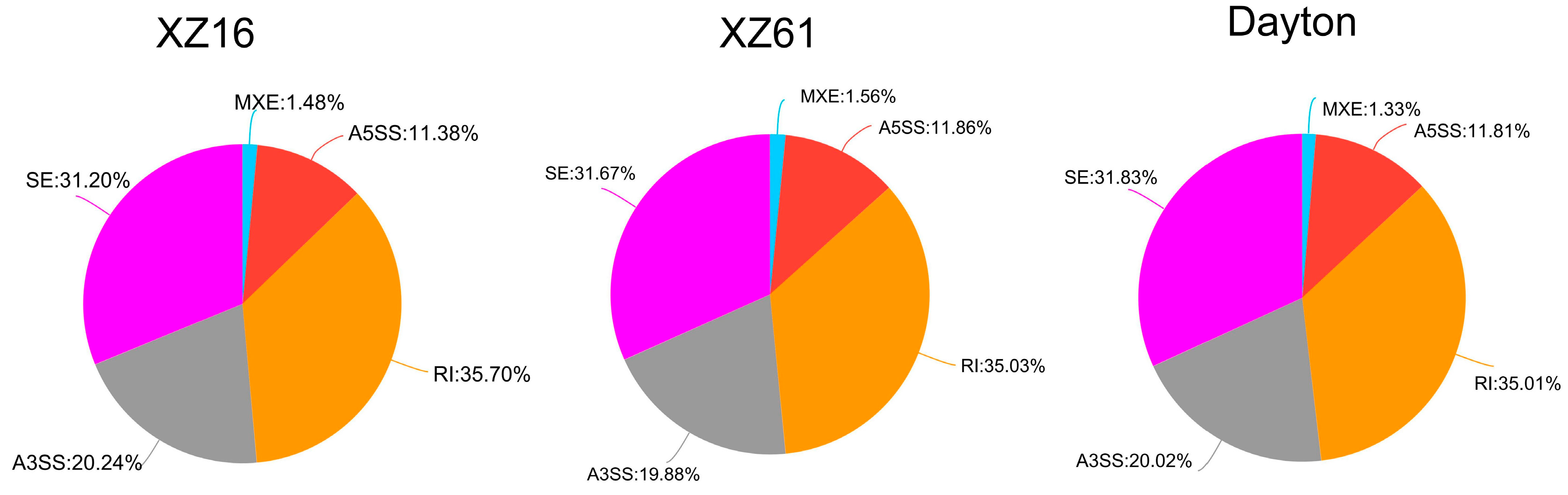
2.6. Alternative Splicing Analysis
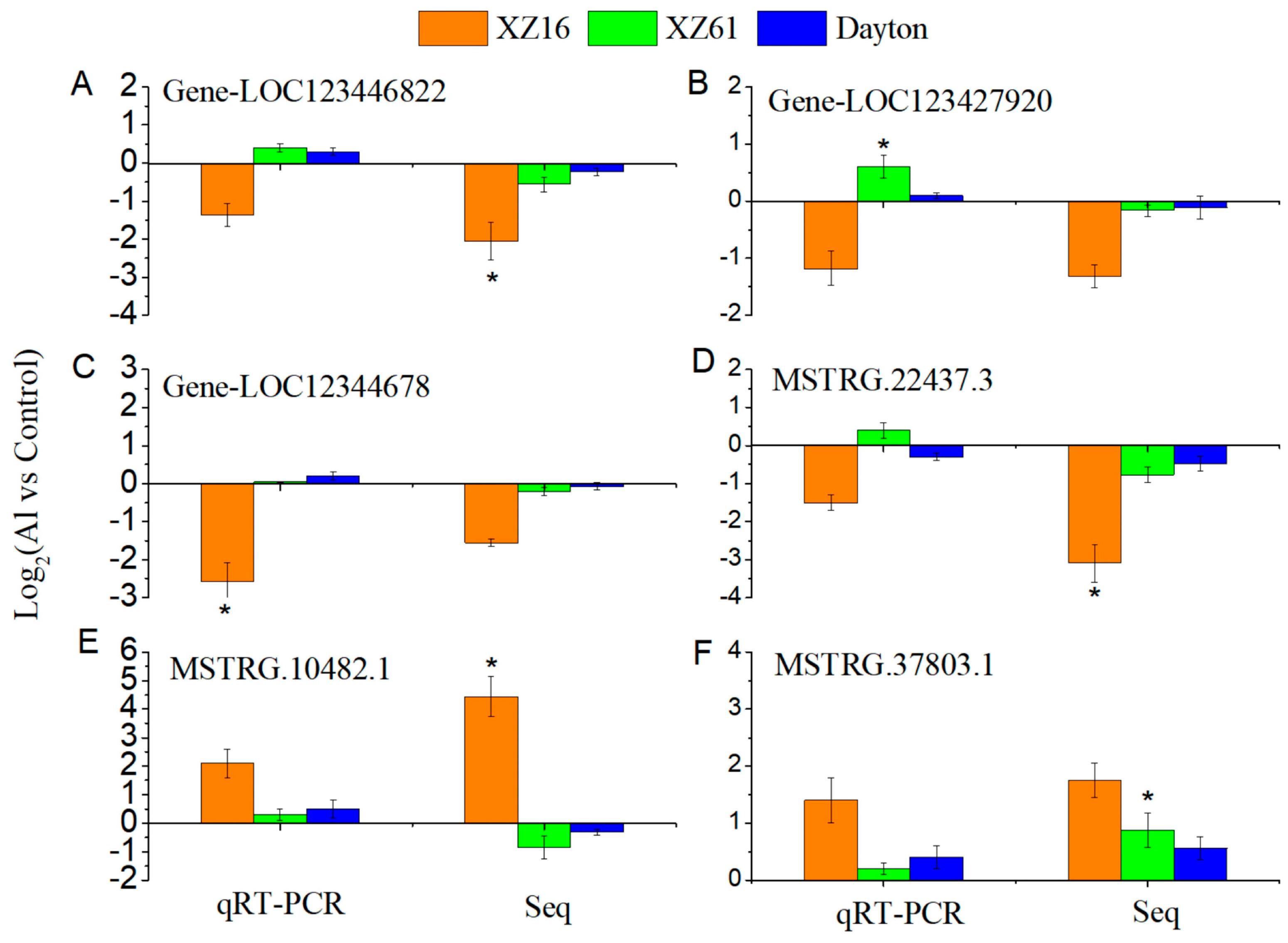
2.7. Validation of LncRNA and mRNA Expression Using qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Treatment
4.2. High-Throughput Sequencing
4.3. Mapping and De Novo Assembly
4.4. Bioinformatics Analysis of Transcriptome Data
4.5. Target Prediction and Family Analysis of LncRNAs
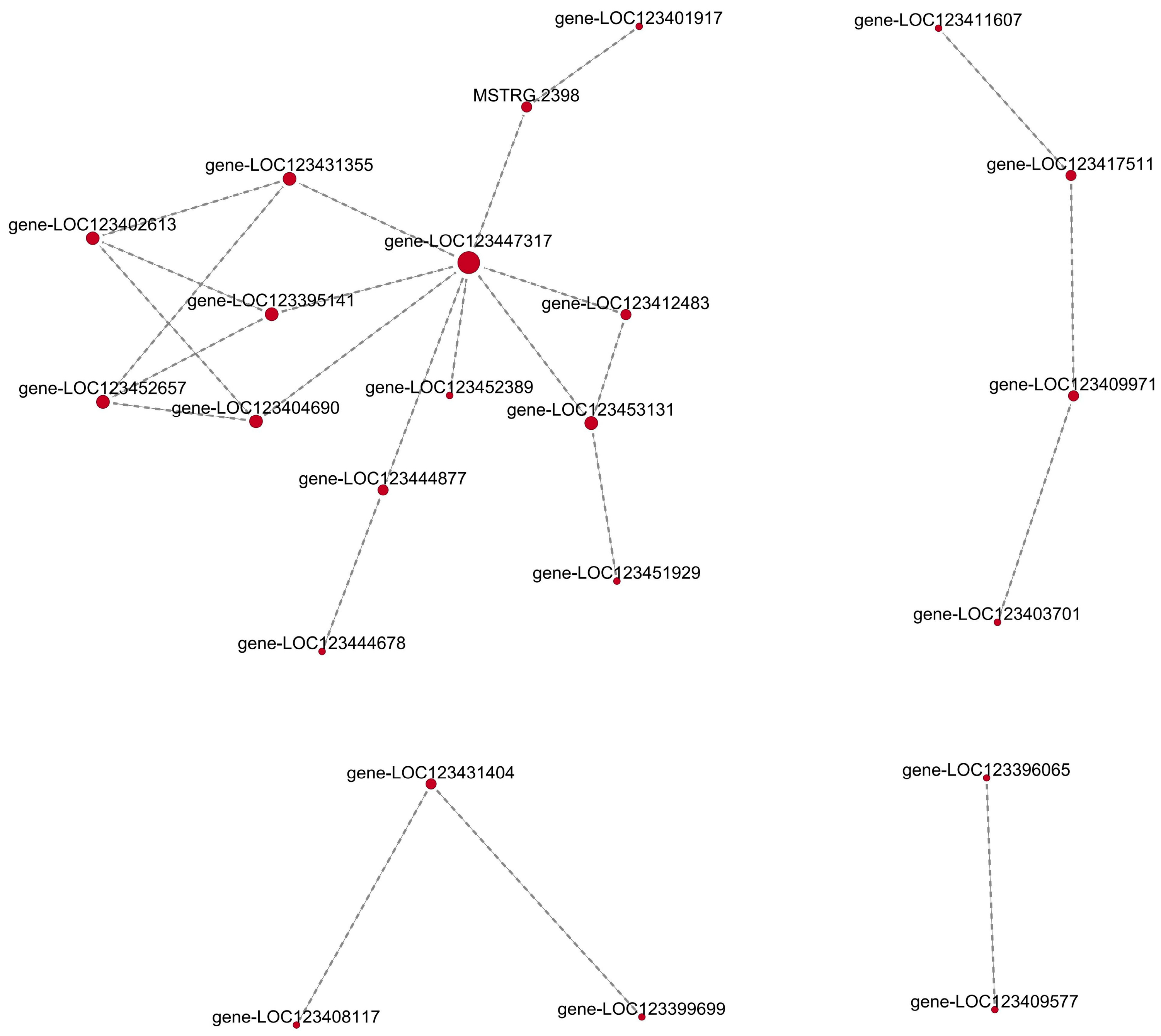
4.6. Protein–Protein Interaction Network Analysis of Differentially Expressed LncRNAs Targeted Genes in XZ16
4.7. sRNA Precursor Prediction and Analysis of Alternative Splicing
4.8. Annotation and Functional Analysis of LncRNA Target Genes
4.9. Quantitative RT-PCR Validation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Y.; Li, C.; Ryan, P.R.; Shabala, S.; You, J.; Liu, J.; Liu, C.; Zhou, M. A new allele for aluminium tolerance gene in barley (Hordeum vulgare L.). BMC Genom. 2016, 17, 186. [Google Scholar] [CrossRef]
- Dai, F.; Nevo, E.; Wu, D.; Comadran, J.; Zhou, M.; Qiu, L.; Chen, Z.; Beiles, A.; Chen, G.; Zhang, G. Tibet is one of the centers of domestication of cultivated barley. Proc. Natl. Acad. Sci. USA 2012, 109, 16969–16973. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kishor, P.B.K. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. BioMetals 2016, 29, 187–210. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Parker, D.R. Assessing the phytotoxicity of mononuclear hydroxyaluminum. Plant Cell Environ. 1989, 12, 479–487. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Guo, J.; Zhou, F.; Singh, S.; Xu, X.; Xie, Q.; Yang, Z.; Huang, C.F. F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 319–327. [Google Scholar] [CrossRef]
- Silva, T.F.; Ferreira, B.G.; Isaias, R.M.D.; Alexandre, S.S.; Franca, M.G.C. Immunocytochemistry and density functional theory evidence the competition of aluminum and calcium for pectin binding in Urochloa decumbens roots. Plant Physiol. Biochem. 2020, 153, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Coller, J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 2013, 14, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, X.; Glass, C.; Rosenfeld, M.G. The long arm of long noncoding RNAs: Roles as sensors regulating gene transcriptional programs. Cold Spring Harb. Perspect. Biol. 2011, 3, a003756. [Google Scholar] [CrossRef]
- Nejat, N.; Mantri, N. Emerging roles of long non-coding RNAs in plant response to biotic and abiotic stresses. Crit. Rev. Biotechnol. 2018, 38, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, X.; Sun, F.; Hu, J.; Zha, X.; Su, W.; Yang, J. Overexpressing lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat. Commun. 2018, 9, 3516. [Google Scholar] [CrossRef]
- Moison, M.; Pacheco, J.M.; Lucero, L.; Fonouni-Farde, C.; Rodríguez-Melo, J.; Mansilla, N.; Christ, A.; Bazin, J.; Benhamed, M.; Ibañez, F.; et al. The lncRNA APOLO interacts with the transcription factor WRKY42 to trigger root hair cell expansion in response to cold. Mol. Plant 2021, 14, 937–948. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, T.; Sun, T.; Yu, X.; Tian, R.; Zhang, W.-H. Identification of tissue-specific and cold-responsive lncRNAs in Medicago truncatula by high-throughput RNA sequencing. BMC Plant Biol. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cells 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 2016, 41, 761–772. [Google Scholar] [CrossRef]
- Kim, D.; Xi, Y.; Sung, S. Modular function of long noncoding RNA, COLDAIR, in the vernalization response. PLoS Genet. 2017, 13, e1006939. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hu, J.; Gao, C.; Chen, G.; Wang, B.; Lin, C.; Song, L.; Ding, Y.; Zhou, G. Genome-wide analysis of long non-coding RNAs unveils the regulatory roles in the heat tolerance of Chinese cabbage (Brassica rapa ssp.chinensis). Sci. Rep. 2019, 9, 5002. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, M.; Zhang, X.; Liu, M.; Yang, C.; Chen, Y.; Chen, R.; Wen, J.; Mysore, K.S.; Zhang, W.H. Novel phosphate deficiency-responsive long non-coding RNAs in the legume model plant Medicago truncatula. J. Exp. Bot. 2017, 68, 5937–5948. [Google Scholar] [CrossRef]
- Wen, X.; Ding, Y.; Tan, Z.; Wang, J.; Zhang, D.; Wang, Y. Identification and characterization of cadmium stress-related LncRNAs from Betula platyphylla. Plant Sci. 2020, 299, 110601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, Z.; Guo, Q.; Liu, Y.; Zheng, Y.; Wu, F.; Jin, W. Identification of maize long non-coding RNAs responsive to drought stress. PLoS ONE 2014, 9, e98958. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, J.; Chen, Q.; Wu, F. Genome-wide characterization of drought stress responsive long non-coding RNAs in Tibetan wild barley. Environ. Exp. Bot. 2019, 164, 124–134. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, N.; Qiu, C.; Luo, L.; Zhang, M.; Zhang, S.; Gao, Z.; Ahmed, I.; Wu, F. Transcriptome profiling uncovers the lncRNA-mediated regulatory networks associated with tolerance to cadmium stress in barley. Environ. Exp. Bot. 2023, 206, 105156. [Google Scholar] [CrossRef]
- Yang, X.; Liu, C.; Niu, X.; Wang, L.; Li, L.; Yuan, Q.; Pei, X. Research on lncRNA related to drought resistance of Shanlan upland rice. BMC Genom. 2022, 23, 336. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, J.; Ahmed, I.M.; Cao, F.; Chen, Z.; Zhang, G.; Li, C.; Wu, F. Differences in physiological features associated with aluminum tolerance in Tibetan wild and cultivated barleys. Plant Physiol. Biochem. 2014, 75, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Liu, J.; Wang, H.; Wong, L.; Chua, N. PLncDB: Plant long non-coding RNA database. Bioinformatics 2013, 29, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Zhang, L.; Liu, X.; Han, G.; Li, J.; Li, X.; Liu, A.; Liao, M.; Zhang, S. PLNlncRbase: A resource for experimentally identified lncRNAs in plants. Gene 2015, 573, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Paytuvi-Gallart, A.; Hermoso Pulido, A.; Martinez De Lagran, I.A.; Sanseverino, W.; Aiese Cigliano, R. GREENC: A Wiki-based database of plant lncRNAs. Nucleic Acids Res. 2016, 44, D1161–D1166. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Q.; Feng, X.; Yang, B.; Zhong, X.; Zhou, Y.; Wang, Q.; Mao, Y.; Xie, W.; Liu, T.; et al. Identification and Functional Analysis of Drought-Responsive Long Noncoding RNAs in Maize Roots. Int. J. Mol. Sci. 2023, 24, 15039. [Google Scholar] [CrossRef]
- Tian, R.; Sun, X.; Liu, C.; Chu, J.; Zhao, M.; Zhang, W.-H. A Medicago truncatula lncRNA MtCIR1 negatively regulates response to salt stress. Planta 2023, 257, 32. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, H.; Cui, P.; Albesher, N.; Xiong, L. A nucleus-localized long non-coding RNA enhances drought and salt stress tolerance. Plant Physiol. 2017, 175, 1321–1336. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.P.; Magalhaes, J.V. Plant adaptation to acid soils, the molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.; Qi, X. LncRNA TCONS_00021861 is functionally associated with drought tolerance in rice (Oryza sativa L.) via competing endogenous RNA regulation. BMC Plant Biol. 2021, 21, 410. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qiu, C.W.; Cao, F.; Liu, L.; Wu, F. Identification and characterization of long noncoding RNAs in two contrasting olive (Olea europaea L.) genotypes subjected to aluminum toxicity. Plant Physiol. Biochem. 2023, 202, 107906. [Google Scholar] [CrossRef]
- Gui, Q.; Yang, Z.; Chen, C.; Yang, F.; Wang, S.; Dong, R. Identification and characterization of long noncoding RNAs involved in the aluminum stress response in Medicago truncatula via genome-wide analysis. Front. Plant Sci. 2022, 13, 1017869. [Google Scholar] [CrossRef]
- Postnikova, O.A.; Nemchinov, L.G. Natural antisense transcripts associated with salinity response in alfalfa. Plant Genome 2015, 8, 2. [Google Scholar] [CrossRef]
- Chen, M.; Wang, C.; Bao, H.; Chen, H.; Wang, Y. Genome-wide identification and characterization of novel lncRNAs in Populus under nitrogen deficiency. Mol. Genet. Genom. 2016, 291, 1663–1680. [Google Scholar] [CrossRef]
- Feng, S.J.; Zhang, X.D.; Liu, X.S.; Tan, S.K.; Chu, S.S.; Meng, J.G.; Zhao, K.X.; Zheng, J.F.; Yang, Z.M. Characterization of long non-coding RNAs involved in cadmium toxic response in Brassica napus. RSC Adv. 2016, 6, 82157–82173. [Google Scholar] [CrossRef]
- Muthusamy, M.; Uma, S.; Backiyarani, S.; Saraswathi, M.S. Genome-wide screening for novel, drought stress-responsive long non-coding RNAs in droughtstressed leaf transcriptome of drought-tolerant and -susceptible banana (Musa spp) cultivars using Illumina high-throughput sequencing. Plant Biotechnol. Rep. 2015, 9, 279–286. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, X.; Wang, W.; Yuan, R.; Shen, F. Identification of Gossypium hirsutum long non-coding RNAs (lncRNAs) under salt stress. BMC Plant Biol. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- López-Galiano, M.J.; García-Robles, I.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Real, M.D.; Rausell, C. Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress. Plants 2019, 8, 201. [Google Scholar] [CrossRef]
- Li, N.; Yang, T.; Guo, Z.; Wang, Q.; Chai, M.; Wu, M.; Li, X.; Li, W.; Li, G.; Tang, J.; et al. Maize microRNA166 Inactivation Confers Plant Development and Abiotic Stress Resistance. Int. J. Mol. Sci. 2020, 21, 9506. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, W.; Dai, H.; Qiu, Y.; Zhang, G.; Chen, Z.H.; Wu, F. HvHOX9, a novel homeobox leucine zipper transcription factor, positively regulates aluminum tolerance in Tibetan wild barley. J. Exp. Bot. 2020, 71, 6057–6073. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Z.; Dong, S.; Li, Z.; Zou, L.; Zhao, P.; Guo, X.; Bao, Y.; Wang, W.; Peng, M. Global identification of full-length cassava lncRNAs unveils the role of cold-responsive intergenic lncRNA 1 in cold stress response. Plant Cell Environ. 2022, 45, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hao, P.; Lv, X.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; Han, Z.; Wu, T. A long non-coding apple RNA, MSTRG.85814.11, acts as a transcriptional enhancer of SAUR32 and contributes to the Fe-deficiency response. Plant J. 2020, 103, 53–67. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Y.; Chen, N.; Liu, Y.; Yang, S.; Du, H.; Wang, W.; Wu, J.; Tai, F.; Chen, F.; et al. A new adenylyl cyclase, putative disease-resistance RPP13-like protein 3, participates in abscisic acid-mediated resistance to heat stress in maize. J. Exp. Bot. 2021, 72, 283–301. [Google Scholar] [CrossRef]
- Linder, J.U. Class III adenylyl cyclases: Molecular mechanisms of catalysis and regulation. Cell. Mol. Life Sci. 2006, 63, 1736–1751. [Google Scholar] [CrossRef]
- Gehring, C.; Turek, I.S. Cyclic nucleotide monophosphates and their cyclases in plant signaling. Front. Plant Sci. 2017, 8, 1704. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Zhao, Y.; Zhao, X.; Xie, W.; Guo, Y.; Wang, Y.; Li, K.; Guo, J.; Zhu, Q.H.; et al. Identification and Characterization of Cinnamyl Alcohol Dehydrogenase Encoding Genes Involved in Lignin Biosynthesis and Resistance to Verticillium dahliae in Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2022, 13, 840397. [Google Scholar] [CrossRef]
- Yan, L.; Li, S.; Cheng, J.; Zhang, Y.; Jiang, C. Boron-mediated lignin metabolism in response to aluminum toxicity in citrus (Poncirus trifoliata (L.) Raf.) root. Plant Physiol. Biochem. 2022, 185, 1–12. [Google Scholar] [CrossRef]
- Vaghela, B.; Vashi, R.; Rajput, K.; Joshi, R. Plant chitinases and their role in plant defense: A comprehensive review. Enzyme Microb. Technol. 2022, 159, 110055. [Google Scholar] [CrossRef]
- de las Mercedes Dana, M.; Pintor-Toro, J.A.; Cubero, B. Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiol. 2006, 142, 722–730. [Google Scholar] [CrossRef]
- Huang, L.; Kuang, L.; Wu, L.; Shen, Q.; Han, Y.; Jiang, L.; Wu, D.; Zhang, G. The HKT Transporter HvHKT1;5 Negatively Regulates Salt Tolerance. Plant Physiol. 2020, 182, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 121–357. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 174–511. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.; Liu, X.; Zhao, S.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long noncoding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2015, 44, D279–D285. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Kornienko, A.E.; Guenzl, P.M.; Barlow, D.P.; Pauler, F.M. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, E.P.; Kolbe, D.L.; Eddy, S.R. Infernal 1.0: Inference of RNA alignments. Bioinformatics 2009, 25, 1713. [Google Scholar] [CrossRef] [PubMed]
- Damian, S.; Gable, A.L.; Nastou, K.C.; David, L.; Rebecca, K.; Sampo, P.; Doncheva, N.T.; Marc, L.; Fang, T.; Peer, B. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2020, 49, 605–612. [Google Scholar]
- Shannon, P.; Markeil, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]

| Sample | Genotype | |||||
|---|---|---|---|---|---|---|
| XZ16 | XZ61 | Dayton | ||||
| Control | Al | Control | Al | Control | Al | |
| Total Raw reads | 105,937,050 | 106,194,624 | 107,972,494 | 102,756,014 | 107,717,356 | 109,715,138 |
| Total clean reads | 102,743,712 | 102,680,488 | 104,662,554 | 99,076,632 | 10,4821,514 | 106,974,122 |
| Total clean reads ratio (%) | 98.61 | 98.61 | 98.62 | 98.47 | 98.58 | 98.61 |
| Total mapped reads ratio (%) | 78.84 | 79.02 | 75.0 | 69.72 | 76.21 | 76.55 |
| Uniquely mapping ratio (%) | 73.05 | 74.05 | 69.11 | 64.45 | 69.53 | 66.35 |
| Novel lncRNA gene | 922 | 925 | 934 | 966 | 890 | 904 |
| Novel lncRNA isoforms | 2177 | 2186 | 2219 | 2279 | 2166 | 2174 |
| Konwn lncRNA gene | 1876 | 1873 | 1889 | 1922 | 1922 | 1923 |
| Known lncRNA isoforms | 2497 | 2515 | 2516 | 2541 | 2564 | 2543 |
| LncRNA ID | Fold Change (Al vs. Control) | Target mRNA | Fold Change (Al vs. Control) | Annotation | ||||
|---|---|---|---|---|---|---|---|---|
| XZ16 | XZ61 | Dayton | XZ16 | XZ61 | Dayton | |||
| MSTRG.22263.1 | 1.40 | 0.49 | 0.00 | MSTRG.22262 | 3.52 | 2.33 | 0.00 | |
| MSTRG.32859.1 | 1.25 | −0.97 | −0.02 | gene-LOC123395388 | 1.01 | 0.01 | 0.36 | Uncharacterized LOC123395388 |
| MSTRG.37803.1 | 1.75 | 0.88 | 0.56 | gene-LOC123404378 | 1.32 | 0.87 | 0.26 | uncharacterized LOC123404378, transcript variant X3 |
| rna-XR_006611989.1 | 1.87 | −0.11 | 0.66 | gene-LOC123404757 | 1.82 | 1.23 | 0.36 | probable cinnamyl alcohol dehydrogenase 5 |
| MSTRG.47952.1 | 1.29 | −0.55 | 1.71 | gene-LOC123409748 | 1.01 | 0.59 | 0.39 | uncharacterized protein At1g15400-like |
| MSTRG.47952.1 | 1.29 | −0.55 | 1.71 | gene-LOC123412807 | 1.03 | 0.07 | 0.94 | phosphopantothenoylcysteine decarboxylase subunit VHS3-like, transcript variant X1 |
| MSTRG.6428.4 | 2.42 | 0.09 | −0.02 | gene-LOC123421803 | 1.39 | 0.52 | 0.41 | uncharacterized LOC123421803 |
| MSTRG.6434.1 | 3.02 | 0.92 | 0.00 | gene-LOC123421889 | 2.81 | 1.49 | 1.00 | uncharacterized LOC123421889 |
| MSTRG.10482.1 | 4.43 | −0.87 | −0.31 | gene-LOC123426388 | 4.22 | −0.29 | −0.26 | uncharacterized LOC123426388 |
| MSTRG.28048.1 | 2.28 | −0.45 | −0.33 | gene-LOC123447317 | 2.06 | −0.36 | −0.31 | endochitinase A-like |
| MSTRG.35329.1 | −1.21 | −0.33 | 0.00 | gene-LOC123452657 | 4.39 | 2.65 | 0.87 | L-type lectin-domain containing receptor kinase SIT2-like |
| MSTRG.19984.2 | −3.33 | 0.00 | 1.37 | gene-LOC123444678 | −1.56 | −0.21 | −0.07 | beta-glucosidase 2-like |
| MSTRG.22437.3 | −3.09 | −0.77 | −0.48 | gene-LOC123446822 | −2.05 | −0.56 | −0.23 | disease resistance RPP13-like protein 4, transcript variant X2 |
| MSTRG.22437.3 | −3.09 | −0.77 | −0.48 | gene-LOC123446823 | −1.98 | −0.22 | −0.27 | disease resistance RPP13-like protein 4 |
| MSTRG.13374.4 | −1.07 | −0.80 | 1.22 | gene-LOC123427920 | −1.32 | −0.16 | −0.11 | putative disease resistance protein RGA4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Chen, X.; Meng, Q.; Song, Z.; Zeng, J.; He, X.; Wu, F.; Ma, W.; Liu, W. Comparative Long Non-Coding Transcriptome Analysis of Three Contrasting Barley Varieties in Response to Aluminum Stress. Int. J. Mol. Sci. 2024, 25, 9181. https://doi.org/10.3390/ijms25179181
Feng X, Chen X, Meng Q, Song Z, Zeng J, He X, Wu F, Ma W, Liu W. Comparative Long Non-Coding Transcriptome Analysis of Three Contrasting Barley Varieties in Response to Aluminum Stress. International Journal of Molecular Sciences. 2024; 25(17):9181. https://doi.org/10.3390/ijms25179181
Chicago/Turabian StyleFeng, Xue, Xiaoya Chen, Quan Meng, Ziyan Song, Jianbin Zeng, Xiaoyan He, Feibo Wu, Wujun Ma, and Wenxing Liu. 2024. "Comparative Long Non-Coding Transcriptome Analysis of Three Contrasting Barley Varieties in Response to Aluminum Stress" International Journal of Molecular Sciences 25, no. 17: 9181. https://doi.org/10.3390/ijms25179181





