The Current State of Realistic Heart Models for Disease Modelling and Cardiotoxicity
Abstract
:1. Introduction
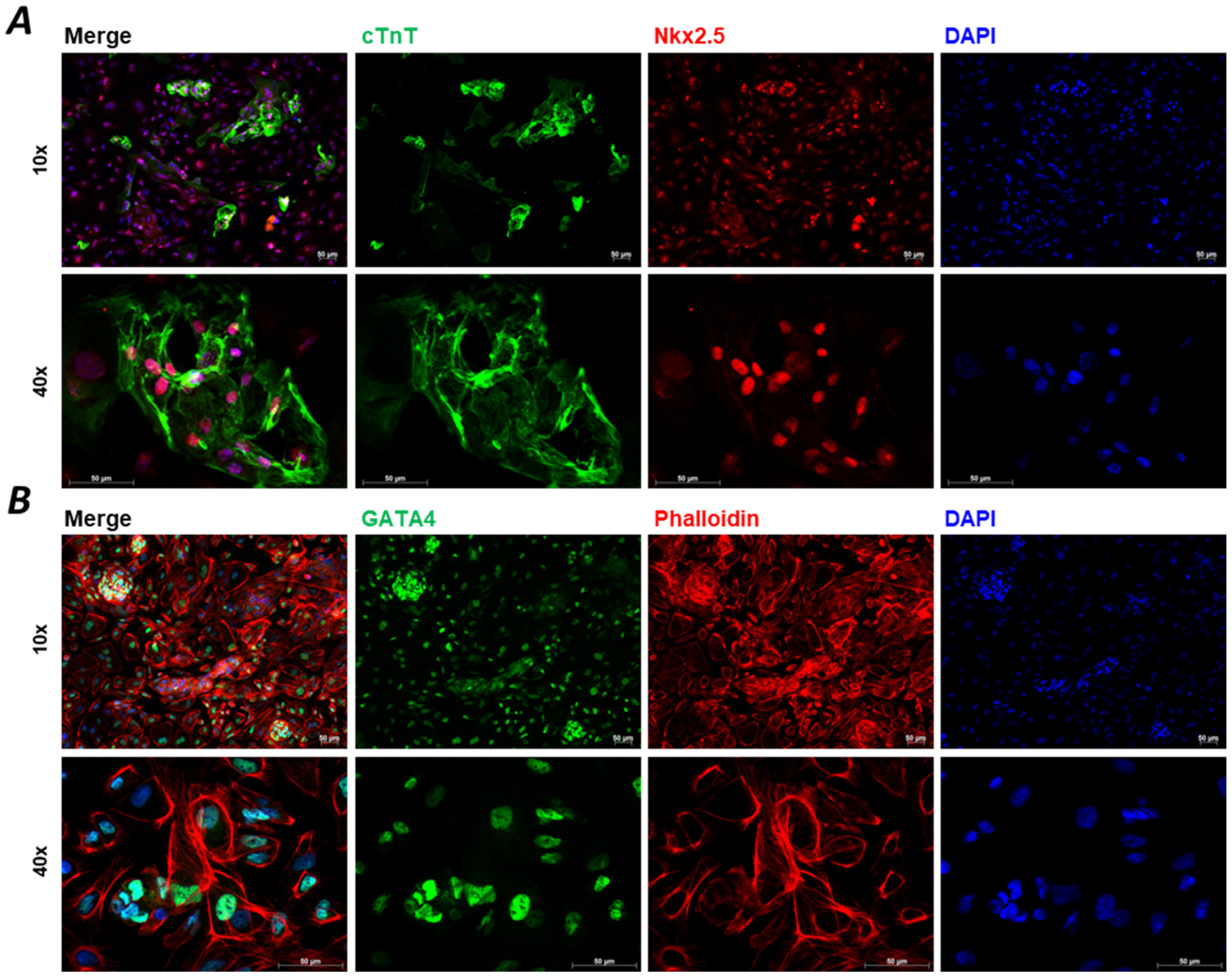
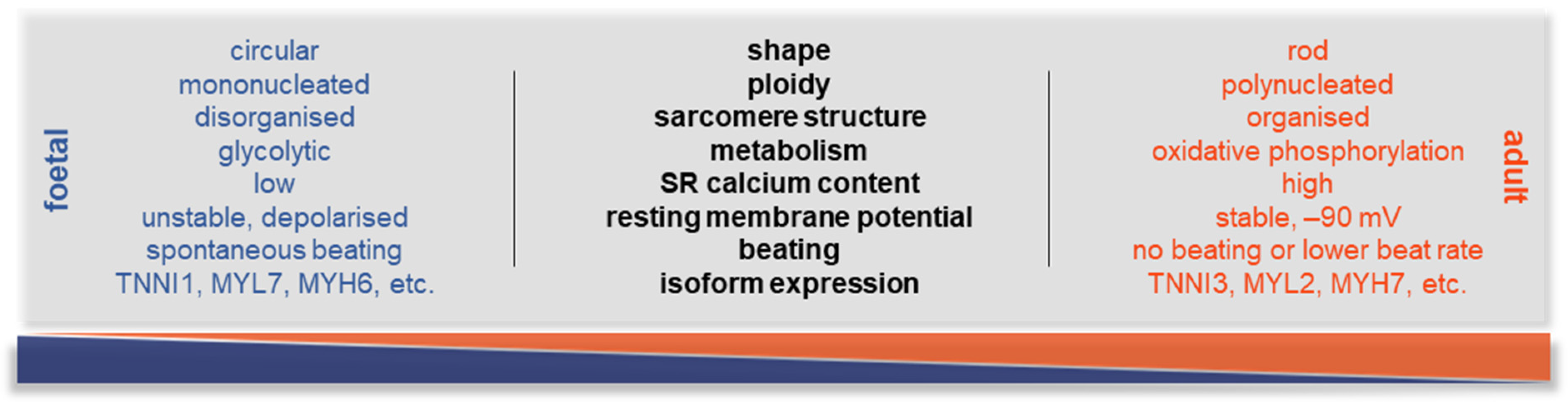
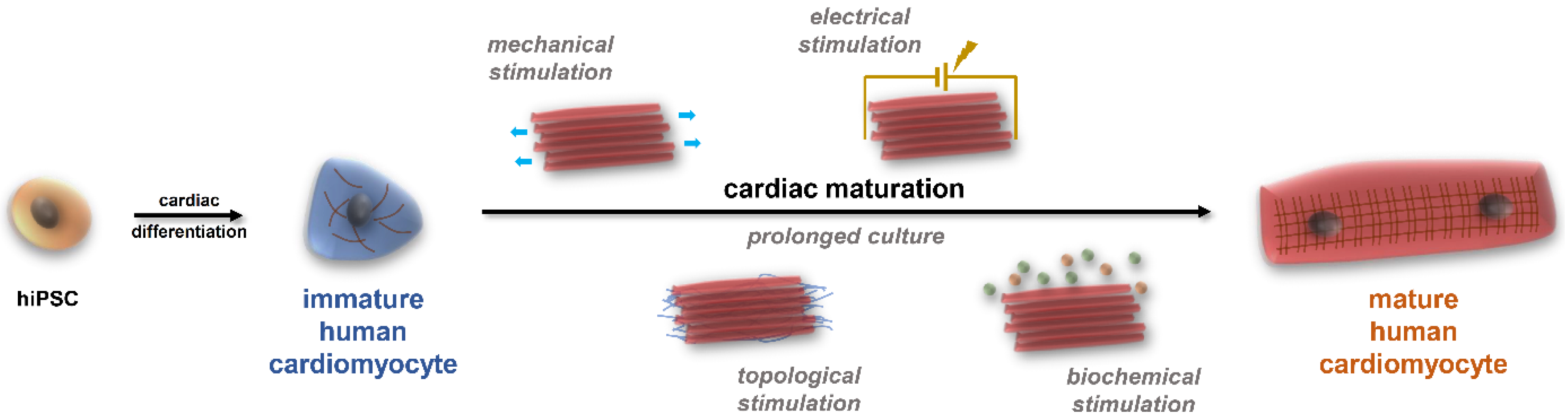
2. The Potential of hiPSC-CMs and the Substantial Issues with Maturation
2.1. Standardisation Procedures
2.2. The Need for Co-Cultures and Subtype-Specific Cultures
2.3. The Benefits of Gene-Editing Technology
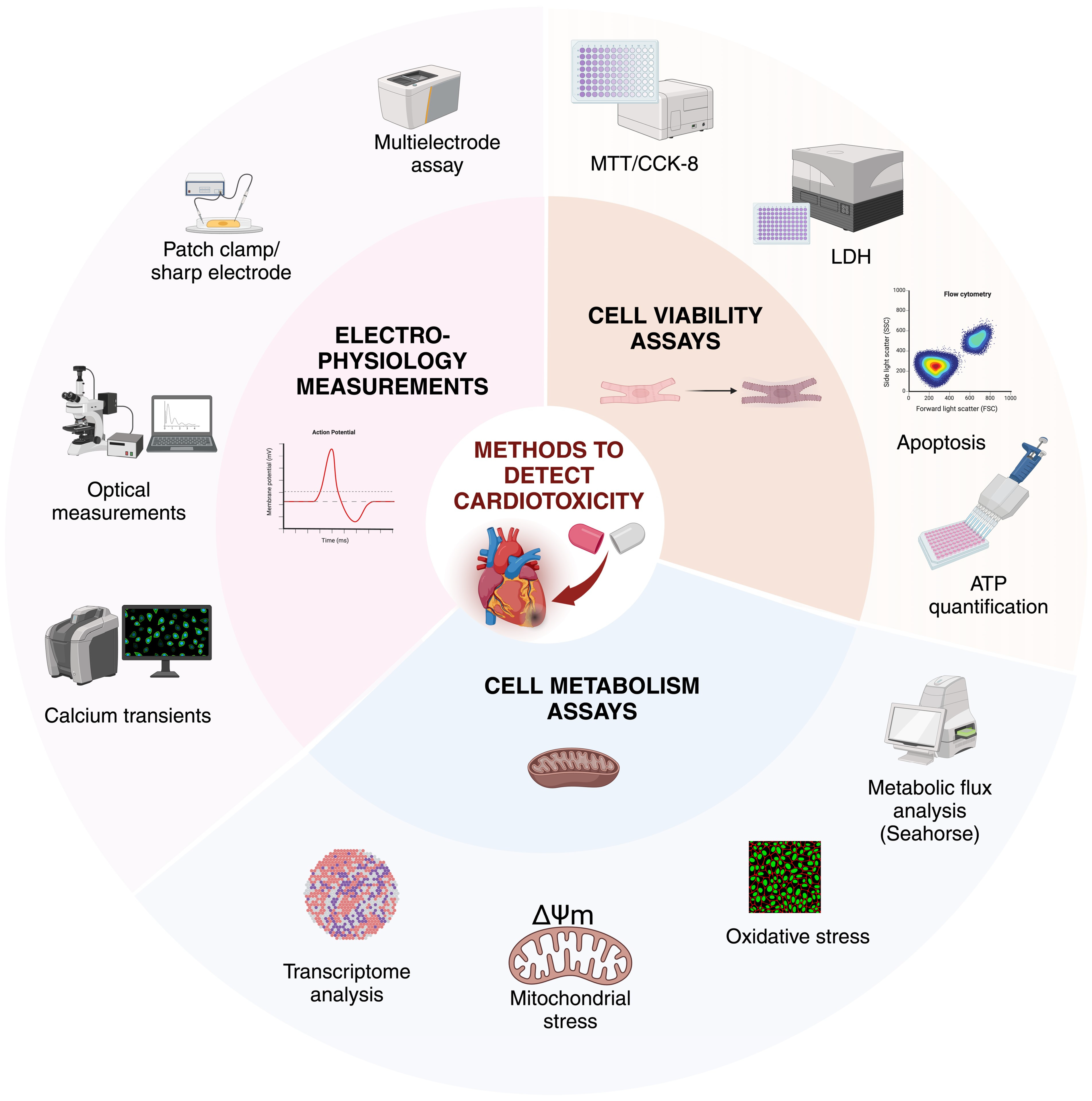
3. In Vitro Cardiotoxicity
Cardiotoxic Effects and Their Detection
4. Scaffolds in Cardiac Applications
5. Heart-on-a-Chip Platforms
6. In Silico Models
7. Disease Modelling
7.1. Atrial Fibrillation
7.2. Hypertrophy
7.3. Channelopathies
7.4. Ischaemia/Hypoxia
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| AI | artificial intelligence |
| AP | action potential |
| APA | action potential amplitude |
| APD | action potential duration |
| BPA | bisphenol A |
| BPAF | bisphenol F |
| BPM | beats per minute |
| BR | beat rate |
| CAST | Cardiac Arrhythmia Suppression Trial |
| CF | cardiac fibroblast |
| CiPA | Comprehensive in vitro Proarrhythmia Assay |
| CL | cycle length |
| CM | cardiomyocyte |
| cTnT | cardiac troponin T |
| CVD | cardiovascular disease |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DL | deep learning |
| ECG | electrocardiogram |
| ECM | extracellular matrix |
| ECT | engineered cardiac tissues |
| EHT | engineered heart tissue |
| EMV | endothelial cell-derived microvesicle |
| ESC | embryonic stem cell |
| FDA | US Food and Drug Administration |
| FPD | field potential duration |
| GATA4 | GATA binding protein 4 |
| hESC-CM | human embryonic stem cell-derived cardiomyocyte |
| hiPSC | human induced pluripotent stem cell |
| hiPSC-CM | human induced pluripotent stem cell-derived cardiomyocyte |
| hiPSC-aCMs | human induced pluripotent stem cell-derived atrial cardiomyocyte |
| HOC | heart-on-a-chip |
| HUVEC | human umbilical vein endothelial cells |
| IDE | interdigitated electrodes |
| LDH | lactate dehydrogenase |
| LQTS | long QT syndrome |
| MCS | maximum contraction speed |
| MEA | multielectrode array |
| ML | machine learning |
| MRS | maximum relaxation speed |
| NFAT | nuclear factor of activated T-cells |
| NGS | next-generation sequencing |
| Nkx2.5 | homeobox protein Nkx2.5 |
| OAP | optical action potential |
| PKC | protein kinase C |
| RMP | resting membrane potential |
| ROS | reactive oxygen species |
| SERCA | sarcopendoplasmic reticulum calcium-ATPase |
| SK | small conductance calcium-activated potassium channel |
| SNP | single nucleotide polymorphism |
| SQTS | short QT syndrome |
| SR | sarcoplasmic reticulum |
| SWORD | Survival with oral d-sotalol Trial |
| TdP | torsade de pointes type polymorphic ventricular tachyarrhythmia |
| VEGF | vascular endothelial growth factor |
References
- Kistamás, K.; Müller, A.; Muenthaisong, S.; Lamberto, F.; Zana, M.; Dulac, M.; Leal, F.; Maziz, A.; Costa, P.; Bernotiene, E.; et al. Multifactorial approaches to enhance maturation of human iPSC-derived cardiomyocytes. J. Mol. Liq. 2023, 387, 122668. [Google Scholar] [CrossRef]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H.L.; et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991, 324, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Cardiac Arrhythmia Suppression Trial, I.I.I. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N. Engl. J. Med. 1992, 327, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.M.; Camm, A.J.; Cooper, W.; Friedman, P.L.; MacNeil, D.J.; Moulton, K.M.; Pitt, B.; Schwartz, P.J.; Veltri, E.P.; Waldo, A.L. Mortality in the Survival with ORal D-sotalol (SWORD) trial: Why did patients die? Am. J. Cardiol. 1998, 81, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Waldo, A.L.; Camm, A.J.; deRuyter, H.; Freidman, P.L.; MacNeil, D.J.; Pitt, B.; Pratt, C.M.; Rodda, B.E.; Schwartz, P.J. Survival with oral d-sotalol in patients with left ventricular dysfunction after myocardial infarction: Rationale, design, and methods (the SWORD trial). Am. J. Cardiol. 1995, 75, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandy, P.; Baczko, I.; Bencsik, P.; Giricz, Z.; Gorbe, A.; Pacher, P.; Varga, Z.V.; Varro, A.; Schulz, R. Definition of hidden drug cardiotoxicity: Paradigm change in cardiac safety testing and its clinical implications. Eur. Heart J. 2019, 40, 1771–1777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- James, S.K.; Roe, M.T.; Cannon, C.P.; Cornel, J.H.; Horrow, J.; Husted, S.; Katus, H.; Morais, J.; Steg, P.G.; Storey, R.F.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: Substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ 2011, 342, d3527. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Creager, M.A.; Olin, J.; Scirica, B.M.; Gilchrist, I.C., Jr.; Murphy, S.A.; Goodrich, E.L.; Braunwald, E.; Morrow, D.A. Peripheral Revascularization in Patients with Peripheral Artery Disease with Vorapaxar: Insights from the TRA 2 degrees P-TIMI 50 Trial. JACC. Cardiovasc. Interv. 2016, 9, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Komajda, M.; Bohm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; Investigators, S. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.J.; Cole, R.; Jensen, B.C.; Pal, J.; Sharma, N.; Yehya, A.; Vader, J. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail. Rev. 2019, 24, 167–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damy, T.; Garcia-Pavia, P.; Hanna, M.; Judge, D.P.; Merlini, G.; Gundapaneni, B.; Patterson, T.A.; Riley, S.; Schwartz, J.H.; Sultan, M.B.; et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur. J. Heart Fail. 2021, 23, 277–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezekowitz, J.A.; O’Connor, C.M.; Troughton, R.W.; Alemayehu, W.G.; Westerhout, C.M.; Voors, A.A.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Emdin, M.; et al. N-Terminal Pro-B-Type Natriuretic Peptide and Clinical Outcomes: Vericiguat Heart Failure with Reduced Ejection Fraction Study. JACC. Heart Fail. 2020, 8, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, M.P.; Bellet, M.; Weber, M.A.; Danaietash, P.; Bakris, G.L.; Flack, J.M.; Dreier, R.F.; Sassi-Sayadi, M.; Haskell, L.P.; Narkiewicz, K.; et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): A multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet 2022, 400, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Kainuma, S.; Kawamura, T.; Suzuki, K.; Ito, Y.; Iseoka, H.; Ito, E.; Takeda, M.; Sasai, M.; Mochizuki-Oda, N.; et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 950829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- A Study of iPS Cell-derived Cardiomyocyte Spheroids (HS-001) in Patients with Heart Failure (LAPiS Study); Heartseed Inc.: Tokyo, Japan, 2022. Available online: https://clinicaltrials.gov/study/NCT04945018 (accessed on 30 July 2024).
- Funakoshi, S.; Yoshida, Y. Recent progress of iPSC technology in cardiac diseases. Arch. Toxicol. 2021, 95, 3633–3650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Lyra-Leite, D.M.; Gutierrez-Gutierrez, O.; Wang, M.; Zhou, Y.; Cyganek, L.; Burridge, P.W. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR Protoc. 2022, 3, 101560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clinical Trial of Human (Allogeneic) iPS Cell-Derived Cardiomyocytes Sheet for Ischemic Cardiomyopathy. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04696328 (accessed on 30 July 2024).
- Safety and Efficacy of Induced Pluripotent Stem Cell-derived Engineered Human Myocardium as Biological Ventricular Assist Tissue in Terminal Heart Failure (BioVAT-HF). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04396899 (accessed on 30 July 2024).
- Treating Heart Failure with hPSC-CMs (HEAL-CHF). 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03763136 (accessed on 30 July 2024).
- Treating Congestive HF with hiPSC-CMs through Endocardial Injection. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04982081 (accessed on 30 July 2024).
- Modeling and Pharmacological Targeting of Genetic Cardiomyopathy in Children via Cardiomyocytes Derived from Induced Pluripotent Stem Cells (DMDstem). 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03696628 (accessed on 30 July 2024).
- Allogeneic iPSC-Derived Cardiomyocyte Therapy in Patients with Worsening Ischemic Heart Failure. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05566600 (accessed on 30 July 2024).
- Vreeker, A.; van Stuijvenberg, L.; Hund, T.J.; Mohler, P.J.; Nikkels, P.G.; van Veen, T.A. Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart. PLoS ONE 2014, 9, e94722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiba, Y.; Fernandes, S.; Zhu, W.Z.; Filice, D.; Muskheli, V.; Kim, J.; Palpant, N.J.; Gantz, J.; Moyes, K.W.; Reinecke, H.; et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012, 489, 322–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elkhoury, K.; Kodeih, S.; Enciso-Martinez, E.; Maziz, A.; Bergaud, C. Advancing Cardiomyocyte Maturation: Current Strategies and Promising Conductive Polymer-Based Approaches. Adv. Healthc. Mater. 2024, 13, e2303288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lami, B.; Ikonomou, L.; Gu, M. Unlocking the potential of induced pluripotent stem cells for neonatal disease modeling and drug development. Semin. Perinatol. 2023, 47, 151729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamberto, F.; Shashikadze, B.; Elkhateib, R.; Lombardo, S.D.; Horanszky, A.; Balogh, A.; Kistamas, K.; Zana, M.; Menche, J.; Frohlich, T.; et al. Low-dose Bisphenol A exposure alters the functionality and cellular environment in a human cardiomyocyte model. Environ. Pollut. 2023, 335, 122359. [Google Scholar] [CrossRef] [PubMed]
- Lamberto, F.; Peral-Sanchez, I.; Muenthaisong, S.; Zana, M.; Willaime-Morawek, S.; Dinnyes, A. Environmental Alterations during Embryonic Development: Studying the Impact of Stressors on Pluripotent Stem Cell-Derived Cardiomyocytes. Genes 2021, 12, 1564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.; Zou, J. Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells in Fully Chemically Defined Conditions. STAR Protoc. 2020, 1, 100015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cofino-Fabres, C.; Passier, R.; Schwach, V. Towards Improved Human In Vitro Models for Cardiac Arrhythmia: Disease Mechanisms, Treatment, and Models of Atrial Fibrillation. Biomedicines 2023, 11, 2355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veerman, C.C.; Kosmidis, G.; Mummery, C.L.; Casini, S.; Verkerk, A.O.; Bellin, M. Immaturity of human stem-cell-derived cardiomyocytes in culture: Fatal flaw or soluble problem? Stem Cells Dev. 2015, 24, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Souidi, M.; Sleiman, Y.; Acimovic, I.; Pribyl, J.; Charrabi, A.; Baecker, V.; Scheuermann, V.; Pesl, M.; Jelinkova, S.; Skladal, P.; et al. Oxygen Is an Ambivalent Factor for the Differentiation of Human Pluripotent Stem Cells in Cardiac 2D Monolayer and 3D Cardiac Spheroids. Int. J. Mol. Sci. 2021, 22, 662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leitolis, A.; Robert, A.W.; Pereira, I.T.; Correa, A.; Stimamiglio, M.A. Cardiomyogenesis Modeling Using Pluripotent Stem Cells: The Role of Microenvironmental Signaling. Front. Cell Dev. Biol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Klos, M.; Wilson, G.F.; Herman, A.M.; Lian, X.; Raval, K.K.; Barron, M.R.; Hou, L.; Soerens, A.G.; Yu, J.; et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: The matrix sandwich method. Circ. Res. 2012, 111, 1125–1136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalkunte, N.G.; Delambre, T.E.; Sohn, S.; Pickett, M.; Parekh, S.; Zoldan, J. Engineering Alignment Has Mixed Effects on Human Induced Pluripotent Stem Cell Differentiated Cardiomyocyte Maturation. Tissue Engineering. Part A 2023, 29, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia, N.A.; Ontoria-Oviedo, I.; Gonzalez-King, H.; Diez-Juan, A.; Sepulveda, P. Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells. PLoS ONE 2015, 10, e0138849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rupert, C.E.; Irofuala, C.; Coulombe, K.L.K. Practical adoption of state-of-the-art hiPSC-cardiomyocyte differentiation techniques. PLoS ONE 2020, 15, e0230001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takaki, T.; Inagaki, A.; Chonabayashi, K.; Inoue, K.; Miki, K.; Ohno, S.; Makiyama, T.; Horie, M.; Yoshida, Y. Optical Recording of Action Potentials in Human Induced Pluripotent Stem Cell-Derived Cardiac Single Cells and Monolayers Generated from Long QT Syndrome Type 1 Patients. Stem Cells Int. 2019, 2019, 7532657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; van Helden, R.W.J.; Krotenberg Garcia, A.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020, 26, 862–879.e11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reilly, L.; Munawar, S.; Zhang, J.; Crone, W.C.; Eckhardt, L.L. Challenges and innovation: Disease modeling using human-induced pluripotent stem cell-derived cardiomyocytes. Front. Cardiovasc. Med. 2022, 9, 966094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parrotta, E.I.; Lucchino, V.; Scaramuzzino, L.; Scalise, S.; Cuda, G. Modeling Cardiac Disease Mechanisms Using Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Progress, Promises and Challenges. Int. J. Mol. Sci. 2020, 21, 4354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kleinsorge, M.; Cyganek, L. Subtype-Directed Differentiation of Human iPSCs into Atrial and Ventricular Cardiomyocytes. STAR Protoc. 2020, 1, 100026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vuckovic, S.; Dinani, R.; Nollet, E.E.; Kuster, D.W.D.; Buikema, J.W.; Houtkooper, R.H.; Nabben, M.; van der Velden, J.; Goversen, B. Characterization of cardiac metabolism in iPSC-derived cardiomyocytes: Lessons from maturation and disease modeling. Stem Cell Res. Ther. 2022, 13, 332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Laksman, Z.; Backx, P.H. The electrophysiological development of cardiomyocytes. Adv. Drug Deliv. Rev. 2016, 96, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Chirikian, O.; Goodyer, W.R.; Dzilic, E.; Serpooshan, V.; Buikema, J.W.; McKeithan, W.; Wu, H.; Li, G.; Lee, S.; Merk, M.; et al. CRISPR/Cas9-based targeting of fluorescent reporters to human iPSCs to isolate atrial and ventricular-specific cardiomyocytes. Sci. Rep. 2021, 11, 3026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, A.; Ramachandra, C.J.A.; Singh, P.; Chitre, A.; Lua, C.H.; Mura, M.; Crotti, L.; Wong, P.; Schwartz, P.J.; Gnecchi, M.; et al. Identification of a targeted and testable antiarrhythmic therapy for long-QT syndrome type 2 using a patient-specific cellular model. Eur. Heart J. 2018, 39, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- James, E.C.; Tomaskovic-Crook, E.; Crook, J.M. Bioengineering Clinically Relevant Cardiomyocytes and Cardiac Tissues from Pluripotent Stem Cells. Int. J. Mol. Sci. 2021, 22, 3005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knollmann, B.C. Induced pluripotent stem cell-derived cardiomyocytes: Boutique science or valuable arrhythmia model? Circ. Res. 2013, 112, 969–976; discussion 976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cyganek, L.; Tiburcy, M.; Sekeres, K.; Gerstenberg, K.; Bohnenberger, H.; Lenz, C.; Henze, S.; Stauske, M.; Salinas, G.; Zimmermann, W.H.; et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight 2018, 3, e99941. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, K.; Bao, X.; Wang, Y.; Lu, T.; Zhang, L. Human induced pluripotent stem cell (hiPSC)-derived cardiomyocyte modelling of cardiovascular diseases for natural compound discovery. Biomed. Pharmacother. 2023, 157, 113970. [Google Scholar] [CrossRef] [PubMed]
- Hanses, U.; Kleinsorge, M.; Roos, L.; Yigit, G.; Li, Y.; Barbarics, B.; El-Battrawy, I.; Lan, H.; Tiburcy, M.; Hindmarsh, R.; et al. Intronic CRISPR Repair in a Preclinical Model of Noonan Syndrome-Associated Cardiomyopathy. Circulation 2020, 142, 1059–1076. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, J.Z.; Itzhaki, I.; Zhang, S.L.; Chen, H.; Haddad, F.; Kitani, T.; Wilson, K.D.; Tian, L.; Shrestha, R.; et al. Determining the Pathogenicity of a Genomic Variant of Uncertain Significance Using CRISPR/Cas9 and Human-Induced Pluripotent Stem Cells. Circulation 2018, 138, 2666–2681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kapplinger, J.D.; Giudicessi, J.R.; Ye, D.; Tester, D.J.; Callis, T.E.; Valdivia, C.R.; Makielski, J.C.; Wilde, A.A.; Ackerman, M.J. Enhanced Classification of Brugada Syndrome-Associated and Long-QT Syndrome-Associated Genetic Variants in the SCN5A-Encoded Na(v)1.5 Cardiac Sodium Channel. Circ. Cardiovasc. Genet. 2015, 8, 582–595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hancox, J.C.; Stuart, A.G.; Harmer, S.C. Functional evaluation of gene mutations in Long QT Syndrome: Strength of evidence from in vitro assays for deciphering variants of uncertain significance. J. Congenit. Cardiol. 2020, 4, 6. [Google Scholar] [CrossRef]
- Kamga, M.V.K.; Reppel, M.; Hescheler, J.; Nguemo, F. Modeling genetic cardiac channelopathies using induced pluripotent stem cells—Status quo from an electrophysiological perspective. Biochem. Pharmacol. 2021, 192, 114746. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Pianezzi, E.; Cervio, E.; Bolis, S.; Biemmi, V.; Benzoni, P.; Camici, G.G.; Moccetti, T.; Barile, L.; Vassalli, G. Human-induced pluripotent stem cell-derived cardiomyocytes from cardiac progenitor cells: Effects of selective ion channel blockade. EP Eur. 2016, 18, iv67–iv76. [Google Scholar] [CrossRef] [PubMed]
- Maurissen, T.L.; Kawatou, M.; Lopez-Davila, V.; Minatoya, K.; Yamashita, J.K.; Woltjen, K. Modeling mutation-specific arrhythmogenic phenotypes in isogenic human iPSC-derived cardiac tissues. Sci. Rep. 2024, 14, 2586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mamoshina, P.; Rodriguez, B.; Bueno-Orovio, A. Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep. Med. 2021, 2, 100216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulla, W.; Murninkas, M.; Levi, O.; Etzion, Y. Incorrectly corrected? QT interval analysis in rats and mice. Front. Physiol. 2022, 13, 1002203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, Y.; Li, Y.N.; Bai, R.; Wu, F.; Ma, S.; Saleem, A.; Zhang, S.; Jiang, Y.; Dong, T.; Guo, T.; et al. hERG-deficient human embryonic stem cell-derived cardiomyocytes for modelling QT prolongation. Stem Cell Res. Ther. 2021, 12, 278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vargas, H.M.; Bass, A.S.; Koerner, J.; Matis-Mitchell, S.; Pugsley, M.K.; Skinner, M.; Burnham, M.; Bridgland-Taylor, M.; Pettit, S.; Valentin, J.P. Evaluation of drug-induced QT interval prolongation in animal and human studies: A literature review of concordance. Br. J. Pharmacol. 2015, 172, 4002–4011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colatsky, T.; Fermini, B.; Gintant, G.; Pierson, J.B.; Sager, P.; Sekino, Y.; Strauss, D.G.; Stockbridge, N. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative—Update on progress. J. Pharmacol. Toxicol. Methods 2016, 81, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Zusterzeel, R.; Johannesen, L.; Ochoa-Jimenez, R.; Mason, J.W.; Sanabria, C.; Kemp, S.; Sager, P.T.; Patel, V.; Matta, M.K.; et al. Assessment of Multi-Ion Channel Block in a Phase I Randomized Study Design: Results of the CiPA Phase I ECG Biomarker Validation Study. Clin. Pharmacol. Ther. 2019, 105, 943–953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blinova, K.; Dang, Q.; Millard, D.; Smith, G.; Pierson, J.; Guo, L.; Brock, M.; Lu, H.R.; Kraushaar, U.; Zeng, H.; et al. International Multisite Study of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Proarrhythmic Potential Assessment. Cell Rep. 2018, 24, 3582–3592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.; Ridder, B.J.; Han, X.; Wu, W.W.; Sheng, J.; Tran, P.N.; Wu, M.; Randolph, A.; Johnstone, R.H.; Mirams, G.R.; et al. Assessment of an In Silico Mechanistic Model for Proarrhythmia Risk Prediction under the CiPA Initiative. Clin. Pharmacol. Ther. 2019, 105, 466–475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crumb, W.J., Jr.; Vicente, J.; Johannesen, L.; Strauss, D.G. An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J. Pharmacol. Toxicol. Methods 2016, 81, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rafatian, N.; Wang, E.Y.; Wu, Q.; Lai, B.F.L.; Lu, R.X.; Savoji, H.; Radisic, M. Towards chamber specific heart-on-a-chip for drug testing applications. Adv. Drug Deliv. Rev. 2020, 165–166, 60–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The evolving role of investigative toxicology in the pharmaceutical industry. Nat. Rev. Drug Discov. 2023, 22, 317–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abulaiti, M.; Yalikun, Y.; Murata, K.; Sato, A.; Sami, M.M.; Sasaki, Y.; Fujiwara, Y.; Minatoya, K.; Shiba, Y.; Tanaka, Y.; et al. Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Sci. Rep. 2020, 10, 19201. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xia, S.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, D.; Hua, Y.; Luo, S. Application of hiPSC as a Drug Tester Via Mimicking a Personalized Mini Heart. Front. Genet. 2022, 13, 891159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, L. Toxicity testing in the era of induced pluripotent stem cells: A perspective regarding the use of patient-specific induced pluripotent stem cell–derived cardiomyocytes for cardiac safety evaluation. Curr. Opin. Toxicol. 2020, 23–24, 50–55. [Google Scholar] [CrossRef]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-based Cardiac Microphysiological System For Drug Screening Applications. Sci. Rep. 2015, 5, 8883. [Google Scholar] [CrossRef]
- Tisdale, J.E.; Chung, M.K.; Campbell, K.B.; Hammadah, M.; Joglar, J.A.; Leclerc, J.; Rajagopalan, B.; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Drug-Induced Arrhythmias: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e214–e233. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.L.; Pettersson, M.; Meyboom, R.H.; Hoes, A.W.; Leufkens, H.G. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur. Heart J. 2005, 26, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, M.; Della Sala, A.; Tocchetti, C.G.; Porporato, P.E.; Ghigo, A. Metabolic Aspects of Anthracycline Cardiotoxicity. Curr. Treat. Options Oncol. 2021, 22, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- L’Ecuyer, T.; Sanjeev, S.; Thomas, R.; Novak, R.; Das, L.; Campbell, W.; Heide, R.V. DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1273–H1280. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, Y.; Chen, T.; Wang, Y.; Guo, T.; Liu, Y.; Li, H.; Yang, L. Cell death regulation in myocardial toxicity induced by antineoplastic drugs. Front. Cell Dev. Biol. 2023, 11, 1075917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doherty, K.R.; Talbert, D.R.; Trusk, P.B.; Moran, D.M.; Shell, S.A.; Bacus, S. Structural and functional screening in human induced-pluripotent stem cell-derived cardiomyocytes accurately identifies cardiotoxicity of multiple drug types. Toxicol. Appl. Pharmacol. 2015, 285, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; McKeithan, W.L.; Serrano, R.; Kitani, T.; Burridge, P.W.; Del Alamo, J.C.; Mercola, M.; Wu, J.C. Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat. Protoc. 2018, 13, 3018–3041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, M.; Tien, N.T.; de Haan, L.; Louisse, J.; Rietjens, I.; Bouwmeester, H. Evaluation of in vitro models of stem cell-derived cardiomyocytes to screen for potential cardiotoxicity of chemicals. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2020, 67, 104891. [Google Scholar] [CrossRef] [PubMed]
- Draghici, A.E.; Taylor, J.A. The physiological basis and measurement of heart rate variability in humans. J. Physiol. Anthropol. 2016, 35, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, D.; Li, B.; Wang, S. Establishment and validation of a torsade de pointes prediction model based on human iPSC-derived cardiomyocytes. Exp. Ther. Med. 2023, 25, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faulkner-Jones, A.; Zamora, V.; Hortigon-Vinagre, M.P.; Wang, W.; Ardron, M.; Smith, G.L.; Shu, W. A Bioprinted Heart-on-a-Chip with Human Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Evaluation. Bioengineering 2022, 9, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Z.; Li, X.; El-Battrawy, I.; Lan, H.; Zhong, R.; Xu, Q.; Huang, M.; Liao, Z.; Lang, S.; Zimmermann, W.H.; et al. Drug Testing in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes from a Patient with Short QT Syndrome Type 1. Clin. Pharmacol. Ther. 2019, 106, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Kiss, D.; Dienes, C.; Hezso, T.; Kovacs, Z.; Szentandrassy, N.; Almassy, J.; Magyar, J.; Banyasz, T.; Nanasi, P.P. Ion current profiles in canine ventricular myocytes obtained by the “onion peeling” technique. J. Mol. Cell. Cardiol. 2021, 158, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Szentandrassy, N.; Kistamas, K.; Hegyi, B.; Horvath, B.; Ruzsnavszky, F.; Vaczi, K.; Magyar, J.; Banyasz, T.; Varro, A.; Nanasi, P.P. Contribution of ion currents to beat-to-beat variability of action potential duration in canine ventricular myocytes. Pflug. Arch. Eur. J. Physiol. 2015, 467, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Zlochiver, V.; Conrad, D.B.; Vaidyanathan, R.; Valiquette, A.M.; Joshi-Mukherjee, R. A Multiwell Cardiac muGMEA Platform for Action Potential Recordings from Human iPSC-Derived Cardiomyocyte Constructs. Stem Cell Rep. 2018, 11, 522–536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Honda, Y.; Li, J.; Hino, A.; Tsujimoto, S.; Lee, J.K. High-Throughput Drug Screening System Based on Human Induced Pluripotent Stem Cell-Derived Atrial Myocytes approximately A Novel Platform to Detect Cardiac Toxicity for Atrial Arrhythmias. Front. Pharmacol. 2021, 12, 680618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eisner, D.A.; Caldwell, J.L.; Kistamas, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sankaranarayanan, R.; Kistamas, K.; Greensmith, D.J.; Venetucci, L.A.; Eisner, D.A. Systolic [Ca2+]i regulates diastolic levels in rat ventricular myocytes. J. Physiol. 2017, 595, 5545–5555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kistamas, K.; Szentandrassy, N.; Hegyi, B.; Vaczi, K.; Ruzsnavszky, F.; Horvath, B.; Banyasz, T.; Nanasi, P.P.; Magyar, J. Changes in intracellular calcium concentration influence beat-to-beat variability of action potential duration in canine ventricular myocytes. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 73–81. [Google Scholar] [PubMed]
- Watanabe, H.; Honda, Y.; Deguchi, J.; Yamada, T.; Bando, K. Usefulness of cardiotoxicity assessment using calcium transient in human induced pluripotent stem cell-derived cardiomyocytes. J. Toxicol. Sci. 2017, 42, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Tadano, K.; Miyagawa, S.; Takeda, M.; Tsukamoto, Y.; Kazusa, K.; Takamatsu, K.; Akashi, M.; Sawa, Y. Cardiotoxicity assessment using 3D vascularized cardiac tissue consisting of human iPSC-derived cardiomyocytes and fibroblasts. Mol. Therapy. Methods Clin. Dev. 2021, 22, 338–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Li, D.; Sun, F.; Rampoldi, A.; Maxwell, J.T.; Wu, R.; Fischbach, P.; Castellino, S.M.; Du, Y.; Fu, H.; et al. Melphalan induces cardiotoxicity through oxidative stress in cardiomyocytes derived from human induced pluripotent stem cells. Stem Cell Res. Ther. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stein, J.M.; Mummery, C.L.; Bellin, M. Engineered models of the human heart: Directions and challenges. Stem Cell Rep. 2021, 16, 2049–2057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasche, P. Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Campostrini, G.; Windt, L.M.; van Meer, B.J.; Bellin, M.; Mummery, C.L. Cardiac Tissues from Stem Cells: New Routes to Maturation and Cardiac Regeneration. Circ. Res. 2021, 128, 775–801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lock, R.I.; Graney, P.L.; Tavakol, D.N.; Nash, T.R.; Kim, Y.; Sanchez, E., Jr.; Morsink, M.; Ning, D.; Chen, C.; Fleischer, S.; et al. Macrophages enhance contractile force in iPSC-derived human engineered cardiac tissue. Cell Rep. 2024, 43, 114302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ronaldson-Bouchard, K.; Yeager, K.; Teles, D.; Chen, T.; Ma, S.; Song, L.; Morikawa, K.; Wobma, H.M.; Vasciaveo, A.; Ruiz, E.C.; et al. Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat. Protoc. 2019, 14, 2781–2817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seguret, M.; Vermersch, E.; Jouve, C.; Hulot, J.S. Cardiac Organoids to Model and Heal Heart Failure and Cardiomyopathies. Biomedicines 2021, 9, 563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rogozinski, N.; Yanez, A.; Bhoi, R.; Lee, M.Y.; Yang, H. Current methods for fabricating 3D cardiac engineered constructs. iScience 2022, 25, 104330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Wu, H.; Yuan, Y.; Hu, B.; Gu, N. Recent fabrications and applications of cardiac patch in myocardial infarction treatment. VIEW 2022, 3, 20200153. [Google Scholar] [CrossRef]
- Cho, S.; Discher, D.E.; Leong, K.A.-O.; Vunjak-Novakovic, G.A.-O.; Wu, J.A.-O. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat. Methods 2022, 19, 1064–1071. [Google Scholar] [CrossRef]
- Murata, K.; Masumoto, H. Systems for the Functional Evaluation of Human Heart Tissues Derived from Pluripotent Stem Cells. Stem Cells 2022, 40, 537–545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Lin, L.; Qiao, L.A.-O. Recent developments in organ-on-a-chip technology for cardiovascular disease research. Anal. Bioanal. Chem. 2023, 415, 3911–3925. [Google Scholar] [CrossRef]
- Christoffersson, J.; Meier, F.; Kempf, H.; Schwanke, K.; Coffee, M.; Beilmann, M.; Zweigerdt, R.; Mandenius, C.F. A Cardiac Cell Outgrowth Assay for Evaluating Drug Compounds Using a Cardiac Spheroid-on-a-Chip Device. Bioengineering 2018, 5, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, Y.; Tian, F.; Miao, X.; Wu, D.; Wang, Y.; Wang, H.; You, K.; Li, Q.; Zhao, S.; Wang, W. Heart-on-a-chip using human iPSC-derived cardiomyocytes with an integrated vascular endothelial layer based on a culture patch as a potential platform for drug evaluation. Biofabrication 2022, 15, 015010. [Google Scholar] [CrossRef] [PubMed]
- Crestani, T.; Steichen, C.; Neri, E.; Rodrigues, M.; Fonseca-Alaniz, M.H.; Ormrod, B.; Holt, M.R.; Pandey, P.; Harding, S.; Ehler, E.; et al. Electrical stimulation applied during differentiation drives the hiPSC-CMs towards a mature cardiac conduction-like cells. Biochem. Biophys. Res. Commun. 2020, 533, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dou, W.; Malhi, M.; Zhao, Q.; Wang, L.; Huang, Z.; Law, J.; Liu, N.; Simmons, C.A.; Maynes, J.T.; Sun, Y. Microengineered platforms for characterizing the contractile function of in vitro cardiac models. Microsyst. Nanoeng. 2022, 8, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, F.; Huang, C.; Lin, Y.D.; Ivanovskaya, A.N.; O’Hara, T.J.; Booth, R.H.; Creek, C.J.; Enright, H.A.; Soscia, D.A.; Belle, A.M.; et al. Simultaneous electrical recording of cardiac electrophysiology and contraction on chip. Lab Chip 2017, 17, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dou, W.; Malhi, M.; Zhu, M.; Liu, H.; Plakhotnik, J.; Xu, Z.; Zhao, Q.; Chen, J.; Chen, S.; et al. Microdevice Platform for Continuous Measurement of Contractility, Beating Rate, and Beating Rhythm of Human-Induced Pluripotent Stem Cell-Cardiomyocytes inside a Controlled Incubator Environment. ACS Appl. Mater. Interfaces 2018, 10, 21173–21183. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.S.; Choi, Y.W.; Shanmugasundaram, A.; Jeong, Y.J.; Park, J.; Oyunbaatar, N.E.; Kim, E.S.; Choi, M.; Lee, D.W. Highly durable crack sensor integrated with silicone rubber cantilever for measuring cardiac contractility. Nat. Commun. 2020, 11, 535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCain, M.L.; Agarwal, A.; Nesmith, H.W.; Nesmith, A.P.; Parker, K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 2014, 35, 5462–5471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, F.; Shang, L.; Chen, Z.; Yu, Y.; Zhao, Y. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, eaar8580. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Z.; Xu, D.; Zhao, Y. Electroconductive and Anisotropic Structural Color Hydrogels for Visual Heart-on-a-Chip Construction. Adv. Sci. 2022, 9, e2105777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, L.; Wang, Y.; Bian, F.; Xu, D.; Zhao, Y. Bioinspired optical and electrical dual-responsive heart-on-a-chip for hormone testing. Sci. Bull. 2023, 68, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, E.Y.; Lai, F.B.L.; Cheung, K.; Radisic, M. Organs-on-a-chip: A union of tissue engineering and microfabrication. Trends Biotechnol. 2023, 41, 410–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hodgkin, A.L.; Huxley, A.F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 1952, 116, 449–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.; Stebbeds, W.; Francis, J.; Pointon, A.; Obrezanova, O.; Beattie, K.A.; Clements, P.; Harvey, J.S.; Smith, G.F.; Bender, A. Deriving waveform parameters from calcium transients in human iPSC-derived cardiomyocytes to predict cardiac activity with machine learning. Stem Cell Rep. 2022, 17, 556–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akwaboah, A.D.; Tsevi, B.; Yamlome, P.; Treat, J.A.; Brucal-Hallare, M.; Cordeiro, J.M.; Deo, M. An in silico hiPSC-Derived Cardiomyocyte Model Built with Genetic Algorithm. Front. Physiol. 2021, 12, 675867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohammadzadeh, S.; Lejeune, E. SarcGraph: A Python package for analyzing the contractile behavior of pluripotent stem cell-derived cardiomyocytes. J. Open Source Softw. 2023, 8, 5322. [Google Scholar] [CrossRef]
- Grancharova, T.; Gerbin, K.A.; Rosenberg, A.B.; Roco, C.M.; Arakaki, J.E.; DeLizo, C.M.; Dinh, S.Q.; Donovan-Maiye, R.M.; Hirano, M.; Nelson, A.M.; et al. A comprehensive analysis of gene expression changes in a high replicate and open-source dataset of differentiating hiPSC-derived cardiomyocytes. Sci. Rep. 2021, 11, 15845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Morgan, W.; Saini, A.; Liu, T.; Lough, J.; Han, L. Single-cell transcriptomic profiling reveals specific maturation signatures in human cardiomyocytes derived from LMNB2-inactivated induced pluripotent stem cells. Front. Cell Dev. Biol. 2022, 10, 895162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunham, C.S.; Mackenzie, M.E.; Nakano, H.; Kim, A.R.; Nakano, A.; Stieg, A.Z.; Gimzewski, J.K. Cardio PyMEA: A user-friendly, open-source Python application for cardiomyocyte microelectrode array analysis. PLoS ONE 2022, 17, e0266647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, B.; Zhang, K.; Chen, C.S.; Lejeune, E. Sarc-Graph: Automated segmentation, tracking, and analysis of sarcomeres in hiPSC-derived cardiomyocytes. PLoS Comput. Biol. 2021, 17, e1009443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toepfer, C.N.; Sharma, A.; Cicconet, M.; Garfinkel, A.C.; Mucke, M.; Neyazi, M.; Willcox, J.A.L.; Agarwal, R.; Schmid, M.; Rao, J.; et al. SarcTrack. Circ. Res. 2019, 124, 1172–1183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grafton, F.; Ho, J.; Ranjbarvaziri, S.; Farshidfar, F.; Budan, A.; Steltzer, S.; Maddah, M.; Loewke, K.E.; Green, K.; Patel, S.; et al. Deep learning detects cardiotoxicity in a high-content screen with induced pluripotent stem cell-derived cardiomyocytes. eLife 2021, 10, e68714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasqualin, C.; Gannier, F.; Yu, A.; Malécot, C.O.; Bredeloux, P.; Maupoil, V. 0426: SarcOptiM, an ImageJ plug-in for cardiomyocyte contractility recording. Arch. Cardiovasc. Dis. Suppl. 2016, 8, 234. [Google Scholar] [CrossRef]
- Maddah, M.; Mandegar, M.A.; Dame, K.; Grafton, F.; Loewke, K.; Ribeiro, A.J.S. Quantifying drug-induced structural toxicity in hepatocytes and cardiomyocytes derived from hiPSCs using a deep learning method. J. Pharmacol. Toxicol. Methods 2020, 105, 106895. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.J.S.; Schwab, O.; Mandegar, M.A.; Ang, Y.S.; Conklin, B.R.; Srivastava, D.; Pruitt, B.L. Multi-Imaging Method to Assay the Contractile Mechanical Output of Micropatterned Human iPSC-Derived Cardiac Myocytes. Circ. Res. 2017, 120, 1572–1583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orita, K.; Sawada, K.; Koyama, R.; Ikegaya, Y. Deep learning-based quality control of cultured human-induced pluripotent stem cell-derived cardiomyocytes. J. Pharmacol. Sci. 2019, 140, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Orita, K.; Sawada, K.; Matsumoto, N.; Ikegaya, Y. Machine-learning-based quality control of contractility of cultured human-induced pluripotent stem-cell-derived cardiomyocytes. Biochem. Biophys. Res. Commun. 2020, 526, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Tohyama, S. Human Engineered Heart Tissue Models for Disease Modeling and Drug Discovery. Front. Cell Dev. Biol. 2022, 10, 855763. [Google Scholar] [CrossRef]
- Brennan, T.; Fink, M.; Rodriguez, B. Multiscale modelling of drug-induced effects on cardiac electrophysiological activity. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2009, 36, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.L.; Wang, K.; Clerx, M.; Johnstone, R.H.; Hortigon-Vinagre, M.P.; Zamora, V.; Allan, A.; Smith, G.L.; Gavaghan, D.J.; Mirams, G.R.; et al. Tailoring Mathematical Models to Stem-Cell Derived Cardiomyocyte Lines Can Improve Predictions of Drug-Induced Changes to Their Electrophysiology. Front. Physiol. 2017, 8, 986. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.H.; Edwards, A.G.; Giles, W.R.; Tveito, A. A computational method for identifying an optimal combination of existing drugs to repair the action potentials of SQT1 ventricular myocytes. PLoS Comput. Biol. 2021, 17, e1009233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaeger, K.H.; Wall, S.; Tveito, A. Computational prediction of drug response in short QT syndrome type 1 based on measurements of compound effect in stem cell-derived cardiomyocytes. PLoS Comput. Biol. 2021, 17, e1008089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnstone, R.H.; Bardenet, R.; Gavaghan, D.J.; Mirams, G.R. Hierarchical Bayesian inference for ion channel screening dose-response data. Wellcome Open Res. 2016, 1, 6. [Google Scholar] [CrossRef]
- Aghasafari, P.; Yang, P.C.; Kernik, D.C.; Sakamoto, K.; Kanda, Y.; Kurokawa, J.; Vorobyov, I.; Clancy, C.E. A deep learning algorithm to translate and classify cardiac electrophysiology. eLife 2021, 10, e68335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juhola, M.; Penttinen, K.; Joutsijoki, H.; Aalto-Setälä, K. Analysis of Drug Effects on iPSC Cardiomyocytes with Machine Learning. Ann. Biomed. Eng. 2021, 49, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Pourrier, M.; Fedida, D. The Emergence of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs) as a Platform to Model Arrhythmogenic Diseases. Int. J. Mol. Sci. 2020, 21, 657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, K.C.; Breitbart, A.; De Lange, W.J.; Hofsteen, P.; Futakuchi-Tsuchida, A.; Xu, J.; Schopf, C.; Razumova, M.V.; Jiao, A.; Boucek, R.; et al. Novel Adult-Onset Systolic Cardiomyopathy Due to MYH7 E848G Mutation in Patient-Derived Induced Pluripotent Stem Cells. JACC. Basic Transl. Sci. 2018, 3, 728–740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, H.; Liu, R.; Maxwell, J.T.; Yang, J.; Xu, C. Machine learning identifies abnormal Ca2+ transients in human induced pluripotent stem cell-derived cardiomyocytes. Sci. Rep. 2020, 10, 16977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seibertz, F.; Sutanto, H.; Dulk, R.; Pronto, J.R.D.; Springer, R.; Rapedius, M.; Liutkute, A.; Ritter, M.; Jung, P.; Stelzer, L.; et al. Electrophysiological and calcium-handling development during long-term culture of human-induced pluripotent stem cell-derived cardiomyocytes. Basic Res. Cardiol. 2023, 118, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kernik, D.C.; Morotti, S.; Wu, H.; Garg, P.; Duff, H.J.; Kurokawa, J.; Jalife, J.; Wu, J.C.; Grandi, E.; Clancy, C.E. A computational model of induced pluripotent stem-cell derived cardiomyocytes incorporating experimental variability from multiple data sources. J. Physiol. 2019, 597, 4533–4564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sacchetto, C.; Vitiello, L.; de Windt, L.J.; Rampazzo, A.; Calore, M. Modeling Cardiovascular Diseases with hiPSC-Derived Cardiomyocytes in 2D and 3D Cultures. Int. J. Mol. Sci. 2020, 21, 3404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kannan, S.; Miyamoto, M.; Zhu, R.; Lynott, M.; Guo, J.; Chen, E.Z.; Colas, A.R.; Lin, B.L.; Kwon, C. Trajectory reconstruction identifies dysregulation of perinatal maturation programs in pluripotent stem cell-derived cardiomyocytes. Cell Rep. 2023, 42, 112330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paci, M.; Hyttinen, J.; Rodriguez, B.; Severi, S. Human induced pluripotent stem cell-derived versus adult cardiomyocytes: An in silico electrophysiological study on effects of ionic current block. Br. J. Pharmacol. 2015, 172, 5147–5160. [Google Scholar] [CrossRef]
- Pir, P.; Le Novere, N. Mathematical Models of Pluripotent Stem Cells: At the Dawn of Predictive Regenerative Medicine. Methods Mol. Biol. 2016, 1386, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Wadkin, L.E.; Orozco-Fuentes, S.; Neganova, I.; Lako, M.; Shukurov, A.; Parker, N.G. The recent advances in the mathematical modelling of human pluripotent stem cells. SN Appl. Sci. 2020, 2, 276. [Google Scholar] [CrossRef]
- Hashizume, T.; Ying, B.W. Challenges in developing cell culture media using machine learning. Biotechnol. Adv. 2024, 70, 108293. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Fathi, A.; Padashi, M.; Abu Osman, N.A. Optimal design of a 3D-printed scaffold using intelligent evolutionary algorithms. Appl. Soft Comput. 2016, 39, 36–47. [Google Scholar] [CrossRef]
- Pueyo, E.; Dangerfield, C.E.; Britton, O.J.; Virag, L.; Kistamas, K.; Szentandrassy, N.; Jost, N.; Varro, A.; Nanasi, P.P.; Burrage, K.; et al. Experimentally-Based Computational Investigation into Beat-To-Beat Variability in Ventricular Repolarization and Its Response to Ionic Current Inhibition. PLoS ONE 2016, 11, e0151461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zang, Y.; Dai, L.; Zhan, H.; Dou, J.; Xia, L.; Zhang, H. Theoretical investigation of the mechanism of heart failure using a canine ventricular cell model: Especially the role of up-regulated CaMKII and SR Ca2+ leak. J. Mol. Cell. Cardiol. 2013, 56, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, X.; Gong, Y.; Zhang, J.; Zang, Y.; Xia, L. Exploring Impaired SERCA Pump-Caused Alternation Occurrence in Ischemia. Comput. Math. Methods Med. 2019, 2019, 8237071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, S.; Nehme, R.; Barrett, L.E. Greater genetic diversity is needed in human pluripotent stem cell models. Nat. Commun. 2022, 13, 7301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varshneya, M.; Mei, X.; Sobie, E.A. Prediction of arrhythmia susceptibility through mathematical modeling and machine learning. Proc. Natl. Acad. Sci. USA 2021, 118, e2104019118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kornej, J.; Borschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Auricchio, A.; Boriani, G.; Braunschweig, F.; Terradellas, J.B.; Burri, H.; Camm, A.J.; Crijns, H.; Dagres, N.; Deharo, J.C.; et al. EHRA White Paper: Knowledge gaps in arrhythmia management-status 2019. EP Eur. 2019, 21, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Voigt, N.; Dobrev, D. New directions in antiarrhythmic drug therapy for atrial fibrillation. Future Cardiol. 2013, 9, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.E.; Han, Y.D.; Michell, A.R.; Ly, O.T.; Vanoye, C.G.; Spanghero, E.; George, A.L., Jr.; Darbar, D.; Khetani, S.R. Engineered cocultures of iPSC-derived atrial cardiomyocytes and atrial fibroblasts for modeling atrial fibrillation. Sci. Adv. 2024, 10, eadg1222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, J.Q.; Ma, Y.; Lee, Y.; Thomson, J.A.; Kamp, T.J. Human embryonic stem cells develop into multiple types of cardiac myocytes: Action potential characterization. Circ. Res. 2003, 93, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yechikov, S.; Kao, H.K.J.; Chang, C.W.; Pretto, D.; Zhang, X.D.; Sun, Y.H.; Smithers, R.; Sirish, P.; Nolta, J.A.; Chan, J.W.; et al. NODAL inhibition promotes differentiation of pacemaker-like cardiomyocytes from human induced pluripotent stem cells. Stem Cell Res. 2020, 49, 102043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Gorp, P.R.R.; Trines, S.A.; Pijnappels, D.A.; de Vries, A.A.F. Multicellular In vitro Models of Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, F.; Long, D.; Huang, W.; Peng, W.; Lan, H.; Zhou, Y.; Dang, X.; Zhou, R. The Biphasic Effect of Retinoic Acid Signaling Pathway on the Biased Differentiation of Atrial-like and Sinoatrial Node-like Cells from hiPSC. Int. J. Stem Cells 2022, 15, 247–257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, M.T.; Shao, N.Y.; Garg, V. Subtype-specific cardiomyocytes for precision medicine: Where are we now? Stem Cells 2020, 38, 822–833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thorpe, J.; Perry, M.D.; Contreras, O.; Hurley, E.; Parker, G.; Harvey, R.P.; Hill, A.P.; Vandenberg, J.I. Development of a robust induced pluripotent stem cell atrial cardiomyocyte differentiation protocol to model atrial arrhythmia. Stem Cell Res. Ther. 2023, 14, 183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulz, C.; Sonmez, M.; Krause, J.; Schwedhelm, E.; Bangfen, P.; Alihodzic, D.; Hansen, A.; Eschenhagen, T.; Christ, T. A critical role of retinoic acid concentration for the induction of a fully human-like atrial action potential phenotype in hiPSC-CM. Stem Cell Rep. 2023, 18, 2096–2107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baena-Montes, J.M.; Krasny, M.J.; O’Halloran, M.; Dunne, E.; Quinlan, L.R. In Vitro Models for Improved Therapeutic Interventions in Atrial Fibrillation. J. Pers. Med. 2023, 13, 1237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sagris, M.; Vardas, E.P.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Tousoulis, D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int. J. Mol. Sci. 2021, 23, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ragab, A.A.Y.; Sitorus, G.D.S.; Brundel, B.; de Groot, N.M.S. The Genetic Puzzle of Familial Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moore, O.M.; Ho, K.S.; Copeland, J.S.; Parthasarathy, V.; Wehrens, X.H.T. Genome Editing and Cardiac Arrhythmias. Cells 2023, 12, 1363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benzoni, P.; Campostrini, G.; Landi, S.; Bertini, V.; Marchina, E.; Iascone, M.; Ahlberg, G.; Olesen, M.S.; Crescini, E.; Mora, C.; et al. Human iPSC modelling of a familial form of atrial fibrillation reveals a gain of function of If and ICaL in patient-derived cardiomyocytes. Cardiovasc. Res. 2020, 116, 1147–1160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, L.; Zhang, M.; Ly, O.T.; Chen, H.; Sridhar, A.; Lambers, E.; Chalazan, B.; Youn, S.W.; Maienschein-Cline, M.; Feferman, L.; et al. Human induced pluripotent stem cell-derived atrial cardiomyocytes carrying an SCN5A mutation identify nitric oxide signaling as a mediator of atrial fibrillation. Stem Cell Rep. 2021, 16, 1542–1554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumer, S.A.; Hoffmann, S.; Laue, S.; Campbell, B.; Raedecke, K.; Frajs, V.; Clauss, S.; Kaab, S.; Janssen, J.W.G.; Jauch, A.; et al. Precise Correction of Heterozygous SHOX2 Mutations in hiPSCs Derived from Patients with Atrial Fibrillation via Genome Editing and Sib Selection. Stem Cell Rep. 2020, 15, 999–1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babini, H.; Jimenez-Sabado, V.; Stogova, E.; Arslanova, A.; Butt, M.; Dababneh, S.; Asghari, P.; Moore, E.D.W.; Claydon, T.W.; Chiamvimonvat, N.; et al. hiPSC-derived cardiomyocytes as a model to study the role of small-conductance Ca2+-activated K+ (SK) ion channel variants associated with atrial fibrillation. Front. Cell Dev. Biol. 2024, 12, 1298007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Kastner, N.; Zlabinger, K.; Spannbauer, A.; Traxler, D.; Mester-Tonczar, J.; Hasimbegovic, E.; Gyongyosi, M. New Insights and Current Approaches in Cardiac Hypertrophy Cell Culture, Tissue Engineering Models, and Novel Pathways Involving Non-Coding RNA. Front. Pharmacol. 2020, 11, 1314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knight, W.E.; Cao, Y.; Lin, Y.H.; Chi, C.; Bai, B.; Sparagna, G.C.; Zhao, Y.; Du, Y.; Londono, P.; Reisz, J.A.; et al. Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes Enables Modeling of Human Hypertrophic Cardiomyopathy. Stem Cell Rep. 2021, 16, 519–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aggarwal, P.; Turner, A.; Matter, A.; Kattman, S.J.; Stoddard, A.; Lorier, R.; Swanson, B.J.; Arnett, D.K.; Broeckel, U. RNA expression profiling of human iPSC-derived cardiomyocytes in a cardiac hypertrophy model. PLoS ONE 2014, 9, e108051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gerdes, A.M.; Liu, Z.; Zimmer, H.G. Changes in nuclear size of cardiac myocytes during the development and progression of hypertrophy in rats. Cardioscience 1994, 5, 203–208. [Google Scholar] [PubMed]
- Chang, C.C.; Cheng, H.C.; Chou, W.C.; Huang, Y.T.; Hsieh, P.L.; Chu, P.M.; Lee, S.D. Sesamin suppresses angiotensin-II-enhanced oxidative stress and hypertrophic markers in H9c2 cells. Environ. Toxicol. 2023, 38, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Ulfenborg, B.; Andersson, C.X.; Heydarkhan-Hagvall, S.; Jeppsson, A.; Sartipy, P.; Synnergren, J. Cardiac hypertrophy in a dish: A human stem cell based model. Biol. Open 2020, 9, bio052381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hesse, M.; Welz, A.; Fleischmann, B.K. Heart regeneration and the cardiomyocyte cell cycle. Pflug. Arch. Eur. J. Physiol. 2018, 470, 241–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolwicz, S.C., Jr.; Tian, R. Glucose metabolism and cardiac hypertrophy. Cardiovasc. Res. 2011, 90, 194–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pohjolainen, L.; Ruskoaho, H.; Talman, V. Transcriptomics reveal stretched human pluripotent stem cell-derived cardiomyocytes as an advantageous hypertrophy model. J. Mol. Cell. Cardiol. Plus 2022, 2, 100020. [Google Scholar] [CrossRef]
- Johansson, M.; Ulfenborg, B.; Andersson, C.X.; Heydarkhan-Hagvall, S.; Jeppsson, A.; Sartipy, P.; Synnergren, J. Multi-Omics Characterization of a Human Stem Cell-Based Model of Cardiac Hypertrophy. Life 2022, 12, 293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leonard, A.; Bertero, A.; Powers, J.D.; Beussman, K.M.; Bhandari, S.; Regnier, M.; Murry, C.E.; Sniadecki, N.J. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J. Mol. Cell. Cardiol. 2018, 118, 147–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Y.; Wu, F.; Yang, Q.; Feng, H.; Xu, D. Resveratrol Inhibits High Glucose-Induced H9c2 Cardiomyocyte Hypertrophy and Damage via RAGE-Dependent Inhibition of the NF-kappaB and TGF-beta1/Smad3 Pathways. Evid.-Based Complement. Altern. Med. Ecam 2022, 2022, 7781910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Troncoso, M.F.; Pavez, M.; Wilson, C.; Lagos, D.; Duran, J.; Ramos, S.; Barrientos, G.; Silva, P.; Llanos, P.; Basualto-Alarcon, C.; et al. Testosterone activates glucose metabolism through AMPK and androgen signaling in cardiomyocyte hypertrophy. Biol. Res. 2021, 54, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gerard, R.; Badi, L.; Kam-Thong, T.; Bu, L.; et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014, 9, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Fandl, H.K.; Garcia, V.P.; Treuth, J.W.; Brewster, L.M.; Greiner, J.J.; Davy, K.P.; Stauffer, B.L.; Desouza, C.A. Endothelial-derived extracellular vesicles from obese/hypertensive adults increase factors associated with hypertrophy and fibrosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H675–H685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, H.; Yang, H.; Rhee, J.W.; Zhang, J.Z.; Lam, C.K.; Sallam, K.; Chang, A.C.Y.; Ma, N.; Lee, J.; Zhang, H.; et al. Modelling diastolic dysfunction in induced pluripotent stem cell-derived cardiomyocytes from hypertrophic cardiomyopathy patients. Eur. Heart J. 2019, 40, 3685–3695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Ross, L.; Grube, C.; Wang, H.S. Toxicity of low dose bisphenols in human iPSC-derived cardiomyocytes and human cardiac organoids—Impact on contractile function and hypertrophy. Chemosphere 2024, 353, 141567. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Garg, V.; Shrestha, R.; Sanguinetti, M.C.; Kamp, T.J.; Wu, J.C. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as Models for Cardiac Channelopathies: A Primer for Non-Electrophysiologists. Circ. Res. 2018, 123, 224–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.B. Channelopathies. Korean J. Pediatr. 2014, 57, 1–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, D.; Wang, X.; Sun, Y.; Fan, H.; Zhou, J.; Yang, Z.; Qiu, H.; Wang, J.; Su, J.; Gong, T.; et al. Patient-specific iPSC-derived cardiomyocytes reveal aberrant activation of Wnt/beta-catenin signaling in SCN5A-related Brugada syndrome. Stem Cell Res. Ther. 2023, 14, 241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Egashira, T.; Yuasa, S.; Suzuki, T.; Aizawa, Y.; Yamakawa, H.; Matsuhashi, T.; Ohno, Y.; Tohyama, S.; Okata, S.; Seki, T.; et al. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovasc. Res. 2012, 95, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lehnart, S.E.; Ackerman, M.J.; Benson, D.W., Jr.; Brugada, R.; Clancy, C.E.; Donahue, J.K.; George, A.L., Jr.; Grant, A.O.; Groft, S.C.; January, C.T.; et al. Inherited arrhythmias: A National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation 2007, 116, 2325–2345. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Behr, E.R.; Hamilton, R.; Probst, V.; Laksman, Z.; Han, H.C. Brugada Syndrome. JACC. Clin. Electrophysiol. 2022, 8, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Liu, T.; Li, K.H.; Laxton, V.; Chan, Y.W.; Keung, W.; Li, R.A.; Yan, B.P. Electrophysiological Mechanisms of Brugada Syndrome: Insights from Pre-clinical and Clinical Studies. Front. Physiol. 2016, 7, 467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belbachir, N.; Portero, V.; Al Sayed, Z.R.; Gourraud, J.B.; Dilasser, F.; Jesel, L.; Guo, H.; Wu, H.; Gaborit, N.; Guilluy, C.; et al. RRAD mutation causes electrical and cytoskeletal defects in cardiomyocytes derived from a familial case of Brugada syndrome. Eur. Heart J. 2019, 40, 3081–3094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cerrone, M.; Lin, X.; Zhang, M.; Agullo-Pascual, E.; Pfenniger, A.; Chkourko Gusky, H.; Novelli, V.; Kim, C.; Tirasawadichai, T.; Judge, D.P.; et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014, 129, 1092–1103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Battrawy, I.; Lan, H.; Cyganek, L.; Zhao, Z.; Li, X.; Buljubasic, F.; Lang, S.; Yucel, G.; Sattler, K.; Zimmermann, W.H.; et al. Modeling Short QT Syndrome Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Am. Heart Assoc. 2018, 7, e007394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yazawa, M.; Hsueh, B.; Jia, X.; Pasca, A.M.; Bernstein, J.A.; Hallmayer, J.; Dolmetsch, R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 2011, 471, 230–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fatima, A.; Xu, G.; Shao, K.; Papadopoulos, S.; Lehmann, M.; Arnaiz-Cot, J.J.; Rosa, A.O.; Nguemo, F.; Matzkies, M.; Dittmann, S.; et al. In vitro modeling of ryanodine receptor 2 dysfunction using human induced pluripotent stem cells. Cell. Physiol. Biochem. 2011, 28, 579–592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nam, Y.W.; Downey, M.; Rahman, M.A.; Cui, M.; Zhang, M. Channelopathy of small- and intermediate-conductance Ca2+-activated K+ channels. Acta Pharmacol. Sin. 2023, 44, 259–267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kistamás, K.; Veress, R.; Horvath, B.; Banyasz, T.; Nanasi, P.P.; Eisner, D.A. Calcium Handling Defects and Cardiac Arrhythmia Syndromes. Front. Pharmacol. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoekstra, M.; Mummery, C.L.; Wilde, A.A.; Bezzina, C.R.; Verkerk, A.O. Induced pluripotent stem cell derived cardiomyocytes as models for cardiac arrhythmias. Front. Physiol. 2012, 3, 346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, Y.; Zheng, Z.; Lian, J. Deciphering Common Long QT Syndrome Using CRISPR/Cas9 in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Cardiovasc. Med. 2022, 9, 889519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feyen, D.A.M.; McKeithan, W.L.; Bruyneel, A.A.N.; Spiering, S.; Hormann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B.; et al. Metabolic Maturation Media Improve Physiological Function of Human iPSC-Derived Cardiomyocytes. Cell Rep. 2020, 32, 107925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lieu, D.K.; Fu, J.D.; Chiamvimonvat, N.; Tung, K.C.; McNerney, G.P.; Huser, T.; Keller, G.; Kong, C.W.; Li, R.A. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Arrhythmia Electrophysiol. 2013, 6, 191–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meijer van Putten, R.M.; Mengarelli, I.; Guan, K.; Zegers, J.G.; van Ginneken, A.C.; Verkerk, A.O.; Wilders, R. Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: A dynamic clamp study with virtual IK1. Front. Physiol. 2015, 6, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gattenlohner, S.; Waller, C.; Ertl, G.; Bultmann, B.D.; Muller-Hermelink, H.K.; Marx, A. NCAM(CD56) and RUNX1(AML1) are up-regulated in human ischemic cardiomyopathy and a rat model of chronic cardiac ischemia. Am. J. Pathol. 2003, 163, 1081–1090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez, B.; Trayanova, N.; Noble, D. Modeling cardiac ischemia. Ann. N. Y. Acad. Sci. 2006, 1080, 395–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rezende, P.C.; Ribas, F.F.; Serrano, C.V., Jr.; Hueb, W. Clinical significance of chronic myocardial ischemia in coronary artery disease patients. J. Thorac. Dis. 2019, 11, 1005–1015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Liang, Y.; Wang, M.; Wang, C.; Wei, H.; Naruse, K.; Takahashi, K. Model of Ischemic Heart Disease and Video-Based Comparison of Cardiomyocyte Contraction Using hiPSC-Derived Cardiomyocytes. J. Vis. Exp. 2020, 159, e61104. [Google Scholar] [CrossRef] [PubMed]
- Hakli, M.; Kreutzer, J.; Maki, A.J.; Valimaki, H.; Lappi, H.; Huhtala, H.; Kallio, P.; Aalto-Setala, K.; Pekkanen-Mattila, M. Human induced pluripotent stem cell-based platform for modeling cardiac ischemia. Sci. Rep. 2021, 11, 4153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaballah, M.; Penttinen, K.; Kreutzer, J.; Maki, A.J.; Kallio, P.; Aalto-Setala, K. Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cells 2022, 11, 1045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forouzandehmehr, M.; Paci, M.; Hyttinen, J.; Koivumaki, J.T. In silico study of the mechanisms of hypoxia and contractile dysfunction during ischemia and reperfusion of hiPSC cardiomyocytes. Dis. Models Mech. 2024, 17, dmm050365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesquita, F.C.P.; King, M.; da Costa Lopez, P.L.; Thevasagayampillai, S.; Gunaratne, P.H.; Hochman-Mendez, C. Laminin Alpha 2 Enhances the Protective Effect of Exosomes on Human iPSC-Derived Cardiomyocytes in an In Vitro Ischemia-Reoxygenation Model. Int. J. Mol. Sci. 2024, 25, 3773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, Y.; Li, J.; Qiu, L.; Jiang, C.; Huang, Y.; Liu, J.; Sun, Q.; Hong, H.; Ye, L. Dexmedetomidine Protects Human Cardiomyocytes Against Ischemia-Reperfusion Injury Through alpha2-Adrenergic Receptor/AMPK-Dependent Autophagy. Front. Pharmacol. 2021, 12, 615424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onodi, Z.; Visnovitz, T.; Kiss, B.; Hambalko, S.; Koncz, A.; Agg, B.; Varadi, B.; Toth, V.E.; Nagy, R.N.; Gergely, T.G.; et al. Systematic transcriptomic and phenotypic characterization of human and murine cardiac myocyte cell lines and primary cardiomyocytes reveals serious limitations and low resemblances to adult cardiac phenotype. J. Mol. Cell. Cardiol. 2022, 165, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Hakli, M.; Kreutzer, J.; Maki, A.J.; Valimaki, H.; Cherian, R.M.; Kallio, P.; Aalto-Setala, K.; Pekkanen-Mattila, M. Electrophysiological Changes of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes during Acute Hypoxia and Reoxygenation. Stem Cells Int. 2022, 2022, 9438281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davis, J.; Chouman, A.; Creech, J.; Monteiro da Rocha, A.; Ponce-Balbuena, D.; Jimenez Vazquez, E.N.; Nichols, R.; Lozhkin, A.; Madamanchi, N.R.; Campbell, K.F.; et al. In vitro model of ischemic heart failure using human induced pluripotent stem cell-derived cardiomyocytes. JCI Insight 2021, 6, e134368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peters, M.C.; Maas, R.G.C.; van Adrichem, I.; Doevendans, P.A.M.; Mercola, M.; Saric, T.; Buikema, J.W.; van Mil, A.; Chamuleau, S.A.J.; Sluijter, J.P.G.; et al. Metabolic Maturation Increases Susceptibility to Hypoxia-induced Damage in Human iPSC-derived Cardiomyocytes. Stem Cells Transl. Med. 2022, 11, 1040–1051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Assay | Method | Measured Parameters | Possible Applications | References |
|---|---|---|---|---|
| Field potential measurement | Multielectrode array systems | stand-alone FPD sodium spike amplitude RR-interval (beat-to-beat interval) spontaneous BR network analysis (syncytium) | direction and magnitude of depolarisation QT interval, beat-to-beat variability heart rate propagation and contractility | [92,93] |
| CardioExcyte 96 | FPD, impedance | QT interval on ECG, prediction of TdP risk | [94] | |
| Action potential measurement | CellOPTIQ | depolarisation time and APD by voltage sensitive dye, spontaneous activity | assessing hiPSC-CM function on hydrogels, drug evaluation | [95] |
| CardioExcyte 96 | myocardial cell activity, BPM, FPD | risk prediction model for TdP in hiPSC-CMs, tool for compound-induced arrhythmias | [94] | |
| Patch-clamp/ Sharp microelectrode | APD, APA, Vmax, ion currents, RMP | electrophysiological characterisation, drug-induced arrhythmias, sequential pharmacological dissection | [96,97,98] | |
| µGMEA | APD, APA, Vmax, FPD, RR-interval | long-term electrophysiological recordings, dynamic changes in transmembrane potential of hiPSC-CMs in network, spatial heterogeneity | [99] | |
| Optical mapping | OAP, CL, d (−F)/dtmax, APD | detection of propensities for drug-induced tachyarrhythmias | [100] | |
| Calcium measurement | CellOPTIQ | intracellular Ca concentration, Ca transient amplitude, Tau | assessing hiPSC-CM function on hydrogels, drug evaluation | [95] |
| Epifluorescence with simultaneous electrophysiology | intracellular Ca concentration, Ca transient amplitude, contractility, Tau, SR content, release kinetics, systolic and diastolic calcium levels | characterisation and drug-induced arrhythmias, Ca flux balance | [101,102,103] | |
| FLIPR Tetra system | Ca transient peak frequency, amplitude, rise time and decay time | cardiotoxicity assessment of a compound (contractility and arrhythmogenic potential) | [104] | |
| Contractile function | CellOPTIQ | contraction amplitude, duration, relaxation duration | assessing hiPSC-CM function on hydrogels, drug evaluation | [95] |
| Cell motion analysis | MCS, MRS, contraction-relaxation duration, BR | detection of drug-induced changes in contractility | [105] | |
| Single cell contraction measurement | single cell shortening, BR | assessing drug effects | [96] | |
| Video-based analysis | BR, beating velocity, maximum contraction and relaxation | detection of dysfunctional CM contractility | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kistamás, K.; Lamberto, F.; Vaiciuleviciute, R.; Leal, F.; Muenthaisong, S.; Marte, L.; Subías-Beltrán, P.; Alaburda, A.; Arvanitis, D.N.; Zana, M.; et al. The Current State of Realistic Heart Models for Disease Modelling and Cardiotoxicity. Int. J. Mol. Sci. 2024, 25, 9186. https://doi.org/10.3390/ijms25179186
Kistamás K, Lamberto F, Vaiciuleviciute R, Leal F, Muenthaisong S, Marte L, Subías-Beltrán P, Alaburda A, Arvanitis DN, Zana M, et al. The Current State of Realistic Heart Models for Disease Modelling and Cardiotoxicity. International Journal of Molecular Sciences. 2024; 25(17):9186. https://doi.org/10.3390/ijms25179186
Chicago/Turabian StyleKistamás, Kornél, Federica Lamberto, Raminta Vaiciuleviciute, Filipa Leal, Suchitra Muenthaisong, Luis Marte, Paula Subías-Beltrán, Aidas Alaburda, Dina N. Arvanitis, Melinda Zana, and et al. 2024. "The Current State of Realistic Heart Models for Disease Modelling and Cardiotoxicity" International Journal of Molecular Sciences 25, no. 17: 9186. https://doi.org/10.3390/ijms25179186






