Human Beta Defensin-2 mRNA and Proteasome Subunit β Type 8 mRNA Analysis, Useful in Differentiating Skin Biopsies from Atopic Dermatitis and Psoriasis Vulgaris Patients
Abstract
1. Introduction
2. Results
2.1. Concentrations of IVL, hBD-2 and Relative Expressions of IVL mRNA, hBD-2 mRNA, TPP2 mRNA, PSMB8 mRNA in Skin Biopsies from Patients with PV, AD and C
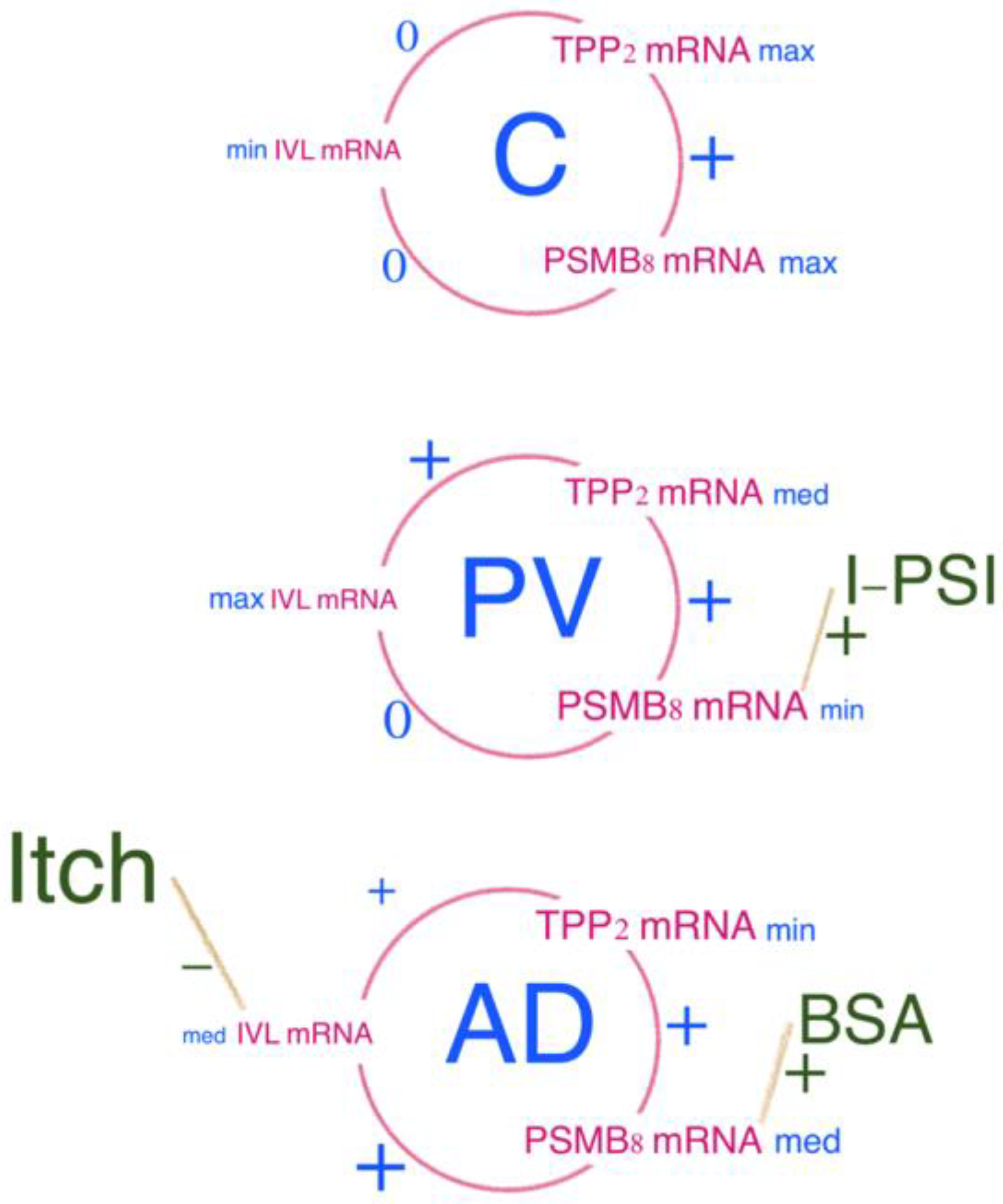
2.2. Correlations between Tested Parameters
- -
- one between the hBD-2 and IVL concentrations,
- -
- and the other between PSMB8 mRNA and TPP2 mRNA.
2.3. Correlations of Tested Parameters and Clinical Data
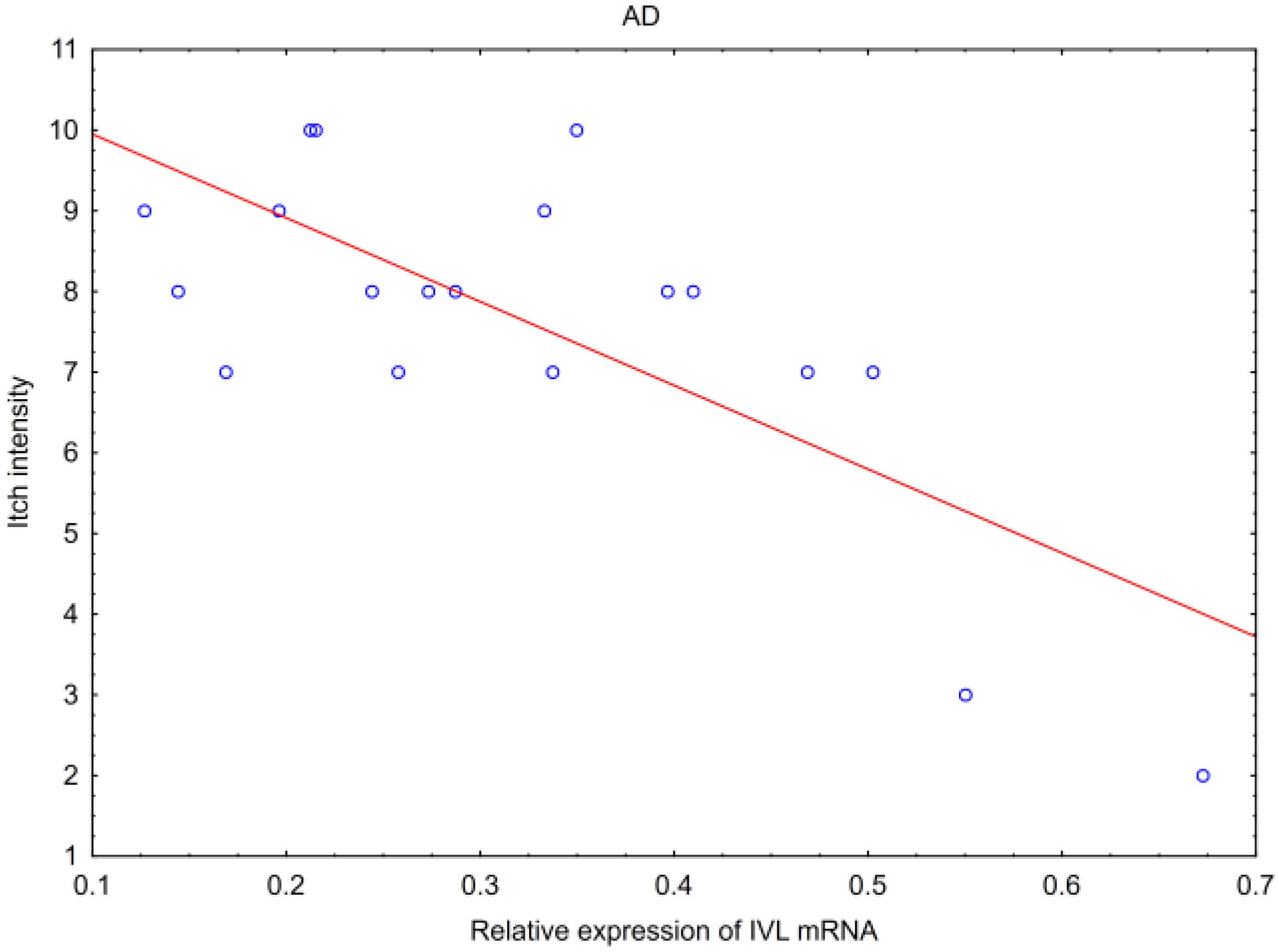
2.4. Correlations of Tested Parameters with Skin Lesions Assessment
- -
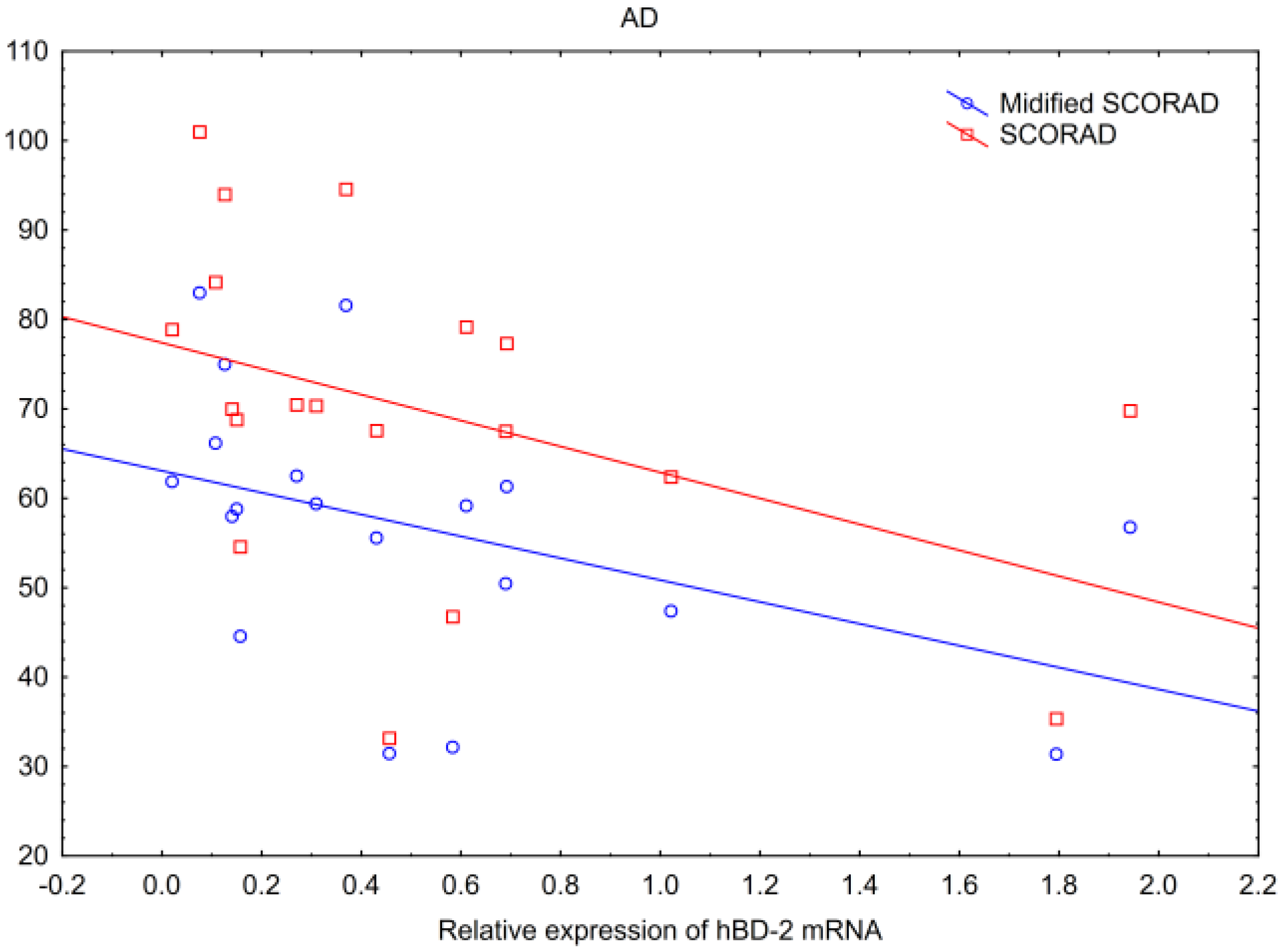
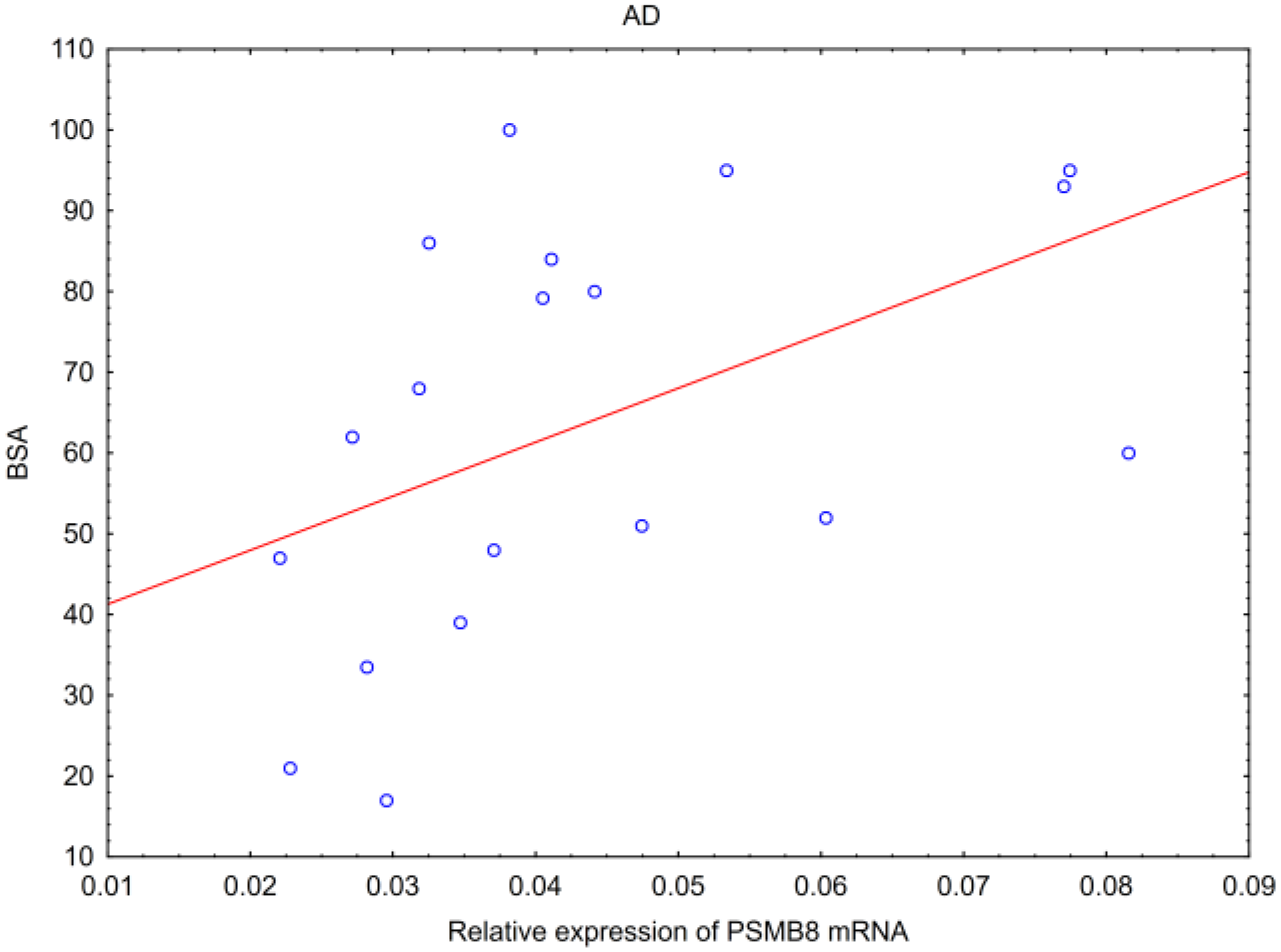
- In AD, hBD-2 mRNA inversely correlated with SCORAD and modified SCORAD scales, and PSMB8 mRNA correlated positively with BSA.
- -
- In PV, hBD-2 mRNA correlated positively with erythema, infiltration and desquamation, and PSMB8 mRNA correlated positively with infiltration in the biopsied lesions.
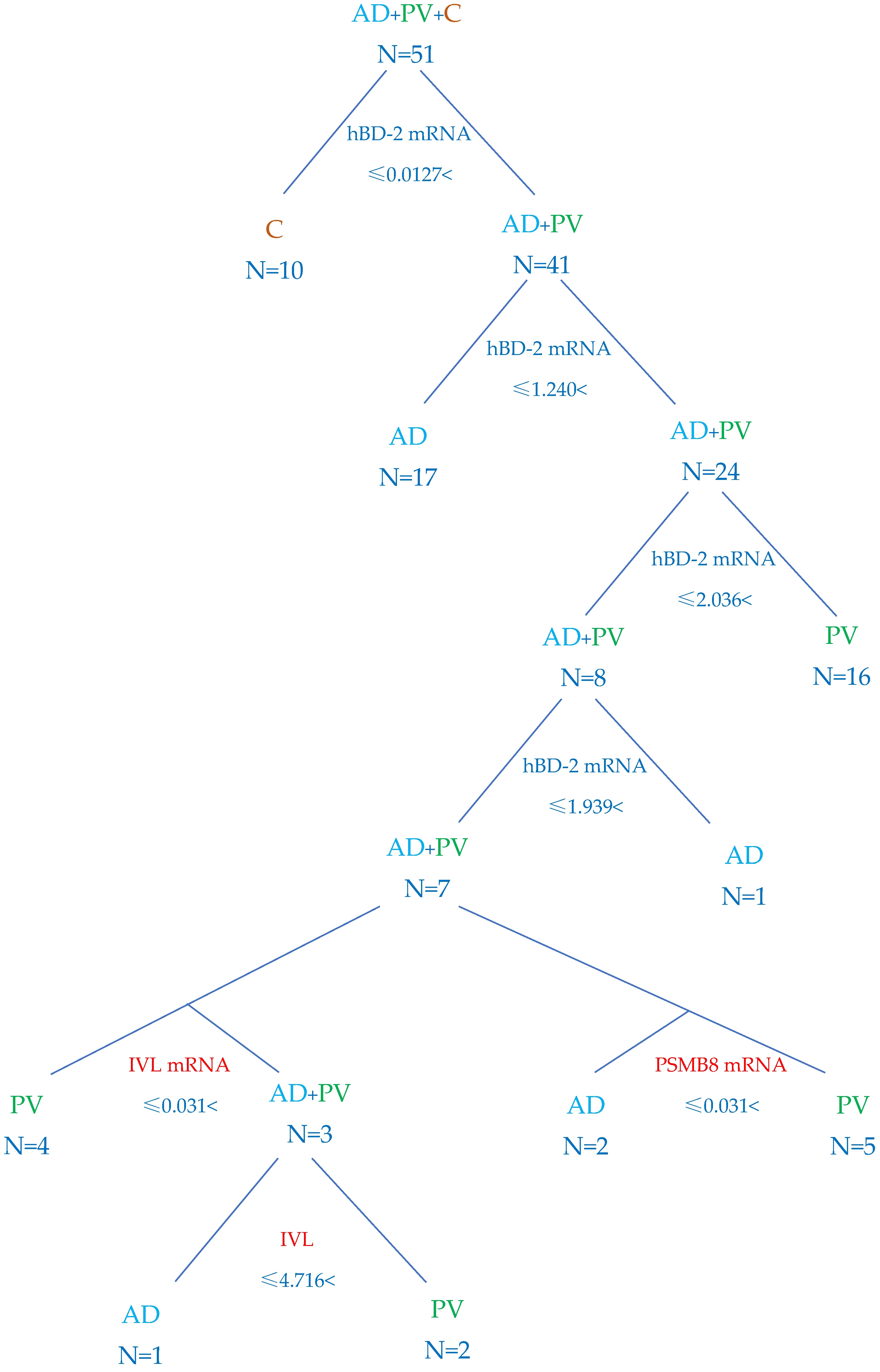
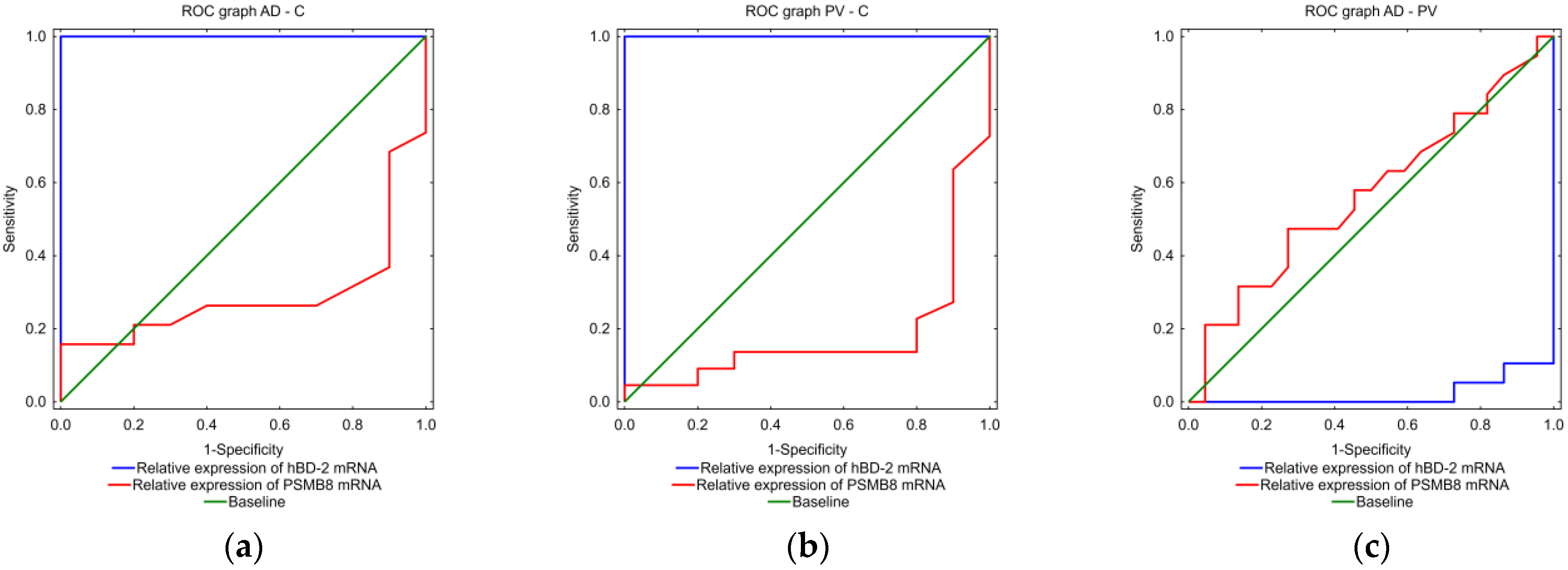
2.5. Algorithm to Differentiate AD and PV
3. Discussion
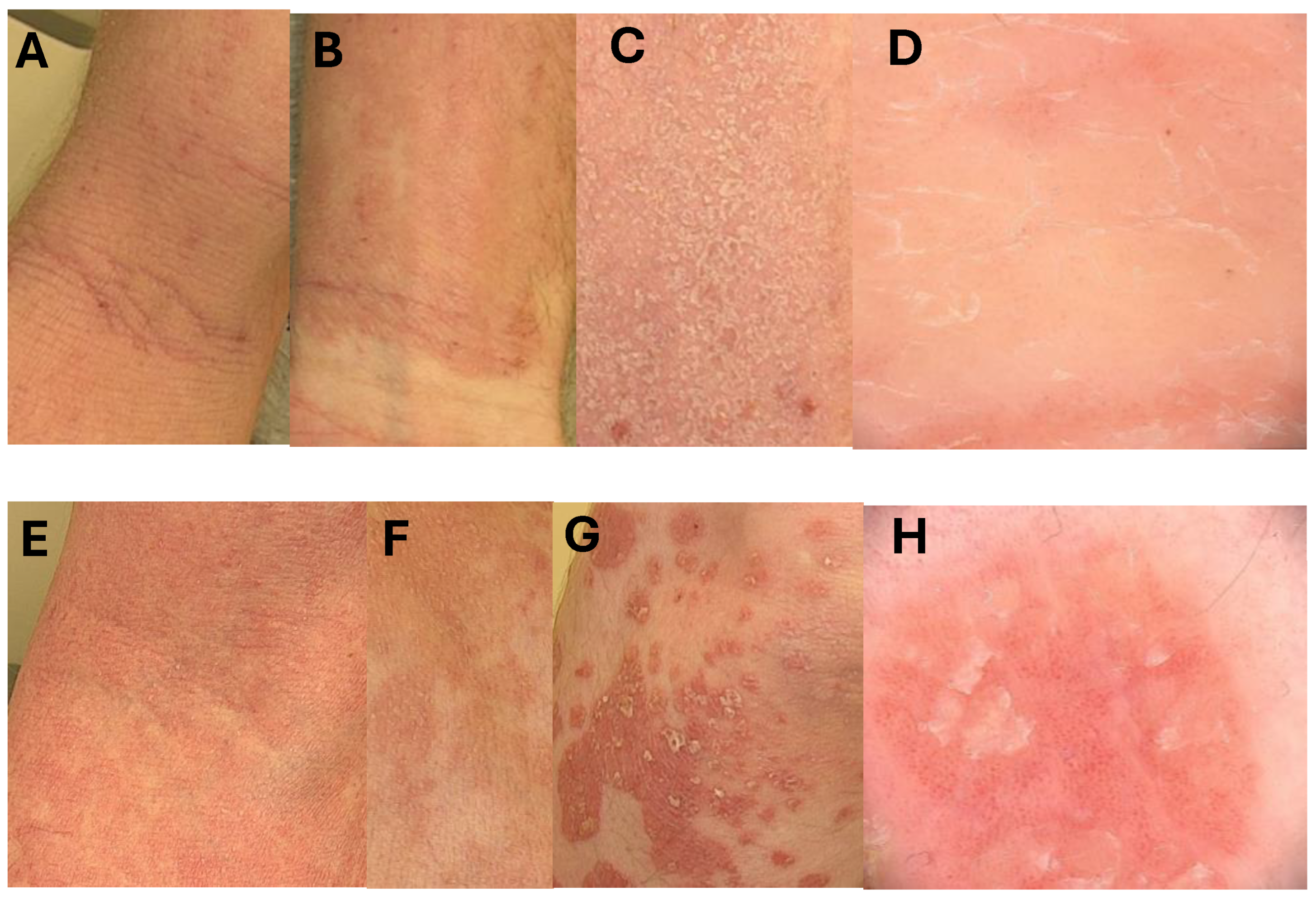
- A—an elbow fossa—classical atopic presentation with lichenification;
- B—an inner surface of the wrist—lesions are sharply demarcated like in psoriasis, but lichenification and scratch marks due to intense pruritus are typical for atopic changes;
- C—same scaling on atopic lesions, scales are thinner than in psoriasis;
- D—dermoscopic picture of AD lesion with irregularly distributed dilated vessels.
- E—confluent psoriatic papules in an elbow fossa resembling atopic dermatitis, but no scratch marks and lichenification are visible;
- F—psoriatic lesions resembling atopic dermatitis with discrete scaling;
- G—typical psoriatic plaques covert with some scales, lesions are sharply demarcated;
- H—classical dermoscopic presentation of psoriasis with regular vessels and some scales.
4. Materials and Methods
4.1. Participants
4.2. Clinical Assessment of Skin Lesions
4.3. Skin Biopsies
4.4. Tissue Homogenate Preparation and ELISA
4.5. Total RNA Isolation, Reverse Transcription (RT) and Real Time PCR
4.6. Statistical Analysis
4.7. Classification Algorithm Based on the Test Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Silvestre Salvador, J.F.; Romero-Perez, D.; Encabo-Duran, B. Atopic Dermatitis in Adults: A Diagnostic Challenge. J. Investig. Allergol. Clin. Immunol. 2017, 27, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- McMenamin, M.E.; Sweeney, E.C. Psoriasis and eczema. Curr. Diagn. Pathol. 1997, 4, 20–27. [Google Scholar] [CrossRef]
- Furue, M.; Kadono, T. “Inflammatory skin march” in atopic dermatitis and psoriasis. Inflamm. Res. 2017, 66, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Hanifin, J.M.; Luger, T.A.; Stevens, S.R.; Pride, H.B. Consensus conference on pediatric atopic dermatitis. J. Am. Acad. Dermatol. 2003, 49, 1088–1095. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef]
- HanifIn, M.J. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 92, 44–47. [Google Scholar] [CrossRef]
- Enamandram, M.; Kimball, A.B. Psoriasis epidemiology: The interplay of genes and the environment. J. Investig. Dermatol. 2013, 133, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Loring, B.; John, S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M.; Global Psoriasis, A. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Tschachler, E. Psoriasis: The epidermal component. Clin. Dermatol. 2007, 25, 589–595. [Google Scholar] [CrossRef]
- Cribier, B.J. Psoriasis under the microscope. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 3–9. [Google Scholar] [CrossRef]
- Menter, A. Psoriasis and psoriatic arthritis treatment. Am. J. Manag. Care 2016, 22, s225–s237. [Google Scholar]
- Oji, V.; Luger, T.A. The skin in psoriasis: Assessment and challenges. Clin. Exp. Rheumatol. 2015, 33, S14–S19. [Google Scholar]
- Docampo-Simon, A.; Belinchon, I.; Sanchez-Pujol, M.J.; Berbegal, L.; Miralles, J.; Lucas, A.; Quecedo, E.; Fuertes, A.; Mateu-Puchades, A.; Betlloch, I. Psoriasis dermatitis, a common phenotype of early forms of both psoriasis and atopic dermatitis in children: A prospective multicenter study. Int. J. Dermatol. 2024. [Google Scholar] [CrossRef]
- Terlikowska Brzósko, A.; Owczarek, W.; Galus, R.; Paluchowska, E.; Kozłowski, W. The role of the pathological examination in the diagnosis of atopic dermatitis and psoriasis vulgaris. Lek. Woj. 2016, 2, 143–147. [Google Scholar]
- Chularojanamontri, L.; Charoenpipatsin, N.; Silpa-Archa, N.; Wongpraparut, C.; Thongboonkerd, V. Proteomics in psoriasis. Int. J. Mol. Sci. 2019, 20, 1141. [Google Scholar] [CrossRef]
- Kromann, B.; Olsson, A.; Zhang, Y.M.; Løvendorf, M.B.; Skov, L.; Dyring-Andersen, B. Proteins in the skin and blood in patients with psoriasis: A systematic review of proteomic studies. J. Dermatol. 2024, 240, 317–328. [Google Scholar] [CrossRef]
- Yang, L.; Guo, W.; Zhang, S.; Wang, G. Ubiquitination-proteasome system: A new player in the pathogenesis of psoriasis and clinical implications. J. Dermatol. Sci. 2018, 89, 219–225. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, E.-K.; Chey, Y.; Song, M.-J.; Jang, H.H. Targeted protein degradation: Principles and applications of the proteasome. Cells 2023, 12, 1846. [Google Scholar] [CrossRef] [PubMed]
- Eklund, S. Interpreting a Giant: Studies of Structure and Function of Tripeptidyl-Peptidase II. Ph.D. Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2011. [Google Scholar]
- Rockel, B.; Kopec, K.O.; Lupas, A.N.; Baumeister, W. Structure and function of tripeptidyl peptidase II, a giant cytosolic protease. Biochim Biophys Acta 2012, 1824, 237–245. [Google Scholar] [CrossRef]
- Fukuda, Y.; Beck, F.; Plitzko, J.M.; Baumeister, W. In situ structural studies of tripeptidyl peptidase II (TPPII) reveal spatial association with proteasomes. Proc. Natl. Acad. Sci. USA 2017, 114, 4412–4417. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Groettrup, M. On the Role of the Immunoproteasome in Protein Homeostasis. Cells 2021, 10, 3216. [Google Scholar] [CrossRef]
- Rana, P.S.; Ignatz-Hoover, J.J.; Driscoll, J.J. Targeting Proteasomes and the MHC Class I Antigen Presentation Machinery to Treat Cancer, Infections and Age-Related Diseases. J. Cancers 2023, 15, 5632. [Google Scholar] [CrossRef]
- Henry, L.; Le Gallic, L.; Garcin, G.; Coux, O.; Jumez, N.; Roger, P.; Lavabre-Bertrand, T.; Martinez, J.; Meunier, L.; Stoebner, P.E. Proteolytic activity and expression of the 20S proteasome are increased in psoriasis lesional skin. Br. J. Dermatol. 2011, 165, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, B. Tripeptidyl-peptidase II: Update on an oldie that still counts. Biochimie 2019, 166, 27–37. [Google Scholar] [CrossRef]
- Reits, E.; Neijssen, J.; Herberts, C.; Benckhuijsen, W.; Janssen, L.; Drijfhout, J.W.; Neefjes, J. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity 2004, 20, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Kloetzel, P.M. Generation of major histocompatibility complex class I antigens: Functional interplay between proteasomes and TPPII. Nat. Immunol. 2004, 5, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Atallah, I.; Quinodoz, M.; Campos-Xavier, B.; Peter, V.G.; Fouriki, A.; Bonvin, C.; Bottani, A.; Kumps, C.; Angelini, F.; Bellutti Enders, F.; et al. Immune deficiency, autoimmune disease and intellectual disability: A pleiotropic disorder caused by biallelic variants in the TPP2 gene. Clin. Genet. 2021, 99, 780–788. [Google Scholar] [CrossRef]
- Hoffjan, S.; Stemmler, S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. Br. J. Dermatol. 2007, 157, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Blunder, S.; Dubrac, S.; Gruber, R.; Moosbrugger-Martinz, V. Epidermal barrier in hereditary ichthyoses, atopic dermatitis, and psoriasis. J. Dtsch. Dermatol. Ges. 2015, 13, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y. Molecular Mechanism of Epidermal Barrier Dysfunction as Primary Abnormalities. Int. J. Mol. Sci. 2020, 21, 1194. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Man, X.-Y.; Li, W.; Zhou, J.; Landeck, L.; Cai, S.-Q.; Zheng, M. Regulation of Involucrin in Psoriatic Epidermal Keratinocytes: The Roles of ERK1/2 and GSK-3β. Cell Biochem. Biophys. 2013, 66, 523–528. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Ogawa, H. The role of human β-defensins in allergic diseases. J. Clin. Exp. Allergy 2016, 46, 1522–1530. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. A peptide antibiotic from human skin. Nature 1997, 387, 861. [Google Scholar] [CrossRef]
- Bernard, F.X.; Morel, F.; Camus, M.; Pedretti, N.; Barrault, C.; Garnier, J.; Lecron, J.C. Keratinocytes under Fire of Proinflammatory Cytokines: Bona Fide Innate Immune Cells Involved in the Physiopathology of Chronic Atopic Dermatitis and Psoriasis. J. Allergy 2012, 2012, 718725. [Google Scholar] [CrossRef]
- Suarez-Farinas, M.; Tintle, S.J.; Shemer, A.; Chiricozzi, A.; Nograles, K.; Cardinale, I.; Duan, S.; Bowcock, A.M.; Krueger, J.G.; Guttman-Yassky, E. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J. Allergy Clin. Immunol. 2011, 127, 954–964. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Ogawa, H.; Niyonsaba, F. Current insights into the role of human β-defensins in atopic dermatitis. Clin. Exp. Immunol. 2017, 190, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.L.; Jungersted, J.; Andersen, P.; Slotved, H.C.; Krogfelt, K.; Agner, T. Human β-defensin-2 as a marker for disease severity and skin barrier properties in atopic dermatitis. Br. J. Dermatol. 2013, 169, 587–593. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Y.; Fan, R.A.; Kirk, C.J. Proteasome Inhibitors and Their Pharmacokinetics, Pharmacodynamics, and Metabolism. Int. J. Mol. Sci. 2021, 22, 11595. [Google Scholar] [CrossRef] [PubMed]
- Seifert, U.; Maranon, C.; Shmueli, A.; Desoutter, J.F.; Wesoloski, L.; Janek, K.; Henklein, P.; Diescher, S.; Andrieu, M.; de la Salle, H.; et al. An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat. Immunol. 2003, 4, 375–379. [Google Scholar] [CrossRef]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schäppi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139, S58–S64. [Google Scholar] [CrossRef]
- Brunner, P.M.; Silverberg, J.I.; Guttman-Yassky, E.; Paller, A.S.; Kabashima, K.; Amagai, M.; Luger, T.A.; Deleuran, M.; Werfel, T.; Eyerich, K.; et al. Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J. Investig. Dermatol. 2017, 137, 18–25. [Google Scholar] [CrossRef]
- Christophers, E. Explaining phenotype heterogeneity in patients with psoriasis. Br. J. Dermatol. 2008, 158, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Andersen, Y.M.; Gislason, G.H.; Skov, L.; Thyssen, J.P. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy 2017, 72, 783–791. [Google Scholar] [CrossRef]
- Egeberg, A.; Skov, L.; Andersen, Y.M.F.; Mallbris, L.; Gislason, G.H.; Silverberg, J.I.; Wu, J.J.; Thyssen, J.P. Ten-year mortality is increased after hospitalization for atopic dermatitis compared with the general population, but reduced compared with psoriasis. J. Am. Acad. Dermatol. 2017, 76, 98–105. [Google Scholar] [CrossRef]
- Girolomoni, G.; de Bruin-Weller, M.; Aoki, V.; Kabashima, K.; Deleuran, M.; Puig, L.; Bansal, A.; Rossi, A.B. Nomenclature and clinical phenotypes of atopic dermatitis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211002979. [Google Scholar] [CrossRef]
- Kamer, B.; Rotsztejn, H.; Kulig, A.; Raczyńska, J.; Piotrowicz, M.; Kulig, K.; Pyziak, K. The coexistence of atopic dermatitis and psoriasis in a 12 months-old girl. Pol. Merkur. Lek. Organ. Pol. Tow. Lek. 2005, 19, 542–544. [Google Scholar]
- Sugiura, K.; Sugiura, M. A Case of Atopic Dermatitis Co-existing with Psoriasis Vulgaris. J. Clin. Exp. Dermatol. Res. 2017, 8, 2. [Google Scholar] [CrossRef]
- Terlikowska-Brzósko, A.; Paluchowska, E.; Owczarek, W.; Koktysz, R.; Galus, R. Coexistence of psoriasis and atopic dermatitis. Our Dermatol. Online 2015, 6, 438. [Google Scholar] [CrossRef]
- Gerger, V.; Rummenigge, C.; Pinter, A.; Kaufmann, R. Safety and efficacy of blocking IL-4/13 and IL-23 in concomitant atopic dermatitis and psoriasis—Two case reports. J. Dtsch. Dermatol. Ges. 2023, 21, 648–650. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Krueger, J.G.; Lebwohl, M.G. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp. Dermatol. 2018, 27, 409–417. [Google Scholar] [CrossRef]
- Eyerich, K.; Eyerich, S. Immune response patterns in non-communicable inflammatory skin diseases. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 692–703. [Google Scholar] [CrossRef]
- Muller, S.; Welchowski, T.; Schmid, M.; Maintz, L.; Herrmann, N.; Wilsmann-Theis, D.; Royeck, T.; Havenith, R.; Bieber, T. Development of a clinical algorithm to predict phenotypic switches between atopic dermatitis and psoriasis (the “Flip-Flop” phenomenon). Allergy 2024, 79, 164–173. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Imanishi, H.; Oshimo, T.; Tsuruta, D. A case of dupilumab-induced psoriasis-like eruption treated with baricitinib. Ski. Health Dis. 2024, 4, e338. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, S.; Li, X.; Mao, T.; Fang, M.; Hu, Y.; Ye, W. Treatment of paradoxical eczematous eruption in psoriasis treated with secukinumab: A case report. Medicine 2023, 102, e32844. [Google Scholar] [CrossRef]
- Thijs, J.L.; de Bruin-Weller, M.S.; Hijnen, D. Current and Future Biomarkers in Atopic Dermatitis. Immunol. Allergy Clin. North. Am. 2017, 37, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.S.; Nierkens, S.; Knol, E.F.; Giovannone, B.; Delemarre, E.M.; van der Schaft, J.; van Wijk, F.; de Bruin-Weller, M.S.; Drylewicz, J.; Thijs, J.L. Confirmation of multiple endotypes in atopic dermatitis based on serum biomarkers. J. Allergy Clin. Immunol. 2021, 147, 189–198. [Google Scholar] [CrossRef]
- de Jongh, G.J.; Zeeuwen, P.L.; Kucharekova, M.; Pfundt, R.; van der Valk, P.G.; Blokx, W.; Dogan, A.; Hiemstra, P.S.; van de Kerkhof, P.C.; Schalkwijk, J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J. Investig. Dermatol. 2005, 125, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002, 347, 1151–1160. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Suárez-Fariñas, M.; Chiricozzi, A.; Nograles, K.E.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Lin, P.; Bergman, R.; Bowcock, A.M. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J. Allergy Clin. Immunol. 2009, 124, 1235–1244.e58. [Google Scholar] [CrossRef]
- Kaczmarska, A.; Kwiatkowska, D.; Skrzypek, K.K.; Kowalewski, Z.T.; Jaworecka, K.; Reich, A. Pathomechanism of Pruritus in Psoriasis and Atopic Dermatitis: Novel Approaches, Similarities and Differences. Int. J. Mol. Sci. 2023, 24, 14734. [Google Scholar] [CrossRef] [PubMed]
- Mlnarczuk, I.; Mroz, P.; Hoser, G.; Nowis, D.; Bialy, L.P.; Ziemba, H.; Grzela, T.; Feleszko, W.; Malejczyk, J.; Wojcik, C.; et al. AAF-cmk sensitizes tumor cells to trail-mediated apoptosis. Leuk. Res. 2004, 28, 53–61. [Google Scholar] [CrossRef]
- Mlynarczuk-Bialy, I. Enigmatic tripeptidylpeptidase II—Protease for special tasks. Postępy Biol. Komórki. 2008, 35, 427–439. [Google Scholar]
- Maruyama, H.; Hirayama, K.; Yamashita, M.; Ohgi, K.; Tsujimoto, R.; Takayasu, M.; Shimohata, H.; Kobayashi, M. Serum 20S proteasome levels are associated with disease activity in MPO-ANCA-associated microscopic polyangiitis. J. BMC Rheumatol. 2020, 4, 36. [Google Scholar] [CrossRef]
 stands for the extreme value,
stands for the extreme value,  denotes an outlier.
denotes an outlier.
 stands for the extreme value,
stands for the extreme value,  denotes an outlier.
denotes an outlier.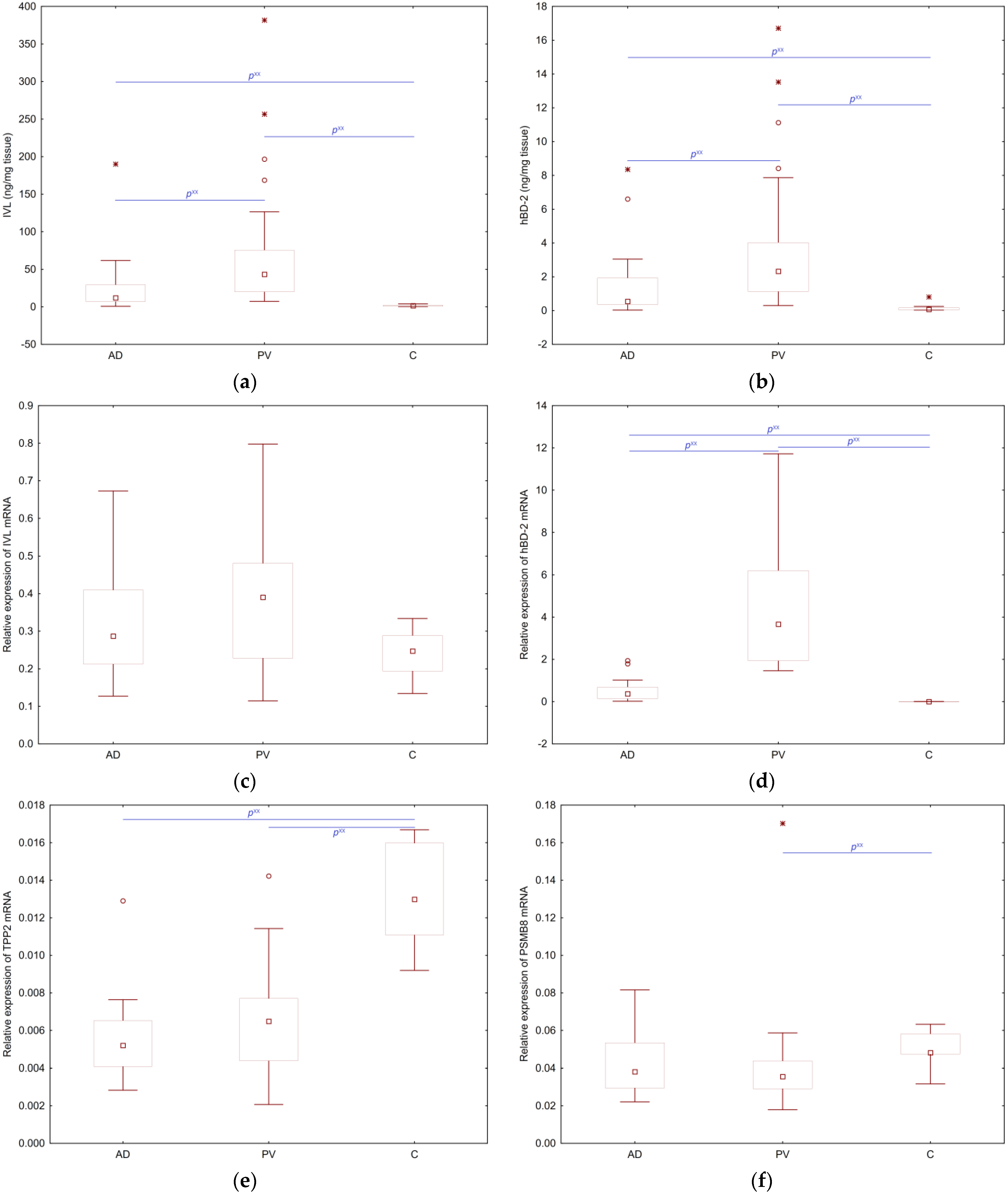
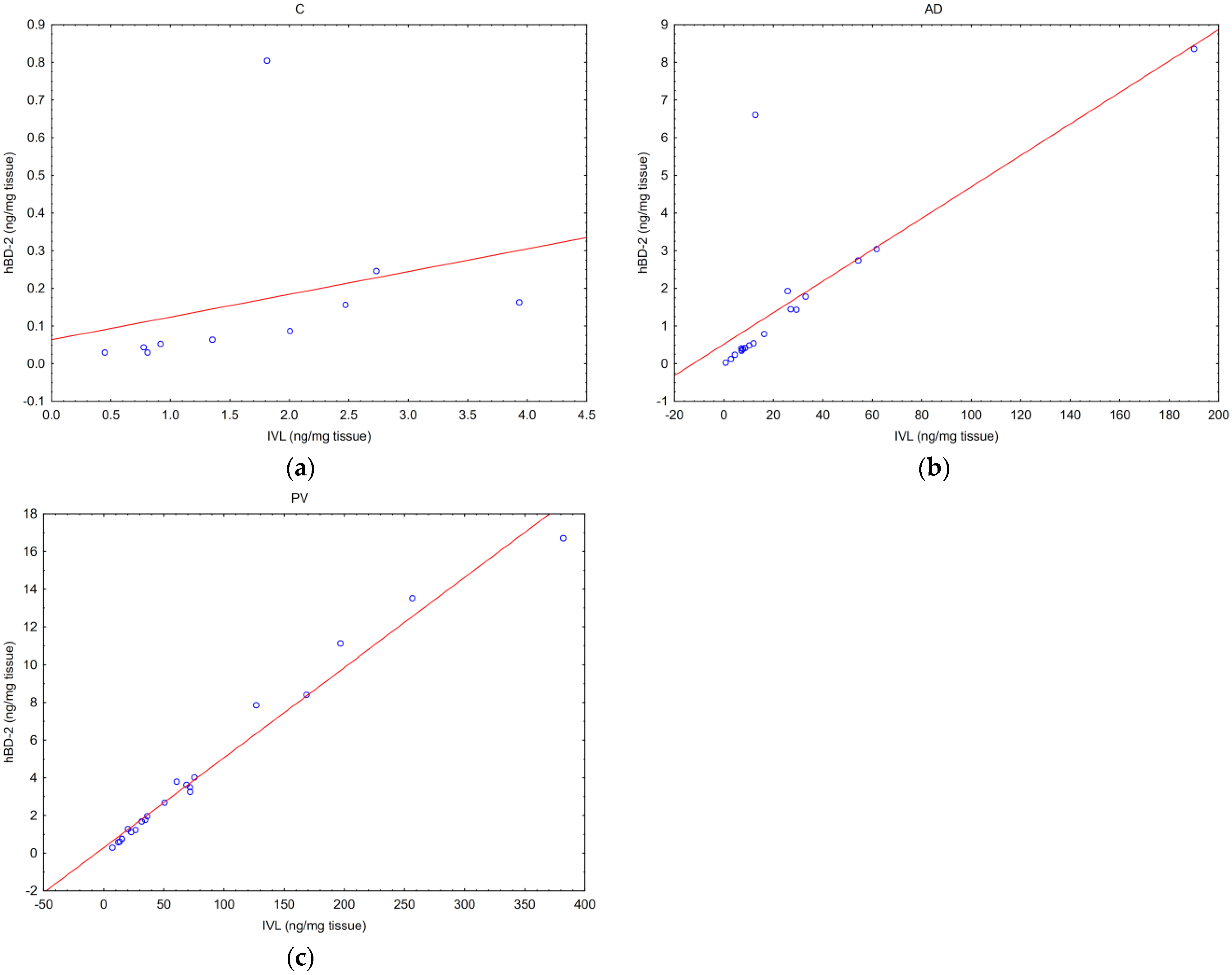

| IVL | hBD-2 | IVL mRNA | hBD-2 mRNA | TPP2 mRNA | PSMB8 | |
|---|---|---|---|---|---|---|
| IVL | AD NA | AD p ** R = 0.93 | AD NS | AD NS | AD NS | AD NS |
| PV NA | PV p ** R = 0.98 | PV NS | PV NS | PV NS | PV NS | |
| C NA | C p ** R = 0.83 | C NS | C NS | C NS | C NS | |
| hBD-2 | AD p ** R = 0.93 | AD NA | AD NS | AD NS | AD NS | AD NS |
| PV p ** R = 0.98 | PV NA | PV NS | PV NS | PV NS | PV NS | |
| C p ** R = 0.83 | C NA | C NS | C NS | C NS | C NS | |
| IVL mRNA | AD NS | AD NS | AD NA | AD NS | AD p ** R = 0.67 | AD p * R = 0.56 |
| PV NS | PV NS | PV NA | PV NS | PV p ** R = 0.60 | PV NS | |
| C NS | C NS | C NA | C NS | C NS | C NS | |
| hBD-2 mRNA | AD NS | AD NS | AD NS | AD NA | AD NS | AD NS |
| PV NS | PV NS | PV NS | PV NA | PV NS | PV NS | |
| C NS | C NS | C NS | C NA | C NS | C NS | |
| TPP2 mRNA | AD NS | AD NS | AD p ** R = 0.67 | AD NS | AD NA | AD p ** R = 0.77 |
| PV NS | PV NS | PV p ** R = 0.60 | PV NS | PV NA | PV p ** R = 0.72 | |
| C NS | C NS | C NS | C NS | C NA | C p ** R = 0.89 | |
| PSMB8 mRNA | AD NS | AD NS | AD p ** R = 0.67 | AD NS | AD p ** R = 0.77 | AD NA |
| PV NS | PV NS | PV NS | PV NS | PV p ** R = 0.72 | PV NA | |
| C NS | C NS | C NS | C NS | C p ** R = 0.89 | C NA | |
| Age Gender | AD NS | |||||
| PV NS | ||||||
| C NS | ||||||
| Disease duration | AD NS | AD NS | AD NS | AD NS | AD NS | AD NS |
| PV NS | PV NS | PV NS | PV p * R = 0.53 | PV NS | PV NS | |
| Single lesion duration | AD NS | |||||
| PV NS | ||||||
| Itch | AD NS | AD NS | AD p * R = −0.53 | AD NS | AD NS | AD NS |
| PV NS | PV NS | PV NS | PV NS | PV NS | PV NS | |
| BSA | AD NS | AD NS | AD NS | AD NS | AD NS | AD p * R = 0.56 |
| PV NS | PV NS | PV NS | PV NS | PV NS | PV NS | |
| SCORAD | AD NS | AD NS | AD NS | AD p * R = −0.53 | AD NS | AD NS |
| Modified SCORAD | AD p * R = −0.60 | |||||
| EASI | AD NS | AD NS | AD NS | AD NS | AD NS | AD NS |
| ESI | ||||||
| ESI-E | ||||||
| ESI-I | ||||||
| ESI-L | ||||||
| PSI | PV NS | PV NS | PV NS | PV p ** R = 0.56 | PV NS | PV NS |
| E-PSI | PV p * R = 0.45 | PV p * | ||||
| I-PSI | PV p * R = 0.42 | R = 0.45 | ||||
| S-PSI | PV p * R = 0.46 | PV NS | ||||
| AD 1 | Variable | Median | Minimum | Maximum | Bottom Quartile | Upper Quartile |
| Age | 32 | 20 | 55 | 23 | 49 | |
| Disease duration (days) | 7665 | 62 | 15,330 | 3650 | 10,950 | |
| Lesion duration (days) | 62 | 2 | 1825 | 21 | 279 | |
| Pruritus | 8 | 2 | 10 | 7 | 9 | |
| BSA | 62% | 17% | 100% | 47% | 86% | |
| SCORAD | 70.00 | 33.20 | 101.00 | 62.40 | 79.20 | |
| Modified SCORAD | 58.80 | 31.40 | 83.00 | 47.40 | 62.50 | |
| EASI | 35.80 | 10.10 | 62.70 | 22.50 | 50.50 | |
| ESI | 9 | 5 | 12 | 8 | 11 | |
| ESI-E | 3 | 1 | 3 | 2 | 3 | |
| ESI-I | 2 | 1 | 3 | 2 | 3 | |
| ESI-Ex | 2 | 1 | 3 | 1 | 3 | |
| ESI-L | 2 | 1 | 3 | 2 | 3 | |
| PV 1 | Variable | Median | Minimum | Maximum | Bottom Quartile | Upper Quartile |
| Age | 36 | 25 | 58 | 28 | 44 | |
| Disease duration (days) | 5840 | 42 | 13,870 | 1825 | 10,220 | |
| Lesion duration (days) | 230.5 | 14.0 | 3650.0 | 124.0 | 730.0 | |
| Pruritus | 4 | 1 | 8 | 3 | 7 | |
| BSA | 19.55% | 3.70% | 76.50% | 9.25% | 29.00% | |
| PASI | 17.15 | 2.60 | 43.90 | 9.30 | 20.20 | |
| PSI | 7 | 3 | 9 | 6 | 8 | |
| E-PSI | 3 | 1 | 3 | 2 | 3 | |
| S-PSI | 2 | 1 | 3 | 2 | 3 | |
| I-PSI | 2 | 1 | 3 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlikowska-Brzósko, A.; Galus, R.; Murawski, P.; Niderla-Bielińska, J.; Młynarczuk-Biały, I.; Paluchowska, E.; Owczarek, W. Human Beta Defensin-2 mRNA and Proteasome Subunit β Type 8 mRNA Analysis, Useful in Differentiating Skin Biopsies from Atopic Dermatitis and Psoriasis Vulgaris Patients. Int. J. Mol. Sci. 2024, 25, 9192. https://doi.org/10.3390/ijms25179192
Terlikowska-Brzósko A, Galus R, Murawski P, Niderla-Bielińska J, Młynarczuk-Biały I, Paluchowska E, Owczarek W. Human Beta Defensin-2 mRNA and Proteasome Subunit β Type 8 mRNA Analysis, Useful in Differentiating Skin Biopsies from Atopic Dermatitis and Psoriasis Vulgaris Patients. International Journal of Molecular Sciences. 2024; 25(17):9192. https://doi.org/10.3390/ijms25179192
Chicago/Turabian StyleTerlikowska-Brzósko, Agnieszka, Ryszard Galus, Piotr Murawski, Justyna Niderla-Bielińska, Izabela Młynarczuk-Biały, Elwira Paluchowska, and Witold Owczarek. 2024. "Human Beta Defensin-2 mRNA and Proteasome Subunit β Type 8 mRNA Analysis, Useful in Differentiating Skin Biopsies from Atopic Dermatitis and Psoriasis Vulgaris Patients" International Journal of Molecular Sciences 25, no. 17: 9192. https://doi.org/10.3390/ijms25179192
APA StyleTerlikowska-Brzósko, A., Galus, R., Murawski, P., Niderla-Bielińska, J., Młynarczuk-Biały, I., Paluchowska, E., & Owczarek, W. (2024). Human Beta Defensin-2 mRNA and Proteasome Subunit β Type 8 mRNA Analysis, Useful in Differentiating Skin Biopsies from Atopic Dermatitis and Psoriasis Vulgaris Patients. International Journal of Molecular Sciences, 25(17), 9192. https://doi.org/10.3390/ijms25179192






