Multi-Omics Integration Analysis Pinpoint Proteins Influencing Brain Structure and Function: Toward Drug Targets and Neuroimaging Biomarkers for Neuropsychiatric Disorders
Abstract
1. Introduction
2. Results
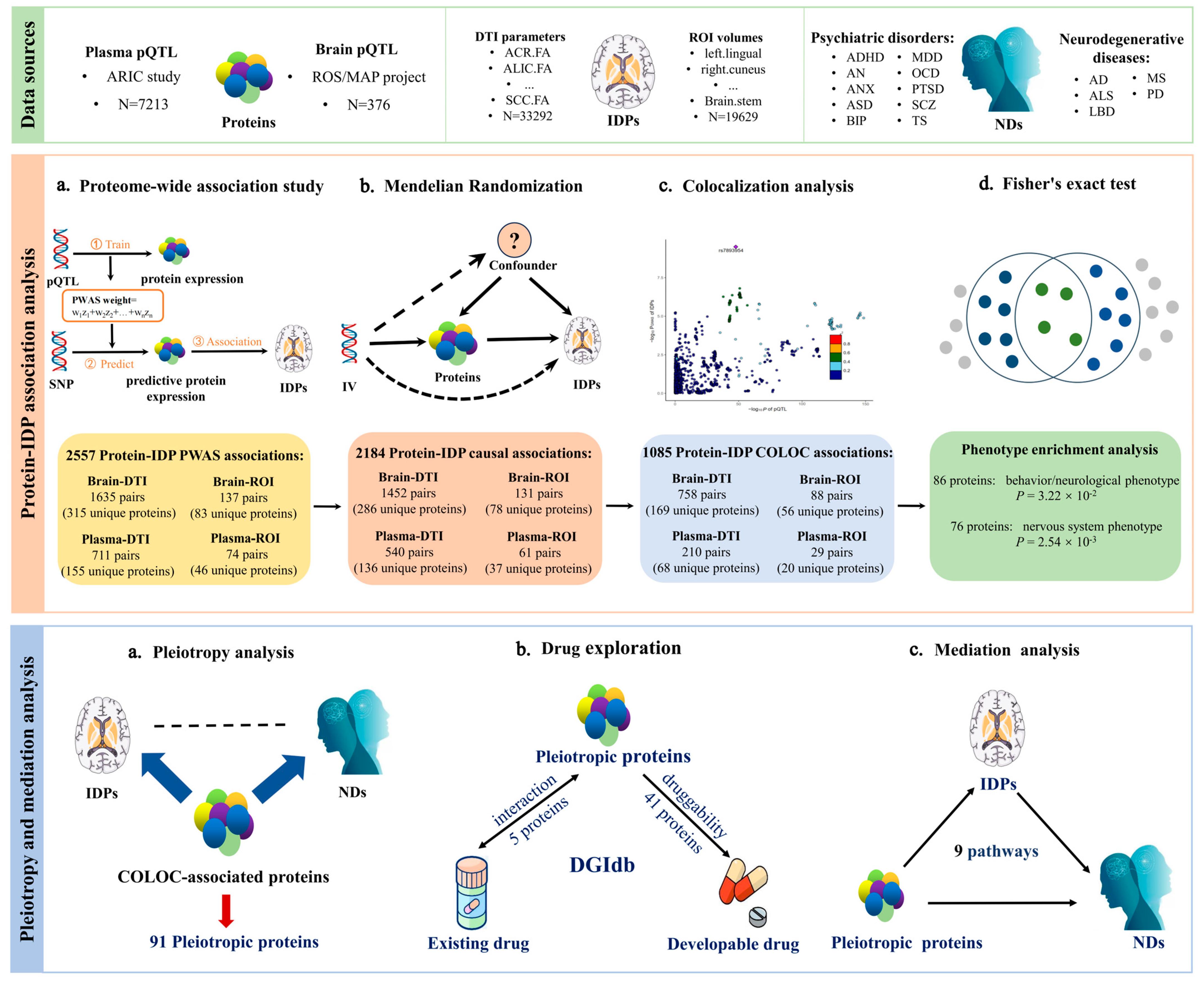
2.1. PWAS Identified 2557 Protein-IDP Associations
2.2. MR Identified 2184 Protein-IDP Associations
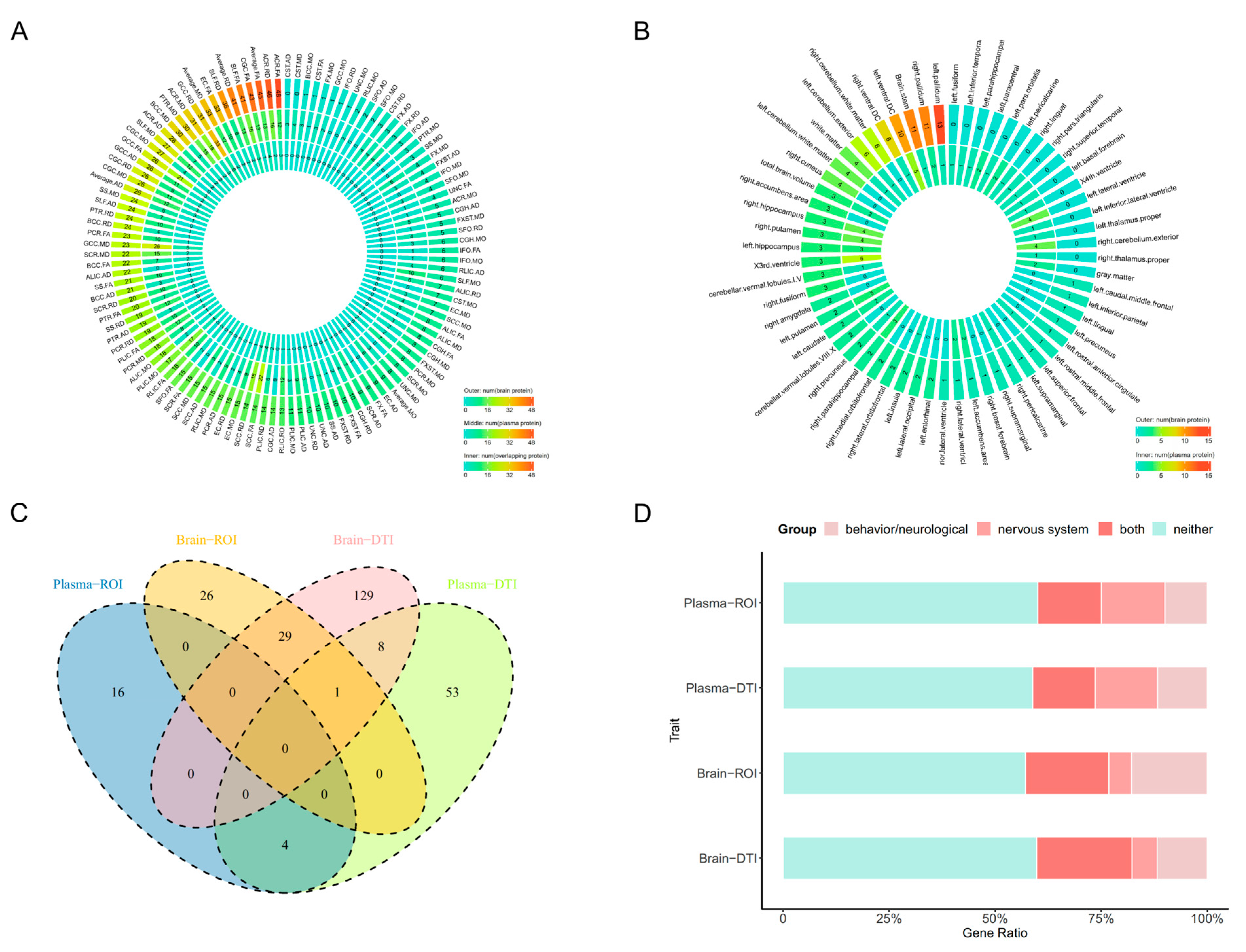
2.3. COLOC Identified 1085 Protein-IDP Associations
2.4. The Phenotype Enrichment Analysis
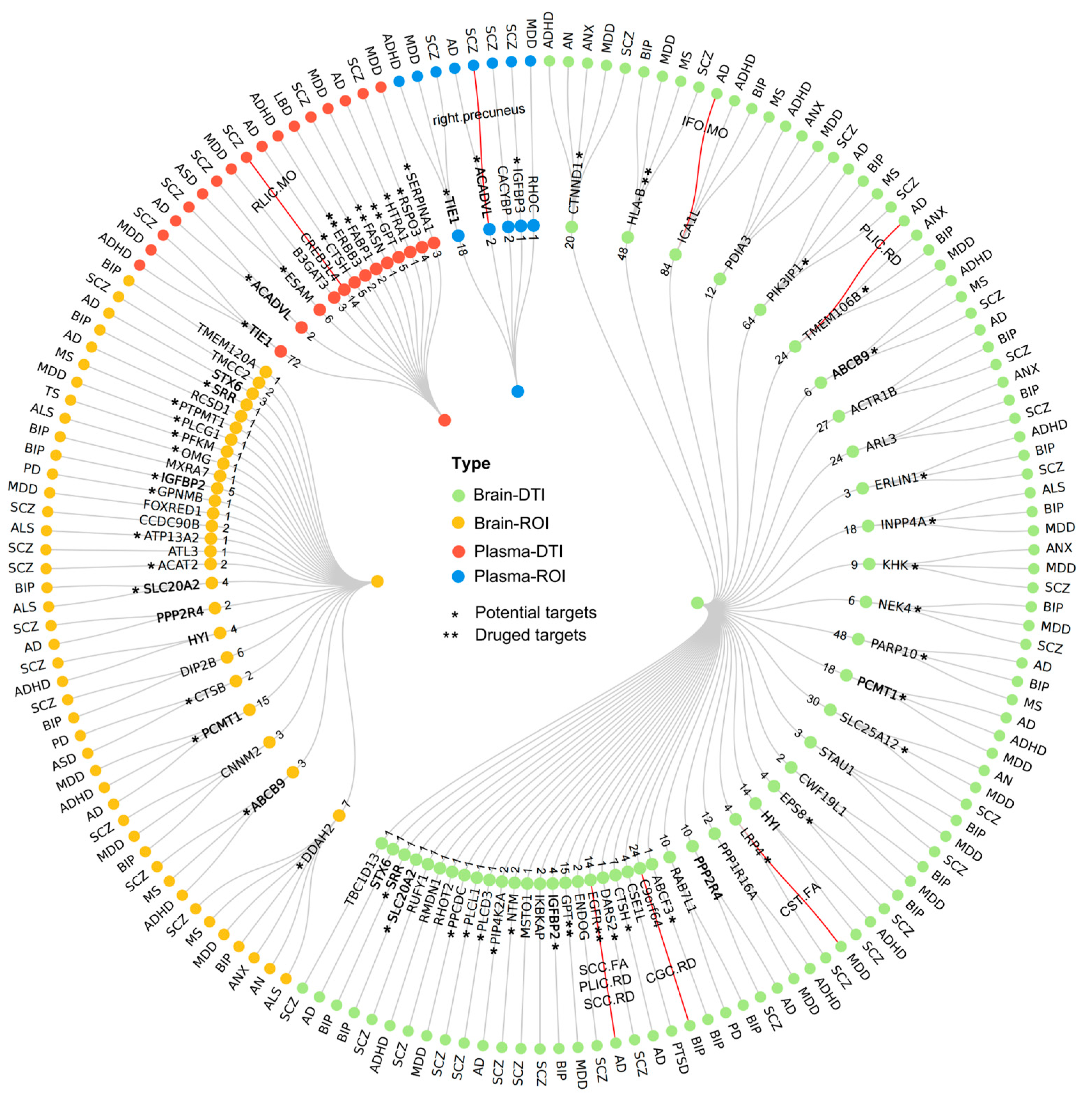
2.5. Pleiotropy Analysis with Neuropsychiatric Disorders
2.6. Druggable Targets Exploration
2.7. Mediation Analysis
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.1.1. GWAS Summary Statistics
4.1.2. Human Brain pQTL Data
4.1.3. Human Plasma pQTL Data
4.2. Statistical Analysis
4.2.1. Proteome-Wide Association Studies
4.2.2. Mendelian Randomization Analysis
4.2.3. Colocalization Analysis
4.2.4. The Phenotype Enrichment Analysis
4.2.5. Pleiotropy Analysis with Neuropsychiatric Disorders
4.2.6. Druggable Targets Exploration
4.2.7. Mediation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fu, J.; Zhang, Q.; Wang, J.; Wang, M.; Zhang, B.; Zhu, W.; Qiu, S.; Geng, Z.; Cui, G.; Yu, Y.; et al. Cross-Ancestry Genome-Wide Association Studies of Brain Imaging Phenotypes. Nat. Genet. 2024, 56, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Savarraj, J.P.J.; Kitagawa, R.; Kim, D.H.; Choi, H.A. White Matter Connectivity for Early Prediction of Alzheimer’s Disease. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2022, 30, 17–28. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Long, J.; Li, W.; Wang, X.; Hu, N.; Zhao, X.; Sun, T. White Matter Changes in Parkinson’s Disease. NPJ Park. Dis. 2023, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Caldiroli, A.; Buoli, M.; van Haren, N.E.M.; de Nijs, J.; Altamura, A.C.; Cahn, W. The Relationship of IQ and Emotional Processing with Insula Volume in Schizophrenia. Schizophr. Res. 2018, 202, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet Lond. Engl. 2022, 399, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, T.; Yang, Y.; Wang, X.; Luo, T.; Shan, Y.; Zhu, Z.; Xiong, D.; Hauberg, M.E.; Bendl, J.; et al. Common Genetic Variation Influencing Human White Matter Microstructure. Science 2021, 372, eabf3736. [Google Scholar] [CrossRef]
- Somasundaram, K.; Kalaiselvi, T. Automatic Brain Extraction Methods for T1 Magnetic Resonance Images Using Region Labeling and Morphological Operations. Comput. Biol. Med. 2011, 41, 716–725. [Google Scholar] [CrossRef]
- Conole, E.L.S.; Stevenson, A.J.; Muñoz Maniega, S.; Harris, S.E.; Green, C.; Valdés Hernández, M.D.C.; Harris, M.A.; Bastin, M.E.; Wardlaw, J.M.; Deary, I.J.; et al. DNA Methylation and Protein Markers of Chronic Inflammation and Their Associations With Brain and Cognitive Aging. Neurology 2021, 97, e2340–e2352. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Wang, Y.; Minn, I.; Bienko, N.; Ambinder, E.B.; Xu, X.; Peters, M.E.; Dougherty, J.W.; Vranesic, M.; Koo, S.M.; et al. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017, 74, 67–74. [Google Scholar] [CrossRef]
- Jin, X.; Dong, S.; Yang, Y.; Bao, G.; Ma, H. Nominating Novel Proteins for Anxiety via Integrating Human Brain Proteomes and Genome-Wide Association Study. J. Affect. Disord. 2024, 358, 129–137. [Google Scholar] [CrossRef]
- Wingo, T.S.; Liu, Y.; Gerasimov, E.S.; Gockley, J.; Logsdon, B.A.; Duong, D.M.; Dammer, E.B.; Lori, A.; Kim, P.J.; Ressler, K.J.; et al. Brain Proteome-Wide Association Study Implicates Novel Proteins in Depression Pathogenesis. Nat. Neurosci. 2021, 24, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.R.; Yates, M.; de Celis, C.R.; Drineas, P.; Jahanshad, N.; Thompson, P.; Paschou, P. Multiomic Approach and Mendelian Randomization Analysis Identify Causal Associations between Blood Biomarkers and Subcortical Brain Structure Volumes. NeuroImage 2023, 284, 120466. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Yi, M.; Xie, S.; Zhang, X.; Tao, D.; Liu, Y.; Yang, Y. Evidence Based on Mendelian Randomization and Colocalization Analysis Strengthens Causal Relationships between Structural Changes in Specific Brain Regions and Risk of Amyotrophic Lateral Sclerosis. Front. Neurosci. 2024, 18, 1333782. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Qian, W.; Wang, W.; Yu, S.; Lin, G.N. Mendelian Randomization Studies of Brain MRI Yield Insights into the Pathogenesis of Neuropsychiatric Disorders. BMC Genom. 2021, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.-J.; Sun, J.; Hua, Q.; Zhang, L.; Zhang, T.; Bai, T.; Wei, L.; Wang, X.; Qiu, B.; Wang, A.; et al. White Matter Dysfunction in Psychiatric Disorders Is Associated with Neurotransmitter and Genetic Profiles. Nat. Ment. Health 2023, 1, 655–666. [Google Scholar] [CrossRef]
- O’Brien, J.T. Clinical Significance of White Matter Changes. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2014, 22, 133–137. [Google Scholar] [CrossRef]
- Ghazi Sherbaf, F.; Rahmani, F.; Jooyandeh, S.M.; Aarabi, M.H. Microstructural Changes in Patients with Parkinson Disease and REM Sleep Behavior Disorder: Depressive Symptoms versus Non-Depressed. Acta Neurol. Belg. 2018, 118, 415–421. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, H.; Shi, S.; Jiang, D.; Tao, B.; Zhao, Y.; Zhang, W.; Gong, Q.; Sweeney, J.A.; Lui, S. White Matter Abnormalities in Never-Treated Patients With Long-Term Schizophrenia. Am. J. Psychiatry 2018, 175, 1129–1136. [Google Scholar] [CrossRef]
- Jiao, Y.; Lin, F.; Wu, J.; Li, H.; Fu, W.; Huo, R.; Cao, Y.; Wang, S.; Zhao, J. Plasticity in Language Cortex and White Matter Tracts after Resection of Dominant Inferior Parietal Lobule Arteriovenous Malformations: A Combined fMRI and DTI Study. J. Neurosurg. 2020, 134, 953–960. [Google Scholar] [CrossRef]
- Fan, C.C.; Loughnan, R.; Makowski, C.; Pecheva, D.; Chen, C.-H.; Hagler, D.J.; Thompson, W.K.; Parker, N.; van der Meer, D.; Frei, O.; et al. Multivariate Genome-Wide Association Study on Tissue-Sensitive Diffusion Metrics Highlights Pathways That Shape the Human Brain. Nat. Commun. 2022, 13, 2423. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, L.; Tian, T.; Cheng, J.; Zhang, B.; Qiu, S.; Geng, Z.; Cui, G.; Zhang, Q.; Liao, W.; et al. Cross-Ancestry Genome-Wide Association Meta-Analyses of Hippocampal and Subfield Volumes. Nat. Genet. 2023, 55, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.; Escott-Price, V.; Legge, S.; Baker, E.; Singh, K.D.; Walters, J.T.R.; Caseras, X.; Anney, R.J.L. Genetic Common Variants Associated with Cerebellar Volume and Their Overlap with Mental Disorders: A Study on 33,265 Individuals from the UK-Biobank. Mol. Psychiatry 2022, 27, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Luo, T.; Li, T.; Li, Y.; Zhang, J.; Shan, Y.; Wang, X.; Yang, L.; Zhou, F.; Zhu, Z.; et al. Genome-Wide Association Analysis of 19,629 Individuals Identifies Variants Influencing Regional Brain Volumes and Refines Their Genetic Co-Architecture with Cognitive and Mental Health Traits. Nat. Genet. 2019, 51, 1637–1644. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Kim, S.H.; Ben-Mahmoud, A.; Kim, O.-H.; Choi, T.-I.; Lee, K.-H.; Ku, B.; Eum, J.; Kee, Y.; Lee, S.; et al. Eif2b3 Mutants Recapitulate Phenotypes of Vanishing White Matter Disease and Validate Novel Disease Alleles in Zebrafish. Hum. Mol. Genet. 2021, 30, 331–342. [Google Scholar] [CrossRef]
- Nasca, A.; Scotton, C.; Zaharieva, I.; Neri, M.; Selvatici, R.; Magnusson, O.T.; Gal, A.; Weaver, D.; Rossi, R.; Armaroli, A.; et al. Recessive Mutations in MSTO1 Cause Mitochondrial Dynamics Impairment, Leading to Myopathy and Ataxia. Hum. Mutat. 2017, 38, 970–977. [Google Scholar] [CrossRef]
- Li, K.; Jin, R.; Wu, X. Whole-Exome Sequencing Identifies Rare Compound Heterozygous Mutations in the MSTO1 Gene Associated with Cerebellar Ataxia and Myopathy. Eur. J. Med. Genet. 2020, 63, 103623. [Google Scholar] [CrossRef]
- Musumeci, O.; Ferlazzo, E.; Rodolico, C.; Gambardella, A.; Gagliardi, M.; Aguglia, U.; Toscano, A. A Family With a Complex Phenotype Caused by Two Different Rare Metabolic Disorders: GLUT1 and Very-Long-Chain Fatty Acid Dehydrogenase (VLCAD) Deficiencies. Front. Neurol. 2020, 11, 514. [Google Scholar] [CrossRef]
- Wang, L.; Chiang, H.-C.; Wu, W.; Liang, B.; Xie, Z.; Yao, X.; Ma, W.; Du, S.; Zhong, Y. Epidermal Growth Factor Receptor Is a Preferred Target for Treating Amyloid-β-Induced Memory Loss. Proc. Natl. Acad. Sci. USA 2012, 109, 16743–16748. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Jeong, Y.J.; Kim, J.; Hoe, H.-S. EGFR Is a Potential Dual Molecular Target for Cancer and Alzheimer’s Disease. Front. Pharmacol. 2023, 14, 1238639. [Google Scholar] [CrossRef]
- Wada, K.; Lee, J.-Y.; Hung, H.-Y.; Shi, Q.; Lin, L.; Zhao, Y.; Goto, M.; Yang, P.-C.; Kuo, S.-C.; Chen, H.-W.; et al. Novel Curcumin Analogs to Overcome EGFR-TKI Lung Adenocarcinoma Drug Resistance and Reduce EGFR-TKI-Induced GI Adverse Effects. Bioorg. Med. Chem. 2015, 23, 1507–1514. [Google Scholar] [CrossRef]
- Tabira, T.; Kawamura, N. A Study of a Supplement Containing Huperzine A and Curcumin in Dementia Patients and Individuals with Mild Cognitive Impairment. J. Alzheimers Dis. JAD 2018, 63, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Healy, B.C.; Liguori, M.; Tran, D.; Chitnis, T.; Glanz, B.; Wolfish, C.; Gauthier, S.; Buckle, G.; Houtchens, M.; Stazzone, L.; et al. HLA B*44: Protective Effects in MS Susceptibility and MRI Outcome Measures. Neurology 2010, 75, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Brucato, N.; Guadalupe, T.; Franke, B.; Fisher, S.E.; Francks, C. A Schizophrenia-Associated HLA Locus Affects Thalamus Volume and Asymmetry. Brain. Behav. Immun. 2015, 46, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Celis, K.; Shuldiner, S.; Haverfield, E.V.; Cappell, J.; Yang, R.; Gong, D.-W.; Chung, W.K. Loss of Function Mutation in Glutamic Pyruvate Transaminase 2 (GPT2) Causes Developmental Encephalopathy. J. Inherit. Metab. Dis. 2015, 38, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Bucci, C. Role of EGFR in the Nervous System. Cells 2020, 9, 1887. [Google Scholar] [CrossRef]
- Hong, S.; Dobricic, V.; Ohlei, O.; Bos, I.; Vos, S.J.B.; Prokopenko, D.; Tijms, B.M.; Andreasson, U.; Blennow, K.; Vandenberghe, R.; et al. TMEM106B and CPOX Are Genetic Determinants of Cerebrospinal Fluid Alzheimer’s Disease Biomarker Levels. Alzheimers Dement. J. Alzheimers Assoc. 2021, 17, 1628–1640. [Google Scholar] [CrossRef]
- Neumann, A.; Ohlei, O.; Küçükali, F.; Bos, I.J.; Timsina, J.; Vos, S.; Prokopenko, D.; Tijms, B.M.; Andreasson, U.; Blennow, K.; et al. Multivariate GWAS of Alzheimer’s Disease CSF Biomarker Profiles Implies GRIN2D in Synaptic Functioning. Genome Med. 2023, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Tian, Y.; Zhou, S.; Li, X.; Wang, K.; Yu, Y. Impaired White Matter Connections of the Limbic System Networks Associated with Impaired Emotional Memory in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 250. [Google Scholar] [CrossRef]
- Klein, A.; Ghosh, S.S.; Bao, F.S.; Giard, J.; Häme, Y.; Stavsky, E.; Lee, N.; Rossa, B.; Reuter, M.; Chaibub Neto, E.; et al. Mindboggling Morphometry of Human Brains. PLoS Comput. Biol. 2017, 13, e1005350. [Google Scholar] [CrossRef]
- Jahanshad, N.; Kochunov, P.V.; Sprooten, E.; Mandl, R.C.; Nichols, T.E.; Almasy, L.; Blangero, J.; Brouwer, R.M.; Curran, J.E.; de Zubicaray, G.I.; et al. Multi-Site Genetic Analysis of Diffusion Images and Voxelwise Heritability Analysis: A Pilot Project of the ENIGMA-DTI Working Group. NeuroImage 2013, 81, 455–469. [Google Scholar] [CrossRef]
- Wang, M.; Beckmann, N.D.; Roussos, P.; Wang, E.; Zhou, X.; Wang, Q.; Ming, C.; Neff, R.; Ma, W.; Fullard, J.F.; et al. The Mount Sinai Cohort of Large-Scale Genomic, Transcriptomic and Proteomic Data in Alzheimer’s Disease. Sci. Data 2018, 5, 180185. [Google Scholar] [CrossRef]
- De Jager, P.L.; Ma, Y.; McCabe, C.; Xu, J.; Vardarajan, B.N.; Felsky, D.; Klein, H.-U.; White, C.C.; Peters, M.A.; Lodgson, B.; et al. A Multi-Omic Atlas of the Human Frontal Cortex for Aging and Alzheimer’s Disease Research. Sci. Data 2018, 5, 180142. [Google Scholar] [CrossRef] [PubMed]
- Wingo, A.P.; Liu, Y.; Gerasimov, E.S.; Gockley, J.; Logsdon, B.A.; Duong, D.M.; Dammer, E.B.; Robins, C.; Beach, T.G.; Reiman, E.M.; et al. Integrating Human Brain Proteomes with Genome-Wide Association Data Implicates New Proteins in Alzheimer’s Disease Pathogenesis. Nat. Genet. 2021, 53, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tam, W.W.; Yu, Y.; Zhuo, Z.; Xue, Z.; Tsang, C.; Qiao, X.; Wang, X.; Wang, W.; Li, Y.; et al. The Application of Aptamer in Biomarker Discovery. Biomark. Res. 2023, 11, 70. [Google Scholar] [CrossRef]
- Zhang, J.; Dutta, D.; Köttgen, A.; Tin, A.; Schlosser, P.; Grams, M.E.; Harvey, B.; CKDGen Consortium; Yu, B.; Boerwinkle, E.; et al. Plasma Proteome Analyses in Individuals of European and African Ancestry Identify Cis-pQTLs and Models for Proteome-Wide Association Studies. Nat. Genet. 2022, 54, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.J.H.; Jansen, R.; de Geus, E.J.C.; Boomsma, D.I.; Wright, F.A.; et al. Integrative Approaches for Large-Scale Transcriptome-Wide Association Studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Gong, W.; Guo, P.; Xue, F.; Zhou, X.; Wang, S.; Yuan, Z. Protein-Centric Omics Integration Analysis Identifies Candidate Plasma Proteins for Multiple Autoimmune Diseases. Hum. Genet. 2023, in press. [CrossRef]
- Gong, W.; Guo, P.; Li, Y.; Liu, L.; Yan, R.; Liu, S.; Wang, S.; Xue, F.; Zhou, X.; Yuan, Z. Role of the Gut-Brain Axis in the Shared Genetic Etiology Between Gastrointestinal Tract Diseases and Psychiatric Disorders: A Genome-Wide Pleiotropic Analysis. JAMA Psychiatry 2023, 80, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomisation (STROBE-MR): Explanation and Elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the Suitability of Summary Data for Two-Sample Mendelian Randomization Analyses Using MR-Egger Regression: The Role of the I2 Statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the Causal Relationship between Imprecisely Measured Traits Using GWAS Summary Data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian Randomization Analysis with Multiple Genetic Variants Using Summarized Data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A Framework for the Investigation of Pleiotropy in Two-Sample Summary Data Mendelian Randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Giambartolomei, C.; Vukcevic, D.; Schadt, E.E.; Franke, L.; Hingorani, A.D.; Wallace, C.; Plagnol, V. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLoS Genet. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Blake, J.A.; Baldarelli, R.; Kadin, J.A.; Richardson, J.E.; Smith, C.L.; Bult, C.J.; Mouse Genome Database Group. Mouse Genome Database (MGD): Knowledgebase for Mouse-Human Comparative Biology. Nucleic Acids Res. 2021, 49, D981–D987. [Google Scholar] [CrossRef] [PubMed]
- Freshour, S.L.; Kiwala, S.; Cotto, K.C.; Coffman, A.C.; McMichael, J.F.; Song, J.J.; Griffith, M.; Griffith, O.L.; Wagner, A.H. Integration of the Drug-Gene Interaction Database (DGIdb 4.0) with Open Crowdsource Efforts. Nucleic Acids Res. 2021, 49, D1144–D1151. [Google Scholar] [CrossRef]
- Carter, A.R.; Sanderson, E.; Hammerton, G.; Richmond, R.C.; Davey Smith, G.; Heron, J.; Taylor, A.E.; Davies, N.M.; Howe, L.D. Mendelian Randomisation for Mediation Analysis: Current Methods and Challenges for Implementation. Eur. J. Epidemiol. 2021, 36, 465–478. [Google Scholar] [CrossRef]
| IDPs | Exposure | Mediator | Outcome | Total Effect | Indirect Effect (95% CI) | Proportion (%) |
|---|---|---|---|---|---|---|
| ROI | ACADVL a | right precuneus | SCZ | −0.343 | −0.187 (−0.316, −0.092) | 54.54 |
| DTI | CREB3L4 a | RLIC.MO | SCZ | 0.144 | 0.021 (0.009, 0.037) | 14.59 |
| ICA1L b | IFO.MO | AD | −0.783 | −0.214 (−0.355, −0.097) | 27.30 | |
| EGFR b | PLIC.RD | AD | 0.807 | 0.153 (0.055, 0.270) | 18.99 | |
| TMEM106B b | PLIC.RD | AD | 0.150 | 0.017 (0.005, 0.032) | 11.18 | |
| EGFR b | SCC.RD | AD | 0.807 | 0.184 (0.087, 0.306) | 22.79 | |
| EGFR b | SCC.FA | AD | 0.807 | 0.161 (0.075, 0.273) | 19.91 | |
| C9orf64 b | CGC.RD | BIP | 0.424 | 0.096 (0.039,0.174) | 22.62 | |
| LRP4 b | CST.FA | MDD | −0.219 | −0.171 (−0.292, −0.070) | 78.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, S.; Gong, W.; Liu, X.; Mo, Q.; Shen, L.; Zhao, Y.; Wang, S.; Yuan, Z. Multi-Omics Integration Analysis Pinpoint Proteins Influencing Brain Structure and Function: Toward Drug Targets and Neuroimaging Biomarkers for Neuropsychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 9223. https://doi.org/10.3390/ijms25179223
Wang Y, Zhang S, Gong W, Liu X, Mo Q, Shen L, Zhao Y, Wang S, Yuan Z. Multi-Omics Integration Analysis Pinpoint Proteins Influencing Brain Structure and Function: Toward Drug Targets and Neuroimaging Biomarkers for Neuropsychiatric Disorders. International Journal of Molecular Sciences. 2024; 25(17):9223. https://doi.org/10.3390/ijms25179223
Chicago/Turabian StyleWang, Yunzhuang, Sunjie Zhang, Weiming Gong, Xinyu Liu, Qinyou Mo, Lujia Shen, Yansong Zhao, Shukang Wang, and Zhongshang Yuan. 2024. "Multi-Omics Integration Analysis Pinpoint Proteins Influencing Brain Structure and Function: Toward Drug Targets and Neuroimaging Biomarkers for Neuropsychiatric Disorders" International Journal of Molecular Sciences 25, no. 17: 9223. https://doi.org/10.3390/ijms25179223
APA StyleWang, Y., Zhang, S., Gong, W., Liu, X., Mo, Q., Shen, L., Zhao, Y., Wang, S., & Yuan, Z. (2024). Multi-Omics Integration Analysis Pinpoint Proteins Influencing Brain Structure and Function: Toward Drug Targets and Neuroimaging Biomarkers for Neuropsychiatric Disorders. International Journal of Molecular Sciences, 25(17), 9223. https://doi.org/10.3390/ijms25179223






