Abstract
Escherichia coli O157:H7 (E. coli O157) is known for causing severe foodborne illnesses such as hemorrhagic colitis and hemolytic uremic syndrome. Although E. coli O157 is typically regarded as an extracellular pathogen and a weak biofilm producer, some E. coli O157 strains, including a clinical strain ATCC 43895, exhibit a notable ability to invade bovine crypt cells and other epithelial cells, as well as to form robust biofilm. This invasive strain persists in the bovine host significantly longer than non-invasive strains. Various surface-associated factors, including lipopolysaccharides (LPS), flagella, and other adhesins, likely contribute to this enhanced invasiveness and biofilm formation. In this study, we constructed a series of LPS-core deletion mutations (waaI, waaG, waaF, and waaC) in E. coli O157 ATCC 43895, resulting in stepwise truncations of the LPS. This approach enabled us to investigate the effects on the biosynthesis of key surface factors, such as flagella and curli, and the ability of this invasive strain to invade host cells. We confirmed the LPS structure and found that all LPS-core mutants failed to form biofilms, highlighting the crucial role of core oligosaccharides in biofilm formation. Additionally, the LPS inner-core mutants ΔwaaF and ΔwaaC lost the ability to produce flagella and curli. Furthermore, these inner-core mutants exhibited a dramatic reduction in adherence to and invasion of epithelial cells (MAC-T), showing an approximately 100-fold decrease in cell invasion compared with the outer-core mutants (waaI and waaG) and the wild type. These findings underscore the critical role of LPS-core truncation in impairing flagella and curli biosynthesis, thereby reducing the invasion capability of E. coli O157 ATCC 43895.
1. Introduction
Escherichia coli O157:H7 (E. coli O157) is a Shiga-toxin-producing pathogen that poses significant public health risks due to its potential to cause severe foodborne illnesses, including hemorrhagic colitis and hemolytic uremic syndrome [1,2]. This bacterium colonizes the bovine recto-anal junction in healthy cattle, which act as primary reservoirs and are common source of foodborne infections [3,4]. The economic impact of E. coli O157 contamination in beef and fresh produce is considerable, with estimates suggesting that medical costs and lost productivity in the United States alone may reach USD 400 million annually [5]. Additionally, the economic burden on meat producers and vegetable growers can include product loss and negative publicity associated with product recalls [6]. Attachment to biological surfaces is a crucial first step in colonization, facilitated by various surface-associated factors such as lipopolysaccharides (LPS) [7], fimbriae [8], flagella, and other adhesins such as intimin [9,10,11], autotransporters [12], and curli [13]. These factors contribute to biofilm formation, cell adherence and invasion, and persistence in the bovine host.
LPS is an essential surface component of the outer membrane in Gram-negative bacteria. E. coli LPS is composed of three distinct regions: the O-antigen, the core oligosaccharide (OS), and lipid A [14]. In E. coli O157, the LPS core-OS is of the R3 type, which differs distinctly from that of E. coli K-12 [15]. The inner core of the R3 type comprises 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) and ADP-heptose residues, while the outer core is constructed of hexoses and 2-acetoamido-2-deoxy-hexoses. ADP-heptose is a crucial component of the LPS inner core, linking the outer part of LPS to Kdo between the Kdo2-lipid A and O-antigen. The R3 core structure involves several genes in the waa operon, including waaC, waaF, waaQ, waaG, waaO, waaR, waaY, waaZ, rfaE, and rfaD [15]. These genes collectively contribute to adding various sugar residues and modifications to the core oligosaccharide, resulting in a stable and functional LPS molecule. Mutations or deletions in these genes can lead to truncated LPS structures, which impact the overall functionality of the bacterial outer membrane [16,17]. The structural integrity of the outer membrane is crucial for the assembling and functionality of other surface structures such as flagella and curli, and for interactions with the host environment [14,18,19,20]. Disruptions in LPS-core synthesis can lead to significant alterations in bacterial behavior and pathogenic potential. Understanding the roles of these genes can provide valuable insights into how modifications in LPS structure can influence the virulence and survival strategies of E. coli O157.
E. coli O157 is typically regarded as an extracellular pathogen and a weak biofilm producer [21,22]. However, some E. coli O157 strains, including a clinical strain ATCC 43895 (E. coli O157 43895), exhibit a notable ability to invade bovine crypt cells and other epithelial cells, as well as to form robust biofilm at 37 °C [23]. The invasive strain E. coli O157 ATCC persists significantly longer in the bovine host than non-invasive E. coli O157 strains [13]. The biofilm formation of E. coli O157 43895 is associated with LPS and curli. Its curli production promotes cell invasion [13]. Curli fimbriae, composed of polymerized amyloid protein, are produced by many E. coli and Salmonella typhimurium strains, preferentially at relatively lower temperatures (25–30 °C) [24,25]. We previously showed that E. coli O157 43895 cells produce curli at 37 °C [13]. The influence of LPS on surface structures such as flagella and curli, and consequently on invasion ability, is not yet fully understood. Our previous study showed that deletion of the genes involved in biosynthesis of E. coli O157 43895 O-antigen does not affect curli production [13]. However, other LPS biosynthesis genes, such as waaG, rfbH, and lpxM have been implicated as important for curli production [26,27,28].
In this study, we constructed derivative strains of E. coli O157 43895 with a series of ordered LPS-core deletion mutations, leading to stepwise truncations of the LPS. We investigated how truncating the LPS core-OS affects the behaviors of invasive E. coli O157 43895, particularly its interaction with epithelial cells. By deleting specific genes involved in LPS core-OS synthesis and comparing these strains with the wild type and previously created O-antigen mutants, we aimed to understand the impacts on key virulence factors, such as flagella and curli biosynthesis, as well as the bacterium’s ability to invade host cells. The findings offer valuable insights into the molecular mechanisms underlying E. coli O157 persistence and pathogenicity, potentially identifying targets for interventions to reduce bovine carriage and human infections caused by this pathogen.
2. Results
Construction of E. coli O157 43895 LPS mutants and comparison of their LPS structures. We generated a series of LPS mutants of invasive E. coli O157 43895, each exhibiting progressively shorter LPS structures, as illustrated in Figure 1. Four genes involved in core biosynthesis were individually deleted from the chromosome of E. coli O157 43895, resulting in the mutants ΔwaaI, ΔwaaG, ΔwaaF, and ΔwaaC. Additionally, three O-antigen mutants (wzy-Tn5, per-Tn5, and manC-Tn5) generated previously by Tn5 mutagenesis were included in this study for their LPS structural analysis and comparison [13]. The expected structures of LPS in these seven E. coli O157 43895 mutants are shown in Figure 1A.
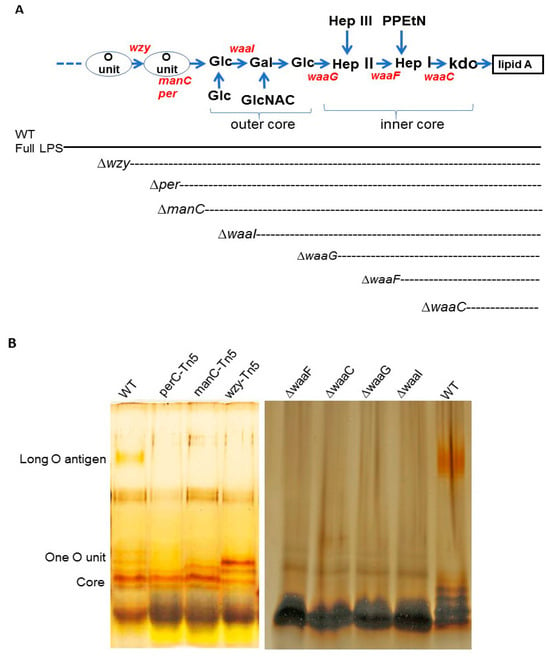
Figure 1.
Structure and analysis of E. coli O157 LPS molecules. (A) Schematic representation of E. coli O157 LPS molecule, showing O-antigen, outer core, inner core, and lipid A. Dotted lines indicate the level of LPS truncation resulting from each mutation (Kdo, 3-deoxy-D-manno-octulosonic acid; PPEtN, pyrophosphorylethanolamine; Hep, heptose; GlcNAc, N-acetylglucosamine; Glc, glucose; Gal, galactose). (B) LPS was isolated from wild-type and mutant cells of E. coli O157 43895 and analyzed by silver-stained Tricine–SDS-PAGE, as described in the Materials and Methods section. The expected location of O-antigen regions and the core is indicated on the left side of the gel.
The mutations commenced with a wzy mutant, characterized by LPS containing only a single O-antigen unit, and culminated in a waaC mutant with an inner core truncated to the Kdo residues (Figure 1A). Each mutation yielded a strain with an LPS length consistent with our expectations. The wild-type strain produced complete LPS molecules with long O-antigens, while the LPS of the waaC mutant comprised only lipid A and Kdo. The LPS synthesized by the other mutants exhibited varying degrees of core and/or O-antigen sugar presence, as visualized in the gel (Figure 1B).
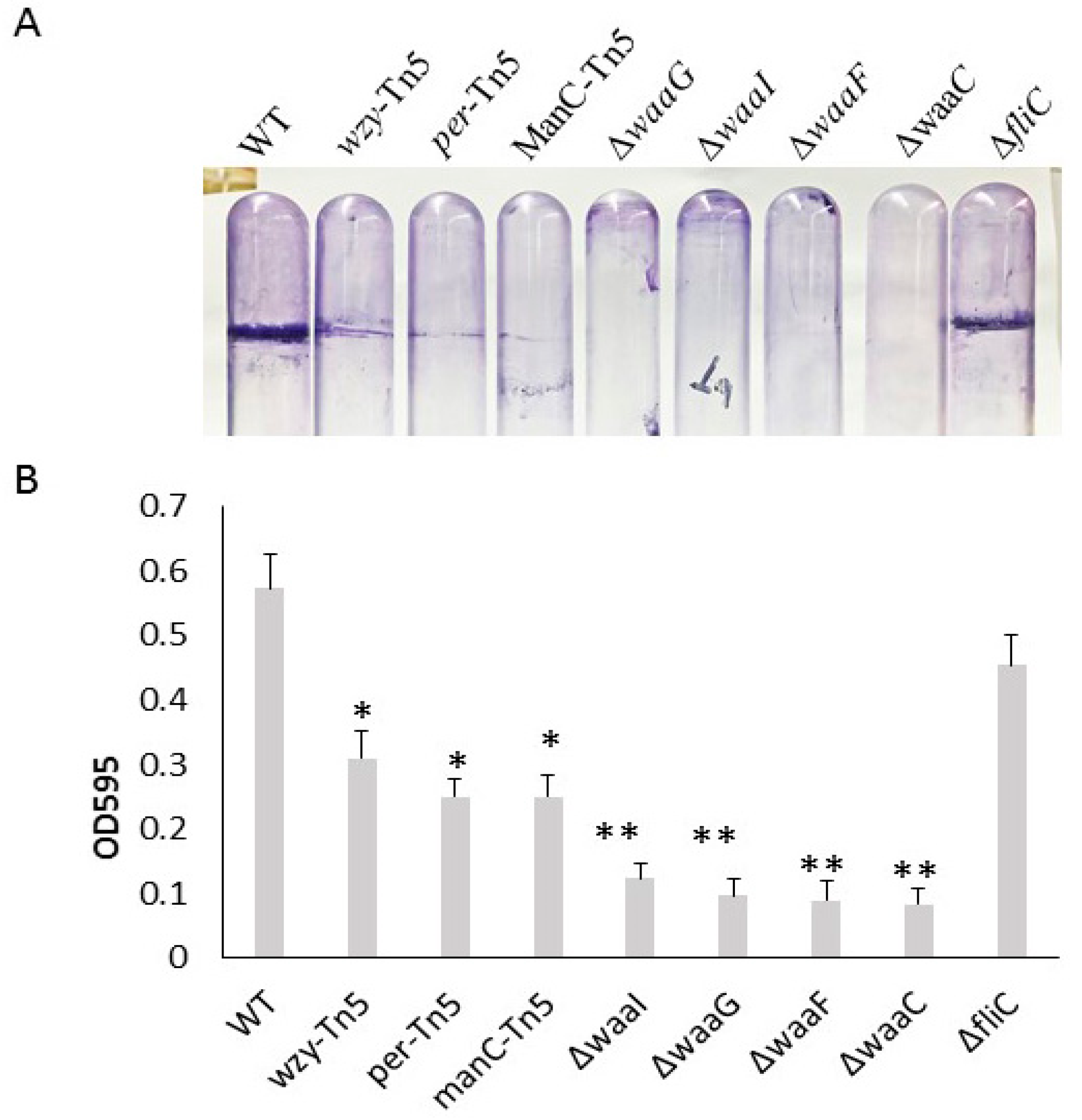
Comparison of biofilm formation of E. coli O157 43895 LPS and flagella mutants. We previously demonstrated that the invasive E. coli O157 43895 forms robust biofilm, even at an elevated temperature of 37 °C, compared with non-invasive strains [13]. In this study, we compared the biofilm-formation abilities of O-antigen, core-OS, and flagella mutants of E. coli O157 43895 with the WT by observing pellicle biofilms and conducting quantification measurement. The WT, along with the O-antigen mutants wzy-Tn5, per-Tn5, and manC-Tn5, the LPS-core mutants ΔwaaI, ΔwaaG, ΔwaaF, and ΔwaaC, and the flagella mutant ΔfliC, were grown in LB broth overnight at 37 °C under static condition. After 48 h of culture, pellicles and rings were visible in the WT and the flagella mutant (Figure 2A). The O-antigen mutants (wzy-Tn5, per-Tn5, and manC-Tn5) also formed visible rings, though they were less pronounced compared with the WT. In contrast, truncation of the LPS core abolished pellicle biofilm formation, as no rings were observed in the cultures of the core mutants (ΔwaaI, ΔwaaG, ΔwaaF, and ΔwaaC) (Figure 2A).
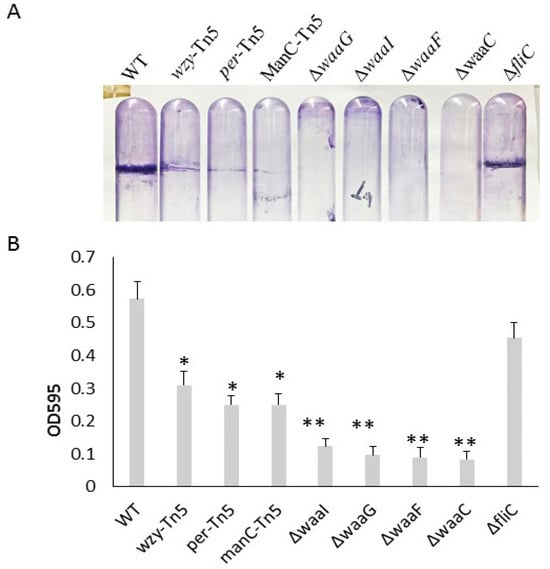
Figure 2.
Biofilm formation of E. coli O157 43895 WT and mutant derivatives. (A) Pellicle formation at 37 °C. Pellicle rings of a 48 h old floating biofilm of E. coli O157 43895. Mutants were detected by crystal violet (CV) staining. (B) Biofilm measurement using a standard microtiter assay. E. coli O157 43895 WT and mutants were incubated at 37 °C in MSM with 4% glucose. Bound CV was solubilized with 95% ethanol and quantified by absorbance at 595 nm. Assays were performed six times for each strain. Mean values with standard deviation are shown. Statistical analysis used one-way ANOVA test and Dunnett post-test. * p < 0.01, ** p < 0.001, compared with the wild type (WT).
In the quantification assay, the WT and mutant strains were grown in a 96-well microtiter plate for 24 h. Biofilms were stained with crystal violet and measured using a spectrophotometer. The ΔfliC mutant demonstrated a biofilm formation ability with an OD595 reading of 0.46, comparable to the WT’s reading of 0.54 (p > 0.05), suggesting that biofilm formation of E. coli O157 43895 is not dependent on flagella. In contrast, biofilm formation by O-antigen mutants (wzy-Tn5, per-Tn5, and manC-Tn5) was significantly reduced compared with the WT (p ≤ 0.01), with the OD595 reading ranging from 0.25 to 0.31 (Figure 2B). Biofilm formation was even further diminished in the LPS-core mutants (ΔwaaI, ΔwaaG, ΔwaaF, and ΔwaaC), with the OD595 reading in the 0.1 range (p ≤ 0.001). The reduction was consistent with the absence of a pellicle ring in the tube assays, indicating that these LPS-core mutants lost their ability to form biofilm. The significant difference in biofilm formation between the LPS core-OS mutants and the O-antigen mutants highlights the importance of an intact of LPS core for biofilm formation in E. coli O157 43895.
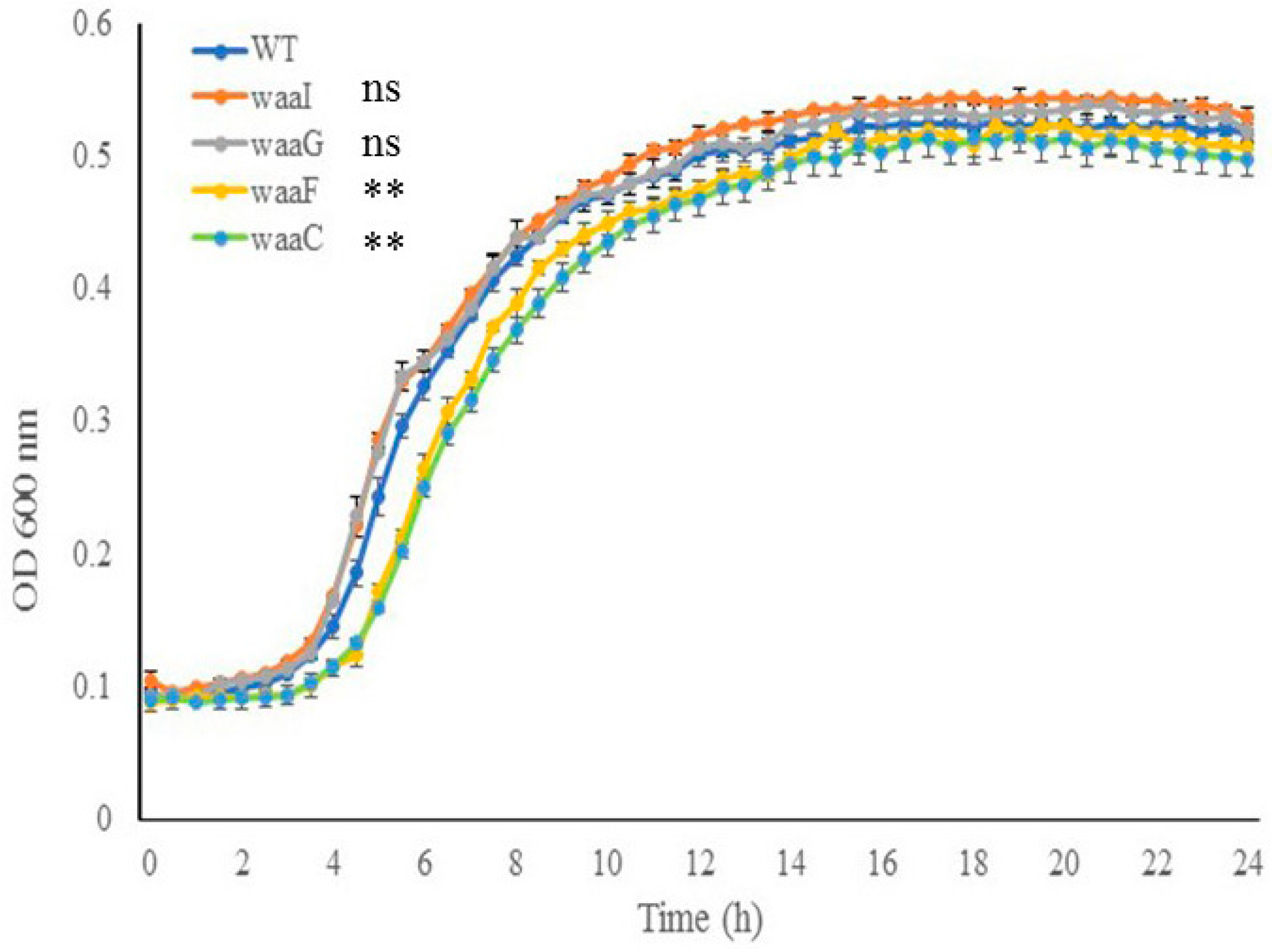
Cell growth of E. coli O157 43895 WT and LPS-core mutants. To investigate the impact of the LPS core on cell growth, core mutants and the WT were grown in MOPS media at 37 °C for 24 h with aeration. As shown in Figure 3, the two outer-core mutants, ΔwaaI and ΔwaaG, displayed growth comparable to the WT strain throughout the 24 h period. In contrast, the two LPS inner-core mutants, ΔwaaF and ΔwaaC, experienced an expanded lag phase and grew significantly slower than the WT during the logarithmic phase (4 h to 12 h) (p < 0.001); however, the two mutants were able to eventually catch up in growth rate during the stationary phase. These results suggest that the absence of the inner LPS core initially affects cell growth.
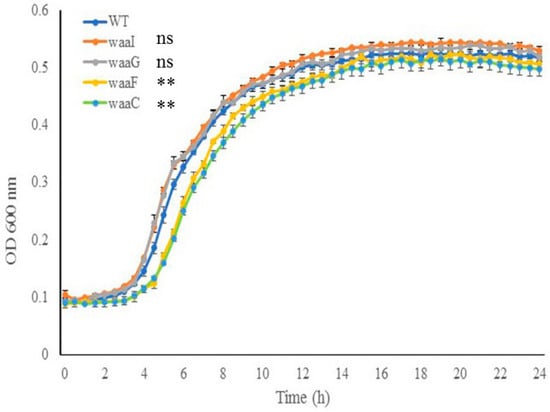
Figure 3.
The effect of LPS-core truncation on cell growth. Growth of wild-type and LPS mutant cells (ΔwaaI, ΔwaaG, ΔwaaF, and ΔwaaC) in MOPS media was monitored using the PowerWave XS plate reader (37 °C with shaking, wavelength 600 nm). Error bars represent standard deviation of the mean for 3 replicates. Error bars not shown for data points where standard deviation is smaller than symbol dot itself. The data of the mutants during log phase (4 h to 12 h) were compared with that of the wild type (WT). ** p < 0.001, ns., nonsignificant via two-way ANOVA with Tukey’s test.
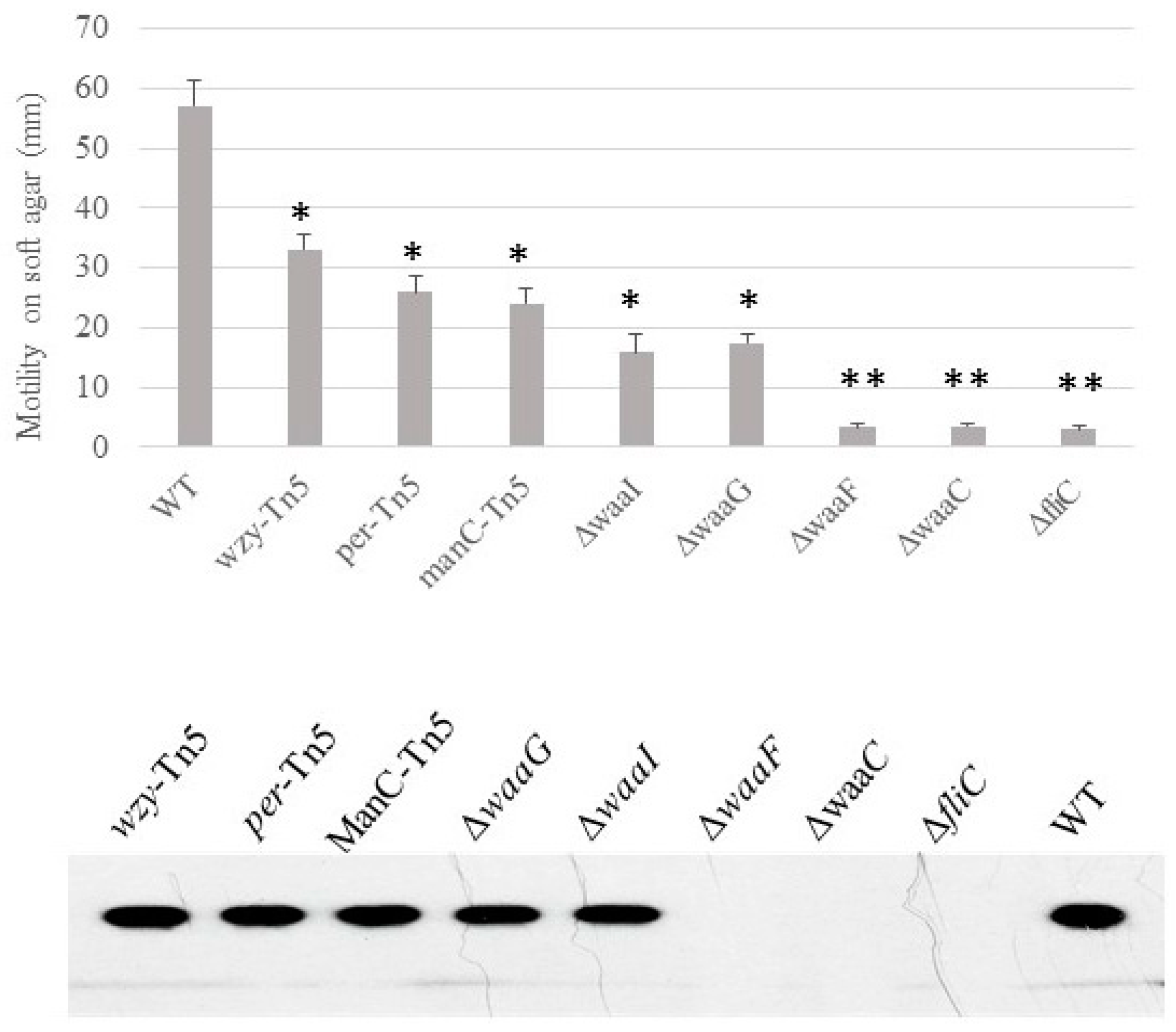
Motility, presence of flagella and curli. We previously demonstrated that the O-antigen mutant Δper of E. coli O157 43895 has impaired motility but can be induced to swim after extended incubation in 0.3% soft agar [7]. Here, we determined the relative ability of LPS-core mutants of E. coli O157 43895 to swim on soft agar and the presence of flagella. After 48 h of prolonged incubation, the O-antigen mutants and one of the outer-core LPS mutants ΔwaaI were able to swim to varying degrees on soft agar, though with impaired motility. In contrast, three core LPS mutants, ΔwaaG, ΔwaaF, and ΔwaaC, lost motility (Figure 4). Western-blot analysis indicated that these three mutants had defective motility due to lack of flagella (Figure 4).
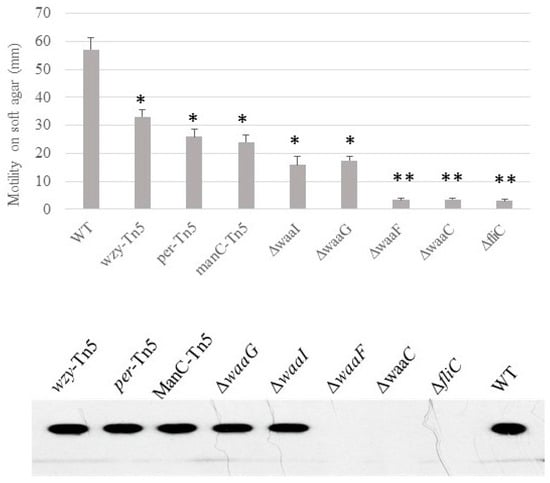
Figure 4.
The effect of LPS-core truncation on the swimming motility and flagella biosynthesis of E. coli O157 43895. Swimming motility of wild-type and LPS mutants was assessed using soft-agar swarm plates. After 40 h incubation, the diameter of the motility halo was measured. Experiments were repeated three times. Presence of flagella was detected by immunoblot (lower panel). Mean values with standard deviation are shown. Statistical analysis used two-tailed unpaired t-test. * p < 0.01, ** p < 0.001, compared with the wild type (WT).
Unlike non-invasive E. coli O157 strains, ATCC 43895 produces curli at 37 °C. To investigate the influence of the core-OS on curli production, we grew the LPS mutants of the invasive strain on CRI plates at 37 °C. After 24 h incubation, the WT and the O-antigen and outer-core mutants (ΔwaaI and ΔwaaG), and flagella mutant (ΔfliC) all produced curli, as evidenced by red coloration of the colonies due to Congo-red binding on the CRI plate. In contrast, the two inner-core LPS mutants (ΔwaaF and ΔwaaC) remained white, indicating a lack of curli production (Figure 5).

Figure 5.
The effect of LPS-core truncation on the curli biosynthesis of E. coli O157 43895. E. coli O157 43895 and mutants were grown on Congo-red (CR) indicator plates at 37 °C for 24 h. Presence of curli on the cell surface was detected by CR binding.
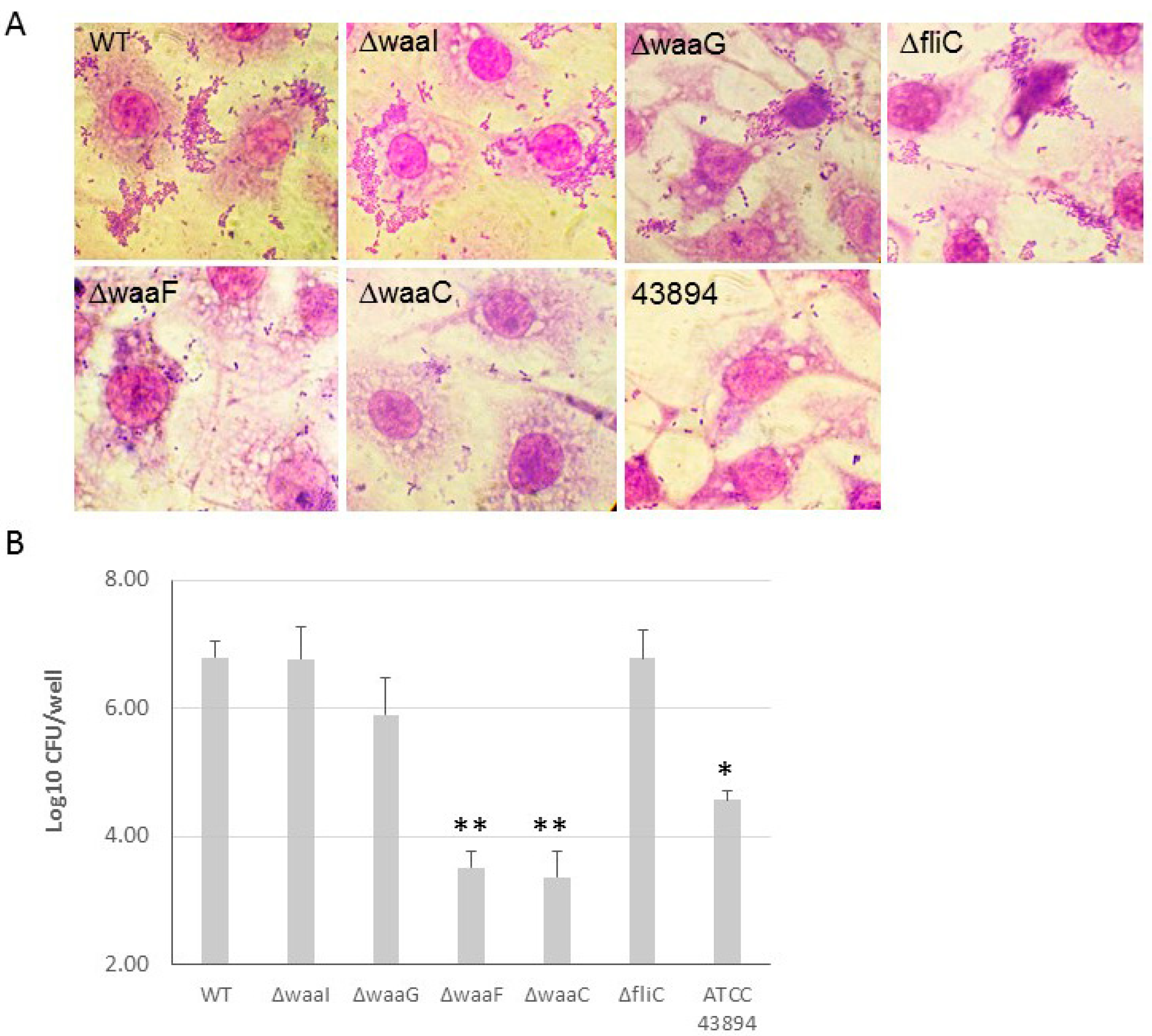
The effect of LPS core-OS of O157 43895 on cell adherence and invasion. E. coli O157 43895 has a strong ability to adhere to and invade epithelial cells compared with other E. coli O157 strains. We previously demonstrated that truncation of O-antigen in E. coli O157 43895 (wzy-Tn5, per-Tn5, and manC-Tn5) did not affect its cell adherence and invasion [13]. In this study, we examined the invasion ability of the isogenic LPS-core mutants ΔwaaI, ΔwaaG, ΔwaaF, and ΔwaaC, as well as the flagella mutant ΔfliC. The WT and mutants were co-cultured with MAC-T cells individually. Giemsa staining revealed that the outer-core mutants ΔwaaI and ΔwaaG, and mutant ΔfliC, exhibited a large number of bacteria adhering to the epithelial cells in an aggregative pattern, similar to WT. However, the two inner-core mutants, ΔwaaF and ΔwaaC, showed dramatically reduced adherence to MAC-T cells, with only a few sporadic bacteria adhering to the cells (Figure 6A). Subsequently, we assessed the ability of these mutants to invade epithelial cells. The WT and mutants were individually co-cultured with a monolayer of MAC-T cells in a 24-well plate at MOI of 10 for 3 h. The number of bacteria recovered after 2 h of gentamicin treatment indicated the number of intracellular bacteria. Among the four LPS-core mutants, ΔwaaF and ΔwaaC showed a more than 100-fold and 10-fold reduction in cell invasion, respectively, compared with WT E. coli O157 43895 and curli-deficient E. coli O157:H7 ATCC 43894. The log value of intracellular bacteria for the two inner-core mutants was significantly lower compared with the WT and the outer-core mutants ΔwaaI and ΔwaaG (p < 0.001), as well as to the non-invasive strain ATCC 43894 (p < 0.01).
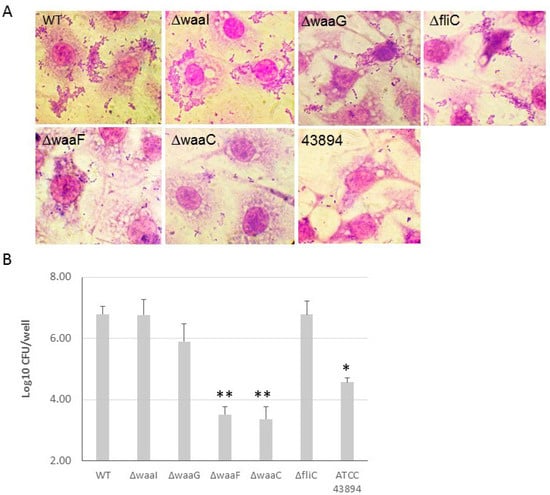
Figure 6.
The effect of LPS core-OS truncation on cell adherence and invasion. (A) The adherence of LPS-core and flagella mutants of E. coli O157 43895 to epithelial cells was examined. The monolayers of MAC-T cells on coverslips in 24-well plates were co-cultured with the bacteria at 37 °C for 3 h. The cells were washed five times with PBS and fixed with 4% paraformaldehyde for 15 min, washed again with PBS, stained with Giemsa stain, and viewed under the microscope (magnification ×1000). (B) E. coli O157 43895 and the mutants were tested for epithelial-cell invasion using a MAC-T gentamicin protection assay, as described in the Materials and Methods section. Bacteria were co-cultured with MAC-T monolayers at a multiplicity of infection (MOI) of 10. The experiment was repeated three times with three replicates per strain, and CFUs were determined by plate count and were transformed to log10 value. Mean values with standard deviation are shown. Statistical analysis used one-way ANOVA test and Dunnett post-test. * p < 0.01, ** p < 0.001, compared with the wild type (WT).
3. Discussion
The LPS core of E. coli O157 belongs to the R3 type, which is preferentially associated with key virulence determinants such as Shiga toxins [29,30,31] and the adhesin intimin and its receptor Tir [32,33]. In this study, we systematically investigated the relationship between O-antigen/LPS-core length and biofilm formation, flagella and curli production, and cell adherence and invasion. Deletion of the waa or disruption of wba genes targeted in this study resulted in truncated LPS structures (Figure 1). The results demonstrate the critical role of the LPS core in various cellular functions of E. coli O157 43895.
Unlike non-invasive E. coli O157 strains, ATCC 43895 forms a robust pellicle biofilm at 37 °C. Pellicle biofilms are bacterial communities at the air–liquid interface, composed of extracellular polymeric substances such as polysaccharides, proteins, flagella, fimbriae, and nucleic acids secreted by the bacteria [34,35]. In some Gram-negative bacteria, LPS is crucial for biofilm matrix construction [36,37,38]. This extracellular matrix can enhance bacterial adherence to host tissues, facilitating colonization in bovine hosts and potentially leading to deeper tissue invasion [23]. Previous research has shown that disrupting the O-antigen biosynthetic genes (per, manC, and wzy) in E. coli O157 43895 and curli fibers contribute to biofilm formation [13]. In this study, we compared the biofilm formation abilities of O-antigen, LPS-core, and flagella mutants of E. coli O157 43895 with the wild type. We found that truncating the LPS core completely abolished biofilm formation, highlighting the critical role of a complete LPS core in maintaining biofilm integrity. The differences in biofilm formation among LPS-core and O-antigen mutants suggest additional factors beyond LPS-core length. Interestingly, despite lacking motility, the flagella fliC mutant still formed a robust pellicle biofilm, which was unexpected.
We analyzed the mobility and flagella presence in O-antigen and LPS-core mutants of invasive E. coli O157 43895. All mutants exhibited significantly reduced motility, with ΔwaaG, ΔwaaF, and ΔwaaC mutants completely losing motility. Western-blot analysis revealed that these mutants were unable to produce flagella. Similar findings were observed in E. coli K-12; LPS-core mutants showed no flagella on their cell surfaces under electron microscopy compared with the wild type [39]. Non-motile phenotypes in LPS-deficient strains have also been reported in other Gram-negative bacteria. For instance, deep rough mutants ΔrfaG and ΔrfaD in Salmonella, which lack outer- and inner-core components, respectively, showed dramatically impaired motility [40,41]. These results suggest that truncation of the LPS core affects flagella assembly in both E. coli and Salmonella. In contrast, truncated LPS-core mutants of Pseudomonas aeruginosa PAO1, such as those with rmlC, migA, and wapR deletions, exhibited reduced motility, but flagella assembly appeared intact [42].
We also assessed curli production in the O-antigen and LPS-core mutants using a Congo-red-binding assay. Compared with O-antigen and outer-core mutants, as well as the wild type, the ΔwaaF and ΔwaaC mutants, which are defective in the inner core, failed to produce curli on their surfaces. This finding is consistent with an earlier study that screened the Keio collection of single-gene deletions in E. coli K-12 using Congo-red indicator plates and identified LPS-core mutants of E. coli K-12 that were defective in curli production [43]. These results support the notion that LPS integrity is crucial for maintaining outer membrane stability and the proper assembly of cell-surface components. Truncation of the LPS core disrupts the outer membrane, leading to leakage of periplasmic contents into the extracellular space and alterations in outer membrane composition [44].
It remains unclear whether the altered LPS structure or the mutated genes involved in LPS-core biosynthesis directly caused the defects in flagella and curli fimbriae. The truncation of the LPS core might induce general cell-envelope stress, downregulating flagella and curli assembly in mutants like ΔwaaF and ΔwaaC, which could affect interactions with epithelial cells. Future studies will focus on these aspects.
We observed that ΔwaaF and ΔwaaC mutants, which lack heptose, experienced an extended lag phage at 37 °C, suggesting that heptose incorporation into the inner core is crucial for optimal cell growth, particularly during the early stages. Interestingly, Murata et al. reported that certain LPS synthetic genes involved in heptose biosynthesis (gmhA, gmhB, and gmhD) or its incorporation into the LPS inner core (waaC and waaF) are essential for bacterial growth at critical high temperatures in E. coli K-12 [45].
Lastly, the reduced adherence and invasion abilities of inner-core mutants ΔwaaF and ΔwaaC underline the necessity of a complete LPS core for effective interaction with host cells. These findings suggest that the LPS-core structure not only contributes to production of flagella and curli and supports growth but also plays a crucial role in pathogenesis by mediating adherence and invasion in invasive E. coli O157 43895.
In summary, this study underscores the multifaceted role of the LPS core in invasive E. coli O157 43895, affecting growth, motility, biofilm formation, and host cell interactions, with significant implications for understanding bacterial persistence and developing targeted interventions.
4. Materials and Methods
Bacterial strains, plasmids, media, and growth conditions. The bacterial strains, plasmids, and primers used in this study are listed in Table 1 and Table 2. Bacteria were grown in Luria–Bertani (LB) broth or agar at 37 °C, unless otherwise stated. When required, antibiotics (Sigma-Aldrich, St. Louis, MO, USA) kanamycin (Kan, 50 mg/mL), chloramphenicol (Cm, 30 mg/mL), or ampicillin (Amp, 100 mg/mL) were added to the media. Congo-red-dye-binding of curli was monitored on Congo-red-indicator (CRI) agar: 10 g Casamino acids, 1 g yeast extract, 20 g agar, 20 mg Congo red, and 10 mg Coomassie brilliant blue G-250/L (Sigma-Aldrich).

Table 1.
E. coli O157 strains and plasmids.

Table 2.
Primers used in this study.
Mutant-strain construction. To create a mutant deficient in expression of LPS core-OS in E. coli O157 43895, the Lambda Red recombinase system [46] was used for gene deletion, as previously described (7). Oligonucleotide primers were purchased from Invitrogen (Carlsbad, CA, USA). The genes of the LPS core-OS of E. coli O157 43895 were independently replaced by a chloramphenicol resistance cassette (the cat gene from pKD3). The genes waaI, waaG, waaF, and waaC were selected for deletion based on their position in the waa cluster. As shown in Figure 1A, waaC encodes a heptosyltransferase I enzyme, which adds the first L-glycero-D-manno-heptose (Hep) residue to the Kdo (3-deoxy-D-manno-oct-2-ulosonic acid) molecule in the LPS core-OS; waaF encodes a heptosyltransferase II enzyme, responsible for the addition of the second Hep residue to the growing LPS core-OS oligosaccharide; both waaG and waaI encode a glucosyltransferase that adds a glucose (Glc) residue to the LPS core-OS (Figure 1A). In addition, H7 flagella mutant was made by deleting fliC for comparison. PCR primers for deletions of waaI, waaG, waaF, and waaC were designed based on the sequence of the waa cluster in the E. coli O157 43895 genome (accession number NZ_CP008957). Chloramphenicol-resistant (CmR) recombinants were selected on LB agar plates containing chloramphenicol. The O-antigen mutants manC-Tn5, per-Tn5, and manC-Tn5, generated previously, were included in this study for comparison [13]. The failure of the LPS core-OS mutants to express the O157 antigen was confirmed by anti-O157 latex agglutination (Pro-Lab Diagnostics, Toronto, ON, Canada). The chloramphenicol resistance gene was eliminated. The replacement of each of the targeted genes by a nonpolar scar structure [46] and the mutations were verified by PCR.
LPS analysis. To confirm the loss of LPS core-OS in the mutants, LPS was isolated and analyzed using a previously described method [47]. Briefly, cells were grown in 3 mL LB broth to an optical density at 600 nm (OD600) of 1.0. The cells were then harvested by centrifugation, resuspended in 1.0 mL of phosphate-buffered saline, and incubated at 60 °C for 30 min. The suspension was then centrifuged at 12,000× g for 30 min, and the supernatant was mixed with an equal volume of Tricine sample buffer (Bio-Rad Laboratories, Hercules, CA, USA) and boiled for 10 min. Proteinase K was added to a final concentration of 0.5 mg/mL, and the sample was incubated at 60 °C for 60 min before being centrifuged at 16,000× g for 30 min. LPS was then analyzed by Tricine–SDS-PAGE and visualized by silver staining, as previously described [7].
Crystal violet (CV) biofilm assay. To assess the ability of E. coli O157 mutants to produce a pellicle biofilm at the air–liquid interface, a fresh colony was grown in LB broth overnight at 37 °C. This culture was then diluted 500-fold with fresh 4 mL LB in a 15 mL polystyrene tube and incubated overnight at 37 °C under static conditions. The content of each tube was removed. The tubes were stained with 5 mL of 1% crystal violet solution for 5 min. The dye was then removed, and the tubes were rinsed with water and air dried.
Biofilm quantification assays were performed using a standard microtiter assay [48]. The overnight culture was inoculated into duplicate 96-well microtiter plates containing minimal salt medium (MSM) with 0.04% glucose and incubated for 24 h without agitation at 37 °C. Each isolate was inoculated in triplicate. After incubation, the titer plates were washed with water and stained with 1% crystal violet for 15 min at room temperature. Plates were then rinsed vigorously with water again to remove unattached cells and residual dye. Biofilm formation was evaluated by measuring the absorbance of the solubilized dye in 95% ethanol at a wavelength of 595 nm using a PowerWave XS reader (Bio-Tek, Winooski, VT, USA).
Growth curves in MOPS media. Three isolates of each strain were cultured overnight in LB broth at 37 °C with shaking at 150 rpm. Overnight cultures were diluted with LB to 0.1 OD600 and grown for 4 h at 37 °C. Cells were pelleted then washed twice with 1× PBS and resuspended in MOPS media [49] (1× MOPS supplemented with 0.5% glucose, 1 µg/mL thiamine, 0.1 mM K2HPO4). The cells in MOPS were diluted to approximately 0.1 OD600. Two hundred microliters of cells was pipetted in technical replicates of 3 in a microtiter plate and growth was monitored using the PowerWave XS reader at 37 °C with continuous shaking. Optical density measurements were collected at a wavelength of 600 nm every 30 min, 5 s after shaking stopped for 24 h.
Motility assays. The swimming motility of the wild-type and the mutant strains was assessed using tryptone swarm plates containing 1% Bacto Tryptone, 0.5% NaCl, and 0.3% agar. Five microliters of an overnight culture was point-inoculated into swarm plates and incubated at 37 °C for up to 40 h. The diameter of the motility halo was measured.
Adherence and invasion assays. The bacterial internalization of MAC-T cells was measured using a standard gentamicin protection assay. First, 24-well tissue culture plates (Corning Costar, NY, USA) were seeded with 104 MAC-T cells and incubated in 5% CO2 at 37 °C until cells were confluent. The MAC-T cell monolayers were then washed twice with Hank’s buffered saline solution (HBSS), and approximately 2 × 106 E. coli O157 cells (multiplicity of infection, MOI, 10:1) in cell culture medium without antibiotics or fetal bovine serum (1 mL) were added. After 3 h at 37 °C, unattached extracellular bacteria were removed by suction, and the monolayers were washed three times with HBSS. Fresh medium containing 100 µg/mL gentamicin was added to kill extracellular bacteria. After an additional 2 h incubation, the monolayers were washed three times with HBSS without Ca2+ or Mg2+. Epithelial cells were lysed by adding 100 µL 0.5% trypsin-EDTA and 900 µL 0.05% Triton X-100/well for 5 min. Bacterial invasion was quantified by counting the CFUs recovered/well on LB agar. To microscopically view the interaction of the bacteria with MAC-T cells, the cells on cover slips were fixed with methanol, stained with Giemsa stain (0.4% w/v, Sigma-Aldrich), and examined microscopically under oil immersion at 100X.
Flagella isolation and immunoblotting. Flagella were sheared from E. coli O157 derivatives using a standard protocol [50] with slight modification. Briefly, E. coli O157 strains were cultured on motility agar for 40 h, as mentioned above. LB was inoculated with 20 µL agar plugs from the edge of the motility halo and incubated at 37 °C with shaking for 30 h. Bacterial pellets were collected by centrifugation (5000× g) for 15 min at 4° C, and resuspended with 1 mL cold PBS with protease inhibitor (Sigma-Aldrich) at appropriate concentration. The microtubes with the bacterial suspension were put on ice for 15 min, and placed a FastPrep FB120 Cell Disruptor (Abiogene, CA, USA) at a 5.5 m/sec speed for 30 s. The vigorous vortex step was repeated 4 times and the tubes were put on ice for 10 min at each interval. The supernatants were transferred into clean tubes after centrifuge (8000× g) for 15 min at 4 °C. The cell fibers were pelleted by centrifugation (41,000× g for 3 h at 4 °C). To verify the presence of flagella, sheared fibers were monomerized according to a published protocol [51]. Flagella preparations were incubated at 70 °C for 15 min and then were examined by Western blotting with the 15D8 monoclonal antibody specific for enteric flagella [52]. Cell suspensions from overnight cultures grown in LB were extracted by boiling, fractionated by SDS-PAGE gel electrophoresis, transferred onto PVDF membranes using a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad, Redmond, WA, USA), and probed with 15D8 (1:2000).
Statistical analysis. Statistical analyses were performed by using GraphPad Prism software 7 (San Diego, CA, USA). Details of sample size, test used, error bars and statistically significant cutoff are presented in the text or figure legends.
Author Contributions
Conceptualization, H.S. and S.W.; methodology, H.S. and R.J.N.A.; software, H.S and R.J.N.A.; validation, H.S. and R.J.N.A. and S,W,; formal analysis, H.S. and R.J.N.A.; investigation, H.S. and R.J.N.A.; resources, H.S. and S.W.; data curation, H.S. and R.J.N.A.; writing—original draft preparation, H.S.; writing—review and editing, H.S. and S.W; supervision, S.W.; project administration, S.W.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the USDA National Institute for Food and Agriculture Foundational and Applied Science Program under award number 2022-67017-36315 and the USDA NIFA Hatch project under award number IDA01723.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.
Acknowledgments
We gratefully acknowledge our former collaborator, Scott A. Minnich for providing the anti-flagellin monoclonal antibody 15D8 used in this study. The authors would also like to acknowledge the University of Idaho P3-R1 Grant Matching Program for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paton, J.C.; Paton, A.W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 1998, 11, 450–479. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A.; Steele, B.T.; Petric, M.; Lim, C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1983, 1, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Naylor, S.W.; Low, J.C.; Besser, T.E.; Mahajan, A.; Gunn, G.J.; Pearce, M.C.; McKendrick, I.J.; Smith, D.G.; Gally, D.L. Lymphoid Follicle-Dense Mucosa at the Terminal Rectum Is the Principal Site of Colonization of Enterohemorrhagic Escherichia coli O157:H7 in the Bovine Host. Infect. Immun. 2003, 71, 1505–1512. [Google Scholar] [CrossRef]
- Sheng, H.; Davis, M.A.; Knecht, H.J.; Hovde, C.J. Rectal administration of Escherichia coli O157:H7: Novel model for colonization of ruminants. Appl. Environ. Microbiol. 2004, 70, 4588–4595. [Google Scholar] [CrossRef]
- Frenzen, P.D.; Drake, A.; Angulo, F.J. Economic cost of illness due to Escherichia coli O157 infections in the United States. J. Food Prot. 2005, 68, 2623–2630. [Google Scholar] [CrossRef]
- Buzby, J.C.; Roberts, T. The economics of enteric infections: Human foodborne disease costs. Gastroenterology 2009, 136, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Lim, J.Y.; Watkins, M.K.; Minnich, S.A.; Hovde, C.J. Characterization of an Escherichia coli O157:H7 O-antigen deletion mutant and effect of the deletion on bacterial persistence in the mouse intestine and colonization at the bovine terminal rectal mucosa. Appl. Environ. Microbiol. 2008, 74, 5015–5022. [Google Scholar] [CrossRef]
- Vogeleer, P.; Tremblay, Y.D.N.; Jubelin, G.; Jacques, M.; Harel, J. Biofilm-Forming Abilities of Shiga Toxin-Producing Escherichia coli Isolates Associated with Human Infections. Appl. Environ. Microbiol. 2015, 82, 1448–1458. [Google Scholar] [CrossRef]
- Lloyd, S.J.; Ritchie, J.M.; Torres, A.G. Fimbriation and curliation in Escherichia coli O157:H7: A paradigm of intestinal and environmental colonization. Gut Microbes 2012, 3, 272–276. [Google Scholar] [CrossRef]
- Mahajan, A.; Currie, C.G.; Mackie, S.; Tree, J.; McAteer, S.; McKendrick, I.; McNeilly, T.N.; Roe, A.; La Ragione, R.M.; Woodward, M.J.; et al. An investigation of the expression and adhesin function of H7 flagella in the interaction of Escherichia coli O157:H7 with bovine intestinal epithelium. Cell. Microbiol. 2009, 11, 121–137. [Google Scholar] [CrossRef]
- Wolfson, E.B.; Elvidge, J.; Tahoun, A.; Gillespie, T.; Mantell, J.; McAteer, S.P.; Rossez, Y.; Paxton, E.; Lane, F.; Shaw, D.J.; et al. The interaction of Escherichia coli O157 :H7 and Salmonella Typhimurium flagella with host cell membranes and cytoskeletal components. Microbiology 2020, 166, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.J.; Sherlock, O.; Rivas, L.; Mahajan, A.; Beatson, S.A.; Torpdahl, M.; Webb, R.I.; Allsopp, L.P.; Gobius, K.S.; Gally, D.L.; et al. EhaA is a novel autotransporter protein of enterohemorrhagic Escherichia coli O157:H7 that contributes to adhesion and biofilm formation. Environ. Microbiol. 2008, 10, 589–604. [Google Scholar] [CrossRef]
- Sheng, H.; Xue, Y.; Zhao, W.; Hovde, C.J.; Minnich, S.A. Escherichia coli O157:H7 Curli Fimbriae Promotes Biofilm Formation, Epithelial Cell Invasion, and Persistence in Cattle. Microorganisms 2020, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [PubMed]
- Kaniuk, N.A.; Vinogradov, E.; Li, J.; Monteiro, M.A.; Whitfield, C. Chromosomal and plasmid-encoded enzymes are required for assembly of the R3-type core oligosaccharide in the lipopolysaccharide of Escherichia coli O157:H7. J. Biol. Chem. 2004, 279, 31237–31250. [Google Scholar] [CrossRef]
- Yethon, J.A.; Vinogradov, E.; Perry, M.B.; Whitfield, C. Mutation of the lipopolysaccharide core glycosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J. Bacteriol. 2000, 182, 5620–5623. [Google Scholar] [CrossRef]
- Frirdich, E.; Whitfield, C. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J. Endotoxin Res. 2005, 11, 133–144. [Google Scholar]
- Cullen, T.W.; Trent, M.S. A link between the assembly of flagella and lipooligosaccharide of the Gram-negative bacterium Campylobacter jejuni. Proc. Natl. Acad. Sci. USA 2010, 107, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Chapman, M.R. Curli biogenesis: Order out of disorder. Biochim. Biophys. Acta 2014, 1843, 1551–1558. [Google Scholar] [CrossRef]
- Romling, U.; Bian, Z.; Hammar, M.; Sierralta, W.D.; Normark, S. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 1998, 180, 722–731. [Google Scholar] [CrossRef]
- Luck, S.N.; Bennett-Wood, V.; Poon, R.; Robins-Browne, R.M.; Hartland, E.L. Invasion of epithelial cells by locus of enterocyte effacement-negative enterohemorrhagic Escherichia coli. Infect. Immun. 2005, 73, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; La, H.J.; Sheng, H.; Forney, L.J.; Hovde, C.J. Influence of plasmid pO157 on Escherichia coli O157:H7 Sakai biofilm formation. Appl. Environ. Microbiol. 2010, 76, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Wang, J.; Lim, J.Y.; Davitt, C.; Minnich, S.A.; Hovde, C.J. Internalization of Escherichia coli O157:H7 by bovine rectal epithelial cells. Front. Microbiol. 2011, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef]
- Collinson, S.K.; Emody, L.; Muller, K.H.; Trust, T.J.; Kay, W.W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J. Bacteriol. 1991, 173, 4773–4781. [Google Scholar] [CrossRef]
- Anriany, Y.; Sahu, S.N.; Wessels, K.R.; McCann, L.M.; Joseph, S.W. Alteration of the rugose phenotype in waaG and ddhC mutants of Salmonella enterica serovar Typhimurium DT104 is associated with inverse production of curli and cellulose. Appl. Environ. Microbiol. 2006, 72, 5002–5012. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.H. Escherichia coli O157:H7 adherence to HEp-2 cells is implicated with curli expression and outer membrane integrity. J. Vet. Sci. 2004, 5, 119–124. [Google Scholar] [CrossRef]
- Zogaj, X.; Nimtz, M.; Rohde, M.; Bokranz, W.; Römling, U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 2001, 39, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Amor, K.; Heinrichs, D.E.; Frirdich, E.; Ziebell, K.; Johnson, R.P.; Whitfield, C. Distribution of core oligosaccharide types in lipopolysaccharides from Escherichia coli. Infect. Immun. 2000, 68, 1116–1124. [Google Scholar] [CrossRef]
- Chart, H.; Perry, N.T.; Jenkins, C. The expression of an R3 lipopolysaccharide-core by pathotypes of Escherichia coli. J. Appl. Microbiol. 2004, 96, 982–986. [Google Scholar] [CrossRef]
- Currie, C.G.; Poxton, I.R. The lipopolysaccharide core type of Escherichia coli O157:H7 and other non-O157 verotoxin-producing E. coli. FEMS Immunol. Med. Microbiol. 1999, 24, 57–62. [Google Scholar] [CrossRef]
- Leclercq, S.O.; Branger, M.; Smith, D.G.E.; Germon, P. Lipopolysaccharide core type diversity in the Escherichia coli species in association with phylogeny, virulence gene repertoire and distribution of type VI secretion systems. Microb. Genom. 2021, 7, 000652. [Google Scholar] [CrossRef]
- Sandner, L.; Eguiarte, L.E.; Navarro, A.; Cravioto, A.; Souza, V. The elements of the locus of enterocyte effacement in human and wild mammal isolates of Escherichia coli: Evolution by assemblage or disruption? Microbiology 2001, 147 Pt 11, 3149–3158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, H.; Chen, J.; Dong, Y.; Wu, L.; He, Z.; Liu, X.; Qiu, G.; Zhou, J. Pellicle formation in Shewanella oneidensis. BMC Microbiol. 2010, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, D.; Ghigo, J.M.; Charbonnel, N.; Haagensen, J.A.; Forestier, C. The characterization of functions involved in the establishment and maturation of Klebsiella pneumoniae in vitro biofilm reveals dual roles for surface exopolysaccharides. Environ. Microbiol. 2008, 10, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Ciornei, C.D.; Novikov, A.; Beloin, C.; Fitting, C.; Caroff, M.; Ghigo, J.M.; Cavaillon, J.M.; Adib-Conquy, M. Biofilm-forming Pseudomonas aeruginosa bacteria undergo lipopolysaccharide structural modifications and induce enhanced inflammatory cytokine response in human monocytes. Innate Immun. 2010, 16, 288–301. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Ren, G.; Li, Y.; Wang, X. Deletion of the genes waaC, waaF, or waaG in Escherichia coli W3110 disables the flagella biosynthesis. J. Basic Microbiol. 2016, 56, 1021–1035. [Google Scholar] [CrossRef]
- Deditius, J.A.; Felgner, S.; Spöring, I.; Kühne, C.; Frahm, M.; Rohde, M.; Weiß, S.; Erhardt, M. Characterization of Novel Factors Involved in Swimming and Swarming Motility in Salmonella enterica Serovar Typhimurium. PLoS ONE 2015, 10, e0135351. [Google Scholar] [CrossRef]
- Frahm, M.; Felgner, S.; Kocijancic, D.; Rohde, M.; Hensel, M.; Curtiss, R., 3rd; Erhardt, M.; Weiss, S. Efficiency of conditionally attenuated Salmonella enterica serovar Typhimurium in bacterium-mediated tumor therapy. mBio 2015, 6, e00254-15. [Google Scholar] [CrossRef] [PubMed]
- Lindhout, T.; Lau, P.C.Y.; Brewer, D.; Lam, J.S. Truncation in the core oligosaccharide of lipopolysaccharide affects flagella-mediated motility in Pseudomonas aeruginosa PAO1 via modulation of cell surface attachment. Microbiology 2009, 155 Pt 10, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Price, J.E.; Burby, P.E.; Blanco, L.P.; Chamberlain, J.; Chapman, M.R. The Production of Curli Amyloid Fibers Is Deeply Integrated into the Biology of Escherichia coli. Biomol. 2017, 7, 75. [Google Scholar] [CrossRef]
- Heinrichs, D.E.; Yethon, J.A.; Whitfield, C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 1998, 30, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Fujimoto, H.; Nishimura, K.; Charoensuk, K.; Nagamitsu, H.; Raina, S.; Kosaka, T.; Oshima, T.; Ogasawara, N.; Yamada, M. Molecular strategy for survival at a critical high temperature in Eschierichia coli. PLoS ONE 2011, 6, e20063. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Møller, A.K.; Leatham, M.P.; Conway, T.; Nuijten, P.J.; de Haan, L.A.; Krogfelt, K.A.; Cohen, P.S. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 2003, 71, 2142–2152. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Pratt, L.A.; Watnick, P.I.; Newman, D.K.; Weaver, V.B.; Kolter, R. Genetic approaches to study of biofilms. Methods Enzymol. 1999, 310, 91–109. [Google Scholar]
- Neidhardt, F.C.; Bloch, P.L.; Smith, D.F. Culture medium for enterobacteria. J. Bacteriol. 1974, 119, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Montie, T.C.; Stover, G.B. Isolation and characterization of flagellar preparations from Pseudomonas species. J. Clin. Microbiol. 1983, 18, 452–456. [Google Scholar] [CrossRef]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.R.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Monday, S.R.; Minnich, S.A.; Feng, P.C.H. A 12-Base-Pair Deletion in the Flagellar Master Control Gene flhC Causes Nonmotility of the Pathogenic German Sorbitol-Fermenting Escherichia coli O157:H- Strains. J. Bacteriol. 2004, 186, 2319–2327. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).