Disturbed Complement Receptor Expression Pattern of B Cells Is Enhanced by Toll-like Receptor CD180 Ligation in Diffuse Cutaneous Systemic Sclerosis
Abstract
1. Introduction
2. Results
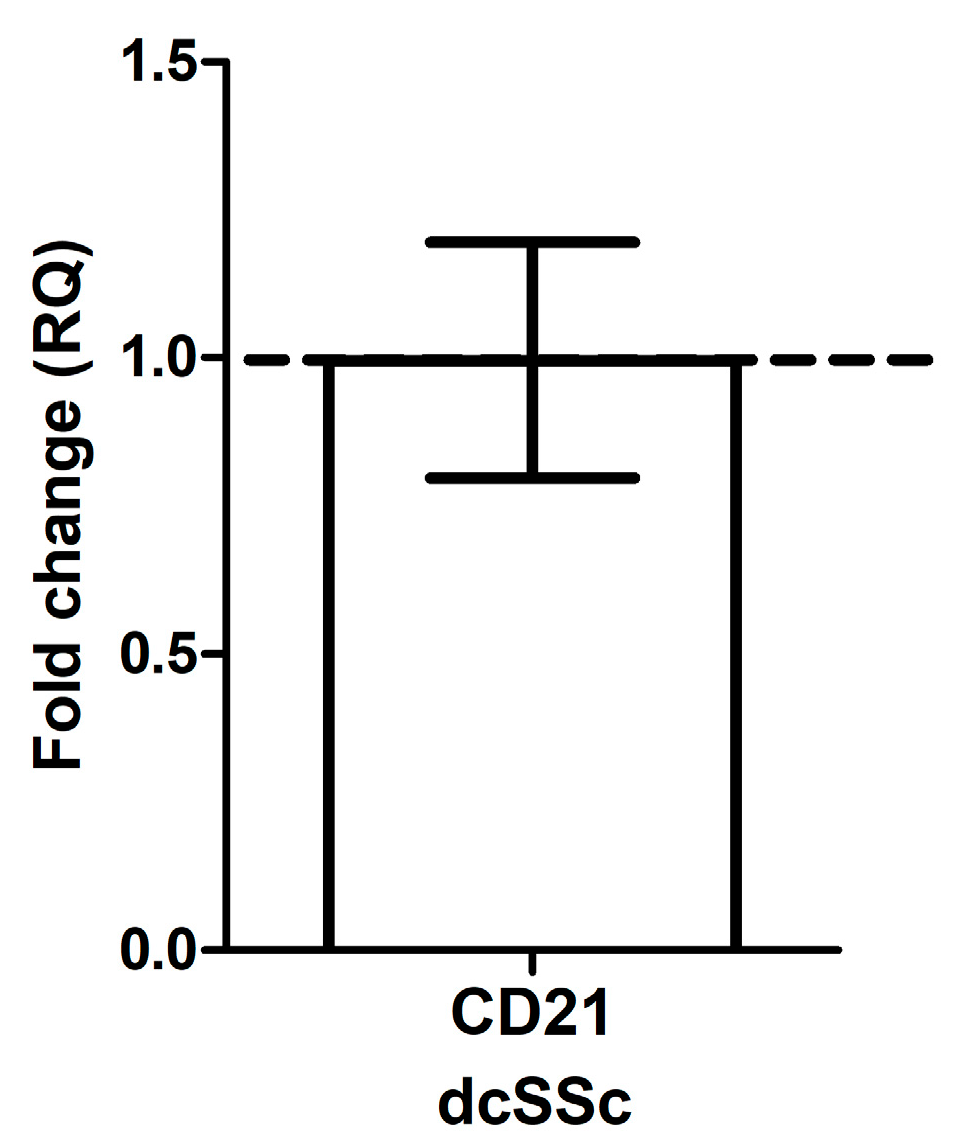
2.1. CD21 mRNA Expression in B Cells in dcSSc Is Similar to HC
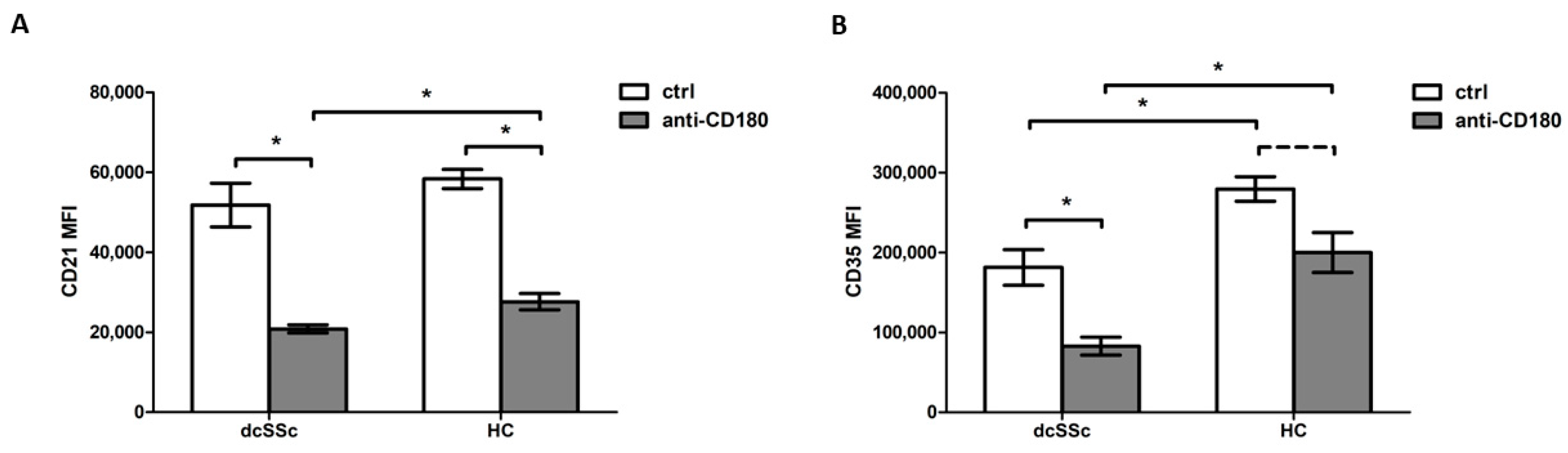
2.2. Decreased CD35 Expression on B Cells in dcSSc
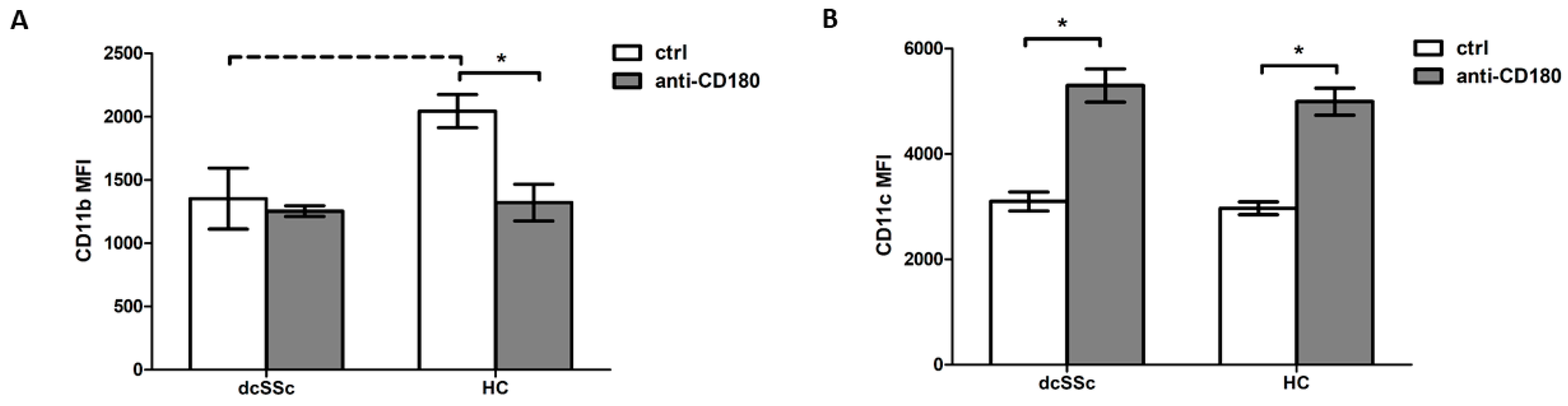
2.3. CD180 Ligation Has Opposite Effects on CD11b and CD11c Expression in B Cells
2.4. Natural IgM Autoantibodies against CS Negatively Correlate with C3 Serum Levels in dcSSc
3. Discussion
4. Materials and Methods
4.1. Enrolled Individuals
4.2. Peripheral Blood Mononuclear Cell Isolation and B-Cell Separation
4.3. RNA Isolation, cDNA Synthesis, and qPCR for the Evaluation of CD21 Expression
4.4. Flow Cytometric Analysis of CRs Expression
4.5. Natural Autoantibodies Measurement
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of Systemic Sclerosis: Recent Insights of Molecular and Cellular Mechanisms and Therapeutic Opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Melissaropoulos, K.; Daoussis, D. B Cells in Systemic Sclerosis: From Pathophysiology to Treatment. Clin. Rheumatol. 2021, 40, 2621–2631. [Google Scholar] [CrossRef]
- Kremlitzka, M.; Mácsik-Valent, B.; Erdei, A. Regulation of B Cell Functions by Toll-like Receptors and Complement. Immunol. Lett. 2016, 178, 37–44. [Google Scholar] [CrossRef]
- Erdei, A.; Kovács, K.G.; Nagy-Baló, Z.; Lukácsi, S.; Mácsik-Valent, B.; Kurucz, I.; Bajtay, Z. New Aspects in the Regulation of Human B Cell Functions by Complement Receptors CR1, CR2, CR3 and CR4. Immunol. Lett. 2021, 237, 42–57. [Google Scholar] [CrossRef]
- Rubtsov, A.V.; Rubtsova, K.; Fischer, A.; Meehan, R.T.; Gillis, J.Z.; Kappler, J.W.; Marrack, P. Toll-like Receptor 7 (TLR7)–Driven Accumulation of a Novel CD11c+ B-Cell Population Is Important for the Development of Autoimmunity. Blood 2011, 118, 1305. [Google Scholar] [CrossRef]
- Brown, G.J.; Cañete, P.F.; Wang, H.; Medhavy, A.; Bones, J.; Roco, J.A.; He, Y.; Qin, Y.; Cappello, J.; Ellyard, J.I.; et al. TLR7 Gain-of-Function Genetic Variation Causes Human Lupus. Nature 2022, 605, 349–356. [Google Scholar] [CrossRef]
- Ding, C.; Ma, Y.; Chen, X.; Liu, M.; Cai, Y.; Hu, X.; Xiang, D.; Nath, S.; Zhang, H.G.; Ye, H.; et al. Integrin CD11b Negatively Regulates BCR Signalling to Maintain Autoreactive B Cell Tolerance. Nat. Commun. 2013, 4, 2813. [Google Scholar] [CrossRef]
- Edwards, K.; Lydyard, P.M.; Kulikova, N.; Tsertsvadze, T.; Volpi, E.V.; Chiorazzi, N.; Porakishvili, N. The Role of CD180 in Hematological Malignancies and Inflammatory Disorders. Mol. Med. 2023, 29, 97. [Google Scholar] [CrossRef]
- Divanovic, S.; Trompette, A.; Atabani, S.F.; Madan, R.; Golenbock, D.T.; Visintin, A.; Finberg, R.W.; Tarakhovsky, A.; Vogel, S.N.; Belkaid, Y.; et al. Negative Regulation of TLR4 Signaling by RP105. Nat. Immunol. 2005, 6, 571. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Midwood, K.S.; Yin, H.; Varga, J. Toll-Like Receptor-4 Signaling Drives Persistent Fibroblast Activation and Prevents Fibrosis Resolution in Scleroderma. Adv. Wound Care 2017, 6, 356. [Google Scholar] [CrossRef]
- Erdő-bonyár, S.; Rapp, J.; Szinger, D.; Minier, T.; Kumánovics, G.; Czirják, L.; Berki, T.; Simon, D. Ligation of TLR Homologue CD180 of B Cells Activates the PI3K/Akt/MTOR Pathway in Systemic Sclerosis and Induces a Pathological Shift in the Expression of BAFF Receptors. Int. J. Mol. Sci. 2022, 23, 6777. [Google Scholar] [CrossRef] [PubMed]
- Erdő-Bonyár, S.; Rapp, J.; Subicz, R.; Filipánits, K.; Minier, T.; Kumánovics, G.; Czirják, L.; Berki, T.; Simon, D. Toll-like Receptor Homologue CD180 Ligation of B Cells Upregulates Type I IFN Signature in Diffuse Cutaneous Systemic Sclerosis. Int. J. Mol. Sci. 2024, 25, 7933. [Google Scholar] [CrossRef] [PubMed]
- Erdő-Bonyár, S.; Rapp, J.; Minier, T.; Ráth, G.; Najbauer, J.; Czirják, L.; Németh, P.; Berki, T.; Simon, D. Toll-like Receptor Mediated Activation of Natural Autoantibody Producing b Cell Subpopulations in an Autoimmune Disease Model. Int. J. Mol. Sci. 2019, 20, 6152. [Google Scholar] [CrossRef] [PubMed]
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Fleming, S.D. Natural Antibodies, Autoantibodies and Complement Activation in Tissue Injury. Autoimmunity 2006, 39, 379–386. [Google Scholar] [CrossRef]
- Herrick, A.L. Pathogenesis of Raynaud’s Phenomenon. Rheumatology 2005, 44, 587–596. [Google Scholar] [CrossRef]
- Narang, A.; Qiao, F.; Atkinson, C.; Zhu, H.; Yang, X.; Kulik, L.; Holers, V.M.; Tomlinson, S. Natural IgM Antibodies That Bind Neoepitopes Exposed as a Result of Spinal Cord Injury, Drive Secondary Injury by Activating Complement. J. Neuroinflammation 2017, 14, 120. [Google Scholar] [CrossRef]
- Scambi, C.; Ugolini, S.; Sakari Jokiranta, T.; De Franceschi, L.; Bortolami, O.; La Verde, V.; Guarini, P.; Caramaschi, P.; Ravagnani, V.; Martignoni, G.; et al. The Local Complement Activation on Vascular Bed of Patients with Systemic Sclerosis: A Hypothesis-Generating Study. PLoS ONE 2015, 10, e0114856. [Google Scholar] [CrossRef]
- Venneker, G.T.; Van den Hoogen, F.H.J.; Boerbooms, A.M.T.; Bos, J.D.; Asghar, S.S. Aberrant Expression of Membrane Cofactor Protein and Decay-Accelerating Factor in the Endothelium of Patients with Systemic Sclerosis. A Possible Mechanism of Vascular Damage. Lab. Invest. 1994, 70, 830–835. [Google Scholar]
- Raschi, E.; Privitera, D.; Bodio, C.; Lonati, P.A.; Borghi, M.O.; Ingegnoli, F.; Meroni, P.L.; Chighizola, C.B. Scleroderma-Specific Autoantibodies Embedded in Immune Complexes Mediate Endothelial Damage: An Early Event in the Pathogenesis of Systemic Sclerosis. Arthritis Res. Ther. 2020, 22, 265. [Google Scholar] [CrossRef]
- Erdei, A.; Isaák, A.; Török, K.; Sándor, N.; Kremlitzka, M.; Prechl, J.; Bajtay, Z. Expression and Role of CR1 and CR2 on B and T Lymphocytes under Physiological and Autoimmune Conditions. Mol. Immunol. 2009, 46, 2767–2773. [Google Scholar] [CrossRef]
- Soto, L.; Ferrier, A.; Aravena, O.; Fonseca, E.; Berendsen, J.; Biere, A.; Bueno, D.; Ramos, V.; Aguillón, J.C.; Catalán, D. Systemic Sclerosis Patients Present Alterations in the Expression of Molecules Involved in B-Cell Regulation. Front. Immunol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Corbi, A.L.; Kishimoto, T.K.; Miller, L.J.; Springer, T.A. The Human Leukocyte Adhesion Glycoprotein Mac-1 (Complement Receptor Type 3, CD11b) Alpha Subunit. Cloning, Primary Structure, and Relation to the Integrins, von Willebrand Factor and Factor B. J. Biol. Chem. 1988, 263, 12403–12411. [Google Scholar] [CrossRef]
- Lamers, C.; Plüss, C.J.; Ricklin, D. The Promiscuous Profile of Complement Receptor 3 in Ligand Binding, Immune Modulation, and Pathophysiology. Front. Immunol. 2021, 12, 662164. [Google Scholar] [CrossRef]
- Vandendriessche, S.; Cambier, S.; Proost, P.; Marques, P.E. Complement Receptors and Their Role in Leukocyte Recruitment and Phagocytosis. Front. Cell Dev. Biol. 2021, 9, 624025. [Google Scholar] [CrossRef]
- Vorup-Jensen, T.; Jensen, R.K. Structural Immunology of Complement Receptors 3 and 4. Front. Immunol. 2018, 9, 2716. [Google Scholar] [CrossRef]
- Golinski, M.L.; Demeules, M.; Derambure, C.; Riou, G.; Maho-Vaillant, M.; Boyer, O.; Joly, P.; Calbo, S. CD11c+ B Cells Are Mainly Memory Cells, Precursors of Antibody Secreting Cells in Healthy Donors. Front. Immunol. 2020, 11, 32. [Google Scholar] [CrossRef]
- Faridi, M.H.; Khan, S.Q.; Zhao, W.; Lee, H.W.; Altintas, M.M.; Zhang, K.; Kumar, V.; Armstrong, A.R.; Carmona-Rivera, C.; Dorschner, J.M.; et al. CD11b Activation Suppresses TLR-Dependent Inflammation and Autoimmunity in Systemic Lupus Erythematosus. J. Clin. Invest. 2017, 127, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, V.; Li, X.; Jimenez, V.; Faridi, H.M.; Gupta, V. CD11b Agonists Offer a Novel Approach for Treating Lupus Nephritis. Transl. Res. 2022, 245, 41–54. [Google Scholar] [CrossRef]
- Sgonc, R.; Gruschwitz, M.S.; Boeck, G.; Sepp, N.; Gruber, J.; Wick, G. Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum. 2000, 43, 2550–2562. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, C.; Bombardieri, M.; Valesini, G. Pathogenic Mechanisms Of Anti-Endothelial Cell Antibodies (AECA): Their Prevalence And Clinical Relevance. Adv. Clin. Chem. 2006, 42, 297–326. [Google Scholar] [CrossRef]
- Holt, C.M.; Lindsey, N.; Moult, J.; Malia, R.G.; Greaves, M.; Hume, A.; Rowell, N.R.; Hughes, P. Antibody-Dependent Cellular Cytotoxicity of Vascular Endothelium: Characterization and Pathogenic Associations in Systemic Sclerosis. Clin. Exp. Immunol. 1989, 78, 359. [Google Scholar] [PubMed]
- Marks, R.M.; Czerniecki, M.; Andrews, B.S.; Penny, R. The Effects of Scleroderma Serum on Human Microvascular Endothelial Cells. Induction Antib.-Depend. Cell. Cytotoxicity. Arthritis Rheum. 1988, 31, 1524–1534. [Google Scholar] [CrossRef]
- Reid, R.R.; Woodcock, S.; Shimabukuro-Vornhagen, A.; Austen, W.G.; Kobzik, L.; Zhang, M.; Hechtman, H.B.; Moore, F.D.; Carroll, M.C. Functional Activity of Natural Antibody Is Altered in Cr2-Deficient Mice. J. Immunol. 2002, 169, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.D.; Shea-Donohue, T.; Guthridge, J.M.; Kulik, L.; Waldschmidt, T.J.; Gipson, M.G.; Tsokos, G.C.; Holers, V.M. Mice Deficient in Complement Receptors 1 and 2 Lack a Tissue Injury-Inducing Subset of the Natural Antibody Repertoire. J. Immunol. 2002, 169, 2126–2133. [Google Scholar] [CrossRef]
- Thurman, J.M.; Ljubanovic, D.; Edelstein, C.L.; Gilkeson, G.S.; Holers, V.M. Lack of a Functional Alternative Complement Pathway Ameliorates Ischemic Acute Renal Failure in Mice. J. Immunol. 2003, 170, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Osthoff, M.; Ngian, G.S.; Dean, M.M.; Nikpour, M.; Stevens, W.; Proudman, S.; Eisen, D.P.; Sahhar, J. Potential Role of the Lectin Pathway of Complement in the Pathogenesis and Disease Manifestations of Systemic Sclerosis: A Case-Control and Cohort Study. Arthritis Res. Ther. 2014, 16, 480. [Google Scholar] [CrossRef]
- Osthoff, M.; Jaeger, V.K.; Heijnen, I.A.F.M.; Trendelenburg, M.; Jordan, S.; Distler, O.; Walker, U.A. Role of Lectin Pathway Complement Proteins and Genetic Variants in Organ Damage and Disease Severity of Systemic Sclerosis: A Cross-Sectional Study. Arthritis Res. Ther. 2019, 21, 76. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Carwile LeRoy, E.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.; Wollheim, F. Scleroderma (Systemic Sclerosis): Classification, Subsets and Pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [CrossRef]
- Szinger, D.; Berki, T.; Németh, P.; Erdo-Bonyar, S.; Simon, D.; Drenjančević, I.; Samardzic, S.; Zelić, M.; Sikora, M.; Požgain, A.; et al. Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation. Int. J. Mol. Sci. 2023, 24, 14961. [Google Scholar] [CrossRef]

| Characteristics | dcSSc Patients (n = 38) |
|---|---|
| Age (years), mean (SD) | 53.34 (13.6) |
| Gender (female), n (%) | 31/38 (81.6%) |
| Disease duration 1 (years), mean (SD) | 7.1 (5.6) |
| Organ involvement | |
| mRSS mean (SD) | 13.93 (9.5) |
| Lung fibrosis 2, n (%) | 30/38 (78.9%) |
| Cardiac involvement 3, n (%) | 24/38 (63.2%) |
| Gastrointestinal involvement 4, n (%) | 20/38 (52.6%) |
| Antibodies | |
| Anti-Scl-70+, n (%) | 15/38 (39.5%) |
| Anti-RNA-polymerase III+, n (%) | 7/38 (18.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdő-Bonyár, S.; Rapp, J.; Subicz, R.; Böröcz, K.; Szinger, D.; Filipánits, K.; Minier, T.; Kumánovics, G.; Czirják, L.; Berki, T.; et al. Disturbed Complement Receptor Expression Pattern of B Cells Is Enhanced by Toll-like Receptor CD180 Ligation in Diffuse Cutaneous Systemic Sclerosis. Int. J. Mol. Sci. 2024, 25, 9230. https://doi.org/10.3390/ijms25179230
Erdő-Bonyár S, Rapp J, Subicz R, Böröcz K, Szinger D, Filipánits K, Minier T, Kumánovics G, Czirják L, Berki T, et al. Disturbed Complement Receptor Expression Pattern of B Cells Is Enhanced by Toll-like Receptor CD180 Ligation in Diffuse Cutaneous Systemic Sclerosis. International Journal of Molecular Sciences. 2024; 25(17):9230. https://doi.org/10.3390/ijms25179230
Chicago/Turabian StyleErdő-Bonyár, Szabina, Judit Rapp, Rovéna Subicz, Katalin Böröcz, Dávid Szinger, Kristóf Filipánits, Tünde Minier, Gábor Kumánovics, László Czirják, Tímea Berki, and et al. 2024. "Disturbed Complement Receptor Expression Pattern of B Cells Is Enhanced by Toll-like Receptor CD180 Ligation in Diffuse Cutaneous Systemic Sclerosis" International Journal of Molecular Sciences 25, no. 17: 9230. https://doi.org/10.3390/ijms25179230
APA StyleErdő-Bonyár, S., Rapp, J., Subicz, R., Böröcz, K., Szinger, D., Filipánits, K., Minier, T., Kumánovics, G., Czirják, L., Berki, T., & Simon, D. (2024). Disturbed Complement Receptor Expression Pattern of B Cells Is Enhanced by Toll-like Receptor CD180 Ligation in Diffuse Cutaneous Systemic Sclerosis. International Journal of Molecular Sciences, 25(17), 9230. https://doi.org/10.3390/ijms25179230





