Genome-Wide Analysis and Expression Profiling of Soybean RbcS Family in Response to Plant Hormones and Functional Identification of GmRbcS8 in Soybean Mosaic Virus
Abstract
1. Introduction
2. Results
2.1. Identification and Physicochemical Characterization of GmRbcS Family Members
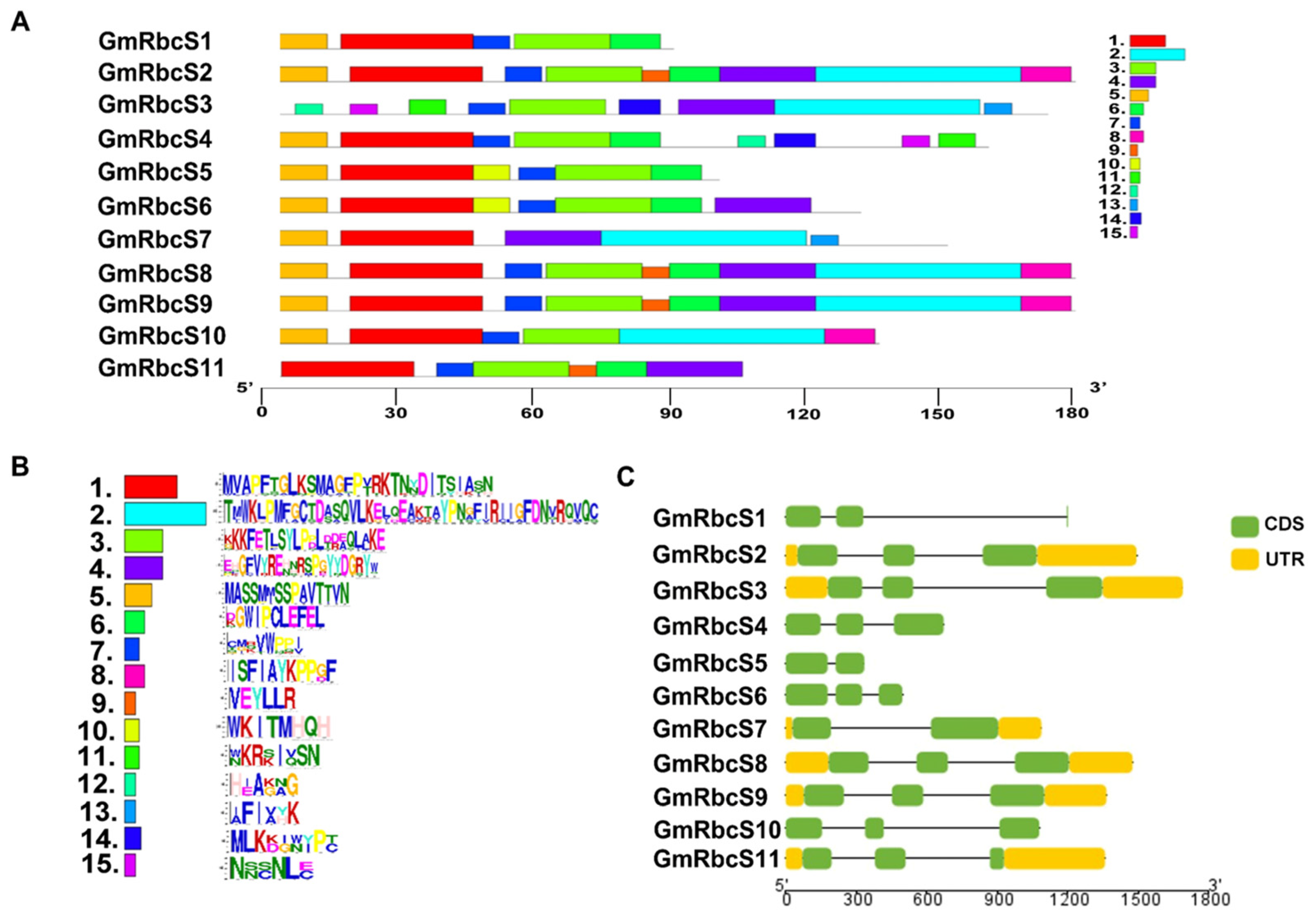
2.2. Conserved Motif, Gene Structure, and Domain Analysis
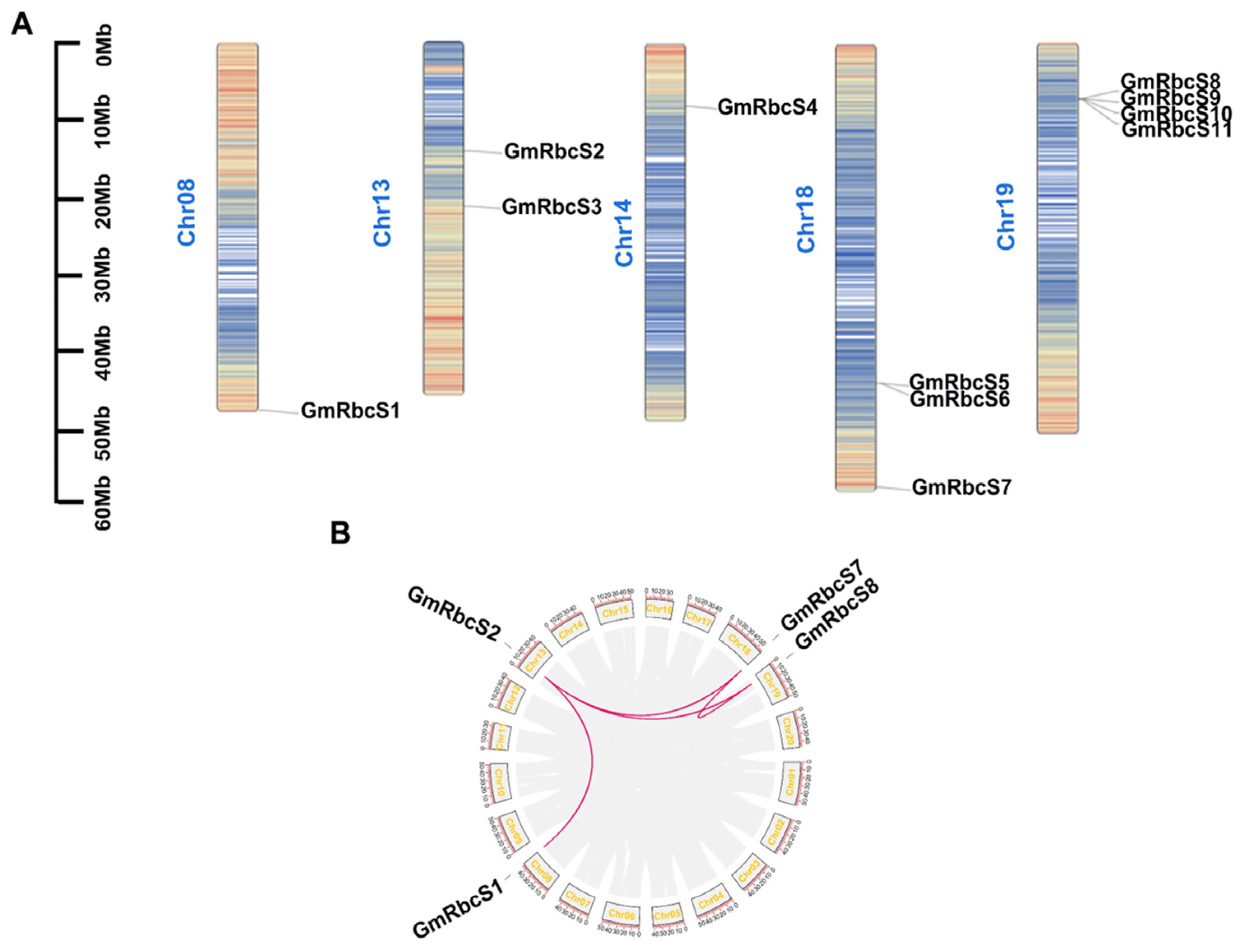
2.3. Phylogenetic Construction, Chromosome Distribution, and Collinearity Analysis
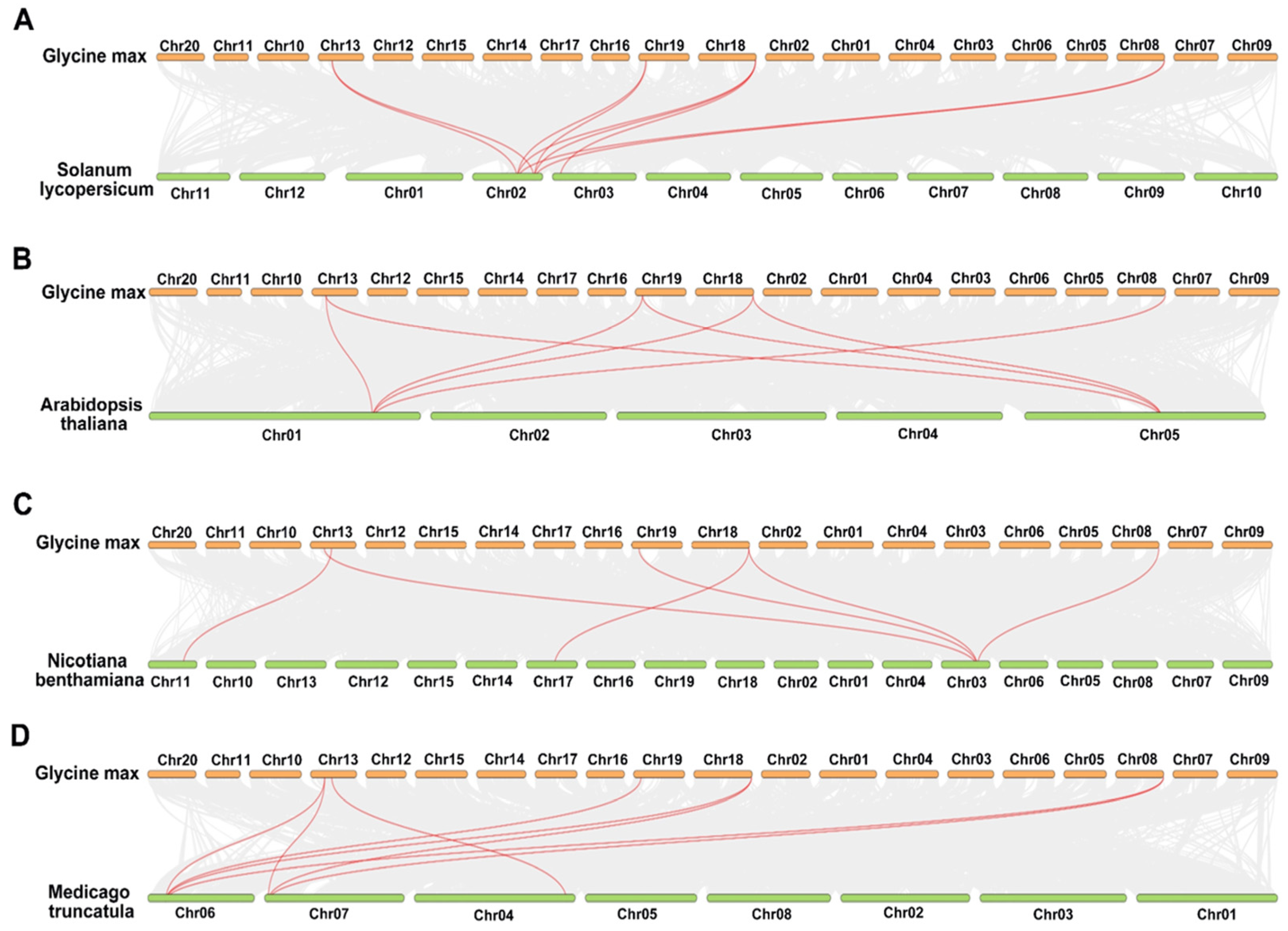
2.4. Comparative Comprehensive Map of RbcSs in Different Species
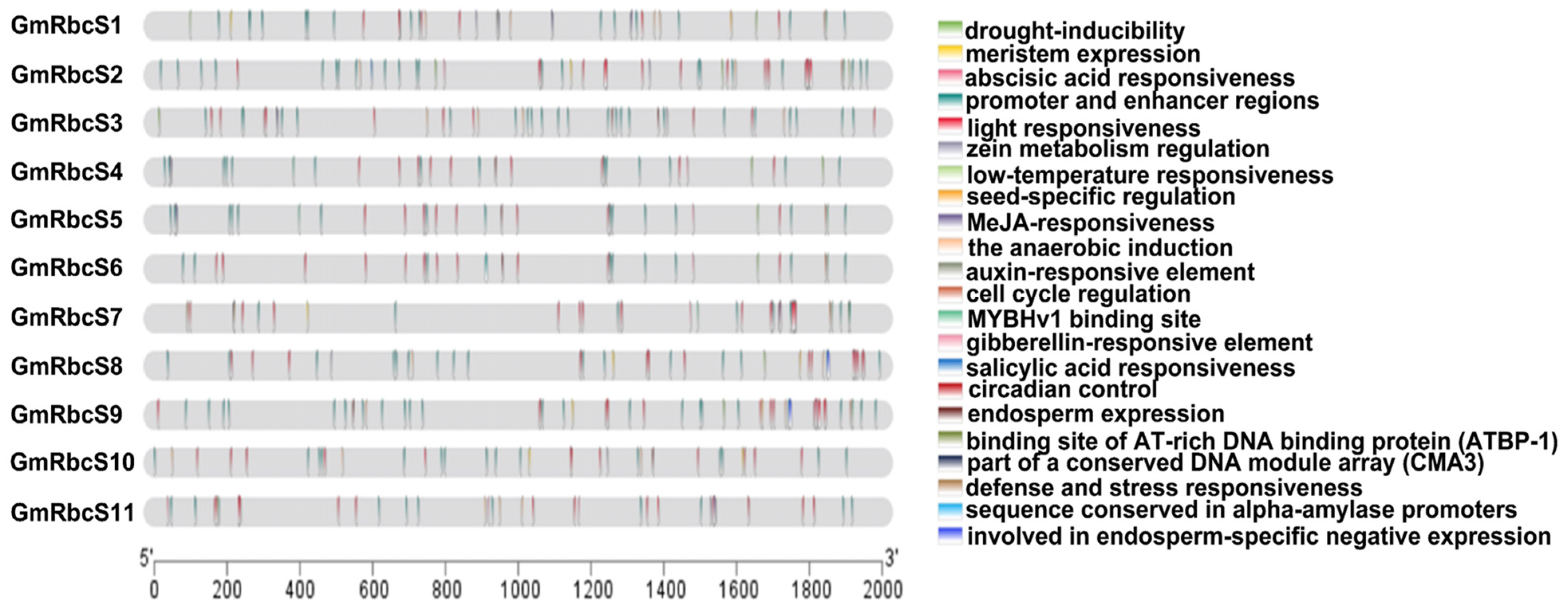
2.5. Cis-Acting Elements Analysis of GmRbcSs Promoter
2.6. Protein–Protein Interaction (PPI) Network in Soybean
2.7. Quantitative Analysis of GmRbcSs Genes in Different Tissues
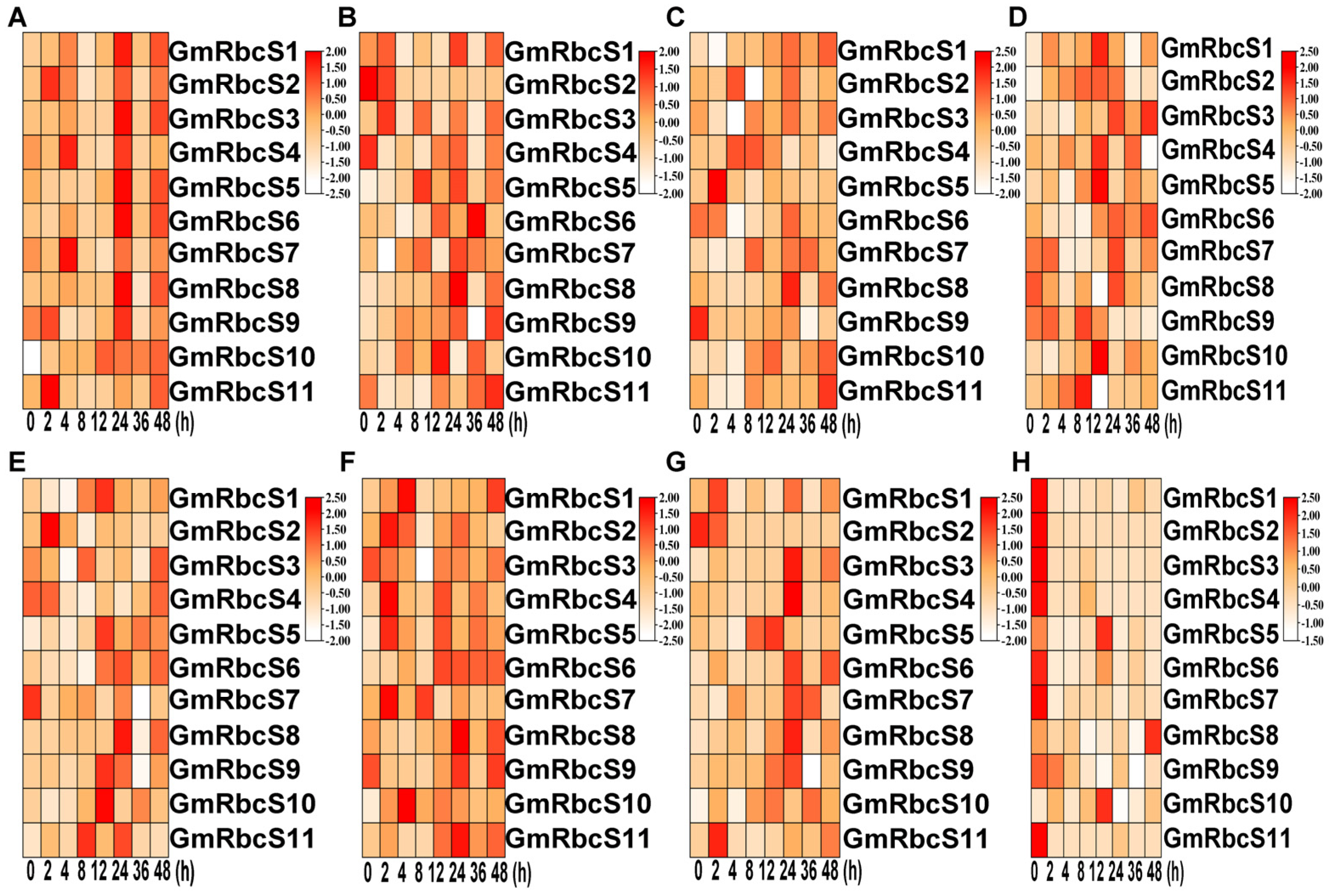
2.8. Expression Patterns of GmRbcSs under Hormone Stresses
2.9. Transient Silencing GmRbcS8 Increases SMV Accumulation in Soybean
3. Discussion
4. Materials and Methods
4.1. Characterization and Physicochemical Properties of the RbcS Family in Soybean
4.2. Phylogenetic and Structure Analysis
4.3. Chromosomal Mapping and Gene Replication
4.4. Gene Regulatory Network Analysis
4.5. Analysis of Cis-Acting Elements in Promoter Sequences
4.6. Spatiotemporal Expression Analysis
4.7. Soybean Materials and Hormone Treatments
4.8. Total RNA Extraction and RT-qPCR Analysis
4.9. Virus-Induced Gene Silencing System (VIGS)
4.10. Assessing the Response of Silenced GmRbcS8 Gene to SMV
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Liu, S.L.; Wang, Z.; Yuan, Y.Q.; Zhang, Z.F.; Liang, Q.J.; Yang, X.; Duan, Z.B.; Liu, Y.C.; Kong, F.J.; et al. Progress in soybean functional genomics over the past decade. Plant Biotechnol. J. 2022, 20, 256–282. [Google Scholar] [CrossRef]
- Du, H.; Fang, C.; Li, Y.; Kong, F.; Liu, B. Understandings and future challenges in soybean functional genomics and molecular breeding. J. Integr. Plant Biol. 2023, 65, 468–495. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.X.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.J.; Thelen, J.J.; Cheng, J.L.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.T.; Liu, J.; Geng, H.Y.; Zhang, J.X.; Liu, Y.C.; Zhang, H.K.; Xing, S.L.; Du, J.C.; Ma, S.S.; Tian, Z.X. De novo assembly of a Chinese soybean genome. Sci. China Life Sci. 2018, 61, 871–884. [Google Scholar] [CrossRef]
- Park, G.T.; Moon, J.K.; Park, S.; Park, S.K.; Baek, J.; Seo, M.S. Genome-wide analysis of KIX gene family for organ size regulation in soybean (Glycine max L.). Front. Plant Sci. 2023, 14, 1252016. [Google Scholar] [CrossRef]
- Liang, M.J.; Du, Z.Y.; Yang, Z.; Luo, T.; Ji, C.L.; Cui, H.L.; Li, R.Z. Genome-wide characterization and expression analysis of MADS-box transcription factor gene family in Perilla frutescens. Front. Plant Sci. 2023, 14, 1299902. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.B.; Zhang, Y.W.; Li, W.; Wang, C.J.; Xu, R.; Dai, H.Y.; Zhang, L.F. Characterization of the pyruvate kinase gene family in soybean and identification of a putative salt responsive gene GmPK21. BMC Genom. 2024, 25, 88. [Google Scholar] [CrossRef]
- George, R.L.; Susan, B.; Nicholas, S.; Murray, G. Chloroplast immunity illuminated. New Phytol. 2020, 229, 3088–3107. [Google Scholar] [CrossRef]
- Witte, C.P.; Herde, M. Nucleotide Metabolism in Plants. Plant Physiol. 2020, 182, 63–78. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Lin, R. Transcriptional regulatory network of the light signaling pathways. New Phytol. 2020, 227, 683–697. [Google Scholar] [CrossRef]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022, 233, 2000–2016. [Google Scholar] [CrossRef]
- Zhuang, X.; Jiang, L. Chloroplast degradation: Multiple routes into the vacuole. Front. Plant Sci. 2019, 10, 359. [Google Scholar] [CrossRef]
- Pipitone, R.; Eicke, S.; Pfister, B.; Glauser, G.; Falconet, D.; Uwizeye, C.; Pralon, T.; Zeeman, S.C.; Kessler, F.; Demarsy, E. A multifaceted analysis reveals two distinct phases of chloroplast biogenesis during de-etiolation in Arabidopsis. eLife 2021, 10, e62709. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.A.; Andralojc, P.J.; Scales, J.C.; Salvucci, M.E.; Carmo-Silva, A.E.; Alonso, H.; Whitney, S.M. Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot. 2013, 64, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.S. The discovery of rubisco. J. Exp. Bot. 2023, 74, 510–519. [Google Scholar] [CrossRef]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and Metabolic Maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef]
- Pottier, M.; Gilis, D.; Boutry, M. The Hidden Face of Rubisco. Trends Plant Sci. 2018, 23, 382–392. [Google Scholar] [CrossRef]
- Moon, K.E.; Thompson, E. Subunits from reduced and s-carboxymethylated ribulose diphosphate carboxylase (fraction i protein). Aust. J. biol. Sci. 1969, 22, 463–470. [Google Scholar] [CrossRef][Green Version]
- Vitlin, G.A.; Feiz, L. Rubisco Assembly in the Chloroplast. Front. Mol. Biosci. 2018, 5, 24. [Google Scholar] [CrossRef]
- Andrews, T.J.; Ballment, B. The function of the small subunits of ribulose bisphosphate carboxylase-oxygenase. J. Biol. Chem. 1983, 258, 7514–7518. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, R.J. Role of the small subunit in ribulose-1, 5-bisphosphate carboxylase/oxygenase. Arch. Biochem. Biophys. 2003, 414, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Genkov, T.; Spreitzer, R.J. Highly conserved small subunit residues influence rubisco large subunit catalysis. J. Biol. Chem. 2009, 284, 30105–30112. [Google Scholar] [CrossRef] [PubMed]
- Genkov, T.; Meyer, M.; Griffiths, H.; Spreitzer, R.J. Functional hybrid rubisco enzymes with plant small subunits and algal large subunits: Engineered rbcS cDNA for expression in chlamydomonas. J. Biol. Chem. 2010, 285, 19833–19841. [Google Scholar] [CrossRef]
- Bracher, A.; Starling-Windhof, A.; Hartl, F.U.; Hayer-Hartl, M. Crystal structure of a chaperone-bound assembly intermediate of form I Rubisco. Nat. Struct. Mol. Biol. 2011, 18, 875–880. [Google Scholar] [CrossRef]
- Studer, R.A.; Christin, P.A.; Williams, M.A.; Orengo, C.A. Stability-activity tradeoffs constrain the adaptive evolution of rubisco. Proc. Natl. Acad. Sci. USA 2014, 111, 2223–2228. [Google Scholar] [CrossRef]
- Bloom, A.J.; Lancaster, K.M. Manganese binding to Rubisco could drive a photorespiratory pathway that increases the energy efficiency of photosynthesis. Nature Plants 2018, 4, 414–422. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakabayashi, K.; Yoshizawa, R.; Mae, T.; Makino, A. Differences in expression of the RBCS multigene family and rubisco protein content in various rice plant tissues at different growth stages. Plant Cell Physiol. 2009, 50, 1851–1855. [Google Scholar] [CrossRef]
- Izumi, M.; Tsunoda, H.; Suzuki, Y.; Makino, A.; Ishida, H. RBCS1A and RBCS3B, two major members within the Arabidopsis RBCS multigene family, function to yield sufficient Rubisco content for leaf photosynthetic capacity. J. Exp. Bot. 2012, 63, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.; Mao, Y.; Orr, D.J.; Carmo-Silva, E.; McCormick, A.J. CRISPR-Cas9-Mediated Mutagenesis of the Rubisco Small Subunit Family in Nicotiana tabacum. Front. Genome Ed. 2020, 2, 605614. [Google Scholar] [CrossRef] [PubMed]
- Shirley, B.W.; Meagher, R.B. A potential role for RNA turnover in the light regulation of plant gene expression: Ribulose-1, 5-bisphosphate carboxylase small subunit in soybean. Nucleic Acids Res. 1990, 18, 3377–3385. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M.; Meagher, R.B. Transcriptional and post-transcriptional processes regulate expression of RNA encoding the small subunit of ribulose-1, 5-biphosphate carboxylase differently in petunia and in soybean. Nucleic Acids Res. 1990, 18, 3621–3629. [Google Scholar] [CrossRef] [PubMed]
- Hanson, T.E.; Tabita, F.R. A ribulose-1, 5-bisphosphate carboxylase/oxygenase (RubisCO)-like protein from Chlorobium tepidum that is involved with sulfur metabolism and the response to oxidative stress. Proc. Natl. Acad. Sci. USA 2001, 98, 4397–4402. [Google Scholar] [CrossRef]
- Feki, S.; Loukili, M.J.; Triki-Marrakchi, R.; Karimova, G.; Old, I.; Ounouna, H.; Nato, A.; Nato, F.; Guesdon, J.L.; Lafaye, P. Interaction between tobacco Ribulose-l, 5-biphosphate Carboxylase/Oxygenase large subunit (RubisCO-LSU) and the PVY Coat Protein (PVY-CP). Eur. J. Plant Pathol. 2005, 112, 221–234. [Google Scholar] [CrossRef]
- Ji, J.; Scott, M.P.; Bhattacharyya, M.K. Light is essential for degradation of ribulose-1, 5-bisphosphate carboxylase-oxygenase large subunit during sudden death syndrome development in soybean. Plant Biol. 2016, 8, 597–605. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Kumar, S.; Karmakar, R.; Gupta, I.; Patel, A.K. Interaction of potyvirus helper component-proteinase (HcPro) with RuBisCO and nucleosome in viral infections of plants. Plant Physiol. Biochem. 2020, 151, 313–322. [Google Scholar] [CrossRef]
- Akbar, S.; Yao, W.; Yu, K.; Qin, L.F.; Ruan, M.H.; Powell, C.A.; Chen, B.S.; Zhang, M.Q. Photosynthetic characterization and expression profiles of sugarcane infected by Sugarcane mosaic virus (SCMV). Photosynth. Res. 2021, 150, 279–294. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Z.; Yan, F.; Lu, Y.; Zheng, H.; Chen, J. Interaction between potyvirus P3 and ribulose-1, 5-bisphosphate carboxylase/oxygenase (RubisCO) of host plants. Virus Genes 2011, 43, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, H.G.; Liu, P.L.; Jiang, L.; Cheng, X.F.; Li, F.F.; Shen, W.T.; Qiu, W.P.; Dai, Z.J.; Cui, H.G. Rubisco small subunit (RbCS) is co-opted by potyvirids as the scaffold protein in assembling a complex for viral intercellular movement. PLoS Pathog. 2024, 20, e1012064. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.W.; Catherall, E.; Díaz-Ramos, A.; Greiff, G.R.L.; Azinas, S.; Gunn, L.; McCormick, A.J. The small subunit of Rubisco and its potential as an engineering target. J. Exp. Bot. 2023, 74, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Riaz, S.; Davey, P.; Zhao, Z.Y.; Sun, Y.Q.; Dykes, G.F.; Zhou, F.; Hartwell, J.; Lawson, T.; Nixon, P.J.; et al. Producing fast and active Rubisco in tobacco to enhance photosynthesis. Plant Cell 2023, 35, 795–807. [Google Scholar] [CrossRef]
- Sharwood, R.E.; Caemmerer, S.; Maliga, P.; Whitney, S.M. The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiol. 2008, 146, 83–96. [Google Scholar] [CrossRef]
- Matsumura, H.; Shiomi, K.; Yamamoto, A.; Taketani, Y.; Kobayashi, N.; Yoshizawa, T.; Tanaka, S.I.; Yoshikawa, H.; Endo, M.; Fukayama, H. Hybrid Rubisco with Complete Replacement of Rice Rubisco Small Subunits by Sorghum Counterparts Confers C Plant-like High Catalytic Activity. Mol. Plant. 2020, 13, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tian, C.; Song, S.; Li, R. Insights on the enhancement of chilling tolerance in Rice through over-expression and knock-out studies of OsRBCS3. Plant Signal. Behav. 2024, 19, 2318514. [Google Scholar] [CrossRef] [PubMed]
- Sugita, M.; Gruissem, W. Developmental, organ-specific, and light-dependent expression of the tomato ribulose-1, 5-bisphosphate carboxylase small subunit gene family. Proc. Natl. Acad. Sci. USA 1987, 84, 7104–7108. [Google Scholar] [CrossRef]
- Galili, S.; Avivi, Y.; Feldman, M. Differential expression of three rbcs subfamilies in wheat. Plant Sci. 1998, 139, 185–193. [Google Scholar] [CrossRef]
- Yeo, T.W.; Mak, Y.M.; Ho, K.K. Rubisco small subunit gene family in cassava. DNA Seq. 1999, 10, 189–194. [Google Scholar] [CrossRef]
- Schwarte, S.; Tiedemann, R. A gene duplication/loss event in the ribulose-1, 5-bisphosphate-carboxylase/oxygenase (rubisco) small subunit gene family among accessions of Arabidopsis thaliana. Mol. Biol. Evol. 2011, 28, 1861–1876. [Google Scholar] [CrossRef]
- Yao, X.; Lai, D.L.; Zhou, M.L.; Ruan, J.J.; Ma, C.; Wu, W.J.; Weng, W.-F.; Fan, Y.; Cheng, J.P. Genome-wide identification, evolution and expression pattern analysis of the GATA gene family in Sorghum bicolor. Front. Plant Sci. 2023, 14, 1163357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, W.; Gao, W.; Zhang, Y.; Zhang, P.; Liu, Y.; Chen, T.; Yang, D.L. Genome-wide identification and analysis of the GGCT gene family in wheat. BMC Genom. 2024, 25, 32. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.L.; Zhang, Z.N.; Gu, Y.H.; Li, W.J.; Wang, W.Y.; Yuan, X.K.; Zhang, Y.X.; Yuan, M.; Du, J.D.; Zhao, Q. Genome-wide identification of the soybean cytokinin oxidase/dehydrogenase gene family and its diverse roles in response to multiple abiotic stress. Front. Plant Sci. 2023, 14, 1163219. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; Chen, Z.Y.; Wu, L.; Liu, X.Q.; Dong, Y.Y.; Wang, F.W.; Li, H.Y. rbcS SRS4 promoter from Glycine max and its expression activity in transgenic tobacco. Genet. Mol. Res. 2015, 14, 7395–7405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Q.; Zhang, H.; Jia, Q.; Hong, Y.; Liu, Y. The rubisco small subunit is involved in tobamovirus movement and Tm-2²-mediated extreme resistance. Plant Physiol. 2013, 161, 374–383. [Google Scholar] [CrossRef]
- Rolland, N.; Curien, G.; Finazzi, G.; Kuntz, M.; Maréchal, E.; Matringe, M.; Ravanel, S.; Seigneurin, B.D. The biosynthetic capacities of the plastids and integration between cytoplasmic and chloroplast processes. Annu. Rev. Genet. 2012, 46, 233–264. [Google Scholar] [CrossRef]
- Moller, I.M.; Rasmusson, A.G.; Van, A.O. Plant mitochondria—Past, present and future. Plant J. 2021, 108, 912–959. [Google Scholar] [CrossRef]
- Dimnet, L.; Salinas, G.T.; Pullara, S.; Moyet, L.; Genevey, C.; Kuntz, M.; Duchêne, A.; Rolland, N. Isolation of Cytosolic Ribosomes Associated with Plant Mitochondria and Chloroplasts. Methods Mol. Biol. 2024, 2776, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Eilon, S. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef]
- Zhang, Y.; Berman, A.; Shani, E. Plant Hormone Transport and Localization: Signaling Molecules on the Move. Annu. Rev. Plant Biol. 2023, 74, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Osipitan, O.A.; Begcy, K.; Werle, R. Cover crops, hormones and herbicides: Priming an integrated weed management strategy. Plant Sci. 2020, 301, 110550. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.L.; Gao, G.Q.; Ali, I.; Wu, X.Y.; Tang, M.Y.; Chen, L.; Jiang, L.L.; Liang, T.F. Effects of various seed priming on morphological, physiological, and biochemical traits of rice under chilling stress. Front. Plant Sci. 2023, 14, 1146285. [Google Scholar] [CrossRef]
- Li, Y.Z.; Chen, H.H.; Wang, Y.W.; Zhu, J.C.; Zhang, X.L.; Sun, J.; Liu, F.; Zhao, Y.Y. Function analysis of GhWRKY53 regulating cotton resistance to verticillium wilt by JA and SA signaling pathways. Front. Plant Sci. 2023, 14, 1203695. [Google Scholar] [CrossRef]
- Moreno, P.A.; Martínez, F.E.; Berg, N.; Pliego, C. Effects of exogenous application of MeJA and SA on the physiological and molecular response of ‘Dusa’ avocado to Rosellinia necatrix. Plant Dis. 2024, 108, 2111–2121. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of Cytokinins for Interactions of Plants With Microbial Pathogens and Pest Insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Svolacchia, N.; Sabatini, S. Cytokinins. Curr. Biol. 2023, 33, R10–R13. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Yang, M.; Derbyshire, M.K.; Yamashita, R.A.; Marchler, B.A. NCBI’s Conserved Domain Database and Tools for Protein Domain Analysis. Curr. Protoc. Bioinform. 2020, 69, e90. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape String App: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Muhammad, I.; Ul, H.S.; Alam, M.; Khattak, A.M.; Akhtar, K.; Ullah, H.; Khan, A.; Lu, G.; Gong, Z.H. The CaChiVI2 Gene of Capsicum annuum L. Confers Resistance Against Heat Stress and Infection of Phytophthora capsici. Front. Plant Sci. 2020, 11, 219. [Google Scholar] [CrossRef]
- Ma, F.F.; Wu, X.Y.; Chen, Y.X.; Liu, Y.N.; Shao, Z.Q.; Wu, P.; Wu, M.; Liu, C.C.; Wu, W.P.; Yang, J.Y.; et al. Fine mapping of the Rsv1-h gene in the soybean cultivar Suweon 97 that confers resistance to two Chinese strains of the soybean mosaic virus. Theor. Appl. Genet. 2016, 129, 2227–2236. [Google Scholar] [CrossRef]




| Gene ID | Gene Name | Protein Length (aa) | Molecular Weight (kDa) | Theoretical Isoelectric Point (pI) | Instability Index | GRAVY | Subcellular Location |
|---|---|---|---|---|---|---|---|
| Glyma.08G365300 | GmRbcS1 | 90 | 9.97 | 8.89 | 43.52 | −0.12 | Chloroplast |
| Glyma.13G046200 | GmRbcS2 | 178 | 20.02 | 8.87 | 30.71 | −0.24 | Chloroplast |
| Glyma.13G097100 | GmRbcS3 | 172 | 19.60 | 8.95 | 53.39 | −0.35 | Chloroplast |
| Glyma.14G089500 | GmRbcS4 | 159 | 17.83 | 9.81 | 45.98 | −0.53 | Chloroplast |
| Glyma.18G182200 | GmRbcS5 | 100 | 11.54 | 9.17 | 49.22 | −0.33 | Chloroplast |
| Glyma.18G182300 | GmRbcS6 | 131 | 15.09 | 9.20 | 50.32 | −0.48 | Chloroplast |
| Glyma.18G296900 | GmRbcS7 | 150 | 16.76 | 9.22 | 43.77 | −0.14 | Chloroplast |
| Glyma.19G046600 | GmRbcS8 | 178 | 20.01 | 8.87 | 31.32 | −0.24 | Chloroplast |
| Glyma.19G046800 | GmRbcS9 | 178 | 20.01 | 8.87 | 31.32 | −0.24 | Chloroplast |
| Glyma.19G046900 | GmRbcS10 | 135 | 14.81 | 9.39 | 33.23 | −0.06 | Chloroplast |
| Glyma.19G047000 | GmRbcS11 | 105 | 12.41 | 7.81 | 36.75 | −0.45 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Feng, W.; Mou, K.; Yu, Z.; Zeng, Y.; Zhang, W.; Zhou, Y.; Li, Y.; Gao, H.; Xu, K.; et al. Genome-Wide Analysis and Expression Profiling of Soybean RbcS Family in Response to Plant Hormones and Functional Identification of GmRbcS8 in Soybean Mosaic Virus. Int. J. Mol. Sci. 2024, 25, 9231. https://doi.org/10.3390/ijms25179231
Zhou F, Feng W, Mou K, Yu Z, Zeng Y, Zhang W, Zhou Y, Li Y, Gao H, Xu K, et al. Genome-Wide Analysis and Expression Profiling of Soybean RbcS Family in Response to Plant Hormones and Functional Identification of GmRbcS8 in Soybean Mosaic Virus. International Journal of Molecular Sciences. 2024; 25(17):9231. https://doi.org/10.3390/ijms25179231
Chicago/Turabian StyleZhou, Fangxue, Wenmi Feng, Kexin Mou, Zhe Yu, Yicheng Zeng, Wenping Zhang, Yonggang Zhou, Yaxin Li, Hongtao Gao, Keheng Xu, and et al. 2024. "Genome-Wide Analysis and Expression Profiling of Soybean RbcS Family in Response to Plant Hormones and Functional Identification of GmRbcS8 in Soybean Mosaic Virus" International Journal of Molecular Sciences 25, no. 17: 9231. https://doi.org/10.3390/ijms25179231
APA StyleZhou, F., Feng, W., Mou, K., Yu, Z., Zeng, Y., Zhang, W., Zhou, Y., Li, Y., Gao, H., Xu, K., Feng, C., Jing, Y., & Li, H. (2024). Genome-Wide Analysis and Expression Profiling of Soybean RbcS Family in Response to Plant Hormones and Functional Identification of GmRbcS8 in Soybean Mosaic Virus. International Journal of Molecular Sciences, 25(17), 9231. https://doi.org/10.3390/ijms25179231





