Immune-Enhancing Effects of Gwakhyangjeonggi-san in RAW 264.7 Macrophage Cells through the MAPK/NF-κB Signaling Pathways
Abstract
1. Introduction
2. Results
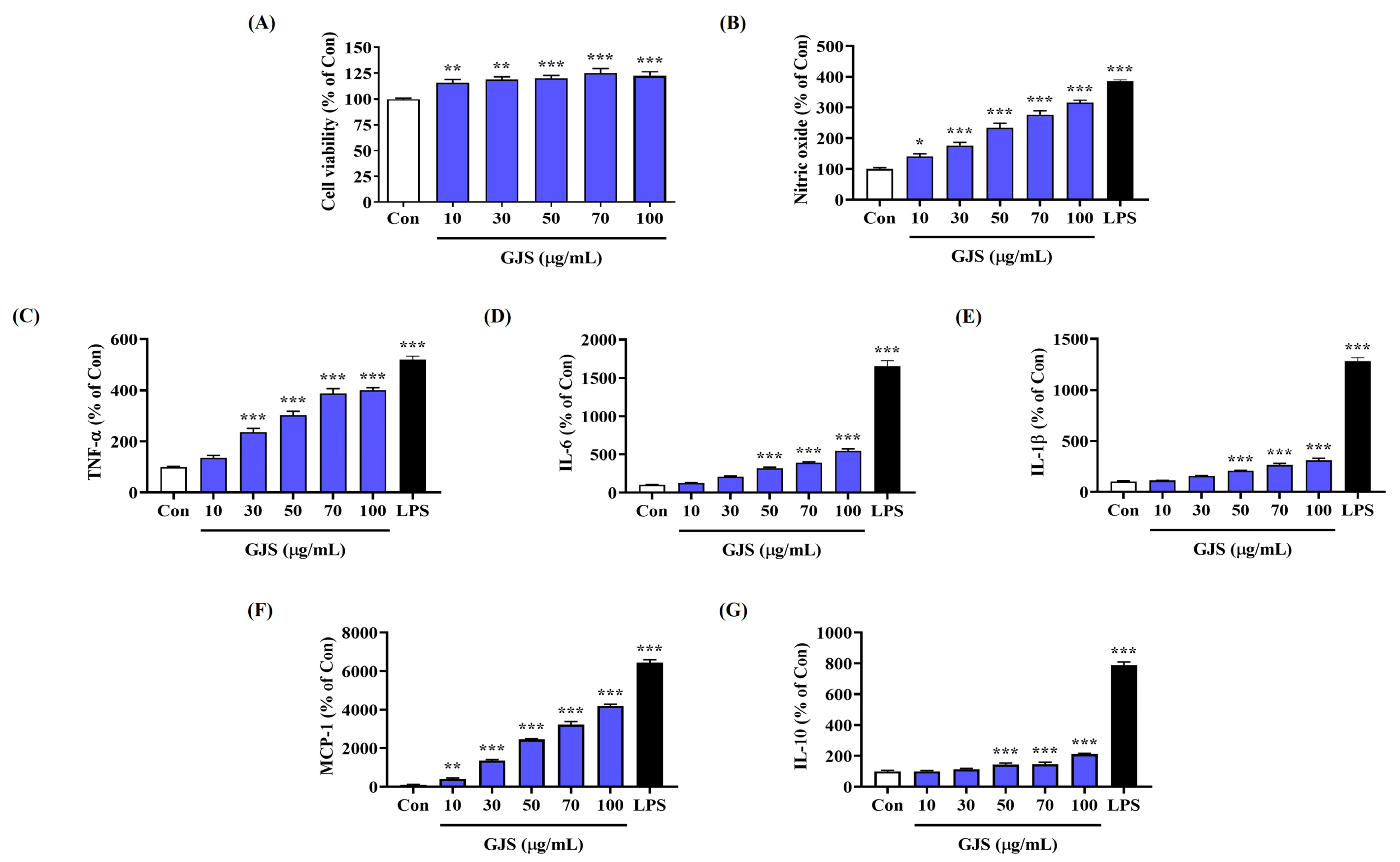
2.1. GJS Induces NO Secretion in Macrophages without Cytotoxicity
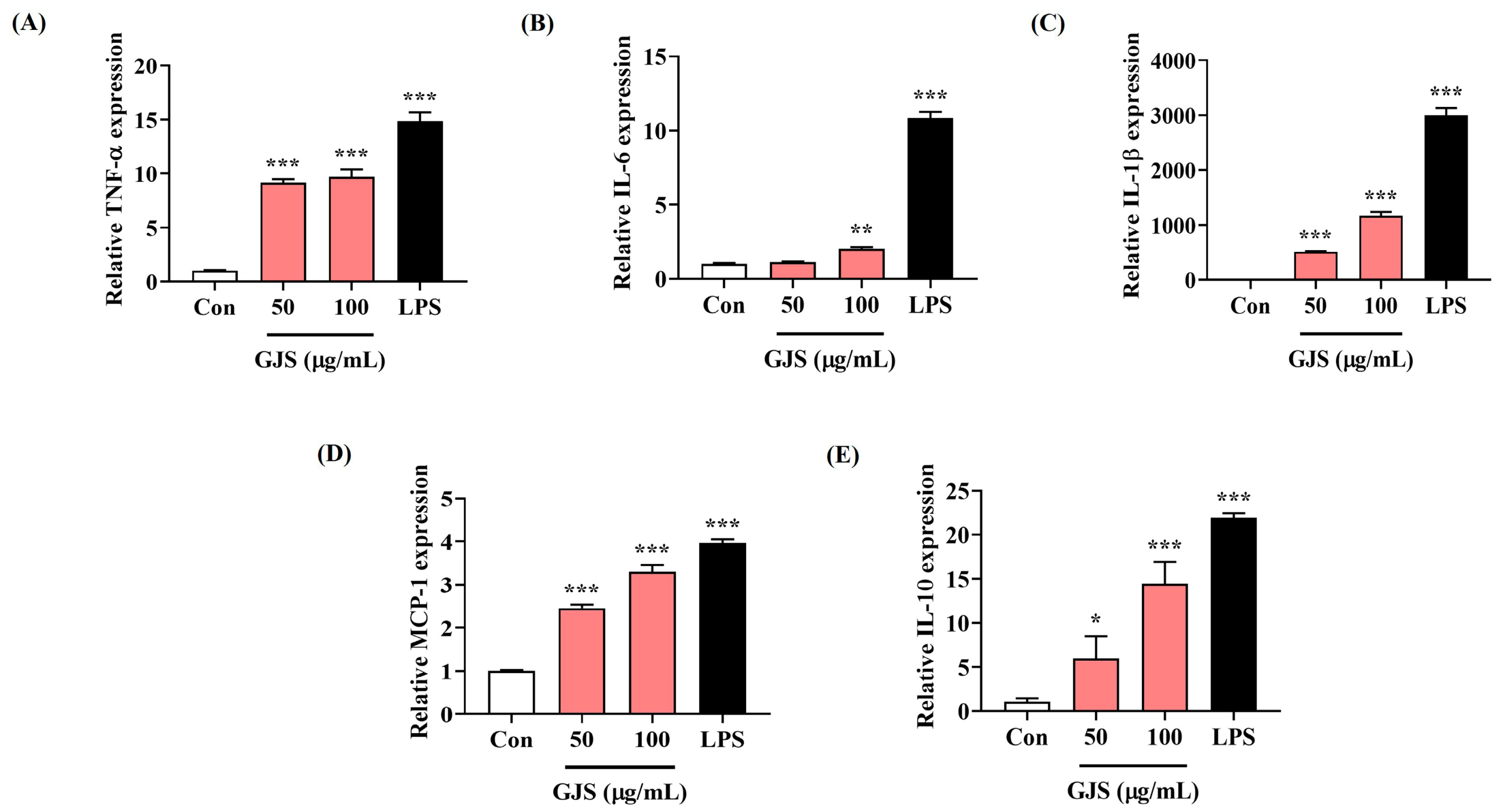
2.2. GJS Induces Cytokine Secretion in Macrophages and Modulates Gene Expression
2.3. GJS Treatment Significantly Increases Intracellular ROS Accumulation in Macrophages
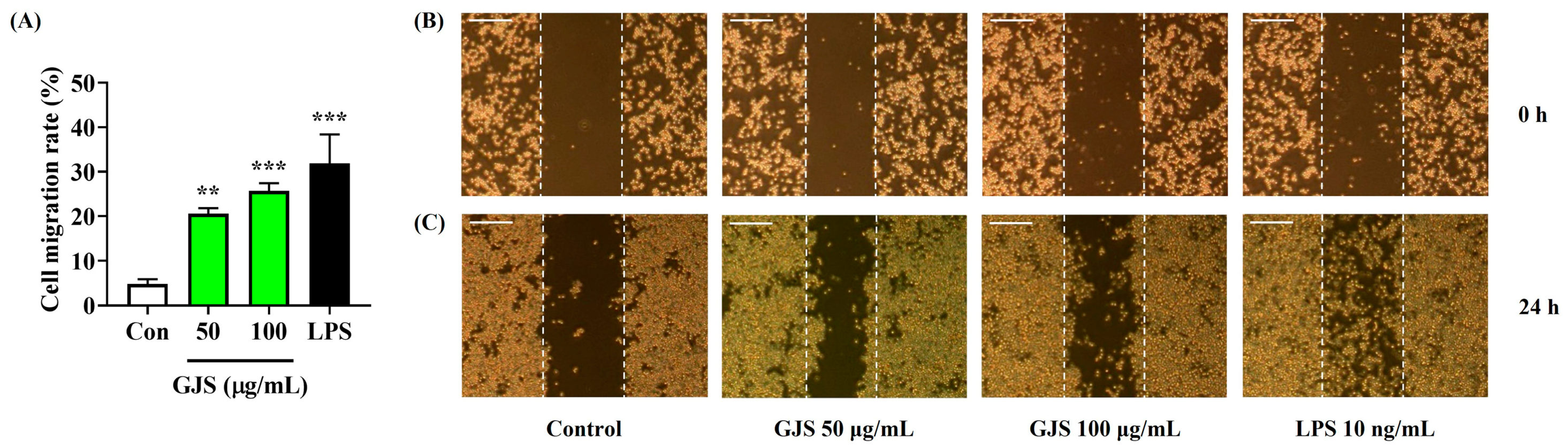
2.4. Strengthening Effects of GJS on the Immune Function of Macrophages
2.5. GJS Activates the Mitogen-Activated Protein Kinase (MAPK) and Nuclear Factor (NF)-κB Signaling Pathways in Macrophages
2.6. GJS Increases Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase (COX)-2 Enzyme Expression in Macrophages
2.7. UHPLC Coupled with Quadrupole-Orbitrap Mass Spectrometry (UHPLC-UV-HRMS) Analysis of Phytochemicals in GJS
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of GJS
4.3. Cell Culture
4.4. Cell Viability Test
4.5. NO Production
4.6. Determination of Cytokines
4.7. RNA Extraction, DNA Synthesis, and qPCR
4.8. Measurement of Intracellular ROS Levels
4.9. Evaluation of Phagocytosis
4.10. Measurement of Adhesion Function
4.11. Measurement of Migration Activity
4.12. Western Blot Analysis
4.13. UHPLC-UV-HRMS Analysis
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Lehman, H.K. Autoimmunity and Immune Dysregulation in Primary Immune Deficiency Disorders. Curr. Allergy Asthma Rep. 2015, 15, 53. [Google Scholar] [CrossRef]
- Files, J.K.; Boppana, S.; Perez, M.D.; Sarkar, S.; Lowman, K.E.; Qin, K.; Sterrett, S.; Carlin, E.; Bansal, A.; Sabbaj, S.; et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J. Clin. Investig. 2021, 131, e140491. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Ketha, K.; Gudipati, M. Immunomodulatory activity of non starch polysaccharides isolated from green gram (Vigna radiata). Food Res. Int. 2018, 113, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.R.; Chen, J.L.; Chen, L.; Huang, X.S.; Cheung, P.C.K. Immunomodulatory Activity of Polysaccharide-Protein Complex from the Mushroom Sclerotia of Polyporus rhinocerus in Murine Macrophages. J. Agric. Food Chem. 2016, 64, 3206–3214. [Google Scholar] [CrossRef]
- Ren, D.; Lin, D.; Alim, A.; Zheng, Q.; Yang, X. Chemical characterization of a novel polysaccharide ASKP-1 from Artemisia sphaerocephala Krasch seed and its macrophage activation via MAPK, PI3k/Akt and NF-κB signaling pathways in RAW264. 7 cells. Food Funct. 2017, 8, 1299–1312. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Chen, J.; Song, D.; Liu, B.; Li, J.; Liu, R.; Niu, J.; Wang, D.; Ling, N. Immune-enhancing effects of a novel glucan from purple sweet potato Ipomoea batatas (L.) Lam on RAW264. 7 macrophage cells via TLR2-and TLR4-mediated pathways. J. Agric. Food Chem. 2021, 69, 9313–9325. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264. 7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, G.B.; Han, I.S.; Shim, Y.S.; Kim, E.G. A clinical report of chronic diarrhea treated with GwakHyang-JungGiSan. J. Int. Korean Med. 2005, 26, 889–896. [Google Scholar]
- Chuan-xing, Y.; Ling, Z. Experimental researches on inhibitory effect of Huoxiang Zhengqi liquid on histamine release. Chin. J. Integr. Med. 2003, 9, 276–280. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.F.; Qi, X.J.; Lu, L.L.; Chan, H.C. Effect of Huoxiang-zhengqi liquid on HCO (3)(-) secretion by intact porcine distal airway epithelium. Sheng Li Xue Bao 2008, 60, 90–96. [Google Scholar] [PubMed]
- Koo, C.M.; Sun, J.K.; Kim, H.H.; Nam, C.G. Effects of GwakHyangJungGiSan on the arterial contraction in rabbit. J. Int. Korean Med. 2003, 24, 260–268. [Google Scholar]
- Zhang, H.; Huang, Y.; Li, K.; Wu, S. Antibacterial material basis and quality control of Huoxiang Zhengqi tincture. Chin. Tradit. Herbal Drugs 2012, 43, 1349–1354. [Google Scholar]
- Kim, J.H.; Shin, H.K.; Seo, C.S. Simultaneous determination of 13 chemical marker compounds in Gwakhyangjeonggi-san, a herbal formula, with validated analytical methods. Nat. Prod. Commun. 2014, 9, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S.; Shin, H.K. Quantitative Analysis of the Twenty Marker Components in Gwakhyangjeonggi-san using Ultra-Performance Liquid Chromatography with Mass Spectrometer. Korean J. Pharmacogn. 2014, 45, 113–120. [Google Scholar]
- Yuan, H.; Cao, M.; Yi, P.; Xie, Q.; Jian, Y.; Li, B.; Qin, Y.; Peng, C.; Wu, H.; Tan, D.; et al. Determination of alkaloids and phenols in the chewable husk products of Areca catechu L. Using HPLC-UV and UHPLC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 612–620. [Google Scholar] [CrossRef]
- Jang, A.K.; Rashid, M.M.; Lee, G.; Kim, D.Y.; Ryu, H.W.; Oh, S.R.; Park, J.; Lee, H.; Hong, J.; Jung, B.H. Metabolites identification for major active components of Agastache rugosa in rat by UPLC-Orbitap-MS: Comparison of the difference between metabolism as a single component and as a component in a multi-component extract. J. Pharm. Biomed. Anal. 2022, 220, 114976. [Google Scholar] [CrossRef]
- Galeas-Pena, M.; McLaughlin, N.; Pociask, D. The role of the innate immune system on pulmonary infections. Biol. Chem. 2019, 400, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sawhney, G.; Nagar, R.K.; Chauhan, N.; Gupta, N.; Kaul, A.; Ahmed, Z.; Sangwan, P.; Kumar, P.S.; Yadav, G. Evaluation of the immunomodulatory and anti-inflammatory activity of Bakuchiol using RAW 264.7 macrophage cell lines and in animal models stimulated by lipopolysaccharide (LPS). Int. Immunopharmacol. 2021, 91, 107264. [Google Scholar] [CrossRef]
- Vazquez-Torres, A.; Jones-Carson, J.; Mastroeni, P.; Ischiropoulos, H.; Fang, F.C. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 2000, 192, 227–236. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Zhao, Y.; Liu, J.; Liu, C.; Zeng, X.; Li, X.; Zhao, J. Anti-inflammatory effects of p-coumaric acid in LPS-stimulated RAW264. 7 cells: Involvement of NF-κB and MAPKs pathways. Med. Chem. 2016, 6, 327–330. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, L.; Yu, B.; Chen, K.; Liu, B.; Liu, J.; Qin, G.; Liu, C.; Liu, H.; Chen, K. Exopolysaccharide from Trichoderma pseudokoningii induces macrophage activation. Carbohydr. Polym. 2016, 149, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Martinvalet, D.; Walch, M. the role of reactive oxygen species in protective immunity. Front. Immunol. 2022, 12, 832946. [Google Scholar] [CrossRef]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front. Immunol. 2014, 5, 113523. [Google Scholar] [CrossRef] [PubMed]
- Scott, O.; Roifman, C.M. NF-κB pathway and the Goldilocks principle: Lessons from human disorders of immunity and inflammation. J. Allergy Clin. Immunol. 2019, 143, 1688–1701. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, H.J. Immunomodulatory effects of fermented Platycodon grandiflorum extract through NF-κB signaling in RAW 264.7 cells. Nutr. Res. Pract. 2020, 14, 453–462. [Google Scholar] [CrossRef]
- Shin, M.S.; Park, S.B.; Shin, K.S. Molecular mechanisms of immunomodulatory activity by polysaccharide isolated from the peels of Citrus unshiu. Int. J. Biol. Macromol. 2018, 112, 576–583. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.H.; Liu, Y.; Wang, Z.; Tang, S.; Zhang, J.; Wang, Y.P. Platycodin D exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. J. Toxicol. Sci. 2016, 41, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Jeong, Y.H.; Pak, M.E.; Go, Y. Banhasasim-Tang Attenuates Lipopolysaccharide-Induced Cognitive Impairment by Suppressing Neuroinflammation in Mice. Nutrients 2020, 12, 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, P.; Fu, W.; Cai, L.; Yu, Y.; Feng, Z.; Wang, Y.; Zhang, F.; Yu, X.; Xu, H.; et al. Ginseng–Astragalus–oxymatrine injection ameliorates cyclophosphamide-induced immunosuppression in mice and enhances the immune activity of RAW264.7 cells. J. Ethnopharmacol. 2021, 279, 114387. [Google Scholar] [CrossRef] [PubMed]
- Pak, M.E.; Park, Y.J.; Yang, H.J.; Hwang, Y.H.; Li, W.; Go, Y. Samhwangsasim-tang attenuates neuronal apoptosis and cognitive decline through BDNF-mediated activation of tyrosin kinase B and p75-neurotrophin receptors. Phytomedicine 2022, 99, 153997. [Google Scholar] [CrossRef]



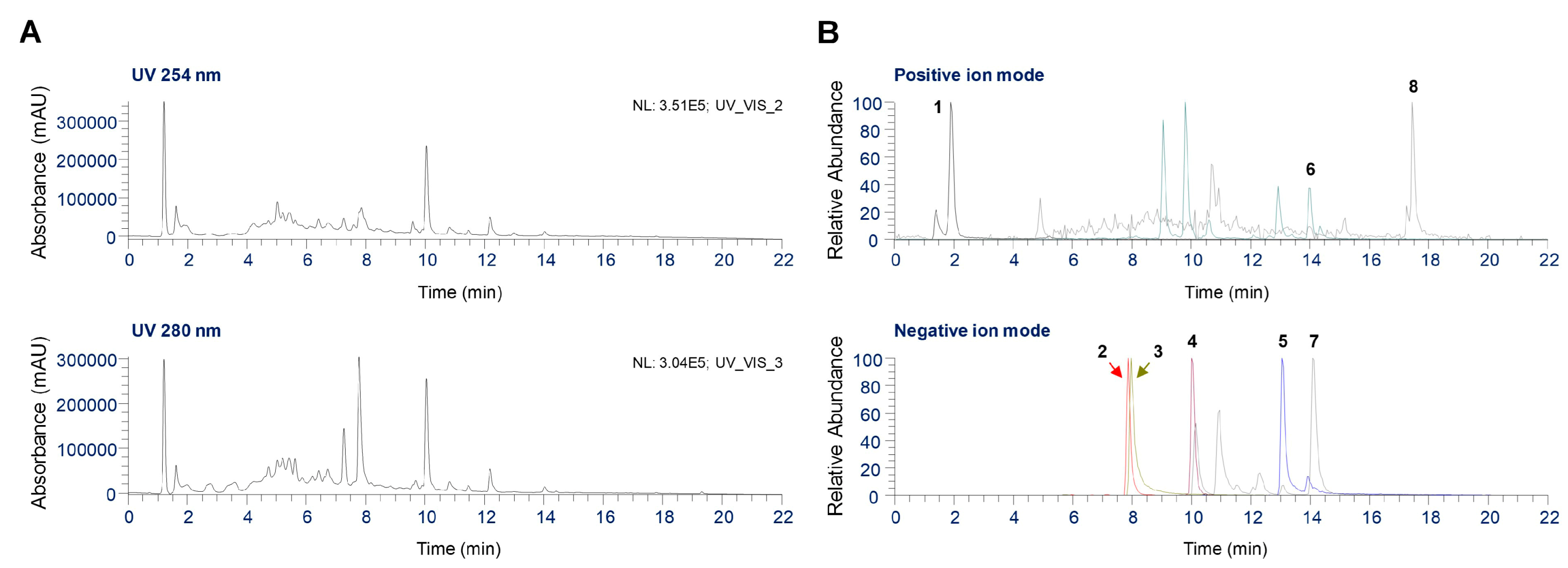
| No | tR(min) | Chemical formula | Adduct | Estimated (m/z) | Calculated (m/z) | Error (ppm) | Identification | Source |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.85 | C8H13NO2 | [M+H]+ | 156.1019 | 156.1021 | 0.9019 | Arecoline hydrobromide | A. catechu |
| 2 | 7.88 | C28H34O15 | [M−H]− | 609.1825 | 609.1830 | 0.9101 | Hesperidin | C. unshiu |
| 3 | 7.97 | C18H16O8 | [M−H]− | 359.0772 | 359.0779 | 1.6998 | Rosmarinic acid | P. frutescens |
| 4 | 10.07 | C57H92O28 | [M−H]− | 1223.5702 | 1223.5704 | 0.1672 | Platycodin D | P.grandiflorum |
| 5 | 13.07 | C42H62O16 | [M−H]− | 821.3965 | 821.3976 | 1.3063 | Glycyrrhizin | G. uralensis |
| 6 | 13.99 | C16H14O5 | [M+H]+ | 287.0914 | 287.0916 | 0.6383 | Oxypeucedanin | A. dahurica |
| 7 | 14.11 | C16H12O5 | [M−H]− | 283.0612 | 283.0614 | 0.6036 | Acacetin | A. rugosa |
| 8 | 17.43 | C15H20O2 | [M+H]+ | 233.1536 | 233.1538 | 0.9827 | Atractylenolide II | A. japonica |
| Scientific Name of Herbs | Medicinal Parts | Composition Ratio (%) |
|---|---|---|
| Agastache rugosa | Herba | 18.75 |
| Perilla frutescens | Leaf | 12.5 |
| Angelica dahurica | Root | 6.25 |
| Areca catechu | Peel of fruit | 6.25 |
| Poria cocos | Sclerotium | 6.25 |
| Magnolia officinalis | Bark | 6.25 |
| Atractylodes japonica | Rhizome | 6.25 |
| Citrus unshiu | Peel of fruit | 6.25 |
| Pinellia ternata | Tuber | 6.25 |
| Platycodon grandiflorum | Root | 6.25 |
| Glycyrrhiza uralensis | Root and rhizome | 6.25 |
| Zingiber officinale | Rhizome (undried) | 6.25 |
| Zizyphus jujuba | Fruit | 6.25 |
| Target Gene | Reference Sequence | Primer Sequence |
|---|---|---|
| TNF-α | NM_013693.3 | F: 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC-3′ |
| R: 5′-GTATGAGATAGCAAATCGGCTGACGGTGTGGG-3′ | ||
| IL-6 | NM_031168.2 | F: 5′-TCCAGTTGCCTTCTTGGGAC-3′ |
| R: 5′-GTGTAATTAAGCCTCCGACTTG-3′ | ||
| IL-1β | NM_008361.4 | F: 5′-ATGGCAACTGTTCCTGAACTCAACT-3′ |
| R: 5′-CAGGACAGGTATAGATTCTTTCCTTT-3′ | ||
| MCP-1 | NM_011333 | F: 5′-GCTACAAGAGGATCACCAGCAG-3′ |
| R: 5′-GTCTGGACCCATTCCTTCTTGG-3′ | ||
| IL-10 | NM_010548 | F: 5′-CGGGAAGACAATAACTGCACCC-3′ |
| R: 5′-CGGTTAGCAGTATGTTGTCCAGC-3′ | ||
| iNOS | NM_010927.4 | F: 5′-GGCAGCCTGTGAGACCTTTG-3′ |
| R: 5′-GCATTGGAAGTGAAGCGTTTC-3′ | ||
| COX-2 | NM_011198.4 | F: 5′-TGAGTACCGCAAACGCTTCTC-3′ |
| R: 5′-TGGACGAGGTTTTTCCACCAG-3′ | ||
| β-actin | NM_007393.5 | F: 5′-AGAGGGAAATCGTGCGTGAC-3′ |
| R: 5′-CAATAGTGATGACCTGGCCGT-3′ |
| Antibody | Corporation | Product No. | RRID | Dilution Rate |
|---|---|---|---|---|
| P-ERK | Cell Signaling | #4377 | AB_331775 | 1:1000 |
| ERK | Cell Signaling | #9102 | AB_330744 | 1:1000 |
| P-p38 | Cell Signaling | #9211 | AB_331641 | 1:1000 |
| P38 | Cell Signaling | #9212 | AB_330713 | 1:1000 |
| P-JNK | Cell Signaling | #9251 | AB_331659 | 1:1000 |
| JNK | Cell Signaling | #9252 | AB_2250373 | 1:1000 |
| β-actin | Cell Signaling | #4970 | AB_2223172 | 1:1000 |
| P-IκBα | Cell Signaling | #2859 | AB_561111 | 1:1000 |
| IκBα | Cell Signaling | #4814 | AB_390781 | 1:1000 |
| P-NF-κB p65 | Cell Signaling | #3033 | AB_331284 | 1:1000 |
| NF-κB p65 | Cell Signaling | #8242 | AB_10859369 | 1:1000 |
| Lamin B1 | Cell Signaling | #13435 | AB_2737428 | 1:1000 |
| iNOS | Cell Signaling | #13120 | AB_2687529 | 1:1000 |
| COX-2 | Cell Signaling | #4842 | AB_2085144 | 1:1000 |
| 2nd anti-mouse | Cell Signaling | #7076 | AB_330924 | 1:5000 |
| 2nd anti-rabbit | Cell Signaling | #7074 | AB_2099233 | 1:5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.H.; Yang, H.J.; Li, W.; Oh, Y.-C.; Choi, J.-G. Immune-Enhancing Effects of Gwakhyangjeonggi-san in RAW 264.7 Macrophage Cells through the MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 9246. https://doi.org/10.3390/ijms25179246
Jeong YH, Yang HJ, Li W, Oh Y-C, Choi J-G. Immune-Enhancing Effects of Gwakhyangjeonggi-san in RAW 264.7 Macrophage Cells through the MAPK/NF-κB Signaling Pathways. International Journal of Molecular Sciences. 2024; 25(17):9246. https://doi.org/10.3390/ijms25179246
Chicago/Turabian StyleJeong, Yun Hee, Hye Jin Yang, Wei Li, You-Chang Oh, and Jang-Gi Choi. 2024. "Immune-Enhancing Effects of Gwakhyangjeonggi-san in RAW 264.7 Macrophage Cells through the MAPK/NF-κB Signaling Pathways" International Journal of Molecular Sciences 25, no. 17: 9246. https://doi.org/10.3390/ijms25179246
APA StyleJeong, Y. H., Yang, H. J., Li, W., Oh, Y.-C., & Choi, J.-G. (2024). Immune-Enhancing Effects of Gwakhyangjeonggi-san in RAW 264.7 Macrophage Cells through the MAPK/NF-κB Signaling Pathways. International Journal of Molecular Sciences, 25(17), 9246. https://doi.org/10.3390/ijms25179246








