The Combination of Natural Compounds Escin–Bromelain–Ginkgo Biloba–Sage Miltiorrhiza (EBGS) Reduces Platelet Adhesion to TNFα-Activated Vascular Endothelium through FAK Signaling
Abstract
1. Introduction
2. Results
2.1. The Pretreatment of HUVEC with Escin, Bromelain, Ginkgo Biloba, or Sage Miltiorrhiza Does Not Affect the Cell Viability of Endothelial Cells
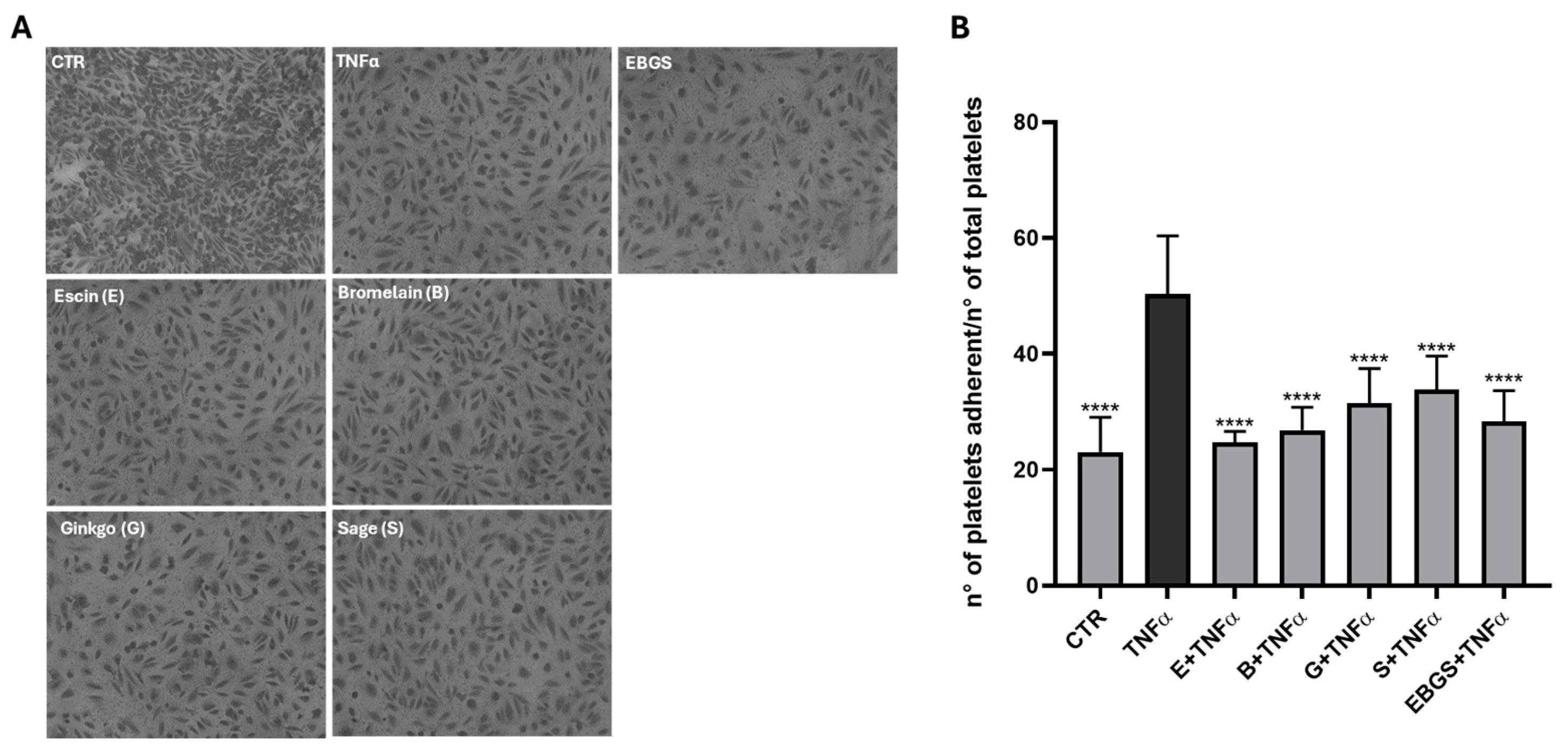
2.2. The Pretreatment of HUVEC with EBGS Decreases Platelet Adhesion on Activated Endothelium
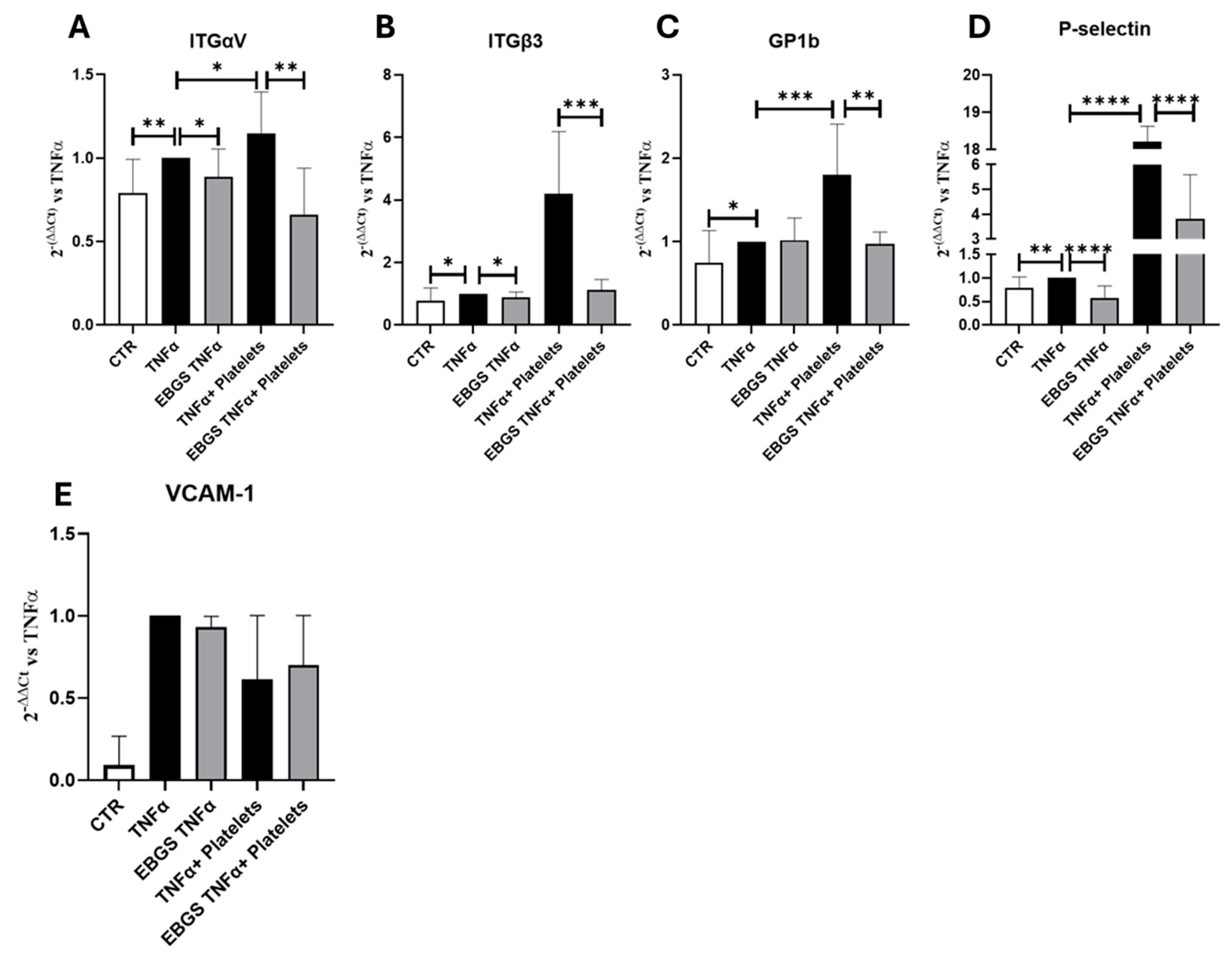
2.3. The Pretreatment of HUVEC with EBGS Reduces the Expression of the Adhesion Molecules Involved in the Platelet Adhesion on Activated Endothelium
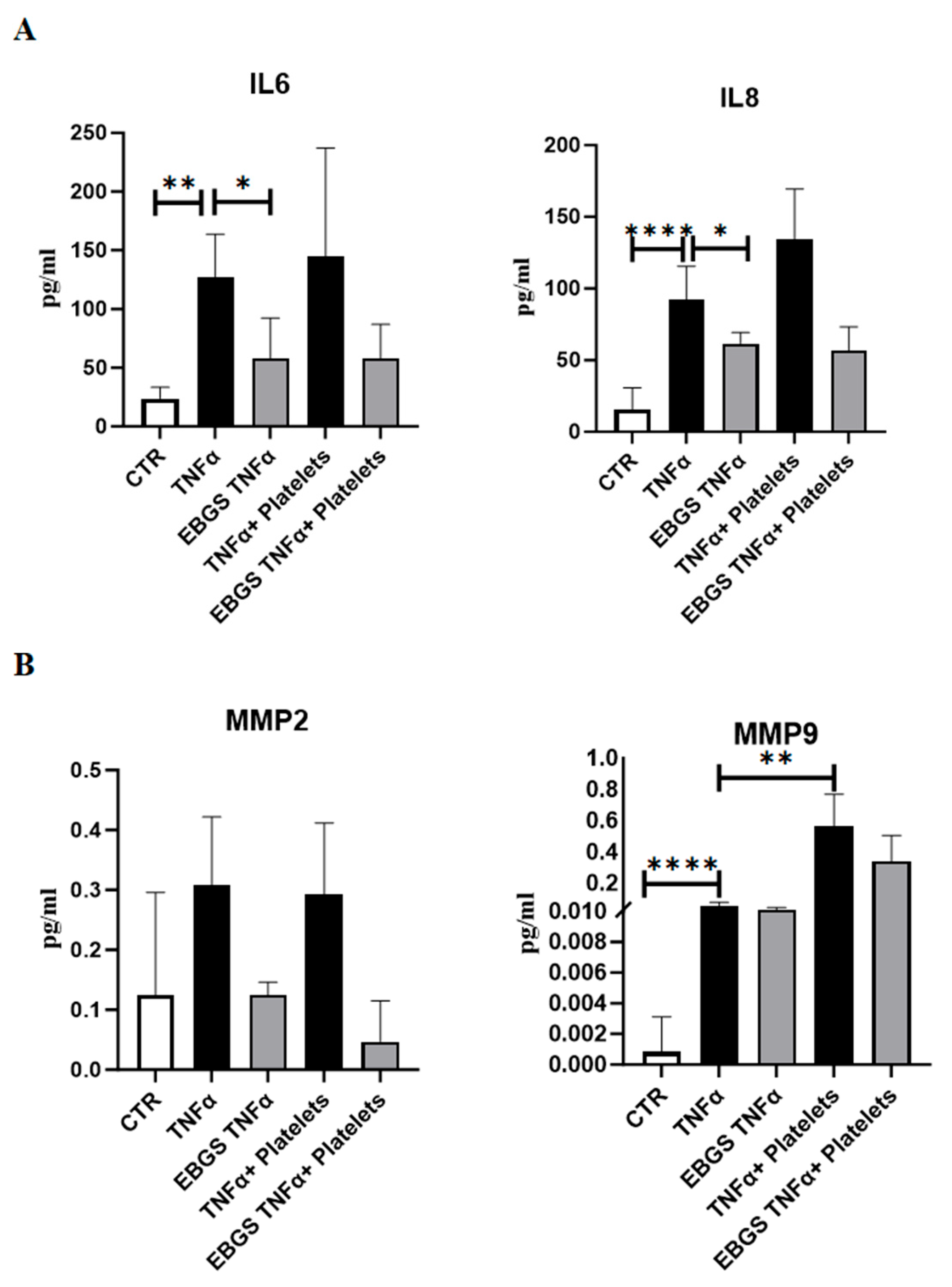
2.4. The Pretreatment of HUVEC with EBGS Reduces the Expression of Inflammatory Chemokines and Metalloproteinases
2.5. The Pretreatment of HUVEC with EBGS Reduces the Expression of the Adhesion Molecules Focal Adhesion Kinase (FAK) and Vascular Cell Adhesion Molecule 1 (VCAM-1), Involved in Platelet–Endothelial Cell Interaction
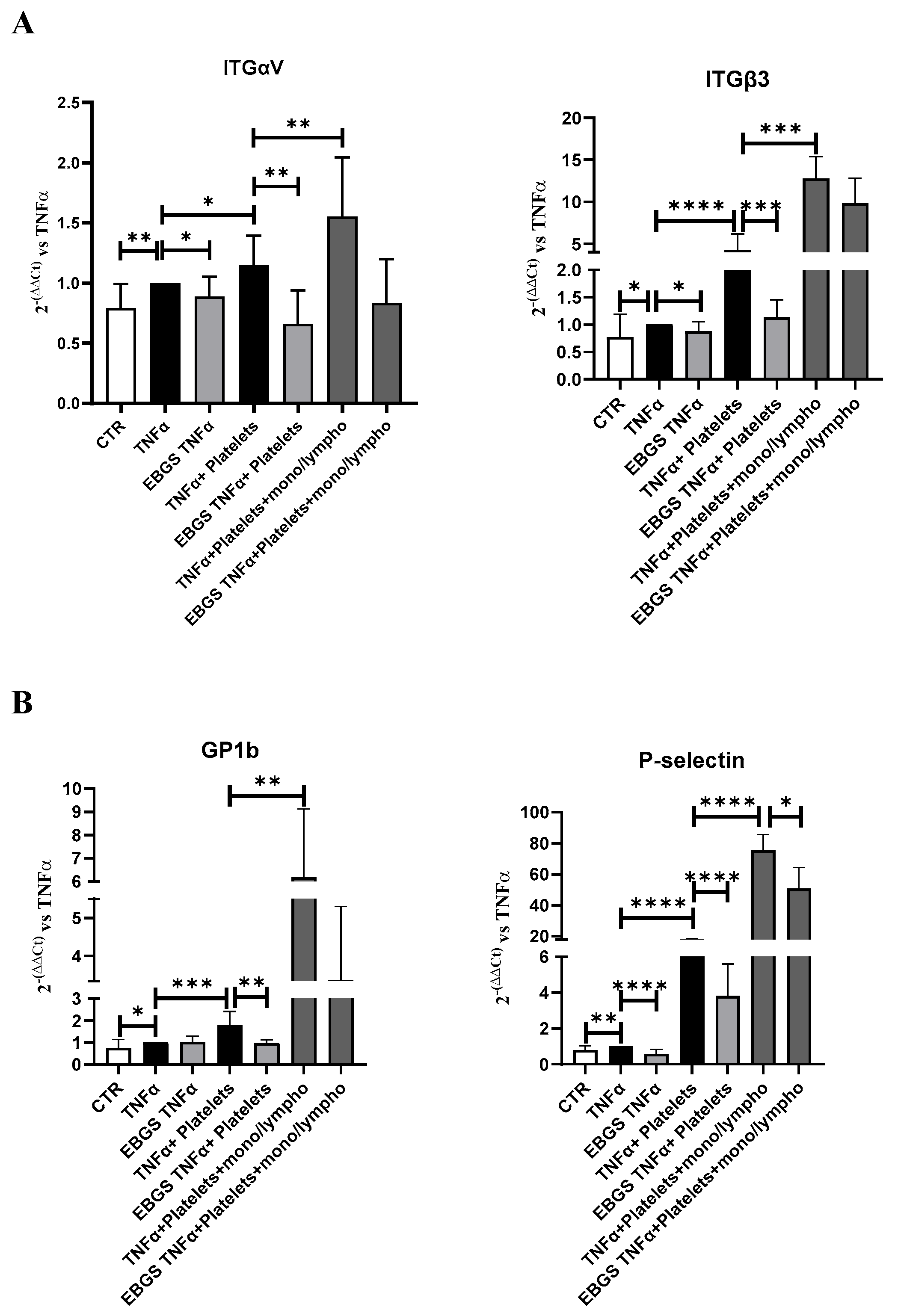
2.6. The Presence of Monocyte–Lymphocyte Interaction Strengthens the Adhesion of Platelets to the Endothelial Monolayer, and EBGS Pretreatment Maintains the Ability to Reduce This Interaction
3. Discussion
4. Materials and Methods
4.1. Preparation of Escin (E), Bromelain (B), Ginkgo Biloba (G), and Sage Miltiorrhiza (S) Extracts and Their Combination EBGS
4.2. Cell Culture and Treatment Procedure
4.3. Viability Assay (MTT Assay)
4.4. RNA Isolation and Real-Time PCR
4.5. Human Platelet Purification
4.6. Adhesion Assay of Human Platelets on HUVEC Monolayer
4.7. Enzyme-Linked ImmunoSorbent Assay (ELISA) Assays
4.8. Western Blot Analysis
4.9. Human Monocyte–Lymphocyte Fraction Purification
4.10. Adhesion Assay of Human Platelets and Human Monocyte–Lymphocyte on HUVEC Monolayer
4.11. Statistical Analysis
5. Conclusions and Limitations
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, S.; Massberg, S.; Engelmann, B. Biological basis and pathological relevance of microvascular thrombosis. Thromb. Res. 2014, 133 (Suppl. S1), S35–S37. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Welborn, M.B., 3rd; Van Zee, K.; Edwards, P.D.; Pruitt, J.H.; Kaibara, A.; Vauthey, J.N.; Rogy, M.; Castleman, W.L.; Lowry, S.F.; Kenney, J.S.; et al. A human tumor necrosis factor p75 receptor agonist stimulates in vitro T cell proliferation but does not produce inflammation or shock in the baboon. J. Exp. Med. 1996, 184, 165–171. [Google Scholar] [CrossRef]
- Jurd, K.M.; Stephens, C.J.; Black, M.M.; Hunt, B.J. Endothelial cell activation in cutaneous vasculitis. Clin. Exp. Dermatol. 1996, 21, 28–32. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Liao, J.; Chen, B.; Zhu, Z.; Du, C.; Gao, S.; Zhao, G.; Zhao, P.; Wang, Y.; Wang, A.; Schwartz, Z.; et al. Long noncoding RNA (lncRNA) H19: An essential developmental regulator with expanding roles in cancer, stem cell differentiation, and metabolic diseases. Genes Dis. 2023, 10, 1351–1366. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-alpha induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Chan, F.K.; Chun, H.J.; Zheng, L.; Siegel, R.M.; Bui, K.L.; Lenardo, M.J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 2000, 288, 2351–2354. [Google Scholar] [CrossRef]
- Durand, M.J.; Gutterman, D.D. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation 2013, 20, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, G.; Salas, E.; Murat, J.; Miszta-Lane, H.; Radomski, M.W. Release of gelatinase A during platelet activation mediates aggregation. Nature 1997, 386, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Fredrickson, B.J.; Dong, J.F.; McIntire, L.V.; Lopez, J.A. Shear-dependent rolling on von Willebrand factor of mammalian cells expressing the platelet glycoprotein Ib-IX-V complex. Blood 1998, 92, 3684–3693. [Google Scholar] [CrossRef]
- Nurden, A.; Nurden, P. Advances in our understanding of the molecular basis of disorders of platelet function. J. Thromb. Haemost 2011, 9 (Suppl. S1), 76–91. [Google Scholar] [CrossRef]
- Nolte, M.A.; Margadant, C. Activation and suppression of hematopoietic integrins in hemostasis and immunity. Blood 2020, 135, 7–16. [Google Scholar] [CrossRef]
- Jiang, P.; Lan, Y.; Luo, J.; Ren, Y.L.; Liu, D.G.; Pang, J.X.; Liu, J.; Li, J.; Wang, C.; Cai, J.P. Rapamycin promoted thrombosis and platelet adhesion to endothelial cells by inducing membrane remodeling. BMC Cell Biol. 2014, 15, 7. [Google Scholar] [CrossRef]
- Van Gils, J.M.; Zwaginga, J.J.; Hordijk, P.L. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J. Leukoc. Biol. 2009, 85, 195–204. [Google Scholar] [CrossRef]
- Huilcaman, R.; Veliz-Olivos, N.; Venturini, W.; Olate-Briones, A.; Treuer, A.V.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Endothelial transmigration of platelets depends on soluble factors released by activated endothelial cells and monocytes. Platelets 2021, 32, 1113–1119. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittala, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.M.; Alessandro, R.; Corrado, C. Effects of Flavonoids on Cancer, Cardiovascular and Neurodegenerative Diseases: Role of NF-kappaB Signaling Pathway. Int. J. Mol. Sci 2023, 24, 9236. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Barreca, M.M.; Raimondo, S.; Diana, P.; Pepe, G.; Basilicata, M.G.; Conigliaro, A.; Alessandro, R. Nobiletin and xanthohumol counteract the TNFalpha-mediated activation of endothelial cells through the inhibition of the NF-kappaB signaling pathway. Cell Biol. Int. 2023, 47, 634–647. [Google Scholar] [CrossRef]
- Rowe, R.W. The structure of rat tail tendon fascicles. Connect. Tissue Res. 1985, 14, 21–30. [Google Scholar] [CrossRef]
- Arnould, T.; Janssens, D.; Michiels, C.; Remacle, J. Effect of aescine on hypoxia-induced activation of human endothelial cells. Eur. J. Pharmacol. 1996, 315, 227–233. [Google Scholar] [CrossRef]
- Montopoli, M.; Froldi, G.; Comelli, M.C.; Prosdocimi, M.; Caparrotta, L. Aescin protection of human vascular endothelial cells exposed to cobalt chloride mimicked hypoxia and inflammatory stimuli. Planta Med. 2007, 73, 285–288. [Google Scholar] [CrossRef]
- Gallelli, L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Des. Dev. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef]
- Varilla, C.; Marcone, M.; Paiva, L.; Baptista, J. Bromelain, a Group of Pineapple Proteolytic Complex Enzymes (Ananas comosus) and Their Possible Therapeutic and Clinical Effects. A Summary. Foods 2021, 10, 2249. [Google Scholar] [CrossRef]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Z.; Wang, P.; Wang, M. Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: A review. Int. J. Biol. Macromol. 2020, 162, 1897–1905. [Google Scholar] [CrossRef]
- Li, Y.; Sheng, Y.; Liu, J.; Xu, G.; Yu, W.; Cui, Q.; Lu, X.; Du, P.; An, L. Hair-growth promoting effect and anti-inflammatory mechanism of Ginkgo biloba polysaccharides. Carbohydr. Polym. 2022, 278, 118811. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Mostafaie, A.; Mansouri, K.; Bidmeshkipour, A.; Motlagh, H.R.; Parvaneh, S. In vitro and ex vivo antiangiogenic activity of Salvia officinalis. Phytother. Res. 2010, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, G. Danshen (Salvia miltiorrhiza Bunge): A Prospective Healing Sage for Cardiovascular Diseases. Curr. Pharm. Des. 2017, 23, 5125–5135. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Feng, Q.; Bai, T.; Wu, L.; Liu, Y.; Zheng, X.; Jia, J.; Pei, J.; Wu, S.; et al. Progress of thrombus formation and research on the structure-activity relationship for antithrombotic drugs. Eur. J. Med. Chem. 2022, 228, 114035. [Google Scholar] [CrossRef]
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef]
- Lichota, A.; Szewczyk, E.M.; Gwozdzinski, K. Factors Affecting the Formation and Treatment of Thrombosis by Natural and Synthetic Compounds. Int. J. Mol. Sci. 2020, 21, 7975. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part Fibre Toxicol. 2018, 15, 1. [Google Scholar] [CrossRef]
- Polla, M.O.; Tottie, L.; Norden, C.; Linschoten, M.; Musil, D.; Trumpp-Kallmeyer, S.; Aukrust, I.R.; Ringom, R.; Holm, K.H.; Neset, S.M.; et al. Design and synthesis of potent, orally active, inhibitors of carboxypeptidase U (TAFIa). Bioorg. Med. Chem. 2004, 12, 1151–1175. [Google Scholar] [CrossRef] [PubMed]
- Vazhappilly, C.G.; Ansari, S.A.; Al-Jaleeli, R.; Al-Azawi, A.M.; Ramadan, W.S.; Menon, V.; Hodeify, R.; Siddiqui, S.S.; Merheb, M.; Matar, R.; et al. Role of flavonoids in thrombotic, cardiovascular, and inflammatory diseases. Inflammopharmacology 2019, 27, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Phang, M.; Lazarus, S.; Wood, L.G.; Garg, M. Diet and thrombosis risk: Nutrients for prevention of thrombotic disease. Semin. Thromb. Hemost. 2011, 37, 199–208. [Google Scholar] [CrossRef]
- Podolnikova, N.P.; Yermolenko, I.S.; Fuhrmann, A.; Lishko, V.K.; Magonov, S.; Bowen, B.; Enderlein, J.; Podolnikov, A.V.; Ros, R.; Ugarova, T.P. Control of integrin alphaIIb beta3 outside-in signaling and platelet adhesion by sensing the physical properties of fibrin(ogen) substrates. Biochemistry 2010, 49, 68–77. [Google Scholar] [CrossRef]
- Shattil, S.J.; Newman, P.J. Integrins: Dynamic scaffolds for adhesion and signaling in platelets. Blood 2004, 104, 1606–1615. [Google Scholar] [CrossRef]
- Law, D.A.; DeGuzman, F.R.; Heiser, P.; Ministri-Madrid, K.; Killeen, N.; Phillips, D.R. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature 1999, 401, 808–811. [Google Scholar] [CrossRef]
- Schaller, M.D. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010, 123, 1007–1013. [Google Scholar] [CrossRef]
- Rubenstein, D.A.; Yin, W. Platelet-Activation Mechanisms and Vascular Remodeling. Compr. Physiol. 2018, 8, 1117–1156. [Google Scholar] [CrossRef]
- Avraham, S.; London, R.; Fu, Y.; Ota, S.; Hiregowdara, D.; Li, J.; Jiang, S.; Pasztor, L.M.; White, R.A.; Groopman, J.E.; et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J. Biol. Chem. 1995, 270, 27742–27751. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin alphaIIbbeta3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.F.; Torti, M.; Canobbio, I. Focal Adhesion Kinases in Platelet Function and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ho, P.C.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Lee, H.S.; Lee, C.H.; Choi, C.I. Pharmacological Activity of Garcinia indica (Kokum): An Updated Review. Pharmaceuticals 2021, 14, 1338. [Google Scholar] [CrossRef]
- Cao, H.; Al Mamun Bhuyan, A.; Umbach, A.T.; Ma, K.; Borst, O.; Gawaz, M.; Zhang, S.; Nurnberg, B.; Lang, F. Garcinol A Novel Inhibitor of Platelet Activation and Apoptosis. Toxins 2019, 11, 382. [Google Scholar] [CrossRef]
- Hsia, C.W.; Huang, W.C.; Jayakumar, T.; Hsia, C.H.; Hou, S.M.; Chang, C.C.; Yen, T.L.; Sheu, J.R. Garcinol acts as a novel integrin alpha(IIb)beta(3) inhibitor in human platelets. Life Sci. 2023, 326, 121791. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Chen, B.; Zhao, Y.; Gong, H.; You, Y.; Qi, R. Ginkgolide B inhibits platelet and monocyte adhesion in TNFalpha-treated HUVECs under laminar shear stress. BMC Complement. Altern. Med. 2018, 18, 220. [Google Scholar] [CrossRef]
- Eligini, S.; Barbieri, S.S.; Arenaz, I.; Tremoli, E.; Colli, S. Paracrine up-regulation of monocyte cyclooxygenase-2 by platelets: Role of transforming growth factor-beta1. Cardiovasc. Res. 2007, 74, 270–278. [Google Scholar] [CrossRef] [PubMed]



| Primers | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| p-selectin | TCCGCTGCATTGACTCTGGACA | CTGAACGCTCTCAAGGATGGAG |
| integrins β3 | CATGGATTCCAGCAATGTCCTCC | TTGAGGCAGGTGGATTGAAGG |
| integrins αV | AGGAGAAGGTGCCTACGAACT | GCACAGGAAAGTCTTGCTAAGGC |
| GP1b | ACCATCCTGGTGTCTGCCACAA | ACGGAGCTTTGGTGGCTGATCA |
| β-actin | CAAGAGATGGCCACGGCTGCT | TCCTTCTGCATCCTGTCGGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreca, M.M.; Raimondo, S.; Conigliaro, A.; Siragusa, S.; Napolitano, M.; Alessandro, R.; Corrado, C. The Combination of Natural Compounds Escin–Bromelain–Ginkgo Biloba–Sage Miltiorrhiza (EBGS) Reduces Platelet Adhesion to TNFα-Activated Vascular Endothelium through FAK Signaling. Int. J. Mol. Sci. 2024, 25, 9252. https://doi.org/10.3390/ijms25179252
Barreca MM, Raimondo S, Conigliaro A, Siragusa S, Napolitano M, Alessandro R, Corrado C. The Combination of Natural Compounds Escin–Bromelain–Ginkgo Biloba–Sage Miltiorrhiza (EBGS) Reduces Platelet Adhesion to TNFα-Activated Vascular Endothelium through FAK Signaling. International Journal of Molecular Sciences. 2024; 25(17):9252. https://doi.org/10.3390/ijms25179252
Chicago/Turabian StyleBarreca, Maria Magdalena, Stefania Raimondo, Alice Conigliaro, Sergio Siragusa, Mariasanta Napolitano, Riccardo Alessandro, and Chiara Corrado. 2024. "The Combination of Natural Compounds Escin–Bromelain–Ginkgo Biloba–Sage Miltiorrhiza (EBGS) Reduces Platelet Adhesion to TNFα-Activated Vascular Endothelium through FAK Signaling" International Journal of Molecular Sciences 25, no. 17: 9252. https://doi.org/10.3390/ijms25179252
APA StyleBarreca, M. M., Raimondo, S., Conigliaro, A., Siragusa, S., Napolitano, M., Alessandro, R., & Corrado, C. (2024). The Combination of Natural Compounds Escin–Bromelain–Ginkgo Biloba–Sage Miltiorrhiza (EBGS) Reduces Platelet Adhesion to TNFα-Activated Vascular Endothelium through FAK Signaling. International Journal of Molecular Sciences, 25(17), 9252. https://doi.org/10.3390/ijms25179252








