Evaluation of Clobetasol and Tacrolimus Treatments in an Imiquimod-Induced Psoriasis Rat Model
Abstract
:1. Introduction
2. Results
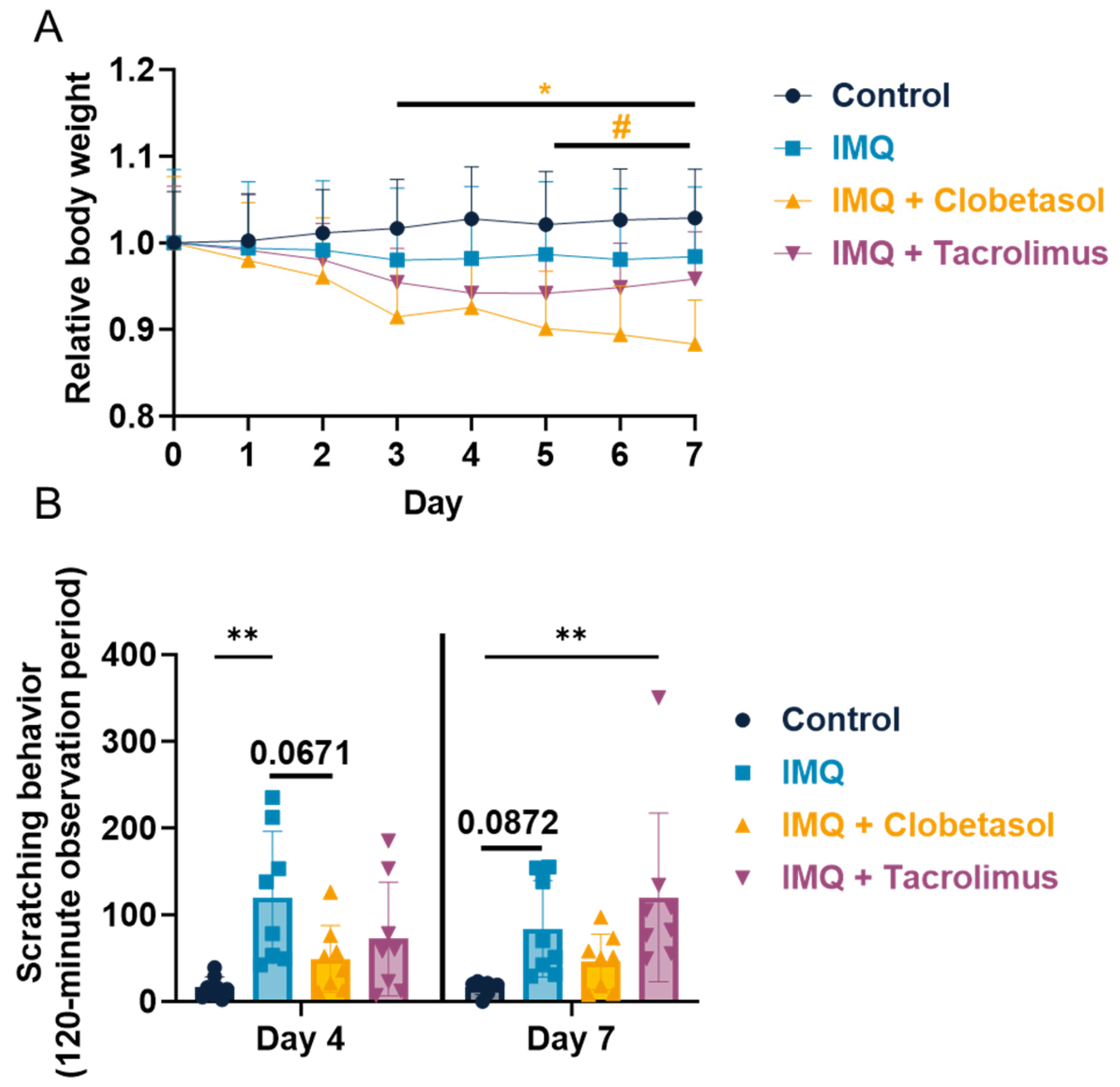
2.1. Evaluation of Imiquimod Effect on Rat Body Weight and Scratching Behavior upon Anti-Psoriatic Drug, Clobetasol, and Tacrolimus
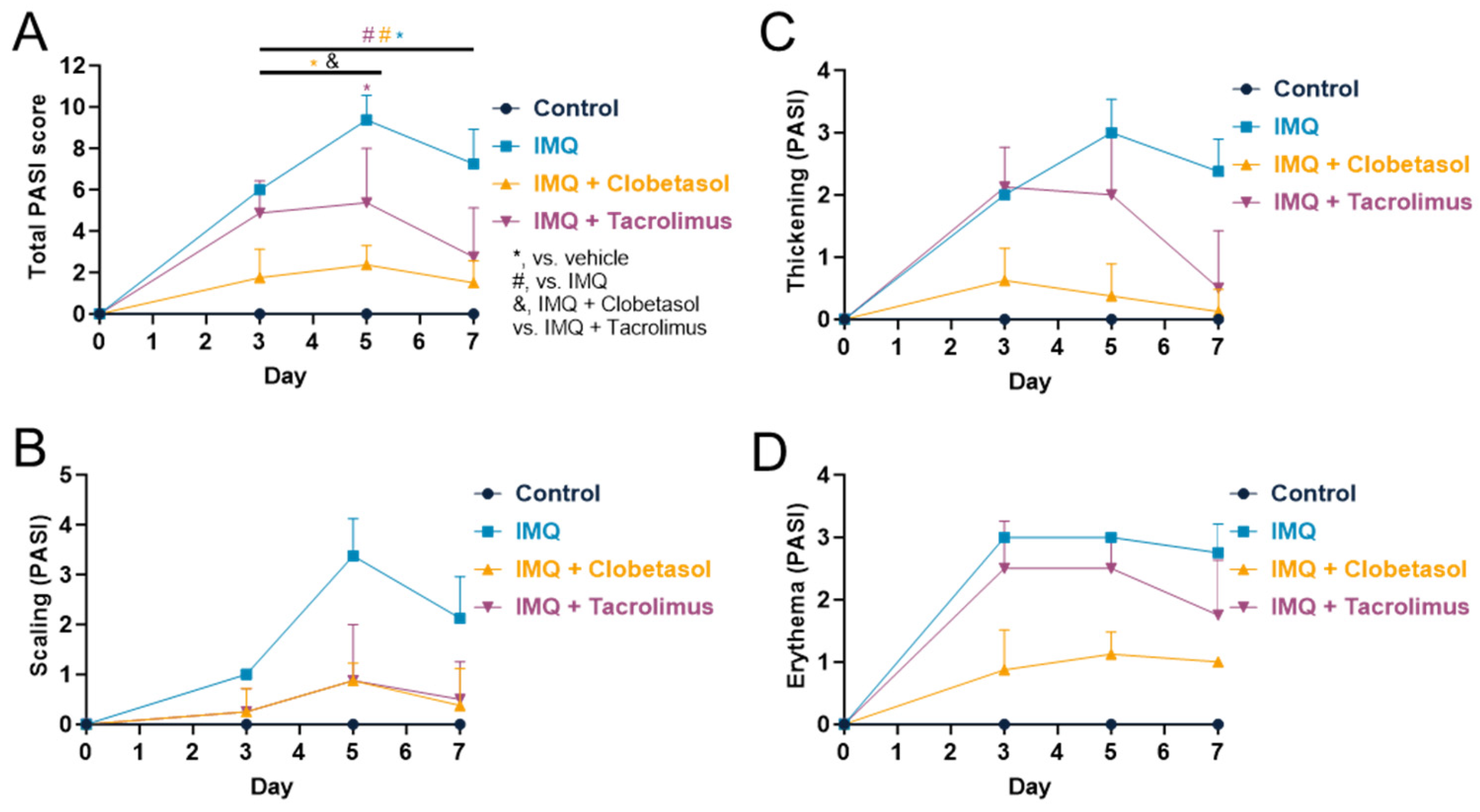
2.2. Evaluation of Psoriasis-like Inflammation Severity Index (PASI)
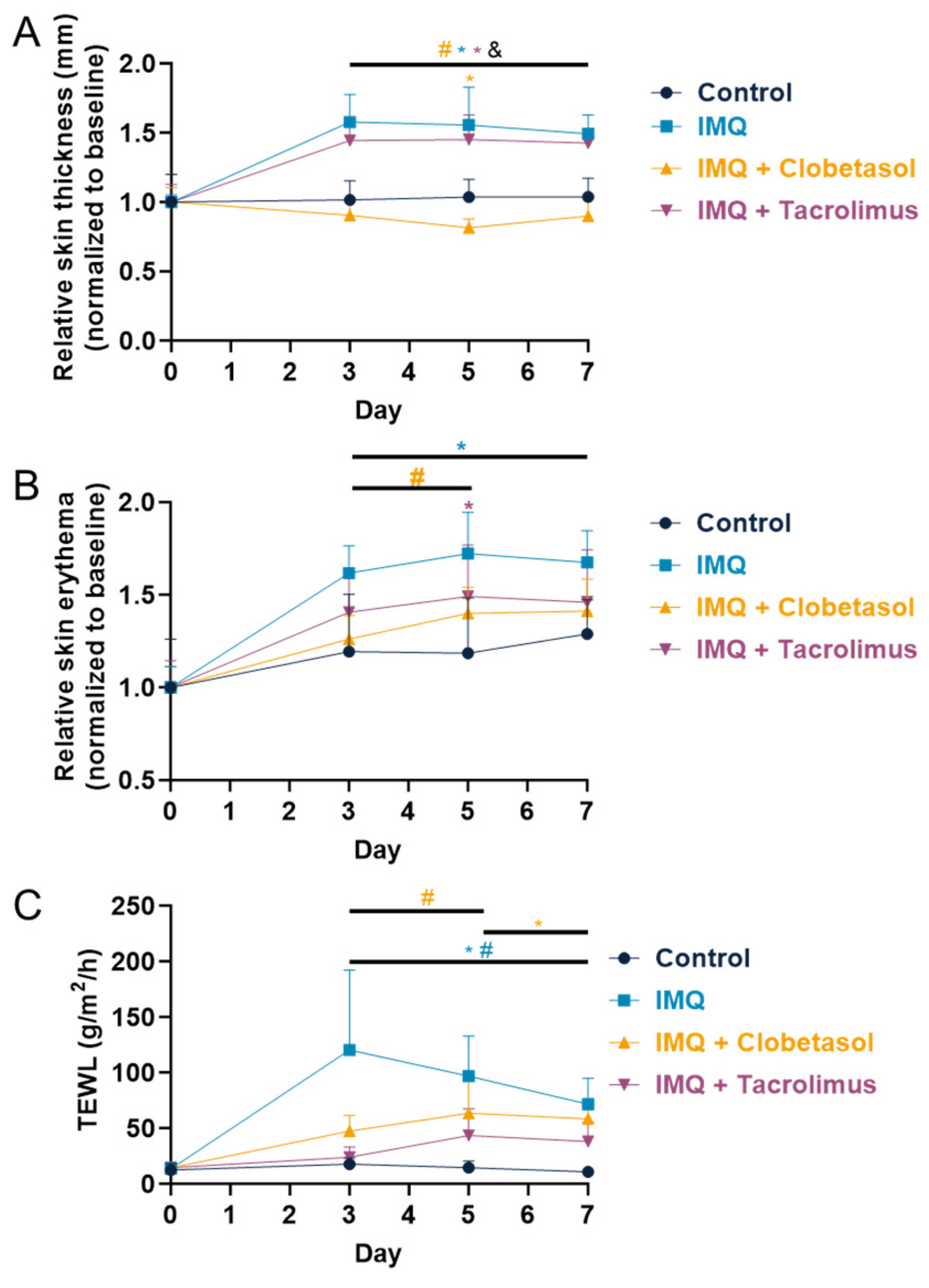
2.3. Evaluation of Skin Thickness, Erythema, and Transepidermal Water Loss (TEWL)
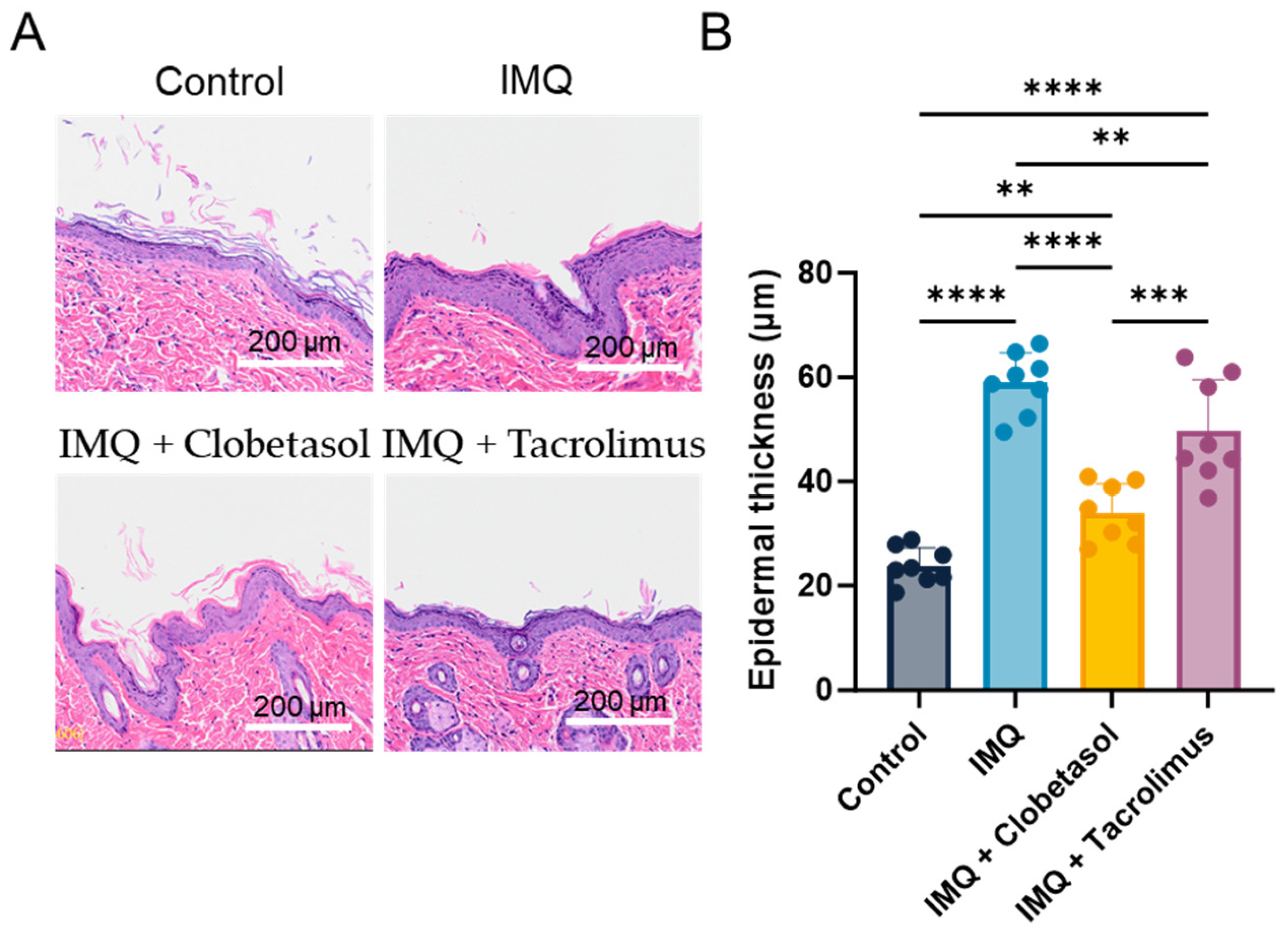
2.4. Evaluation of Epidermal Thickness (Histology)
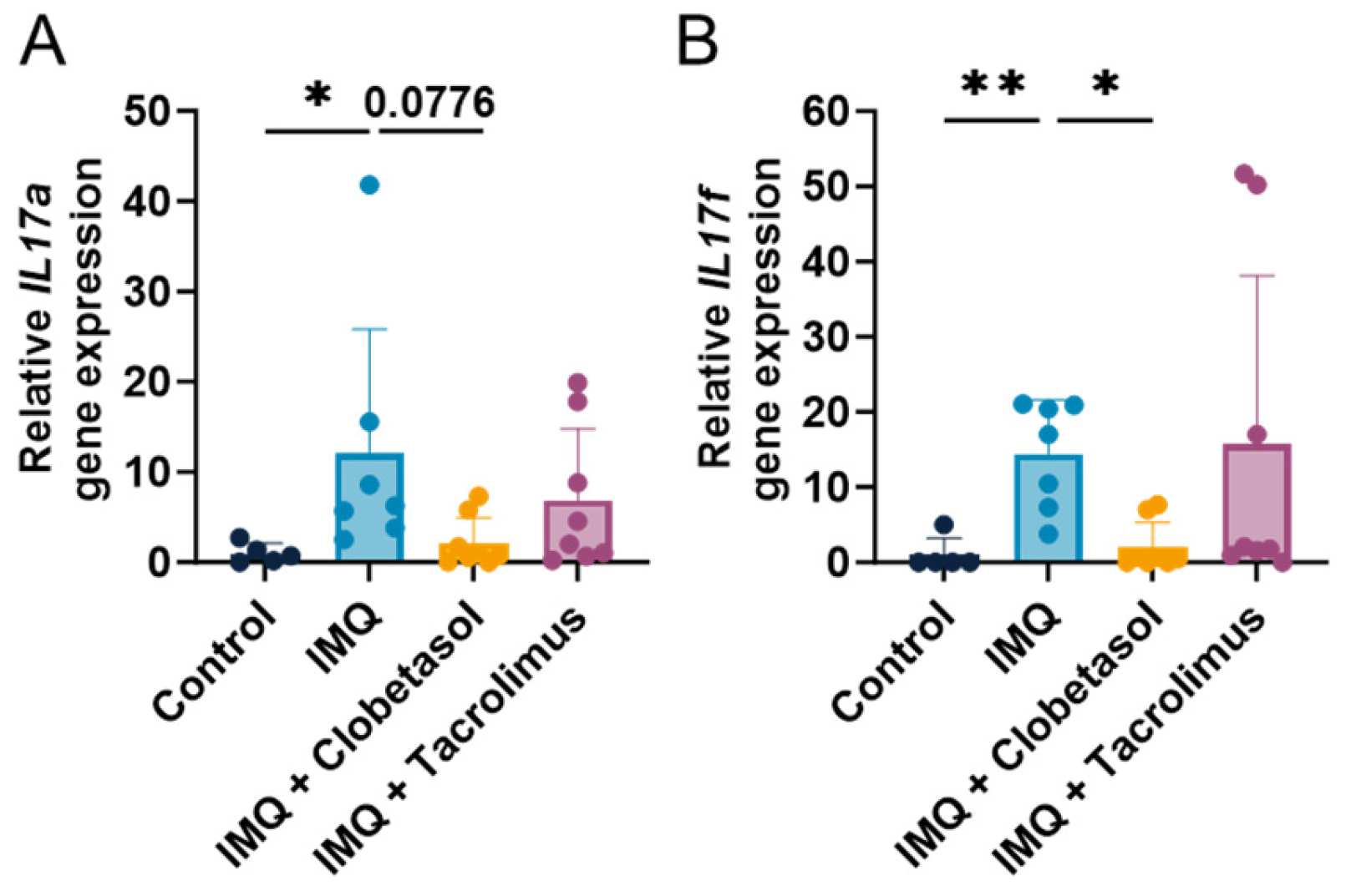
2.5. Cytokine Profile
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Ethical Consideration and Limit Points
4.3. Psoriasis Induction
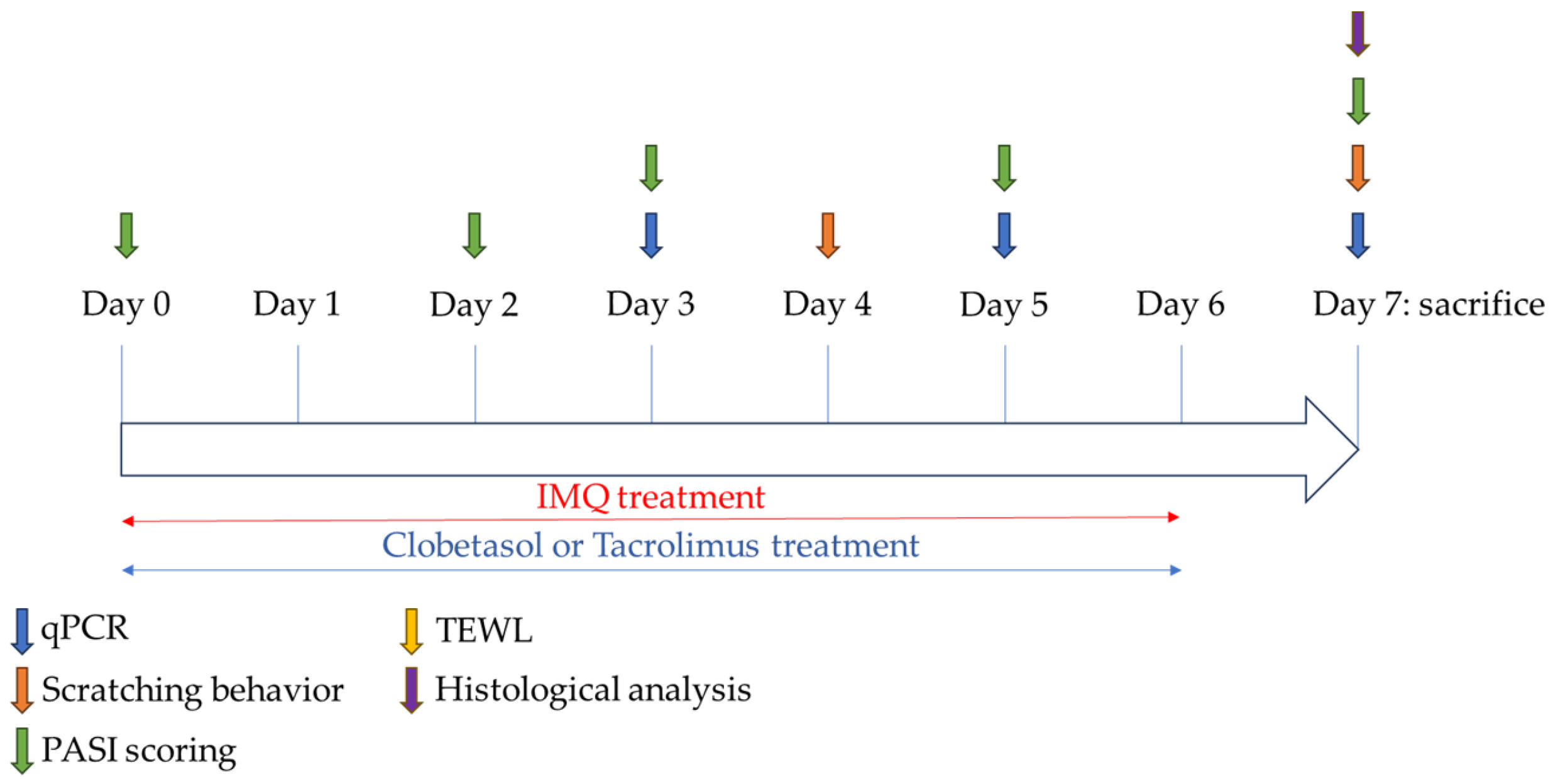
4.4. Study Design
- -
- Group 1 (Control group): no IMQ + no treatment
- -
- Group 2 (IMQ-treated group): IMQ + no treatment
- -
- Group 3 (Clobetasol group): IMQ + Clobetasol propionate
- -
- Group 4 (Tacrolimus group): IMQ + Tacrolimus monohydrate
4.5. Evaluation of Psoriasis-like Inflammation Severity Index (PASI)
4.6. Evaluation of Skin Erythema and Transepidermal Water Loss (TEWL)
4.7. Evaluation of Scratching Behavior
4.8. Evaluation of Epidermal Thickness by Histology
4.9. Quantification of Inflammatory Cytokine in Skin Samples by qPCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, A.W.; Mehta, M.D.; Schupp, C.W.; Gondo, G.C.; Bell, S.J.; Griffiths, C.E.M. Psoriasis Prevalence in Adults in the United States. JAMA Dermatol. 2021, 157, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, Regional, and Worldwide Epidemiology of Psoriasis: Systematic Analysis and Modelling Study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.P.; Madke, B. The Current Advancement in Psoriasis. Cureus 2023, 15, e47006. [Google Scholar] [CrossRef] [PubMed]
- Vissers, W.H.P.M.; van Vlijmen, I.; van Erp, P.E.J.; de Jong, E.M.G.J.; van de Kerkhof, P.C.M. Topical Treatment of Mild to Moderate Plaque Psoriasis with 0.3% Tacrolimus Gel and 0.5% Tacrolimus Cream: The Effect on SUM Score, Epidermal Proliferation, Keratinization, T-Cell Subsets and HLA-DR Expression. Br. J. Dermatol. 2008, 158, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Manzke, V.; Erpenbeck, L. Animal Models of Psoriasis—Highlights and Drawbacks. J. Allergy Clin. Immunol. 2021, 147, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, R.S.; Gudjonsson, J.E.; Ward, N.L. Mouse Models of Psoriasis—A Comprehensive Review. J. Investig. Dermatol. 2022, 142, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Shahine, Y.; El-Aal, S.A.A.; Reda, A.M.; Sheta, E.; Atia, N.M.; Abdallah, O.Y.; Ibrahim, S.S.A. Diosmin Nanocrystal Gel Alleviates Imiquimod-Induced Psoriasis in Rats via Modulating TLR7,8/NF-κB/Micro RNA-31, AKT/mTOR/P70S6K Milieu, and Tregs/Th17 Balance. Inflammopharmacology 2023, 31, 1341–1359. [Google Scholar] [CrossRef]
- Parmar, K.M.; Jagtap, C.S.; Katare, N.T.; Dhobi, M.; Prasad, S.K. Development of a Psoriatic-like Skin Inflammation Rat Model Using Imiquimod as an Inducing Agent. Indian J. Pharmacol. 2021, 53, 125–131. [Google Scholar] [CrossRef]
- Lariosa-Willingham, K.; Leonoudakis, D.; Simon, F.; Walker, K.; Guillaume, P.; Warren, L.; Stratton, J. Imiquimod-Induced Pruritus in Female Wild-Type and Knockin Wistar Rats: Underscoring Behavioral Scratching in a Rat Model for Antipruritic Treatments. BMC Res. Notes 2023, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, D.M.; Wan Po, A.L.; Williams, H.C.; Griffiths, C.E. Clinical Measures of Disease Severity and Outcome in Psoriasis: A Critical Appraisal of Their Quality. Br. J. Dermatol. 1999, 141, 185–191. [Google Scholar] [CrossRef]
- Gourraud, P.-A.; Le Gall, C.; Puzenat, E.; Aubin, F.; Ortonne, J.-P.; Paul, C.F. Why Statistics Matter: Limited Inter-Rater Agreement Prevents Using the Psoriasis Area and Severity Index as a Unique Determinant of Therapeutic Decision in Psoriasis. J. Investig. Dermatol. 2012, 132, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The Role of IL-23 and the IL-23/TH17 Immune Axis in the Pathogenesis and Treatment of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the Pathogenesis of Psoriasis: From Keratinocyte Perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef]
- Smajlović, A.; Haverić, A.; Alić, A.; Hadžić, M.; Smajlović, A.; Mujezinović, I.; Lojo-Kadrić, N.; Ramić, J.; Elez-Burnjaković, N.; Haverić, S.; et al. Molecular and Histopathological Profiling of Imiquimod Induced Dermatosis in Swiss Wistar Rats: Contribution to the Rat Model for Novel Anti-Psoriasis Treatments. Mol. Biol. Rep. 2021, 48, 4295–4303. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, Q.; Xu, H.; Dai, X.; Yu, Y.; Cui, L.; Chen, Y.; Gu, J.; Zhang, X.; Guo, C.; et al. IL-17A Promotes Psoriasis-Associated Keratinocyte Proliferation through ACT1-Dependent Activation of YAP-AREG Axis. J. Investig. Dermatol. 2022, 142, 2343–2352. [Google Scholar] [CrossRef]
- Palamara, F.; Meindl, S.; Holcmann, M.; Lührs, P.; Stingl, G.; Sibilia, M. Identification and Characterization of pDC-like Cells in Normal Mouse Skin and Melanomas Treated with Imiquimod. J. Immunol. 2004, 173, 3051–3061. [Google Scholar] [CrossRef]
- Kono, T.; Kondo, S.; Pastore, S.; Shivji, G.M.; Tomai, M.A.; McKenzie, R.C.; Sauder, D.N. Effects of a Novel Topical Immunomodulator, Imiquimod, on Keratinocyte Cytokine Gene Expression. Lymphokine Cytokine Res. 1994, 13, 71–76. [Google Scholar]
- Fujisawa, H.; Shivji, G.M.; Kondo, S.; Wang, B.; Tomai, M.A.; Miller, R.L.; Sauder, D.N. Effect of a Novel Topical Immunomodulator, S-28463, on Keratinocyte Cytokine Gene Expression and Production. J. Interferon Cytokine Res. 1996, 16, 555–559. [Google Scholar] [CrossRef]
- Satake, T.; Suetsugu, A.; Nakamura, M.; Hasegawa, K.; Kunisada, T.; Shimizu, M.; Saji, S.; Moriwaki, H.; Hoffman, R.M. Differential Organ-Targeting and Cellular Characteristics of Metastatic Human Pancreatic Cancer Cell Lines in Mouse Models. Anticancer Res. 2018, 38, 1927–1935. [Google Scholar] [CrossRef]
- Imbertson, L.M.; Couture, A.M.; Gibson, S.J.; Smith, R.M.A.; Miller, R.L.; Reiter, M.J.; Wagner, T.L.; Tomai, M.A.; Beaurline, J.M. Cytokine Induction in Hairless Mouse and Rat Skin After Topical Application of the Immune Response Modifiers Imiquimod and S-28463. J. Investig. Dermatol. 1998, 110, 734–739. [Google Scholar] [CrossRef]
- Van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis1. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, Z.; Hou, S.; Liu, X. Influence of Different Types of Contact Hypersensitivity on Imiquimod-Induced Psoriasis-like Inflammation in Mice. Mol. Med. Rep. 2016, 14, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin Inhibits Imiquimod-Induced Psoriasis-like Inflammation by Inhibiting IL-1beta and IL-6 Production in Mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef] [PubMed]
- National Clinical Guideline Centre (UK). Psoriasis: Assessment and Management of Psoriasis; National Institute for Health and Clinical Excellence: Guidance; Royal College of Physicians (UK): London, UK, 2012. [Google Scholar]
- Jabeen, M.; Boisgard, A.-S.; Danoy, A.; El Kholti, N.; Salvi, J.-P.; Boulieu, R.; Fromy, B.; Verrier, B.; Lamrayah, M. Advanced Characterization of Imiquimod-Induced Psoriasis-like Mouse Model. Pharmaceutics 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dou, W.; Zhao, Y.; Hu, J. A Comparison of the Effects of Topical Treatment of Calcipotriol, Camptothecin, Clobetasol and Tazarotene on an Imiquimod-Induced Psoriasis-like Mouse Model. Immunopharmacol. Immunotoxicol. 2014, 36, 17–24. [Google Scholar] [CrossRef]
- Rather, I.A.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K.; Bhat, E.A.; Lim, J.; Paek, W.K.; Park, Y.-H. Probiotic Lactobacillus Sakei proBio-65 Extract Ameliorates the Severity of Imiquimod Induced Psoriasis-like Skin Inflammation in a Mouse Model. Front. Microbiol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Khafaji, A.W.M.; Al-Zubaidy, A.A.K.; Farhood, I.G.; Salman, H.R. Ameliorative Effects of Topical Ramelteon on Imiquimod-Induced Psoriasiform Inflammation in Mice. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 6231–6248. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Topical Corticosteroid Therapy for Psoriasis—A Review of Clobetasol Propionate 0.025% Cream and the Clinical Relevance of Penetration Modification. J. Clin. Aesthet. Dermatol. 2020, 13, 22–29. [Google Scholar] [PubMed]
- Pels, R.; Sterry, W.; Lademann, J. Clobetasol Propionate—Where, When, Why? Drugs Today 2008, 44, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Sofen, H.; Hudson, C.P.; Cook-Bolden, F.E.; Preston, N.; Colón, L.E.; Colón, L.E.; Caveney, S.W.; Gottschalk, R.W. Clobetasol Propionate 0.05% Spray for the Management of Moderate-to-Severe Plaque Psoriasis of the Scalp: Results from a Randomized Controlled Trial. J. Drugs Dermatol. 2011, 10, 885–892. [Google Scholar] [PubMed]
- Salwa, F.; Badanthadka, M.; D’Souza, L. Differential Psoriatic Effect of Imiquimod on Balb/c and Swiss Mice. J. Health Allied Sci. NU 2021, 11, 170–177. [Google Scholar] [CrossRef]
- Filippopoulou, F.; Habeos, G.I.; Rinotas, V.; Sophocleous, A.; Sykiotis, G.P.; Douni, E.; Chartoumpekis, D.V. Dexamethasone Administration in Mice Leads to Less Body Weight Gain over Time, Lower Serum Glucose, and Higher Insulin Levels Independently of NRF2. Antioxidants 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Zhang, L.; Fu, J.; Di, T.; Li, N.; Meng, Y.; Guo, J.; Zhao, J. Stress Aggravates and Prolongs Imiquimod-Induced Psoriasis-like Epidermal Hyperplasis and IL-1β/IL-23p40 Production. J. Leukoc. Biol. 2020, 108, 267–281. [Google Scholar] [CrossRef]
- Takanami, K.; Morishita, M.; Sakamoto, T.; Sakamoto, H. Chronic Corticosterone Exposure Evokes Itch Hypersensitivity and Sexual Dysfunction in Male Rats: Relationship between the Two Distinct Gastrin-Releasing Peptide Systems in the Spinal Cord. Gen. Comp. Endocrinol. 2023, 339, 114289. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Choo, Y.-K.; Jeon, S.H.; Jang, W.-G.; Lee, J.-H.; Park, J.-H.; Kim, C.-H. Therapeutic Anti-Psoriatic Effects of Myeloid-Derived Suppressor Cells in Combination with Systemic Tacrolimus (FK-506) in an Imiquimod-Induced Mouse Model of Psoriasis. Int. Immunopharmacol. 2020, 86, 106553. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.W. Topical Calcineurin Inhibitors for Atopic Dermatitis: Review and Treatment Recommendations. Paediatr. Drugs 2013, 15, 303–310. [Google Scholar] [CrossRef]
- Malecic, N.; Young, H. Tacrolimus for the Management of Psoriasis: Clinical Utility and Place in Therapy. Psoriasis 2016, 6, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, H.; Panchal, S.; Mehta, T. Formulation Strategies for Drug Delivery of Tacrolimus: An Overview. Int. J. Pharm. Investig. 2012, 2, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, I.M.; Rubins, A.; Jablonska, S.; Dobozy, A.; Ruzicka, T.; Kind, P.; Dubertret, L.; Bos, J.D. Topical Tacrolimus Is Not Effective in Chronic Plaque Psoriasis. A Pilot Study. Arch. Dermatol. 1998, 134, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.-P.; van de Kerkhof, P.C.M.; Prinz, J.C.; Bieber, T.; Lahfa, M.; Rubins, A.; Wozel, G.; Lorette, G.; European Tacrolimus Psoriasis Study Group. 0.3% Tacrolimus Gel and 0.5% Tacrolimus Cream Show Efficacy in Mild to Moderate Plaque Psoriasis: Results of a Randomized, Open-Label, Observer-Blinded Study. Acta Derm. Venereol. 2006, 86, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Boulter, N.; Suarez, F.G.; Schibeci, S.; Sunderland, T.; Tolhurst, O.; Hunter, T.; Hodge, G.; Handelsman, D.; Simanainen, U.; Hendriks, E.; et al. A Simple, Accurate and Universal Method for Quantification of PCR. BMC Biotechnol. 2016, 16, 27. [Google Scholar] [CrossRef] [PubMed]
| Target | Sequence (5′->3′) | Product Length |
|---|---|---|
| IL-17a | F: CCCTTAGCTCAAAAGCGAGC R: TCATGTGGTGGTCCAACTTCC P: CACAGTGCCGCCACCAGCGC | 174 |
| IL-17f | F: TCAATCAAAACCAGGGCATT R: TGATACAGCCTGAGTGTCTG P: ACCCGAGACCCCGACCGGTTCCCC | 141 |
| GAPDH | F: TTCAACGGCACAGTCAAG R: CCAGTAGACTCCACGACATA P: CCCATCACCATCTTCCAGGAGC | 134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillaume, P.; Rupp, T.; Froget, G.; Goineau, S. Evaluation of Clobetasol and Tacrolimus Treatments in an Imiquimod-Induced Psoriasis Rat Model. Int. J. Mol. Sci. 2024, 25, 9254. https://doi.org/10.3390/ijms25179254
Guillaume P, Rupp T, Froget G, Goineau S. Evaluation of Clobetasol and Tacrolimus Treatments in an Imiquimod-Induced Psoriasis Rat Model. International Journal of Molecular Sciences. 2024; 25(17):9254. https://doi.org/10.3390/ijms25179254
Chicago/Turabian StyleGuillaume, Philippe, Tristan Rupp, Guillaume Froget, and Sonia Goineau. 2024. "Evaluation of Clobetasol and Tacrolimus Treatments in an Imiquimod-Induced Psoriasis Rat Model" International Journal of Molecular Sciences 25, no. 17: 9254. https://doi.org/10.3390/ijms25179254






