The Influence of Flavonoids with -Br, -Cl Atoms and -NO2, -CH3 Groups on the Growth Kinetics and the Number of Pathogenic and Probiotic Microorganisms
Abstract
:1. Introduction
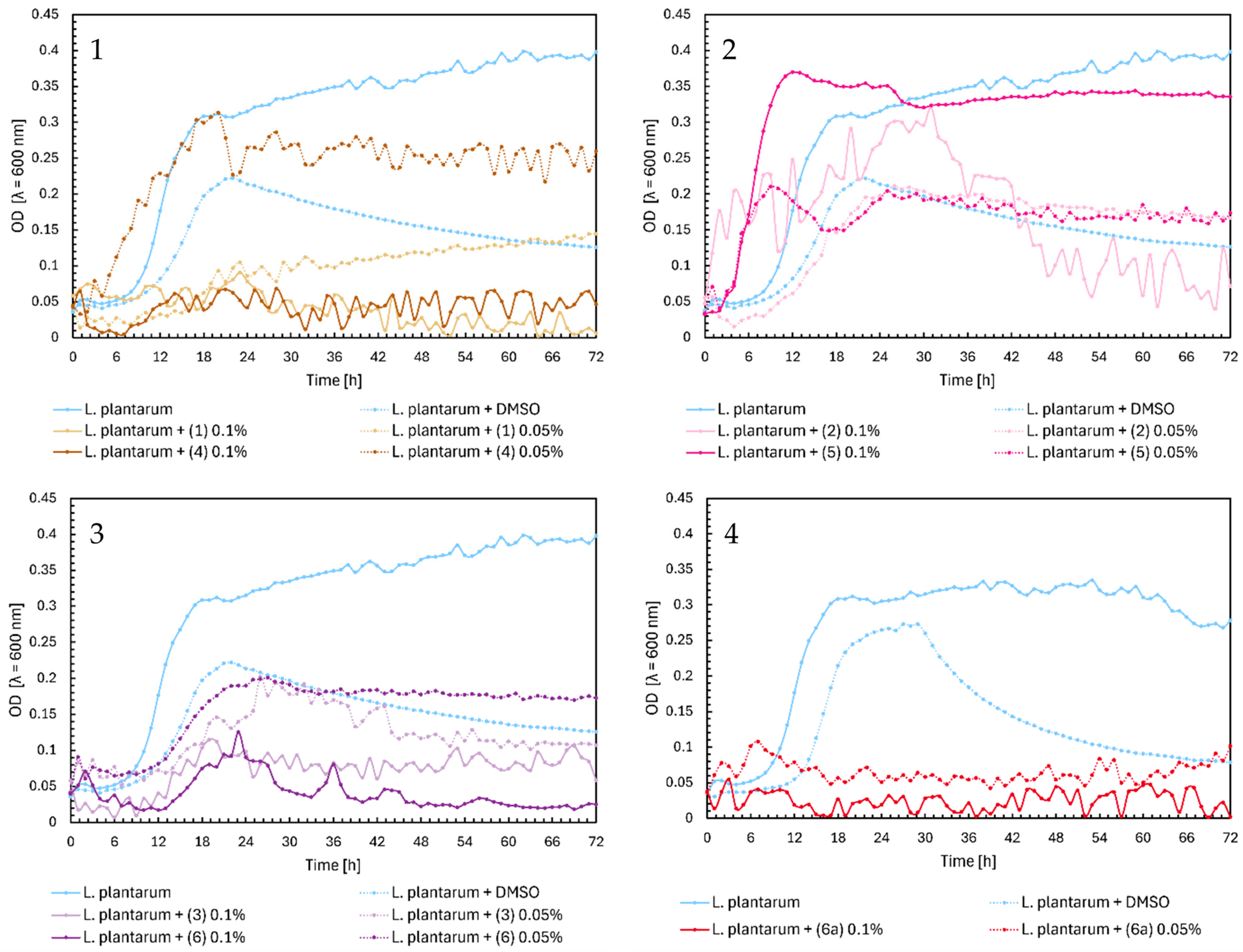
2. Results
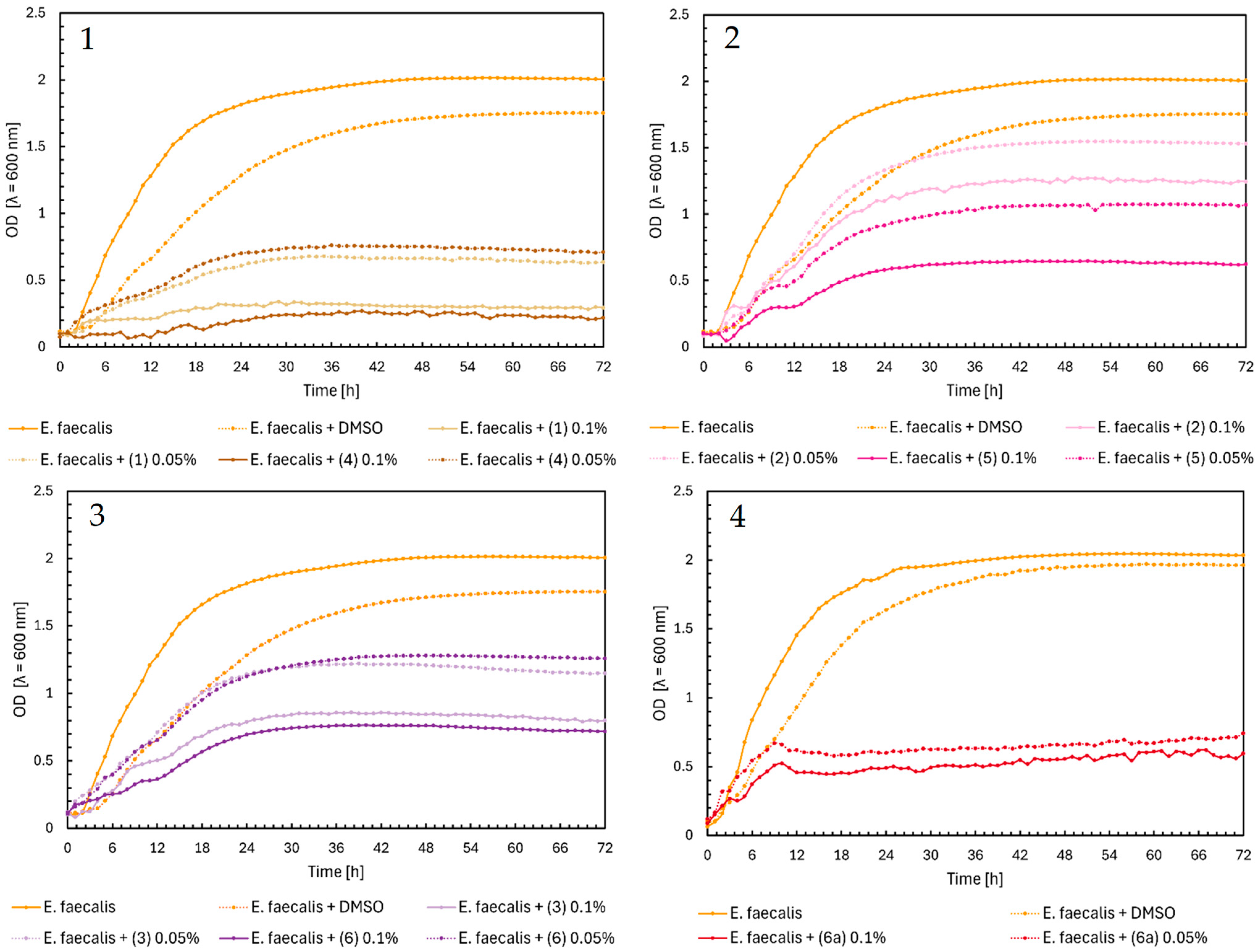
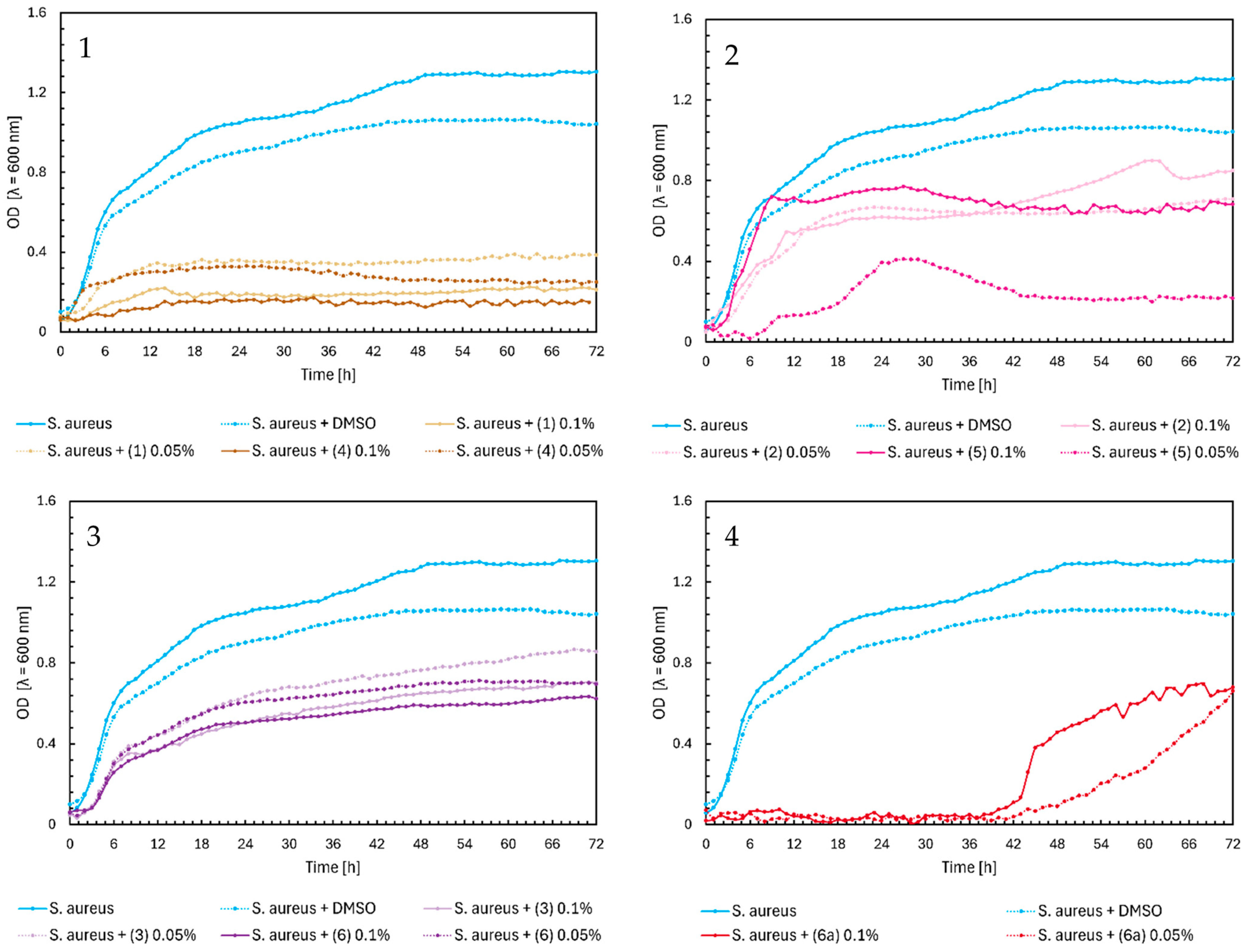
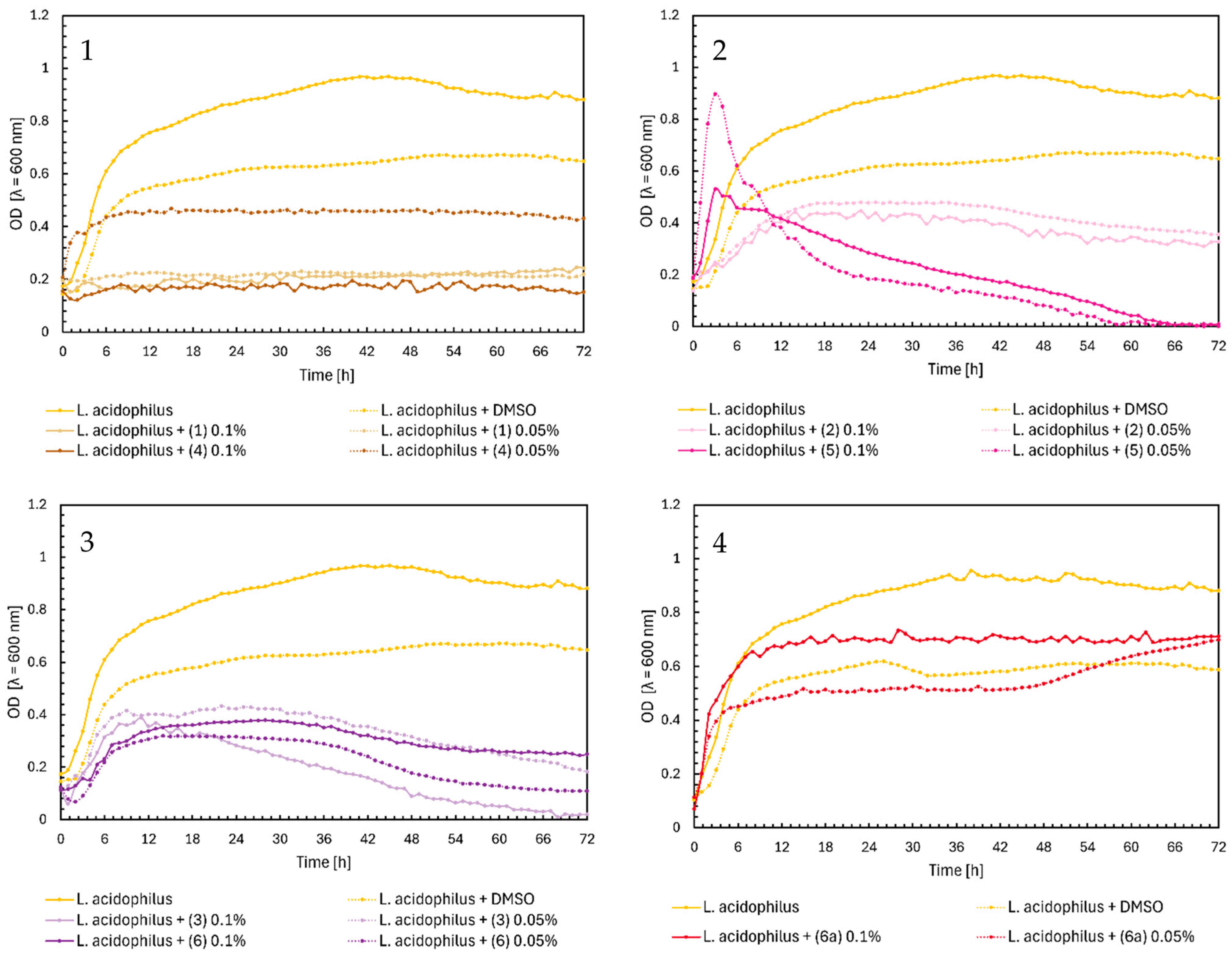
2.1. Microbial Research
2.2. Digestion In Vitro
2.3. Activity Predictions In Silico
3. Discussion
4. Materials and Methods
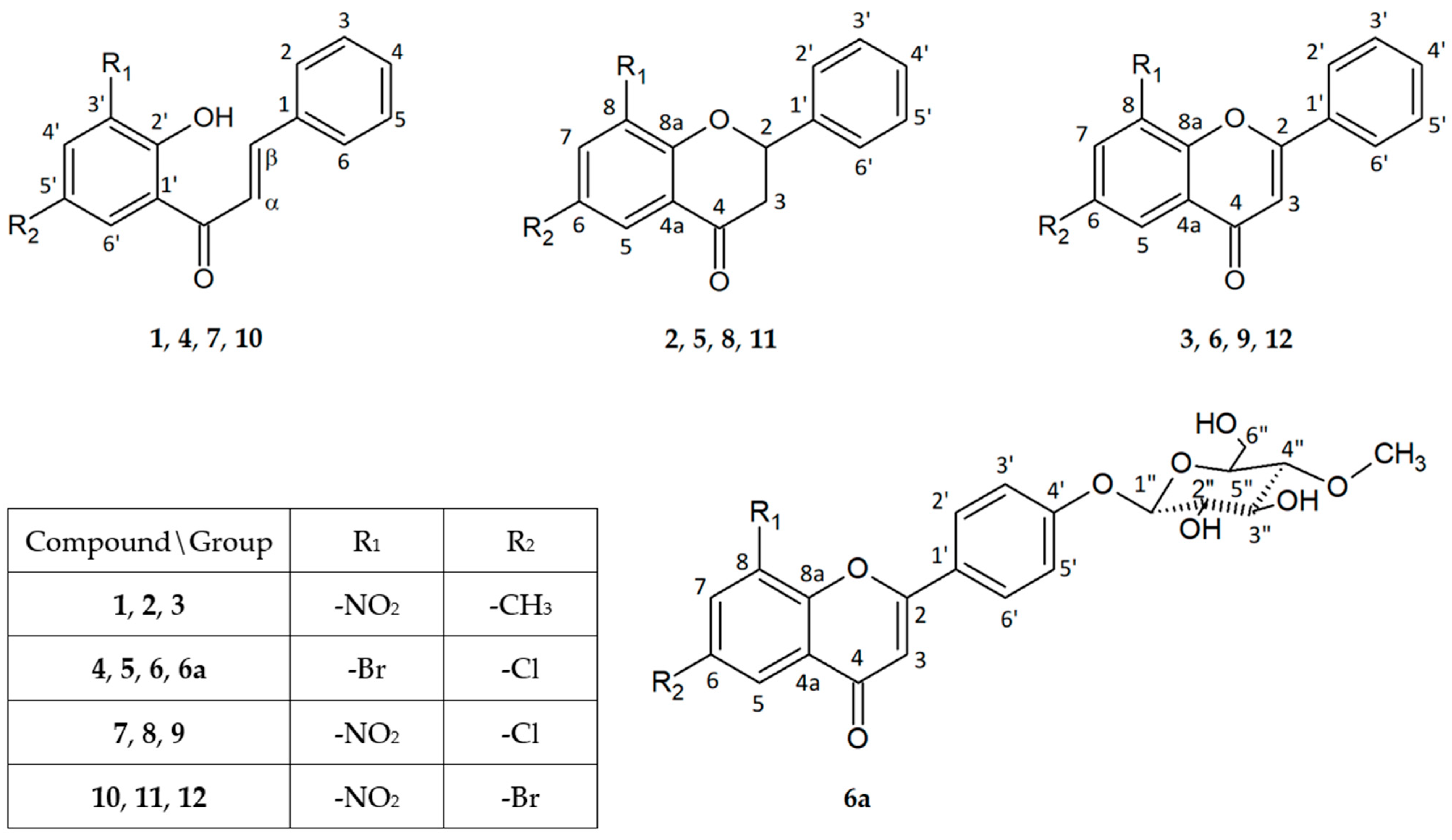
4.1. Flavonoid Compunds
4.2. Microorganisms
4.3. Antimicrobial Analysis
4.4. Digestion In Vitro Model
4.5. Determining the Number of Specific Microorganisms
4.6. In Silico Studies
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure-Antioxidant Activity Relationships of Flavonoids Isolated from Different Plant Species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Justino, G.C.; Rodrigues, M.; Florêncio, M.H.; Mira, L. Structure and Antioxidant Activity of Brominated Flavonols and Flavanones. J. Mass Spectrom. 2009, 44, 1459–1468. [Google Scholar] [CrossRef]
- Proença, C.; Ribeiro, D.; Soares, T.; Tomé, S.M.; Silva, A.M.S.; Lima, J.L.F.C.; Fernandes, E.; Freitas, M. Chlorinated Flavonoids Modulate the Inflammatory Process in Human Blood. Inflammation 2017, 40, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, Z. A Review of Anti-Infective and Anti-Inflammatory Chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Thebti, A.; Meddeb, A.; Ben Salem, I.; Bakary, C.; Ayari, S.; Rezgui, F.; Essafi-Benkhadir, K.; Boudabous, A.; Ouzari, H.I. Antimicrobial Activities and Mode of Flavonoid Actions. Antibiotics 2023, 12, 225. [Google Scholar] [CrossRef]
- Ekalu, A.; Habila, D. Flavonoids: Isolation, Characterization, and Health Benefits. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Jirillo, E.; Santacroce, L. Current View on Major Natural Compounds Endowed with Antibacterial and Antiviral Effects. Antibiotics 2024, 13, 603. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and Gut Health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic Flavonoids with Antimicrobial Activity: A Review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Lewin, G.; MacIuk, A.; Moncomble, A.; Cornard, J.P. Enhancement of the Water Solubility of Flavone Glycosides by Disruption of Molecular Planarity of the Aglycone Moiety. J. Nat. Prod. 2013, 76, 8–12. [Google Scholar] [CrossRef]
- Gonçavales, B.M.F.; Cardoso, D.S.P.; Ferreira, M.U. Overcoming Multidrug Resistance: Flavonoid and Terpenoid Nitrogen-Containing Derivatives as ABC Transporter Modulators. Molecules 2020, 25, 3364. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Jovel, J.; Dieleman, L.A.; Kao, D.; Mason, A.L.; Wine, E. The Human Gut Microbiome in Health and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The Microbiome: Stress, Health and Disease. Mamm. Genome 2014, 25, 49–74. [Google Scholar] [CrossRef]
- Pan, L.; Ye, H.; Pi, X.; Liu, W.; Wang, Z.; Zhang, Y.; Zheng, J. Effects of Several Flavonoids on Human Gut Microbiota and Its Metabolism by in Vitro Simulated Fermentation. Front. Microbiol. 2023, 14, 1092729. [Google Scholar] [CrossRef]
- Klinder, A.; Shen, Q.; Heppel, S.; Lovegrove, J.A.; Rowland, I.; Tuohy, K.M. Impact of Increasing Fruit and Vegetables and Flavonoid Intake on the Human Gut Microbiota. Food Funct. 2016, 7, 1788–1796. [Google Scholar] [CrossRef]
- Perz, M.; Krawczyk-Łebek, A.; Kostrzewa-Susłow, E.; Dymarska, M.; Janeczko, T. Biotransformation of Flavonoids with -NO2, -CH3 Groups and -Br, -Cl Atoms by Entomopathogenic Filamentous Fungi. Int. J. Mol. Sci. 2023, 24, 9500. [Google Scholar] [CrossRef]
- Perz, M.; Szymanowska, D.; Janeczko, T.; Kostrzewa-Susłow, E. Antimicrobial Properties of Flavonoid Derivatives with Bromine, Chlorine, and Nitro Group Obtained by Chemical Synthesis and Biotransformation Studies. Int. J. Mol. Sci. 2024, 25, 5540. [Google Scholar] [CrossRef]
- Binsack, R.; Boersma, B.J.; Patel, R.P.; Kirk, M.; White, C.R.; Darley-Usmar, V.; Barnes, S.; Zhou, F.; Parks, D.A. Enhanced Antioxidant Activity After Chlorination of Quercetin by Hypochlorous Acid. Alcohol. Clin. Exp. Res. 2001, 25, 434–443. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Nat. Publ. Gr. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Satokata, A.A.C.; Souza, J.H.; Silva, L.L.O.; Santiago, M.B.; Ramos, S.B.; de Assis, L.R.; dos Santos Theodoro, R.; e Oliveira, L.R.; Regasini, L.O.; Martins, C.H.G. Chalcones with Potential Antibacterial and Antibiofilm Activities against Periodontopathogenic Bacteria. Anaerobe 2022, 76, 102588. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phyther. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Hurtová, M.; Káňová, K.; Dobiasová, S.; Holasová, K.; Čáková, D.; Hoang, L.; Biedermann, D.; Kuzma, M.; Cvačka, J.; Křen, V.; et al. Selectively Halogenated Flavonolignans—Preparation and Antibacterial Activity. Int. J. Mol. Sci. 2022, 23, 15121. [Google Scholar] [CrossRef]
- Marzec, E.; Świtalska, M.; Winiewska-Szajewska, M.; Wójcik, J.; Wietrzyk, J.; Maciejewska, A.M.; Poznański, J.; Mieczkowski, A. The Halogenation of Natural Flavonoids, Baicalein and Chrysin, Enhances Their Affinity to Human Protein Kinase CK2. IUBMB Life 2020, 72, 1250–1261. [Google Scholar] [CrossRef]
- Ullah Mughal, E.; Ayaz, M.; Hasan, A.; Sadiq, A.; Riaz, M.; Malik, A.; Hussain, S.; Choudhary, M.I.; Hussain, Z. Synthesis and Antibacterial Activity of Substituted Flavones, 4-Thioflavones and 4-Iminoflavones. Bioorganic Med. Chem. 2006, 14, 4704–4711. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.B.; Yu, Y.; Liang, Y.Z.; Zeng, B. In Vitro Antioxidant and Antimicrobial Activities of the Extract of Pericarpium Citri Reticulatae of a New Citrus Cultivar and Its Main Flavonoids. LWT-Food Sci. Technol. 2008, 41, 597–603. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A Structure-Activity Relationship Study of Flavonoids as Inhibitors of E. Coli by Membrane Interaction Effect. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.P.; de Fátima Albino Smânia, E.; Monache, F.D.; Smânia, A. Structure–Activity Relationship of Antibacterial Chalcones. Bioorganic Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Alcaráz, L.E.; Blanco, S.E.; Puig, O.N.; Tomás, F.; Ferretti, F.H. Antibacterial Activity of Flavonoids against Methicillin-Resistant Staphylococcus Aureus Strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-Activity and Lipophilicity Relationships of Selected Antibacterial Natural Flavones and Flavanones of Chilean Flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef]
- Zhou, S.-F. Drugs Behave as Substrates, Inhibitors and Inducers of Human Cytochrome P450 3A4. Curr. Drug Metab. 2008, 9, 310–322. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The Metabolic Fate of Dietary Polyphenols in Humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic Metabolism of Dietary Polyphenols: Influence of Structure on Microbial Fermentation Products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Dey, P. Gut Microbiota in Phytopharmacology: A Comprehensive Overview of Concepts, Reciprocal Interactions, Biotransformations and Mode of Actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.T.; Monteleone, G. Immunity, Inflammation, and Allergy in the Gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef]
- Liao, Z.L.; Zeng, B.H.; Wang, W.; Li, G.H.; Wu, F.; Wang, L.; Zhong, Q.P.; Wei, H.; Fang, X. Impact of the Consumption of Tea Polyphenols on Early Atherosclerotic Lesion Formation and Intestinal Bifidobacteria in High-Fat-Fed ApoE−/− Mice. Front. Nutr. 2016, 3, 42. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef]
- Ciemniak, K.; Maciejewska, P.; Cielecka-Piontek, J.; Kobus-Cisowska, J.; Soporowski, J.; Szymanowska, D. Wpływ Preparatu Probiotycznego Na Dysbiozę u Osób Starszych. Acta Sci. Pol. 2017, 16, 93–99. [Google Scholar]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Szczepaniak, O.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. Enriching Novel Dark Chocolate with Bacillus Coagulans as a Way to Provide Beneficial Nutrients. Food Funct. 2019, 10, 997–1006. [Google Scholar] [CrossRef] [PubMed]




| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Total Number of Microorganisms | Bifidobacterium sp. | Lactobacillus sp. | Nonpathogenic E. coli | Enterococcus sp. | ||||||
| Microbiome of healthy people | (2.85 ± 2.36) × 1011 | (7.75 ± 1.95) × 1011 | (6.95 ± 0.25) × 109 | (3.18 ± 2.22) × 1011 ↑ | (3.25 ± 0.95) × 105 | (6.75 ± 1.15) × 106 | (2.70 ± 0.40) × 106 | (8.65 ± 0.95) × 105 | (3.45 ± 2.25) × 106 | (6.40 ± 3.20) × 105 |
| Microbiome of people over 75 y.o. | (8.15 ± 3.85) × 109 | (4.50 ± 1.10) × 1010 | (1.31 ± 1.19) × 108 | (5.55 ± 0.05) × 108 | (5.10 ± 1.60) × 104 | (9.15 ± 2.85) × 107 ↑ | (6.80 ± 1.00) × 106 | (7.35 ± 0.45) × 105 | (4.68 ± 3.72) × 105 | (9.20 ± 0.60) × 105 |
| Microbiome of people after antibiotic therapy | (3.65 ± 0.85) × 107 | (3.84 ± 3.06) × 1011 ↑ | (2.30 ± 1.10) × 105 | (5.05 ± 0.55) × 108 ↑ | (2.69 ± 2.02) × 103 | (1.59 ± 0.61) × 107 ↑ | (5.95 ± 3.85) × 104 | (8.15 ± 1.45) × 105 | (4.20 ± 3.00) × 104 | (7.15 ± 1.55) × 105 |
| Microbiome of people after chemotherapy | (2.99 ± 2.61) × 107 | (3.19 ± 2.41) × 109 ↑ | (3.30 ± 1.20) × 104 | (2.85 ± 1.65) × 107 ↑ | (5.00 ± 2.80) × 103 | (3.47 ± 3.24) × 108 ↑ | (6.70 ± 2.20) × 103 | (2.40 ± 1.20) × 103 | (4.05 ± 0.45) × 102 | (5.60 ± 1.10) × 103 |
| Microbiome of obese people | (3.29 ± 2.51) × 108 | (3.10 ± 0.80) × 1011 ↑ | (2.75 ± 0.65) × 106 | (2.37 ± 2.14) × 108 ↑ | (2.01 ± 1.80) × 107 | (4.05 ± 0.45) × 106 | (6.35 ± 0.95) × 106 | (4.20 ± 2.10) × 105 | (3.00 ± 1.80) × 105 | (7.50 ± 1.20) × 105 |
| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Clostridium sp. | E. coli | Proteolytic Bacteria | Yeast-like Fungi | |||||
| Microbiome of healthy people | (2.31 ± 2.19) × 103 | (3.75 ± 2.55) × 102 | (4.60 ± 0.30) × 103 | (5.05 ± 3.85) × 102 | (1.31 ± 1.19) × 103 | (1.60 ± 0.50) × 102 | absence | absence |
| Microbiome of people over 75 y.o. | (3.45 ± 1.35) × 105 | (2.75 ± 0.45) × 103 ↓ | (1.41 ± 1.39) × 105 | (6.45 ± 2.25) × 103 ↓ | (3.38 ± 3.32) × 104 | (1.38 ± 0.93) × 102 ↓ | (1.16 ± 1.04) × 103 | absence ↓ |
| Microbiome of people after antibiotic therapy | (4.50 ± 2.20) × 105 | (2.30 ± 0.00) × 103 ↓ | (5.95 ± 1.85) × 102 | (2.30 ± 1.10) × 102 | (4.16 ± 4.05) × 103 | (1.90 ± 0.70) × 102 | (8.80 ± 3.20) × 104 | absence ↓ |
| Microbiome of people after chemotherapy | (1.28 ± 1.03) × 103 | (2.30 ± 0.00) × 102 | (4.20 ± 1.00) × 102 | (3.90 ± 2.80) × 102 | (4.00 ± 2.80) × 102 | (1.70 ± 0.60) × 102 | (5.00 ± 0.50) × 102 | absence ↓ |
| Microbiome of obese people | (2.90 ± 2.01) × 107 | (3.95 ± 1.65) × 103 ↓ | (4.52 ± 4.18) × 104 | (1.54 ± 0.76) × 103 | (3.55 ± 2.05) × 102 | (2.30 ± 1.10) × 102 | absence | absence |
| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Total Number of Microorganisms | Bifidobacterium sp. | Lactobacillus sp. | Nonpathogenic E. coli | Enterococcus sp. | ||||||
| Microbiome of healthy people | (2.85 ± 2.36) × 1011 | (4.47 ± 4.43) × 1010 | (6.95 ± 0.25) × 109 | (6.70 ± 1.10) × 109 | (3.25 ± 0.95) × 105 | (3.63 ± 3.07) × 108 ↑ | (2.70 ± 0.40) × 106 | (7.25 ± 1.65) × 105 | (3.45 ± 2.25) × 106 | (4.90 ± 3.70) × 105 |
| Microbiome of people over 75 y.o. | (8.15 ± 3.85) × 109 | (3.74 ± 2.96) × 1010 | (1.31 ± 1.19) × 108 | (7.25 ± 1.65) × 106 ↓ | (5.10 ± 1.60) × 104 | (4.50 ± 2.20) × 106 ↑ | (6.80 ± 1.00) × 106 | (5.05 ± 0.55) × 106 | (4.68 ± 3.72) × 105 | (1.15 ± 0.05) × 104 |
| Microbiome of people after antibiotic therapy | (3.65 ± 0.85) × 107 | (5.60 ± 1.10) × 108 | (2.30 ± 1.10) × 105 | (3.35 ± 2.25) × 107 ↑ | (2.69 ± 2.02) × 103 | (6.25 ± 0.65) × 106 ↑ | (5.95 ± 3.85) × 104 | (4.95 ± 1.75) × 105 | (4.20 ± 3.00) × 104 | (5.05 ± 3.85) × 105 |
| Microbiome of people after chemotherapy | (2.99 ± 2.61) × 107 | (6.70 ± 0.90) × 108 | (3.30 ± 1.20) × 104 | (3.15 ± 2.46) × 106 ↑ | (5.00 ± 2.80) × 103 | (4.40 ± 3.20) × 106 ↑ | (6.70 ± 2.20) × 103 | (7.80 ± 2.00) × 105 ↑ | (4.05 ± 0.45) × 102 | (2.95 ± 0.65) × 104 ↑ |
| Microbiome of obese people | (3.29 ± 2.51) × 108 | (4.84 ± 4.06) × 109 | (2.75 ± 0.65) × 106 | (7.05 ± 1.25) × 107 | (2.01 ± 1.80) × 107 | (3.95 ± 1.65) × 106 | (6.35 ± 0.95) × 106 | (1.15 ± 0.05) × 105 | (3.00 ± 1.80) × 105 | (4.70 ± 0.00) × 105 |
| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Clostridium sp. | E. coli | Proteolytic Bacteria | Yeast-like Fungi | |||||
| Microbiome of healthy people | (2.31 ± 2.19) × 103 | (3.40 ± 1.10) × 103 | (4.60 ± 0.30) × 103 | (7.10 ± 1.50) × 102 | (1.31 ± 1.19) × 103 | (2.30 ± 1.10) × 102 | absence | absence |
| Microbiome of people over 75 y.o. | (3.45 ± 1.35) × 105 | (4.40 ± 1.20) × 104 | (1.41 ± 1.39) × 105 | (2.84 ± 2.76) × 104 | (3.38 ± 3.32) × 104 | (3.57 ± 3.23) × 103 | (1.16 ± 1.04) × 103 | (5.20 ± 0.00) × 102 |
| Microbiome of people after antibiotic therapy | (4.50 ± 2.20) × 105 | (6.70 ± 3.10) × 105 | (5.95 ± 1.85) × 102 | (9.75 ± 5.25) × 102 | (4.16 ± 4.05) × 103 | absence ↓ | (8.80 ± 3.20) × 104 | (5.60 ± 3.30) × 102 ↓ |
| Microbiome of people after chemotherapy | (1.28 ± 1.03) × 103 | (4.05 ± 0.65) × 104 | (4.20 ± 1.00) × 102 | (1.15 ± 0.05) × 103 | (4.00 ± 2.80) × 102 | absence ↓ | (5.00 ± 0.50) × 102 | absence ↓ |
| Microbiome of obese people | (2.90 ± 2.01) × 107 | (1.70 ± 0.50) × 104 ↓ | (4.52 ± 4.18) × 104 | (4.90 ± 2.70) × 102 ↓ | (3.55 ± 2.05) × 102 | (1.75 ± 0.45) × 102 | absence | absence |
| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Total Number of Microorganisms | Bifidobacterium sp. | Lactobacillus sp. | Nonpathogenic E. coli | Enterococcus sp. | ||||||
| Microbiome of healthy people | (2.85 ± 2.36) × 1011 | (8.70 ± 0.20) × 1011 | (6.95 ± 0.25) × 109 | (5.15 ± 0.65) × 109 | (3.25 ± 0.95) × 105 | (5.95 ± 2.55) × 105 | (2.70 ± 0.40) × 106 | (3.50 ± 2.30) × 105 | (3.45 ± 2.25) × 106 | (8.15 ± 0.35) × 106 |
| Microbiome of people over 75 y.o. | (8.15 ± 3.85) × 109 | (5.40 ± 0.20) × 1010 | (1.31 ± 1.19) × 108 | (4.60 ± 1.20) × 1010 ↑ | (5.10 ± 1.60) × 104 | (7.05 ± 1.85) × 106 ↑ | (6.80 ± 1.00) × 106 | (6.50 ± 1.30) × 106 | (4.68 ± 3.72) × 105 | (3.85 ± 1.35) × 105 |
| Microbiome of people after antibiotic therapy | (3.65 ± 0.85) × 107 | (8.15 ± 0.35) × 108 | (2.30 ± 1.10) × 105 | (6.50 ± 2.00) × 107 ↑ | (2.69 ± 2.02) × 103 | (4.37 ± 4.14) × 104 | (5.95 ± 3.85) × 104 | (4.85 ± 3.65) × 105 | (4.20 ± 3.00) × 104 | (5.60 ± 2.20) × 104 |
| Microbiome of people after chemotherapy | (2.99 ± 2.61) × 107 | (5.95 ± 3.65) × 107 | (3.30 ± 1.20) × 104 | (4.50 ± 3.30) × 105 | (5.00 ± 2.80) × 103 | (4.55 ± 3.35) × 105 ↑ | (6.70 ± 2.20) × 103 | (2.25 ± 1.36) × 104 | (4.05 ± 0.45) × 102 | (6.10 ± 1.60) × 104 ↑ |
| Microbiome of obese people | (3.29 ± 2.51) × 108 | (5.20 ± 1.30) × 109 | (2.75 ± 0.65) × 106 | (6.05 ± 2.65) × 107 | (2.01 ± 1.80) × 107 | (4.70 ± 0.80) × 108 | (6.35 ± 0.95) × 106 | (4.30 ± 3.10) × 107 | (3.00 ± 1.80) × 105 | (5.55 ± 1.05) × 106 |
| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Clostridium sp. | E. coli | Proteolytic Bacteria | Yeast-like Fungi | |||||
| Microbiome of healthy people | (2.31 ± 2.19) × 103 | (4.60 ± 2.10) × 102 | (4.60 ± 0.30) × 103 | (4.58 ± 4.33) × 104 | (1.31 ± 1.19) × 103 | (1.16 ± 0.64) × 103 | absence | absence |
| Microbiome of people over 75 y.o. | (3.45 ± 1.35) × 105 | (7.35 ± 1.55) × 103 ↓ | (1.41 ± 1.39) × 105 | (3.20 ± 2.00) × 103 ↓ | (3.38 ± 3.32) × 104 | (4.05 ± 1.55) × 103 | (1.16 ± 1.04) × 103 | (4.55 ± 0.65) × 102 |
| Microbiome of people after antibiotic therapy | (4.50 ± 2.20) × 105 | (5.25 ± 4.36) × 105 | (5.95 ± 1.85) × 102 | (6.05 ± 2.85) × 102 | (4.16 ± 4.05) × 103 | absence ↓ | (8.80 ± 3.20) × 104 | (3.07 ± 2.73) × 104 |
| Microbiome of people after chemotherapy | (1.28 ± 1.03) × 103 | (4.02 ± 3.79) × 103 | (4.20 ± 1.00) × 102 | (1.90 ± 0.40) × 102 | (4.00 ± 2.80) × 102 | absence ↓ | (5.00 ± 0.50) × 102 | absence ↓ |
| Microbiome of obese people | (2.90 ± 2.01) × 107 | (6.05 ± 2.65) × 106 | (4.52 ± 4.18) × 104 | (5.05 ± 2.75) × 103 | (3.55 ± 2.05) × 102 | (2.56 ± 2.35) × 103 | absence | absence |
| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Total Number of Microorganisms | Bifidobacterium sp. | Lactobacillus sp. | Nonpathogenic E. coli | Enterococcus sp. | ||||||
| Microbiome of healthy people | (2.85 ± 2.36) × 1011 | (5.20 ± 3.70) × 1011 | (6.95 ± 0.25) × 109 | (2.90 ± 1.60) × 109 | (3.25 ± 0.95) × 105 | (5.90 ± 3.00) × 105 | (2.70 ± 0.40) × 106 | (2.05 ± 0.25) × 106 | (3.45 ± 2.25) × 106 | (1.16 ± 1.04) × 106 |
| Microbiome of people over 75 y.o. | (8.15 ± 3.85) × 109 | (5.05 ± 0.55) × 1011 ↑ | (1.31 ± 1.19) × 108 | (4.95 ± 2.85) × 108 | (5.10 ± 1.60) × 104 | (3.90 ± 0.60) × 104 | (6.80 ± 1.00) × 106 | (3.20 ± 2.00) × 106 | (4.68 ± 3.72) × 105 | (6.65 ± 1.15) × 105 |
| Microbiome of people after antibiotic therapy | (3.65 ± 0.85) × 107 | (3.60 ± 1.10) × 108 | (2.30 ± 1.10) × 105 | (3.50 ± 2.30) × 105 | (2.69 ± 2.02) × 103 | (5.85 ± 0.05) × 103 | (5.95 ± 3.85) × 104 | (3.53 ± 3.17) × 105 | (4.20 ± 3.00) × 104 | (5.90 ± 3.00) × 104 |
| Microbiome of people after chemotherapy | (2.99 ± 2.61) × 107 | (3.35 ± 2.46) × 106 | (3.30 ± 1.20) × 104 | (4.42 ± 4.19) × 104 | (5.00 ± 2.80) × 103 | (6.60 ± 1.20) × 103 | (6.70 ± 2.20) × 103 | (2.85 ± 1.96) × 103 | (4.05 ± 0.45) × 102 | (4.05 ± 2.85) × 102 |
| Microbiome of obese people | (3.29 ± 2.51) × 108 | (5.15 ± 0.65) × 108 | (2.75 ± 0.65) × 106 | (3.87 ± 3.53) × 106 | (2.01 ± 1.80) × 107 | (3.96 ± 3.84) × 107 | (6.35 ± 0.95) × 106 | (1.83 ± 1.38) × 106 | (3.00 ± 1.80) × 105 | (7.15 ± 1.55) × 105 |
| Origin of the Intestinal Microbiome | The Number of Microorganisms CFU/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | With Compound | Control | With Compound | Control | With Compound | Control | With Compound | |
| Clostridium sp. | E. coli | Proteolytic Bacteria | Yeast-like Fungi | |||||
| Microbiome of healthy people | (2.31 ± 2.19) × 103 | (1.25 ± 0.05) × 102 | (4.60 ± 0.30) × 103 | (2.25 ± 1.15) × 104 | (1.31 ± 1.19) × 103 | (6.20 ± 0.30) × 103 | absence | absence |
| Microbiome of people over 75 y.o. | (3.45 ± 1.35) × 105 | (7.70 ± 1.90) × 104 | (1.41 ± 1.39) × 105 | (3.50 ± 2.30) × 105 | (3.38 ± 3.32) × 104 | (7.25 ± 1.65) × 104 | (1.16 ± 1.04) × 103 | (7.70 ± 4.30) × 102 |
| Microbiome of people after antibiotic therapy | (4.50 ± 2.20) × 105 | (2.35 ± 0.85) × 105 | (5.95 ± 1.85) × 102 | (4.05 ± 3.76) × 103 | (4.16 ± 4.05) × 103 | (3.50 ± 2.30) × 103 | (8.80 ± 3.20) × 104 | (1.29 ± 0.51) × 104 |
| Microbiome of people after chemotherapy | (1.28 ± 1.03) × 103 | (3.55 ± 1.25) × 102 | (4.20 ± 1.00) × 102 | (3.00 ± 1.50) × 102 | (4.00 ± 2.80) × 102 | (7.80 ± 2.00) × 102 | (5.00 ± 0.50) × 102 | (4.70 ± 3.10) × 102 |
| Microbiome of obese people | (2.90 ± 2.01) × 107 | (3.14 ± 2.36) × 107 | (4.52 ± 4.18) × 104 | (5.25 ± 4.36) × 104 | (3.55 ± 2.05) × 102 | (2.75 ± 0.65) × 102 | absence | absence |
| Compound | Probability | Antibacterial | Anticarcinogenic | Vasoprotector | Chemopreventive | CDP-glycerol Glycerophosphotransferase Inhibitor | Bacterial Efflux Pomp Inhibitor | Antifungal |
|---|---|---|---|---|---|---|---|---|
| (1) | Pa | 0.373 | 0.309 | 0.491 | 0.316 | 0.336 | x | 0.453 |
| Pi | 0.037 | 0.055 | 0.042 | 0.034 | 0.264 | x | 0.039 | |
| (2) | Pa | 0.216 | 0.322 | 0.372 | 0.280 | x | x | 0.346 |
| Pi | 0.104 | 0.050 | 0.093 | 0.040 | x | x | 0.064 | |
| (3) | Pa | 0.212 | 0.302 | 0.707 | 0.239 | x | x | 0.280 |
| Pi | 0.107 | 0.057 | 0.010 | 0.052 | x | x | 0.090 | |
| (4) | Pa | 0.312 | 0.210 | 0.340 | 0.212 | x | 0.124 | 0.539 |
| Pi | 0.056 | 0.109 | 0.116 | 0.062 | x | 0.082 | 0.025 | |
| (5) | Pa | 0.212 | 0.233 | 0.256 | 0.177 | x | 0.133 | 0.397 |
| Pi | 0.107 | 0.092 | 0.232 | 0.076 | x | 0.050 | 0.050 | |
| (6) | Pa | 0.209 | 0.216 | 0.497 | 0.146 | x | 0.133 | 0.324 |
| Pi | 0.109 | 0.104 | 0.041 | 0.096 | x | 0.049 | 0.072 | |
| (6a) | Pa | 0.538 | 0.719 | 0.828 | 0.514 | 0.920 | x | 0.664 |
| Pi | 0.013 | 0.008 | 0.004 | 0.014 | 0.007 | x | 0.012 | |
| (7) | Pa | 0.332 | 0.247 | 0.355 | 0.242 | x | 0.127 | 0.499 |
| Pi | 0.049 | 0.084 | 0.104 | 0.051 | x | 0.071 | 0.031 | |
| (8) | Pa | 0.160 | 0.247 | 0.265 | 0.208 | x | 0.135 | 0.384 |
| Pi | 0.155 | 0.084 | 0.214 | 0.063 | x | 0.043 | 0.053 | |
| (9) | Pa | x | 0.228 | 0.516 | 0.169 | x | 0.136 | 0.313 |
| Pi | x | 0.096 | 0.035 | 0.080 | x | 0.042 | 0.076 | |
| (10) | Pa | 0.365 | 0.207 | 0.268 | 0.188 | x | x | 0.464 |
| Pi | 0.039 | 0.112 | 0.208 | 0.070 | x | x | 0.037 | |
| (11) | Pa | 0.207 | 0.216 | x | 0.169 | x | x | 0.325 |
| Pi | 0.111 | 0.104 | x | 0.081 | x | x | 0.072 | |
| (12) | Pa | 0.203 | 0.202 | 0.381 | 0.139 | x | x | 0.263 |
| Pi | 0.114 | 0.118 | 0.087 | 0.102 | x | x | 0.099 |
| Compound | GI Absorption | BBB Permeant | P-gp Substrate | Water Solubility (Ali) | Bioavailability Score | PAINS |
|---|---|---|---|---|---|---|
| (1) | High | No | No | Moderately soluble | 0.55 | 0 alert |
| (2) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (3) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (4) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (5) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (6) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (6a) | High | No | Yes | Moderately soluble | 0.55 | 0 alert |
| (7) | High | No | No | Moderately soluble | 0.55 | 0 alert |
| (8) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (9) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (10) | High | No | No | Moderately soluble | 0.55 | 0 alert |
| (11) | High | Yes | No | Moderately soluble | 0.55 | 0 alert |
| (12) | High | No | No | Moderately soluble | 0.55 | 0 alert |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perz, M.; Szymanowska, D.; Kostrzewa-Susłow, E. The Influence of Flavonoids with -Br, -Cl Atoms and -NO2, -CH3 Groups on the Growth Kinetics and the Number of Pathogenic and Probiotic Microorganisms. Int. J. Mol. Sci. 2024, 25, 9269. https://doi.org/10.3390/ijms25179269
Perz M, Szymanowska D, Kostrzewa-Susłow E. The Influence of Flavonoids with -Br, -Cl Atoms and -NO2, -CH3 Groups on the Growth Kinetics and the Number of Pathogenic and Probiotic Microorganisms. International Journal of Molecular Sciences. 2024; 25(17):9269. https://doi.org/10.3390/ijms25179269
Chicago/Turabian StylePerz, Martyna, Daria Szymanowska, and Edyta Kostrzewa-Susłow. 2024. "The Influence of Flavonoids with -Br, -Cl Atoms and -NO2, -CH3 Groups on the Growth Kinetics and the Number of Pathogenic and Probiotic Microorganisms" International Journal of Molecular Sciences 25, no. 17: 9269. https://doi.org/10.3390/ijms25179269





