Anti-Cancer Potential of Isoflavone-Enriched Fraction from Traditional Thai Fermented Soybean against Hela Cervical Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Genistein and Daidzein in Thua Nao Ethyl Acetate Fraction (TN-EA)-Induced Human Cervical Carcinoma Cell (HeLa) Death
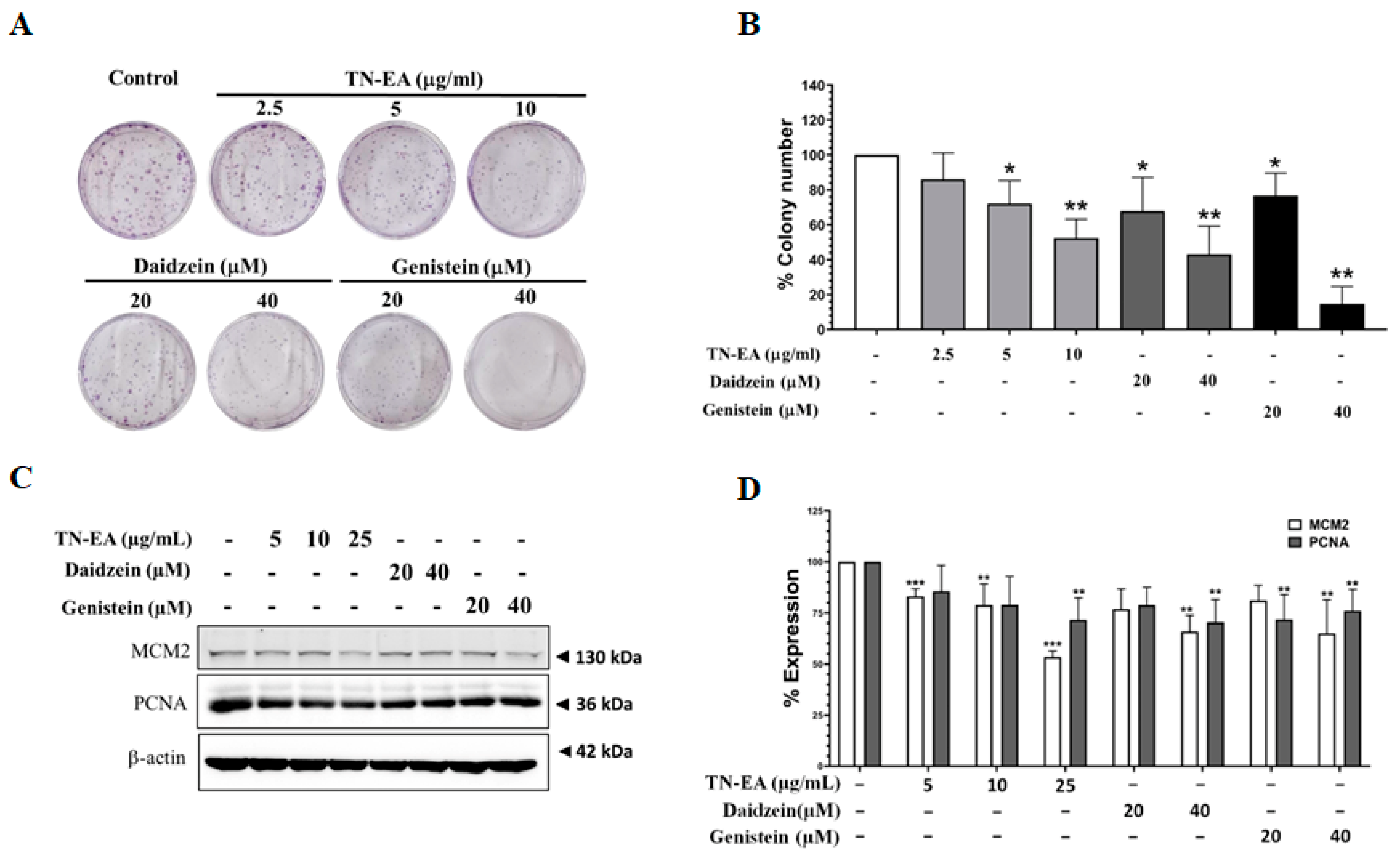
2.2. Anti-Proliferative Activity of TN-EA and Its Active Compounds on HeLa Cells
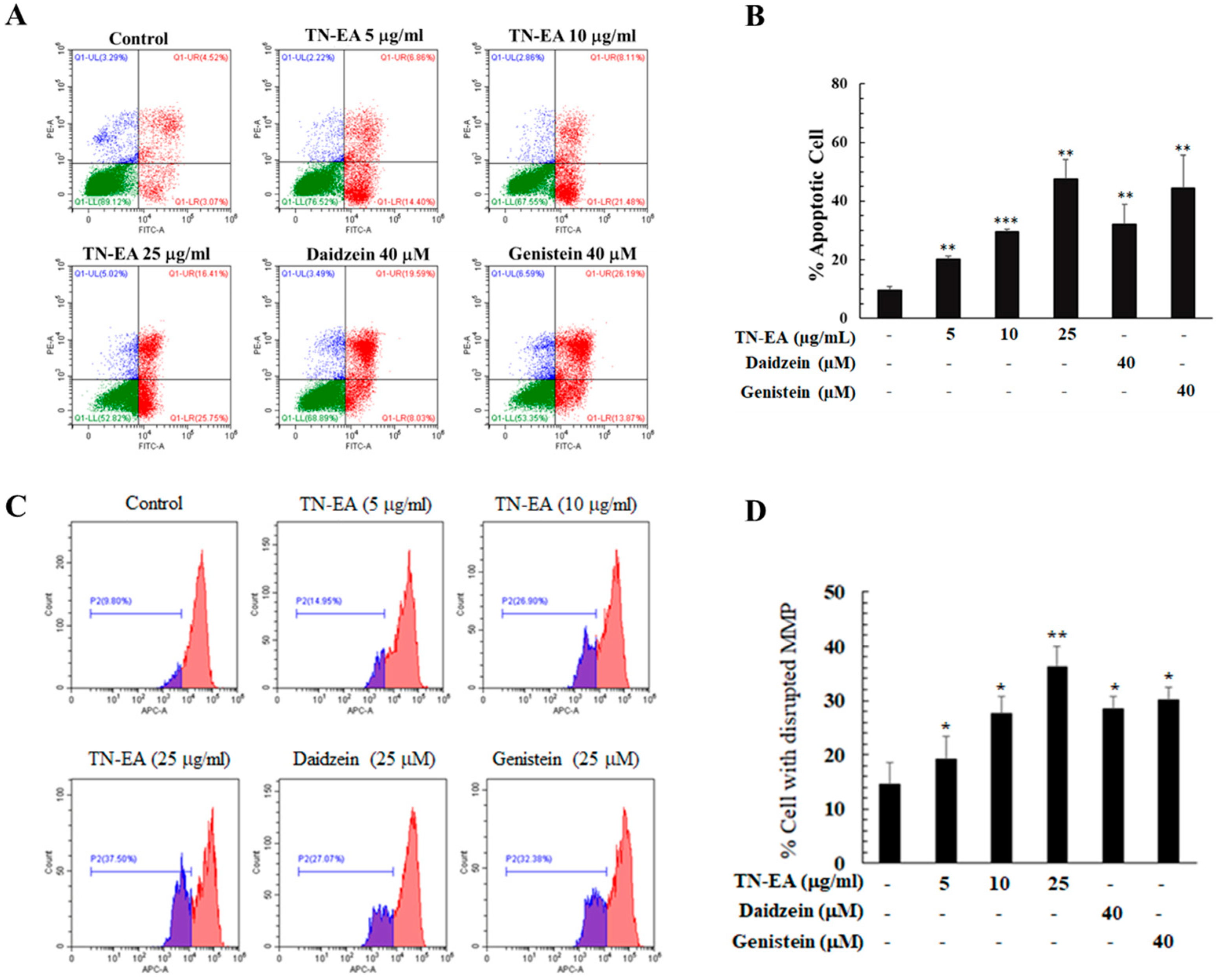
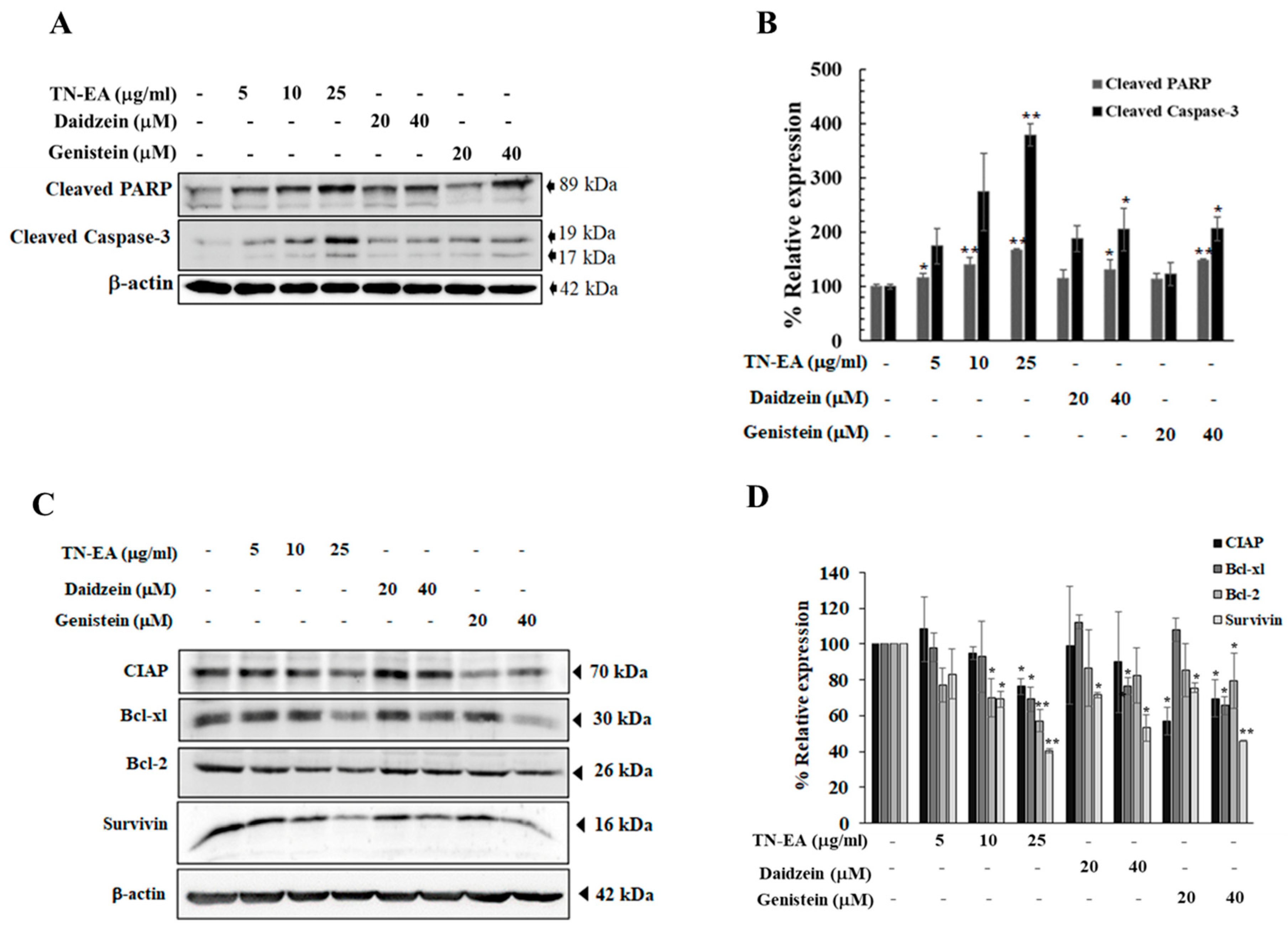
2.3. TN-EA, Daidzein, and Genistein Promote the Apoptosis of HeLa Cells
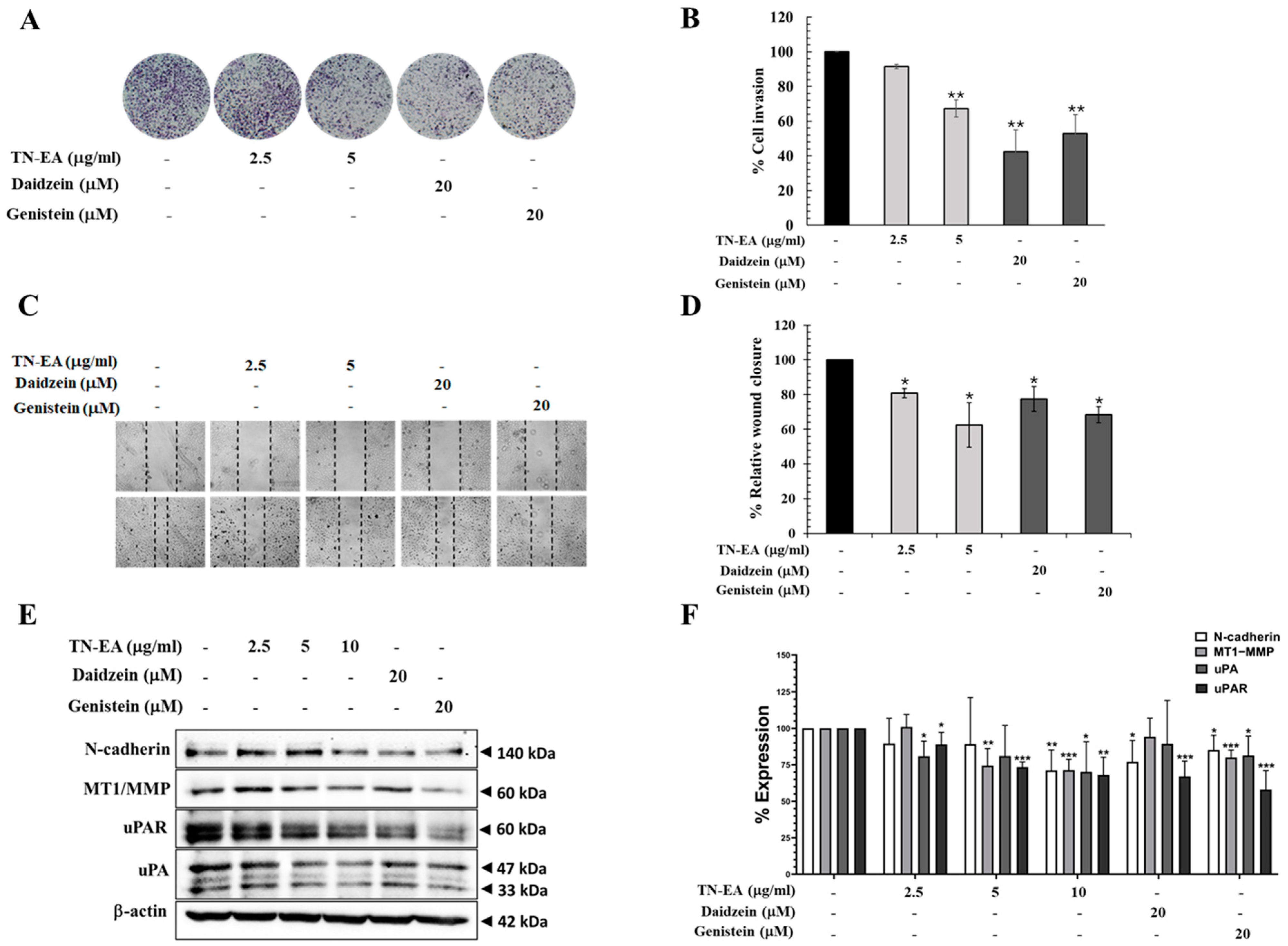
2.4. TN-EA, Daidzein, and Genistein Suppress the Migration and Invasion of HeLa Cells
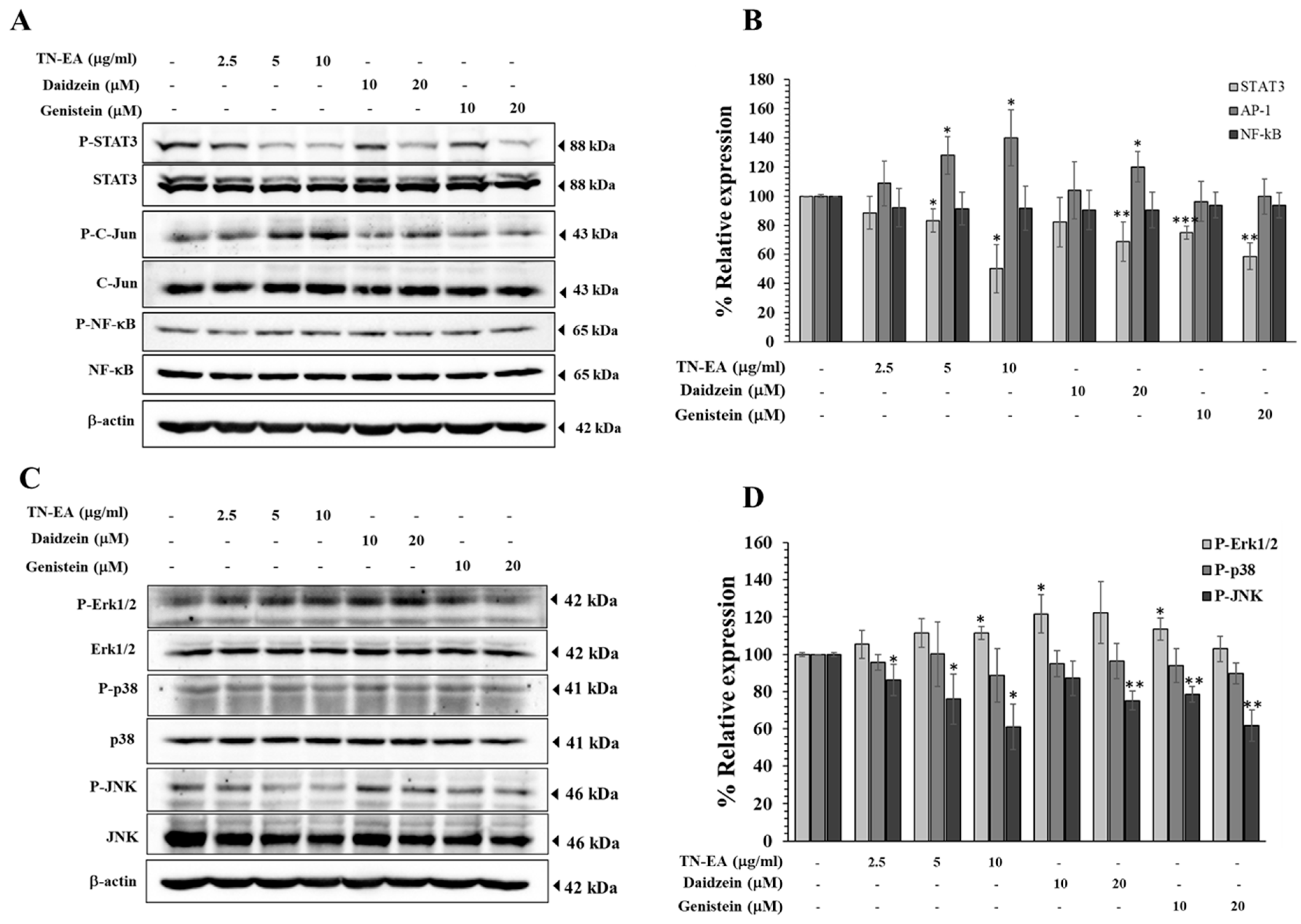
2.5. Effect of TN-EA, Daidzein, and Genistein on Oncogenic Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Thua Nao Extract Fractions
4.3. High-Performance Liquid Chromatography (HPLC) Analysis
4.4. Cells and Cell Cultures
4.5. Cell Viability Assay
4.6. Colony Formation
4.7. Cell Cycle Assay
4.8. Apoptosis Assay
4.9. Mitochondrial Membrane Potential
4.10. Wound-Healing Assay
4.11. Invasion Assay
4.12. Western Blot Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Wongpratate, M.; Bumrungthai, S. Cervical cancer in Thailand: 2023 update. Obstet. Gynecol. Sci. 2024, 67, 261–269. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Applegate, C.C.; Rowles, J.L.; Ranard, K.M.; Jeon, S.; Erdman, J.W. Soy Consumption and the Risk of Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, K.; Zhang, Z.; Zhang, C.; Sun, Y.; Feng, Z. Fermented soybean foods: A review of their functional components, mechanism of action and factors influencing their health benefits. Food Res. Int. 2022, 158, 111575. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Fermented soybean foods and diabetes. J. Diabetes Investig. 2023, 14, 1329–1340. [Google Scholar] [CrossRef]
- do Prado, F.G.; Pagnoncelli, M.G.B.; de Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented Soy Products and Their Potential Health Benefits: A Review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-S.; Qi, W.-T.; Guo, W.; Wang, C.-L.; Hu, Z.-B.; Li, A.-K. Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets. Food Nutr. Res. 2018, 62, 1384. [Google Scholar] [CrossRef]
- Ghasemi, A.; Soleimanjahi, H.; Razeghi, S.; Gorji, A.; Tabaraei, A.; Moradi, A.; Alizadeh, A.; Vakili, M.A. Genistein induces a protective immunomodulatory effect in a mouse model of cervical cancer. Iran. J. Immunol. 2012, 9, 119–127. [Google Scholar]
- Pejčić, T.; Zeković, M.; Bumbaširević, U.; Kalaba, M.; Vovk, I.; Bensa, M.; Popović, L.; Tešić, Ž. The role of isoflavones in the prevention of breast cancer and prostate cancer. Antioxidants 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Jiang, C. Soy and isoflavones consumption and breast cancer survival and recurrence: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 3079–3090. [Google Scholar] [CrossRef]
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, B.; Hammarström, C.; Yang, J.; Ramberg, H.; Diep, L.M.; Karlsen, S.J.; Kucuk, O.; Saatcioglu, F.; Taskèn, K.A.; Svindland, A. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br. J. Nutr. 2012, 108, 2138–2147. [Google Scholar] [CrossRef]
- Chukeatirote, E. Thua nao: Thai fermented soybean. J. Ethn. Foods 2015, 2, 115–118. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Sutheeworapong, S.; Jenjaroenpun, P.; Charoensiddhi, S.; Khoiri, A.N.; Topanurak, S.; Sutthikornchai, C.; Jintaridth, P. Microbiome analysis of thai traditional fermented soybeans reveals short-chain fatty acid-associated bacterial taxa. Sci. Rep. 2023, 13, 7573. [Google Scholar] [CrossRef] [PubMed]
- Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Paradee, N.; Prommaban, A.; Ounjaijean, S.; Rerkasem, K.; Parklak, W.; Prakit, K.; Saengsitthisak, B.; et al. Antioxidant Potential and Cytotoxic Effect of Isoflavones Extract from Thai Fermented Soybean (Thua-Nao). Molecules 2021, 26, 7432. [Google Scholar] [CrossRef]
- Chia, J.S.; Du, J.L.; Wu, M.S.; Hsu, W.B.; Chiang, C.P.; Sun, A.; Lu, J.J.; Wang, W.B. Fermentation product of soybean, black bean, and green bean mixture induces apoptosis in a wide variety of cancer cells. Integr. Cancer Ther. 2013, 12, 248–256. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef]
- Chang, W.-H.; Liu, J.-J.; Chen, C.-H.; Huang, T.-S.; Lu, F.-J. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by fermented soy milk. Nutr. Cancer 2002, 43, 214–226. [Google Scholar] [CrossRef]
- Bemis, D.L.; Capodice, J.L.; Desai, M.; Buttyan, R.; Katz, A.E. A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin. Cancer Res. 2004, 10, 5282–5292. [Google Scholar] [CrossRef]
- Su, C.-L.; Wu, C.-J.; Chen, F.-N.; Wang, B.-J.; Sheu, S.-R.; Won, S.-J. Supernatant of bacterial fermented soybean induces apoptosis of human hepatocellular carcinoma Hep 3B cells via activation of caspase 8 and mitochondria. Food Chem. Toxicol. 2007, 45, 303–314. [Google Scholar] [CrossRef]
- Naeem, H.; Momal, U.; Imran, M.; Shahbaz, M.; Hussain, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Umar, M.; Mujtaba, A.; El-Ghorab, A.H. Anticancer perspectives of genistein: A comprehensive review. Int. J. Food Prop. 2023, 26, 3305–3341. [Google Scholar] [CrossRef]
- Yao, Z.; Xu, X.; Huang, Y. Daidzin inhibits growth and induces apoptosis through the JAK2/STAT3 in human cervical cancer HeLa cells. Saudi J. Biol. Sci. 2021, 28, 7077–7081. [Google Scholar] [CrossRef]
- Yashar, C.M.; Spanos, W.J.; Taylor, D.D.; Gercel-Taylor, C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.H.; Lee, S.C.; Song, Y.S. Involvement of both extrinsic and intrinsic apoptotic pathways in apoptosis induced by genistein in human cervical cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 196–201. [Google Scholar] [CrossRef]
- Jha, A.; Nikbakht, M.; Parashar, G.; Shrivastava, A.; Capalash, N.; Kaur, J. Reversal of hypermethylation and reactivation of the RARbeta2 gene by natural compounds in cervical cancer cell lines. Folia Biol 2010, 56, 195–200. [Google Scholar]
- Hedlund, T.E.; Johannes, W.U.; Miller, G.J. Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. Prostate 2003, 54, 68–78. [Google Scholar] [CrossRef]
- Esposito, F.; Giuffrida, R.; Raciti, G.; Puglisi, C.; Forte, S. Wee1 kinase: A potential target to overcome tumor resistance to therapy. Int. J. Mol. Sci. 2021, 22, 10689. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Kitazumi, I.; Tsukahara, M. Regulation of DNA fragmentation: The role of caspases and phosphorylation. FEBS J. 2011, 278, 427–441. [Google Scholar] [CrossRef]
- Yang, Y.; An, Y.; Ren, M.; Wang, H.; Bai, J.; Du, W.; Kong, D. The mechanisms of action of mitochondrial targeting agents in cancer: Inhibiting oxidative phosphorylation and inducing apoptosis. Front. Pharmacol. 2023, 14, 1243613. [Google Scholar] [CrossRef]
- Liu, N.; Huang, L.; Xu, H.; He, X.; He, X.; Cao, J.; Xu, W.; Wang, Y.; Wei, H.; Wang, S. Phosphatidylserine decarboxylase downregulation in uric acid-induced hepatic mitochondrial dysfunction and apoptosis. MedComm 2023, 4, e336. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef] [PubMed]
- Cetraro, P.; Plaza-Diaz, J.; MacKenzie, A.; Abadía-Molina, F. A review of the current impact of inhibitors of apoptosis proteins and their repression in cancer. Cancers 2022, 14, 1671. [Google Scholar] [CrossRef]
- Park, H.J.; Jeon, Y.K.; You, D.H.; Nam, M.J. Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2 family in human hepatic cancer cells. Food Chem. Toxicol. 2013, 60, 542–549. [Google Scholar] [CrossRef]
- Bi, Y.-l.; Min, M.; Shen, W.; Liu, Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 2018, 39, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.G.; Holloway, R.W.; Miller, V.A.; Waisman, D.M. Plasmin and plasminogen system in the tumor microenvironment: Implications for cancer diagnosis, prognosis, and therapy. Cancers 2021, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Celià-Terrassa, T.; Kang, Y. How important is EMT for cancer metastasis? PLoS Biol. 2024, 22, e3002487. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Qin, J.; Chen, J.X.; Zhu, Z.; Teng, J.A. Genistein inhibits human colorectal cancer growth and suppresses miR-95, Akt and SGK1. Cell. Physiol. Biochem. 2015, 35, 2069–2077. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Chen, S.; Ruan, X.; Liao, H.; Zhang, Y.; Sun, J.; Gao, J.; Deng, G. Genistein inhibits migration and invasion of cervical cancer HeLa cells by regulating FAK-paxillin and MAPK signaling pathways. Taiwan. J. Obstet. Gynecol. 2020, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kayisli, U.A.; Aksu, C.A.H.; Berkkanoglu, M.; Arici, A. Estrogenicity of isoflavones on human endometrial stromal and glandular cells. J. Clin. Endocrinol. Metab. 2002, 87, 5539–5544. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Lin, I.; Oka, C.; Kumazoe, M.; Komatsu, S.; Murata, M.; Kamachi, S.; Tachibana, H. Soy isoflavone metabolite equol inhibits cancer cell proliferation in a PAP associated domain containing 5-dependent and an estrogen receptor-independent manner. J. Nutr. Biochem. 2022, 100, 108910. [Google Scholar] [CrossRef]
- Thakur, K.; Janjua, D.; Aggarwal, N.; Chhokar, A.; Yadav, J.; Tripathi, T.; Chaudhary, A.; Senrung, A.; Shrivastav, A.; Bharti, A.C. Physical interaction between STAT3 and AP1 in cervical carcinogenesis: Implications in HPV transcription control. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166817. [Google Scholar] [CrossRef]
- Vishnoi, K.; Viswakarma, N.; Rana, A.; Rana, B. Transcription factors in cancer development and therapy. Cancers 2020, 12, 2296. [Google Scholar] [CrossRef]
- Liu, Y.; Sepich, D.S.; Solnica-Krezel, L. Stat3/Cdc25a-dependent cell proliferation promotes embryonic axis extension during zebrafish gastrulation. PLoS Genet. 2017, 13, e1006564. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Li, R. Progress in research on correlation among STAT3, CyclinD1, P21 genes and tumors. J. Otol. 2012, 7, 19–24. [Google Scholar]
- Bhardwaj, A.; Sethi, G.; Vadhan-Raj, S.; Bueso-Ramos, C.; Takada, Y.; Gaur, U.; Nair, A.S.; Shishodia, S.; Aggarwal, B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κB–regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007, 109, 2293–2302. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, S.; Li, S.; Wang, Y.; Cui, W. Role of STAT3 in cancer cell epithelial-mesenchymal transition. Int. J. Oncol. 2024, 64, 106331. [Google Scholar] [CrossRef]
- Ibrahim, S.A.E.-F.; Abudu, A.; Jonhson, E.; Aftab, N.; Conrad, S.; Fluck, M. The role of AP-1 in self-sufficient proliferation and migration of cancer cells and its potential impact on an autocrine/paracrine loop. Oncotarget 2018, 9, 34259. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Xu, C.-J.; Nair, S.S.; Chen, C.; Hebbar, V.; Kong, A.-N.T. Modulation of activator protein-1 (AP-1) and MAPK pathway by flavonoids in human prostate cancer PC3 cells. Arch. Pharmacal Res. 2006, 29, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef] [PubMed]
- Quadros, M.R.; Peruzzi, F.; Kari, C.; Rodeck, U. Complex regulation of signal transducers and activators of transcription 3 activation in normal and malignant keratinocytes. Cancer Res. 2004, 64, 3934–3939. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Rosemblit, C.; Rivas, M.A.; Proietti Anastasi, C.J.; Díaz Flaqué, M.C.; Mercogliano, M.F.; Beguelin, W.; Maronna, E.; Guzmán, P.; Gercovich, F.G. p42/p44 MAPK-mediated Stat3 Ser727 phosphorylation is required for progestin-induced full activation of Stat3 and breast cancer growth. Endocr.-Relat. Cancer 2013, 20, 197–212. [Google Scholar] [CrossRef]
- Xue, P.; Zhao, Y.; Liu, Y.; Yuan, Q.; Xiong, C.; Ruan, J. A novel compound RY10-4 induces apoptosis and inhibits invasion via inhibiting STAT3 through ERK-, p38-dependent pathways in human lung adenocarcinoma A549 cells. Chem.-Biol. Interact. 2014, 209, 25–34. [Google Scholar] [CrossRef]
- Gkouveris, I.; Nikitakis, N.; Karanikou, M.; Rassidakis, G.; Sklavounou, A. JNK1/2 expression and modulation of STAT3 signaling in oral cancer. Oncol. Lett. 2016, 12, 699–706. [Google Scholar] [CrossRef]
- Guo, W.; Wu, S.; Wang, L.; Wei, X.; Liu, X.; Wang, J.; Lu, Z.; Hollingshead, M.; Fang, B. Antitumor activity of a novel oncrasin analogue is mediated by JNK activation and STAT3 inhibition. PLoS ONE 2011, 6, e28487. [Google Scholar] [CrossRef]
- Vergne, S.; Titier, K.; Bernard, V.; Asselineau, J.; Durand, M.; Lamothe, V.; Potier, M.; Perez, P.; Demotes-Mainard, J.; Chantre, P. Bioavailability and urinary excretion of isoflavones in humans: Effects of soy-based supplements formulation and equol production. J. Pharm. Biomed. Anal. 2007, 43, 1488–1494. [Google Scholar] [CrossRef]
- Jang, H.-H.; Noh, H.; Kim, H.-W.; Cho, S.-Y.; Kim, H.-J.; Lee, S.-H.; Lee, S.-H.; Gunter, M.J.; Ferrari, P.; Scalbert, A. Metabolic tracking of isoflavones in soybean products and biosamples from healthy adults after fermented soybean consumption. Food Chem. 2020, 330, 127317. [Google Scholar] [CrossRef]
- Bolca, S.; Urpi-Sarda, M.; Blondeel, P.; Roche, N.; Vanhaecke, L.; Possemiers, S.; Al-Maharik, N.; Botting, N.; De Keukeleire, D.; Bracke, M. Disposition of soy isoflavones in normal human breast tissue. Am. J. Clin. Nutr. 2010, 91, 976–984. [Google Scholar] [CrossRef]
- Dijkstra, S.C.; Lampe, S.C.; Ray, R.M.; Brown, R.; Wu, C.; Li, W.; Chen, C.; King, I.B.; Gao, D.; Hu, Y. Biomarkers of dietary exposure are associated with lower risk of breast fibroadenomas in Chinese women. J. Nutr. 2010, 140, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Rannikko, A.; Petas, A.; Rannikko, S.; Adlercreutz, H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. Prostate 2006, 66, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, B.; Boezelijn, G.; Diep, L.M.; Kvernrod, K.; Ogren, O.; Ramberg, H.; Moen, A.; Wessel, N.; Berg, R.E.; Egge-Jacobsen, W. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer 2011, 63, 889–898. [Google Scholar] [CrossRef] [PubMed]
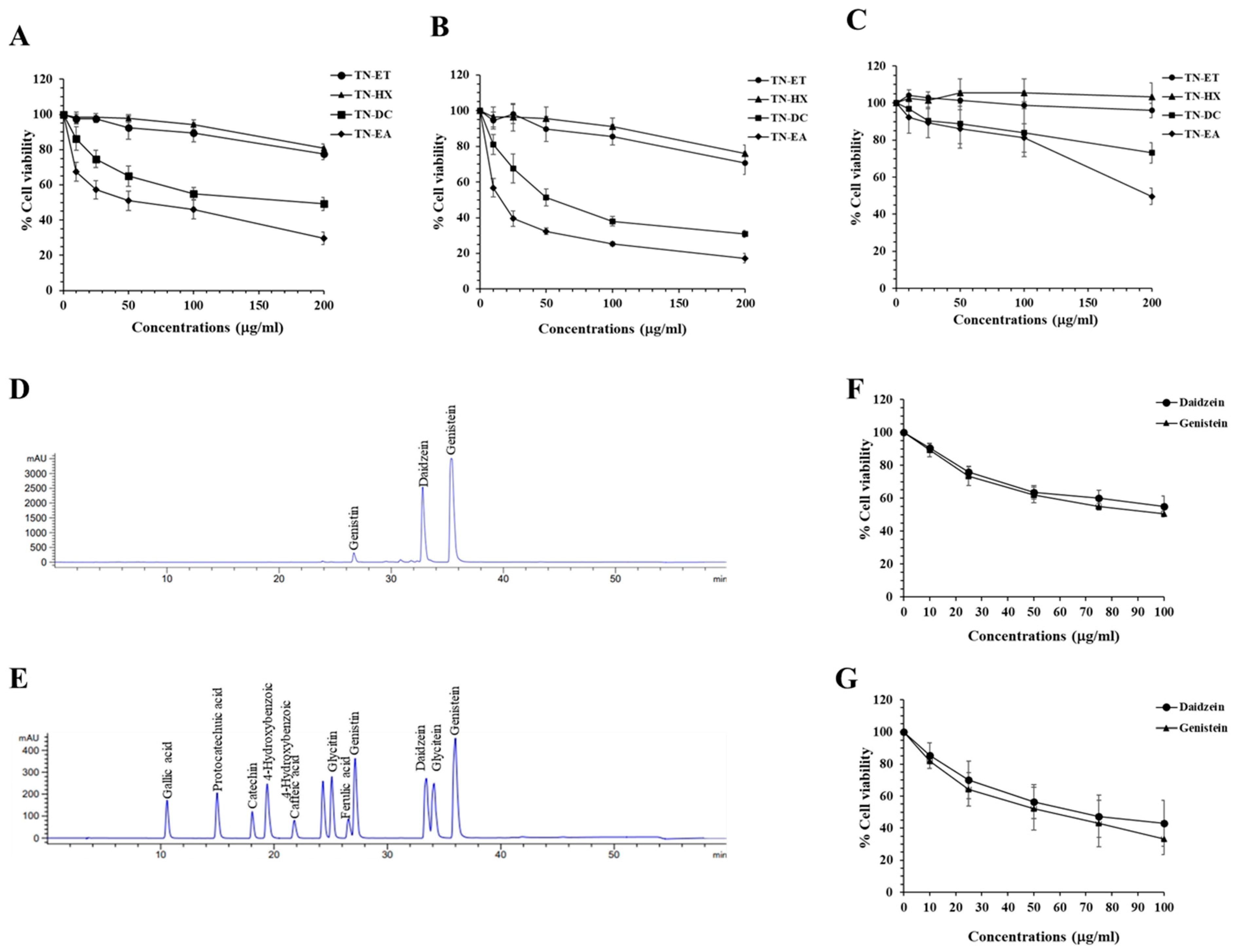

| Standard | Concentration in TN-EA (μg/mg Extract) |
|---|---|
| Gallic acid | ND |
| Protocatechuic acid | ND |
| Catechin hydrate | ND |
| 4-Hydroxybenzoic acid | ND |
| Caffeic acid | ND |
| Daidzin | ND |
| Glycitin | ND |
| Ferulic acid | ND |
| Genistin | 8.702 |
| Daidzein | 57.172 |
| Glycitein | ND |
| Genistein | 59.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhamwang, A.; Inthanon, S.; Dejkriengkraikul, P.; Semangoen, T.; Yodkeeree, S. Anti-Cancer Potential of Isoflavone-Enriched Fraction from Traditional Thai Fermented Soybean against Hela Cervical Cancer Cells. Int. J. Mol. Sci. 2024, 25, 9277. https://doi.org/10.3390/ijms25179277
Sukhamwang A, Inthanon S, Dejkriengkraikul P, Semangoen T, Yodkeeree S. Anti-Cancer Potential of Isoflavone-Enriched Fraction from Traditional Thai Fermented Soybean against Hela Cervical Cancer Cells. International Journal of Molecular Sciences. 2024; 25(17):9277. https://doi.org/10.3390/ijms25179277
Chicago/Turabian StyleSukhamwang, Amonnat, Sirinada Inthanon, Pornngarm Dejkriengkraikul, Tistaya Semangoen, and Supachai Yodkeeree. 2024. "Anti-Cancer Potential of Isoflavone-Enriched Fraction from Traditional Thai Fermented Soybean against Hela Cervical Cancer Cells" International Journal of Molecular Sciences 25, no. 17: 9277. https://doi.org/10.3390/ijms25179277
APA StyleSukhamwang, A., Inthanon, S., Dejkriengkraikul, P., Semangoen, T., & Yodkeeree, S. (2024). Anti-Cancer Potential of Isoflavone-Enriched Fraction from Traditional Thai Fermented Soybean against Hela Cervical Cancer Cells. International Journal of Molecular Sciences, 25(17), 9277. https://doi.org/10.3390/ijms25179277







