Pretreatment of Melanoma Cells with Aqueous Ethanol Extract from Madhuca longifolia Bark Strongly Potentiates the Activity of a Low Dose of Dacarbazine
Abstract
1. Introduction
2. Results
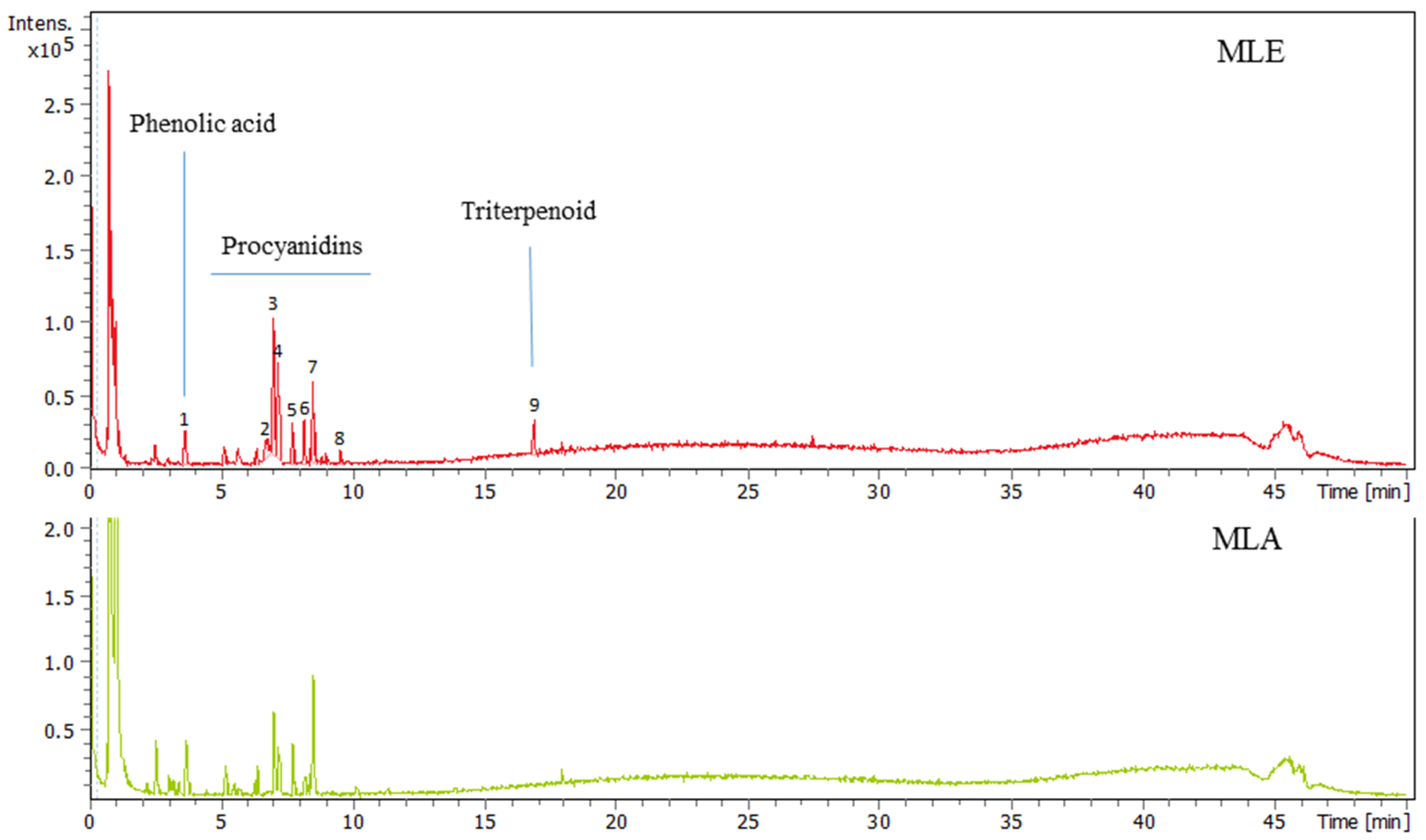
2.1. Analysis of Madhuca longifolia Extracts
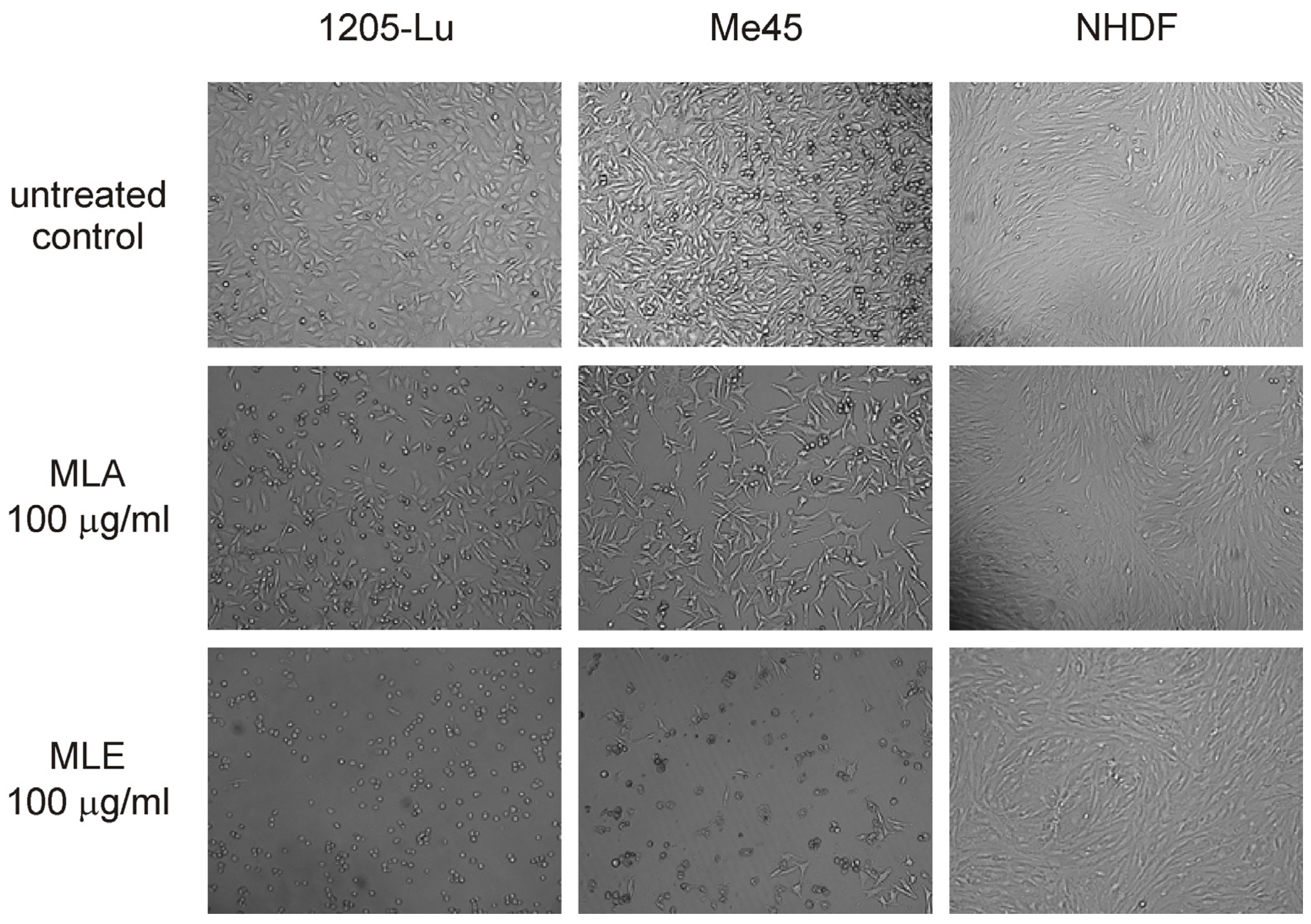
2.2. Influence of the Extracts on Cell Viability
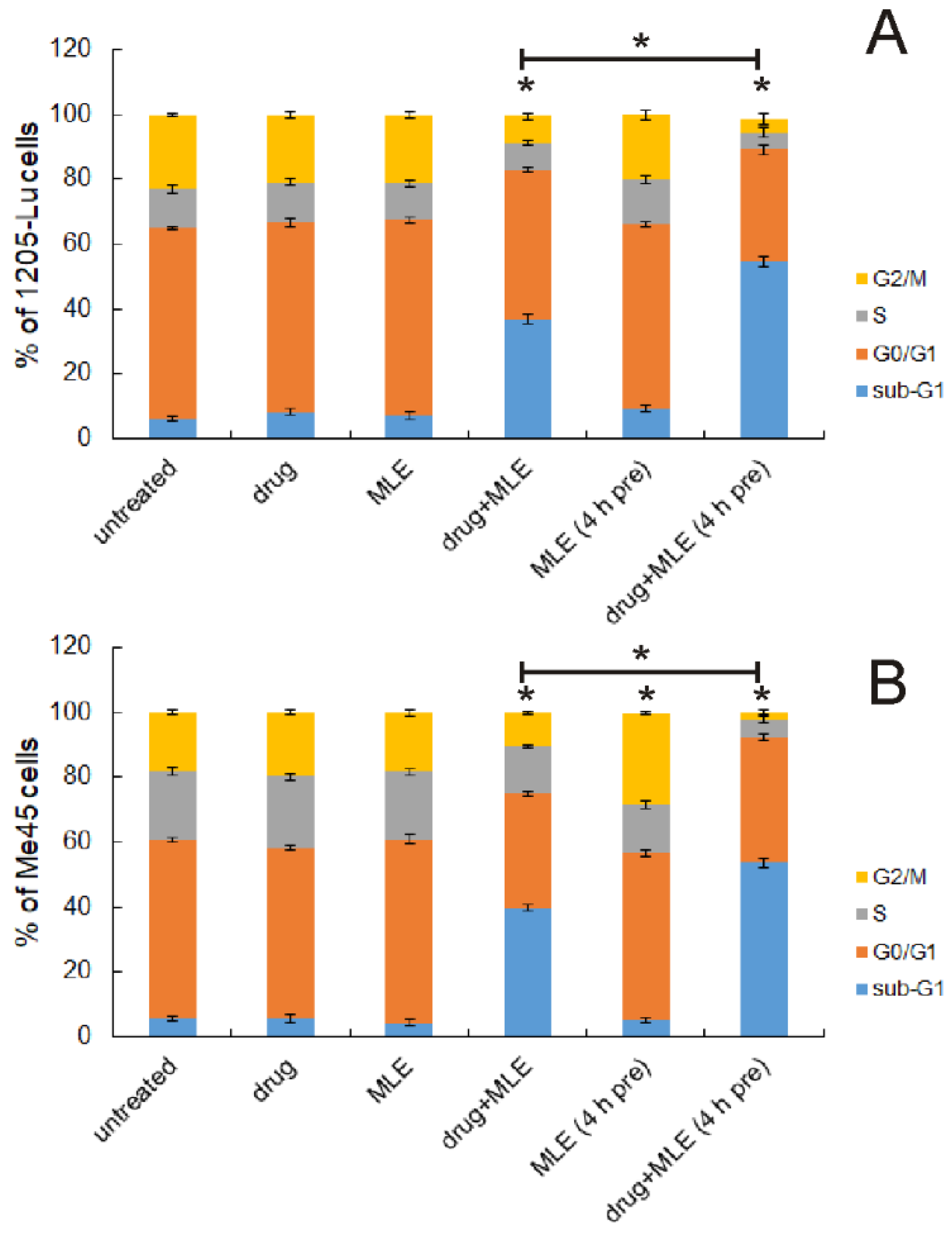
2.3. Influence of MLE on Cell Cycle
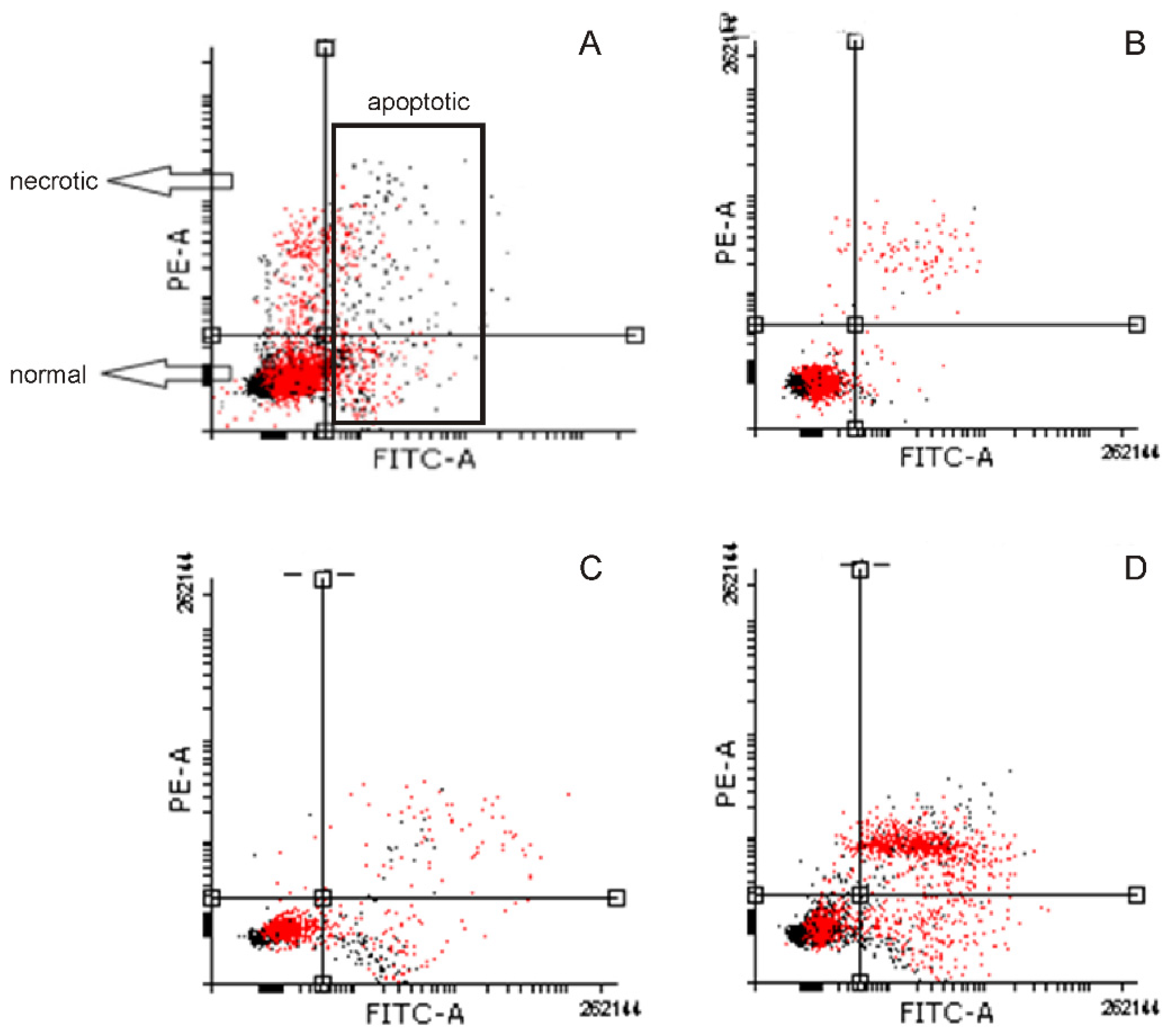
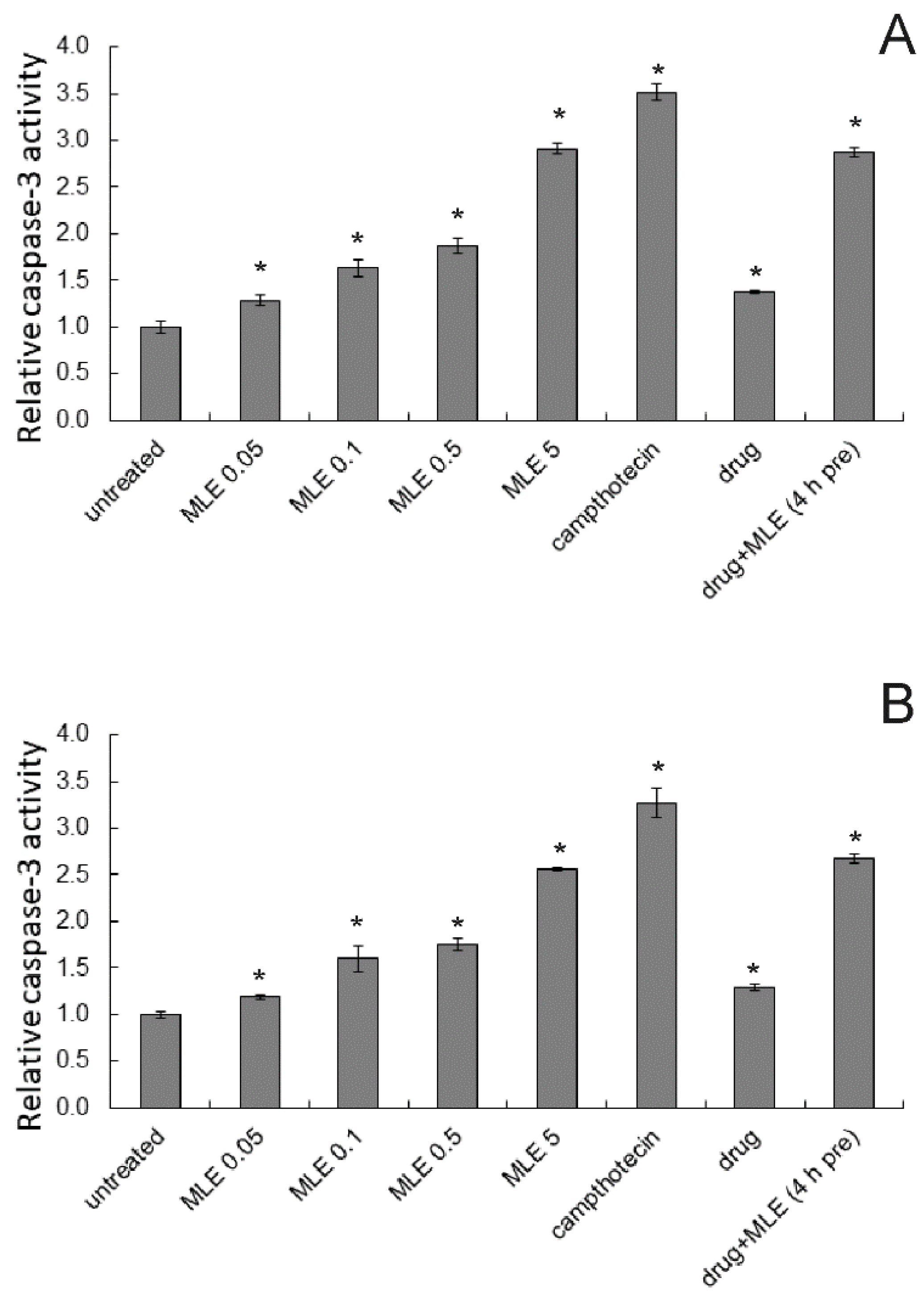
2.4. Induction of Apoptosis
2.5. Influence of MLE on Intracellular ROS Level
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Extraction
4.4. UHPLC-ESI-MS and MS/MS Analysis
4.5. Cell Culture
4.6. Cytotoxicity Assay
4.7. Flow Cytometry
4.8. Caspase 3 Acitvity
4.9. Combination Index Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, P.; Singh, D.; Mallik, A.; Nayak, S. Madhuca longifolia (Sapotaceae): A review of its traditional uses, phytochemistry and pharmacology. Int. J. Biomed. Res. 2012, 3, 291–305. [Google Scholar] [CrossRef]
- Sinha, J.; Singh, V.; Singh, J.; Rai, A.K. Phytochemistry, ethnomedical uses and future prospects of Mahua (Madhuca longifolia) as a food: A review. J. Nutr. Food Sci. 2017, 7, 1000573. [Google Scholar] [CrossRef]
- Chinnueswaraiah, M.; Elumalai, A.; Rahman, H. Isolation of phytocheical constituents from steam barks of Madhuca longifolia. Int. Res. J. Pharm. App. Sci. 2011, 1, 43–60. [Google Scholar]
- Akshatha, K.N.; Mahadeva Murthy, S.; Lakshmidevi, N. Ethnomedical uses of Madhuca longifolia—A review. Life Sci. Microbiol. 2013, 3, 44–53. [Google Scholar]
- Bürkel, P.; Rajbhandari, M.; Jürgenliemk, G. Bassia longifolia (= Madhuca longifolia): Isolation of flavan-3-ols and their contribution to the antibacterial and antidiabetic activity in vitro. Heliyon 2023, 9, e21134. [Google Scholar] [CrossRef]
- Gaikwad, R.D.; Ahmed, M.L.; Khalid, M.S.; Swamy, P. Anti-inflammatory activity of Madhuca longifolia seed saponin mixture. Pharm. Biol. 2009, 47, 592–597. [Google Scholar] [CrossRef]
- Shaban, A.; Verma, S.K.; Nautiyal, R.; Singh, S.K.; Purohit, R.; Chimata, M.L. In vitro cytotoxicity of Madhuca indica against different human cancer cell lines. Int. J. Pharm. Sci. Res. 2012, 3, 1385–1387. [Google Scholar] [CrossRef]
- Jha, D.; Mazumder, P.M. Biological, chemical and pharmacological aspects of Madhuca longifolia. Asian Pacif. J. Trop. Med. 2018, 11, 9–14. [Google Scholar] [CrossRef]
- Jose, A.G.R.; Govindarajulu, B.; Abirami, T.; Kavitha, V.; Saritha, G.; Karthikeyan, J. In vitro antioxidant and cytotoxic activity of ethanol bark extract of Madhuca longifolia on MCF-7 and Vero cell lines. J. Pharmacogn. Phytochem. 2018, 7, 1368–1371. [Google Scholar]
- Sarkar, M.K.; Vadivel, V.; Charan Raja, M.R.; Mahapatra, S.K. Potential anti-proliferative activity of AgNPs synthesized using M. longifolia in 4T1 cell line through ROS generation and cell membrane damage. J. Photochem. Photobiol. B 2018, 186, 160–168. [Google Scholar] [CrossRef]
- Dubey, I.; Gilhotra, R.M.; Saluja, M.S. In-vitro antioxidant and anticancer activity of Madhuca longifolia. Int. J. Emerg. Techno. Innov. Res. 2019, 6, 1302–1313. [Google Scholar]
- Ernst, M.; Giubellino, A. The current state of treatment and future directions in cutaneous malignant melanoma. Biomedicines 2022, 10, 822. [Google Scholar] [CrossRef]
- Palathinkal, D.M.; Sharma, T.R.; Koon, H.B.; Bordeaux, J.S. Current systemic therapies for melanoma. Dermatol. Surg. 2014, 40, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Amini, E.; Nikdel, N.; Salek-Abdollahi, F. The cytotoxicity of dacarbazine potentiated by sea cucumber saponin in resistant B16F10 melanoma cells through apoptosis induction. Avicenna J. Med. Biotechnol. 2016, 8, 112–119. [Google Scholar] [PubMed]
- Karakaya, G.; Ercan, A.; Oncul, S.; Aytemir, M.D. Synthesis and cytotoxic evaluation of kojic acid derivatives with inhibitory activity on melanogenesis in human melanoma cells. Anticancer Agents MedChem. 2018, 18, 2137–2148. [Google Scholar] [CrossRef]
- Maldini, M.; Montoro, P.; Piacente, S.; Pizza, C. Phenolic compounds from Bursera simaruba Sarg. bark: Phytochemical investigation and quantitative analysis by tandem mass spectrometry. Phytochemistry 2009, 70, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Tanaka, M.; Arihara, S.; Pal, B.C.; Roy, S.K.; Matsumura, E.; Katayama, S. New oleanene triterpenoid saponins from Madhuca longifolia. J. Nat. Prod. 2000, 63, 1679–1681. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMSn profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Romanczyk, L.J.; Rodriguez, C.A.; Kwik-Uribe, C.; Keen, C.L. Cytotoxic effects of digalloyl dimer procyanidins in human cancer cell lines. J. Nutr. Biochem. 2008, 19, 797–808. [Google Scholar] [CrossRef]
- Hah, Y.S.; Kim, J.G.; Cho, H.Y.; Park, J.S.; Heo, E.P.; Yoon, T.J. Procyanidins from Vitis vinifera seeds induce apoptotic and autophagic cell death via generation of reactive oxygen species in squamous cell carcinoma cells. Oncol. Lett. 2017, 14, 1925–1932. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Murata, K.; Fujimura, Y.; Tachibana, H. Procyanidin C1 inhibits melanoma cell growth by activating 67-kDa laminin receptor signaling. Mol. Nutr. Food Res. 2020, 64, e1900986. [Google Scholar] [CrossRef]
- Sun, Y.S.; Wang, Z.W.; Gao, Z.; Zhao, W.; Thakur, K.; Zhong, Q.; Wei, Z.J. Proanthocyanidin oligomers extract from hawthorn mediates cell cycle arrest, apoptosis, and lysosome vacuolation on HCT116 cells. Curr. Res. Food Sci. 2022, 5, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal medicine: An introduction to its history, usage, regulation, current trends, and research needs. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Wachtel-Galor, S., Benzie, I.F.F., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 1. [Google Scholar]
- Bhaumik, A.; Kumar, M.U.; Khan, K.A.; Srinivas, C. The bioactive compounds obtained from the fruit-seeds of Madhuca longifolia (L) act as potential anticancer agents. Sch. J. App. Med. Sci. 2014, 2, 1235–1238. [Google Scholar]
- Yadav, S.; Sharma, M.; Ganesh, N.; Srivastava, S.; Srivastava, M.M. Anti-melanoma bio-efficacy of the plant Madhuca longifolia and its enhancement using bioactive principle loaded gold nanoparticle. Asian J. Pharm. Clin. Res. 2020, 13, 142–149. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Gulino, G.R.; Volante, M.; Ghigo, D.; Kopecka, J. Two repeated low doses of doxorubicin are more effective than a single high dose against tumors overexpressing P-glycoprotein. Cancer Lett. 2015, 360, 219–226. [Google Scholar] [CrossRef]
- Juhasz, I.; Albelda, S.M.; Elder, D.E.; Murphy, G.F.; Adachi, K.; Herlyn, D.; Valyi-Nagy, I.T.; Herlyn, M. Growth and invasion of human melanomas in human skin grafted to immunodeficient mice. Am. J. Pathol. 1993, 143, 528–537. [Google Scholar]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]



| Cell Line | Concentration (μg/mL) | Ratio | Combination Index (CI) | |
|---|---|---|---|---|
| MLE | Dacarbazine | |||
| 0.05 | 1 | 1:20 | 0.976 | |
| 1205-Lu | 0.10 | 1 | 1:10 | 0.654 |
| 0.50 | 1 | 1:2 | 0.892 | |
| 5.00 | 1 | 5:1 | 1.345 | |
| 0.05 | 1 | 1:20 | 0.954 | |
| Me45 | 0.10 | 1 | 1:10 | 0.695 |
| 0.50 | 1 | 1:2 | 0.912 | |
| 5.00 | 1 | 5:1 | 1.327 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Środa-Pomianek, K.; Barycka, A.; Gleńsk, M.; Rajbhandari, M.; Skonieczna, M.; Palko-Łabuz, A.; Wesołowska, O. Pretreatment of Melanoma Cells with Aqueous Ethanol Extract from Madhuca longifolia Bark Strongly Potentiates the Activity of a Low Dose of Dacarbazine. Int. J. Mol. Sci. 2024, 25, 7220. https://doi.org/10.3390/ijms25137220
Środa-Pomianek K, Barycka A, Gleńsk M, Rajbhandari M, Skonieczna M, Palko-Łabuz A, Wesołowska O. Pretreatment of Melanoma Cells with Aqueous Ethanol Extract from Madhuca longifolia Bark Strongly Potentiates the Activity of a Low Dose of Dacarbazine. International Journal of Molecular Sciences. 2024; 25(13):7220. https://doi.org/10.3390/ijms25137220
Chicago/Turabian StyleŚroda-Pomianek, Kamila, Anna Barycka, Michał Gleńsk, Meena Rajbhandari, Magdalena Skonieczna, Anna Palko-Łabuz, and Olga Wesołowska. 2024. "Pretreatment of Melanoma Cells with Aqueous Ethanol Extract from Madhuca longifolia Bark Strongly Potentiates the Activity of a Low Dose of Dacarbazine" International Journal of Molecular Sciences 25, no. 13: 7220. https://doi.org/10.3390/ijms25137220
APA StyleŚroda-Pomianek, K., Barycka, A., Gleńsk, M., Rajbhandari, M., Skonieczna, M., Palko-Łabuz, A., & Wesołowska, O. (2024). Pretreatment of Melanoma Cells with Aqueous Ethanol Extract from Madhuca longifolia Bark Strongly Potentiates the Activity of a Low Dose of Dacarbazine. International Journal of Molecular Sciences, 25(13), 7220. https://doi.org/10.3390/ijms25137220






