Genome-Wide Identification of microRNAs Associated with Starch Biosynthesis and Endosperm Development in Foxtail Millet
Abstract
1. Introduction
2. Results
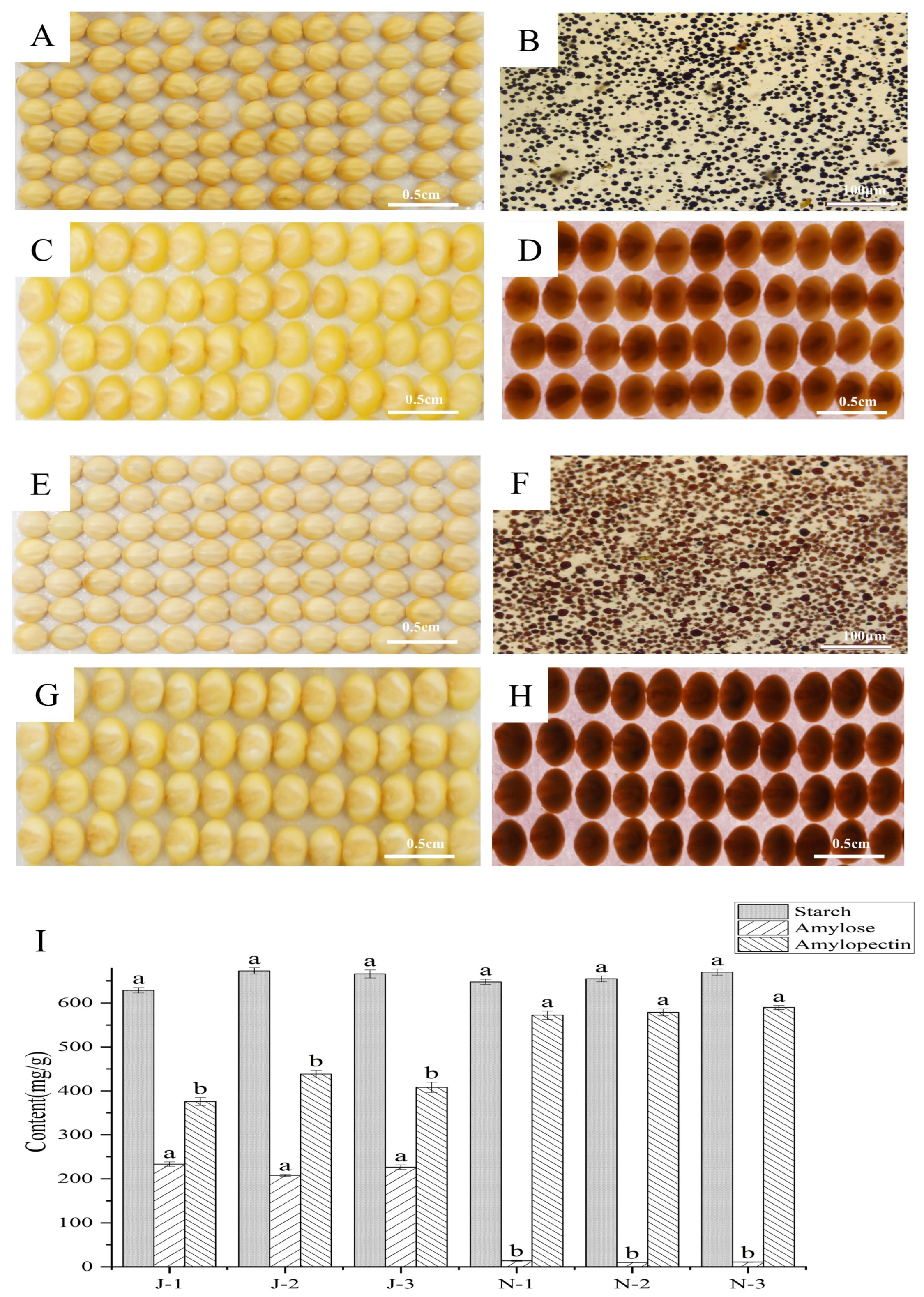
2.1. Phenotyping and Amylose Determination
2.2. Morphological Characteristics of Starch
2.3. XRD Patterns and Pasting Properties of Starch
2.4. Summary of Transcriptome Data during Grain Filling in Foxtail Millet
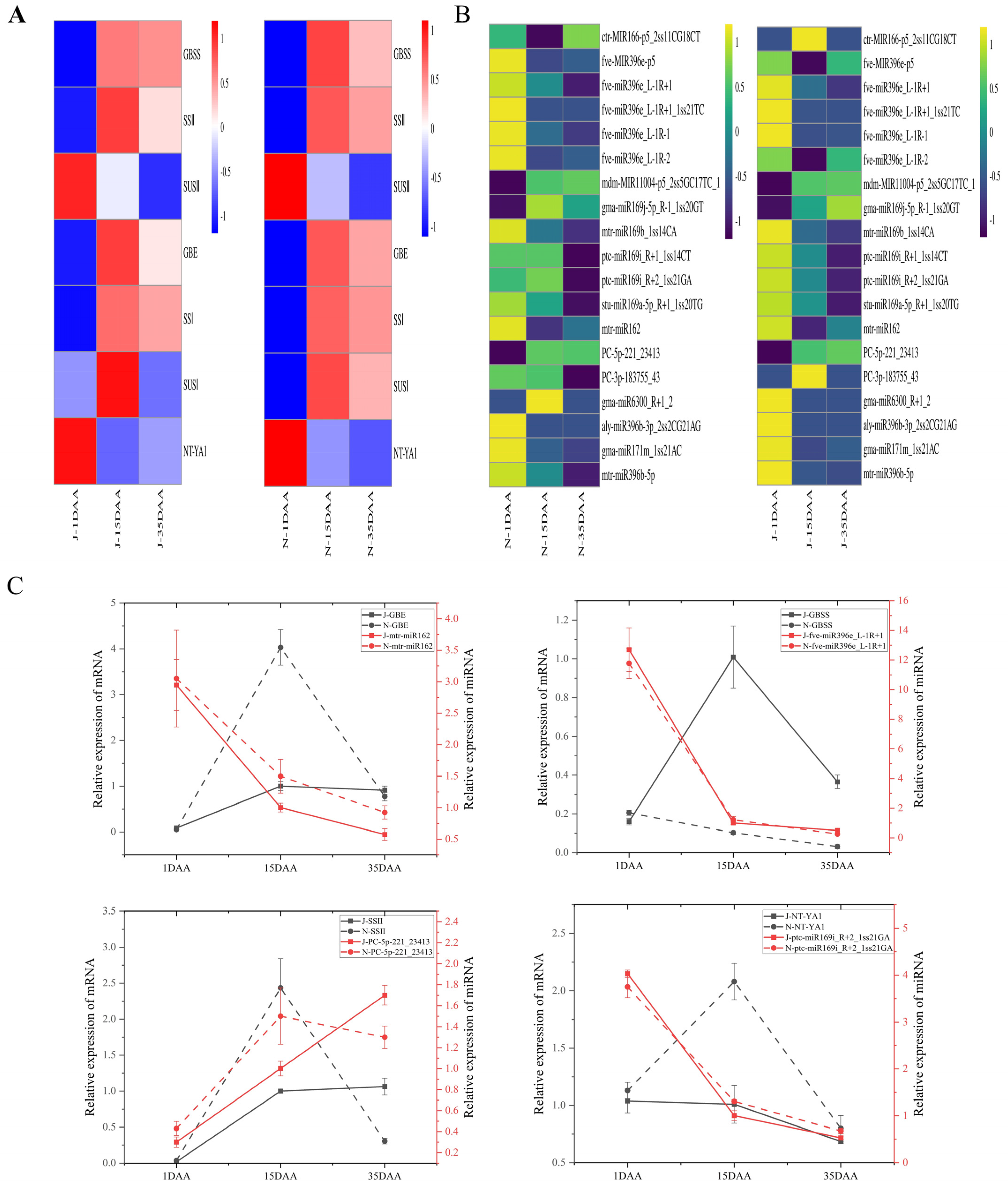
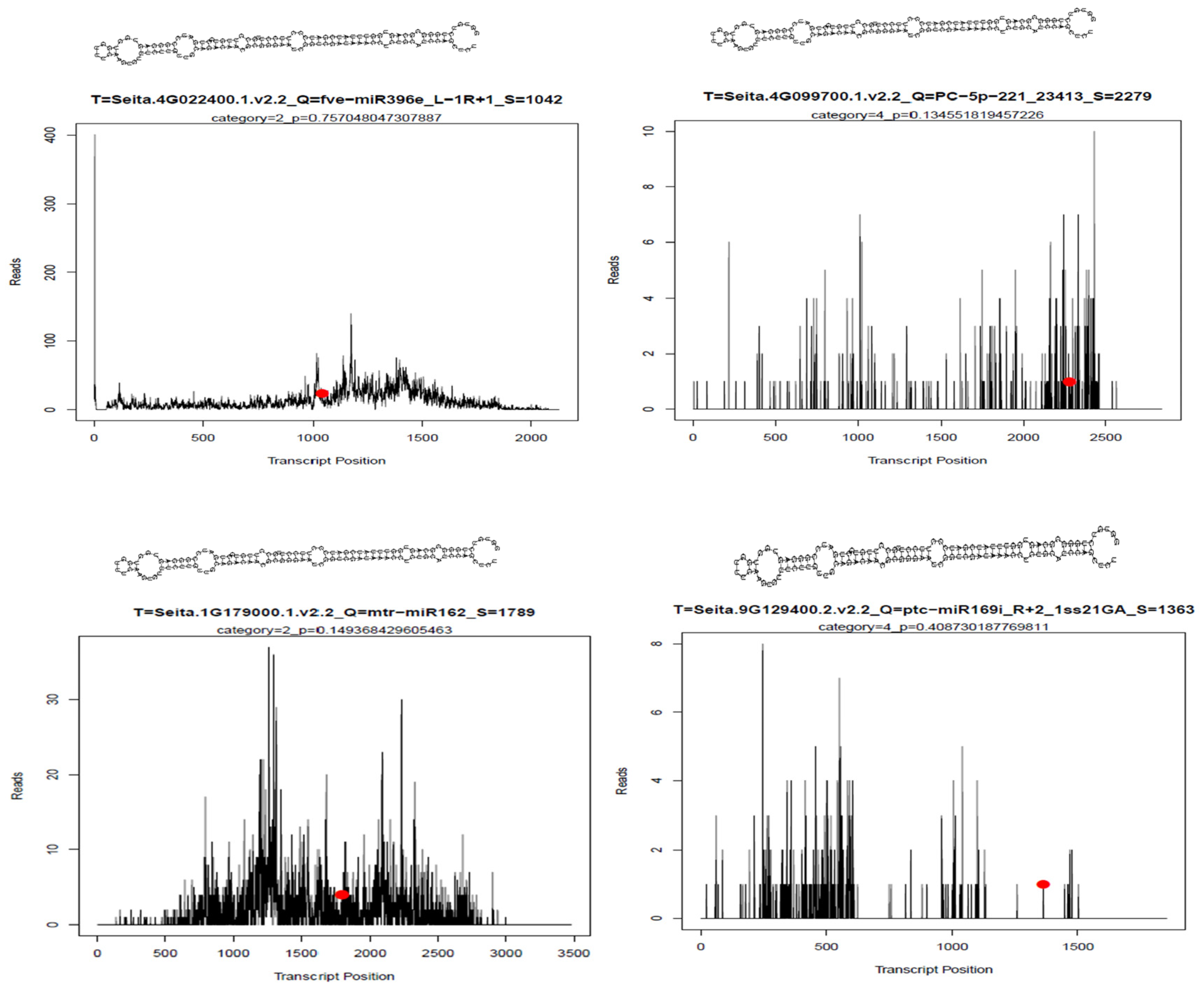
2.5. Identified Differential Expression of miRNAs in Developing Endosperm
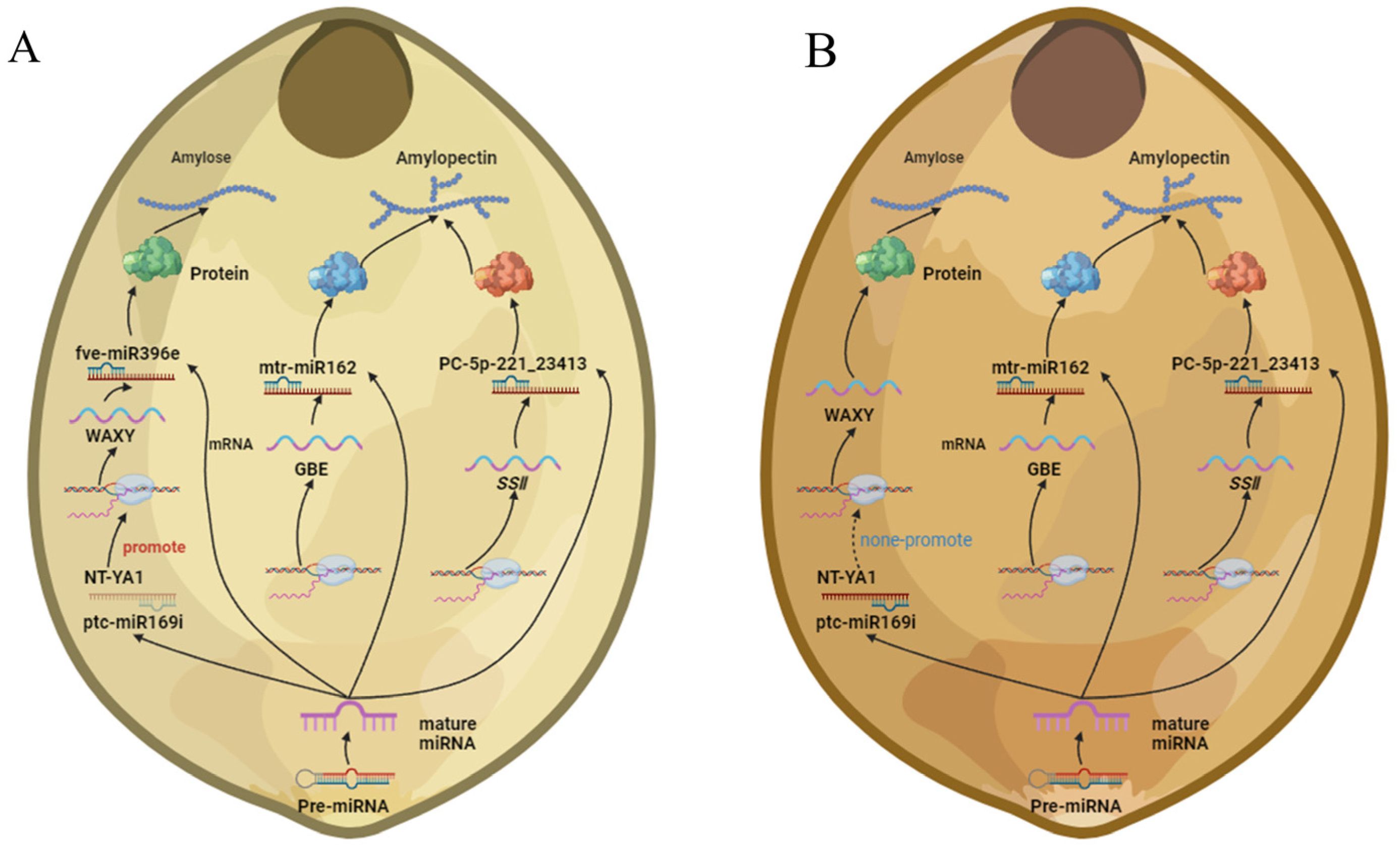
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phenotypic Evaluation
4.3. Determination of Starch Crystal Structure, Morphological Characteristics and Pasting Properties
4.4. Transcriptome and Small RNA Library Construction and Sequencing
4.5. Quantitative Real-Time PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Wan, Z.; Perry, L.; Lu, H.; Wang, Q.; Zhao, C.; Li, J.; Xie, F.; Yu, J.; Cui, T.; et al. Early millet use in northern China. Proc. Natl. Acad. Sci. USA 2012, 109, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, J.; Liu, K.; Wu, N.; Li, Y.; Zhou, K.; Ye, M.; Zhang, T.; Zhang, H.; Yang, X.; et al. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Lata, C.; Gupta, S.; Prasad, M. Foxtail millet: A model crop for genetic and genomic studies in bioenergy grasses. Crit. Rev. Biotechnol. 2013, 33, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, N.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. Insights into structure-function relationships of starch from foxtail millet cultivars grown in China. Int. J. Biol. Macromol. 2020, 155, 1176–1183. [Google Scholar] [CrossRef]
- Nakayama, H.; Afzal, M.; Okuno, K. Intraspecific differentiation and geographical distribution of Wx alleles for low amylose content in endosperm of foxtail millet, Setaria italica (L.) Beauv. Euphytica 1998, 102, 289–293. [Google Scholar] [CrossRef]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W.; et al. Accelerated ex situ breeding of GBSS- and PTST1-edited cassava for modified starch. Sci. Adv. 2018, 4, eaat6086. [Google Scholar] [CrossRef]
- Huang, L.; Sreenivasulu, N.; Liu, Q. Waxy editing: Old meets new. Trends Plant Sci. 2020, 25, 963–966. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Cai, L.; Ge, B.; Xu, S.; Chen, X.; Yang, H. Up-regulation of circARF3 reduces blood-brain barrier damage in rat subarachnoid hemorrhage model via miR-31-5p/MyD88/NF-κB axis. Aging-US 2021, 13, 21345–21363. [Google Scholar] [CrossRef]
- John, E.; Wienecke-Baldacchino, A.; Liivrand, M.; Heinäniemi, M.; Carlberg, C.; Sinkkonen, L. Dataset integration identifies transcriptional regulation of microRNA genes by PPARγ in differentiating mouse 3T3-L1 adipocytes. Nucleic Acids Res. 2012, 40, 4446–4460. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Weng, J.; Liu, H.; Yu, G.; Liu, Y.; Xiao, Q.; Huang, H.; Wang, Y.; Wei, B.; et al. Coordinated regulation of starch synthesis in maize endosperm by microRNAs and DNA methylation. Plant J. 2021, 105, 108–123. [Google Scholar] [CrossRef]
- Feng, M.; Lu, M.; Long, J.; Yin, Z.; Jiang, N.; Wang, P.; Liu, Y.; Guo, W.; Wu, X. miR156 regulates somatic embryogenesis by modulating starch accumulation in citrus. J. Exp. Bot. 2022, 73, 6170–6185. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, S.; Deng, K.; Wu, P.; Feng, K.; Li, L. Integrated mRNA and Small RNA Sequencing Reveals a microRNA Regulatory Network Associated with Starch Biosynthesis in Lotus (Nelumbo nucifera Gaertn.) Rhizomes. Int. J. Mol. Sci. 2022, 9, 7605. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Sun, H.; Du, Y.; Zhang, J.; Li, J.; Liu, Y.; Zhao, Y.; Zhao, Q. Characterization and expression patterns of microRNAs involved in rice grain filling. PLoS ONE. 2013, 8, e54148. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, N.; Kang, F.; Wang, J.; Wang, X. Cultivar replacement increases water use efficiency in foxtail millet in Shaanxi Province, China. Plant Physiol. Biochem. 2021, 164, 73–81. [Google Scholar] [CrossRef]
- Mahajan, P.; Bera, M.B.; Panesar, P.S.; Chauhan, A. Millet starch: A review. Int. J. Biol. Macromol. 2021, 180, 61–79. [Google Scholar] [CrossRef]
- Xing, B.; Yang, X.; Zou, L.; Liu, J.; Liang, Y.; Li, M.; Zhang, Z.; Wang, N.; Ren, G.; Zhang, L.; et al. Starch chain-length distributions determine cooked foxtail millet texture and starch physicochemical properties. Carbohydr. Polym. 2023, 320, 121240. [Google Scholar] [CrossRef]
- Gong, D.; Xu, X.; Wu, L.; Dai, G.; Zheng, W.; Xu, Z. Effect of biochar on rice starch properties and starch-related gene expression and enzyme activities. Sci. Rep. 2020, 10, 16917. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, Y.; Hao, C.; Li, T.; Liu, H.; Zhang, X. Starch metabolism in wheat: Gene variation and association analysis reveal additive effects on kernel weight. Front. Plant Sci. 2020, 11, 562008. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Kawagoe, Y. Septum formation in amyloplasts produces compound granules in the rice endosperm and is regulated by plastid division proteins. Plant Cell Physiol. 2010, 51, 1469–1479. [Google Scholar] [CrossRef]
- Sattler, S.E.; Singh, J.; Haas, E.J.; Guo, L.; Sarath, G.; Pedersen, J.F. Two distinct waxy alleles impact the granule-bound starch synthase in sorghum. Mol. Breed. 2009, 24, 349. [Google Scholar] [CrossRef]
- Matsushima, R.; Maekawa, M.; Kusano, M.; Kondo, H.; Fujita, N.; Kawagoe, Y.; Sakamoto, W. Amyloplast-localized substandard starch grain 4 protein influences the size of starch grains in rice endosperm. Plant Physiol. 2014, 164, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet grains: Nutritional quality, processing, and potential health benefits. Compr. Rev. Food. Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.; Xu, B. High-amylose maize starch: Structure, properties, modifications and industrial applications. Carbohydr. Polym. 2023, 1, 120185. [Google Scholar] [CrossRef]
- Adegoke, T.V.; Wang, Y.; Chen, L.; Wang, H.; Liu, W.; Liu, X.; Cheng, Y.C.; Tong, X.; Ying, J.; Zhang, J. Posttranslational modification of waxy to genetically improve starch quality in rice grain. Int. J. Mol. Sci. 2021, 22, 4845. [Google Scholar] [CrossRef]
- Guzmán, C.; Alvarez, J.B. Wheat waxy proteins: Polymorphism, molecular characterization and effects on starch properties. Theor. Appl. Genet. 2016, 129, 1–16. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.; Chen, X. The ‘how’ and ‘where’ of plant microRNAs. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, S.; Zhao, J.; Xiong, H.; An, P.; Wang, J.; Zhang, H.; Zhang, L. Identification of miRNAs and their target genes in Larix olgensis and verified of differential expression miRNAs. BMC Plant Biol. 2019, 19, 247. [Google Scholar] [CrossRef]
- Samad, A.F.A.; Sajad, M.; Nazaruddin, N.; Fauzi, I.A.; Murad, A.M.A.; Zainal, Z.; Ismail, I. MicroRNA and transcription factor: Key players in plant regulatory network. Front. Plant Sci. 2017, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, S.; Han, J.; Zhou, Y.; Liu, C.; Zhou, Z.; Wang, F.; Cheng, Z.; Zhang, J.; Hu, Y.; et al. Integrated transcriptome, small RNA, and degradome analysis reveals the complex network regulating starch biosynthesis in maize. BMC Genomics 2019, 20, 574. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Liu, J.; Ma, Q.; Wu, E.; Gao, J.; Yang, Q.; Feng, B. Transcriptome analysis reveals the mechanism of nitrogen fertilizers in starch synthesis and quality in waxy and non-waxy proso millet. Carbohydr. Polym. 2024, 323, 121372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yuan, Y.; Liu, J.; Han, M.; Li, J.; Jin, F.; Feng, B. Transcriptome analysis reveals new insights in the starch biosynthesis of non-waxy and waxy broomcorn millet (Panicum miliaceum L.). Int. J. Biol. Macromol. 2023, 230, 123155. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shi, N.; Fei, H.; Liu, Y.; Zhang, Y.; Li, Z.; Ruan, C.; Zhang, D. Physicochemical, structural, and digestive properties of pea starch obtained via ultrasonic-assisted alkali extraction. Ultrason. Sonochem. 2022, 89, 106136. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Q.; Liu, P.; Jia, W.; Chen, Z.; Xu, Z. Integration of mRNA and miRNA analysis reveals the molecular mechanism underlying salt and alkali stress tolerance in tobacco. Int. J. Mol. Sci. 2019, 20, 2319. [Google Scholar] [CrossRef]
- Seo, M.; Kim, K.; Yoon, J.; Jeong, J.Y.; Lee, H.J.; Cho, S.; Kim, H. RNA-seq analysis for detecting quantitative trait-associated genes. Sci. Rep. 2016, 6, 24375. [Google Scholar] [CrossRef]
- Hong, Y.; Ni, S.; Zhang, G. Transcriptome and metabolome analysis reveals regulatory networks and key genes controlling barley malting quality in responses to drought stress. Plant Physiol. Biochem. 2020, 152, 1–11. [Google Scholar] [CrossRef]
- Addo-Quaye, C.; Miller, W.; Axtell, M.J. CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 2009, 25, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, M.; Panigrahi, K.C.S.; Poli, Y.; Ranga, A.; Majeed, N. Integrated Expression Analysis of Small RNA, Degradome and Microarray Reveals Complex Regulatory Action of miRNA during Prolonged Shade in Swarnaprabha Rice. Biology 2022, 23, 798. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Gong, Z.; Zheng, J.; Li, Z.; Zhou, G.; Xu, Y.; Li, X. Genomic and epigenomic insights into the mechanism of cold response in upland cotton (Gossypium hirsutum). Plant Physiol. Biochem. 2023, 206, 108206. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, D.; Guo, S.; Yu, X. Genome-Wide Identification of microRNAs Associated with Starch Biosynthesis and Endosperm Development in Foxtail Millet. Int. J. Mol. Sci. 2024, 25, 9282. https://doi.org/10.3390/ijms25179282
Li Q, Li D, Guo S, Yu X. Genome-Wide Identification of microRNAs Associated with Starch Biosynthesis and Endosperm Development in Foxtail Millet. International Journal of Molecular Sciences. 2024; 25(17):9282. https://doi.org/10.3390/ijms25179282
Chicago/Turabian StyleLi, Qiang, Dongming Li, Shihua Guo, and Xiaofang Yu. 2024. "Genome-Wide Identification of microRNAs Associated with Starch Biosynthesis and Endosperm Development in Foxtail Millet" International Journal of Molecular Sciences 25, no. 17: 9282. https://doi.org/10.3390/ijms25179282
APA StyleLi, Q., Li, D., Guo, S., & Yu, X. (2024). Genome-Wide Identification of microRNAs Associated with Starch Biosynthesis and Endosperm Development in Foxtail Millet. International Journal of Molecular Sciences, 25(17), 9282. https://doi.org/10.3390/ijms25179282




