FABP5 Is a Possible Factor for the Maintenance of Functions of Human Non-Pigmented Ciliary Epithelium Cells
Abstract
1. Introduction
2. Results
2.1. Origin of FABP5 within the Intraocular Environment
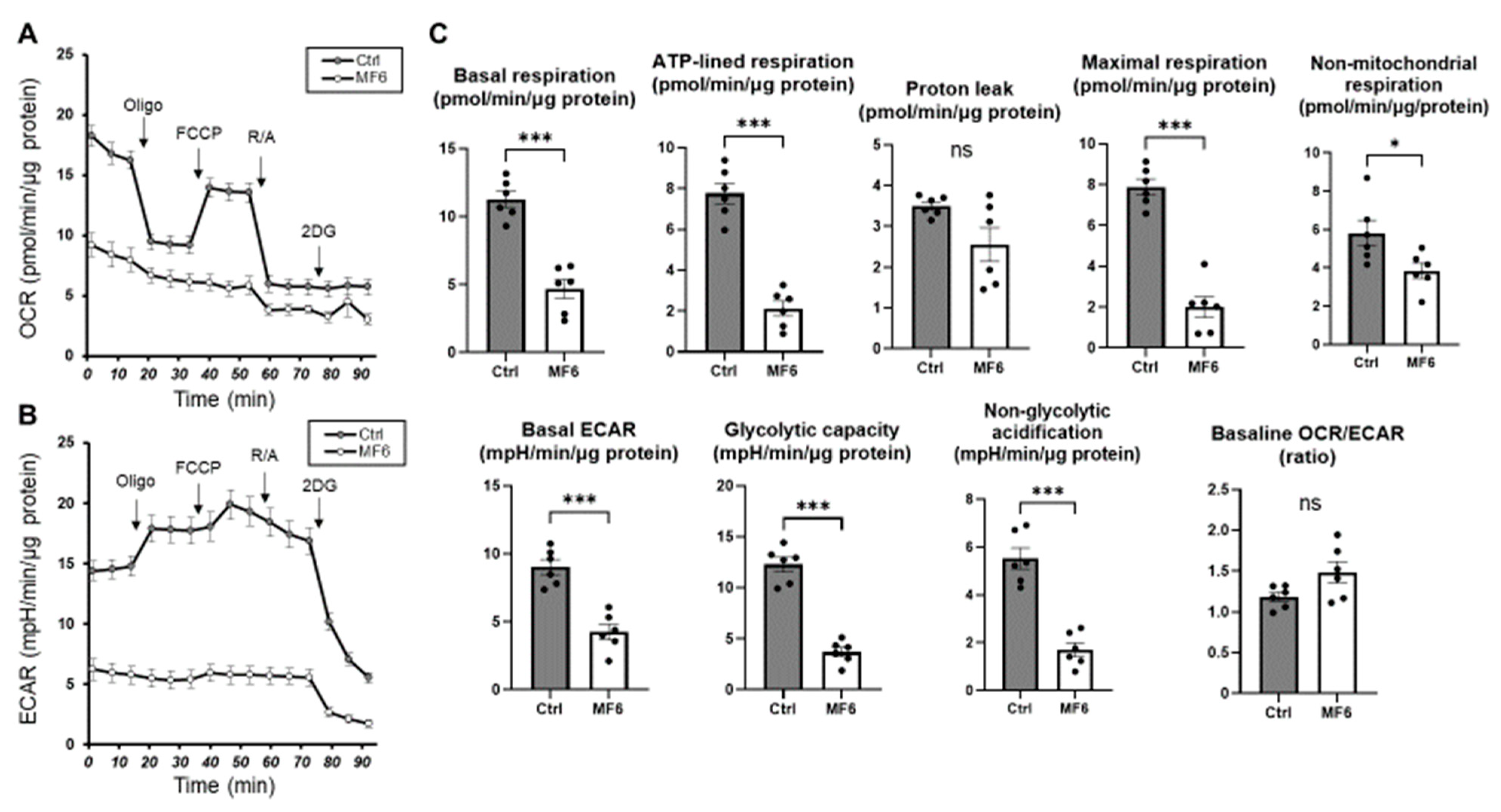
2.2. Cellular Metabolic Analysis to Elucidate Possible Biological Roles of FABP5 in HNPCE Cell Line
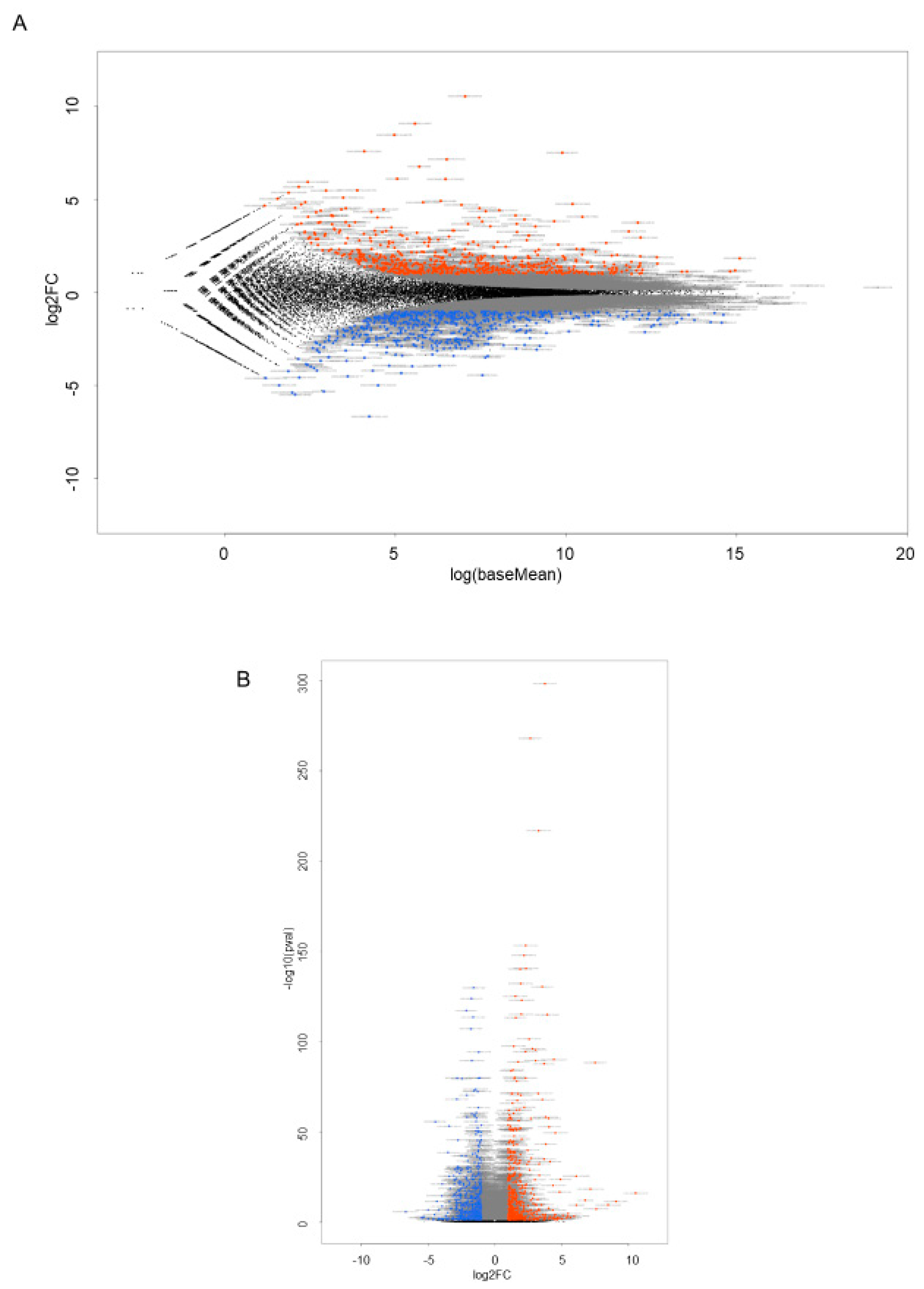
2.3. RNA Sequencing Analysis to Elucidate Underlying Mechanisms of FABP5-Related Physiological Roles in HNPCE Cell Line
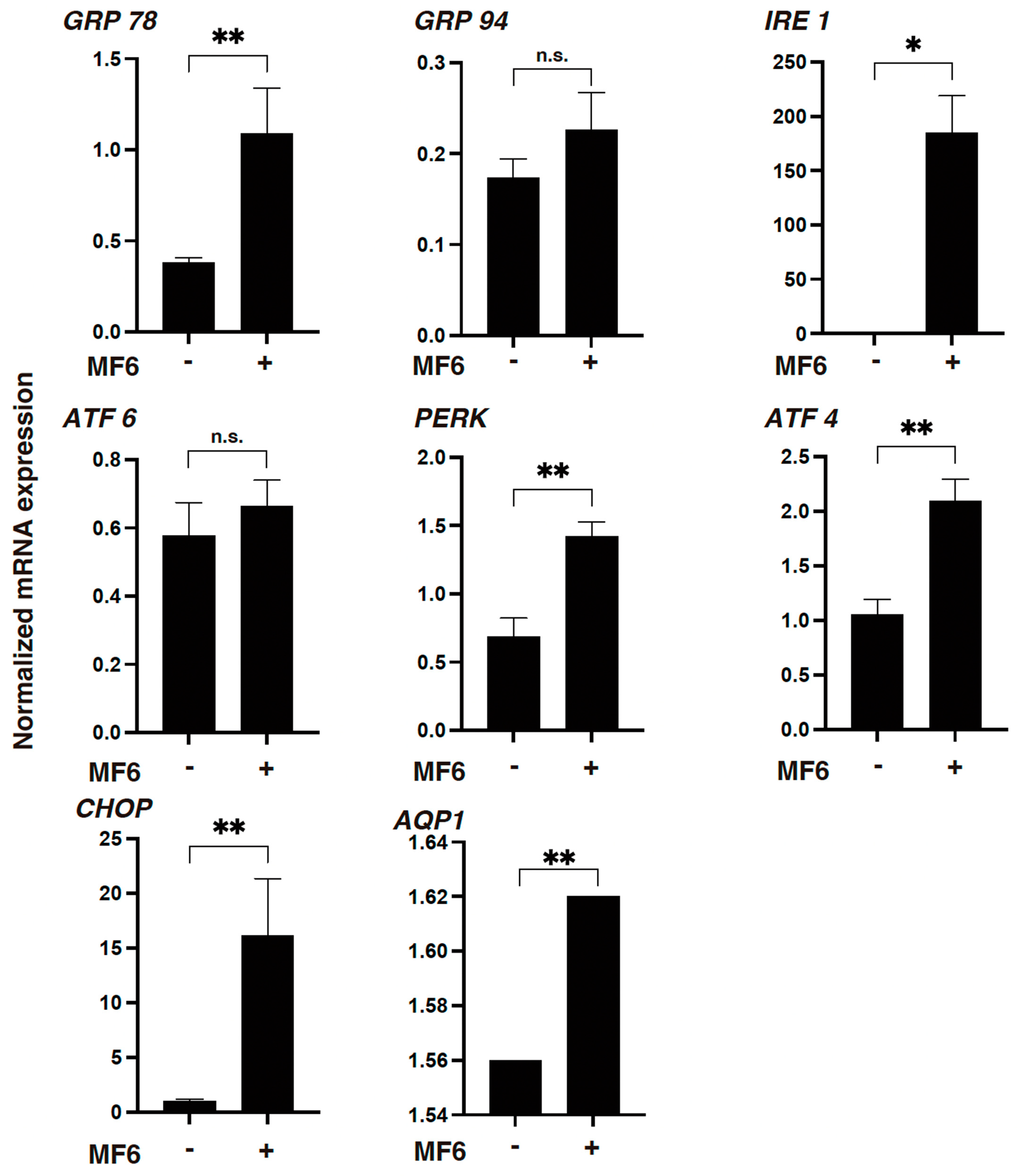
2.4. mRNA Expression of UPR-Related Factor and Aquaporin 1 (AQP1)
3. Discussion
4. Materials and Methods
4.1. Two-Dimensional (2D) Culture of HOCF Cells, HNPCE Cells, RB Cells and ARPE19 Cells
4.2. Seahorse Assay
4.3. RNA Sequencing Analysis of Gene Functions and Analysis of Pathways
4.4. Other Analytical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs--mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.M.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Plasma fatty acid binding protein 4 is associated with atherogenic dyslipidemia in diabetes. J. Lipid. Res. 2008, 49, 1746–1751. [Google Scholar] [CrossRef]
- Shi, M.; Ma, L.; Fu, P. Role of Fatty Acid Binding Protein 4 (FABP4) in Kidney Disease. Curr. Med. Chem. 2020, 27, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calvo, R.; Girona, J.; Alegret, J.M.; Bosquet, A.; Ibarretxe, D.; Masana, L. Role of the fatty acid-binding protein 4 in heart failure and cardiovascular disease. J. Endocrinol. 2017, 233, R173–R184. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, X. Therapeutic Implications of FABP4 in Cancer: An Emerging Target to Tackle Cancer. Front. Pharmacol. 2022, 13, 948610. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Song, P.; Xu, Y.; Zha, M.; Zhang, Y.; Rudan, I. Global epidemiology of retinal vein occlusion: A systematic review and meta-analysis of prevalence, incidence, and risk factors. J. Glob. Health 2019, 9, 010427. [Google Scholar] [CrossRef]
- Rogers, S.; McIntosh, R.L.; Cheung, N.; Lim, L.; Wang, J.J.; Mitchell, P.; Kowalski, J.W.; Nguyen, H.; Wong, T.Y. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010, 117, 313–319. [Google Scholar] [CrossRef]
- Umetsu, A.; Furuhashi, M.; Watanabe, M.; Ohkawa, E.; Tsugeno, Y.; Suzuki, S.; Itoh, K.; Ida, Y.; Hikage, F.; Ohguro, H. Fatty acid metabolism is involved in both retinal physiology and the pathology of retinal vascular diseases. Prostaglandins Leukot. Essent. Fat. Acids 2022, 183, 102473. [Google Scholar] [CrossRef]
- Ohguro, H.; Watanabe, M.; Sato, T.; Nishikiori, N.; Umetsu, A.; Higashide, M.; Ogawa, T.; Furuhashi, M. FABP4 Is an Indispensable Factor for Regulating Cellular Metabolic Functions of the Human Retinal Choroid. Bioengineering 2024, 11, 584. [Google Scholar] [CrossRef]
- Freddo, T.F. A contemporary concept of the blood-aqueous barrier. Prog. Retin. Eye Res. 2013, 32, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Delamere, N.A. Ciliary Body and Ciliary Epithelium. Adv. Organ Biol. 2005, 10, 127–148. [Google Scholar] [CrossRef] [PubMed]
- González-Marrero, I.; Hernández-Abad, L.G.; Carmona-Calero, E.M.; Castañeyra-Ruiz, L.; Abreu-Reyes, J.A.; Castañeyra-Perdomo, A. Systemic Hypertension Effects on the Ciliary Body and Iris. An Immunofluorescence Study with Aquaporin 1, Aquaporin 4, and Na⁺, K⁺ ATPase in Hypertensive Rats. Cells 2018, 7, 210. [Google Scholar] [CrossRef]
- Fernández-Vigo, J.I.; Kudsieh, B.; Shi, H.; De-Pablo-Gómez-de-Liaño, L.; Fernández-Vigo, J.; García-Feijóo, J. Diagnostic imaging of the ciliary body: Technologies, outcomes, and future perspectives. Eur. J. Ophthalmol. 2022, 32, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Raviola, G.; Raviola, E. Intercellular junctions in the ciliary epithelium. Investig. Ophthalmol. Vis. Sci. 1978, 17, 958–981. [Google Scholar]
- Coca-Prados, M.; Ghosh, S.; Gilula, N.B.; Kumar, N.M. Expression and cellular distribution of the alpha 1 gap junction gene product in the ocular pigmented ciliary epithelium. Curr. Eye Res. 1992, 11, 113–122. [Google Scholar] [CrossRef]
- Ghosh, S.; Hernando, N.; Martín-Alonso, J.M.; Martin-Vasallo, P.; Coca-Prados, M. Expression of multiple Na+,K(+)-ATPase genes reveals a gradient of isoforms along the nonpigmented ciliary epithelium: Functional implications in aqueous humor secretion. J. Cell. Physiol. 1991, 149, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Martin-Alonso, J.M.; Ghosh, S.; Hernando, N.; Crabb, J.W.; Coca-Prados, M. Differential expression of the cellular retinaldehyde-binding protein in bovine ciliary epithelium. Exp. Eye Res. 1993, 56, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Hernando, N.; Martín-Alonso, J.M.; Ghosh, S.; Coca-Prados, M. Isolation of a cDNA encoding a glutathione S-transferase (GST) class-pi from the bovine ocular ciliary epithelium. Exp. Eye Res. 1992, 55, 711–718. [Google Scholar] [CrossRef]
- Rowland, J.M.; Potter, D.E.; Reiter, R.J. Circadian rhythm in intraocular pressure: A rabbit model. Curr. Eye Res. 1981, 1, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Neuronal death in glaucoma. Prog. Retin. Eye Res. 1999, 18, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alonso, J.M.; Ghosh, S.; Coca-Prados, M. Cloning of the bovine plasma selenium-dependent glutathione peroxidase (GP) cDNA from the ocular ciliary epithelium: Expression of the plasma and cellular forms within the mammalian eye. J. Biochem. 1993, 114, 284–291. [Google Scholar] [CrossRef]
- Ortego, J.; Escribano, J.; Crabb, J.; Coca-Prados, M. Identification of a neuropeptide and neuropeptide-processing enzymes in aqueous humor confers neuroendocrine features to the human ocular ciliary epithelium. J. Neurochem. 1996, 66, 787–796. [Google Scholar] [CrossRef]
- Coca-Prados, M.; Escribano, J.; Ortego, J. Differential gene expression in the human ciliary epithelium. Prog. Retin. Eye Res. 1999, 18, 403–429. [Google Scholar] [CrossRef] [PubMed]
- Sellner, P. Fatty acid-binding protein from embryonic chick retina resembles mammalian heart fatty acid-binding protein. Investig. Ophthalmol. Vis. Sci. 1994, 35, 443–452. [Google Scholar]
- Adamson, J.; Morgan, E.A.; Beesley, C.; Mei, Y.; Foster, C.S.; Fujii, H.; Rudland, P.S.; Smith, P.H.; Ke, Y. High-level expression of cutaneous fatty acid-binding protein in prostatic carcinomas and its effect on tumorigenicity. Oncogene 2003, 22, 2739–2749. [Google Scholar] [CrossRef]
- Jing, C.; Beesley, C.; Foster, C.S.; Chen, H.; Rudland, P.S.; West, D.C.; Fujii, H.; Smith, P.H.; Ke, Y. Human cutaneous fatty acid-binding protein induces metastasis by up-regulating the expression of vascular endothelial growth factor gene in rat Rama 37 model cells. Cancer Res. 2001, 61, 4357–4364. [Google Scholar]
- Morgan, E.A.; Forootan, S.S.; Adamson, J.; Foster, C.S.; Fujii, H.; Igarashi, M.; Beesley, C.; Smith, P.H.; Ke, Y. Expression of cutaneous fatty acid-binding protein (C-FABP) in prostate cancer: Potential prognostic marker and target for tumourigenicity-suppression. Int. J. Oncol. 2008, 32, 767–775. [Google Scholar] [PubMed]
- Forootan, S.S.; Bao, Z.Z.; Forootan, F.S.; Kamalian, L.; Zhang, Y.; Bee, A.; Foster, C.S.; Ke, Y. Atelocollagen-delivered siRNA targeting the FABP5 gene as an experimental therapy for prostate cancer in mouse xenografts. Int. J. Oncol. 2010, 36, 69–76. [Google Scholar] [PubMed]
- Teresa Borrello, M.; Rita Emma, M.; Listi, A.; Rubis, M.; Coslet, S.; Augello, G.; Cusimano, A.; Cabibi, D.; Porcasi, R.; Giannitrapani, L.; et al. NUPR1 protects liver from lipotoxic injury by improving the endoplasmic reticulum stress response. Faseb. J. 2021, 35, e21395. [Google Scholar] [CrossRef] [PubMed]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- López, I.; Tournillon, A.S.; Nylander, K.; Fåhraeus, R. p53-mediated control of gene expression via mRNA translation during Endoplasmic Reticulum stress. Cell Cycle (Georget. Tex.) 2015, 14, 3373–3378. [Google Scholar] [CrossRef]
- Kobiita, A.; Silva, P.N.; Schmid, M.W.; Stoffel, M. FoxM1 coordinates cell division, protein synthesis, and mitochondrial activity in a subset of β cells during acute metabolic stress. Cell Rep. 2023, 42, 112986. [Google Scholar] [CrossRef]
- Duvigneau, J.C.; Luís, A.; Gorman, A.M.; Samali, A.; Kaltenecker, D.; Moriggl, R.; Kozlov, A.V. Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine 2019, 124, 154577. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ma, Y.; Tong, X.; Yang, W.; Dai, Y.; Pan, Y.; Ren, P.; Liu, L.; Fan, H.Y.; Zhang, Y.; et al. Metformin inhibits testosterone-induced endoplasmic reticulum stress in ovarian granulosa cells via inactivation of p38 MAPK. Hum. Reprod. (Oxf. Engl.) 2020, 35, 1145–1158. [Google Scholar] [CrossRef]
- Borsu, L.; Presse, F.; Nahon, J.L. The AROM gene, spliced mRNAs encoding new DNA/RNA-binding proteins are transcribed from the opposite strand of the melanin-concentrating hormone gene in mammals. J. Biol. Chem. 2000, 275, 40576–40587. [Google Scholar] [CrossRef]
- Furuhashi, M.; Sakuma, I.; Morimoto, T.; Higashiura, Y.; Sakai, A.; Matsumoto, M.; Sakuma, M.; Shimabukuro, M.; Nomiyama, T.; Arasaki, O.; et al. Independent and Distinct Associations of FABP4 and FABP5 with Metabolic Parameters in Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 575557. [Google Scholar] [CrossRef] [PubMed]
- Higashide, M.; Furuhashi, M.; Watanabe, M.; Itoh, K.; Suzuki, S.; Umetsu, A.; Tsugeno, Y.; Ida, Y.; Hikage, F.; Ohguro, H. Fatty Acid-Binding Proteins 4 and 5 Are Involved in the Pathogenesis of Retinal Vascular Diseases in Different Manners. Life 2022, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, A.; Tanaka, M.; Sato, T.; Akiyama, Y.; Endo, K.; Mori, K.; Ohnishi, H.; Watanabe, M.; Ohguro, H.; Hanawa, N.; et al. High Intraocular Pressure Is Independently Associated with New-Onset Systemic Hypertension over a 10-Year Period. Circ. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Erbay, E.; Babaev, V.R.; Mayers, J.R.; Makowski, L.; Charles, K.N.; Snitow, M.E.; Fazio, S.; Wiest, M.M.; Watkins, S.M.; Linton, M.F.; et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009, 15, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Higashide, M.; Furuhashi, M.; Ohguro, H. TGF-β-3 Induces Different Effects from TGF-β-1 and -2 on Cellular Metabolism and the Spatial Properties of the Human Trabecular Meshwork Cells. Int. J. Mol. Sci. 2023, 24, 4181. [Google Scholar] [CrossRef]
- Ichioka, H.; Hirohashi, Y.; Sato, T.; Furuhashi, M.; Watanabe, M.; Ida, Y.; Hikage, F.; Torigoe, T.; Ohguro, H. G-Protein-Coupled Receptors Mediate Modulations of Cell Viability and Drug Sensitivity by Aberrantly Expressed Recoverin 3 within A549 Cells. Int. J. Mol. Sci. 2023, 24, 771. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinform. (Oxf. Engl.) 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, K.; Zhang, S.; Yuan, J.; Liao, X.; Wu, C.; Zou, Y.; Ha, Y.; Shen, Z.; Guo, J.; et al. Ingenuity pathway analysis of differentially expressed genes involved in signaling pathways and molecular networks in RhoE gene-edited cardiomyocytes. Int. J. Mol. Med. 2020, 46, 1225–1238. [Google Scholar] [CrossRef]
- Alimadadi, A.; Aryal, S.; Manandhar, I.; Joe, B.; Cheng, X. Identification of Upstream Transcriptional Regulators of Ischemic Cardiomyopathy Using Cardiac RNA-Seq Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3472. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]


| Up-Regulation | Down-Regulation | ||
|---|---|---|---|
| Molecules | p-Value | Molecules | p-Value |
| DUSP15 | 10.544 | SCEL-AS1 | −6.667 |
| LAMP3 | 9.076 | INHBB | −5.489 |
| KLHDC7B | 8.471 | LOC124902532 | −5.380 |
| SLC30A2 | 7.591 | AC1230231 | −5.323 |
| NGFR | 7.513 | FAM111B | −4.993 |
| NGFR-AS1 | 7.156 | IGLON5 | −4.992 |
| RP11_81K22 | 6.769 | RP5_1028K72 | −4.602 |
| CTD_3118D112 | 6.112 | ANXA8/ANXA8L1 | −4.571 |
| ATP6V0D2 | 6.097 | MT-TT | −4.505 |
| LOC105369568 | 5.942 | SCEL | −4.448 |
| Name | p-Value |
|---|---|
| Cell Cycle Checkpoints | 3.15 × 10−23 |
| Kinetochore Metaphase Signaling Pathway | 1.32 × 10−21 |
| Mitotic Prometaphase | 9.90 × 10−21 |
| Mitotic Metaphase and Anaphase | 1.63 × 10−20 |
| RHO GTPase Activate Formins | 6.27 × 10−20 |
| Name | p-Value Range |
|---|---|
| Cellular Development | 3.00 × 10−6–2.31 × 10−30 |
| Cellular Growth and Proliferation | 3.00 × 10−6–2.31 × 10−30 |
| Cell Cycle | 2.58 × 10−6–5.10 × 10−30 |
| Cellular Assembly and Organization | 2.79 × 10−6–9.10 × 10−29 |
| DNA Replication, Recombination, and Repair | 2.38 × 10−6–9.10 × 10−29 |
| Name | p-Value Range |
|---|---|
| Organismal Survival | 2.71 × 10−7–7.48 × 10−37 |
| Cardiovascular System Development and Function | 2.60 × 10−6–4.03 × 10−21 |
| Organismal Development | 3.00 × 10−6–4.03 × 10−21 |
| Tissue Morphology | 2.60 × 10−6–4.44 × 10−20 |
| Connective Tissue Development and Function | 1.68 × 10−6–2.28 × 10−13 |
| Upstream Regulator | Expr Log/Ratio | Molecule Type | Activation z-Score |
|---|---|---|---|
| NUPR1 | 4.115 | transcription regulator | 6.199 |
| ATF4 | 1.570 | transcription regulator | 5.362 |
| CDKN1A | 1.551 | kinase | 3.367 |
| FOXM1 | −1.823 | transcription regulator | −5.520 |
| PCLAF | −2.458 | other | −4.266 |
| IL6 | 1.557 | cytokine | 1.576 |
| AREG | 1.215 | growth factor | −2.557 |
| Master Regulator | Expr Log/Ratio | Molecule Type | Activation z-Score |
|---|---|---|---|
| NUPR1 | 4.115 | transcription regulator | 6.353 |
| HEY1 | 1.093 | transcription regulator | 4.932 |
| PARPBP | −1.108 | other | −5.647 |
| CKAP2L | −2.625 | other | −6.245 |
| Associated Network Functions | Score |
|---|---|
| Cellular, Movement, Reproductive System Development and Function, Nutritional Disease | 46 |
| Cancer, Hematological Disease, Immunological Disease | 39 |
| Amino Acid Metabolism, Small Molecule Biochemistry, Molecular Transeport | 39 |
| Cell Morphology, Cell Cycle, Cellular Assembly and Organization | 37 |
| Lipid Metabolism, Small Molecule Biochemistry, Post-Translational Modification | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashide, M.; Watanabe, M.; Sato, T.; Umetsu, A.; Nishikiori, N.; Ogawa, T.; Furuhashi, M.; Ohguro, H. FABP5 Is a Possible Factor for the Maintenance of Functions of Human Non-Pigmented Ciliary Epithelium Cells. Int. J. Mol. Sci. 2024, 25, 9285. https://doi.org/10.3390/ijms25179285
Higashide M, Watanabe M, Sato T, Umetsu A, Nishikiori N, Ogawa T, Furuhashi M, Ohguro H. FABP5 Is a Possible Factor for the Maintenance of Functions of Human Non-Pigmented Ciliary Epithelium Cells. International Journal of Molecular Sciences. 2024; 25(17):9285. https://doi.org/10.3390/ijms25179285
Chicago/Turabian StyleHigashide, Megumi, Megumi Watanabe, Tatsuya Sato, Araya Umetsu, Nami Nishikiori, Toshifumi Ogawa, Masato Furuhashi, and Hiroshi Ohguro. 2024. "FABP5 Is a Possible Factor for the Maintenance of Functions of Human Non-Pigmented Ciliary Epithelium Cells" International Journal of Molecular Sciences 25, no. 17: 9285. https://doi.org/10.3390/ijms25179285
APA StyleHigashide, M., Watanabe, M., Sato, T., Umetsu, A., Nishikiori, N., Ogawa, T., Furuhashi, M., & Ohguro, H. (2024). FABP5 Is a Possible Factor for the Maintenance of Functions of Human Non-Pigmented Ciliary Epithelium Cells. International Journal of Molecular Sciences, 25(17), 9285. https://doi.org/10.3390/ijms25179285








